ABSTRACT
The purpose of this study is to mine CAR-T patents and therapies under development, to design a landscape of the sector and to understand key therapy segments and their current trends. The study analyzed the entire market, consisting of 1624 patent families and 509 biologics under development, to depict an overview of the CAR-T therapies and their state of the art. Our results showed cutting-edge inventions, the major players, the dynamics of cooperation among institutions, the progress of the therapies’ generation over the years and future innovation pathways. CAR-T therapies are transforming the current scenario for cancer treatment, and this study reveals the picture of what we can likely expect ahead in order to assist scientists at the academy and industry to improve their research strategies.
KEYWORDS: Patent landscape, technology forecasting, clinical trials, CAR-T cells
1. Introduction
Chimeric antigen receptor (CAR) T cell therapy is an innovative and revolutionary type of immunotherapy for cancer treatment, in which patient’s T cells are isolated, genetically engineered, expanded, and then infused back into patients where they continue to expand and destroy cancer cells.1 This field was launched in 1989, when Zelig Eshhar, an Israeli immunologist from The Weizmann Institute of Science, created the engineered recombinant receptor molecule known as a chimeric antigen receptor (CAR).2,3 This was the proof of concept that allowed for the generation of genetically engineered patient-derived T cells targeting a specific antigen, independently of MHC-TCR interaction. In the 1990s, researchers began to use these genetically engineered T cells to ht cancer.4,5
CAR specificity comes from the extracellular domain, derived from the antigen-binding site of a monoclonal antibody. A single-chain variable fragment is generated by linking the variable region heavy and light chains. A flexible peptide linker is included, and this extracellular domain is fused to an intracellular domain via a transmembrane sequence.6,7 The intracellular domain is designed to recapitulate the normal series of events by which T cells are activated. In addition, the incorporation of costimulatory signals to the CAR makes CAR-T cells less susceptible to negative regulation by the host cells, which occurs in normal immune responses against cancer. Therefore, a CAR construct reprograms T cells into “killer cells”, targeting a specific cancer antigen and these cells can persist in the patient’s body for years helping immune surveillance.6,7
The process of modifying the intracellular domain to increase effectiveness led to the development of first-, second-, third-, and, recently, fourth-generation CARs. First-generation CARs consisted of only the TCR complex CD3-zeta chain domain and antigen recognition domains.2,3 The use of first-generation CARs was explored in clinical trials that included patients with several types of cancers. These studies, however, showed only modest efficacy mainly due to insufficient T-cell persistence in vivo.8-11 Subsequently, second-generation CARs were developed, which incorporated costimulatory domains, such as CD28 or 4-1BB (CD137), thus increasing CAR-T cell survival and proliferation, ultimately leading to improved antitumor efficacy.5-14 Third-generation CARs combine the CD3-zeta domain with more than two costimulatory domains; nonetheless, its superiority over second-generation CARs has not yet been completely demonstrated.15 Finally, the fourth CAR generation, still in its early development, incorporates other genes to empower the antitumor activity of CAR-T cells16 (Table 1). Recently, other genetic modifications (i.e. receptor design or different vectors used for gene delivery) have been evaluated in pre-clinical (in vitro or in vivo biological testing) and clinical trial.15
Table 1.
The evolution of CARs' design.
| CAR-T generation | Description |
|---|---|
| First | The first-generation has a modular design, including a specific cancer-targeting antibody outside the cell, a transmembrane component, and an intracellular costimulatory signaling domain (i.e. CD3z) that amplifies the activation of the CAR T cells.1,2 |
| Second | Second-generation CARs contain an extra intra cellular costimulatory domain (i.e. CD28, 41-BB, CD27, or OX-40).5,12-14 |
| Third | Third-generation CARs have two costimulatory domains within the cells.15 |
| Fourth | Fourth-generation CARs incorporate other genes to empower the antitumor activity of CAR-T cells. They are second-generation CARs with the addition of gene cassettes for cytokines or other genes that are controlled through antigen binding to the CARs.16 |
On August 30, 2017, a historic milestone was achieved when CAR-T cell therapy was officially approved by the FDA, announcing a new era of cancer treatment. The Novartis CAR-T cell product, tisagenlecleucel (Kymriah®), was approved for children, young adults aged 25, and under who relapsed or were not responding to conventional therapy for acute lymphoblastic leukemia (ALL). Subsequently, on October 18, 2017, the FDA approved axicabtagene ciloleucel (Yescarta®), for patients with relapsed or refractory diffuse large B cell lymphoma (DLBCL) and other rare large B-cell lymphomas. Recently, The European Commission (EC) has also approved both products.
Based on this initial success, currently, CAR-T cell therapies are being developed and tested for other hematologic neoplasms and for many different solid tumors. Figure 1 shows a timeline of CAR-T cell therapy development, highlighting the main milestones.
Figure 1.
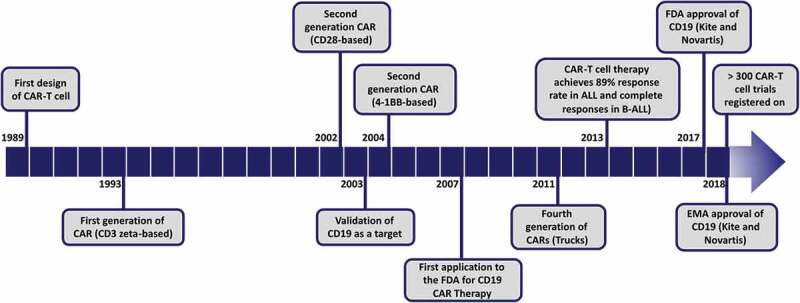
Timeline of CAR-T cell therapy development and progress. The main milestones from the first design of genetically modified T cells with a chimeric antigen receptor (CAR) to the first products approved by FDA and EMA are represented in the timeline.
The intense development of new CAR-T therapies is marked by an active use of the intellectual property rights by the companies along with robust R&D investments. Herein, we provide the patent and clinical landscape of CAR-T technologies with the current scenario of clinical trials, the cutting-edge CAR-related inventions, the main players and how they interact with each other to develop new technologies. This study also depicts a snapshot of the CAR-T cell therapies motivated by their applications and their intrinsic development. Finally, we provide a technological route showing the emerging trends in the face of current R&D challenges. The Derwent Innovation database, from Clarivate Analytics, was used to search specifically for Car-T cell patents and for CAR-T Cell therapies that are under development and for lead compounds; we used the Integrity Database from Clarivate Analytics (described in the supplementary material).
2. Evaluation of the global CAR-T cell therapy patent landscape
CAR-T cell patents were selected from the Derwent Innovation Database (Clarivate Analytics), a complete patent database delivering access to globally trusted documents from more than 40 jurisdictions and includes expert English translations of patent content. For this propose, we used a subject-specific search query (Supplementary material). We identified 1624 patent families worldwide in regard to the application years ranging from 1998 to 2017. Since CAR-T cell therapy is a rising trend in biotechnology, most of these patents have not reached clinical trials yet, as they represent early-stage technologies. The patent application process is usually prior to the pre-clinical phase, so, some of the technologies selected have a true potential to reach the market if they perform well in clinical trials. Most of the selected patents deal with different aspects of CAR-T cell technology, including usage, preparation methods and composition of matter. The top CAR-T inventor is Carl June, the pioneer researcher in the field of immunotherapy (Figure S1). June appears in first place with 69 patents followed by Martin Pulé, the founder of Autolus Therapeutics, with 57 patents. Regarding the main assignees, The University of Pennsylvania appears in first place with 144 patents, followed by NIH (represented by the US Depart of Health and Human Services) with 83 patents, and Novartis and Memorial Sloan-Kettering Cancer Center in third place with 74 patents each (Figure 1A). Among the top 20 assignees, only two do not demonstrate any cooperation, which is measured by co-ownership of patents, Shanghai UniCAR Therapy Co Ltd and Achillion Pharm. In contrast, the leading top four assignees of CAR-T cell patents showed rates of cooperation of up to 40%, which means that an intensive collaborative effort to generate new technologies is somehow important in this domain. Novartis has only five proprietary patents and 93% of its CAR-T patent portfolio are in cooperation (Figure 2B). Collaboration between universities, companies, and hospitals in the field of immunology is not very frequent, because their interests are different. Campos E. et al. showed that the majority of patent applications in immunology are applied for by only one entity.17 However, CAR-T cell treatment is a complex process and requires a great level of collaboration between doctors, health centers and manufacturers and the patents reveal this high rate of cooperation. We also analyzed the countries where CAR-T cell patent applications were first filed (Figure 2B). The geographical distribution according to the priority countries showed that the largest concentration of patents comes from the United States and China. Although both countries are actively developing new technologies and clinical trials, the United States stands out by presenting nearly three times more patent applications than China.
Figure 2.
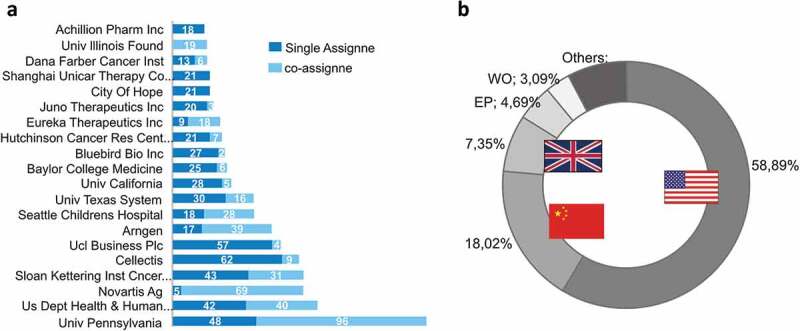
The top patent assignees and priority countries related to CAR-T cell technology. (a) Top 15 assignees in terms of a number of patent families split by the proprietary technology as single assignee and co-assignee (in cooperation), showing that most of the companies establish partnerships that resulted in patent application. (b) Number of patent applications per priority country. PCT Patents are represented as WO and patent applied to the European office, as EP.
Innovative CAR-T cell therapies are strongly dependent on cooperation among institutions, as mentioned before. In order to clarify how cooperation occurs, a network using patent information from assignees was generated (Figure 3). Overall, the assignees that compose the largest component of the network show five different clusters, the biggest cluster has the US Depart of Health and Human Services, the University of Pennsylvania and the General Hospital as central actors. The connections among different partners are not so intensive since assignees have an average of two partners (average degree = 2.6) resulting in an average of around seven shared patents (average weighted degree = 7.18). The most influential players according to the eigenvector centrality (EC) are the US. Depart. Of Health and Human Services (EC = 1) and the University of Pennsylvania (EC = 0.86). Among the interactions, Sloan Kettering has an important role within the network since it forms a cooperation bridging two influential players, the US Depart. Of Health and Human Services, and the General Hospital, this is the link with the highest betweenness.
Figure 3.
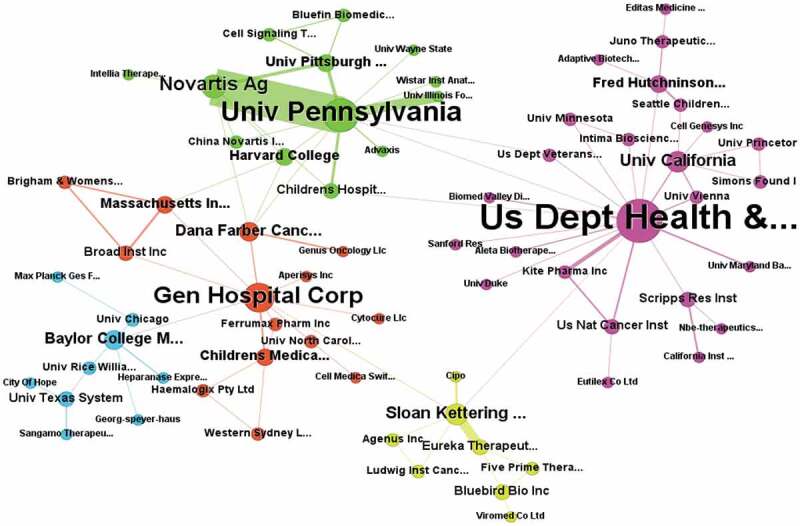
Cooperation network among assignees of CAR-T cell technologies. Information of co-assignment patents was used as a proxy to build the network. Different colors and the node’s size represent the communities and numbers of different partners, respectively.
Lately, the intensive research and investment efforts to discover new CAR-T therapies make the sector one of the most dynamic. From 2005 to 2009, only a few CAR-T cell patents were filed, most of them revealing the CAR concept and the first steps of its clinical applications. Since 2012, however, the number of patent filings has been growing quickly, which has led to an increase in the number of registered clinical trials (2014 to 2018) (Figure 4). The clinical translation process has also been accelerating along with therapy with CAR-T cells. This process begins with pre-clinical development and then goes onto increasingly complex and demanding phases of clinical trials. Invariably, in addition to the expertise of researchers, a robust research infrastructure and significant funds are needed for the clinical application of new cellular technologies. In the case of CAR-T cell therapy, clinical studies in children and adults with acute lymphoblastic leukemia (ALL) have shown impressive initial response rates, ranging from 70% to 90%, showing the great efficiency of treatment.18 What is more, good results have been achieved for B-cell non-Hodgkin’s lymphoma (NHL), especially for aggressive B-cell lymphomas. Single-center and multicenter clinical trials with CAR T-cell therapy anti-CD19 have shown long-term remissions in poor-risk diffuse large B-cell lymphoma (DLBCL).19
Figure 4.
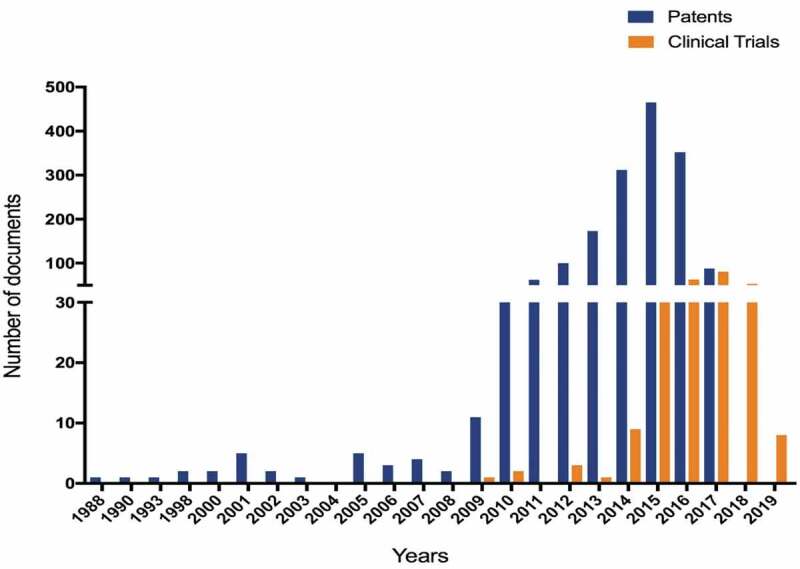
Number of patent applications and clinical trials being conducted over the years.
3. Preclinical studies and clinical trials of CAR-T cell therapy: current scenario
To identify major trends in the discovery and development of novel “CAR-T candidate drugs”, we have analyzed available data from the Integrity database (Clarivate Analytics, USA) (Supplementary information). On September 15, 2018, there were 679 candidate drugs registered, which were subdivided into groups according to their development status and main target antigen explored (Figure 5A and B). The majority of them, representing 40%, are in biological testing phase, i.e. the earliest stage of development. Candidate drugs at this stage include products from patents and studies reporting preliminary pharmacology data (in vitro testing). Progressing to a later phase occurs only if there is information published (annual reports or clinical data) indicating that they have progressed. A high percentage of the studies is in the pre-clinical development phase (in vivo testing), 34%. Phase I and Phase I/II represent 14% and 9% of the studies reported, respectively (Table S1).
Figure 5.
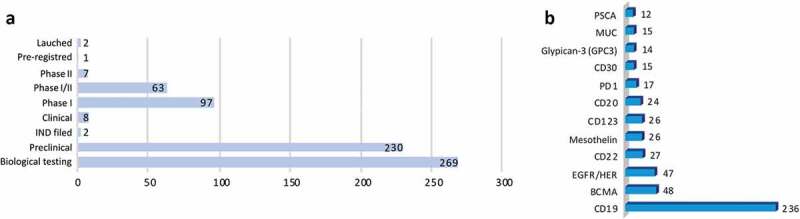
(a) Development status and (b) main target antigen explored in the CAR-T drugs. Analysis performed with data available in the Integrity database (Clarivate Analytics, USA) on September 15, 2018.
The most studied target antigen is the CD19 (32%, 218 candidate drugs), a cell surface protein restricted to B cells and B cell precursors, which is also expressed on the surface of malignant B-cells in patients with B-cell leukemias or lymphomas.1,20,21 Following CD19 antigen, the other two closely studied targets are the B cell maturation antigen (BCMA) (7%, 48 candidate drugs) and epidermal growth factor receptor (EGFR/HER) (7% 47 candidate drugs). BCMA is a target related to plasma cell cancers and has been used to treat myeloma patients. EGFR is used for solid tumors, such as childhood sarcoma, kidney cancer, and neuroblastoma. We also analyzed the characteristics of CAR constructs that are under preclinical and clinical investigation. Among the 679 candidate drugs, 435 describe the CAR generation that is being used (Figure 6A and Table S1). Of these, the majority are from second generation, which have only one co-stimulatory signal (72%). The third generation appears in second place (19.5%) followed by first (6.7%) and fourth (2%).
Figure 6.
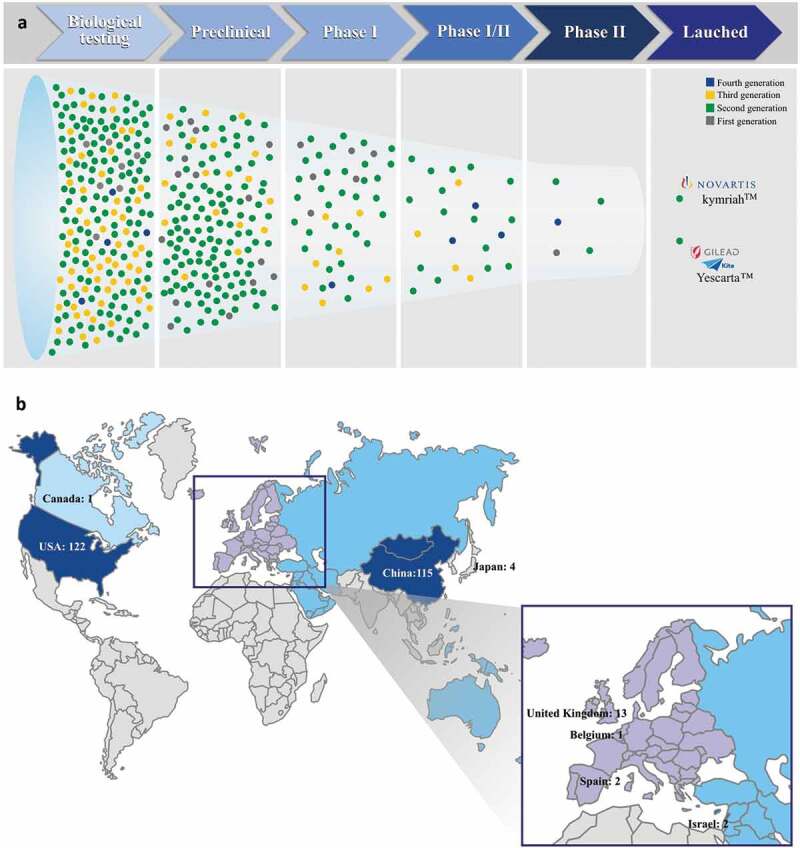
(a) Generation of CAR constructs that are under pre-clinical and clinical investigation. (b) Number of clinical studies being conducted worldwide. Analysis performed with data available in the Integrity database (Thomson Reuters, USA) on September 15, 2018.
The majority of CARs in clinical late phase (Phase I/II and II) are also from the second generation (Figure 6A). The current two FDA-approved CAR-T products, Kymriah® (Novartis) and Yescarta® (Kite Pharma), are also second-generation products and target CD19+ cancer B cells.
Four clinical trials at Phase I/II are being conducted with CAR-T cells containing a third-generation construct (Chinese PLA General Hospital, Janssen/Nanjing Legend, Autolus/UCLB, and Uppsala University). Three of them contain the co-stimulatory molecules CD28 and CD137 and one CD28 and OX-40. In addition, three trials (Phase I/II) use a fourth-generation CAR. These CARs have incorporated as an additional element, an inducible caspase-9 gene, which can lead to the self-destruction of CAR-T cells by apoptosis, thus increasing the safety of the clinical trials.
In late-phase Phase II, besides the trials using thesecond generation, there is one first-generation CAR (Great Ormond Street Hospital Child NHS/UCL). In this trial an anti-CD19 CAR construct is used to transduce Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes (CTL) for treatment of pediatric ALL. The group uses a first-generation CD19CAR, relying on the endogenous T-cell receptor for proliferation.22
The late-phase trials (Phase I/II and II) are being conducted by academic institutions and pharmaceutical companies (Figure 6A), with a close collaboration between them. Fred Hutchinson Cancer Research Center and Juno Therapeutics are involved in three second-generation CAR-T trials each. Celgene, Chinese PLA General Hospital and Shanghai Genechem are involved in two second-generation CAR-T trials each. Academic institutions, either alone or in collaboration with industry players, have demonstrated a critical role in the target discovery, design, and development of novel CAR-T therapies. This strong collaboration between clinical and basic scientists is crucial to move forward the proof-of-concept stage through to the translational stage toward clinical implementation.
Most clinical studies in progress use autologous CAR-T cells. Nevertheless, recently allogeneic CAR-T cells have appeared as an attractive approach to allow for a greater number of patients to have access to CAR-T cell therapies. Since CAR-T cell therapies are autologous cellular products, customized for each patient, to enable successful clinical progression, “ready-to-use” allogeneic universal cellular products should be developed. In this context, Cellectis S.A. and Celyad S.A., two biopharmaceutical companies focusing on the development of CAR-T therapies, have invested in allogeneic CAR-T therapies. Cellectis has developed universal CARs (UCAR), with the first clinical trial using UCRA19 starting in Europe in June 2016 and in the U.S. in 2017. More recently, Cellectis received FDA approval for Phase 1 clinical trial for UCAR-T22, Cellectis’ second controlled TALEN® gene-edited product candidate for the treatment of B-cell acute lymphoblastic leukemia (B-ALL) in adult patients (http://www.cellectis.com/en/products/ucarts). This year, the FDA also accepted Celyad IND (CYAD-101) the first non-gene-edited allogeneic CAR engineered to express the chimeric antigen receptor NKG2D, a receptor expressed in natural killer (NK) cells that binds to eight distinct ligands expressed on tumor cells (https://www.celyad.com).
Clinical trials represent a critical step for the translation of newly developed therapies to clinical practice. By September 2018, 11 years after the first clinical study with the new experimental drug (IND) had been registered, 261 clinical trials were being conducted employing 180 candidate drugs, according to Integrity. Just from January 2018 to September 2018, 28 new CAR-T cell clinical trials were registered in the same database, which reflects the enormous activity of academy and industry in this area. Among the ongoing trials the vast majority are being conducted in the USA (122 clinical trials) and China (115 clinical trials) (Figure 6B), corroborating with the data of patent applications per priority country. As previously mentioned, the USA and China are also the countries that register most patents in this area.
As previously emphasized, cooperation is fundamental to implement CAR-T cell therapies in the clinic. Therefore, we also analyzed the cooperation networks of the 679 candidate drugs under development. Thus, we generated a network including assignees and the related candidate drugs under development (Supplementary Information Figure 2). The University of Pennsylvania, Baylor College of Medicine (BCM) and Memorial Sloan-Kettering Cancer Center (MSKCC) were highlighted in the network due to the volume of CAR-T candidate drugs under development. From these, 155 are co-developed by two or more institutions, a rate of 37%. The two main interconnected components of the cooperation network have 53 institutions (nodes) and 77 collaborations among them (edges) (Figure 7).
Figure 7.
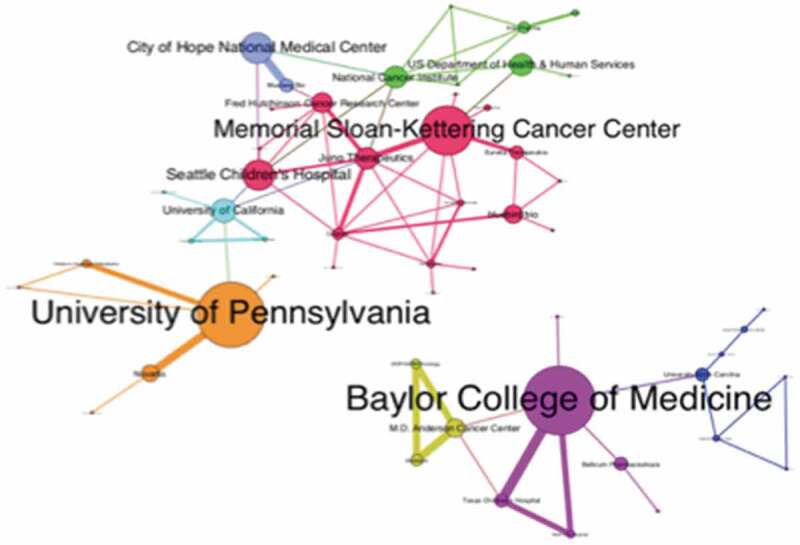
Cooperation network in CAR-T cell technology development. The giant component of the cooperation network showing different communities identified by the metrics of modularity.
The University of Pennsylvania and Novartis entered into a collaboration in 2012 to develop and commercialize CAR-T therapies. This successful collaboration resulted in the first therapy based on gene transfer approved by the FDA.23 The collaboration between NCI (National Cancer Institute/NIH) and Kite allowed for the translation of NCI CAR-T, developed in the mid- to late-2000s, into the clinic, being the second CAR-T Cell Therapy approved by the FDA.24 Figure 5 also highlights a close collaboration between BCM, MD Anderson Cancer Center and ZIOPHARM Oncology for the development of non-viral adoptive cellular cancer immunotherapies. BCM has also collaborated with Bellicum, which exclusively licensed the worldwide rights to the invention CaspaCIDe® platform from BCM. The invention relates to the on-demand in vivo elimination of therapeutic cells, such as CAR-T, which have been modified to express the iCasp9 protein which is responsive to rimiducid, enabling the elimination of the cells. MSKCC has a close interaction with Juno, BlueBird, and Eureka Therapeutics focusing on CAR-T research for Myelo6ma. These collaborations mainly take place between universities and industry experts looking to conduct well-designed research to generate high-value patents that can be used in clinical trials and lead to an efficient treatment.
4. Patent technological route
In order to identify emerging technologies among the 1624 patents, we used the technological route (TR) methodology previously described.25 Thus, we use the backward citation data from all the CAR-T cell patents to map the technological route and identify emerging technologies. The resulting technological route includes 19 patents and the arrow directions show the relationship between citing patent and cited patent (Figure 8). The route reveals some interesting information about three trends in CAR-T inventions. The oldest patents in the TR deal with technologies describing the mechanism and concepts of CAR-T cells, represented by the eight patents at the end of TR, further enabled the emergence of the highly cited patent US7446190B2, which describes the engineering of chimeric T cell receptors. This technology is the basis for the evolution of the most cited patent WO2012079000A1, which is essential for the emergence of therapeutic applications for the treatment of cancer, especially lymphocytic leukemia and lymphomas. As the route continues to more recent patents, five PCT patents (Patent Cooperation Treaty) with applications in 2017, are at the top of the route, relating to improving CAR-T therapies which show promising trends: WO2017015427A1 describes a new method for improving T cell expansion; WO2017040930A2 describes biomarkers for predicting the patient’s risk of developing cytokine release syndrome (CRS); WO2017149515A1 developed CAR-T cells with multiple CAR molecules with enhanced efficacy and which avoid off-target toxicities; WO2017165683A1 relates to compositions and methods for using a PD-L1 blocking mini-antibody as a strategy to enhance CAR-T cell killing; and the patent WO2017181119A2 is related to enabling selective expression of a desired protein of interest and controlling CAR expression. Clearly, the route shows five potential strategies to improve CAR-T cell efficacy and safety, including (1) enhancing the expansion and functional capacity of ex vivo–expanded, (2) new strategies to manage CRS to avoid or alleviate this adverse event; (3) multiple CAR molecules with enhanced efficacy and which avoid off-target toxicities; (4) strengthening the potency of CAR-T cells, modulation of the immunosuppressive tumor microenvironment with immune-checkpoint blockade (PD-L1 blocking mini-antibody); and (5) Functional control of the intensity or toxicity of T-cell activation including of a “switch-on or switch-off” possibility in CAR design.
Figure 8.
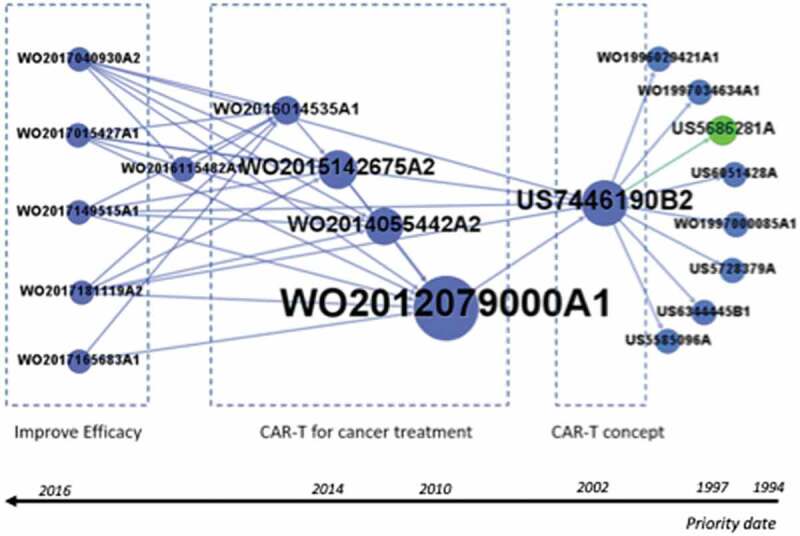
The emerging technological route of CAR-T cell patents. The main path of the global patent citation network was patents which compose the route ranked by priority country.
Although this therapy is already on the market, further investigations still need to be performed so as to safely target tumor antigen, optimize cell manufacturing, outline optimal combinatorial strategies to improve therapeutic treatment and control CAR-T cells in vivo and also for the development of strategies to predict CRS.
It should be noted that initially there was a diversity of actors participating in the development of CAR-T technologies that are in the main TR, especially Georgetown University, Sloan Kettering Inst Cancer and Cantab Pharma. However, in 2012, the University of Pennsylvania developed a new product that would change cancer treatment with the patent WO2012079000A1, and from 2015 there has been an expansion of the players with the entry of Novartis in cooperation with the University of Pennsylvania, who have begun to determine the direction of the development of this technology. It can be inferred that the University of Pennsylvania during its drug discovery process sought partners in the business environment to continue the development of its candidates, and Novartis was more effective in perceiving this as a strategic opportunity for cooperation in order to gain a competitive advantage against the other players. Additional information about the patents composing the TR are shown in Supplementary material.
5. Limitations of this study
This study has some limitations due to the scope of the search query terms used and the availability of information, if we consider that this is a dynamic and rapidly evolving technological field. Our analyses are based on patent classifications. These classifications depend on public reporting of information which varies in quality and timing between academia and industries from different geographic locations, with the possibility that some groups did not fully report their status for competitive reasons or over the report to attract more funding. Another limitation is the classification of clinical studies, some groups may use the terms ‘Phase I’, Phase I/II, Phase II, Phase II/III rather loosely and not according to regulatory standards, in part to attract more attention and financing as it is a very competitive field. In addition, the type of analysis performed in this study is not able to evaluate patent quality, so many patents may have limited or low value. This aspect cannot be reflected in the quantification of patents – where a strong patent can surpass more than 100 weak patents. It is also important to state that our analysis is tracking published patent applications and not issued patents, and only some unknown % of filed applications will result in issued patents with specific claims protecting the product. We believe that the limitations are minimized by our group having a deep understanding of the technical field and also having professional patent research skills to optimize search queries that maximize the integrity of the analyses presented in this study.
6. Conclusions
CAR-T cell therapy is an innovative therapy, resulting from more than 30 years of intense research, employing knowledge about immune system cells, tumor immunology, cellular signaling, genetic engineering, antibody structure and a wide understanding of neoplasia conditions. In the past few years, CAR-T cells have revolutionized the cancer treatment scenario achieving remission rates never reached before, including complete remissions in clinical trials for B cell malignancies. Even though CAR-T-based approaches have demonstrated impressive efficacies, they still require further optimization to achieve similar effects with solid tumors.
This work showed the intense collaboration between academic institutions and biotechnology companies, demonstrating a milestone in this sector. In addition, collaboration has enabled many advances that have made CAR-T cell therapy faster, cheaper and more efficient.
The increasing number of patent applications and, consequently, the increasing number of ongoing clinical trials will allow for substantial advances in the coming years. The biggest challenge that CAR-T cell therapies will face in the next decade is how to make them accessible to a larger number of patients. Currently, only two products are available in the market and they have high prices (Kymriah® US$475,000 and Yescarta® US$373,000).
CD19 is still the most studied target, and despite the success of the clinical studies, many challenges remain to improve outcomes. In this study, we showed the path of technological trends that includes patents with greater future exploratory potential. The most recent patents in the technological route indicate exciting new developments that will improve five major R&D challenges that companies are starting to address: i) new methods for CAR-T in vitro expansion, ii) methods to predict CRS, the most prevalent adverse effect following infusion of CAR-T cells, iii) development of CAR-T cells that recognize multi-antigen signatures, which will dramatically improve tumor recognition specificity, iv) creation of CAR-T cells that are active in an immunosuppressive microenvironment with an immune-checkpoint blockade, such as PD-L1 blocking mini-antibody, which can be very useful for solid tumors and, finally, v) development of methods to control CAR-T cell activity in vivo.
Tracking emerging technologies can help in the choice of promising R&D projects and in making strategic decisions about technology exploration and further licensing. The present study demonstrates patents with greater future exploratory potential but does not rule out the uncertainty of technological changes. In addition, this study can contribute to decision-making in strategic research planning by government, academia, and industry, providing technological trends of CAR-T cell therapy.
Supplementary Material
Acknowledgments
This study was supported by research funds from the São Paulo Research Grants Foundation (FAPESP): grant #2013/08135-2, grant #2014/50947-7, grant #2015/13816-4, grant #2017/25364-6 and grant #2014/22500-8. We also acknowledge the National Council for Scientific and Technological Development (CNPq) grant #465539/2014-9.
The authors would like to thank Andy Cumming for the English review and Sandra Navarro for the figure design.
Funding Statement
This study was supported by research funds from the São Paulo Research Grants Foundation (FAPESP): grant [#2013/08135-2], grant [#2014/50947-7], grant [#2015/13816-4], grant [#2017/25364-6] and grant [#2014/22500-8]; National Council for Scientific and Technological Development (CNPq) grant [#465539/2014-9].
Author contributions
V.C., C.G., K.S. and K.M. designed the experiments, analyzed the data, and wrote the manuscript; G.P. and D.C. discussed the data and wrote the manuscript
Disclaimer
Given the complexity of the patent landscape surrounding CARs, we suggest utilizing this publication for informative purposes only.
Disclosure of potential conflicts of interest
The authors confirm that this article’s contents have no conflicts of interest.
Supplementary Materials
Supplemental data for this article can be accessed on https://doi.org/10.1080/21645515.2019.1689744.
References
- 1.Fesnak AD, June CH, Levine BL.. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16:566–81. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci. 1989;86:10024–28. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90(2):720–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darcy PK, Kershaw MH, Trapani JA, Smyth MJ. Expression in cytotoxic T lymphocytes of a single-chain anti-carcinoembryonic antigen antibody. Redirected Fas ligand-mediated lysis of colon carcinoma. Eur J Immunol. 1998;28:1663–72. doi:. [DOI] [PubMed] [Google Scholar]
- 5.Finney HM, Lawson AD, Bebbington CR, Weir AN. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol. 1998. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 6.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.June CH, Maus MV, Plesa G, Johnson LA, Zhao Y, Levine BL, Grupp SA, Porter DL. Engineered T cells for cancer therapy. Cancer Immunol Immunother. 2014;63:969–75. doi: 10.1007/s00262-014-1568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–15. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamers CHJ, Sleijfer S, Vulto AG, Kruit WHJ, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 10.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ. Adoptive immunotherapy for indolent non-hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–71. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–26. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 13.Sadelain M, Rivière I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 14.Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–98. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kershaw MH, Westwood JA, Slaney CY, Darcy PK. Clinical application of genetically modified T cells in cancer therapy. Clin Transl Immunol. 2014;3:e16. doi: 10.1038/cti.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budde LE, Berger C, Lin Y, Wang J, Lin X, Frayo SE, Brouns SA, Spencer DM, Till BG, Jensen MC. Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma. PLoS One. 2013;8:e82742. doi: 10.1371/journal.pone.0082742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campos E, Campos A. Effect of the economic crisis on the production of immunology patents managed through the Patent Cooperation Treaty agreement from 2004–2011. JRSM Open. 2015;6:1–8. doi: 10.1177/2054270415593449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, Reaman GH, Carroll WL. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30:1663–69. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavez JC, Bachmeier C, Kharfan-Dabaja MA. CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Ther Adv Hematol. 2019;10:204062071984158. doi: 10.1177/2040620719841581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheuermann RH, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk Lymphoma. 1995;18:385–97. doi: 10.1080/14719037.2015.1029350. [DOI] [PubMed] [Google Scholar]
- 21.Maus MV, Levine BL. Chimeric antigen receptor T-cell therapy for the community oncologist. Oncologist. 2016;21:608–17. doi: 10.1634/theoncologist.2015-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossig C, Pule M, Altvater B, Saiagh S, Wright G, Ghorashian S, Clifton-Hadley L, Champion K, Sattar Z, Popova B. Vaccination to improve the persistence of CD19CAR gene-modified T cells in relapsed pediatric acute lymphoblastic leukemia. Leukemia. 2017;31:1087–95. doi: 10.1038/leu.2017.39. [DOI] [PubMed] [Google Scholar]
- 23.Infanti J, Burkholder A. Penn medicine news. August 30, 2017. https://www.pennmedicine.org/news/news-releases/2017/august/fda-approves-personalized-cellular-therapy-for-advanced-leukemia.
- 24.Fischer A FDA news release. October 18, 2017. https://www.fda.gov/news-events/press-announcements/fda-approves-car-t-cell-therapy-treat-adults-certain-types-large-b-cell-lymphoma.
- 25.Pereira CG, Picanco-Castro V, Covas DT, Porto GS. Patent mining and landscaping of emerging recombinant factor VIII through network analysis. Nat Biotechnol. 2018;36:585–90. doi: 10.1038/nbt.4178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Infanti J, Burkholder A. Penn medicine news. August 30, 2017. https://www.pennmedicine.org/news/news-releases/2017/august/fda-approves-personalized-cellular-therapy-for-advanced-leukemia.


