Abstract
Background
Zinc is a trace element with potent immunoregulatory and antiviral properties, and is utilized in the treatment of coronavirus disease 2019 (COVID-19). However, we do not know the clinical significance of serum Zinc levels in COVID-19 patients. The aim of this study was to determine the clinical significance of serum zinc in COVID-19 patients and to establish a correlation with disease severity.
Methods
This was a prospective study of fasting zinc levels in COVID-19 patients at the time of hospitalization. An initial comparative analysis was conducted between COVID-19 patients and healthy controls. COVID-19 patients with zinc deficiency were compared to those with normal zinc levels.
Results
COVID-19 patients (n = 47) showed significantly lower zinc levels when compared to healthy controls (n = 45): median 74.5 (interquartile range 53.4–94.6) μg/dl vs 105.8 (interquartile range 95.65–120.90) μg/dl (p < 0.001). Amongst the COVID-19 patients, 27 (57.4%) were found to be zinc deficient. These patients were found to have higher rates of complications (p = 0.009), acute respiratory distress syndrome (18.5% vs 0%, p = 0.06), corticosteroid therapy (p = 0.02), prolonged hospital stay (p = 0.05), and increased mortality (18.5% vs 0%, p = 0.06). The odds ratio (OR) of developing complications was 5.54 for zinc deficient COVID-19 patients.
Conclusions
The study data clearly show that a significant number of COVID-19 patients were zinc deficient. These zinc deficient patients developed more complications, and the deficiency was associated with a prolonged hospital stay and increased mortality.
Keywords: SARS-CoV-2, Zinc, COVID-19
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a major healthcare problem around the world, with significantly higher morbidity and mortality in patients with coexisting conditions such as diabetes mellitus and hypertension (Yang et al., 2020, Adhikari et al., 2020). The clinical presentation can be heterogeneous, ranging from asymptomatic to severe disease, which can be associated with a cytokine storm. The pathogenesis of COVID-19 is not fully understood, but is probably multifactorial, resulting in a systemic hyperinflammatory response and associated thromboembolic complications in severe cases (Yazdanpanah et al., 2020, Galimberti et al., 2020).
Zinc is a trace element with potent immunoregulatory and antiviral properties. Zinc is essential for growth, reproductive health, immunity, and neurobehavioral development (International Zinc Nutrition Consultative Group (IZiNCG) et al., 2004). The recommended daily intake of zinc ranges between 3 mg and 16 mg. Under physiological conditions, zinc is essential for cellular growth and the maturation of immune cells, particularly in the development and activation of T-lymphocytes (Wintergerst et al., 2006). Studies have shown that around 10% of our body proteins utilize zinc and that zinc is a cofactor in at least 200 immunomodulatory and antioxidant reactions (Iddir et al., 2020). Prolonged deficiency is associated with immune system dysfunction, sterility in males, neurosensory disorders, and decreased body mass (Prasad, 2008). Studies have also shown increased viral infection in patients with zinc deficiency (Read et al., 2019).
The antiviral property of zinc has been studied extensively in various viral infections, including coronavirus, hepatitis C virus, and HIV (Barocas et al., 2019). However, the exact role of zinc in SARS-CoV-2 is not well studied. The proposed mechanisms of the antiviral property of zinc include the inhibition of RNA synthesis, topoisomerase, and viral replication (Skalny et al., 2020).
To date, there is no definitive curative therapy for COVID-19. Therefore, the current treatment involves a multimodal approach with corticosteroids, antivirals, and anticoagulation therapy. Multivitamin supplements are not uncommon in ‘flu’ prescriptions. Supplementation with zinc is increasingly recommended in the management of COVID-19 patients (Alexander et al., 2020, Kumar et al., 2020). However, it is unclear whether these patients are actually deficient in zinc.
The aim of this study was to determine the clinical significance of serum zinc levels in COVID-19 patients and to establish a correlation with disease severity.
Methods
A prospective observational study was conducted from May 17 to May 27, 2020, in which serum zinc levels were tested in all consecutive SARS-CoV-2 RT-PCR-positive patients referred to Dr. Rela Institute and Medical Centre, Chennai, for the secondary and tertiary care management of COVID-19. This is a multi-speciality tertiary care institution, currently managing a significant volume of patients with COVID-19. After informed consent had been obtained, about 5 mL of blood was collected in a BD gel vacutainer following 6 h of fasting since the time of hospital admission. The biochemical analysis was performed on the serum sample after separation and the zinc level was measured with a fully automated Indiko Plus analyser (Thermo Scientific, USA) using a colorimetric method. The reference range used for the zinc concentration was 80–120 μg/dl. To verify the accuracy of the method, two levels of random controls were analysed (Randox chemistry control: Human Assay Control-2 LOT-1369 UN, Human Assay Control-3 LOT-1066 UE; Randox Immunoassay Control: level-1 LOT-1862, level-2 LOT-1877, and level-3 LOT 1867). Method performance was monitored by the analysis of the same control serum within the batches. The result obtained agreed with the certified values.
This study was performed following approval from the hospital ethics committee. Only SARS-CoV-2-positive adult patients admitted to the hospital during the study period were included. Patients already on zinc supplements, those who did not require hospital admission, and those who were unwilling to participate in the study were excluded from enrolment. Controls were hospital staff members from the outpatient department with no underlying comorbidities, who underwent a blood test to estimate zinc levels following informed consent.
A comparative analysis was conducted between COVID-19 patients and healthy volunteers. COVID-19 patients were further stratified according to their serum zinc concentration. A zinc level <80 μg/dl was defined as ‘deficient’. COVID-19 patients with zinc deficiency were identified and compared to those with normal zinc levels. Corticosteroid therapy was initiated in patients with ‘moderate’ disease, defined as the presence of any of hypoxia (saturation <92%) measured by pulse oximetry, the requirement for oxygen therapy, tachycardia, or tachypnoea, and in patients with ‘severe’ disease, defined as any of oxygen saturation <90%, hypotension, acute respiratory distress syndrome (ARDS), or end organ damage. All patients received hydroxychloroquine, antibiotics, and multivitamins, including vitamin C 500 mg twice a day and zinc 150 mg once a day (after the test), Patients with moderate and severe disease received additional subcutaneous anticoagulation for the duration of their hospital stay as the standard of care. Patients were managed in the intensive care unit in case of clinical deterioration causing haemodynamic instability and invasive ventilation.
A descriptive statistical analysis was performed for all variables using IBM SPSS Statistics version 21.0 (IBM Corp., Armonk, NY, USA), consisting of mean, standard deviation, percentage, median, and interquartile range (IQR; 25–75%). Proportions and associations between characteristics of the study groups were compared by Fisher’s exact test. The Mann–Whitney U-test and t-test were used to compare continuous variables between the study groups. A univariate logistic regression analysis was conducted to determine the odds ratio (OR) and 95% confidence intervals (95% CI). Results were considered statistically significant when the p-value was <0.05.
Results
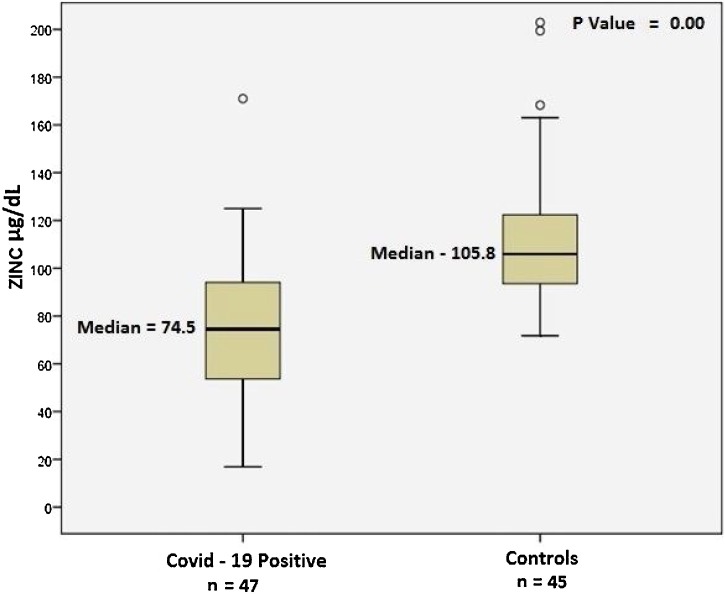
The comparative analysis of COVID-19 patients (n = 47) and healthy controls (n = 45) showed a median age of 34.0 years (range 18–77 years) versus 32.0 years (18–60 years) (p = 0.067) and a male to female sex ratio of 1.6:1 versus 2.1:1 (p = 0.09), respectively. COVID-19 patients had significantly lower zinc levels in comparison to the healthy controls: median 74.5 μg/dl (IQR 53.4–94.6 μg/dl) versus 105.8 μg/dl (IQR 95.65–120.90 μg/dl), p<0.001) (Figure 1). Five of the 45 healthy controls had low zinc levels (range 71.8–79.6 μg/dl).
Figure 1.
Serum zinc levels in patients with COVID-19 and healthy controls.
COVID-19 patients: zinc deficiency versus normal levels
Amongst COVID-19 (n = 47) patients, 27 (57.4%) were found to be zinc deficient. A comparative analysis was conducted between COVID-19 patients with zinc deficiency and those with normal zinc levels. Majority of patients presented with fever and cough, and there was no statistically significant difference in these symptoms between the groups (p = 0.481 and p = 0.121). Other symptoms included sore throat, myalgia, and gastrointestinal symptoms, which were observed in both groups with no significant difference between them. Pre-existing comorbid conditions such as age >60 years (7.4% vs 10%, p = 1.0), diabetes mellitus (14.8% vs 15%, p = 1.0), hypertension (14.8% vs 25%, p = 0.40), coronary artery disease (3.7% vs 20%, p = 0.70), pregnancy (7.4% vs 0, p = 1.0), hypothyroidism (3.7% vs 0, p = 0.5), rheumatoid arthritis (3.7% vs 0, p = 1.0), obesity (0 vs 5%, p = 0.42), and bronchial asthma (0 vs 5%, p = 0.42) did not differ significantly between the zinc deficient COVID-19 patients and those with normal zinc levels. At the time of hospitalization, four (8.5%) patients required non-invasive oxygen therapy ranging from 2 to 8 L and four (8.5%) required mechanical ventilation. The disease severity of COVID-19 on admission was mild, moderate, and severe in 21 (77.8%) vs 18 (90%), 1 (3.7%) vs 2 (10%), and 5 (18.5%) vs 0 (p = 0.09) patients with zinc deficiency and those with normal zinc levels, respectively (Table 1 ).
Table 1.
Comparison of variables in COVID-19 patients on admission: zinc deficient versus normal zinc level.
| Variables | Zinc deficient COVID-19 patients (n = 27) (57.4%) |
Normal zinc level COVID-19 patients (n = 20) (42.6%) |
p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years), median (IQR) | 33 (18–75) | 35 (27–77) | 0.546 | ||
| Male to female sex ratio | 1.7:1 | 3:1 | 0.529 | ||
| Asymptomatic | 1 | 3.7 | 2 | 10 | 0.567 |
| Fever | 20 | 74.1 | 17 | 85 | 0.481 |
| Cough | 12 | 44.4 | 4 | 20 | 0.121 |
| Sore throat | 5 | 18.5 | 1 | 5 | 0.221 |
| Loose stools | 4 | 14.8 | 4 | 20 | 0.707 |
| Myalgia | 6 | 22.2 | 4 | 25 | 0.5 |
| Nausea | 0 | 0 | 1 | 5 | 0.426 |
| Anosmia | 1 | 3.7 | 0 | 0 | 1.0 |
| Dyspnoea | 4 | 14.8 | 3 | 15 | 1.0 |
| Comorbidities | |||||
| Diabetes mellitus | 4 | 14.8 | 3 | 15 | 1.0 |
| Systemic hypertension | 4 | 14.8 | 5 | 25 | 0.405 |
| Coronary artery disease | 1 | 3.7 | 4 | 20 | 0.707 |
| Pregnancy | 2 | 7.4 | 0 | 0 | 1.0 |
| Hypothyroidism | 1 | 3.7 | 0 | 0 | 0.5 |
| Rheumatoid arthritis | 1 | 3.7 | 0 | 0 | 1.0 |
| Obesity | 0 | 0 | 1 | 5 | 0.426 |
| Age >60 years | 2 | 7.4 | 2 | 10 | 1.0 |
| Bronchial asthma | 0 | 0 | 1 | 5 | 0.426 |
| Laboratory indices, median (IQR) | |||||
| Bilirubin (mg/dl) (Normal 0.2–1.2) | 0.48 (0.35–0.48) | 0.57 (0.38–0.90) | 0.241 | ||
| AST (U/l) (Normal 0–45) | 28 (18–34) | 25 (18–32) | 0.639 | ||
| ALT (U/l) (Normal 0–47) | 18 (11–32) | 22 (19–28) | 0.517 | ||
| Creatinine (mg/dl) (Normal 0.5–1.3) | 0.80 (0.69–0.93) | 0.96 (0.64–1.05) | 0.166 | ||
| LDH (U/l) (Normal 135–225) | 264 (206.5–417.5) | 200 (169–242) | 0.006 | ||
| Ferritin (ng/mL) (Normal 28–397) | 216.0 (70.5–511.2) | 202.3 (98.7–313.4) | 0.622 | ||
| CRP (mg/l) (Normal <5) | 11.0 (3.5–48.5) | 3.6 (1.3–35.8) | 0.144 | ||
| D-dimer (ng/mL) (Normal <250) | 499.0 (237–603) | 158.5 (106.75–487.5) | 0.108 | ||
| Fasting glucose (mg/dl) (Normal 70–100) | 110 (93–128) | 101.5 (92.7–142.5) | 0.780 | ||
| Triglyceride (mg/dl) (Normal <150) | 103 (76–167) | 124 (101.2–190.2) | 0.165 | ||
| Vitamin D (ng/mL) (Normal 0–30) | 13.6 (11.3–25.7) | 19.3 (12.9–22.2) | 0.533 | ||
| Disease severity on admission | 0.09 | ||||
| Mild | 21 (77.8%) | 18 (90%) | |||
| Moderate | 1 (3.7%) | 2 (10%) | |||
| Severe | 5 (18.5%) | 0 | |||
AST, aspartate aminotransferase; ALT, alanine aminotransferase; CRP, C-reactive protein; IQR, interquartile range; LDH, lactate dehydrogenase.
In total, 14 (29.7%) patients received corticosteroids, commenced on day 5 (median 1–7 days) from the time of admission, of whom 12 (85.7%) had a zinc deficiency. Twelve patients received oxygen therapy during the hospital stay, including six patients on invasive mechanical ventilation.
Complications
Overall, zinc deficient patients developed more complications than those with normal levels: 19 (70.4%) vs 6 (30.0%), respectively (p = 0.009). A subgroup analysis showed that a higher number of patients in the zinc deficient group had ARDS (18.5% vs 0%, p = 0.063), hypotension (14.8% vs 0%, p = 0.126), and elevated interleukin-6 (IL-6) (33.3% vs 15%, p = 0.110) when compared to those with normal zinc levels (Table 2). Interestingly, the median peak IL-6 level was 67.8 pg/mL (IQR 23.8–158.1 pg/mL) vs 10.4 pg/mL (IQR 3.05–44.03 pg/mL) (p = 0.029) for zinc deficient and normal zinc level COVID-19 patients, respectively.
Table 2.
Complications in COVID-19 patients during hospital stay: zinc deficient versus normal zinc level.
| Complications | Zinc deficient COVID-19 patients (n = 27) (57.4%) |
Normal zinc levelCOVID-19 patients ( n = 20) (42.6%) |
p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Corticosteroids | 12 | 44.48 | 2 | 10 | 0.022 |
| ARDS | 5 | 18.5 | 0 | 0 | 0.063 |
| Hypotension | 4 | 14.8 | 0 | 0 | 0.126 |
| Sepsis | 1 | 3.7 | 0 | 0 | 1.0 |
| IL-6 > 7 pg/mL | 9 | 33.3 | 3 | 15 | 0.110 |
| Othersa | 2 | 7.4 | 1 | 5 | 1.0 |
| ICU | 7 | 25.9 | 2 | 10 | 0.266 |
| Hospital stay ≥7 days | 16 | 59.3 | 6 | 30 | 0.047 |
| Death | 5 | 18.5 | 0 | 0 | 0.06 |
ARDS, acute respiratory distress syndrome; ICU, intensive care unit; IL-6, interleukin 6.
Others: melena, hyponatremia, and hypokalemia; one in each patient.
A higher number of zinc deficient COVID-19 patients had prolonged hospital stay (≥7 days) when compared to those with normal zinc levels (59.2% vs 30.0%, p = 0.047); the mean hospital stay was 7.9 days and 5.7 days, respectively (t = 2.036, df = 44.7, p = 0.048). Similarly, more proportion of patients in the zinc deficient group received corticosteroids (44.4% vs 10%, p = 0.022) and required intensive care unit (ICU) care (25.9% vs 10%, p = 0.266) when compared to patients with normal zinc levels, and the recorded deaths were higher in the zinc deficient group: 5 (18.5%) vs 0 (0%), p = 0.06. The clinical and treatment characteristics of the patients who died are shown in Table 3 .
Table 3.
Clinical and treatment characteristics of patients with COVID-19 who died.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Age (years)/sex | 40/M | 51/F | 50/F | 72/F | 75/F |
| Comorbidities | CAD | DM, HT | DM, HT, hypothyroid | DM, HT | Nil |
| Initial symptoms | Fever Myalgia Cough |
Fever Anorexia |
Fever Dyspnoea |
Dyspnoea | Fever Dyspnoea |
| Duration of symptoms (days) | 2 | 5 | 7 | 3 | 2 |
| Complications | ARDS | Sepsis, ARDS | ARDS, MODS | ARDS | ARDS |
| Treatment | Methylprednisolone SupplementsaAntibiotics Enoxaparin |
Methylprednisolone SupplementsaPiperacillin –tazobactam Enoxaparin |
Methylprednisolone SupplementsaPiperacillin –tazobactam Enoxaparin |
Methylprednisolone SupplementsaPiperacillin –tazobactam Enoxaparin |
Methylprednisolone SupplementsaM eropenem Enoxaparin |
| Hospital stay (days) | 3 | 7 | 3 | 8 | 18 |
| Admission zinc level (μg/dl) | 36.4 | 47 | 57 | 59 | 81 |
| WBC count (×109/l) | 4.6 | 16.8 | 13.3 | 9.07 | 18.2 |
| Lymphocyte count (×109/l) | 0.73 | 0.67 | 1.33 | 1.18 | 1.27 |
| CRP (ng/mL) | 32.5 | 108.3 | 227 | 193.3 | 300.9 |
| Ferritin (ng/dl) | 979.8 | 203.6 | 636.5 | 514.5 | 1441 |
ARDS, acute respiratory distress syndrome; CAD, coronary artery disease; CRP, C-reactive protein; DM, type 2 diabetes mellitus; F, female; HT, systemic hypertension; M, male; MODS, multi-organ dysfunction syndrome; WBC, white blood cell.
Supplements: vitamin C 500 mg twice a day and zinc 150 mg once a day.
The OR of developing any complications in zinc deficient COVID-19 patients was 5.54 (95% CI 1.56–19.6, p = 0.008). On further analysis, the OR was 7.2 (95% CI 1.39–37.35, p = 0.02) for corticosteroid use, 3.39 (95% CI 0.99–11.57, p = 0.076) for hospital stay ≥7 days, 3.15 (95% CI 0.58–17.67, p = 0.266) for ICU admission, and 5.48 (95% CI 0.61–49.35, p = 0.129) for mortality.
Discussion
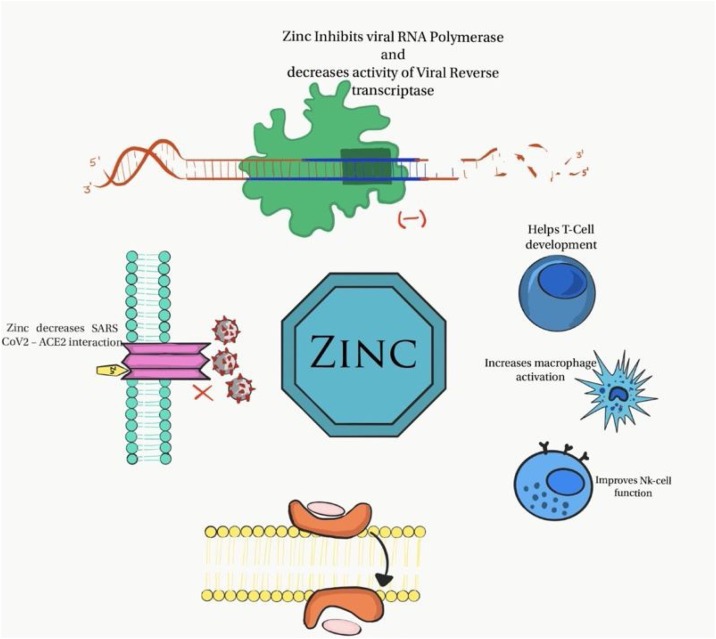
This appears to be the first clinical study correlating lower baseline zinc levels in patients with COVID-19 compared to healthy controls (74.5 μg/dl vs 105.8 μg/dl, p <0.001). Amongst COVID-19 patients, 57.4% (n = 27) were zinc deficient. However, we do not know whether zinc deficiency in these patients is a causation or an epiphenomenon. In vitro studies have shown that SARS-CoV-2 viral spike protein interacts with angiotensin-converting enzyme 2 (ACE2) and the serine protease transmembrane protease serine 2 (TMPRSS2) in the alveoli, permitting its entry into the cells. Interestingly, ACE2 is a zinc-dependent peptidyl dipeptide hydrolase composed of two subdomains (I and II), of which N-terminal containing subdomain I and C-terminus containing subdomain II are involved with zinc binding (Reeves and O’Dell, 1985). This process is facilitated and coordinated by amino acids His374, His378, Glu402 (HEXXH + E motif) and a molecule of water at subdomain I and by amino acids Arg169, Trp477, and Lys481 with a chloride ion at subdomain two (Towler et al., 2004). Earlier studies demonstrated that a decreased zinc level favours this interaction of ACE2 with SARS-CoV-2 spike protein and likewise that an increased zinc level inhibits ACE2 expression resulting in reduced viral interaction (Devaux et al., 2020, Li et al., 2020).
The zinc–viral particle interplay has been studied previously with other RNA viruses such as hepatitis C virus, coronavirus, HIV, and influenza virus (te Velthuis et al., 2010, Ferrari et al., 1999, Haraguchi et al., 1999, Ghaffari et al., 2019). Zinc supplements are traditionally prescribed for common cold ailments, usually caused by coronaviruses. Zinc supplements have been associated with a shortened duration of symptoms, reduced severity of illness, and more importantly with reduced morbidity and mortality in children (Wessels et al., 2020).
Zinc has been shown to exhibit antiviral properties by inhibition of RNA synthesis, viral replication, DNA polymerase, reverse transcriptase, and viral proteases (Read et al., 2019, Ko et al., 2018, Xue et al., 2014). However, the literature is unclear regarding SARS-CoV-2 and zinc. Interestingly, hydroxychloroquine, a drug used initially in the management of COVID-19, is an ionophore that transports zinc across the hydrophobic cell membrane (Xue et al., 2014, Rahman and Idid, 2020). Moreover, evidence specifically suggests that zinc supplements with antiviral drugs containing zinc ionophores precisely target and bind to SARS-CoV-2 preventing its replication within the infected host cells (te Velthuis et al., 2010). Intracellularly, zinc binds with RNA-dependent RNA polymerase causing elongation inhibition and decreased template binding of the viral mRNA (Rahman and Idid, 2020, te Velthuis et al., 2010).
Zinc plays a major role at various levels in the process of immune development and acts as an immunomodulator. Zinc deficiency has been associated with poor development of lymphoid tissue and reduced natural killer (NK) cell function leading to poor innate immunity (Shankar and Prasad, 1998). Zinc deficiency is associated with reduced macrophage activation and cytokine generation. Zinc is involved in T-cell and B-cell function. Thymulin, a zinc-dependent thymus hormone, binds to T-cell receptor and promotes T-cell maturation and cytotoxicity (Prasad, 2008). In addition, zinc deficiency is associated with downregulation of interferon gamma, resulting in severe impairment of cell-mediated immunity. Also, it enhances the production of interleukins, particularly IL-2, via activation of nuclear factor kappa B (NF-κB) (Prasad et al., 2001). The above in vitro studies indicate that zinc deficiency is associated with immune dysfunction and the risk of infection. The role of zinc in the immunology of SARS-CoV-2 infection definitely warrants further clinical research.
ADAM enzymes (A disintegrin and metalloproteinase) are zinc-dependent cell surface proteins of the adamalysin protein family known to play a major role in inflammation. ADAM 17 catalyses the activation of the proinflammatory cytokine tumour necrosis factor alpha (TNF-α) and conversion of membrane-bound (m)IL-6 to soluble (s)-IL6. Targeting the inhibition of ADAM 17 at the zinc cofactor site inhibits the enzyme, causing downregulation of inflammation by inhibiting these two pathways (Kato et al., 2006, Henry et al., 2020a).
These in vitro evidence suggests that zinc may have a pivotal role in COVID-19 (Figure 2). Therefore, zinc deficiency in COVID-19 patients may not be just a mere association. More studies are required to ascertain the relationship between COVID-19 and zinc.
Figure 2.
Illustration of antiviral and immunomodulatory properties of zinc in COVID-19.
The present study data clearly demonstrate a higher complication rate (70.4% vs 30.0%, p = 0.009) in zinc deficient COVID-19 patients, with an OR of 5.54. In addition, these patients showed an increased trend towards the development of ARDS (18.5% vs 0%, p = 0.06), longer hospital stays (mean 7.9 vs 5.7 days, p = 0.048), were more likely to have received corticosteroids (44.4% vs 10%, p = 0.02), and had increased mortality (5 (18.5%) vs 0 (0%), p = 0.06), indicative of a severe disease spectrum in these patients. This study showed an association between the baseline zinc level and COVID-19 disease course, such that zinc deficient patients encounter more complications and mortality.
Lactate dehydrogenase (LDH) is an intracellular enzyme, present in most cells, that catalyses the interconversion of pyruvate and lactate. LDH is a marker of organ injury, particularly related to hypoxia (Shi et al., 2020). A pooled analysis of 1532 COVID-19 patients showing elevated LDH found that this was associated with a 6-fold increased risk of severe disease and 16-fold increased risk of death (Henry et al., 2020b). The elevated LDH in the present study was probably indicative of severe disease as a result of zinc deficiency.
This was a single-centre study with a limited number of patients requiring hospitalization for COVID-19. It would be interesting to study the zinc level and its role across the entire spectrum of the disease, including asymptomatic patients with no comorbid conditions who are otherwise managed with home isolation. Moreover, it is unclear whether low zinc is a simple association or a causative factor in COVID-19. The literature and our understanding of zinc in COVID-19 patients is currently limited. Clearly, a multi-centre study is required to throw more light on this specific issue.
In conclusion, this study clearly demonstrated that COVID-19 patients were zinc deficient when compared to healthy adults. It is convincing that low baseline zinc levels in these patients were associated with more complications, leading to prolonged hospitalization and increased mortality. It is not clear whether zinc supplementation after admission to hospital helps to reduce the severity of disease. It is worth exploring the exact role of zinc in COVID-19 patients and establishing the appropriate dosage to improve their survival. With more research, zinc could provide a cost-effective therapy for COVID-19, certainly the need of the hour in this pandemic.
Funding
None declared.
Ethical approval
Ethical approval was obtained.
Conflict of interest
None.
CRediT authorship contribution statement
Dinesh Jothimani: Conceptualisation, Methodology, Formal analysis, Writing - original draft. Ezhilarasan Kailasam: Methodology, Investigation. Silas Danielraj: Data curation, Formal analysis, Writing - review & editing. Balaji Nallathambi: Data curation, Writing - review & editing. Hemalatha Ramachandran: Formal analysis, Software. Padmini Sekar: Writing - original draft. Shruthi Manoharan: Visualization. Vidyalakshmi Ramani: Writing - review & editing. Gomathy Narasimhan: Writing - review & editing. Ilankumaran Kaliamoorthy: Writing - review & editing. Mohamed Rela: Supervision, Validation, Writing - review & editing.
References
- Adhikari S.P., Meng S., Wu Y.J. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. doi: 10.1186/s40249-020-00646-x. Published 2020 March 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J., Tinkov A., Strand T.A., Alehagen U., Skalny A., Aaseth J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients. 2020;12(8):E2358. doi: 10.3390/nu12082358. Published 2020 August 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barocas J.A., So-Armah K., Cheng D.M. Zinc deficiency and advanced liver fibrosis among HIV and hepatitis C co-infected anti-retroviral naïve persons with alcohol use in Russia. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0218852. Published 2019 June 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.M., Raoult D. ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect. 2020;53(3):425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari E., Wright-Minogue J., Fang J.W., Baroudy B.M., Lau J.Y., Hong Z. Characterization of soluble hepatitis C virus RNA-dependent RNA polymerase expressed in Escherichia coli. J Virol. 1999;73(2):1649–1654. doi: 10.1128/JVI.73.2.1649-1654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti S., Baldini C., Baratè C. The CoV-2 outbreak: how hematologists could help to fight Covid-19. Pharmacol Res. 2020;157:104866. doi: 10.1016/j.phrs.2020.104866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffari H., Tavakoli A., Moradi A. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J Biomed Sci. 2019;26(1):70. doi: 10.1186/s12929-019-0563-4. Published 2019 September 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi Y., Sakurai H., Hussain S., Anner B.M., Hoshino H. Inhibition of HIV-1 infection by zinc group metal compounds. Antiviral Res. 1999;43(2):123–133. doi: 10.1016/s0166-3542(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Henry B.M., Aggarwal G., Wong J. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis [published online ahead of print, 2020 May 27] Am J Emerg Med. 2020;38:1722–1726. doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., Aggarwal G., Wong J. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.05.073. Epub ahead of print. PMCID: PMC7251362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iddir M., Brito A., Dingeo G. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12(6):E1562. doi: 10.3390/nu12061562. Published 2020 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Zinc Nutrition Consultative Group (IZiNCG), Brown K.H., Rivera J.A. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull. 2004;25(1 Suppl 2):S99–S203. [PubMed] [Google Scholar]
- Kato G.J., McGowan V., Machado R.F. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107(6):2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y.L., Morihara D., Shibata K. Factors attenuating zinc deficiency improvement in direct-acting antiviral agent-treated chronic hepatitis C virus infection. Nutrients. 2018;10(11):1620. doi: 10.3390/nu10111620. Published 2018 November 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Kubota Y., Chernov M., Kasuya H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19 [published online ahead of print, 2020 May 25] Med Hypotheses. 2020;144:109848. doi: 10.1016/j.mehy.2020.109848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x. Published 2020 April 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A.S. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14(5–6):353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A.S., Bao B., Beck F.W., Sarkar F.H. Zinc activates NF-kappaB in HUT-78 cells. J Lab Clin Med. 2001;138(4):250–256. doi: 10.1067/mlc.2001.118108. [DOI] [PubMed] [Google Scholar]
- Rahman M.T., Idid S.Z. Can Zn Be a critical element in COVID-19 treatment? [published online ahead of print, 2020 May 26] Biol Trace Elem Res. 2020:1–9. doi: 10.1007/s12011-020-02194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves P.G., O’Dell B.L. An experimental study of the effect of zinc on the activity of angiotensin converting enzyme in serum. Clin Chem. 1985;31(4):581–584. [PubMed] [Google Scholar]
- Shankar A.H., Prasad A.S. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68(2 Suppl):447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- Shi J., Li Y., Zhou X. Lactate dehydrogenase and susceptibility to deterioration of mild COVID-19 patients: a multicenter nested case-control study. BMC Med. 2020;18(1):168. doi: 10.1186/s12916-020-01633-7. Published 2020 June 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalny A.V., Rink L., Ajsuvakova O.P. Zinc and respiratory tract infections: perspectives for COVID-19 (Review) Int J Mol Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001176. Published 2010 November 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler P., Staker B., Prasad S.G. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J Biol Chem. 2004;279(17):17996–18007. doi: 10.1074/jbc.M311191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels I., Rolles B., Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. Published 2020 July 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintergerst E.S., Maggini S., Hornig D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50(2):85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- Xue J., Moyer A., Peng B., Wu J., Hannafon B.N., Ding W.Q. Chloroquine is a zinc ionophore. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109180. Published 2014 October 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanpanah F., Hamblin M.R., Rezaei N. The immune system and COVID-19: Friend or foe? [published online ahead of print, 2020 June 2] Life Sci. 2020;256:117900. doi: 10.1016/j.lfs.2020.117900. [DOI] [PMC free article] [PubMed] [Google Scholar]