Abstract
Background
The aim of this study was to establish a predictive model for prognostic factors and overall survival (OS) in nasopharyngeal lymphoepithelial carcinoma (NLEC) patients.
Material/Methods
The data of 538 NLEC patients diagnosed between 1988 and 2015 were extracted from the Surveillance, Epidemiology, and End Results database. Patients who were diagnosed from 1988 to 1999 were included in the validation cohort, and those diagnosed from 2000 to 2015 in the primary cohort. Least absolute shrinkage and selection operator and multivariate Cox regression analyses were performed. The discrimination and calibration capabilities of the predictive models were evaluated using the receiver operating characteristic (ROC) curve and calibration plot, respectively.
Results
Radiotherapy (P<0.0001), early-stage cancer based on the American Joint Committee on Cancer (AJCC) staging system (P<0.0001), younger age (P=0.0005) were associated with better OS rates. In the primary cohort, the areas under the ROC curves (AUC) of the nomogram for predicting 1-, 10-, and 15-year OS were 0.749, 0.754, and 0.81, respectively. Meanwhile, in the validation cohort, the AUC of the nomogram for predicting 1-, 10-, and 15-year OS were 0.692, 0.692, and 0.682, respectively. Furthermore, the calibration plot exhibited optimal agreements between the nomogram-predicted and actual 1-, 10-, and 15-year OS in both cohorts. The 1-, 10-, and 15-year OS rates were 93.6%, 62.7%, and 49.9%, respectively.
Conclusions
Age, early-stage cancer based on the AJCC staging system, radiotherapy, and gender can be used to predict OS in nasopharyngeal lymphoepithelial carcinoma patients.
MeSH Keywords: Head and Neck Neoplasms, Nasopharyngeal Neoplasms, Prognosis
Background
Nasopharyngeal lymphoepithelial carcinoma is a rare type of neoplasm, and it was first described in 1921 [1,2]. Lymphoepithelial carcinoma was believed to be a peculiar tumor with a close connection between blastomatous proliferative epithelium and lymphocytes. In 2005, the World Health Organization classified nasopharyngeal carcinomas into 2 general types, namely, keratinizing and non-keratinizing squamous cell carcinoma. The latter was further classified into differentiated and undifferentiated subtypes. Lymphoepithelial carcinoma is a non-keratinizing undifferentiated type [3], which is characterized by aggregates of undifferentiated carcinoma with rich non-neoplastic lymphocytic components [4]. These cells have indistinct cell borders. Moreover, syncytial growth is usually evident, and moderate to marked nuclear pleomorphism as well as increased mitotic activity and necrosis are often observed [3]. Based on our knowledge, data on the prognosis of nasopharyngeal lymphoepithelial carcinoma are limited. Hence, the current study aimed to identify the risk factors associated with OS in patients with nasopharyngeal lymphoepithelial carcinoma clinical using information from the Surveillance, Epidemiology, and End Results (SEER) database. In particular, a predictive model for the individual risk factors of overall survival (OS) was established.
Material and Methods
Study design
The data used in this study were obtained using the SEER*Stat software (version 8.3.6). The SEER database covers about 30% of the population in the United States [5]. Data from 1975 to 2015 were obtained using the International Classification of Diseases for Oncology, 3rd Edition, histology/behavior code for lymphoepithelial carcinoma (8082/3). Then, the following data were obtained: patient’s age, gender, race, pathological grade, year of diagnosis, primary site of the tumor and stage based on the American Joint Committee on Cancer (AJCC) staging system, historic stage A, summary stage, collaborative stage (CS) extension, CS lymph node, extent of disease (EOD) 10-extent, EOD 10-nodes, surgical treatment, radiation, radiation sequence with surgery, chemotherapy, survival months, and vital status. Tumor staging was reevaluated based on the criteria from the 7th edition of the AJCC Staging Manual. In addition, only cases in which the nasopharynx is the primary tumor site were included (C11.0, C11.1, C11.2, C11.3, C11.8, and C11.9). The exclusion criteria were as follows: 1) unclear TNM staging; 2) uncertain radiation sequence with surgery; and 3) without information on surgery, autopsy, and death certificate. Finally, 538 patients diagnosed between 1988 and 2015 were included in the study. Among them, those diagnosed from 1988 to 1999 were included in the validation cohort, and those diagnosed from 2000 to 2015 in the primary cohort.
Statistical analysis
All analyses were conducted using R software version 3.6.2. (http://www.r-project.org/). The least absolute shrinkage and selection operator (LASSO) regression method was utilized to identify the optimal predictive variables of the risk factors. Then, a multivariate Cox regression analysis was performed to identify the independent predictors of survival by incorporating the variables selected in the LASSO regression model; then, a predictive model was developed. The variables were presented as hazard ratio (HR) with 95% confidence interval (CI). Sociodemographic variables (P<0.05) were included in the model. Based on the results of the multivariate Cox regression analysis, a nomogram was established. The discrimination capability of the nomogram was assessed using the receiver operating characteristic (ROC) curve, which is equivalent to the concordance statistic [6]. The accuracy of the predictive model was presented as the area under the ROC curve [7], and a higher value indicates better outcomes. A calibration curve was used to evaluate the model. Finally, the ROC and calibration plot were developed based on the results of the regression analysis. A P value <0.05 was considered statistically significant.
Ethics
This population study did not include identifiable information throughout the analyses. Moreover, it did not require informed consent from the institutional research committee of the first affiliated hospital of Xian Jiaotong University.
Results
Characteristics of the participants
After screening patients using the inclusion and exclusion criteria, 538 were finally included in our study. The participants were divided into 2 groups according to the year of diagnosis. Among them, 392 patients were included in the primary cohort, and 146 patients in the validation cohort. The data of the primary cohort were utilized to establish the nomogram, and the clinical information of the validation cohort was used to validate the capability of the nomogram. The primary cohort comprised 281 males (72%) and 111 females (28%). Meanwhile, the validation cohort was composed of 35 males (24%) and 111 females (76%). According to the patient’s age at diagnosis, the participants were divided into 4 groups: children (<18 years old), adolescents and young adults (18–34 years old), adults (35–64 years old), and elderly (≥65 years old). The tumor pathological grade was categorized into 4 groups: 1) grade II: moderately differentiated, 2) grade III: poorly differentiated, 3) grade IV: undifferentiated, and 4) unknown. In addition, lymphoepithelial carcinoma is a non-keratinizing undifferentiated type, most patients had an undifferentiated grade, and the proportion of patients with grade I disease was low, and they did not meet the inclusion criteria. The clinical characteristics of the participants are summarized in Table 1.
Table 1.
Clinical characteristics of patients with nasopharyngeal lymphoepithelial carcinoma.
| Characters | Primary cohort (n=392) | Validation cohort (n=146) | ||
|---|---|---|---|---|
| No. of patients | % | No. of patients | % | |
| Gender | ||||
| Male | 281 | 72 | 35 | 24 |
| Female | 111 | 28 | 111 | 76 |
| Age (years) | ||||
| Children (<18) | 25 | 6 | 10 | 7 |
| AYAs (18–34) | 54 | 14 | 21 | 14 |
| Adults (35–64) | 251 | 64 | 95 | 65 |
| Elderly (≥65) | 62 | 16 | 20 | 14 |
| Race | ||||
| White | 159 | 40 | 47 | 32 |
| Black | 57 | 15 | 11 | 8 |
| Other/unknow | 176 | 45 | 88 | 60 |
| 7th AJCC stage | ||||
| I | 66 | 17 | 39 | 27 |
| II | 110 | 28 | 41 | 28 |
| III | 114 | 29 | 55 | 37 |
| IVA | 32 | 8 | 7 | 5 |
| IVB | 47 | 12 | 4 | 3 |
| IVC | 23 | 6 | 0 | 0 |
| Grade | ||||
| II | 2 | 1 | 0 | 0 |
| III | 57 | 14 | 36 | 25 |
| IV | 252 | 64 | 70 | 48 |
| Unknow | 81 | 21 | 40 | 27 |
| Radiation sequence with surgery | ||||
| No radiation or surgery | 260 | 66 | 119 | 82 |
| Radiation after surgery | 129 | 33 | 25 | 17 |
| Radiation prior to surgery | 3 | 1 | 2 | 1 |
| Surgery | ||||
| Yes | 72 | 18 | 30 | 21 |
| No | 320 | 82 | 116 | 79 |
| Radiation | ||||
| Yes | 362 | 92 | 139 | 95 |
| No | 30 | 8 | 7 | 5 |
| Chemotherapy | ||||
| Yes | 337 | 86 | 71 | 49 |
| No | 55 | 14 | 75 | 51 |
AYAs – adolescents and young adults; AJCC – American Joint Committee on Cancer.
Prognostic factors in the primary cohort
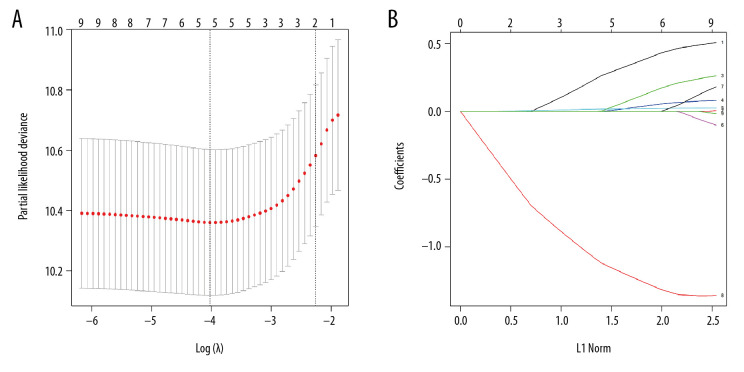
Based on the LASSO regression analysis, the non-zero coefficient variables were age, gender, grade, disease stage based on the AJCC staging system, and radiotherapy (Figure 1A, 1B). These variables were analyzed using the multivariate Cox proportional hazards regression model. Results showed that age, disease stage based on AJCC staging system, and radiotherapy were independent prognostic factors of OS in patients with nasopharyngeal lymphoepithelial carcinoma (Table 2). Based on clinical experience, gender was also included in establishing the predictive model.
Figure 1.
The clinical variables were screened using the least absolute shrinkage and selection operator (LASSO) Cox regression model. (A) The selection of the optimal parameters in the LASSO model used a cross-validation method based on the minimum criterion. The partial likelihood deviation curves were plotted in accordance with log (lambda). A dashed vertical line was drawn at the optimal value using the minimum criterion and 1-SE criterion of the minimum criterion. (B) LASSO coefficient profiles of the nine features. Generating a coefficient profile based on the log (lambda) sequence. Cross validation was performed to obtain vertical lines over the selected values, where the optimal lambda yields the characteristic of five non-zero coefficients.
Table 2.
Multivariate Cox regression analyses for factors predicting overall survival.
| Variate | HR | 95% CI | P value |
|---|---|---|---|
| Gender | |||
| Female | Reference | ||
| Male | 1.24 | 0.83–1.83 | 0.29 |
| Age | |||
| Children | Reference | ||
| AYAs | 2.84 | 0.95–8.50 | 0.06 |
| Adults | 2.95 | 1.07–8.16 | 0.04 |
| Elderly | 7.11 | 2.45–20.62 | 0.00 |
| 7th AJCC stage | |||
| I | Reference | ||
| II | 1.70 | 0.94–3.07 | 0.08 |
| III | 2.25 | 1.26–4.02 | 0.01 |
| IVA | 2.52 | 1.06–5.97 | 0.04 |
| IVB | 4.82 | 2.46–9.44 | 0.00 |
| IVC | 7.02 | 3.25–15.18 | 0.00 |
| Metastasis | |||
| M0 | Reference | ||
| M1 | 2.08 | 1.20–3.60 | 0.01 |
| Radiation | |||
| No | Reference | ||
| Yes | 0.33 | 0.21–0.53 | 0.00 |
The result is retained to 2 decimal places. HR – hazard ratio; CI – confidence interval; AYAs – adolescents and young adults; AJCC – American Joint Committee on Cancer.
OS and risk factors in the primary cohort
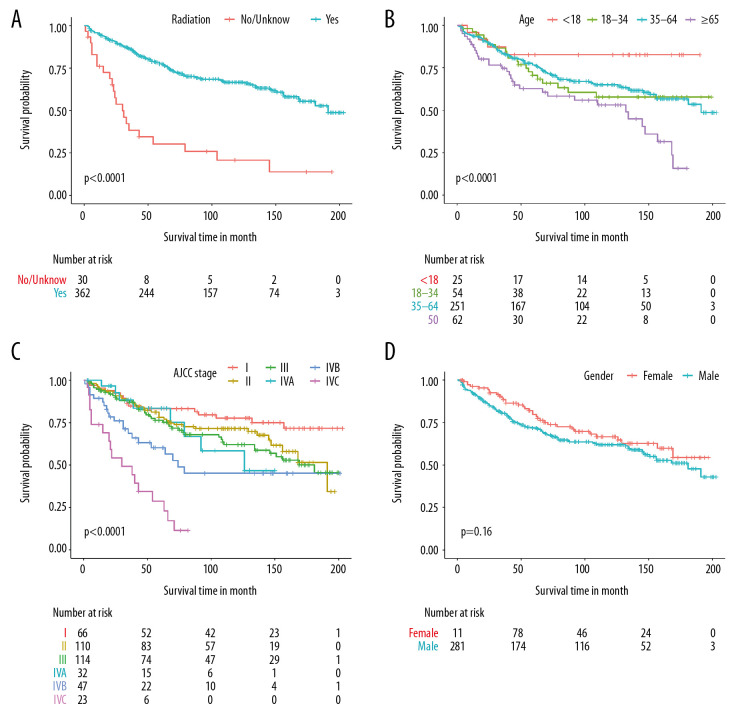
The median follow-up time was 75 months (range, 1–203 months), and the median survival time was 181 months. OS was defined as time from diagnosis to death from any causes. The 1-, 10-, and 15-year OS rates were 93.6%, 62.7%, and 49.9%, respectively (Figure 2). The OS differed among the groups with various characteristics, and those who received radiotherapy had a better OS than those who did not (P<0.0001, Figure 3A). The OS of younger patients was significantly better than that of older patients (P=0.005, Figure 3B). Moreover, patients with early-stage cancer based on the AJCC staging system had a better prognosis than those with advanced-stage cancer, and patients with stage IV disease had a better OS than those with diseases of other stages (P<0.0001, Figure 3C). However, the survival rate did not significantly differ between women and men (P=0.150, Figure 3D).
Figure 2.
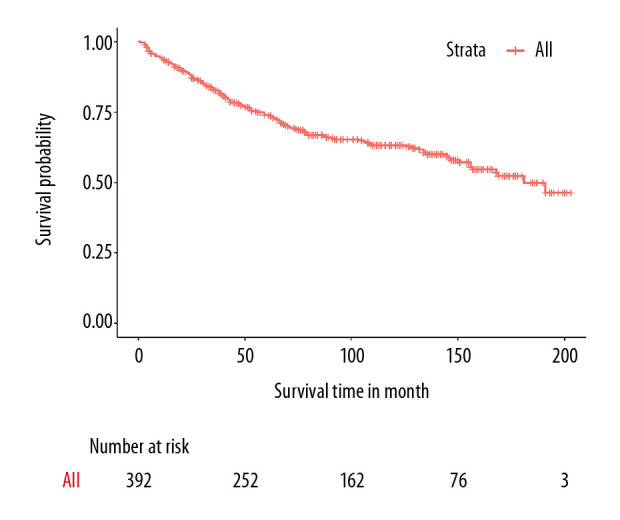
Overall survival curves in the primary cohort based on the Kaplan-Meier analysis.
Figure 3.
Kaplan-Meier survival analysis of the risk factors in the primary cohort. Time values were measured in months. (A) Survival according to radiotherapy. (B) Survival according to age. (C) Survival according to the AJCC stage. (D) Survival according to gender.
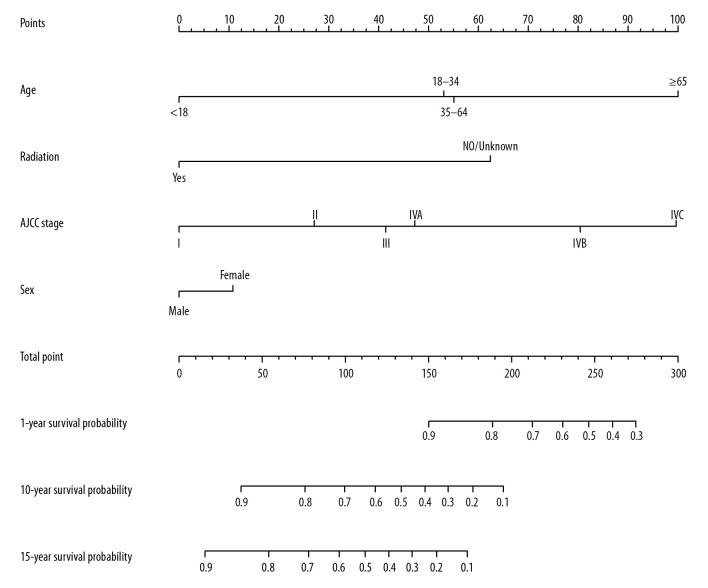
Prognostic nomogram for OS
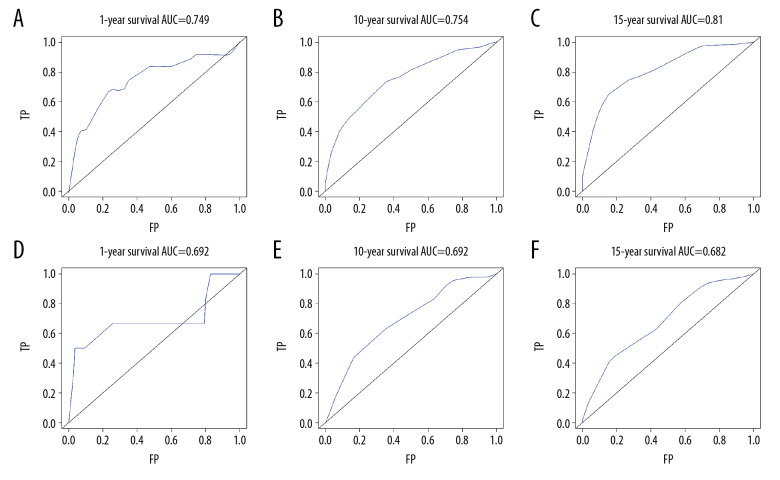
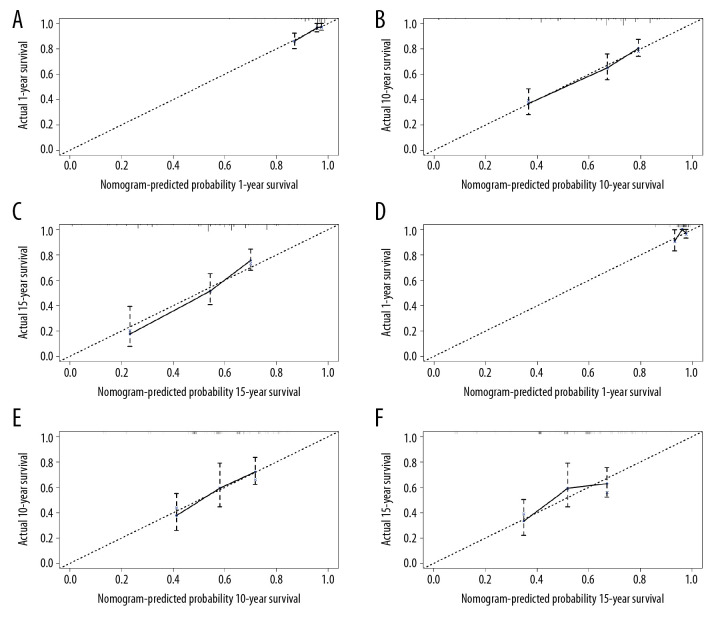
All significant independent factors for OS in the primary cohort were integrated in the prognostic nomogram (Figure 4). The ROC for predicting the 1-, 10-, and 15-year OS were 0.749, 0.754, and 0.810, respectively (Figure 5A–5C). The calibration plot exhibited a perfect agreement between the nomogram-predicted and actual OS at 1, 10, and 15 years (Figure 6A–6C).
Figure 4.
Prognostic nomogram for nasopharyngeal lymphoepithelial carcinoma. The points for each variable were summed up to obtain the total points, and the final scores were used to estimate the 1-, 10-, and 15-year overall survival.
Figure 5.
Receiver operating characteristic for predicting overall survival in the primary cohort at (A) 1 year, (B) 10 years, and (C) 15 years. Receiver operating characteristic for predicting overall survival in the validation cohort at (D) 1 year, (E) 10 years, and (F) 15 years.
Figure 6.
The calibration plot for predicting patient survival in the primary cohort at (A) 1 year, (B) 10 years, and (C) 15 years. The calibration plot for predicting patient survival in the validation cohort at (D) 1 year, (E) 10 years, and (F) 15 years. The nomogram-predicted probability of OS was plotted on the x-axis, and the actual overall survival on the y-axis.
Predictive accuracy of the nomogram for OS in the validation cohort
In the validation cohort, the median follow-up time was 183.5 months (range, 6–341 months), the median OS was 232 months, and the 1-, 10-, and 15-year OS rates were 95.9%, 58.7%, and 53%, respectively. The ROC for predicting the 1-, 10-, and 15-year OS were 0.692, 0.692, and 0.682, respectively (Figure 5D–5F). Furthermore, the calibration plot exhibited a perfect agreement between the nomogram-predicted and actual OS at 1, 10, and 15 years (Figure 6D–6F).
Discussion
Summary of key findings
Nasopharyngeal lymphoepithelial carcinoma (NLEC) is similar to non-keratinizing undifferentiated carcinoma. The tumor cells are arranged in nests or in isolated patterns. Moreover, intense lymphoplasmacytic cell infiltration in which the cellular borders are indistinct is observed, and the tumor cells often have a syncytial pattern. The tumor cell nuclei are round, oval, or elongated, with mild irregular nuclear borders, delicate chromatin, and 1 or 2 distinct eosinophilic nucleoli [8]. The incidence and prevalence of NLEC are low. To the best of our knowledge, the study first explored the clinicopathological characteristics and independent prognostic factors of NLEC based on information in the SEER database. According to the LASSO regression model, a multivariate Cox regression analysis was performed to identify prognostic factors, including age, tumor stage, and radiotherapy. Although the analysis showed that gender was not a significant factor (HR: 1.24, 95%CI: 0.83–1.83, P=0.29), it was still included in establishing this model based on experience. The discrimination and calibration capabilities of the nomogram were evaluated using the ROC and calibration curve, respectively.
Comparisons with other studies
Similar to previous studies [9], tumor stage was considered an important predictor of survival (P<0.0001), and advanced tumor stage was correlated with decreased survival, particularly in patients with stage IVC who have a significantly lower survival time due to distal metastasis. Nasopharyngeal carcinoma is highly sensitive to ionizing radiation, and radiation is the main treatment for nonmetastatic diseases [10]. Radiotherapy has significantly improved the survival time of patients with NLEC (P<0.0001). Our study showed that age was an independent prognostic factor, and children had a better prognosis than adults because they were significantly more likely to undergo radiotherapy and chemotherapy. In addition, more aggressive treatment regimens might have contributed to the overall improvement in survival [11]. Elderly patients have the poorest prognosis, which might be attributed to the following: 1) these patients may develop diseases associated with age; hence, the mortality risk is higher. 2) The systemic condition and immunity of these patients are relatively poor, and tolerance to radio chemotherapy is poor. Patel et al. [12] found that African American and Asian patients had better survival rates than Caucasian patients. However, our study showed that race was not associated with survival. Most participants in this study were white or black, and only a small proportion of our study patients were Asian patients and those of other descents. Thus, racial differences could have attributed to the differences in different study results. Unlike the study of Nakanishi et al. [13], our results showed that chemotherapy had no impact on prognosis. Whether chemotherapy is effective for lymphoepithelial carcinoma remains controversial [9,14]. Therefore, large randomized clinical trials must be performed to validate our study finding.
Methods of data analysis
Unlike other clinical articles that conducted a single-factor analysis for the preliminary screening of data, this study performed the LASSO and Cox proportional hazards regression analyses to evaluate and set up the predictive model. LASSO regression is a statistical method that can perform both variable selection and regularization [15]. It uses the absolute value function of the model coefficients as a penalty strategy to compress the model coefficients. Hence, some regression coefficients were found to have extremely weak effects on value, even directly zero. Mathematical procedures that tune and select the preferred level of model complexity were applied to enhance the predictive accuracy, interpretability, and generalizability of the statistical model [16]. Moreover, it was utilized to establish the predictive model using a data set with common intercorrelated independent variables in biological and medical research [17]. Thus, the LASSO regression has important statistical features that can assess the association between several risk factors and clinical characteristics. From a statistical standpoint, it could be a very promising method for the assessment of the association between the characteristics of patients with NLEC and OS in a more predictive manner, unlike in previous studies that used factor analysis and structural equation modeling.
Limitations
The current study had several limitations. The AUCs were not large, which might be attributed to the following causes. First, the information in the database was recorded according to the 7th or earlier edition of the AJCC Staging Manual; the staging guidance of the 8th edition cannot be used in numerous places [10,18]. Therefore, the 7th edition of the manual was used. The prognostic value is lower in the 7th edition of the AJCC Staging Manual than in the 8th edition [19]. Second, some data on potential risk factors were not available in the database. Nasopharyngeal carcinoma is believed to be associated with Epstein-Barr virus (EBV) infection [3], and this infection is a risk factor in patients with lymphoepithelioma-like carcinoma (LELC) [20–23]. Patients with human papilloma virus (HPV) infection, particularly HPV-16 infection, have a worse OS than those without [24,25]. A high Ki-67 expression leads to radiation resistance, which is considered a poor prognostic indicator in patients with locally advanced disease [26]. Information about smoking and drinking was not available in the database, and both smoking and drinking are important prognostic factors. That is, smokers have lower OS and locoregional recurrent-free survival than non-smokers [27], and a higher cumulative smoking indicates a lower survival rate [28]. Drinkers often have a poorer prognosis than non-drinkers [29]. Moreover, pretreatment lactate dehydrogenase (LDH) level is a biomarker for predicting survival rates [30]. Third, this type of tumor is rare. Hence, only a small sample size was included this trial, and this is considered another limitation of the current study. Hence, future studies with a larger sample size must be conducted.
Conclusions
This study developed a novel nomogram that can predict OS in patients with NLEC and can be used as a reference by clinicians. Younger age, early-stage cancer based on the AJCC staging system, and radiotherapy were associated with a better prognosis. However, further research must be conducted to validate whether this nomogram can be applied to other patient groups.
Acknowledgements
We would like to thank the staff of the Surveillance, Epidemiology, and End Results (SEER) program for providing open access to the SEER database.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Schmincke A. On the subject of lymphoepithelial tumours. Beitr Pathol Anat. 1921;68:161–70. [Google Scholar]
- 2.Regaud C, Reverchon L. A case of squamous epithelioma in the body of the superior maxillary. Rev Laryngol Otol Rhinol. 1921;42:369–78. [Google Scholar]
- 3.Wenig BM. Lymphoepithelial-like carcinomas of the head and neck. Semin Diagn Pathol. 2015;32:74–86. doi: 10.1053/j.semdp.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Hipp JA, Jing X, Zarka MA, et al. Cytomorphologic characteristics and differential diagnoses of lymphoepithelial carcinoma of the parotid. J Am Soc Cytopathol. 2016;5:93–99. doi: 10.1016/j.jasc.2015.09.216. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics. Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 6.Kidd AC, McGettrick M, Tsim S, et al. Survival prediction in mesothelioma using a scalable Lasso regression model: instructions for use and initial performance using clinical predictors. BMJ Open Respir Res. 2018;5:e000240. doi: 10.1136/bmjresp-2017-000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang EJ, Nandram B, Ko Y, Kim DH. Small mall area estimation of receiver operating characteristic curves for ordinal data under stochastic ordering. Stat Med. 2020;39:1514–28. doi: 10.1002/sim.8493. [DOI] [PubMed] [Google Scholar]
- 8.Yu X, Wen Y, Qin R, et al. Prognosis and distribution of lymph nodes metastases in resectable primary pulmonary lymphoepithelioma-like carcinoma: A large cohort from a single center. Thorac Cancer. 2018;9:360–67. doi: 10.1111/1759-7714.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Challapalli SD, Simpson MC, AdjeiBoakye E, et al. Survival differences in nasopharyngeal carcinoma among racial and ethnic minority groups in the United States: A retrospective cohort study. Clin Otolaryngol. 2019;44:14–20. doi: 10.1111/coa.13225. [DOI] [PubMed] [Google Scholar]
- 10.Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet. 2019;394:64–80. doi: 10.1016/S0140-6736(19)30956-0. [DOI] [PubMed] [Google Scholar]
- 11.Richards MK, Dahl JP, Gow K, et al. Factors associated with mortality in pediatric vs. adult nasopharyngeal carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:217–22. doi: 10.1001/jamaoto.2015.3217. [DOI] [PubMed] [Google Scholar]
- 12.Patel VJ, Chen NW, Resto VA. Racial and ethnic disparities in nasopharyngeal cancer survival in the United States: A SEER Study. Otolaryngol Head Neck Surg. 2017;156:122–31. doi: 10.1177/0194599816672625. [DOI] [PubMed] [Google Scholar]
- 13.Nakanishi Y, Wakisaka N, Kondo S, et al. Progression of understanding for the role of Epstein-Barr virus and management of nasopharyngeal carcinoma. Cancer Metastasis Rev. 2017;36:435–47. doi: 10.1007/s10555-017-9693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan JY, Wong EW, Ng SK, et al. Non-nasopharyngeal head and neck lymphoepithelioma-like carcinoma in the United States: A population-based study. Head Neck. 2016;24215:1294–300. doi: 10.1002/hed.24215. [DOI] [PubMed] [Google Scholar]
- 15.Pripp AH, Stanišić M. Association between biomarkers and clinical characteristics in chronic subdural hematoma patients assessed with lasso regression. PLoS One. 2017;12:e0186838. doi: 10.1371/journal.pone.0186838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tibshirani R. Regression shrinkage and selection via the Lasso. Journal of the Royal Statistical Society Series B – Statistical Methodology. 1996;58:267–88. [Google Scholar]
- 17.Tibshirani R. Regression shrinkage and selection via the lasso: A retrospective. Journal of the Royal Statistical Society Series B – Statistical Methodology. 2011;73:273–82. [Google Scholar]
- 18.Amin MB American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed. New York: Springer; 2017. [Google Scholar]
- 19.Yang XL, Wang Y, Liang SB, et al. Comparison of the seventh and eighth editions of the UICC/AJCC staging system for nasopharyngeal carcinoma: Analysis of 1317 patients treated with intensity-modulated radiotherapy at two centers. BMC Cancer. 2018;18:606–16. doi: 10.1186/s12885-018-4419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min BH, Tae CH, Ahn SM, et al. Epstein-Barr virus infection serves as an independent predictor of survival in patients with lymphoepithelioma-like gastric carcinoma. Gastric Cancer. 2016;19:852–59. doi: 10.1007/s10120-015-0524-x. [DOI] [PubMed] [Google Scholar]
- 21.Yip TT, Ngan RK, Fong AH, et al. Application of circulating plasma/serum EBV DNA in the clinical management of nasopharyngeal carcinoma. Oral Oncology. 2014;50:527–38. doi: 10.1016/j.oraloncology.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Tang LQ, Li CF, Li J, et al. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. JNCI J Natl Cancer Inst. 2016;108:djv291. doi: 10.1093/jnci/djv291. [DOI] [PubMed] [Google Scholar]
- 23.He Q, Zhou Y, Fu C, et al. Lymphoepithelioma is a nonkeratinizing squamous cell carcinoma with Epstein-Barr virus infection in China. J Cancer Res Ther. 2017;13:807–12. doi: 10.4103/jcrt.JCRT_280_17. [DOI] [PubMed] [Google Scholar]
- 24.Petersson F. Nasopharyngeal carcinoma: A review. Semin Diagn Pathol. 2015;32:54–73. doi: 10.1053/j.semdp.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Cardesa A, Nadal A. Carcinoma of the head and neck in the HPV era. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:161–73. [PubMed] [Google Scholar]
- 26.Zhao Y, Shen L, Huang X, et al. High expression of Ki-67 acts a poor prognosis indicator in locally advanced nasopharyngeal carcinoma. Biochem Biophys Res Commun. 2017;494:390–96. doi: 10.1016/j.bbrc.2017.09.118. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Shen LJ, Li BF, et al. Smoking is a poor prognostic factor for male nasopharyngeal carcinoma treated with radiotherapy. Radiother Oncol. 2014;110:409–15. doi: 10.1016/j.radonc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Guo SS, Huang PY, Chen QY, et al. The impact of smoking on the clinical outcome of locoregionally advanced nasopharyngeal carcinoma after chemoradiotherapy. Radiat Oncol. 2014;9:246–54. doi: 10.1186/s13014-014-0246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Zhao BC, Chen C, et al. Alcohol drinking as an unfavorable prognostic factor for male patients with nasopharyngeal carcinoma. Sci Rep. 2016;6:19290. doi: 10.1038/srep19290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen ZH, Guo QJ, Lu TZ, et al. Pretreatment serum lactate dehydrogenase level as an independent prognostic factor of nasopharyngeal carcinoma in the intensity-modulated radiation therapy era. Med Sci Monit. 2017;23:437–45. doi: 10.12659/MSM.899531. [DOI] [PMC free article] [PubMed] [Google Scholar]