The recently published Multi-Society Document from American Heart Association/American College of Cardiology/Heart Rhythm Society by Roden et al1 conveys an important alert on heart rate corrected QT interval (QTc) prolongation and Torsades-de-pointes risk associated with exploratory coronavirus disease 2019 (COVID-19) treatments. Indeed, accumulating evidence indicates that COVID-19 patients are burdened by a higher risk of malignant ventricular arrhythmias, with a potential contributing role of repurposed antiviral therapies.1,2 In particular, the authors underlined as among the exploratory COVID-19 treatments are included antimalarials (chloroquine and hydroxychloroquine), protease inhibitors (lopinavir/ritonavir), and azithromycin,1 all drugs listed as definite or possible causes of Torsades-de-pointes at https://www.crediblemeds.org.
In this view, recommendations about electrocardiographic/QTc monitoring along with a decisional guide for optimizing risk/benefit ratio when exploratory drugs are administered is of crucial importance.1 Moreover, the document also highlights as severely ill patients with COVID-19 are frequently burdened by comorbidities, specifically electrolyte imbalances, concomitant QT-prolonging drugs, and the high-grade systemic inflammatory state,1–3 further increasing Torsades-de-pointes susceptibility.1
However, while correcting hypokalemia and hypomagnesemia is recommended, the possibility of targeting inflammation to reduce arrhythmia risk has not been addressed, although supported by several data: (1) cytokine levels, particularly IL-6 (interleukin 6), are markedly elevated in severe COVID-19, where the anti-IL-6 receptor monoclonal-antibody tocilizumab seems to be able to reduce mortality2; (2) it has been demonstrated that IL-6 directly blocks the human-ether-a-go-go-related potassium channel2 and that high circulating IL-6 levels (≥10 pg/mL) due to different inflammatory diseases associate with QTc prolongation and Torsades-de-pointes development3; (3) IL-6 can also potently inhibit cytochrome p450-3A potentially increasing bioavailability of several QT-prolonging drugs (macrolides, azole antifungals, antidepressants, and antihistamines), as well as induce central hypothalamus-mediated cardiac sympathetic system hyperactivation, a well-recognized trigger for life-threatening arrhythmic events in patients with long-QT syndrome2; (4) in active rheumatoid arthritis, tocilizumab rapidly reversed QTc prolongation by controlling systemic inflammation.4
A recent study performed on a large cohort of hospitalized patients in the New York City area supports the inherent relevance of these mechanisms in COVID-19. In fact, at admission, that is, before treatment with exploratory antiviral drugs, a high percentage of patients who underwent ECG showed marked QTc prolongation, >500 ms (6.1%, 260/4250), along with elevated C-reactive protein levels (median 13.0 mg/dL).5
As a complement to the Multi-Society Document,1 we propose the perspective to consider administration of anti-IL-6 targeted drugs (tocilizumab and sarilumab) in COVID-19, not only in patients with signs of multi-organ dysfunction, but also in those with QTc>500 ms, particularly when IL-6≥10 pg/mL (Figure). In these subjects, by specifically dampening inflammation-driven arrhythmic risk, IL-6 blockade could reduce the need of withholding/withdrawing potentially useful COVID-19 repurposed pharmacotherapies. Although it is not currently known how to select patients in whom correction of QTc prolongation will improve overall outcome, nevertheless evidence indicates that a short-term anti-IL-6 treatment is safe, also potentially decreasing the extent of myocardial injury frequently observed in COVID-19. A phase II clinical trial evaluating for the first time the impact of tocilizumab in nonrheumatoid arthritis subjects with an acute cardiac damage demonstrated how in these patients, a single administration of tocilizumab reduced the inflammatory response and myocardial injury (troponin levels), with no safety concerns (including infections) in the following 6 months follow-up period.2
Figure.
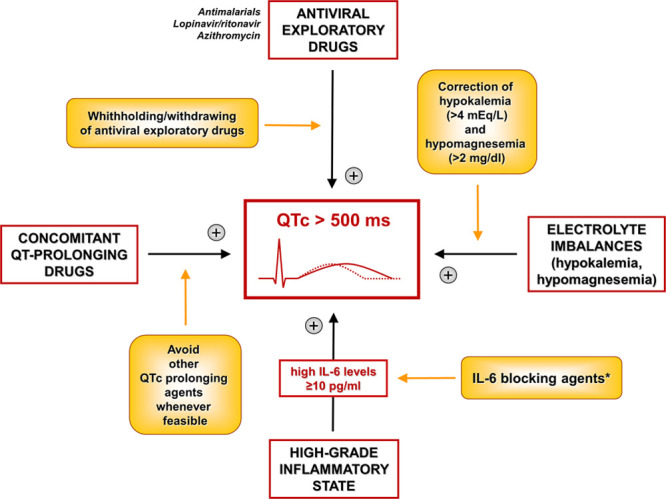
Proposal for an integrated management of heart rate corrected QT interval (QTc) prolongation in coronavirus disease 2019 (COVID-19), also including inflammation targeting with IL (interleukin)-6 blocking agents. Yellow boxes represent methods to reverse the mechanisms that related arrows point. *When all other more conventional reversible causes are addressed.
Sources of Funding
This work was funded by Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR), Progetti di Rilevante Interesse Nazionale (PRIN), Bando 2017, protocollo 2017XZMBYX.
Disclosures
Dr Lazzerini received a grant (minor funding) from Roche Italia S.p.A. in 2018. The other authors report no conflicts.
Nonstandard Abbreviations and Acronyms
- COVID-19
- coronavirus disease 2019
- IL-6
- interleukin 6
- QTc
- heart rate corrected QT interval
Drs Boutjdir and Leopoldo Capecchi contributed equally to this work.
Dr Laghi-Pasini retired.
For Sources of Funding and Disclosures, see page 1071.
References
- 1.Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for drug interactions on QTc in exploratory COVID-19 (Coronavirus Disease 2019) treatment. Circulation 2020141e906–e907doi: 10.1161/CIRCULATIONAHA.120.047521 [DOI] [PubMed] [Google Scholar]
- 2.Lazzerini PE, Boutjdir M, Capecchi PL. COVID-19, Arrhythmic risk, and inflammation: mind the gap! Circulation 20201427–9doi: 10.1161/CIRCULATIONAHA.120.047293 [DOI] [PubMed] [Google Scholar]
- 3.Lazzerini PE, Laghi-Pasini F, Bertolozzi I, Morozzi G, Lorenzini S, Simpatico A, Selvi E, Bacarelli MR, Finizola F, Vanni F, et al. Systemic inflammation as a novel QT-prolonging risk factor in patients with torsades de pointes. Heart 20171031821–1829doi: 10.1136/heartjnl-2016-311079 [DOI] [PubMed] [Google Scholar]
- 4.Lazzerini PE, Acampa M, Capecchi PL, Fineschi I, Selvi E, Moscadelli V, Zimbone S, Gentile D, Galeazzi M, Laghi-Pasini F. Antiarrhythmic potential of anticytokine therapy in rheumatoid arthritis: tocilizumab reduces corrected QT interval by controlling systemic inflammation. Arthritis Care Res (Hoboken) 201567332–339doi: 10.1002/acr.22455 [DOI] [PubMed] [Google Scholar]
- 5.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen S, et al. ; and the Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020232052–2059doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]


