Abstract
Objective
To observe the changes in blood pressure (BP) over 10 years and to investigate current BP association to serum uric acid (SUA) levels and cardiovascular risk factors in the epidemiological data of a target group of patients with prehypertension in 2007.
Design
Cross-sectional study.
Setting
Mlati Subdistrict, Sleman District, Yogyakarta Province, Indonesia.
Participants
A total of 733 patients from ‘Mlati Study Database’ in 2007 were selected by simple random sampling using statistical software. Subjects had both physical and laboratory examinations.
Outcome measures
Morning home BP and laboratory examination of urine (uric acid excretion and creatinine) and blood samples (SUA, blood urea nitrogen, creatinine, a lipid profile and fasting blood glucose levels).
Results
About 31.1% of 733 subjects with prehypertension became hypertensive after 10 years, 24.6% returned to normal tension and the rest of it remained in prehypertensive state. Mean (SD) of SUA levels in 2017 was significantly higher in men than in women (5.78 (1.25) mg/dL vs 4.52 (1.10) mg/dL, p<0.001). Furthermore, men tended to have high-normal (5–7 mg/dL) or high SUA levels (≥7 mg/dL) compared with women (p<0.001, Relative Risk (RR)=2.60). High-normal and high SUA levels in population with a history of prehypertension were significantly associated with current prehypertension and hypertension only in women (p=0.001, RR=1.21). Age and body mass index was found to be significantly associated with both systolic and diastolic BP in men, but only with systolic BP in women. Fasting blood glucose and SUA levels were significantly associated with systolic and diastolic BP only in women.
Conclusion
We concluded that after 10 years, of 733 subjects with prehypertension, 31.1% became hypertensive. The SUA levels in men are significantly higher than those in women. Moreover, high-normal and high SUA levels were significantly associated with prehypertension and hypertension in women but not in men.
Keywords: hypertension, public health, epidemiology
Strengths and limitations of this study.
This study followed up the changes in blood pressure on subjects for over 10 years.
The associations between serum uric acid, blood pressure and cardiovascular risk factors were analysed based on gender.
The analysis was also performed by using both Seventh Report of Joint National Committee and 2017 American College of Cardiology/American Heart Association guidelines.
This study could not present the changes of all measured values over a 10-year period because, in the prior study in 2007, these laboratory values were not examined, except for blood pressure.
Introduction
Hypertension is still a major problem worldwide, as reflected by a meta-analysis report in 2016 stating that in 2010, 31.1% of the world’s population was hypertension.1 In Indonesia, the prevalence of hypertension in 2013 was 25.8%, based on the Indonesian Ministry of Health report.2 Therefore, it is important to facilitate the early diagnosis and treatment of hypertension and its possible effects. Patients with prehypertension were hypothesised to eventually become hypertensive after 10 years, and thus have a poorer quality of life.3 4 During the last two decades, it has been repeatedly published that the incidence of hypertension is associated with even moderate increases in levels of serum uric acid (SUA) and an increased risk of cardiovascular diseases (CVD).5 6 Current findings based on a systematic review and meta-analysis suggested that hyperuricemia is associated with the increased risk of incident hypertension.7 The Framingham Heart Study reported an increased risk of blood pressure (BP) progression in 3157 subjects with hyperuricemia. Serum uric acid was positively associated with increases in both systolic blood pressure (SBP) and diastolic blood pressure (DBP) after 4 years with no antihypertensive treatment.8 8 A meta-analysis by Jiang et al indicated that SUA was possibly associated with prehypertension but still found conflicting results.9 The associations among SUA, hypertension, cardiovascular risk factors and gender remain controversial. SUA levels have been known to have an association with BP and hypertension.10–12 Some studies reported that hyperuricemia has higher susceptibility of developing hypertension especially in men,10 13 while the other study reported vice versa.14 Lee et al. also showed that SUA levels in woman had stronger association with BP than in men.15 In terms of the association of SUA and cardiovascular risk, SUA did not have a causal role in the development of cardiovascular outcomes.16 Another study stated that the SUA level was an independent predictive factor for cardiovascular risk in individuals without hypertension and diabetes.17 SUA also being reported to have a stronger association with cardiovascular risk18 and risk of cardiovascular disease mortality19 in women than in men. Another study also stated that higher SUA levels are asociated with higher cardiovascular death risk score in patients with no cardiovascular disease but have cardiovascular risk factor.20
Therefore, the aim of this study was to observe the progression from prehypertension to hypertension after 10 years (2007–2017) and the association of BP with SUA as well as other cardiovascular risk factors in 2017.
Methods
Study design
This study was a cross-sectional study conducted in Mlati Subdistrict, Sleman District in the Yogyakarta Special Region, Indonesia.
Study population
We pooled data from participants enrolled in the 2007 Mlati Study Database. The sample of the Mlati Study included 12 073 people aged 20–69 years who lived in three villages in Mlati (Tirtoadi, Sumberadi and Tlogoadi), Sleman, Yogyakarta, Indonesia. The inclusion criteria for the prehypertensive subgroup of the study sample were SBP of 120–139 mm Hg and/or DBP of 80–89 mm Hg, no proteinuria, no glycosuria, and age between 20 and 49 years; this subgroup included 4190 participants (current age was 30–59 years). In 2017, of the 4190 individuals with a history of prehypertension in 2007, 1500 subjects were selected as participants in the current study by simple random sampling using statistical software. All 1500 subjects were invited to have a physical and laboratory examination; however, only 733 subjects who participated in the sampling were examined (the other subjects who did not show up during the laboratory examination were due to the change of residential area or death or any other unknown reasons and were excluded from this study). All subjects did not take any drugs lowering BP and SUA. All subjects were provided informed consent at the beginning of the study (figure 1).
Figure 1.
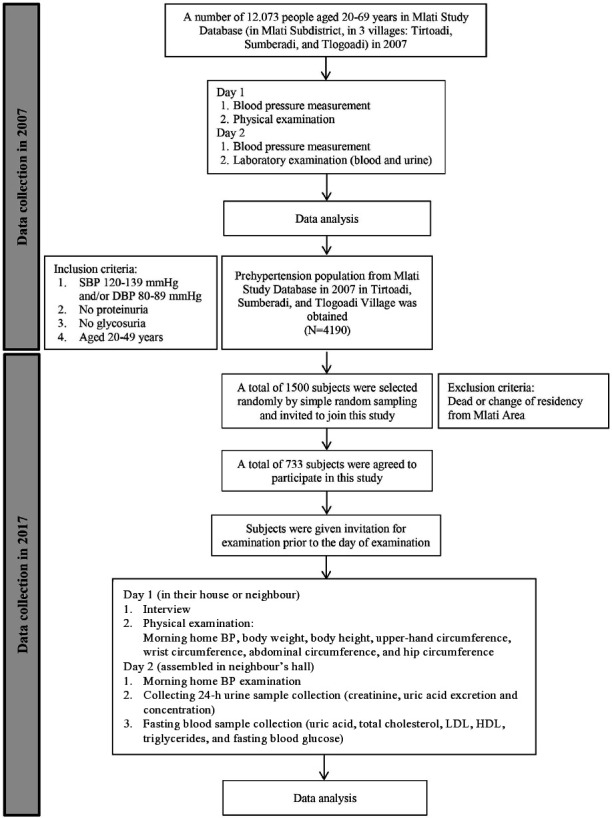
Study flow chart. Data collection was conducted two times in 2007 (resulting in a collection of prehypertensive population of 4190 patients) and 2017 (to collect the study sample of 733 and obtain physical and laboratory examinations data). BP, blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
Patient and public involvement
Patients were not involved in any of the design, analysis and presentation of the study results.
Definition of prehypertension and hypertension
The definitions of prehypertension and hypertension were based on the Seventh Report of Joint National Committee (JNC 7) because the newer JNC 8 report renewed only their treatment targets, not their classifications. The SBP of 120–139 mm Hg and/or DBP of 80–89 mm Hg are defined as prehypertension, while SBP of ≥140 mm Hg and/or DBP of ≥90 mm Hg are defined as hypertension.21
For further analysis, we applied the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline, which classifies BP as follows: (1) normal BP=SBP <120 mm Hg and DBP <80 mm Hg, (2) elevated BP=SBP 120–129 mm Hg and DBP <80 mm Hg, (3) stage 1 hypertension=SBP 130–139 mm Hg or DBP 80–89 mm Hg and (4) stage 2 hypertension=SBP ≥140 mm Hg or DBP ≥90 mm Hg.22
SUA cut-off point
Based on the study by Sja’bani (2014), the cut-off point of SUA was divided into three categories: normal (<5 mg/dL), high-normal (5–7 mg/dL) and high (≥7 mg/dL).23
Data collection
The data collection was conducted two times during the study period. The first data collection was conducted in 2007 to collect the prehypertension population (n=4190). The second data collection was performed in 2017 to collect samples from the Mlati Study Database by the random sampling method (n=733).
In 2007, interviews were conducted on 12 073 subjects to obtain demographic data (eg, sex and age), family history of hypertension and diabetes mellitus, patients’ history of diabetes mellitus, patients’ history of consuming hypertension and uric acid drugs, and to perform physical and laboratory examinations. Physical examinations, which included measurements of morning home BP (measured by using sphygmomanometer), body weight, body height, upper-hand circumference, wrist circumference, abdominal circumference and hip circumference, were conducted on day 1 in subjects’ house or their neighbour. BP measurements were performed in the morning (at 06:00–08:00) by the medical team for two times (or until stable BP was obtained) while subjects in sitting position. On day 2, we examined morning home BP and took urine and blood samples.
In 2017, we collected data from 733 subjects, including interviews of demographic data, physical and laboratory examinations. On the 1st day, subjects were interviewed, physically examined and given urine containers for one-time urine samples, as well as for a 24-hour urine collection that had to be submitted on day 2, in their home or neighbour. The physical examination was performed by the medical team, consisting of a morning home BP measurement in the morning (at 06:00–08:00) for two times (or until stable BP was obtained), while subjects in sitting position, using the Omron HEM-907 digital automatic BP monitor (Omron Healthcare Co., Kyoto, Japan) and measurements of body weight, body height, upper-hand circumference, wrist circumference, abdominal circumference and hip circumference. On the 2nd day, subjects who were in fasting condition were invited to come to the neighbour’s hall in the morning and physically examined for BP again (at 06:00–08:00) and drawn for their blood. Urine and blood samples were examined in the laboratory (Prodia Laboratory, Yogyakarta, Indonesia). A 24-hour urine sample was collected to measure uric acid excretion and creatinine, and a blood sample was collected to measure SUA, blood urea nitrogen, creatinine, a lipid profile (total cholesterol, low-density lipoprotein/LDL-C, high-density lipoprotein/HDL-C and triglycerides) and fasting blood glucose levels.
Statistical analysis
The outcomes of this study were presented in two primary analyses which were (1) BP changes during the period of 2007–2017 to measure the progression from prehypertension (2007) to hypertension (2017) and (2) the association of current BP with SUA levels and cardiovascular risk factors. Additional analyses were also performed to observe the SUA association with cardiovascular risk factors. The data analyses were mostly performed based on gender to know about the gender differences in the analyses mentioned above.
Data presented later in the results section were collected in 2007 and 2017. Data were analysed using IBM SPSS Statistics for Windows V.22.0.24 The data consisted of continuous and categorical data, which were expressed as the mean (SD) for continuous data and as numbers and percentages for categorical data. The continuous variables were analysed and compared by independent sample t-tests and nonparametric Mann-Whitney U tests. The categorical variables were analysed and compared by Pearson χ2 tests. BP changes during the period of 2007–2017 were presented using frequencies and percentages. The associations of current BP with SUA levels and gender were analysed using the Pearson χ2. Multivariable analysis was performed using multiple linear regressions to describe the association between SUA levels and BP, with adjustment for age and cardiovascular risk factors. Additional analysis to observe the association between SUA levels and cardiovascular risk factors and gender was performed using independent sample t-tests and nonparametric Mann-Whitney U tests. The significance of associations between categorical and numerical variables was determined using 95% CIs.
Results
The subjects of this study consisted of 733 adults (aged 30–59 years) living in the Mlati Subdistrict; 306 (41.75%) and 427 (58.25%) were men and women, respectively. The characteristics of the subjects (by gender) are presented in table 1. There was no significant difference in age, SBP, DBP, total cholesterol, LDL and fasting blood glucose between men and women (p>0.05). Significant differences were found in body mass index (BMI) (p<0.001), SUA levels (p<0.001), HDL (p<0.001) and triglycerides (p<0.001). BMI and HDL were significantly higher in women, whereas SUA levels and triglycerides were significantly higher in men.
Table 1.
Characteristics of subjects by gender in 2017 in mean (SD)
| Variables | Men (n=306) and Women (n=427) | P value |
| Age (years) | 46 (7.71) 46 (7.76) | 0.431 |
| 30–39 | 35 (2.86) 36 (2.63) | 0.093 |
| 40–49 | 45 (2.89) 45 (2.67) | 0.372 |
| 50–59 | 54 (3.18) 54 (2.77) | 0.779 |
| BMI (kg/m2) | 23.5 (3.70) 25.7 (4.81) | <0.001* |
| SBP (mm Hg) | 132 (17.26) 134 (21.62) | 0.595 |
| DBP (mm Hg) | 78 (11.96) 79 (12.32) | 0.091 |
| Uric acid (mg/dL) | 5.8 (1.25) 4.5 (1.10) | <0.001* |
| Total cholesterol (mg/dL) | 167 (36.86) 166 (41.59) | 0.559 |
| LDL (mg/dL) | 109 (29.59) 106 (33.27) | 0.155 |
| HDL (mg/dL) | 41 (10.02) 47 (12.20) | <0.001* |
| Triglyceride (mg/dL) | 129 (79.09) 103 (63.84) | <0.001* |
| Fasting blood glucose (mg/dL) | 100 (37.22) 97 (33.70) | 0.101 |
*Significant (p<0.05).
BMI, body mass index; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
After 10 years, among the 733 subjects with prehypertension, 180 (24.6%) returned to normal BP, 325 (44.3%) remained in a prehypertensive state and 228 (31.1%) became hypertensive. For SUA levels, 50.3% had normal SUA, 43.1% were high-normal, and only 6.6% had high SUA levels (table 2).
Table 2.
BP changes after 10 years and SUA frequency distribution (n=733)
| Variables | Frequency (%) | |
| 2007 | 2017 | |
| BP* | ||
| Normal | 0 | 180 (24.6) |
| Prehypertension | 733 (100) | 325 (44.3) |
| Hypertension | 0 | 228 (31.1) |
| SUA† | ||
| Normal | – | 369 (50.3) |
| High-normal | – | 316 (43.1) |
| High | – | 48 (6.6) |
*BP, normal: SBP of <120 mm Hg and DBP of <80 mm Hg, prehypertension: SBP of 120–139 mm Hg and/or DBP of 80–89 mm Hg, hypertension: SBP of ≥140 mm Hg and/or DBP of ≥90 mm Hg).
†SUA, normal: <5 mg/dL, high-normal: 5–7 mg/dL, high: ≥7 mg/dL.
BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure; SUA, serum uric acid.
In men, 32.3% of the subjects had high-normal or high levels of SUA, while in women, only 17.3% had high-normal or high levels of SUA. There was a significant difference in SUA between men and women (p<0.001, RR=2.60, 95% CI=2.22–3.05). When gender was further analysed by age distribution, age was significantly associated with SUA levels only in women aged 50–59 years (p<0.001, RR=2.08, 95% CI=1.36–3.15). Additionally, there was a significant association between SUA levels and uric acid excretion by 24-hour urine (p=0.002, RR=1.32, 95% CI=1.10–1.57). On the other hand, no significant association was observed between SUA levels and BMI (p=0.153, RR=1.1, 95% CI=0.96–1.32) or between SUA levels and uric acid concentration (p=0.100, RR=0.786, 95% CI=0.59–1.05) (table 3).
Table 3.
Association between gender, age, BMI, uric acid excretion and uric acid concentration to SUA level
| Variables | SUA* | P value | RR | 95% CI | |
| High-normal and high (%) | Normal (%) | ||||
| Gender | |||||
| Men | 237 (32.3) | 69 (9.4) | <0.001 | 2.60 | 2.22–3.05 |
| Women | 127 (17.3) | 300 (40.9) | |||
| Age (years) | |||||
| Men | |||||
| 30–39† | 52 (17.0) | 11 (3.6) | – | 1 | – |
| 40–49 | 104 (34.0) | 22 (7.2) | 1000 | 1.00 | 0.87–1.15 |
| 50–59 | 81 (26.5) | 36 (11.8) | 0.053 | 0.84 | 0.71–0.99 |
| Women | |||||
| 30–39† | 22 (5.2) | 85 (19.9) | – | 1 | – |
| 40–49 | 40 (9.4) | 128 (30.0) | 0.530 | 1.16 | 0.73– 1.84 |
| 50–59 | 65 (15.2) | 87 (20.4) | <0.001 | 2.08 | 1.37–3.15 |
| BMI‡ | |||||
| Overweight–obese | 171 (23.3) | 154 (21.0) | 0.153 | 1.13 | 0.96–1.32 |
| Underweight–normal | 193 (26.3) | 215 (29.3) | |||
| Uric acid excretion (24 hours)§ | |||||
| High | 169 (23.1) | 130 (17.7) | 0.002 | 1.32 | 1.10–1.57 |
| Normal | 195 (26.6) | 239 (32.6) | |||
| Uric acid concentration¶ | |||||
| Normal | 200 (27.3) | 202 (27.5) | 0.956 | 1.00 | 087–1.16 |
| High | 164 (22.4) | 167 (22.8) | |||
*SUA, normal: <5 mg/dL, high-normal: 5–7 mg/dL, high: ≥7 mg/dL.
†Reference category.
‡BMI, underweight: <18.5 kg/m2, normal: 18.5–24.9 kg/m2, overweight: 25–29.9 kg/m2, obese: >30 kg/m2.
§Uric acid excretion, normal: <435.08 mg/day, high: ≥435.08 mg/day.
¶Uric acid concentration (mg per 100 mL of urine), normal: <46.63 mg%, high: ≥46.63 mg%.
BMI, body mass index; SUA, serum uric acid.
The associations between gender and SUA levels on BP are shown in table 4. There was no significant association between gender and BP (p=0.584). To examine the association between uric acid and hypertension, we compared SUA levels and morning home BP. The association between SUA levels and BP was statistically significant (p=0.008, RR=1.12, 95% CI=1.03–1.22). In subjects with previous history of prehypertension, high-normal SUA or high SUA levels were associated with current prehypertension or hypertension. Furthermore, the association between SUA levels and BP in men and women is also described in table 4. In men, SUA levels were not significantly associated with BP (p=0.805, RR=1.02, 95% CI=0.88–1.19). However, there was a significant association between SUA levels and BP in women (p=0.001, RR=1.21, 95% CI=1.09–1.34). In women, the risk of having prehypertension or hypertension was 1.21 times higher in those who had high-normal or high SUA levels than those with normal SUA levels. Additional analysis using the 2017 ACC/AHA guideline for observing the associations between gender and SUA levels on BP also showed similar results with the previous analysis using JNC 7 guideline regarding the significant associations between SUA levels and BP.
Table 4.
Association between gender and SUA on BP
| Variables | BP | |||||||
| JNC 7* | 2017 ACC/AHA† | |||||||
| Pre-HT and HT (%) | Normal (%) | P value | RR (95% CI) | HT-1 and HT-2 (%) | Normal and elevated (%) | P value | RR (95% CI) | |
| Gender | ||||||||
| Men | 234 (31.9) | 72 (9.8) | 0.584 | 1.02 (0.94 to 1.11) | 159 (21.7) | 147 (20.1) | 0.129 | 0.9 (0.79 to 1.03) |
| Women | 319 (43.5) | 108 (14.7) | 246 (33.6) | 181 (24.7) | ||||
| SUA‡ | ||||||||
| High-normal and high | 290 (39.6) | 74 (10.1) | 0.008* | 1.12 (1.03 to 1.22) | 224 (30.6) | 140 (19.1) | 0.001* | 1.26 (1.10 to 1.43) |
| Normal | 263 (35.9) | 106 (14.5) | 181 (24.7) | 188 (25.6) | ||||
| SUA | ||||||||
| Men | ||||||||
| High-normal and high | 182 (59.5) | 55 (18.0) | 0.805 | 1.02 (0.88 to 1.19) | 129 (42.2) | 108 (35.3) | 0.109 | 1.25 (0.93 to 1.68) |
| Normal | 52 (17.0) | 17 (5.6) | 30 (9.8) | 39 (12.7) | ||||
| Women | ||||||||
| High-normal and high | 108 (25.3) | 19 (4.4) | 0.001* | 1.21 (1.09 to 1.34) | 95 (22.2) | 32 (7.5) | 0.000* | 1.49 (1.28 to 1.73) |
| Normal | 211 (49.4) | 89 (20.8) | 151 (35.4) | 149 (34.9) | ||||
*BP was categorised using the JNC 7 guideline (prehypertension: SBP of 120–139 mm Hg and/or DBP of 80–89 mm Hg, hypertension: SBP of ≥140 mm Hg and/or DBP of ≥90 mm Hg).
†BP was categorised using the 2017 ACC/AHA guideline (normal BP=SBP <120 mm Hg and DBP <80 mm Hg, elevated BP=SBP 120–129 mm Hg and DBP <80 mm Hg, stage 1 hypertension=SBP 130–139 mm Hg or DBP 80–89 mm Hg, stage 2 hypertension=SBP ≥140 mm Hg or DBP ≥90 mm Hg).
‡SUA, normal: <5 mg/dL, high-normal: 5–7 mg/dL, high: ≥7 mg/dL.
ACC/AHA, American College of Cardiology/American Heart Association; BP, blood pressure; DBP, diastolic blood pressure; HT, hypertension; JNC 7, Seventh Report of Joint National Committee; SBP, systolic blood pressure; SUA, serum uric acid.
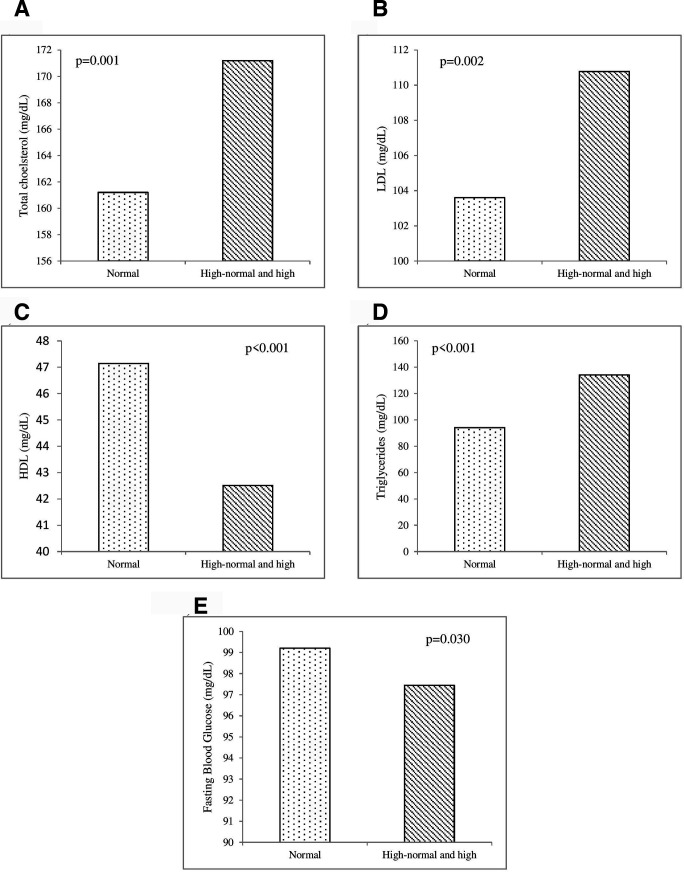
Figure 2 shows the association between SUA and cardiovascular risk factors. The SUA levels were significantly associated with total cholesterol (p=0.001), LDL (p=0.002), HDL (p<0.001), triglycerides (p<0.001) and fasting blood glucose (p=0.030). Subjects with high-normal and high SUA levels had significantly higher total cholesterol, LDL and triglyceride levels than subjects with normal SUA levels. On the other hand, HDL and fasting blood glucose were statistically lower among subjects with high-normal and high SUA levels than among those with normal SUA levels.
Figure 2.
Mean of cardiovascular risk factors in different serum uric acid (SUA) levels. The SUA levels were significantly associated with total cholesterol (p=0.001), LDL/low-density lipoprotein (p=0.002), HDL/high- density lipoprotein (p<0.001), triglycerides (p<0.001) and fasting blood glucose (p=0.030). The SUA category: normal (<5 mg/dL), high-normal (5–7 mg/dL) and high (≥7 mg/dL).
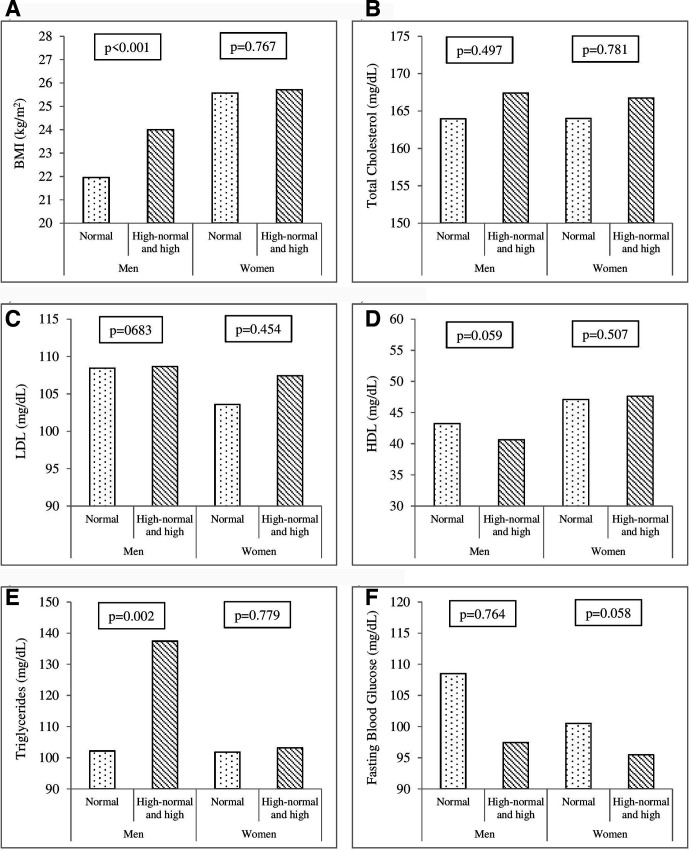
The relationships between SUA levels and cardiovascular risk factors among men and women are presented in figure 3. In men, there were significant differences in BMI (p<0.001) and triglycerides (p=0.002) between subjects with normal SUA levels and those with high-normal and high SUA levels. In women, there were no significant differences in all cardiovascular risk factors (p>0.05) between subjects with normal SUA levels and those with high-normal and high SUA levels.
Figure 3.
A - F) Mean of cardiovascular risk factors in men and women between normal and high-normal/high SUA levels. A and E) Significant differences were found in BMI (p<0.001) and triglycerides (p=0.002) between subjects with normal SUA levels and those with high-normal and high SUA levels in men. B, C, D, F) There were not significant differences (p>0.05) between subjects with normal SUA levels and those with high-normal and high SUA levels in men and women. The SUA category: normal (<5 mg/dL), high-normal (5–7 mg/dL) and high (≥7 mg/dL). BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SUA, serum uric acid.
Multivariable analysis was conducted to describe the association between SUA levels and BP, with adjustment for age and cardiovascular risk factors. Cardiovascular risk factors such as BMI, total cholesterol, LDL, HDL, triglycerides and fasting blood glucose were all taken into account for adjustment in multiple linear regression (table 5). Age, BMI, fasting blood glucose and SUA levels were significantly associated with BP. Significant associations were found between age and SBP both in men (p<0.001) and women (p<0.001), and DBP only in men (p<0.001). BMI was significantly associated with SBP and DBP both in men (p<0.001 and p<0.001) and women (p<0.001 and p<0.001). In addition, fasting blood glucose was found to be associated with SBP and DBP in women (p=0.001 and p=0.020). Regarding SUA levels, SUA was significantly associated with both SBP and DBP in women (p<0.001 and p<0.001, respectively) but such association was not found in men.
Table 5.
Multiple linear regression of association of age, cardiovascular risk factors and SUA on blood pressure
| Variables | Blood pressure of men | Blood pressure of women | ||||||
| Systolic | Diastolic | Systolic | Diastolic | |||||
| Coef. β | P value | Coef. β | P value | Coef. β | P value | Coef. β | P value | |
| Age | 0.704 | <0.001* | 0.336 | <0.001* | 0.674 | <0.001* | −0.017 | 0.817 |
| SUA | −0.247 | 0.745 | 0.582 | 0.267 | 4.527 | <0.001* | 2.223 | <0.001* |
| BMI | 1.602 | <0.001* | 1.295 | <0.001* | 0.929 | <0.001* | 0.722 | <0.001* |
| Total cholesterol | 0.044 | 0.696 | 0.042 | 0.591 | 0.119 | 0.279 | 0.030 | 0.643 |
| LDL | −0.074 | 0.529 | −0.036 | 0.657 | −0.102 | 0.365 | −0.014 | 0.828 |
| HDL | 0.184 | 0.174 | 0.042 | 0.653 | −0.054 | 0.656 | −0.049 | 0.483 |
| Triglycerides | −0.005 | 0.828 | −0.001 | 0.941 | −0.029 | 0.280 | 0.006 | 0.721 |
| Fasting blood glucose | 0.032 | 0.213 | −0.001 | 0.941 | 0.098 | 0.001* | 0.040 | 0.020* |
*Significant (p<0.05).
BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SUA, serum uric acid.
Discussion
This study consisted of two parts of data collection. The first data collection was performed in 2007 to gather data on the prehypertension population (n=4190); this study was later called the ‘Mlati Study Database’. In 2017, after 10 years, the second data collection was performed to gather samples from the Mlati Study Database by a random sampling method (n=733) to show the change in BP status from prehypertension to hypertension. The data collection in 2017 also aimed to show the association between uric acid (serum, urinary excretion and concentrate) and hypertension.
The results of our study showed that gender and uric acid excretion (by 24-hour urine) were significantly associated with SUA levels. The mean SUA levels in men were significantly higher than those in women. In addition, subjects with high-normal and high SUA levels had a risk of having prehypertension and hypertension that was 1.12 times higher than those with normal SUA levels. When analysed by gender, high-normal and high SUA levels were significantly associated with prehypertension and hypertension only in women. The relationship between SUA levels and the development of hypertension or renal disease had been shown in several previous studies. This relationship was significantly higher in women than in men.15 25
The study by Kawabe et al revealed that in women, the older the age was, the higher the quartile of SUA, but in men, the quartile of SUA did not increase with age. However, an increase in the quartile of SUA along with higher BMI was only found in men but not in women. Additionally, the mean value of SUA in men was higher than in women.26 These results were consistent with our finding that SUA levels were significantly higher in men and that SUA levels were significantly associated with higher BMI in men. However, the study populations in this study and the study by Kawabe et al were different in terms of the age group examined, which were adults (30–59 years old) and elderly adults, respectively.26 A similar finding was also found by Zhang, et al which reported that SUA levels were statistically higher in men than in women, though the SUA level did not increase with the age both in men and women.27 These studies’ results were consistent with our finding which stated that SUA level was significantly higher in men and SUA level was significantly associated with higher BMI also in men. However, the study population in this study and the study by Kawabe et al was different in the age group which was adults (30–59 years) and elderly, respectively.
Chen et al reported a different result in a cross-sectional analysis regarding the association between SUA levels and the presence of hypertension when analysed by gender. For the total population, SUA levels had significant associations with hypertension. The levels of SUA had a significant relationship with hypertension in men aged <30 years, 30–40 years and >40 years but only in women aged >40 years.10 This situation could be explained in table 3, which provides the age distribution of women and its association with SUA levels. In table 3, the proportion of women aged 40–49 years combined with those aged 50–59 years having high-normal and high SUA levels was 24.6%. This age range in women is associated with menopausal problems. A study by Hak et al stated that menopause was associated with an increased risk of incident gout, which may help explain why the age of the women in this study could play a significant role in their SUA levels.28
Regarding the cardiovascular risk factors, the result of this study found that the SUA levels were significantly associated with total cholesterol (p=0.001), LDL (p=0.002), HDL (p<0.001), triglycerides (p<0.001) and fasting blood glucose (p=0.030), regardless of gender. When the data were analysed by gender, significant differences were found only in BMI and triglycerides and only in men (p<0.001 and p=0.002, respectively). Another study has shown a stronger association between the increase of SUA level and cardiovascular mortality among women in healthy subjects compared with men.29 Meta-analysis showed that there was a significant association between hyperuricemia and cardiovascular mortality in women, but not in men.30 Chen et al reported that SUA levels were significantly associated with the occurrence of metabolic syndrome and hypertension in the total population. In men, SUA levels had a positive association with the occurrence of metabolic syndrome in the age groups of <30 and 30–40. In women, SUA levels were significantly associated with the occurrence of metabolic syndrome in the age groups of <30 and>40.10
In this study, BMI was significantly associated with SBP and DBP in both genders. This finding was in line with those of a previous study by Droyvold et al, in which the authors reported that an increase in BMI was associated with increased BP in men and women.31 With regard to the association between BMI and SUA levels, our findings were different from those of a report by Rodrigues et al, in which the authors reported a significant correlation between BMI and SUA levels in both men and women.32 The link between BMI and hyperuricemia has not been well elucidated; however, insulin resistance might be the bridging gap. Obese people are more likely to have metabolic syndrome, and the metabolic syndrome itself is associated with insulin resistance. It is thought that insulin resistance impairs the ability of the kidney to excrete uric acid and therefore leads to hyperuricemia.33
This study found that fasting blood glucose was associated with SBP and DBP only in women. The same result was observed in a study by Yan et al, which revealed that fasting plasma glucose was independent of both SBP and DBP.34 Fasting blood glucose was also associated with SUA levels, but when analysed by gender, no significant difference was found. This finding is contradictory to those of a study by Kawamoto et al, which revealed that SUA levels were associated with fasting plasma glucose in women.35 The mechanism of how this phenomenon occurred remains unclear, and further study is needed to observe a cause-effect relationship. Serum triglycerides were also associated with SUA levels in this study. The relationship between SUA levels and lipid profiles has been described in various studies, but the exact mechanism remains unclear. A study by Peng et al revealed that all lipid profile parameters, including triglycerides but not HDL cholesterol, were associated with SUA levels.36 SUA levels were associated with both SBP and DBP but only in women. This result is similar to those of previous studies.35 37 It has been suggested that the mechanism by which uric acid causes hypertension is due to endothelial dysfunction after oxidative stress damage to the endothelium during excessive uric acid formation.37
There were several limitations to this study. First, subjects in this study were collected from the database made in 2007. From 1500 subjects randomly selected at the beginning of this study, only 733 subjects joined and attend the 2-day examination. More than half of the selected subjects did not attend the examination invitation due to several reasons, thus, this had lessened the total samples of subjects of this study. However, a total sample of 733 has met the minimum sample requirement for this study based on sample size calculation (a minimum sample size of 661 subjects are needed for this study). We invited 1500 subjects at the beginning of this study to anticipate any subjects that could not participate in this study due to any reasons, so that the minimum number of samples could still be met. This was one of the difficulties we met since this study was a community-based study. The findings of this study were expected to be generalised to the 4190 patients with prehypertension who were collected from Mlati Study Database in 2007. Second, this study could not present the changes of all measured values over a 10-year period because, in the prior study in 2007, these laboratory values were not examined, except for BP. Therefore, only the changes in BP can be presented on the results. Third, the instruments used to measure BP in 2007 and 2017 were different that might cause instrument bias. In 2007, we used sphygmomanometers, whereas in 2017 we used digital automatic BP monitors. Thus, this may lead to bias in BP data measurement between 2007 and 2017. Nevertheless, we tried to minimise the bias by calibrating both the sphygmomanometers and digital automatic BP monitors before data collection, so that, the BP data were all accurate.
Conclusion
In conclusion, after 10 years of follow-up (2007–2017), of 733 subjects with prehypertension, 180 (24.6%) returned to normal BP, 325 (44.3%) remained in a prehypertensive state and 228 (31.1%) got hypertension. In the cross-sectional analyses of SUA in 2017, the SUA levels in men were significantly higher than those in women. Moreover, high-normal and high SUA levels were significantly associated with prehypertension and hypertension in women but not in men.
Supplementary Material
Acknowledgments
We would like to thank the Mlati Study Group, which assisted in this research. We would also like to thank Prodia Laboratory for performing laboratory examinations.
Footnotes
Contributors: LAB, MS and YT composed the idea of the study and arranged the study’s design. MS, FI, AW and AK obtained the data. ZZ led the statistical analysis with the supervision of MS. MS, LAB and ZZ wrote the first draft of this paper and all authors read, revised and approved the final manuscript.
Funding: This research was supported by the Mlati Study Group, Faculty of Medicine, University of Gadjah Mada, Indonesia (No: MSG-008-2017).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The protocol of this study was approved by the Medical and Health Research Ethics Committee of Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia with the ID approval of KE/FK/0961/EC/2017.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author, [MS], upon reasonable request.
References
- 1.Mills KT, Bundy JD, Kelly TN, et al. . Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation 2016;134:441–50. 10.1161/CIRCULATIONAHA.115.018912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annual Health Research Report Division of research and health development: Indonesian Ministry of health 2013.
- 3.Ruchira P, Gajendra Singh M. Ps 15-11 impact of hypertension on quality of life among people living in an urban area of Delhi, India. J Hypertens 2016;34:e462. [Google Scholar]
- 4.Carvalho MVde, Siqueira LB, Sousa ALL, et al. . The influence of hypertension on quality of life. Arq Bras Cardiol 2013;100:164–74. 10.5935/abc.20130030 [DOI] [PubMed] [Google Scholar]
- 5.Cicero AFG, Rosticci M, Fogacci F, et al. . High serum uric acid is associated to poorly controlled blood pressure and higher arterial stiffness in hypertensive subjects. Eur J Intern Med 2017;37:38–42. 10.1016/j.ejim.2016.07.026 [DOI] [PubMed] [Google Scholar]
- 6.Jin M, Yang F, Yang I, et al. . Uric acid, hyperuricemia and vascular diseases. Front Biosci 2012;17:656–69. 10.2741/3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grayson PC, Kim SY, LaValley M, et al. . Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res 2011;63:102–10. 10.1002/acr.20344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sundström J, Sullivan L, D'Agostino RB, et al. . Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 2005;45:28–33. 10.1161/01.HYP.0000150784.92944.9a [DOI] [PubMed] [Google Scholar]
- 9.Jiang M, Gong D, Fan Y. Serum uric acid levels and risk of prehypertension: a meta-analysis. Clin Chem Lab Med 2016. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y-Y, Kao T-W, Yang H-F, et al. . The association of uric acid with the risk of metabolic syndrome, arterial hypertension or diabetes in young subjects- an observational study. Clinica Chimica Acta 2018;478:68–73. 10.1016/j.cca.2017.12.038 [DOI] [PubMed] [Google Scholar]
- 11.Ali N, Mahmood S, Islam F, et al. . Relationship between serum uric acid and hypertension: a cross-sectional study in Bangladeshi adults. Sci Rep 2019;9:9061. 10.1038/s41598-019-45680-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Q, Yin Y-J, Chen W-Y, et al. . Assessment of the association between serum uric acid levels and the incidence of hypertension in nonmetabolic syndrome subjects: a prospective observational study. Medicine 2018;97:6. 10.1097/MD.0000000000009765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin X, Wang X, Li X, et al. . Gender- and age-specific differences in the Assocoation of hyperuricemia and hypertension: a cross-sectional study. Int J Endocrinol 2019. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishio S, Maruyama Y, Sugano N, et al. . Gender interaction of uric acid in the development of hypertension. Clin Exp Hypertens 2018;40:446–51 https://doi.org/ 10.1080/10641963.2017.1392556 [DOI] [PubMed] [Google Scholar]
- 15.Lee JJ, Ahn J, Hwang J, et al. . Relationship between uric acid and blood pressure in different age groups. Clin Hypertens 2015;21:14. 10.1186/s40885-015-0022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Culleton BF, Larson MG, Kannel WB, et al. . Serum uric acid and risk for cardiovascular disease and death: the Framingham heart study. Ann Intern Med 1999;131:7–13. 10.7326/0003-4819-131-1-199907060-00003 [DOI] [PubMed] [Google Scholar]
- 17.Chang C-C, Wu C-H, Liu L-K, et al. . Association between serum uric acid and cardiovascular risk in nonhypertensive and nondiabetic individuals: the Taiwan I-Lan longitudinal aging study. Sci Rep 2018;8:5234. 10.1038/s41598-018-22997-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Høieggen A, Alderman MH, Kjeldsen SE, et al. . The impact of serum uric acid on cardiovascular outcomes in the life study. Kidney Int 2004;65:1041–9. 10.1111/j.1523-1755.2004.00484.x [DOI] [PubMed] [Google Scholar]
- 19.Borghi C, Rodriguez-Artalejo F, De Backer G, et al. . Serum uric acid levels are associated with cardiovascular risk score: a post hoc analysis of the EURIKA study. Int J Cardiol 2018;253:167–73. 10.1016/j.ijcard.2017.10.045 [DOI] [PubMed] [Google Scholar]
- 20.Rahimi-Sakak F, Maroofi M, Rahmani J, et al. . Serum uric acid and risk of cardiovascular mortality: a systematic review and dose-response meta-analysis of cohort studies of over a million participants. BMC Cardiovasc Disord 2019;19:218. 10.1186/s12872-019-1215-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chobanian AV, Bakris GL, Black HR, et al. . The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289:2560–72. 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 22.Whelton PK, Carey RM, Aronow WS, et al. . 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. Hypertension 2018;71:1269–324. 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 23.Sja’bani M. Hypertension and renoprotective effects of high serum uric acid treatment. in annual scientific meeting of Indonesian nephrology in Palembang. South Sumatra, Indonesia: Lembaga Penerbit Ilmu Penyakit Dalam, Bagian Ilmu Penyakit Dalam Fakultas Kedokteran UNSRI, Palembang, 2014. [Google Scholar]
- 24.IBM Corp. Released Ibm SPSS statistics for windows, version 22.0. Armonk, NY: IBM Corp, 2013. [Google Scholar]
- 25.Zhang W, Sun K, Yang Y, et al. . Plasma uric acid and hypertension in a Chinese community: prospective study and metaanalysis. Clin Chem 2009;55:2026–34. 10.1373/clinchem.2009.124891 [DOI] [PubMed] [Google Scholar]
- 26.Kawabe M, Sato A, Hoshi T, et al. . Gender differences in the association between serum uric acid and prognosis in patients with acute coronary syndrome. J Cardiol 2016;67:170–6. 10.1016/j.jjcc.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, Liu R, Yuan J, et al. . Gender-Related differences in the association between serum uric acid and left ventricular mass index in patients with obstructive hypertrophic cardiomyopathy. Biol Sex Differ 2016;7:22. 10.1186/s13293-016-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hak AE, Curhan GC, Grodstein F, et al. . Menopause, postmenopausal hormone use and risk of incident gout. Ann Rheum Dis 2010;69:1305–9. 10.1136/ard.2009.109884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedman DS, Williamson DF, Gunter EW, et al. . Relation of serum uric acid to mortality and ischemic heart disease. The NHANES I epidemiologic follow-up study. Am J Epidemiol 1995;141:637–44. 10.1093/oxfordjournals.aje.a117479 [DOI] [PubMed] [Google Scholar]
- 30.Kim SY, Guevara JP, Kim KM, et al. . Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res 2010;62:NA–80. 10.1002/acr.20065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drøyvold WB, Midthjell K, Nilsen TIL, et al. . Change in body mass index and its impact on blood pressure: a prospective population study. Int J Obes 2005;29:650–5. 10.1038/sj.ijo.0802944 [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues SL, Baldo MP, Capingana DP, et al. . Gender difference of serum uric acid and cardiovascular risk factors: population based study. Arq Bras Cardiol 2011. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Hsieh MC, Chang SJ, et al. . Diabetes, and hyperuricemia. Curr Opin Rheumatol 2013;25:210–6. [DOI] [PubMed] [Google Scholar]
- 34.Yan Q, Sun D, Li X, et al. . Association of blood glucose level and hypertension in elderly Chinese subjects: a community based study. BMC Endocr Disord 2016;16:22. 10.1186/s12902-016-0119-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawamoto R, Tabara Y, Kohara K, et al. . Serum uric acid is more strongly associated with impaired fasting glucose in women than in men from a community-dwelling population. PLoS One 2013;8:e65886–5. 10.1371/journal.pone.0065886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng T-C, Wang C-C, Kao T-W, et al. . Relationship between hyperuricemia and lipid profiles in US adults. Biomed Res Int 2015;2015:1–7. 10.1155/2015/127596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maruhashi T, Nakashima A, Soga J, et al. . Hyperuricemia is independently associated with endothelial dysfunction in postmenopausal women but not in premenopausal women. BMJ Open 2013;3:e003659. 10.1136/bmjopen-2013-003659 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.