Abstract
Background: Studies show that telestroke (TS) improves rural access to care and outcome for stroke patients receiving TS services, but population health impacts of TS are not known. We examine impacts associated with South Carolina's (SC) statewide TS network on an entire state population of patients suffering acute ischemic stroke (AIS) as TS became available across SC counties.
Methods: A population health study using Donabedian's conceptual model and an ecological design to describe the change observed over time in use of thrombolysis and endovascular therapy (EVT) as the SC TeleStroke Network (SCTN) diffused across SC counties. Changes in county rates of stroke mortality and discharge destination are reported. The unit of interest is the population rate for AIS patients living in a SC county. Patients’ county of residence at the time of hospitalization defined county cohorts. Relative risks were estimated using logistic regression adjusted for age >75 years.
Results: Overall tissue plasminogen activator (tPA) rate was 6.28%, and EVT rate was 1.10%. Patients living where SCTN was available had a 25% higher likelihood of receiving tPA (adjusted relative risk [ARR] = 1.25, 95% confidence interval [CI] = 1.15–1.36) and lower risks of mortality (ARR = 0.91; 95% CI = 0.84–0.99) or discharge to skilled nursing (ARR = 0.93; 95% CI = 0.89–0.97).
Conclusions: TS diffusion affects the structure of the health system serving a county, as well as the processes of care delivered in the emergency department; these changes are associated with measurable population health improvements. Results support a population benefit of TS implementation.
Keywords: telemedicine, telehealth, telestroke, population health, tPA, EVT stroke outcomes
Introduction
For patients with acute ischemic stroke (AIS), tissue plasminogen activator (tPA) and endovascular therapy (EVT) are the standard and highly time-sensitive treatments.1–6 However, many AIS patients lack access to these timely treatments affecting patient outcomes and quality of life. Due to multiple factors, only 3–5% of patients receive tPA within the required 4.5-h treatment window.7,8 Furthermore, complication rates have been shown to increase when untrained or inexperienced physicians administer intravenous tPA.7,9,10
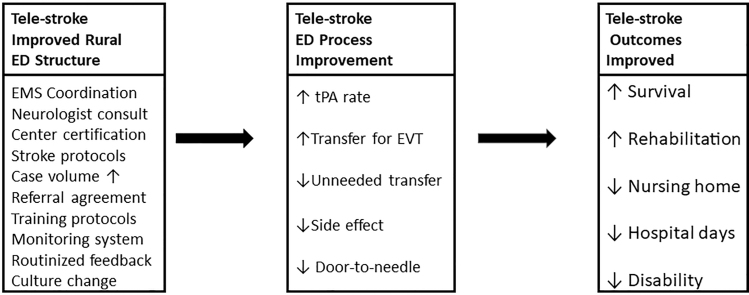
These problems are exacerbated in remote or rural areas where patients may not have access to stroke specialists.11,12 Fortunately, telestroke (TS) programs have demonstrated that by changing the structure of the stroke care delivery system in rural areas (Fig. 1) we are able to improve key process of care measures such as tPA administration, which then improves outcome measures such as post-AIS functional status and safety.7,13–17 The American Heart Association recently released a statement summarizing the key quality and outcome measures that TS programs should monitor and report.7
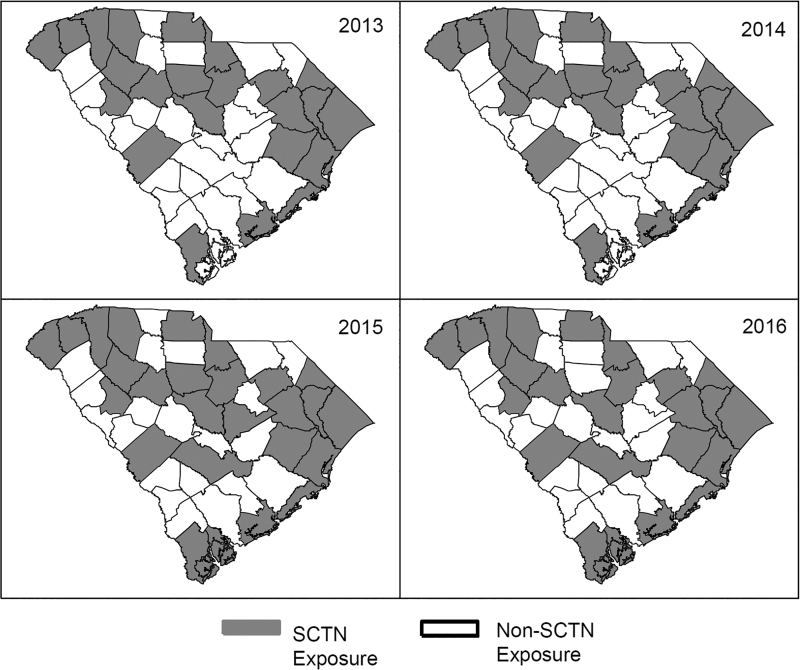
Fig. 1.
SCTN coverage map by year. County-level SCTN coverage did not change from 2013 to 2014. SCTN, South Carolina Telestroke Network.
High quality stroke care is especially important in South Carolina (SC), which ranks sixth in the nation for stroke death rates.18 SC has large regions designated as fully or partially medically underserved19; thus, many patients lack access to timely stroke care. In 2011, only 77% of SC residents had ground transportation access within 60 min to a hospital capable of tPA administration, and <30% had access to a facility capable of EVT.20 To mitigate these disparities, the Medical University of South Carolina (MUSC) led the development of a statewide SC Telestroke Network (SCTN). The SCTN operates in a “hub-and-spoke” model in which larger centers with comprehensive stroke resources provide expert stroke consultation to smaller, often rural hospitals that lack such resources.14,21–23
Between 2008 to present, the SCTN has expanded to include 3 hubs connected to 40 spokes, with the number of consultations increasing over time. Video-based telemedicine carts located in the spoke emergency department (ED) connect a stroke neurologist at the hub to the spoke ED.
In addition to improvements in processes and outcomes of stroke care associated with the SCTN, we anecdotally observed the diffusion of knowledge and information sharing across SC providers and acute care hospitals. We hypothesized that expansion of SCTN has changed the structural environment for stroke care and, thus, benefits patients with stroke who are not receiving a tele-consult, amplifying the benefits of the Network beyond direct patient consultation (Appendix Fig. A1). Given the depth, breadth, and robust case volumes of the SCTN, we hypothesized that this structural change of the rural stroke care system may result in a demonstrable population health benefit. Thus, we sought to examine differences in care processes and outcomes for populations defined as all hospitalized AIS cases for a calendar year in each of 46 SC counties.
Using an ecological design, we classified AIS patients by year and county of residence. An AIS event was counted as unexposed to SCTN for all county-years without SCTN in place for at least 6 months and exposed if the event occurred to a resident of a county which had SCTN at any county hospital for at least 6 months of the calendar year. Thus, counties changed exposure status as the SCTN diffused across the state, and (in a few cases) lost exposure status if the SCTN program was discontinued.
This assignment of exposure uses the counties as their own controls and measures the population-level influence of the SCTN. The rationale for this assignment is that the SCTN is a structural change in the stroke care delivery system within a county which may be expected to improve measures of the care process (tPA administration, EVT rates), which in turn will affect outcome measures (in-hospital mortality, discharge destination) (Appendix Fig. A1) based on where you live.
This is akin to measuring the effect of bringing medical care to an underserved area and examining effects on population health as county-level services for patients with AIS improve with increases in SCTN access from 2013 to 2016 (Fig. 1). This is a fairly weak measure of exposure to SCTN because even under optimal conditions only about 20% of AIS patients are eligible for tPA and fewer are eligible to receive EVT; thus, observing an improvement in two process measures (tPA and EVT) over and above the general rate of improvement observed for all SC counties over time is an important measure of a program effect.
Methods
Study Design and Cohort Description
We conducted a retrospective population analysis using billing data, coded according to the International Classification of Diseases, Ninth Revision (ICD-9) in addition to Diagnostic Related Groups (DRGs), from 2013 to 2016 calendar years from the South Carolina Office of Research and Statistics (SC ORS) hospital claims data. SC maintains this database to track population health outcomes, and all inpatient hospitalizations are included. We identified all individuals over age 18 admitted for AIS (Primary diagnosis ICD-9 codes 434.01, 434.11, 434.91, 436.xx within DRG codes 61, 62, 63, 64, 65, 66, 67, 68 in years 2013–2016). ICD coding changed to tenth revision in October of 2015. However, SC ORS data include both ICD-9 and ICD-10 codes before and after this transition between versions and, thus, to be consistent we used revision nine codes with DRGs for cohort identification.
DRG codes were included in the stroke specification to ensure capture of all EVT cases as these stroke cases are sometimes coded as Craniotomies and not as primary AIS. Individuals with primary diagnoses of hemorrhagic stroke were excluded. This approach allows the identification of hemorrhagic stroke coded as an intracranial hemorrhage complication of tPA use for an ischemic stroke. SCTN tracking and patient registry data were used to designate county-level exposure to examine the development and growth of the SCTN over time. We chose the years 2013–2016 as our study years because these are the most recent claims data available for all SC hospitalizations.
SCTN Exposure Definition
We defined patients as having exposure to the SCTN if the patient's residential county was a SC county with a hospital that was either a SCTN hub or spoke for at least 6 months of the year at the time of the AIS hospitalization (Fig. 1). Aggregation of data by county (as opposed by zip code) was chosen because several rural zip codes in SC cut across counties with different SCTN implementation times.24 The choice of aggregation by county was also better able to capture any effects of SCTN spokes’ training and collaboration with ambulance services due to the county-level organization of emergency medical service in rural underserved areas.25
Outcomes of Interest
Process of care measures
To operationalize the definition of the outcome receipt of tPA, a combination of ICD-9, ICD-10, and DRGs was used. tPA was designated as received if any of these codes in any primary or secondary procedure category were indicated (ICD-9; 99.10, ICD-10; 3E03317, 3E04317, 3E05317, 3E06317, Z92.82) or if there was a DRG code of 61, 62, or 63. Receipt of EVT was defined as having an ICD-9 or ICD-10 procedure code in any position of 39.74 (ICD-9 procedure) or 03CG3ZZ (ICD-10 procedure) in any position.26–28
Clinical outcome measures
Clinical outcomes included in-hospital mortality or hospice (died) as indicated in discharge destination. Additional outcomes included discharge to an inpatient rehabilitation facility (IRF), discharge to a skilled nursing facility (SNF), or discharge to home within the hospital coding of discharge destination.
Statistical Analyses
Descriptive statistics include count and percentage for categorical variables. Unadjusted differences between SCTN and non-SCTN exposure patients were calculated using chi-square tests. All outcomes were dichotomous and, thus, were examined for year and SCTN exposure effect using multivariable logistic regression models and Poisson regression models with robust error variance in Proc Genmod to estimate effect sizes as adjusted relative risk (ARR) ratios.29 Outcomes are reported as ARR as is recommended for cohort studies where the outcome is not a rare event.30
Our main outcomes of interest were measures of access to time-sensitive stroke interventions. Since the use of tPA and EVT is recommended for all patients without contraindications, we only controlled for year and patients over 75 years of age because these older patients may be more likely to have medical conditions that made them ineligible for tPA or EVT.
It is important to note that since our primary measures were indicators of access to care, it was not appropriate to control for population characteristics, such as race, insurance, or income variables, because eligibility for use of tPA and EVT is not determined by these variables, and controlling for their effect may cancel out any effect that SCTN may be expected to have on disparities in access to care. Our secondary measures (in-hospital death and discharge destination) are used as measures of population outcomes associated with improved access to care and are not risk adjusted beyond year and age >75.
We chose this approach because we are not comparing hospital performance or direct clinical effectiveness, where risk adjustment is essential for internal validity, but using them to estimate population outcome effects observed across geographic areas.31 We did not adjust for comorbid conditions because the presence of comorbid conditions is not a contraindication for use of tPA or EVT (our process measures). Indeed, good stroke care assures that the right patient gets the right treatment at the right time, with due considerations of their comorbid conditions.
Thus, controlling for comorbidities may nullify an important factor associated with good stroke care. Control for variations in comorbid conditions is important mainly to guard against bias from healthier patients being in the exposure group. Since our counties serve as their own controls, they are unlikely to gain a much healthier patient base over the four exposure years, because even the most effective stroke prevention program is unlikely to improve a county's stroke risk factors for its residents substantially over a 4-year time period. Interaction effects between SCTN exposure and year were investigated in each outcome model for potential exposure differences by year of the study and were considered significant at α < 0.1. No significant SCTN exposure by year interactions was found.
Because all data were deidentified by the SC ORS, the study was deemed to be nonhuman research by the MUSC Institutional Review Board. Statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Inc.; Cary, NC). Two-sided statistical significance was set at α < 0.05.
Results
The AIS population included 39,364 patients hospitalized in SC between 2013 and 2016, 27,042 (68.70%) resided in counties with SCTN exposure and 12,322 (31.30%) did not. Exposure groups were evenly distributed on age ≥75 (37.68% SCTN vs. 35.72% non-SCTN, p-value = 0.10), male sex (49.23% SCTN vs. 49.63 non-SCTN, p-value = 0.46), and race (p = 0.16) (Table 1). Patient distribution over time changed from there being a higher proportion of non-SCTN exposed AIS patients in 2013 and 2014 to higher proportions of AIS SCTN exposure patients in 2015 and 2016. This coincides with expansion in the SCTN's geographical coverage over time (Fig. 2).
Table 1.
Demographics and Unadjusted Outcomes Among South Carolina Patients with Acute Ischemic Stroke: 2013–2016
| OVERALL (n = 39,364) | SCTN EXPOSURE (n = 27,042) | NON-SCTN EXPOSURE (n = 12,322) | p* | |
|---|---|---|---|---|
| Age (≥75) | 14,430 (36.66) | 10,028 (37.08) | 4,402 (35.72) | 0.10 |
| Male | 19,430 (49.36) | 13,314 (49.23) | 6,116 (49.63) | 0.46 |
| Race | 0.16 | |||
| White | 24,440 (62.09) | 16,717 (61.82) | 7,723 (62.68) | |
| Black | 13,878 (35.26) | 9,616 (35.56) | 4,262 (34.59) | |
| Other | 1,046 (2.66) | 709 (2.62) | 337 (2.73) | |
| Year | <0.0001 | |||
| 2013 | 9,326 (23.69) | 6,074 (22.46) | 3,252 (26.39) | |
| 2014 | 9,705 (24.65) | 6,320 (23.37) | 3,385 (27.47) | |
| 2015 | 9,995 (25.39) | 7,370 (27.25) | 2,625 (21.30) | |
| 2016 | 10,338 (26.26) | 7,278 (26.91) | 3,060 (24.83) | |
| Unadjusted outcomes | ||||
| tPA | 2,472 (6.28) | 1,813 (6.70) | 659 (5.35) | <0.0001 |
| EVT | 434 (1.10) | 315 (1.16) | 119 (0.97) | 0.08 |
| Mortalitya | 2,530 (6.43) | 1,689 (6.25) | 841 (6.83) | 0.03 |
| Discharge destination | 0.003 | |||
| Home | 12,670 (34.46) | 8,783 (34.71) | 3,887 (33.91) | |
| Home with home health | 6,617 (18.00) | 4,526 (17.89) | 2,091 (18.24) | |
| IRF | 6,712 (18.26) | 4,714 (18.63) | 1,998 (17.43) | |
| SNF | 6,730 (18.30) | 4,536 (17.93) | 2,194 (19.14) | |
| Transfer | 1,151 (3.13) | 763 (3.02) | 388 (3.38) | |
| Other | 2,887 (7.85) | 3,641 (13.81) | 1,843 (14.17) |
Statistics are reported as count (percentage).
p-values were calculated to compare characteristic differences between groups using chi-square tests.
Mortality indicates patients who died in a freestanding medical facility, including hospital or hospice.
EVT, endovascular treatment; IRF, inpatient rehabilitation facility; SCTN, South Carolina Telestroke Network; SNF, skilled nursing facility; tPA, tissue Plasminogen Activator.
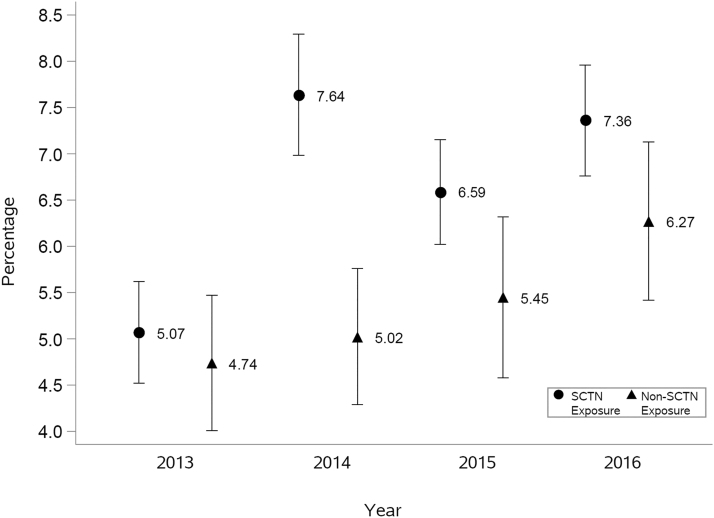
Fig. 2.
Unadjusted percentage of tPA administration by year. tPA, tissue plasminogen activator.
All unadjusted crude study outcomes were statistically different between the SCTN and non-SCTN exposure groups with SCTN having higher proportions of patients receiving tPA (p < 0.0001) and EVT (marginal p = 0.08) and non-SCTN exposure patients having higher mortality (p = 0.03) (Table 1). SCTN exposure patients were also more likely to be discharged to home and independent rehabilitation facilities than non-SCTN patients and less likely to be discharged to a SNF or transferred to long-term acute care (p = 0.003) (Table 1).
The unadjusted tPA rates in 2013 were similar between SCTN and non-SCTN exposure patients (5.07% vs. 4.74%, respectively) (Fig. 2). However, while rates tended to increase in both exposure groups from 2013 to 2016 (time p-value <0.0001), rates increased by a greater amount in patients living in SCTN exposure counties (Fig. 2).
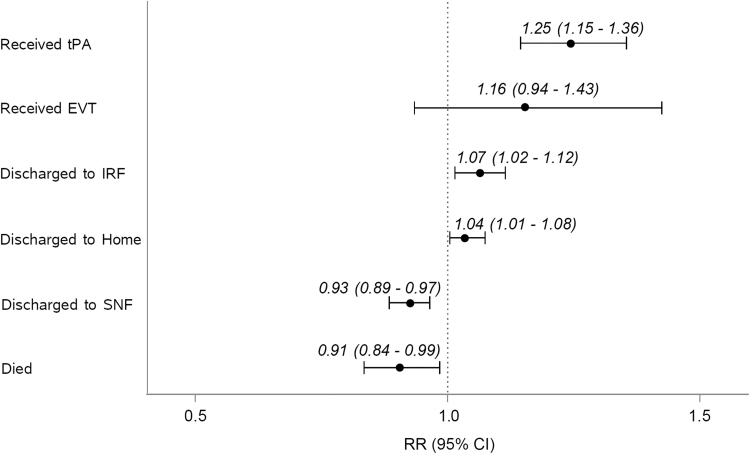
In adjusted analysis, patients living in SCTN exposure counties have a 24.9% higher likelihood of receiving tPA than patients in non-SCTN exposure counties (ARR = 1.25, 95% confidence interval [CI] = 1.15–1.36, p < 0.0001) (Fig. 3).
Fig. 3.
ARR of process and clinical outcomes on SCTN exposure versus non-SCTN exposure. Each relative risk estimate and 95% confidence interval, between the exposure groups, are generated by a separate multivariable regression model adjusted for year and age >75. ARR, adjusted relative risk; EVT, endovascular treatment; IRF, inpatient rehabilitation facility; SNF, skilled nursing facility.
Risk of death decreased over the study period, with a 9% lower risk of death in SCTN exposure compared with non-SCTN exposure patients (ARR = 0.91, 95% CI = 0.84–0.99, p = 0.03) (Fig. 3). Likelihood of discharge to a SNF was 7% lower in SCTN exposure patients (ARR = 0.93, 95% CI = 0.89–097, p = 0.001), but was 7% higher to IRF (ARR = 1.07, 95% CI = 1.02–1.12, p = 0.005) and 4% higher to home (ARR = 1.04, 95% CI = 1.01–1.08, p = 0.006), compared with non-SCTN exposure patients (Fig. 3).
Discussion and Conclusions
The SCTN, initiated by MUSC in 2008, is a well-established hub-and-spoke TS network.14,21–23 During the years of this study, the SCTN experienced rapidly increasing utilization at established spokes. Important indicators of efficacy (e.g., door to needle time and so on) are monitored and reported as measures of program quality.14 In the current research, we sought to characterize associations between the SCTN and population health impact among South Carolinians’ with AIS between 2013 and 2016.
The objective was to ascertain whether there might be discernible population health benefits associated with program diffusion and improvements in overall quality of stroke care. To accomplish this, we utilized a large archival data source available through the SC ORS and developed AIS population cohorts for our study years.
The cohorts were stratified into groups with SCTN exposure and groups without SCTN exposure based on patient county of residence and SCTN programmatic data indicating which counties had hospitals that were either a hub or spoke participant in the SCTN during that year. Therefore, as the SCTN was implemented counties switched from unexposed to exposed status.
Therefore, counties serve as their own controls because they delivered AIS cases to the control population until they became part of the SCTN when their AIS cases began to be counted in the exposed group. No attempts were made to identify AIS patients who actually received TS services because we were interested in measuring the population impact of an intervention that has a well-documented benefit at the individual recipient level.
We observed that exposure to the SCTN at the patient residential county level was significantly associated with important stroke quality of care indicators, including receipt of tPA (ARR = 1.25). In addition, major clinical outcomes, including reduced mortality (ARR = 0.91), increased discharge to inpatient rehabilitation and home, with lower discharge to a SNF (ARR = 1.07, 1.04, and 0.93, respectively), were significantly associated with SCTN exposure. When considered in the context of Donabedian's Model for quality in health care, in which structure of health care drives the processes and outcomes of care,32 the combination of improved stroke care processes and improved stroke outcomes offers important evidence for the positive impact of the SCTN on population health.
It could be posited that improvements in processes and outcomes of stroke care among the SCTN exposure group were due primarily to direct TS consultations. However, given the large cohort of AIS patients during our study period (n = 39,364) relative to the total number of patients who both received a TS consult and had an AIS (n = ∼3,970), this seems unlikely. Furthermore, there were annual improvements in stroke care processes, including tPA administration and EVT for both the SCTN exposure and nonexposure cohorts, although these were significantly greater in the SCTN cohort. Since ∼90% of the overall AIS cohort was treated without TS consultation, we presume that these treatment plans were according to local standards and processes of care.
Thus, we believe factors associated with the SCTN, in addition to the consultative function, may be driving stroke care improvements. Potential mechanisms are likely multifaceted. For example, during implementation and subsequent operations, emergency transportation teams and ED staff receive education and training in acute stroke care both on-site and through remote webinars. This training is reinforced with local educational materials and routine data reporting and benchmarking.
Within the SCTN, the key process of care measures is tracked and participating sites provided with benchmarked performance reports. These activities translate into increasing awareness of stroke symptoms, protocol development between the SCTN and spoke sites, training on stroke assessment and treatments, and pursuit of various stroke center designations all of which could contribute to improved baseline quality of stroke care independent from a stroke specialist consultation. Indeed, since the SCTN inception in 2008, the number of SC hospitals designated as Primary Stroke Centers has increased from 0 to 15 as of 2018.
Other effects of the SCTN might be related to health care provider migration between SCTN sites and non-SCTN sites, a phenomenon we have anecdotally observed as both common and an unanticipated pathway for the diffusion of knowledge. ED providers may work in multiple acute care hospitals in adjacent counties in rural communities and thus may bring the knowledge and skills acquired in a SCTN spoke site to a non-SCTN county.
Ultimately, after over a decades’ experience in TS network development and sustainment, our view is that the combination of telehealth coupled with robust education and quality improvement efforts is synergistic. Providing practical support in a time of acute patient need coupled with regular quality monitoring and reporting is instrumental to driving patient care improvements over time. Either of these, in isolation, is less likely to achieve demonstrable population health impacts associated with the SCTN.
TS is one of the most common uses of telemedicine in neurology.33 This is driven by specialty shortages and related lack of access, need for time sensitive treatments, and technological capabilities.33 However, there are extensive challenges to robust examination of TS programs, including the often small and siloed nature of many TS programs and general reluctance to share patient outcome data across organizations. It is also rare for TS programs to collect rich treatment data on patients who did not receive telehealth, making comparisons with valid control groups challenging. In fact, a recent meta-analysis found that of 26 included studies, only 2 were randomized trials and the remainder were observational studies.34
Use of archival claims data could theoretically overcome challenges of small, singe-center TS programs but unfortunately identifying patient-level TS care in claims data is problematic due to coding errors and rurality reimbursement restrictions, which can impact the validity of the data.35 Our approach offers a novel strategy to evaluating the impact of a mature TS network by applying an epidemiologic, population health strategy to investigate potential benefits of a TS network over time.
While we believe our findings offer compelling evidence for the value of TS networks, we acknowledge some important limitations. First, our approach identified important associations with SCTN exposure but given the retrospective nature of our study design we cannot prove causation. Second, due to the deidentified nature of our archival data we are unable to determine which patients did or did not receive a TS consultation and thus cannot match our cohorts to further explore any attenuations on the population measures associated with the “dose” of telehealth measured in the programmatic data collected by the SCTN. This is an important limitation of our study design, because ecological studies are susceptible to the “ecological fallacy,” a well-documented weakness of ecological studies where group measure is not reflective of individual effects. Future studies should measure SCTN “dose” variations and use patient data linked to site of care to explore this further.
Finally, we attributed SCTN exposure based on patient county of residence and, due to hospital confidentiality rules within the SC ORS, were unable to ascertain which specific hospital provided a patient's treatment. It is quite likely that a differential effect is associated with differences in hospital performance. This is an important issue to explore in future studies.
In summary, we utilized a large archival claims data source to conduct a population level analysis of all hospitalized patients in SC suffering from AIS from 2013 to 2016. We found that SCTN exposure was associated with significant improvements in both processes and outcomes of stroke care providing further evidence regarding the value of such networks. Ongoing clinical trials will add further insights into this important modality of delivering expert stroke care.
Appendix Figure A1
Appendix Figure A1.
Conceptual model of Tele-Stroke effects. Modification of Donabedian's model (1988).
Disclaimer
The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by HRSA, HHS, or the U.S. Government.
Disclosure Statement
None of the authors has any conflicts of interest to disclose. The authors have no financial relationships relevant to this article to disclose.
Funding Information
This publication was supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) as part of the National Telehealth Center of Excellence Award (U66 RH31458-01-00). Data analytic support for the study was provided through the CEDAR core funded by the MUSC Office of the Provost and by the South Carolina Clinical and Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, through NIH grant number UL1 RR029882.
References
- 1. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587 [DOI] [PubMed] [Google Scholar]
- 2. Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D EC ASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329 [DOI] [PubMed] [Google Scholar]
- 3. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama a Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20 [DOI] [PubMed] [Google Scholar]
- 4. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Davalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millan M, Davis SM, Roy D, Thornton J, Roman LS, Ribo M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–1731 [DOI] [PubMed] [Google Scholar]
- 5. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG DA WN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21 [DOI] [PubMed] [Google Scholar]
- 6. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, Johnston KC, Johnston SC, Khalessi AA, Kidwell CS, Meschia JF, Ovbiagele B, Yavagal DR, American Heart Association Stroke Council. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:3020–3035 [DOI] [PubMed] [Google Scholar]
- 7. Wechsler LR, Demaerschalk BM, Schwamm LH, Adeoye OM, Audebert HJ, Fanale CV, Hess DC, Majersik JJ, Nystrom KV, Reeves MJ, Rosamond WD, Switzer JA, American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Quality of Care and Outcomes Research. Telemedicine quality and outcomes in stroke: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017;48:e3–e25 [DOI] [PubMed] [Google Scholar]
- 8. Kleindorfer D, Xu Y, Moomaw CJ, Khatri P, Adeoye O, Hornung R. US geographic distribution of rt-PA utilization by hospital for acute ischemic stroke. Stroke 2009;40:3580–3584 [DOI] [PubMed] [Google Scholar]
- 9. Heuschmann PU, Kolominsky-Rabas PL, Roether J, Misselwitz B, Lowitzsch K, Heidrich J, Hermanek P, Leffmann C, Sitzer M, Biegler M, Buecker-Nott HJ, Berger K, German Stroke Registers Study Group. Predictors of in-hospital mortality in patients with acute ischemic stroke treated with thrombolytic therapy. JAMA 2004;292:1831–1838 [DOI] [PubMed] [Google Scholar]
- 10. Katzan IL, Furlan AJ, Lloyd LE, Frank JI, Harper DL, Hinchey JA, Hammel JP, Qu A, Sila CA. Use of tissue-type plasminogen activator for acute ischemic stroke: The cleveland area experience. JAMA 2000;283:1151–1158 [DOI] [PubMed] [Google Scholar]
- 11. Bladin CF, Cadilhac DA. Effect of telestroke on emergent stroke care and stroke outcomes. Stroke 2014;45:1876–1880 [DOI] [PubMed] [Google Scholar]
- 12. Joubert J, Prentice LF, Moulin T, Liaw ST, Joubert LB, Preux PM, Ware D, Medeiros de Bustos E, McLean A. Stroke in rural areas and small communities. Stroke 2008;39:1920–1928 [DOI] [PubMed] [Google Scholar]
- 13. Al Kasab S, Orabi MY, Harvey JB, Turner N, Aysse P, Debenham E, Holmstedt CA. Rate of symptomatic intracerebral hemorrhage related to intravenous tPA administered over telestroke within 4.5-hour window. Telemed J E Health 2018;24:749–752 [DOI] [PubMed] [Google Scholar]
- 14. Al Kasab S, Harvey JB, Debenham E, Jones DJ, Turner N, Holmstedt CA. Door to needle time over telestroke-A comprehensive stroke center experience. Telemed J E Health 2018;24:111–115 [DOI] [PubMed] [Google Scholar]
- 15. Nguyen-Huynh MN, Klingman JG, Avins AL, Rao VA, Eaton A, Bhopale S, Kim AC, Morehouse JW, Flint AC KP NC Stroke FORCE Team. Novel telestroke program improves thrombolysis for acute stroke across 21 hospitals of an integrated healthcare system. Stroke 2018;49:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khan K, Shuaib A, Whittaker T, Saqqur M, Jeerakathil T, Butcher K, Crumley P. Telestroke in northern alberta: A two year experience with remote hospitals. Can J Neurol Sci 2010;37:808–813 [DOI] [PubMed] [Google Scholar]
- 17. Almallouhi E, Holmstedt CA, Harvey J, Reardon C, Guerrero WR, Debenham E, Turner N, Aysse P, Al Kasab S. Long-term functional outcome of telestroke patients treated under drip-and-stay paradigm compared with patients treated in a comprehensive stroke center: A single center experience. Telemed J E Health 2019;25:724–729 [DOI] [PubMed] [Google Scholar]
- 18. SCDHEC. Stroke in South Carolina. Available at https://www.scdhec.gov/sites/default/files/Library/ML-002149.pdf (Last accessed June17, 2019)
- 19. South Carolina DHEC. SC Medically Underserved Areas. Available at https://scdhec.gov/sites/default/files/docs/Health/docs/SC%20Medically%20Underserved%20Areas%20-%20Map.pdf (Last accessed March26, 2019)
- 20. Adeoye O, Albright KC, Carr BG, Wolff C, Mullen MT, Abruzzo T, Ringer A, Khatri P, Branas C, Kleindorfer D. Geographic access to acute stroke care in the United States. Stroke 2014;45:3019–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al Kasab S, Adams RJ, Debenham E, Jones DJ, Holmstedt CA. Medical University of South Carolina telestroke: A telemedicine facilitated network for stroke treatment in South Carolina-A progress report. Telemed J E Health 2017;23:674–677 [DOI] [PubMed] [Google Scholar]
- 22. Kazley AS, Wilkerson RC, Jauch E, Adams RJ. Access to expert stroke care with telemedicine: REACH MUSC. Front Neurol 2012;3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adams R, Debenham E, Chalela J, Chimowitz M, Hays A, Hill C, Holmstedt C, Jauch E, Kitch A, Lazarids C, Turan T. REACH MUSC: A telemedicine facilitated network for stroke: Initial operational experience. Front Neurol 2012;2:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. South Carolina Digital Vector Map with Counties & Zip Codes. Available at https://digital-vector-maps.com/state-maps-detail/5506/South-Carolina-Map-with-Counties—Zip-Codes-Adobe-Illustrator.htm (Last accessed March14, 2019)
- 25. SC Association of Counties. EMS Sites—South Carolina Counties. Available at www.sccounties.org/ems-websites (Last accessed March13, 2019)
- 26. Simpson KN, Simpson AN, Mauldin PD, Hill MD, Yeatts SD, Spilker JA, Foster LD, Khatri P, Martin R, Jauch EC, Kleindorfer D, Palesch YY, Broderick JP IM S III Investigators. Drivers of costs associated with reperfusion therapy in acute stroke: The interventional management of stroke III trial. Stroke 2014;45:1791–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of diagnostic codes for acute stroke in administrative databases: A systematic review. PLoS One 2015;10:e0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stryker Stroke Coding Guideline. 2017. hospital and physician hemorrhagic & acute ischemic stroke inpatient coding and payment guide. Fremont, CA: Stryker, 2017. Available at https://www.strykerneurovascular.com/downloads/AP001300.AA_2017_Stroke_Coding_Guide_FINAL.pdf (Last accessed June17, 2019)
- 29. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706 [DOI] [PubMed] [Google Scholar]
- 30. Lemeshow S, Sturdivant RX, Hosmer DW. Applied logistic regression (Wiley Series in Probability and Statistics). New York, NY: Wiley, 2013 [Google Scholar]
- 31. Katzan IL, Spertus J, Bettger JP, Bravata DM, Reeves MJ, Smith EE, Bushnell C, Higashida RT, Hinchey JA, Holloway RG, Howard G, King RB, Krumholz HM, Lutz BJ, Yeh RW, American Heart Association Stroke Council, Council on Quality of Care and Outcomes Research, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Radiology and Intervention, Council on Cardiovascular Surgery and Anesthesia, Council on Clinical Cardiology. Risk adjustment of ischemic stroke outcomes for comparing hospital performance: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2014;45:918–944 [DOI] [PubMed] [Google Scholar]
- 32. Donabedian A. The quality of care. How can it be assessed? JAMA 1988;260:1743–1748 [DOI] [PubMed] [Google Scholar]
- 33. Wechsler LR. Advantages and limitations of teleneurology. JAMA Neurol 2015;72:349–354 [DOI] [PubMed] [Google Scholar]
- 34. Baratloo A, Rahimpour L, Abushouk AI, Safari S, Lee CW, Abdalvand A. Effects of telestroke on thrombolysis times and outcomes: A meta-analysis. Prehosp Emerg Care 2018;22:472–484 [DOI] [PubMed] [Google Scholar]
- 35. Office of Inspector General. CMS paid practitioners for telehealth services that did not meet Medicare requirements. 2018. Available at https://oig.hhs.gov/oas/reports/region5/51600058.pdf (Last accessed June17, 2019)