Abstract
Pyrophosphate (PPi) serves as a potent and physiologically important regulator of mineralization, with systemic and local concentrations determined by several key regulators, including: tissue-nonspecific alkaline phosphatase (ALPL gene; TNAP protein), the progressive ankylosis protein (ANKH; ANK), and ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1; ENPP1). Results to date have indicated important roles for PPi in cementum formation, and we addressed several gaps in knowledge by employing genetically edited mouse models where PPi metabolism was disrupted and pharmacologically modulating PPi. in a PPi-deficient mouse model. We demonstrate that acellular cementum growth is inversely proportional to PPi levels, with reduced cementum in Alpl KO (increased PPi levels) mice and excess cementum in Ank KO mice (decreased PPi levels). Moreover, simultaneous ablation of Alpl and Ank results in reestablishment of functional cementum in dKO mice. Additional reduction of PPi by dual deletion of Ank and Enpp1 does not further increase cementogenesis, and PDL space is maintained in part through bone modeling/remodeling by osteoclasts. Our results provide insights into cementum formation and expand our knowledge of how PPi regulates cementum. We also demonstrate for the first time that pharmacologic manipulation of PPi through an ENPP1-Fc fusion protein can regulate cementum growth, supporting therapeutic interventions targeting PPi metabolism.
Keywords: Cementum, Mineralized Tissue/Development, Tooth Development, Bone
INTRODUCTION
Modulators of the mineralization inhibitor pyrophosphate (PPi) are critical toward maintaining mineralized tissue homeostasis. Extracellular PPi concentration is determined by several key regulators, including: tissue-nonspecific alkaline phosphatase (ALPL gene; TNAP protein), an enzyme that promotes mineralization by PPi hydrolysis; the progressive ankylosis protein (ANKH; ANK), a membrane protein that mediates intracellular to extracellular PPi transport; and ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1; ENPP1), an enzyme that produces adenosine monophosphate (AMP) and PPi from adenosine triphosphate (ATP). Loss of function of any of these regulators leads to dysregulated mineralization [1].
The oral cavity is home to four mineralized tissues, the only such place in the body. The bulk of teeth is composed of dentin, which extends to the tooth root. Enamel covers the coronal portion of the tooth, and cementum is found along the root surface. The coronal 2/3 of cementum consists of the acellular cementum, while the apical 1/3 is composed of the cellular cementum. The periodontal complex includes cementum and alveolar bone, joined into a functional unit by the periodontal ligament (PDL). Periodontal disease can progressively destroy all periodontal tissues. Current therapies to promote their repair are unpredictable, particularly in regards to the acellular cementum, which is the key tissue anchoring the PDL to the tooth root. Insights into the developmental biology of cementum may provide novel approaches to promote its regeneration and the return of periodontal function.
Previous work has revealed that acellular cementum is exceptionally sensitive to alterations in PPi metabolism. Loss-of-function mutations in ALPL (the disease hypophosphatasia, HPP; OMIM#241500, 241510, 146300) increase systemic PPi levels and inhibit cementogenesis, phenocopied by the Alpl knockout (Alpl KO) mouse model of HPP [2, 3]. Loss-of-function mutations in ENPP1 (the disease generalized arterial calcification in infancy, GACI; OMIM#208000) decrease systemic PPi levels and promote rapid cementogenesis and expanded cementum, evident in humans and Enpp1 mutant mice [4]. Loss-of-function mutations in ANKH (the disease craniometaphyseal dysplasia, CMD; OMIM#123000) also decrease systemic PPi, and Ank− KO mice exhibit a hypercementosis phenotype remarkably similar to Enpp1 loss-of-function mice [3, 5].
Current data suggest that PPi directly regulates cementum mineralization [3], however many aspects of PPi and mineral interactions remain unresolved. Important remaining questions include whether dual genetic ablation of PPi regulatory genes can correct or exacerbate the cementum phenotype, i.e. testing the limits of PPi influence on cementogenesis; whether extreme reduction in levels of PPi within tissues would promote ectopic calcification in the PDL or bone-tooth ankylosis; and whether pharmacologic interventions are capable of modulating ongoing cementum formation. We aimed to address these questions by employing several genetically edited mouse models.
RESULTS
Acellular Cementum Growth Is Inversely Proportional to PPi Levels
To further define the role of PPi in directing development of dentoalveolar mineralized tissues, particularly focusing on cementum, we employed several mouse models where PPi levels were altered by global gene ablation. Extracellular and intracellular PPi profiles have previously been generated in these mouse models [6]. Ank KO mice have reduced extracellular PPi levels (e.g., 60% extracellular PPi reduction in Ank KO osteoblasts compared to WT osteoblasts) and previously were shown to rapidly form unusually thick acellular cementum, while other dentoalveolar tissues were relatively unaffected [3, 5, 6]. Conversely, Alpl KO mice have increased PPi levels (e.g., 30–130% extracellular PPi increase in Alpl KO osteoblasts compared to WT osteoblasts) and display a paucity of acellular cementum, as well as additional severe mineralization defects that phenocopy the inherited condition, hypophosphatasia (HPP), [2, 3, 7–9]. We hypothesized that dual genetic modulation of PPi metabolism by Ank and Alpl knockout and subsequent relative correction of PPi levels [6] would normalize cementum as well as other mineralized tissues. Due to the shortened lifespan of Alpl KO mice, tooth development was examined only at 26 dpn.
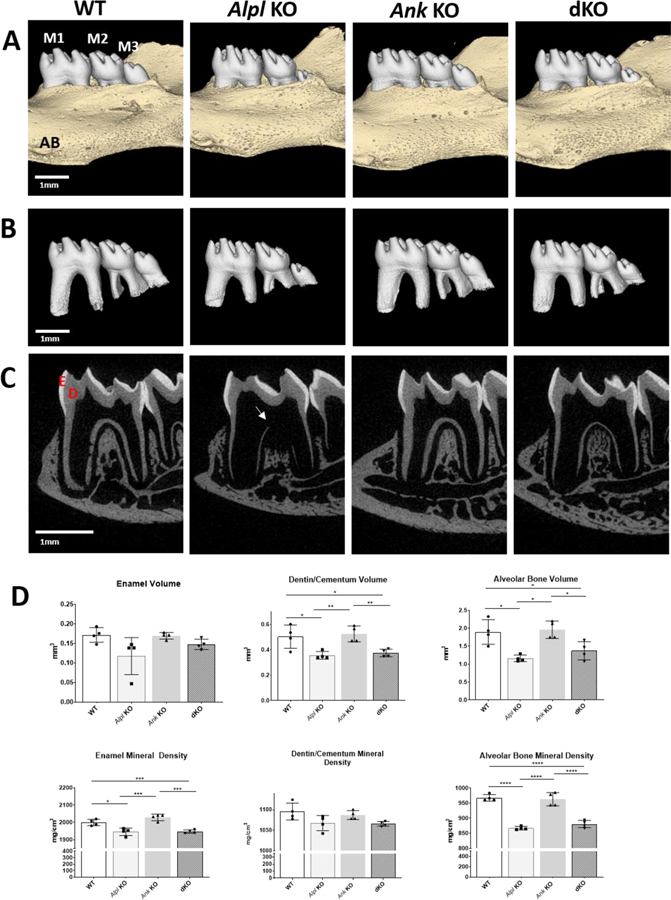
Micro-CT analysis revealed that Alpl KO dentoalveolar defects were ameliorated with deletion of the Ank allele in Alpl, Ank dKO mice. Decreased molar root length and delayed tooth eruption of mandibular third molars were partially improved in dKO mice (Figure 1A–C), however, reduced dentin and alveolar bone volumes remained unimproved in Alpl, Ank dKO mice (Figure 1D). Hypomineralized regions of dentin in Alpl KO mice (Figure 1C, arrow) appeared to be improved in Alpl, Ank dKO mice, however quantitative measurements indicted no significant improvement in dentin/cementum volumes of dKO vs. Alpl KO mice (Figure 1D). Histology of the first molar showed dramatically increased acellular cementum in Ank KO mice and absence of cementum and proper PDL insertions in Alpl KO mice as reported previously (Figure 2A) [8, 9]. Histology revealed that decreasing PPi via deletion of Ank in Alpl KO mice enabled formation of acellular cementum along the root surface, and PDL attachment was reestablished (Figure 2A, B), although cementum thickness was noticeably greater than in WT molars.
Figure 1. Ablation of Ank in Alpl KO Mice Partially Improves Dentoalveolar Mineralization in Alpl KO Mice.
Three-dimensional reconstructions of (A) mouse mandibles and (B) isolated molar teeth show comparable crown morphology and eruption pattern of mandibular first (M1) and second (M2) molars in WT, Alpl KO, Ank KO, and Alpl, Ank dKO mice at 26 dpn. Compared to WT and Ank KO, both Alpl KO and Alpl, Ank dKO mice exhibit delayed eruption and underdeveloped roots of mandibular 3rd molars (M3). Sagittal 2D images with enamel (E) in white and dentin (D) and alveolar bone (AB) in gray (C) reveal thin, hypomineralized dentin and alveolar bone mineralization defects (yellow arrow) in Alpl KO that are partially ameliorated in Alpl, Ank dKO mice. (D) Quantitative analysis indicates dentin/cementum and alveolar bone volumes and enamel and alveolar bone mineral density defects in Alpl KO molars are not corrected in Alpl, Ank dKO molars.
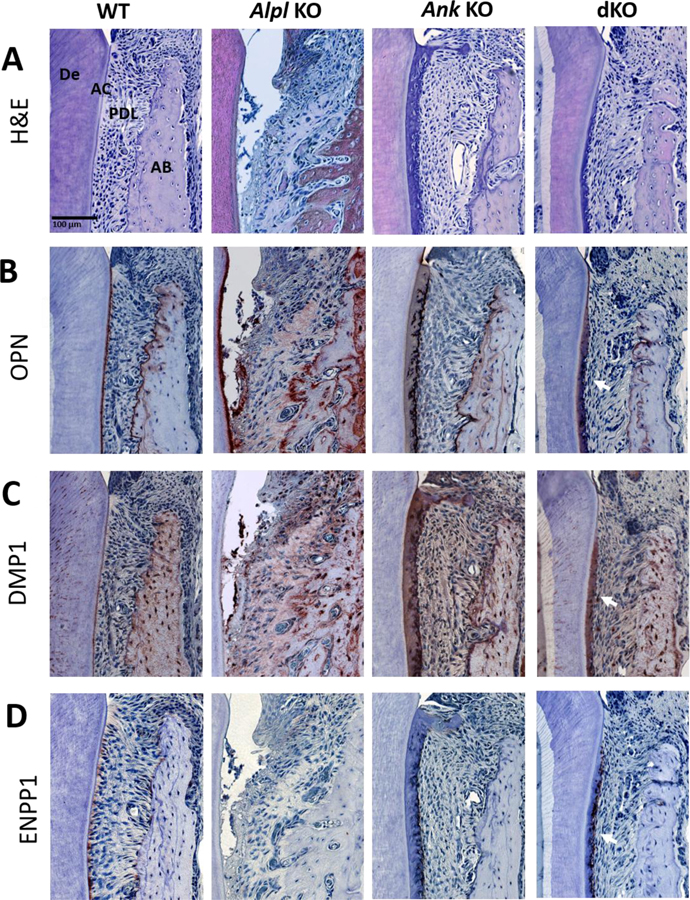
Figure 2. Acellular Cementum Growth Is Inversely Proportional to PPi Levels.
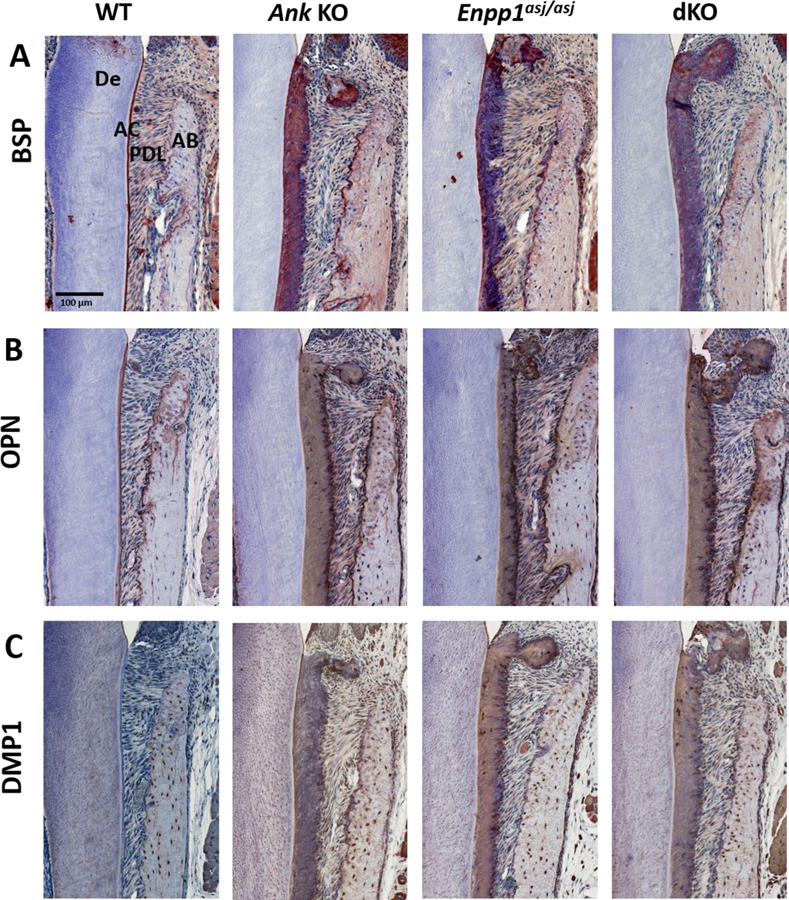
H&E staining (A) shows a layer of acellular cementum (AC) covering root dentin (De) in WT mice, lack of AC in Alpl KO mice, and increased AC in Ank KO mice at 26 dpn. In Alpl, Ank dKO mice, AC is reestablished along the root surface and exhibits increased width (arrowheads). (B) OPN serves as a marker for AC on root surfaces. (C) DMP1 immunolocalization increases with thick AC in Ank KO and Alpl, Ank dKO mice. (D) ENPP1 is expressed by cementoblasts lining root surfaces and is reduced in Alpl KO tissues and elevated in Ank KO samples (arrows). In areas of hypercementosis, Alpl, Ank dKO samples exhibit increased Enpp1 expression (arrows). AB: Alveolar bone; PDL: Periodontal ligament.
Immunolocalization of cementum marker osteopontin (OPN) confirmed formation of acellular cementum on the dKO molar root surface (Figure 2B). Dentin matrix protein-1 (DMP1) was localized to the thick Ank KO and dKO cementum with less staining in WT cementum (Figure 2C). ENPP1 functions in parallel to ANK to produce extracellular PPi and is expressed in cementoblasts (Figure 2D). ENPP1 was absent in Alpl KO cementoblasts and over-expressed in Ank KO cementoblasts as previously reported [2]. In dKO molars, ENPP1 over-expression was observed only adjacent to regions of thickened cementum, suggesting a relationship between cementum growth and PPi interaction via induction of ENPP1 expression.
Additional Reduction of Pyrophosphate Does Not Increase Cementogenesis
Results of experiments described above provide evidence that increasing, decreasing, or normalizing PPi levels through genetic ablation of Alpl and/or Ank can alter expression of cementum genes/proteins and predictably determine acellular cementum thickness. However, it remained unclear whether further reduction in PPi levels beyond those in Ank KO mice would result in additional acellular cementum formation, reduction of PDL space, and/or tooth-bone ankylosis. Similar to ANK, ENPP1 increases extracellular PPi levels, and Enpp1asj/asj mice exhibit reduced local and systemic PPi levels and thick acellular cementum, while other dentoalveolar tissues are relatively unaffected [2–4]. We genetically ablated both Ank and Enpp1 functions in mice, hypothesizing additional cementum growth beyond the respective Ank KO and Enpp1asj/asj mice.
We confirmed effects of single and double gene ablation on selected markers of mineralization (Table 1). Sera from adult mice were obtained, and compared to WTs and Ank KOs, circulating alkaline phosphatase levels (ALP) in Enpp1asj/asj mice were significantly higher (p<0.05), whereas in dKO mice ALP levels trended higher (Table 1). This is consistent with clinical case reports of GACI individuals experiencing secondary hypophosphatemic rickets [10], further supporting a relationship between ENPP1 and TNAP. Serum calcium, phosphorous, sodium, potassium, and magnesium levels were comparable between WTs and all KOs. Furthermore, as previously described, single KO and dKO mice exhibited prominent ectopic calcification of the joints [6] (Appendix Figure 1A). In particular, periarticular calcifications and thinning of digits (Appendix Figure 1A, arrowheads) were apparent in Ank KO, Enpp1asj/asj, and dKO mice at 60dpn. While Enpp1asj/asj mice exhibited reduced cortical bone thickness [11], dKO mice additionally showed reduced femur length (Appendix Figure 1B). Cortical area fraction and trabecular number were decreased in all KOs compared to WTs.
Table 1: Summary of serum chemistry analyses from loss of ANK and/or ENPP1.
Sera from 60dpn male and female mice were analyzed. Data are presented as mean± standard deviation. Compared to WTs and Ank KOs, alkaline phosphatase levels in Enpp1asj/asj mice were significantly higher (p<0.05), whereas dKO mice alkaline phosphatase levels trended higher. Serum calcium, phosphorous, sodium, potassium, and magnesium levels were comparable between WTs and all KOs.
| WT | Ank KO | Enpp1asj/asj | dKO | |
|---|---|---|---|---|
| Alkaline Phosphatase (U/L) | 233.00 ± 50.06 | 239.20 ± 51.03 | 320.50 ± 70.83* | 287.60 ± 58.08 |
| Calcium (mmol/L) | 2.05 ± 0.51 | 1.91 ± 0.35 | 1.96 ± 0.61 | 1.86 ± 0.38 |
| Phosphorous (mg/dL) | 16.38 ± 2.97 | 13.74 ± 3.49 | 14.79 ± 5.50 | 16.81 ± 6.62 |
| Sodium (mmol/L) | 617.60 ± 18.24 | 617.50 ± 10.01 | 618.90 ± 11.48 | 615.20 ± 13.39 |
| Potassium (mmol/L) | 16.48 ± 5.50 | 16.25 ± 6.39 | 17.43 ± 7.74 | 20.56 ± 9.10 |
| Magnesium (mmol/L) | 1.71 ± 0.31 | 1.52 ± 0.19 | 1.76 ± 0.26 | 1.73 ± 0.23 |
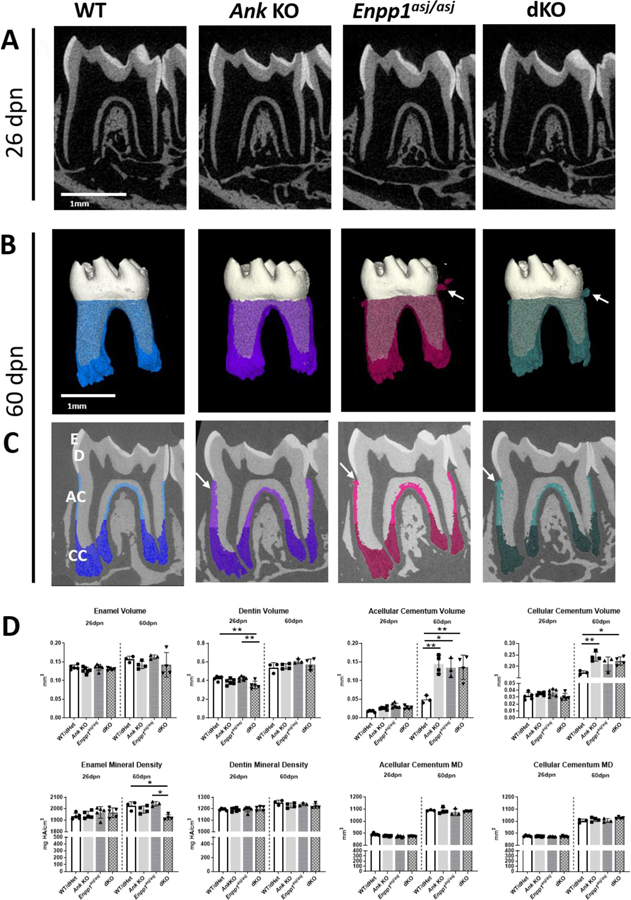
MicroCT analysis indicated reduced enamel density in dKO mice compared to WTs, Ank KOs, and Enpp1asj/asj mice. Reduced dentin volume was observed in dKO mice at 26 dpn, which normalized by 60 dpn (Figure 3A–D). Acellular cementum volume was increased vs. WT by about 300% in 60 dpn single and dKO mice (p<0.05 or 0.01), with no differences detected amongst the latter three genotypes. Cellular cementum was increased by about 15–30% in only Ank KO and dKO vs. WT mice at 60dpn (p<0.05 or 0.01). No differences were found in single or double KO tissue mineral densities (Figure 3).
Figure 3. Additional Reduction of Pyrophosphate Does Not Increase Cementogenesis.
At 26dpn, 2D reconstructions (A) of mouse mandibles exhibit comparable crown morphology, root structures, and alveolar bone (AB) between WT, Ank KO, Enpp1 mutant, and dKO mice. Compared to WT at 60dpn (B, C), cementum is increased in Ank KO, Enpp1asj/asj, and dKO mice (cementum colored in B, C). Cementicles (B, C, white arrows) are evident in Ank KO, Enpp1asj/asj, and dKO mandibles with more prominent cementicles in Enpp1asj/asj and dKO vs. Ank KO mice. Cementum was subdivided into acellular cementum (AC) (lighter color, coronal 2/3 of root in panel C) and cellular cementum (CC) (darker color, apical 1/3 of root in panel C). (D) Quantitative analysis at 26 and 60 dpn shows differences in Ank KO, Enpp1asj/asj, and dKO vs. WT in acellular cementum volume and cellular cementum volume. The dKO mouse does not develop further increased acellular or cellular cementum compared to individual KO mice. De: Dentin; E: Enamel
Double Knockout of Ank and Enpp1 Does Not Result in Dental Ankylosis
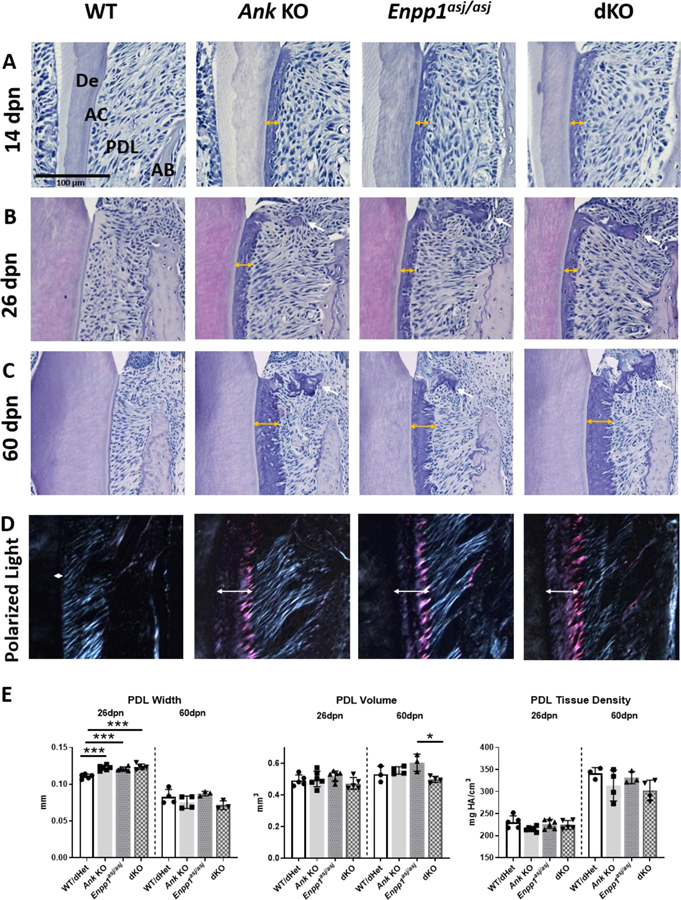
Surprisingly, dKO mice did not experience increased acellular cementum growth vs. Ank KO or Enpp1asj/asj mice. We next examined other aspects of the periodontal tissues to determine effects of double gene ablation, investigating potential loss of functional PDL through inappropriate mineralization. Histological analyses revealed dramatic and continuous expansion of acellular cementum in Ank KO, Enpp1asj/asj, and dKO mice (Figure 4A–C). Sharpey’s fiber insertions into cementum remained oblique, functionally positioned, and of similar fiber density, even in the expanded cementum of KO and dKO mice (Figure 4D). MicroCT analysis revealed that PDL width was increased in Ank KO, Enpp1asj/asj, and dKO mice vs. WT at 26 dpn (p<0.001, Figure 4E), even in the face of expanded cementum (Figure 4). Overall PDL volume and mineral density did not change in Ank KO, Enpp1asj/asj, or dKO mice vs. WT mice at 26dpn. At 60dpn, dKO PDL volume decreased in comparison to Enpp1asj/asj mutants (Figure 4E).
Figure 4. Double Knockout of Ank and Enpp1 Does Not Result in Dental Ankylosis.
At 14dpn (A), 26dpn (B), and 60dpn (C), first molar acellular cementum (AC) width grows to over 10-fold greater thickness vs. WT in Ank and Enpp1asj/asj and dKO mice (double-headed arrows). Cementicles are absent at 14dpn, but present at later stages in the periodontal ligament (PDL) when the root is fully formed (B, C, single-headed white arrows). Microscopic observation of H&E stained sections between crossed polarizers revealed that Ank KO, Enpp1asj/asj, and dKO mice exhibit normal appearing and functionally oriented Sharpey’s fiber insertion into the cementum (D). Quantitative analysis reveals minimal changes in PDL dimensions and no differences in density in Ank KO, Enpp1asj/asj, and dKO mice vs. WT (E). AB: Alveolar bone; De: Dentin.
Inappropriate calcification within the PDL space was not observed in Ank KO, Enpp1asj/asj, or dKO mice, with one exception. Cementicles were regularly present at the buccal aspect of the cervical PDL around the mandibular first molar (also present around second and third molars). Cementicles were generally attached to acellular cementum adjacent to the cementoenamel junction (Figure 3B).
Altered Cementum Extracellular Matrix Proteins in the Absence of ANK and ENPP1
Based on altered cementogenesis in KO and dKO mice featuring low PPi, we performed IHC to examine presence and distribution of cementum markers. Bone sialoprotein (BSP), the most commonly used marker for acellular cementum, marked thick KO and dKO cervical cementum, although less uniformly than in WT molars (Figure 5A). OPN, a widely distributed mineral regulator, was stained with slightly higher intensity in cervical cementum adjacent to PDL insertions of KOs compared to WTs (Figure 5B, Appendix Figure 2B). DMP1, not normally found at high levels in acellular cementum, intensely localized to cervical cementum of Ank KO, Enpp1asj/asj, and dKO mice and as expected, in osteocytes in all phenotypes (Figure 5C, Appendix Figure 2C). Cementicles stained prominently for all three markers. IHC for BSP, OPN, and DMP1 in PDL and alveolar bone did not reveal any major differences, and cellular cementum also stained similarly for these markers (Figure 5A–C, Appendix Figure 2).
Figure 5. Altered Extracellular Matrix Markers in Acellular Cementum of KO and dKO Mice.
Expanded acellular cementum (AC) of Ank KO, Enpp1asj/asj, and dKO mice feature strong immunolocalization of bone sialoprotein (BSP) and osteopontin (OPN). Ank KO, Enpp1asj/asj, and dKO mice exhibit increased dentin matrix protein 1 (DMP1) compared to WT (C). Cementicles stained prominently for BSP, OPN, and DMP1. De: Dentin; PDL: Periodontal ligament; AB: Alveolar bone.
Maintenance of PDL Space by Alveolar Bone Modeling in Mice with Hypercementosis
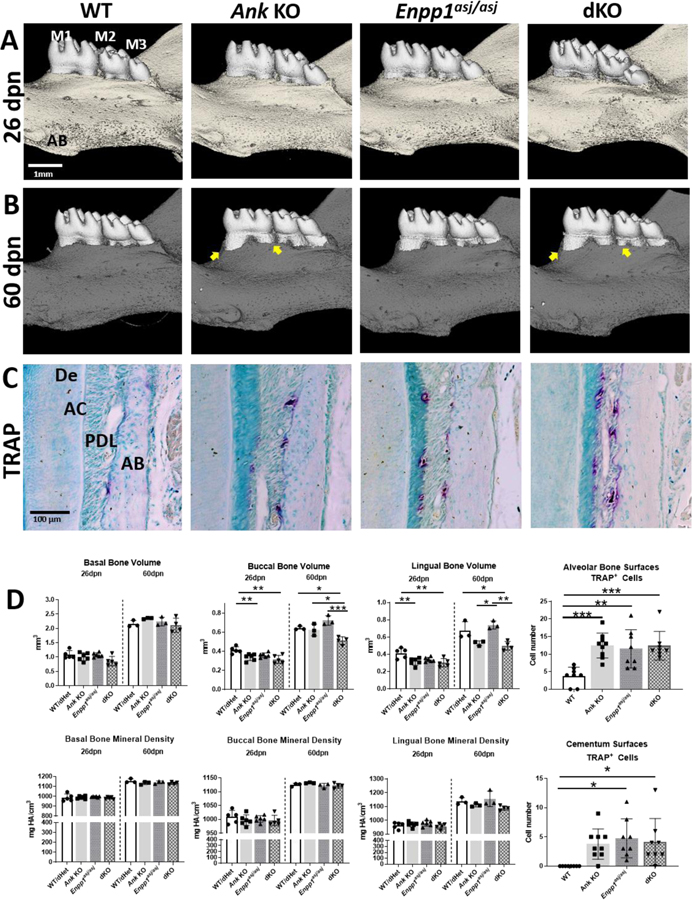
Despite rapid cementogenesis, the PDL space was not diminished in single KO and dKO mice and thus, we further examined alveolar bone. At 26dpn, alveolar bone crest heights were comparable between all genotypes (Figure 7A). By 60dpn, compared to WT and Enpp1asj/asj mice, alveolar bone crest heights were reduced in Ank KO and dKO (Figure 6B, arrows). Buccal and lingual alveolar bone volumes were reduced in Ank KO and dKO mice vs. WT at 26 and 60 dpn (p<0.05 to 0.001) and not in Enpp1asj/asj mice (Figure 6D). Basal bone (inferior and distant to the molars) did not show volume differences between genotypes, suggesting alveolar bones differences arose from active modeling (e.g., from occlusal forces) rather than developmental defects inherent to osteogenesis. No differences in alveolar bone density were noted between genotypes (6D).
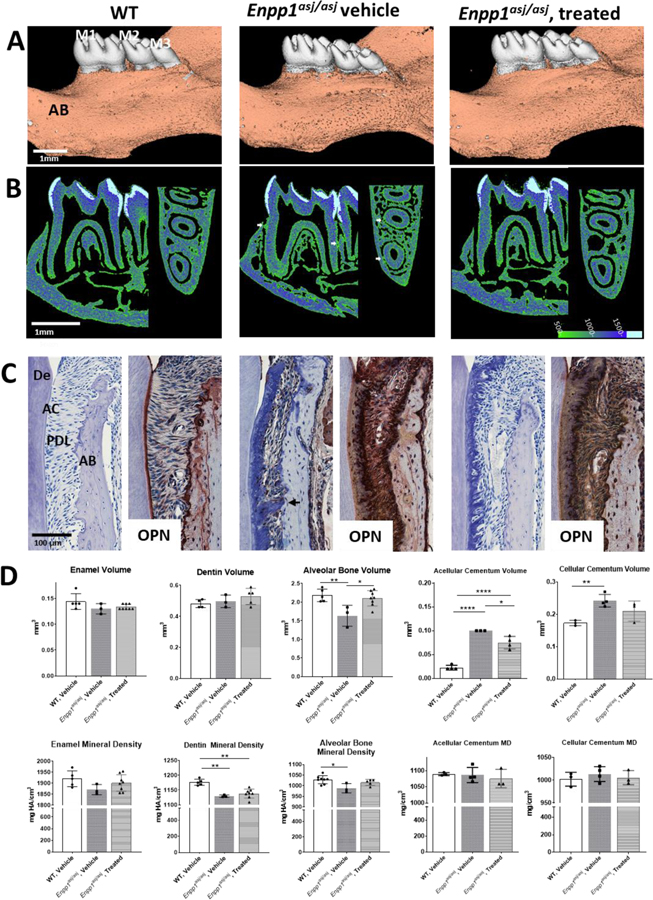
Figure 7. Pharmacologic Modulation of Pyrophosphate Regulates Ongoing Cementogenesis.
3D reconstructions (A) of mouse mandibles and molars (M1–M3) at 35–37dpn after a high phosphate and low magnesium “acceleration” diet, including WT control, untreated Enpp1asj/asj, and Enpp1asj/asj mice treated with the ENPP1-Fc protein for 3 weeks. In untreated Enpp1asj/asj mice, ankylosis (white arrows) between alveolar bone (AB) and cementum is evident in 2D heat map reconstructions (B) and histological sections (C), but ankylosis is absent in ENPP1-Fc treated Enpp1asj/asj mice. Heat map images correspond to hydroxyapatite mineral densities (mg/cm3) defined by the color bar in B. Both untreated and treated Enpp1asj/asj mice feature increased OPN immunolocalization in the periodontal ligament (PDL). ENPP1-Fc treated Enpp1asj/asj mice exhibit lower acellular and cellular cementum volumes (AC and CC, respectively) compared to vehicle-treated Enpp1asj/asj mice (D). Alveolar bone volume is normalized in ENPP1-Fc-treated vs. untreated Enpp1asj/asj mice PDL. De: Dentin.
Figure 6. Maintenance of PDL Space by Alveolar Bone Modeling in Mice with Hypercementosis.
3D reconstructions (A, B) of mouse mandibles indicate similar alveolar bone (AB) levels at 26 dpn but AB loss in Ank KO and dKO by 60dpn (B, arrows). TRAP staining (C) reveals osteoclast-like cells (red-purple staining) on alveolar bone and first molar (M1) root surfaces. (D) Quantitative analysis indicates reduced alveolar bone volumes in Ank KO and dKO vs. WT mandibles at 26dpn and/or 60dpn. No differences in alveolar bone density between genotypes are observed. Ank KO, Enpp1asj/asj and dKO mice feature increased numbers of osteoclast-like cells on AB surfaces and odontoclast-like cells on acellular cementum (AC) surfaces. PDL: Periodontal ligament; De: Dentin.
We performed TRAP staining to quantify osteoclast-like and odontoclast-like cells in the periodontal tissues at 60dpn (Figure 6C). Ank KO, Enpp1asj/asj, and dKO mice exhibited about 2-fold increased numbers of osteoclast-like cells on bone surfaces (p<0.05), while all the single KO and dKO mice displayed increased numbers of odontoclast-like cells on root surfaces (p<0.05) compared to WT mice. (Figure 6D).
Pharmacologic Modulation of Pyrophosphate Regulates Ongoing Cementogenesis
In the experiments described above, we observed that genetic modulation of PPi by deletion of Alpl, Ank, and/or Enpp1 markedly affected acellular cementum growth during development. We next investigated whether ENPP1 pharmacologic intervention aimed at regulating PPi levels could have a similar effect, an approach with potential for clinical application to promote cementogenesis in conditions of periodontal loss or protect against ankylosis. We hypothesized that systemic restoration of ENPP1 would have local effects on the periodontium, i.e., inhibit cementogenesis. An ENPP1-Fc fusion protein restored PPi levels and prevented ectopic vascular calcification in Enpp1asj/asj mice [12] and we investigated the effects of this protein on cementogenesis and dentoalveolar development.
All mice were fed an “acceleration diet” featuring high inorganic phosphate (Pi) and low magnesium to promote ectopic calcification. In this context, two-thirds (n=3) of untreated Enpp1asj/asj mice developed bone-tooth ankylosis (Figure 7B–C, arrows). Ankylosis was not observed in any Enpp1asj/asj mice treated with ENPP1-Fc (n=8). We observed increased PDL width variability in Enpp1asj/asj mice receiving the vehicle versus WT and ENPP1-Fc dosed Enpp1asj/asj mice (Supplemental Figure 3). Furthermore, whereas Enpp1asj/asj mice on a normal diet exhibited a higher baseline PDL width compared to WT mice (Figure 4), Enpp1asj/asj mice receiving the acceleration diet exhibited lower or comparable PDL widths versus WT mice (Supplemental Figure 3C). In Enpp1asj/asj mutants receiving the acceleration diet, OPN expression was further enhanced (Figure 7C). Acellular cementum volume was significantly decreased by about 25% in Enpp1asj/asj mice treated by ENPP1-Fc vs. vehicle (Figure 7D), though this was still greater than in WT mice (p<0.05 to 0.001). Cellular cementum volume of Enpp1asj/asj mice was decreased about 10% with ENPP1-Fc compared to vehicle. Alveolar bone volume was increased to normal in treated Enpp1asj/asj mice (p<0.05 to 0.01), indicating reduced modeling in response to cementum expansion. Enamel and dentin volumes and densities remained unchanged in ENPP1-Fc treated Enpp1asj/asj mice vs. vehicle treated Enpp1asj/asj or WT (Figure 7D).
DISCUSSION
Pyrophosphate (PPi) is a physiologically important regulator of mineralization, with systemic and local concentrations determined by TNAP, ANK, and ENPP1, among other factors. Acellular cementum thickness is inversely proportional to PPi levels, with reduced cementum in Alpl KO mice (increased PPi), and excess cementum in Ank KO mice (decreased PPi). Genetic correction of PPi by deletion of the Ank allele in Alpl, Ank dKO mice ameliorated Alpl KO dentoalveolar mineralization defects, particularly restoring acellular cementum and associated PDL attachment and function. Simultaneous ablation of both Ank and Enpp1 did not expand acellular cementum growth beyond that observed in single KO mice, but also did not result in ankylosis, in part due to increased presence of osteoclasts that modeled alveolar bone to allow for the expanding cementum layer. While these mouse models provide insights into cementum formation during development, we additionally demonstrated for the first time that pharmacologic manipulation of PPi through an ENPP1-Fc fusion protein can regulate cementum growth. These studies provide new insights into how PPi developmentally regulates cementum, additionally supporting interventions targeting PPi metabolism as novel therapeutic approaches.
Acellular Cementum is Exceptionally Sensitive to Modulation by Pyrophosphate
As reported in previous publications and extended by experiments reported here, development and maintenance of mineralized tissues within the oral cavity are strongly influenced by perturbations in PPi metabolism. Excess PPi in Alpl KO mice had deleterious effects on all of the dentoalveolar tissues: enamel, dentin, cementum, and alveolar bone, as previously described [2, 3, 8, 9, 13]. Alpl KO mice phenocopy the severe infantile form of HPP, where extensive skeletal and other systemic manifestations reflect perturbation of mineralization [14]. All clinical forms of HPP are associated with dental defects, the most universal being premature loss of teeth from reduced acellular cementum [15, 16]. Additionally, HPP individuals and Alpl KO mice (Figure 2) exhibit apical migration of the junctional epithelium. The conversion of junctional epithelium to pocket epithelium is a hallmark of periodontal disease in humans and can be caused by a multitude of factors, including altered inflammatory responses and biofilms in the oral cavity [17]. As a result, clinical case studies have investigated the presence of periodontal pathogens in HPP individuals [18, 19]. These studies did not yield a common microbial profile among studied HPP subjects, suggesting that apical junctional epithelium migration is a consequence of inadequate acellular cementum attachment.
Strikingly, when PPi levels were normalized by ablation of Ank in the Alpl, Ank dKO mice, acellular cementum was restored, as well as periodontal attachment and function. In contrast, enamel, dentin, and alveolar bone exhibited very limited or no improvement in dKO mice, with substantial deficits in tissue volumes and mineral densities apparent. These effects are consistent with findings that Alpl, Ank dKO mice and primary osteoblasts in vitro did not show complete correction of the mineralization phenotype [6]. These novel observations underscore the exceptional sensitivity of acellular cementum to direct modulation by PPi, mirroring early studies using first generation bisphosphonates (e.g. etidronate), PPi analogues, that profoundly disrupted cementum formation in rodents [20–23].
We previously reported that single ablation of Ank or Enpp1 in mice caused expansion of acellular cementum [2–5]. Dual ablation of Ank and Enpp1 results in further reduction of PPi levels and increased severity of ectopic calcifications, evidence that these regulators have distinct effects and operate on different pathways [6]. However, we found that simultaneous ablation of Ank and Enpp1 did not lead to further expansion of acellular cementum growth. Based on this, we conclude there is a limit to the effects that reduction of PPi can have on acellular cementum growth, with ANK and ENPP1 both making similar and somewhat redundant contributions, rather than an additive or synergistic effect. Whether ANK and ENPP1 operate by some sort of interdependent mechanism is an important remaining question deserving additional studies.
While the focus of this study was on acellular cementum, as the most important cementum type for tooth attachment and periodontal function, novel insights into cellular cementum biology were gained. We confirmed in all mouse models with reduced PPi that cellular cementum is less sensitive than acellular cementum to perturbations in PPi levels. For example, while acellular cementum volumes increased by 300% in Ank KO vs. WT mice, cellular cementum volumes increased only by about 25%. Effects of Enpp1 ablation on cellular cementum were even less than in Ank KO mice, indicating potentially unique roles for ANK vs. ENPP1 in cellular cementogenesis.
Periodontal Function is Not Compromised by Absence of ANK and ENPP1
For the periodontal apparatus to remain functional, the hard-soft tissue borders at cementum-PDL and bone-PDL interfaces must be carefully maintained. Despite decades of studies, the factor(s) regulating these PDL borders remain to be fully clarified. These regulators must be potent in order to maintain the PDL as an unmineralized connective tissue despite it being composed of a collagenous matrix nearly identical to that in bone and cementum. In addition, the PDL exhibits high expression of pro-mineralization enzyme TNAP and harbors stem and progenitor cells that can differentiate into osteogenic and cementogenic cells [24–26].
Previously we established that loss-of-function of either ANK or ENPP1 in mice did not disrupt the cementum-PDL-bone borders of the PDL or bulk properties of the PDL [3–5, 27, 28]. Here we confirmed that despite dramatically increased cementum in Ank KO, Enpp1asj/asj, and dKO mice compared to WT controls, PDL insertions remained normal, PDL width and volume were largely unchanged or increased, and no differences in PDL tissue density were observed. There are two notable exceptions to these observations. First, cementicles were observed in Ank KO, Enpp1asj/asj, and dKO periodontia. These were tooth-attached or free floating in the PDL and always localized to cervical buccal regions of PDL, however were not observed to bridge tooth and bone, and did not appear to be exacerbated in dKO vs. single KO mice. Second, ankylosis developed between cementum and alveolar bone in Enpp1 mutant mice receiving high phosphate acceleration diet, paralleling exacerbation of ectopic calcification elsewhere in the body, e.g. in arteries [12]. WT mice administered the acceleration diet did not exhibit ankylosis. These findings on cementicles and ankylosis under challenge support that ANK and ENPP1, and by extension PPi levels, play a role in maintaining mineralized tissue borders in the periodontium.
Altered expression and distribution of ECM proteins in periodontia were observed in association with reduced PPi levels. BSP localization was altered in the thick cementum of Ank, Enpp1, and dKO mouse molars. BSP is important in cementum formation and Ibsp KO mice exhibit a periodontal phenotype resembling Alpl KO mice: cementum hypoplasia, PDL detachment, and periodontal destruction [29, 30]. While the connection between BSP and PPi metabolism remains unclear, Ibsp KO mice exhibit increased ALP levels and increased circulating PPi levels, at early stages of root development, compared to WT mice [5]. Ablation of Ank in Ibsp KO mice reestablished acellular cementum, albeit with reduced growth compared to Ank KO mice, suggesting an additive genetic effect between Ank and Ibsp [5]. OPN coordinates with PPi to regulate calcification in the body [6, 31, 32], in some cases contributing to skeletal and dental pathologies of mineralization disorders such as HPP and X-linked hypophosphatemia (XLH) [9, 33]. Increased OPN in the thick cementum of Ank, Enpp1, single and dKO mice likely represents a back-up mechanism to try and control exuberant cementum growth. Spp1 KO mice featured reduced PDL volume, suggesting OPN as a candidate factor maintaining the PDL space, though ablation of Spp1 in Ank KO mice did not exacerbate the hypercementosis [24]. DMP1 expression is dramatically increased in all models of reduced PPi and associated with the unusual inclusion of cementocyte-like cells in the increased acellular cementum. It remains to be seen whether these cementum ECM changes are directly related to genetic ablation, reduced PPi levels, increased cementogenesis, or some combination of these, and in vitro experiments with genetically edited cementoblast cell lines are ongoing to dissect the underlying mechanisms and functional consequences.
The modeling/remodeling action of osteoclasts on alveolar bone contributes to maintenance of PDL space. There were increased numbers of osteoclasts on alveolar bone surfaces in Ank KO, Enpp1asj/asj, and dKO mice, apparently successfully modeling bone away from the expanding molar roots and maintaining PDL width and volume. As the bone modeling/remodeling process involves osteocytes, osteoblasts, and osteoclasts [34, 35], and all these cells express Ank and Enpp1, direct effects on cell functions may be involved. Ank mutations are reported to affect osteoblast and osteoclast differentiation [36, 37], however effects of gene deletion on these cells have not been explored in detail. Enpp1 ablation is reportedly important in osteocyte maintenance, with little to no effect on osteoclastogenesis and osteoclast resorption potential [38]. While some differences in alveolar bone volumes were noted between single KO and dKO mice, which may be attributed to different contributions of each gene to modeling/remodeling, it is telling that no differences were found in basal bone. These results suggest alveolar bone differences are secondary to local events such as hypercementosis. When challenged by orthodontic tooth movement, Enpp1 mutant mice featured reduced molar movement associated with increased osteoclast numbers on root surfaces and decreased osteoclast numbers on alveolar bone surfaces compared to WT [39]. It is intriguing to speculate that inclusion of cementocyte-like cells in cervical cementum expressing OPN and DMP1 may be influencing osteoclast/odontoclast recruitment to root surfaces, a factor that may help explain increased odontoclast numbers reported here.
In humans, loss-of-function mutations in ENPP1 or ANKH (respectively, the conditions GACI and CMD) are associated with ankylosis, delayed tooth eruption, disturbed primary tooth exfoliation, malocclusion, and slow orthodontic movement [4, 40]. While we failed to observe several of these changes in Ank KO, Enpp1asj/asj, and dKO mouse models, we and others have demonstrated slower orthodontic treatment in these mice [39, 40], indicating altered periodontal biology that may emerge more fully under challenge.
Potential to Promote Cementum Regeneration by Pharmacologic Regulation of Pyrophosphate
These findings support modulation of PPi as a novel therapeutic approach to protect, repair, or regenerate acellular cementum. We showed previously in a proof-of principle study of healing after periodontal fenestration that reduced PPi in Ank KO mice encouraged increased regeneration of cementum compared to WT mice [41]. We extend those findings here by reporting that administration of ENPP1-Fc relatively corrected PPi levels and attenuated acellular cementogenesis, demonstrating that the tissue is amenable to PPi modulation throughout life. Notably, ankylosis was ameliorated in Enpp1 mutants dosed with ENPP1-Fc, showing potential for this approach to control ectopic calcification in periodontal tissues. This is in line with previous studies where administration of etidronate profoundly affected cementogenesis [20–23].
However, to create a positive environment for cementogenesis, PPi levels should be targeted for reduction by antagonizing ANK/ANKH or ENPP1 function, or by increasing TNAP function. An engineered, mineral-targeted form of TNAP (asfotase alfa) is currently used in enzyme replacement therapy for HPP, and we have shown that early administration in Alpl KO mice can completely prevent cementum defects [14, 42]. Further studies are planned to determine whether increasing TNAP or modulating activities of other PPi regulators have therapeutic potential to improve cementum repair/regeneration and restore periodontal function.
EXPERIMENTAL PROCEDURES
Mice
Animal studies were approved by the NIH Animal Care and Use Committee (Bethesda, MD). Preparation of mice genetically ablated for Alpl (Alpl KO) or Ank (Ank KO), or with Enpp1 loss-of-function mutation (Enpp1asj/asj) have been described previously [5, 39, 43, 44]. Alpl, Ank double KO (dKO) and Ank, Enpp1 dKO mice were generated by crossing double heterozygous males and females. Mice were maintained on a C57BL/6 genetic background and fed a normal rodent diet (NIH-31). Time points were selected based on stages of root development: 14 days postnatal (dpn) corresponding to acellular cementum formation prior to eruption; 26 dpn following completion of root formation and with cellular cementum formation; and 60 dpn, after the first molar has been in occlusion for over a month. No differences between males and females were noted, and data presented include males and females.
Serum Analysis
Mice (n =4–8 per genotype) were euthanized by cervical dislocation, and blood was obtained via cardiac puncture and placed in anti-coagulant tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Sera were separated by centrifugation at 5,000 G-force for 10 min at room temperature, aliquoted, and were stored at −80°C. Serum biochemistry was analyzed at the NIH Veterinary Services Clinical Chemistry (Bethesda, MD, USA).
ENPP1-Fc Fusion Protein
Development and testing of a recombinant ENPP1-Fc fusion protein was previously described [12]. Enpp1asj/asj mice were treated daily with subcutaneous injections of 8mg/kg ENPP1-Fc from 14 dpn until termination at 35–37 dpn. All mice (WT, treated and untreated Enpp1asj/asj) were fed an acceleration diet rich in phosphate and restricted in magnesium (Envigo, TD.00442 diet) [12]. Both males and females were used in these studies, and no differences were detected.
Histology
Tissues for histology were fixed in Bouin’s solution for 24 hours, decalcified in an acetic acid/formalin/sodium chloride solution, and paraffin embedded for serial 5 µm frontal (buccolingual) sections. Hematoxylin and eosin (H&E) staining was used to assess morphology. Immunohistochemistry (IHC) procedures have been described previously [24]. Antibodies included: Polyclonal rabbit anti-mouse bone sialoprotein (BSP) IgG (Dr. Renny Franceschi, University of Michigan); polyclonal rabbit anti-rat dentin matrix protein-1 (DMP1) IgG (Takara, Shiga, Japan); polyclonal goat anti-human ENPP1 IgG (Abcam, Cambridge, MA, USA); and polyclonal LF-175 rabbit anti-mouse osteopontin (OPN) IgG (Dr. Larry Fisher, NIDCR/NIH, Bethesda, MD). Tartrate-resistant acid phosphatase (TRAP) staining was used to identify osteoclast/odontoclast-like cells as performed previously [39].
Micro-computed Tomography (Micro-CT)
Hemi-mandibles and limbs were scanned in a µCT 50 scanner (Scanco Medical, Bassersdorf, Switzerland) at 70 kVp, 76 µA, 0.5 Al filter, 900 ms integration time, and 2 or 6 µm voxel dimension. Reconstructed images were calibrated to 5 known densities of hydroxyapatite and analyzed using AnalyzePro (version 1.0; AnalyzeDirect, Overland Park, KS). Cementum was traced as previously described [4]. In brief, reconstructed images underwent a median filter, 11 kernel size, and a mask of cementum was generated with a density range of 450–1175 mg HA/cm3, this mask was then overlaid onto the original scan and then cementum is defined as mineralized tissue above 650 mg HA/cm3 in masked area. Based on previous histological and microCT analyses of WT mice, the cervical 2/3 of cementum was designated as acellular cementum, and the apical 1/3 was designated as cellular cementum [4].
Statistical Analysis
Mean ± standard deviation (SD) are shown in graphs. Data were analyzed using one-way ANOVA with post-hoc Tukey test for pairwise comparisons (Prism version 7.04; GraphPad Software, La Jolla, CA), where p<0.05 was considered statistically significant. Significance is designated with asterisks (*: p<0.05, **: p<0.01, ***: p<0.001).
Supplementary Material
Supplemental Figure 1. Ectopic Calcification and Long Bone Changes in Ank KO, Enpp1asj/asj, and dKO Mice.Periarticular calcifications and thinning of digits (arrowheads) observed in Ank and Enpp1asj/asj and dKO mice at 60dpn. (A) 3D reconstructions and cortical and trabecular regions of interest for femurs (cortical bone: grey, trabecular bone: orange, and cortical pores: green) of 60 dpn mice. (B) Quantitative analysis of bone length and cortical and trabecular bone parameters in all genotypes.
Supplemental Figure 2. Comparable Extracellular Matrix Markers in Cellular Cementum of KO and dKO Mice. Cellular cementum (CC) of all genotypes exhibits comparable BSP, OPN, and DMP1 immunolocalization. De: Dentin; PDL: Periodontal ligament; AB: Alveolar bone.
Supplemental Figure 3. Hypercementosis Compromises PDL Space in Untreated Enpp1asj/asj Mice Receiving a High Phosphate Diet. (A). Heat map 2D microCT reconstructions of the mandibular first molar mesial and distal roots show ankylosis between cementum and bone in Enpp1asj/asj vehicle mice. Heat maps correspond to hydroxyapatite mineral densities (mg/cm3) defined by color bar. (B, C) A closer examination of PDL thicknesses in mesial and distal roots show increased PDL width variability in Enpp1asj/asj mice receiving the vehicle versus WT and ENPP1-Fc dosed Enpp1asj/asj mice. To measure PDL thickness, 50 axial slices from each root were sampled, and the width from each slice was plotted on a frequency graph (B). Slices analyzed from Enpp1asj/asj mice receiving the vehicle trended toward reduced PDL widths compared to WT and ENPP1-Fc dosed Enpp1asj/asj mice. Because ankylosis did not occur along the entire circumference of tooth roots and PDL widths did not decrease uniformly, we did not observe a significant PDL width difference between Enpp1asj/asj and WT mice on a high phosphate diet when all slices were averaged (C).
Highlights.
Ablation of Ank in Alpl KO mice partially improves dentoalveolar mineralization.
Acellular cementum growth is inversely proportional to pyrophosphate levels.
Double knockout of Ank and Enpp1 does not increase cementogenesis and does not result in dental ankylosis.
PDL space is maintained by alveolar bone modeling in mice with hypercementosis.
Pharmacologic modulation of pyrophosphate regulates ongoing cementogenesis.
ACKNOWLEDGMENTS
We thank Dr. Vardit Kram for assistance with microCT scanning (National Institute of Dental and Craniofacial Research [NIDCR]/NIH) as well as Dr. Evelyn Ralston and Ms. Aster Kinea (Light Imaging Section, National Institute of Arthritis and Musculoskeletal and Skin Diseases [NIAMS]/NIH) for assistance in slide scanning. EYC, TDV, MBC, AN, EM, FHN, SFA, DK, KZ, XL, PRS, JLM, BLF, and MJS report no conflicts of interest. DTB is an inventor of patents owned by Yale University which describe therapeutics for ENPP1 deficiency. DTB is an equity holder and receives research and consulting support from Inozyme Pharma, Inc. This work was funded by K99/R00 grant AR073926 and NIAMS DIR to EYC; Japan Society for the Promotion of Science to AN, R01 grant DE12889 to JLM, R03 grants DE028411 and DE028632 and R01 grant DE027639 to BLF, and Inozyme Pharma to DTB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Millan JL, The role of phosphatases in the initiation of skeletal mineralization. Calcif Tissue Int, 2013. 93(4): p. 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zweifler LE, et al. , Counter-regulatory phosphatases TNAP and NPP1 temporally regulate tooth root cementogenesis. Int J Oral Sci, 2015. 7(1): p. 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster BL, et al. , Central role of pyrophosphate in acellular cementum formation. PLoS One, 2012. 7(6): p. e38393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thumbigere-Math V, et al. , Hypercementosis Associated with ENPP1 Mutations and GACI. J Dent Res, 2018. 97(4): p. 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ao M, et al. , Overlapping functions of bone sialoprotein and pyrophosphate regulators in directing cementogenesis. Bone, 2017. 105: p. 134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harmey D, et al. , Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol, 2004. 164(4): p. 1199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowden SA and Foster BL, Profile of asfotase alfa in the treatment of hypophosphatasia: design, development, and place in therapy. Drug Des Devel Ther, 2018. 12: p. 3147–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beertsen W, VandenBos T, and Everts V, Root development in mice lacking functional tissue non-specific alkaline phosphatase gene: inhibition of acellular cementum formation. J Dent Res, 1999. 78(6): p. 1221–9. [DOI] [PubMed] [Google Scholar]
- 9.Foster BL, et al. , Tooth root dentin mineralization defects in a mouse model of hypophosphatasia. J Bone Miner Res, 2013. 28(2): p. 271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira CR, et al. , Treatment of hypophosphatemic rickets in generalized arterial calcification of infancy (GACI) without worsening of vascular calcification. Am J Med Genet A, 2016. 170A(5): p. 1308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackenzie NC, et al. , Altered bone development and an increase in FGF-23 expression in Enpp1(−/−) mice. PLoS One, 2012. 7(2): p. e32177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albright RA, et al. , ENPP1-Fc prevents mortality and vascular calcifications in rodent model of generalized arterial calcification of infancy. Nat Commun, 2015. 6: p. 10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav MC, et al. , Enzyme replacement prevents enamel defects in hypophosphatasia mice. J Bone Miner Res, 2012. 27(8): p. 1722–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowden SA and Foster BL, Alkaline Phosphatase Replacement Therapy for Hypophosphatasia in Development and Practice. Adv Exp Med Biol, 2019. 1148: p. 279–322. [DOI] [PubMed] [Google Scholar]
- 15.Whyte MP, Wenkert D, and Zhang F, Hypophosphatasia: Natural history study of 101 affected children investigated at one research center. Bone, 2016. 93: p. 125–138. [DOI] [PubMed] [Google Scholar]
- 16.Reibel A, et al. , Orodental phenotype and genotype findings in all subtypes of hypophosphatasia. Orphanet J Rare Dis, 2009. 4: p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosshardt DD and Lang NP, The junctional epithelium: from health to disease. J Dent Res, 2005. 84(1): p. 9–20. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto E, et al. , Clinical and microbiological evaluations of childrenwith hypophosphatasia affected by periodontitis. Pediatric Dental Journal, 2007. 17(1): p. 84–92. [Google Scholar]
- 19.Watanabe H, et al. , Clinical and laboratory studies of severe periodontal disease in an adolescent associated with hypophosphatasia. A case report. J Periodontol, 1993. 64(3): p. 174–80. [DOI] [PubMed] [Google Scholar]
- 20.Takano Y, et al. , Possible role of dentin matrix in region-specific deposition of cellular and acellular extrinsic fibre cementum. J Electron Microsc (Tokyo), 2003. 52(6): p. 573–80. [DOI] [PubMed] [Google Scholar]
- 21.Alatli I and Hammarstrom L, Root surface defects in rat molar induced by 1-hydroxyethylidene-1,1-bisphosphonate. Acta Odontol Scand, 1996. 54(1): p. 59–65. [DOI] [PubMed] [Google Scholar]
- 22.Alatli-Kut I, Hultenby K, and Hammarstrom L, Disturbances of cementum formation induced by single injection of 1-hydroxyethylidene-1,1-bisphosphonate (HEBP) in rats: light and scanning electron microscopic studies. Scand J Dent Res, 1994. 102(5): p. 260–8. [DOI] [PubMed] [Google Scholar]
- 23.Beertsen W, Niehof A, and Everts V, Effects of 1-hydroxyethylidene-1, 1-bisphosphonate (HEBP) on the formation of dentin and the periodontal attachment apparatus in the mouse. Am J Anat, 1985. 174(1): p. 83–103. [DOI] [PubMed] [Google Scholar]
- 24.Foster BL, et al. , Osteopontin regulates dentin and alveolar bone development and mineralization. Bone, 2018. 107: p. 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Jong T, et al. , The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J Periodontal Res, 2017. 52(6): p. 965–974. [DOI] [PubMed] [Google Scholar]
- 26.Beertsen W, McCulloch CA, and Sodek J, The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000, 1997. 13: p. 20–40. [DOI] [PubMed] [Google Scholar]
- 27.Foster BL, et al. , The progressive ankylosis protein regulates cementum apposition and extracellular matrix composition. Cells Tissues Organs, 2011. 194(5): p. 382–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nociti FH Jr., et al. , Cementum: a phosphate-sensitive tissue. J Dent Res, 2002. 81(12): p. 817–21. [DOI] [PubMed] [Google Scholar]
- 29.Foster BL, et al. , Mineralization defects in cementum and craniofacial bone from loss of bone sialoprotein. Bone, 2015. 78: p. 150–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster BL, et al. , Deficiency in acellular cementum and periodontal attachment in bsp null mice. J Dent Res, 2013. 92(2): p. 166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harmey D, et al. , Elevated skeletal osteopontin levels contribute to the hypophosphatasia phenotype in Akp2(−/−) mice. J Bone Miner Res, 2006. 21(9): p. 1377–86. [DOI] [PubMed] [Google Scholar]
- 32.Addison WN, et al. , Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem, 2007. 282(21): p. 15872–83. [DOI] [PubMed] [Google Scholar]
- 33.Boukpessi T, et al. , Osteopontin and the dento-osseous pathobiology of X-linked hypophosphatemia. Bone, 2017. 95: p. 151–161. [DOI] [PubMed] [Google Scholar]
- 34.Bellido T, Osteocyte-driven bone remodeling. Calcif Tissue Int, 2014. 94(1): p. 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crockett JC, et al. , Bone remodelling at a glance. J Cell Sci, 2011. 124(Pt 7): p. 991–8. [DOI] [PubMed] [Google Scholar]
- 36.Dutra EH, Chen IP, and Reichenberger EJ, Dental abnormalities in a mouse model for craniometaphyseal dysplasia. J Dent Res, 2013. 92(2): p. 173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen IP, et al. , A Phe377del mutation in ANK leads to impaired osteoblastogenesis and osteoclastogenesis in a mouse model for craniometaphyseal dysplasia (CMD). Hum Mol Genet, 2011. 20(5): p. 948–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajjawi MO, et al. , Mineralisation of collagen rich soft tissues and osteocyte lacunae in Enpp1(−/−) mice. Bone, 2014. 69: p. 139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf M, et al. , Reduced Orthodontic Tooth Movement in Enpp1 Mutant Mice with Hypercementosis. J Dent Res, 2018. 97(8): p. 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen IP, et al. , Dental Anomalies Associated with Craniometaphyseal Dysplasia. J Dent Res, 2014. 93(6): p. 553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodrigues TL, et al. , Modulation of phosphate/pyrophosphate metabolism to regenerate the periodontium: a novel in vivo approach. J Periodontol, 2011. 82(12): p. 1757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKee MD, et al. , Enzyme replacement therapy prevents dental defects in a model of hypophosphatasia. J Dent Res, 2011. 90(4): p. 470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fedde KN, et al. , Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J Bone Miner Res, 1999. 14(12): p. 2015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gurley KA, et al. , Mineral formation in joints caused by complete or joint-specific loss of ANK function. J Bone Miner Res, 2006. 21(8): p. 1238–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Ectopic Calcification and Long Bone Changes in Ank KO, Enpp1asj/asj, and dKO Mice.Periarticular calcifications and thinning of digits (arrowheads) observed in Ank and Enpp1asj/asj and dKO mice at 60dpn. (A) 3D reconstructions and cortical and trabecular regions of interest for femurs (cortical bone: grey, trabecular bone: orange, and cortical pores: green) of 60 dpn mice. (B) Quantitative analysis of bone length and cortical and trabecular bone parameters in all genotypes.
Supplemental Figure 2. Comparable Extracellular Matrix Markers in Cellular Cementum of KO and dKO Mice. Cellular cementum (CC) of all genotypes exhibits comparable BSP, OPN, and DMP1 immunolocalization. De: Dentin; PDL: Periodontal ligament; AB: Alveolar bone.
Supplemental Figure 3. Hypercementosis Compromises PDL Space in Untreated Enpp1asj/asj Mice Receiving a High Phosphate Diet. (A). Heat map 2D microCT reconstructions of the mandibular first molar mesial and distal roots show ankylosis between cementum and bone in Enpp1asj/asj vehicle mice. Heat maps correspond to hydroxyapatite mineral densities (mg/cm3) defined by color bar. (B, C) A closer examination of PDL thicknesses in mesial and distal roots show increased PDL width variability in Enpp1asj/asj mice receiving the vehicle versus WT and ENPP1-Fc dosed Enpp1asj/asj mice. To measure PDL thickness, 50 axial slices from each root were sampled, and the width from each slice was plotted on a frequency graph (B). Slices analyzed from Enpp1asj/asj mice receiving the vehicle trended toward reduced PDL widths compared to WT and ENPP1-Fc dosed Enpp1asj/asj mice. Because ankylosis did not occur along the entire circumference of tooth roots and PDL widths did not decrease uniformly, we did not observe a significant PDL width difference between Enpp1asj/asj and WT mice on a high phosphate diet when all slices were averaged (C).