Abstract
Aim: This study investigated the effects of e-waste occupational exposure on lipid profile and atherogenic indices in Waste Electrical and Electronic Workers in South-South Nigeria.
Matrials and methods: Whole blood levels of lead and cadmium were analyzed using ICPMS (Inductively Coupled Plasma Mass Spectrometry). Total serum cholesterol (TC), high density lipoprotein (HDL) cholesterol and triglycerides (TG) were determined using spectrophotometric method. Low density lipoprotein (LDL) cholesterol value was calculated by the Friedewald equation using analyzed values of TC, HDL cholesterol and TG. Atherogenic index of plasma (AIP) was calculated as log TG/HDLc, atherogenic coefficient (AC) as [(TC-HDLc)/HDLc], Castelli risk index (CRI-1) as (TC/HDLc) and CRI-II as (LDLc/HDLc).
Results: Total cholesterol, LDL cholesterol, AC, CRI-1 and CRI-11 significantly increased in the e-waste exposed participants compared to the unexposed group. Significant positive correlations between lead and cadmium, cadmium and total cholesterol as well as between cadmium and LDL-cholesterol were observed
Conclusion: Occupational exposure to e-waste borne chemicals may cause changes in lipid levels and increase risk of cardiovascular disease in the Nigerian e-waste workers included in the present study. The level of artisanal involvement in crude e-waste reprocessing should be considered critical in cardiovascular health risk assessment.
Keywords:cardiovascular disease, lead, cadmium, lipid profile, atherogenic indices, e-waste.
INTRODUCTION
Electronic waste (e-waste) has emerged as a critical global environmental health issue because of its massive production volume and insufficient management policy in many countries (1). Africa is reportedly a destination for several tonnes of uncontrolled e-waste (1, 24). E-waste products contain intricate blends of plastics and chemicals, which when not properly handled can be harmful to humans and pose environmental hazard (2). E-waste contains more than 1 000 different chemical substances, including toxic elements like lead, arsenic, cadmium, mercury, selenium and hexavalent chromium and flame retardants. The presence of toxic substances in e-waste classifies them as hazardous waste (3). Occupational exposure to heavy metals predisposes workers to metal toxicity. Human exposure to heavy metals has risen dramatically in the past 50 years as a result of an exponential increase in the use of heavy metals in industrial processes and products. The level of metals in blood depends on the bioaccessiblity rate (4) and is considered as an index of biologically active metals in the body reflecting the environmental exposure of a population.
Because of the long-term and widespread use of lead, it is one of the most ubiquitous of the toxic metals. The main targets of lead toxicity are the hematopoietic system and the nervous system. Several of the enzymes involved in the synthesis of haem are sensitive to inhibition by lead, the two most susceptible enzymes being ALA (5-aminolaevulinic acid) synthase and haem synthetase (HS). Lead also causes damage to the arterioles and capillaries, resulting in cerebral edema and neuronal degeneration. Another system affected by lead is the reproductive system. Lead exposure can cause male and female reproductive toxicity, miscarriages, and degenerate offspring (5). Another heavy metal that is of serious concern is cadmium. Cadmium is an extremely toxic substance and the major hazard is from inhalation of cadmium metal or cadmium oxide. Although it is present in food, significant oral ingestion is rare and absorption from the gut is poor (5–8%). However, various dietary and other factors may enhance absorption from the gastrointestinal tract (6). Cadmium toxicity can occur in industrial workers exposed to cadmium fumes (7). Cadmium may act on blood pressure (BP) through mechanisms related to oxidative stress (8), endothelial dysfunction, and partial agonism of calcium channels, increased vasoconstriction, and activation of the sympathetic nervous system (9).
Lipids are a group of fats and fat-like substances that are important constituents of cells and sources of energy when metabolized. Different plasma lipids vary greatly in various populations due to differences in geographical, cultural, dietary habits and genetic make-up (10, 11). Both experimental and epidemiological studies indicate that exposure to cadmium (Cd) may alter lipid metabolism and contribute to the development of cardiovascular diseases (CVD), including atherosclerosis, hypertension, stroke and cardiac arrest (12-14). Prabu et al (15) found out that the levels of total cholesterol (TC), triglycerides (TG), phospholipidis (PL), free fatty acids (FFA), low density lipoprotein (LDL) cholesterol and very low density lipoprotein (VLDL) cholesterol were significantly increased, while the level of HDL cholesterol was significantly decreased in the serum of Cd-treated rats. Studies have also shown that lead mediates the elevation of serum lipids and lipid peroxide through the mechanism of enzyme inhibition resulting in their accumulation in blood vessel walls (16-18). Heavy metal induced hyperlipidemia is a risk factor for the development of various cardiovascular related diseases and the impairment of liver function. Lipid profile and Atherogenic Indices in Nigerians Occupationally Exposed to e-waste: A Cardiovascular Risk Assessment Study Conclusion: Occupational exposure to e-waste borne chemicals may cause changes in lipid levels and increase risk of cardiovascular disease in the Nigerian e-waste workers included in the present study. The level of artisanal involvement in crude e-waste reprocessing should be considered critical in cardiovascular health risk assessment. Keywords: cardiovascular disease, lead, cadmium, lipid profile, atherogenic indices, e-waste. 198 Maedica A Journal of Clinical Medicine, Volume 15, No. 2, 2020 Ubani et al (19) observed that serum levels of LDL-cholesterol was elevated in individuals exposed to gas flaring containing heavy metals, and was suggested to be due to heavy metal mediated impairment of receptor-mediated endocytosis in the liver which prevents the binding of LDL to specific receptors that could lead to its degradation and release of cholesterol. LDL cholesterol transports cholesterol and phospholipids from the liver to the peripheral tissues and organs like the heart. It is responsible for the deposition of cholesterol on the walls of arteries causing atherosclerosis (18).
Furthermore, in the absence of an abnormal lipid profile the possibility of coronary artery disease (CAD) cannot be ruled out. It has been suggested that the different combinations of these lipid profile parameters can be used to identify such high risk individuals. Atherogenic index of plasma (AIP), atherogenic coefficient (AC) and Castelli risk index (CRI) are atherogenic indices which have been used in predicting the risk of CAD. These are the calculated fractions which can be used in the clinical setting for assessing the risk of cardiovascular disease beyond the routinely done lipid profile. AIP is based on two important parameters, TG and HDL cholesterol, both of which are independent risk factors for CAD (20), and it can be calculated as log TG/HDLc. Atherogenic coefficient is yet another ratio relying on the significance of HDL cholesterol in predicting the risk of CAD and it can be calculated as [(TC- HDLc)/HDLc]. Castelli risk index, calculated as (TC/HDLc) and (CRI-II) as (LDLc/HDLc), is another fraction which involves independent risk factors for CAD (21-23).
Thus, the present study aimed to investigate the effects of e-waste occupational exposure on lipid profiles and atherogenic indices in Nigerian workers in Benin City.
METHOD
Study population
The population of this study was centred on occupationally exposed e-waste workers in Benin City with at least five years duration of exposure and control subjects were recruited from apparently healthy non-exposed subjects residing in Ugbowo community, Benin City, Nigeria. Subjects’ mean age was 31 in the exposed group (n=63) and 49 in the unexposed group (n=41). All study participants were males, based on the fact that the e-waste reprocessing vocation is male dominated. The unequal number of participants in the two study groups was as a result of the number of participants who gave consent and satisfied the inclusion and exclusion criteria within the time frame of the study.
Selection criteria
Inclusion criteria: The exposed subjects comprised of electronic technicians carrying out informal (primitive) e-waste recycling, processing and dismantling/repair of electronic and electrical equipment. Subjects who were occupationally exposed to e-waste for a period of five years and above at the time of sample collection were selected for the study. The five years duration of exposure used in this study was based on the E-waste Risk Assessment Report (24). Control subjects were healthy male individuals with minimal or no occupational exposure to e-waste toxic metals and no hobby involving e-waste exposure or other toxic substances. The non-exposed participants had no previous demographic and medical history of hypertension (24).
Exclusion criteria: e-waste workers with less than five years exposure to e-waste toxic metals at the time of sample collection were excluded from the study. Subjects with history of hypertension, tobacco smoking and alcohol ingestion have been also excluded. A history of previous cardiovascular disease (myocardial infarction/ angina/ cerebrovascular accident) was an exclusion criterion. Tobacco smoking and alcohol consumption have also served as a basis of exclusion for recruiting the apparently healthy control subjects (24).
Data and sample collection
A pretested structured questionnaire containing both open and closed ended questions was administered to all subjects to obtain socio-demographic information and information about exposure burden. Blood pressure measurement was taken manually with a sphygmomanometer and then documented. Approximately 10 millilitres of venous blood was collected from test subjects (e-waste workers and control subjects) using standard phlebotomy technique. Blood samples obtained were dispensed into EDTA anticoagulant specimen bottles (5 millilitres). Another five millilitres was dispensed into anticoagulant free specimen bottles to obtain serum. All samples were appropriately preserved till time of analysis (25). Height and weight were measured using a measuring tape and spring balance respectively and the body mass index (BMI) was calculated from both using the formula (26): BMI = mass (kg)/[(height(m)]².
Reagent and sample preparation for cadmium analysis
Whole blood dilution was made (1 in 7 dilutions) with 0.2% HNO₃ and 0.1% Triton X-100. Cadmium standard working solution (1 µg/L) was made by dilution with distilled water. Matrix modifier solution was prepared by mixing 50 µL of 0.3 g/L of Mg(NO₃)₂ and 1 mL of 0.33 g/L of Pd(NO₃)₂ and 2 mL of 0.2% v/v nitric acid 50 µL of 0.1% Triton X-100.
Study population
The population of this study was centred on occupationally exposed e-waste workers in Benin City with at least five years duration of exposure and control subjects were recruited from apparently healthy non-exposed subjects residing in Ugbowo community, Benin City, Nigeria. Subjects’ mean age was 31 in the exposed group (n=63) and 49 in the unexposed group (n=41). All study participants were males, based on the fact that the e-waste reprocessing vocation is male dominated. The unequal number of participants in the two study groups was as a result of the number of participants who gave consent and satisfied the inclusion and exclusion criteria within the time frame of the study.
Procedure for cadmium analysis
Cadmium was analyzed using electrothermal atomic absorption spectrometer, ETAAS (Perkin Elmer analyst 800, Norwalk, U.S.A) method (27). The light source (hollow cathode lamp) specific for cadmium was inserted into the ETAAS. The wavelength was adjusted to 228.8 nm. The instrument was standardized with the standard blank (1% HNO3) and cadmium standard. Aliquot of 20 µL of whole blood was injected directly into the graphite furnace. Equal volume of matrix modifier was injected into the graphite furnace. The concentration of cadmium was displayed (in µg/L)on the screen after the run time (four minutes).
Reagent and sample preparations for lead analysis
Lead standard working solution was diluted with distilled water and 1 in 13 dilution of whole blood was made with 0.2% HNO₃ and 0.1% Triton X-100. Matrix modifier solution 50 µL of 10 g/L of NH₄H₂PO₄, was mixed with 2 mL of 0.2% v/v Nitric acid and 0.1% Triton X-100.
Procedure for lead analysis
Lead was analyzed using electrothermal atomic absorption spectrometer (Perkin Elmer analyst 800, Norwalk, U.S.A) method (27). The hollow cathode lamp specific for lead was inserted and the wavelength was adjusted to 283.3 nm. The instrument was standardized and calibrated with standard blank and lead standard. An aliquot of 20 µL of whole blood was injected directly into the graphite furnace. Equal volume of matrix modifier solution was also injected into the graphite furnace. The concentration lead in the sample was displayed on the screen after the run time (three minutes).
Estimation of total cholesterol
Total serum cholesterol was measured enzymatically (28) in the serum sample. The principle is based on series of coupled reactions that hydrolyse cholesteryl esters and oxidize 3-OH group of cholesterol. One of the reaction by-products, hydrogen peroxide (H₂O₂), is measured quantitatively in a peroxidase catalyzed reaction that produces a colour (pink). Absorbance is read at 500 nm. Colour intensity is proportional to cholesterol concentration. The cholesterol reagent (Agappe Diagnostic, Switzerland) comprised of pipes buffer (50 mmol/L, pH 6.9), phenol (24 mmol/L), sodium cholate (0.5 mmol/L), cholesterol esterase (⩾200 U/L), cholesterol oxidase (⩾250 U/L), peroxidase (⩾1 000U/L) and 4-aminoantipyrine (0.5 mmol/L). Concentration of standard solution was 5.13 mmol/L.
Procedure for total cholesterol
One thousand µL of cholesterol reagent was dispensed into two clean test tubes respectively. Ten µL of serum sample and 10 µL of cholesterol standard were then added into the tests respectively. The set up was mixed gently and incubated at room temperature for 10 minutes. Absorbance was read at 500 nm against reagent blank using a spectrophotometer. The concentration of cholesterol (mmol/L) in the sample was calculated using this formula: = Absorbance of test/Absorbance of standard×Concentration of standard
Estimation of HDL cholesterol
HDL cholesterol was estimated by direct method (29). The principle is based on the fact that lipoproteins present in the specimen (LDL, VLDL and chylomicrons) are precipitated quantitatively by the addition of phoshotungstic acid in the presence of magnesium ions. This renders them non-reactive and effectively excluded from the assay. Thus, after centrifugation, only HDL cholesterol was detected. HDL cholesterol reagent (Agappe Diagnostic, Switzerland) comprised of phosphotungstate (14 mmol/L) and magnesium chloride (1 mmol/L). HDL cholesterol standard concentration is 0.28 mmol/L.
Procedure for HDL cholesterol
Equal volumes of serum sample and HDL cholesterol reagent (300 µL each) were mixed in a test tube and allowed to for 10 minutes at room temperature. The tube content was mixed again and centrifuged for 10 minutes at 4 000 revolution per minute. After centrifugation, the clear supernatant was separated from the precipitate and the HDL cholesterol concentration was determined using the above described cholesterol reagent (29).
The concentration of HDL (mmol/L) in the sample is calculated using this formula: = Absorbance of test/Absorbance of standard×Concentration of standard
Triglyceride (TG) estimation
TG concentration was estimated by Gpo-pap method (30). The principle is based on enzymatic technique of measuring TG in serum using a series of coupled reactions in which TG are hydrolysed to produce glycerol. Glycerol is then oxidized using glycerol oxidase and hydrogen peroxide (H₂O₂), one of the reaction products, is measured as described for cholesterol. Absorbance is measured at 500 nm. TG reagent (Randox, UK) is comprised of reagent A and reagent B. Reagent A is made up of pipes buffer (40 mmol/L, pH 7.60), 4-chloro-phenol (5.5 mmol/L) and magnesium ions (17.5 mmol/L), while reagent B is comprised of 4-aminophenazone (0.5 mmol/L), adenosine triphosphate (1.0 mmol/L), lipases with activity of .1.5 U/mL glycerol-kinase (⩾0.4 U/mL), glycerol-3-phosphate oxidase (⩾1.5 U/mL) and peroxidase (⩾0.5 U/mL).
Procedure for TG
Equal volumes of reagent A and B (1 000 µL) was mixed and dispensed into two clean pre-labelled test tubes. 10µl of serum sample and 10µl of TG standard was added respectively. The set up was mixed gently and incubated at room temperature for 10 minutes. Absorbance was read at 500 nm against reagent blank using a spectrophotometer.
Low density lipoprotein
Low density lipoprotein (LDL) cholesterol value was calculated using the Friedewald equation (31). This was calculated based on the measured values of total cholesterol, triglycerides and HDL-cholesterol according to the following relationship: LDL-cholesterol = TC – HDL – [TG]/ 5.
Atherogenic (lipid) indices
Atherogenic ratios were calculated from the lipid profile parameters.
Atherogenic index of plasma (AIP) = log TG/HDLc (20)
Atherogenic coefficient (AC) = (TC– HDLc)/HDLc (23)
Castelli’s risk index (CRI-I) = TC/HDLc (21)
Castelli’s risk index (CRI-II) = LDLc/HDLc (21)
Statistical analysis
Results were analyzed using Student’s t-test and Pearson’s correlation coefficient with SPSS software, Version 16. A p value < 0.05 was considered as significant.
RESULTS
Basic health indices of participants
Basic health indices were compiled for both workers exposed to e-waste and non-exposed participants (Table 1). The mean blood pressure and mean pulse rate were higher in exposed subjects compared to the non-exposed. A higher level of BMI of exposed group was also observed compared to the non-exposed group.
Participants’ blood lead and cadmium levels
Blood lead and cadmium levels of e-waste exposed workers and non-exposed participants are shown in Table 2. Blood lead and cadmium levels were significantly higher in subjects who were occupationally exposed to e-waste compared to non-exposed ones.
Lipid profile and atherogenic ratios of participants
The lipid profile results showed a significant increase in total cholesterol and LDL cholesterol in exposed subjects compared to the non-exposed group. There was no significant difference in triglyceride and HDL cholesterol concentrations in both exposed and non-exposed groups. No significant increase of AIP was observed in the exposed group compared to the non-exposed one. AC, CRI-1 and CRI-11 were significantly increased in exposed subjects
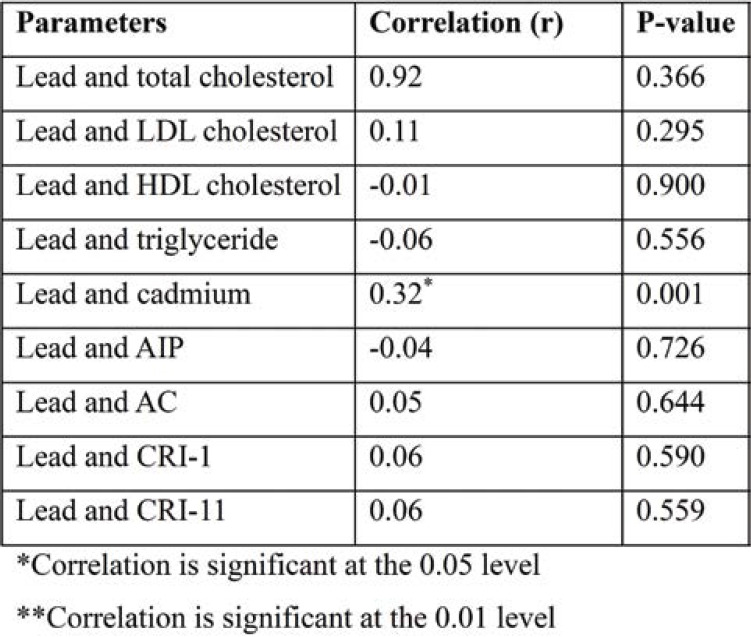
Correlation of lead with lipid profile and atherogenic indices
Table 4 shows Pearson correlation of lead levels with lipid profile and atherogenic indices. There was a significant positive correlation between lead and cadmium. Other correlation results were not significant.
Correlation of cadmium with lipid profile and atherogenic indices
Correlations between cadmium and lipid profile as well as atherogenic indices are shown in Table 5. There was a significant positive correlation between cadmium and total cholesterol as well as between cadmium and LDL cholesterol. Other correlation results were not significant.
DISCUSSIONS
The effects of heavy metals toxicity on occupationally exposed subjects have been well documented (8, 32). These metals cause oxidative stress through the generation of reactive oxygen species (ROS) and damage to lipids, proteins and DNA (33). This study was designed to determine the effects of lead and cadmium on serum lipid profile and atherogenic ratios of workers occupationally exposed to e-waste in the assessment of risk for cardiovascular disease.
From the health indices of the present study, the mean blood pressure in exposed subjects (systolic 125.23±2.15 and diastolic 75.53+1.77) was higher compared to the non-exposed subjects (systolic 119.84±1.90 and diastolic 70.34±1.67). The mean pause rate was also higher in the exposed group (79.86±1.65) compared to the unexposed group (73.98±1.6). A higher level of BMI of the exposed group (24.24) has been also observed compared to the non-exposed group (21.72). Other researchers have demonstrated that increasing atherogenic index associated with an elevation in BMI was an independent predictor of cardiovascular diseases (34).
Blood lead and cadmium levels were significantly higher in subjects occupationally exposed to e-waste than non-exposed ones. These increased levels may be attributed to prolonged exposure to these metals (at least five years). Lead and cadmium do not have any known beneficial biological roles; rather their detrimental effects on physiological, biochemical, and behavioural dysfunctions have been documented in both animals and humans by several researchers (35, 36).
stress, metals are classified into redox-active and redox-inactive metals. Redox active metals include iron, copper, chromium, manganese, and other transition metals, while redox inactive metals include lead, cadmium, mercury, and arsenic. Both groups deplete major cellular antioxidants by different mechanisms. Redox-active metals are able to undergo fenton-like reaction to exaggerated oxidative stress, while redox- inactive metals deplete antioxidants especially thiol-containing antioxidants and enzymes (32).
Cadmium has been known to stimulate the formation of metallothioneins and reactive oxygen species (ROS), thus causing oxidative damage to erythrocytes and various tissues resulting in loss of membrane functions (37). Lead induces oxidative stress as a result of an imbalance between generation and removal of ROS in tissues and cellular components, causing damage to membranes, DNA and proteins (1989).
Reactive intermediates produced under conditions of oxidative stress cause the oxidation of polyunsaturated fatty acids (PUFAs) in membrane lipid bilayers, eventually leading to the formation of aldehydes (39). Among these, the most abundant aldehydes are 4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA), while acrolein is the most reactive one. MDA, acrolein and HNE are α, β-unsaturated electrophilic compounds, which preferentially form 1,4-Michael type adducts with nucleophiles, such as proteins and DNA (39). The formation of aldehyde adducts with apolipoprotein B (Apo B) in LDL converted the latter to an atherogenic form that was taken up by macrophages, leading to the formation of foam cells (40, 41). In this way, lipid peroxidation products formed from oxidative stress caused by toxic metals can reduce blood vessel diameter, increase pulse rate and blood pressure.
The lipid profile results showed a significant increase in total cholesterol and LDL cholesterol in exposed subjects compared to the non-exposed group. There was no significant difference in triglycerides and HDL cholesterol concentrations in both exposed and non-exposed groups (0.40±0.04 and 0.41±0.05; 2.09±0.12 and 2.13±0.14, respectively). The elevated level of total cholesterol and the insignificant level of TG agreed with that reported in occupational workers exposed to lead reported by Kristal-Boneh et al (42). However, there was inconsistency in the levels of LDL cholesterol and HDL cholesterol (42). A significant increase in TG has been also reported by Afolabi et al (6) in rats exposed to cadmium. According to Lariegle et al (14) exposure to cadmium caused increase in both TG and LDL. Extensive evidence of an association between serum lipid and lipoprotein levels and coronary artery disease (CAD) has been well documented (43-45). The elevated level of total cholesterol may be due to an increased synthesis of cholesterol through stimulation of the rate limiting enzyme, 3-hydroxyl-3-methyl-glutaryl coenzyme A (HMG COA) reductase by cadmium or lead (46). The significant increase in LDL may be attributed to increased synthesis, reduced activity of lipoprotein lipase or loss of LDL receptor function, thus leading to reduced catabolism and elevated LDL (46). LDL cholesterol has been shown to play an important role in the development of atherosclerosis, which is a risk factor for cardiovascular (CVS) diseases (47). LDL cholesterol atherogenic property is of clinical relevance because studies have also shown its major role in foam cell formation as a result of its oxidative modification (48). The HDL cholesterol level of e-waste exposed group was not significantly different from that of the non-exposed group. HDL cholesterol has been shown to have protective property against CAD (49).
In the present study, after the evaluation of the atherogenic indices, no significant increase in AIP was observed in the exposed group compared to the non-exposed one. AC, CRI-1 and CRI-11 were significantly increased in exposed subjects compared to non-exposed ones. Atherogenic indices have been found to be useful in predicting risk of developing CAD even in the absence of abnormalities in the lipid profile (50). For instance, studies have shown that AIP ratio is a strong predictor of myocardial infarction (51). However, in the current study, AIP was not significantly different between the exposed and non-exposed groups, and the obtained value (-0.74±0.06) was below the reference range used in assessing the risk of developing cardiovascular disease. A range of -0.3 -0.1 is classified as low risk, 0.1-0.24 is regarded as medium risk and above 0.24 is classified as high risk (50). Atherogenic coefficient (AC) was significantly higher in the exposed group compared to the non-exposed one. AC index has been shown to reflect the atherogenic potential of entire spectrum of lipoprotein fractions and is useful for therapy management (50). In the present study, CRI-1 and CRI-11 have also shown a significant increase in the exposed group compared to the non-exposed one. Previous studies have shown that CRI-1 and CRI-11 was associated with the formation of coronary plaques (52). However, lipid indices obtained for CRI-1 and CRI-11 were below the reference ranges (>4 and >3, respectively). This does not rule out the fact that these exposed subjects have the tendency of developing CAD, as the effect may become paramount in the future following longer period of exposure to these heavy metals, since they are chronically and persistently exposed.
Pearson correlation findings reveal a significant positive correlation between lead and cadmium as well as between cadmium and total cholesterol, and between cadmium and LDL cholesterol. Also, our findings suggested that continuous exposure to cadmium would cause a corresponding increase in total cholesterol and LDL cholesterol concentrations. It could be inferred that this may lead to oxidative stress through generation of bioactive aldehydes (such as 4 hydroxyalkenals, malondialdehyde, MDA and acrolein), which are toxic messengers and are capable of propagating and amplifying oxidative injury in these participants (50, 53).
CONCLUSION
Occupational exposure to e-waste borne chemicals may cause changes in lipid levels and an increased risk of cardiovascular disease in Nigerian e-waste workers. The potential clinical implication and benefit of this study is that the level of artisanal involvement in crude e-waste reprocessing should be considered critical in cardiovascular health risk assessment.
Financial support: This work was supported by TET Fund (Tertiary Education Trust) Fund – TET Fund Institutional Support Research Grant University of Benin), 2014/2015.
Conflicts of interest: none declared.
Acknowledgments: I wish to acknowledge the efforts of Edouwaye Lina Igharo, Dr. S. U. Ezenkwa, Uche Chukwumelie Zedech and Dr Nnenna Linda Nwobi in the course of this research, as well as those of Mr Kingley Ikeke, Igiewe Osaze and David Aiyanyor. Also, the authors would like to thank the e-waste workers and unexposed participants in Benin City, Nigeria, for giving their informed consent to participate in the study.
TABLE 1.
Basic health indices of e-waste exposed workers and non-exposed participants
TABLE 2.
Blood lead and cadmium levels of e-waste exposed workers and unexposed participants
TABLE 3.
Lipid profile and atherogenic indices of e-waste exposed and unexposed participants
TABLE 4.
Association between positive reassessment, positive refocusing and putting into perspective and time to medical consultation
TABLE 5.
Association between positive reassessment, positive refocusing and putting into perspective and time to medical consultation
Contributor Information
Osaretin Godwin IGHARO, Department of Medical Laboratory Sciences, School of Basic Medical Sciences, College of Medical Sciences, University of Benin, Benin City, Nigeria.
Yinka AKINFENWA, Department of Community Health, College of Medical Sciences, University of Benin, Benin City, Nigeria.
Alphonsus R. ISARA, Department of Community Health, College of Medical Sciences, University of Benin, Benin City, Nigeria Department of Community Health, University of Benin Teaching Hospital, Benin City, Edo State, Nigeria.
Festus Aigbokheo IDOMEH, Department of Medical Laboratory Sciences, School of Basic Medical Sciences, College of Medical Sciences, University of Benin, Benin City, Nigeria.
Nnenna L. NWOBI, Department of Chemical pathology, Ben Carson School of Medicine, Babcock University, Ilisan Remo, Ogun State, Nigeria
John I. ANETOR, Department of Chemical Pathology, Toxicology and Micronutrient Metabolism Unit, College of Medicine, University of Ibadan, Ibadan, Nigeria
Oladele OSIBANJO, Department of Chemistry and Basel Convention Coordinating Centre for Training and Technology Transfer for the Africa Region, University of Ibadan, Oyo State, Nigeria.
References
- 1.Ogunseitan OA, Schoenung JM, Saphores JD, Shapiro AA. Science and regulation. The electronics revolution: from e-wonderland to e-wasteland. Science. 2009;326:670–671. doi: 10.1126/science.1176929. [DOI] [PubMed] [Google Scholar]
- 2.Leung AW, Duzgoren-Aydin NS, Cheung KC, Wong MH. Heavy metal concentrations of surface dust from e-waste recycling and its human health implications in Southeast China. Science Technology Environmental. 2008;42:2674–2680. doi: 10.1021/es071873x. [DOI] [PubMed] [Google Scholar]
- 3.Widmer R. Global perspectives on e-waste. Environmental Impact Assessment Review. 2005;25:436–458. [Google Scholar]
- 4.Khan S, Lin AJ, Zhang SZ, et al. Accumulation of polycyclic aromatic hydrocarbons and heavy metals in lettuce grown in the soils contaminated with long-term wastewater irrigation. J Hazard Mater. 2008;152:506–515. doi: 10.1016/j.jhazmat.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Cope WG, Leidy RB, Hodgson E. Classes of Toxicants: Use Classes. A Textbook of Modern Toxicology. 3rd edition. John Wiley and Sons, Inc., Hoboken, New Jersey. 2004. pp. 49–74.
- 6.Afolabi OK, Oyewo EB, Adekunle AS, et al. Impaired Lipid Levels and Inflammatory Response in Rats Exposed to Cadmium. EXCLI Journal. 2012;211:677–687. [PMC free article] [PubMed] [Google Scholar]
- 7.Crook MA. Disorders of Haem Metabolism: Iron and Porphyrias. Clinical Biochemistry and Metabolic Medicine. 8th ed. Hodder and Stoughton Ltd, London. 2012. pp. 224–234.
- 8.Valko M, Morris H, Cronin MT. Metals, toxicity and oxida-tive stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 9.Varoni MV, Palomba D, Gianorso S, Anania V. Cadmium as an environmental factor of hypertension in animals: New perspectives on mechanisms. Vet Res Commun. 2003;1:807–810. doi: 10.1023/b:verc.0000014277.06785.6f. [DOI] [PubMed] [Google Scholar]
- 10.Abubakar A, Mabruok MA, Gerie AB, et al. Relation of body mass index with lipid profile and blood pressure in healthy female of lower socioeconomic group, in Kaduna, Northern Nigeria. Asian Journal of Medical Sciences. 2009;3:94. [Google Scholar]
- 11.Hart C, Ecob R, Smith GD. People, Places and Coronary Heart Disease Risk Factors: a Multilevel Analysis of the Scotish Heart Health Study. Arch Soc Sci Med. 1997;45:893. doi: 10.1016/s0277-9536(96)00431-5. [DOI] [PubMed] [Google Scholar]
- 12.Satarug S, Baker JK, Urbenjapol S, et al. A global perspective on cadmium pollution and toxicity in nonoccupationally exposed population. Toxicol Lett. 2003;137:65. doi: 10.1016/s0378-4274(02)00381-8. [DOI] [PubMed] [Google Scholar]
- 13.Subramanyan G, Bhaskar M, Govindappa S. The role of cadmium in induction of atherosclerosis in rabbits. Indian Heart J. 1992;44:177–180. [PubMed] [Google Scholar]
- 14.Larregle EV, Varas SM, Olivreos LB, et al. Lipid metabolism in liver of rat exposed to cadmium. Food Chem.Toxicol. 2008;46:1786–1792. doi: 10.1016/j.fct.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Prabu SM, Muthumani M, Shagirtha M. Quercetin potentially attenuates cadmium induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. European Review for Medical and Pharmacological Sciences. 2013;17:582–595. [PubMed] [Google Scholar]
- 16.Alvares AP, Kapelner S, Sassa S, et al. Lead intoxication: effects on cytochrome P‐450 mediated hepatic oxidations. Clin Pharmacol Ther. 1976;19:183–190. doi: 10.1002/cpt1976192183. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Niiya Y, Kurita H, Shima S, Sarai S. Serum lipid peroxide level and blood superoxide dismutase activity in workers with occupational exposure to lead. Int Arch Occup Environ Health. 1985;56:119–127. doi: 10.1007/BF00379383. [DOI] [PubMed] [Google Scholar]
- 18.Egwurugwu JN, Nwafor A, Chinko BC, et al. Effects of Prolonged Exposure to Gas flares on the lipid profile of Humans in the Niger Delta region, Nigeria. American Journal of Research Communication. 2013;5:115–145. [Google Scholar]
- 19.Ubani CS, Joshua EP, Amiara VO. Toxicological effects of Kerosene contaminated diet on the lipid profile of Albino Rats. Journal of Pharmacy Research. 2010;2:292–297. [Google Scholar]
- 20.Milada D. Atherogenic Index of Plasma [Log(Triglycerides/HDLCholesterol)]: Theoretical and Practical Implications. Clin Chem. 2004;7:1113–1115. doi: 10.1373/clinchem.2004.033175. [DOI] [PubMed] [Google Scholar]
- 21.Stampfer MJ, Sacks FM, Salvini S, et al. A. Prospective Study of Cholesterol, Apolipoproteins, and the Risk of Myocardial Infarction. N Engl.J Med. 1991;325:373–381. doi: 10.1056/NEJM199108083250601. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 23.Brehm A, Pfeiler G, Pacini G, et al. Relationship between Serum Lipoprotein Ratios and Insulin Resistance in Obesity. Clin Chem. 2004;50:2316–2322. doi: 10.1373/clinchem.2004.037556. [DOI] [PubMed] [Google Scholar]
- 24.Adaramodu AA, Osuntogun BA, Ehi-Eromosele CO. Heavy Metal Concentration of Surface Dust Present in E-waste Components: The Westminister Electronic Market, Lagos Case Study. ARPN Journal of Science and Technology. 2012;2:247–270. [Google Scholar]
- 25.Cheesbrough, M. Blood Collection: District Laboratory Practice in Tropical Countries, Part 2. University Press, New York, Cambridge, 2006. pp. 234–251.
- 26.Romero-Corral A, Somers V, Sierra-Johnson J, Thomas R, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J of Obesity. 2008;32:959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olmedo P, Pia A, Hernández AFO, et al. Validation of a method to quantify chromium, cadmium, manganese, nickel and lead in human whole blood, urine, saliva and hair samples by electrothermal atomic absorption spectrometry. Analytica Chimica Acta. 2010;2:60–67. doi: 10.1016/j.aca.2009.11.056. [DOI] [PubMed] [Google Scholar]
- 28.Abell LL, Levy BB, Brodie BB, Kendall FE. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952;195:357. [PubMed] [Google Scholar]
- 29.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1958;6:24–27. [Google Scholar]
- 30.Tietz NW. Serum triglyceride determination: Clinical Guide to Laboratory Tests. Saunders Co, Philadelphia. 1990. pp. 554–556.
- 31.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 32.Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic Metals and Oxidative Stress Part 1: Mechanisms Involved in Metal Induced Oxidative Damage. Cur Tropics in Medicinal Chem. 2001;6:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 33.Patra RC, Rautray AK, Swarup D. Oxidative Stress in Lead and Cadmium Toxicity and Its Amelioration. Veterinary Medicine International. 2011. pp. 1–9. [DOI] [PMC free article] [PubMed]
- 34.Oladipo A. Plasma lipid profile, atherogenic and coronary risk indices in some residents of Abeokuta in south-western Nigeria. Biokemistry. 2008;2:85–91. [Google Scholar]
- 35.Goyer RA, Cherian MG. Ascorbic acid and EDTA treatment of lead toxicity in rats. Life Sciences. 1979;5:433–438. doi: 10.1016/0024-3205(79)90215-7. [DOI] [PubMed] [Google Scholar]
- 36.Ruff HA, Markowitz ME, Bijur PE, Rosen JF. Relationships among blood lead levels, iron deficiency, and cognitive development in two-year-old children. Environmental Health Perspectives. 1997;2:180–185. doi: 10.1289/ehp.96104180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patra RC, Swarup D, Senapat SK. Effects of cadmium on lipid peroxides and superoxide dismutasein hepatic, renal and testicular tissue of rats. Veterinary and Human Toxicology. 1999;2:65–67. [PubMed] [Google Scholar]
- 38.Halliwell B, Gutteridge JMC. Protection against oxidants in biologyogical systems: the superoxide theory of oxygen toxicity. Free Radical in Biology and Medicine, B. Halliwell and J. M. C. Gutteridge (Eds.), Clarendon Press, Oxford, UK. 1989. pp. 86–123.
- 39.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg D, Parthasarathy S, Carew TE. Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N EngI J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg D. In celebration of the 100th anniversary of the lipid hypothesis of atherosclerosis. J Lipid Res. 2013;54:2946–2949. doi: 10.1194/jlr.R043414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kristal-Boneh E, Coller D, MOccH PF, et al. The Association Between Occupational Lead Exposure and Serum Cholesterol and Lipoprotein Levels. American Journal of Public Health. 1999;7:1085. doi: 10.2105/ajph.89.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawber TR, Kannel WB, Revotskie N, Kagan A. The epidemiology of coronary heart disease. The Framingham inquiry. Proc R Soc Med. 1962;58:551–565. doi: 10.1177/003591576205500403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stamler J. Diet, serum lipids, and coronary heart disease: the epidemiologic evidence. Levy RI, Riflind BM, Dennis BH, Ernst N (Eds.). Nutrition, Lipids, and Coronary Heart Disease-A Global View. Raven Press, New York, NY. 1979. pp. 34–36.
- 45.Lin RC, Dai J, Lumeng L, Zhang M. Serum Low Density Lipoprotein of Alcoholic Patients is Chemically Modified in vivo and Indices Apolipoprotein E Synthesis by Macrophages. J Clin Invest. 1999;5:1979–1986. doi: 10.1172/JCI117882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Twisk J, Gillian-Daniel DL, Tebon A, et al. The role of the LDL receptor in apolipoprotein B secretion. J Clin Invest. 2000;105:521–532. doi: 10.1172/JCI8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown G, Albers JJ, Fisher LD, et al. Regression of the coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 48.Witztum JL, Steinberg D. Role of oxidized LDL in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Von Eckardstein A, Nofer JR, Assmann G. High density lipoproteins and arteriosclerosis: role of cholesterol efflux and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 1991;21:13–27. doi: 10.1161/01.atv.21.1.13. [DOI] [PubMed] [Google Scholar]
- 50.Bhardwaj J, Bhattacharjee MK, Bhatnagar ST. Atherogenic Index of Plasma, Castelli Risk Index and Atherogenic Coefficient – New Parameters in Assessing Cardiovascular Risk. International Journal of Pharmacy and Biological Sciences. 2013;3:359–364. [Google Scholar]
- 51.Gaziano JM, Hennekens CHO, Donnell CJ, et al. Fasting triglycerides, high density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96:2520–2525. doi: 10.1161/01.cir.96.8.2520. [DOI] [PubMed] [Google Scholar]
- 52.Nair D, Carrigan TP, Curtin RJ, et al. Association of total cholesterol/ high-density lipoprotein cholesterol ratio with proximal coronary atherosclerosis detected by multislice computed tomography. Prev Cardiol. 2009;1:19–26. doi: 10.1111/j.1751-7141.2008.00011.x. [DOI] [PubMed] [Google Scholar]
- 53.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]