ABSTRACT
Enterovirus 71 (EV71) is one of the major causative agents for hand, foot and mouth disease (HFMD) in children. Although there are three inactivated virus-based HFMD vaccines licensed in China, alternative approaches have been taken to produce an effective and safer vaccine that is easier to manufacture in large scale. Among these, a virus-like particles (VLPs) based EV71 vaccine is under active development. For this purpose, an efficient methodology for the production of EV71-VLPs by recombinant technology is needed. We here report the construction and expression of the P1 and 3C genes of EV71 in Pichia pastoris for producing VLP-based EV71 vaccine antigen with a high yield and simple manufacturing process. Based on codon-optimized P1 and 3C genes, EV71-VLPs were efficiently expressed in Pichia pastoris system, and the expression level reached 270 mg/L. Biochemical and biophysical analyses showed that the produced EV71-VLPs consisted of processed VP0, VP1, and VP3 present as ~35nm spherical particles. The immune response as a function of EV71-VLPs and adjuvant dose ratio was investigated for vaccine development. Immunization with EV71-VLPs of 1–5 µg/dose and adjuvant of 225 µg/dose induced robust neutralizing antibody responses in mice and provided effective protection against lethal challenge in both maternally transferred antibody and passive transfer protection mouse models. Therefore, the yeast produced EV71-VLPs antigen is a promising candidate for the development of a vaccine against HFMD.
KEYWORDS: Enterovirus 71, hand foot and mouth disease, vaccine, immunogenicity, Pichia pastoris, virus-like particles
Introduction
Hand, foot and mouth disease (HFMD) has been prevalent in the Asia-Pacific region over the last decade, causing seasonal morbidity and mortality in children. Enterovirus 71 (EV71) and coxsackievirus A16 (CVA16) are the major causative agents of this disease.1–4 Phylogenetically, EV71 is closely related to CVA16, while EV71 causes neurological disease and is responsible for major deaths and severe sequelae during epidemics.5–7 Therefore, prophylactic EV71 vaccine has subsequently brought great attentions for the prevention of HFMD.
Three inactivated EV71 vaccines had been proven to be effective for preventing EV71 infection in children and have been approved for marketing in mainland China,8–10 however, these vaccines have some disadvantages such as potentially epitope-damaging, the risk of being inactivated incompletely and high production cost.11 VLPs are considered a very attractive platform for viral vaccine development because of their high immunogenicity and excellent safety. VLPs formed through the self-assembly of envelope or capsid proteins of viruses are structurally similar to the corresponding infectious viral particles but are non-infectious given that no viral RNA is incorporated. VLPs have thus been widely used in developing novel vaccines for many viruses including hepatitis B virus, human papillomavirus, norwalk virus, coxsackievirus A16, and hepatitis C virus.12 Pichia pastoris is an efficient platform for the expression of heterologous proteins due to its high cell density fermentation, high protein yield, and simple manufacturing procedure, and thus has been used in the industrial manufacture of biopharmaceutical proteins.13 Recently, the co-expression of P1 and 3C by baculovirus-insect cell,14–22 Pichia pastoris23 or Saccharomyces cerevisiae expression system24 has been examined as an alternative novel vaccine antigen candidate for preventing the EV71 infection. However, the VLPs expression level in baculovirus-insect cell,22 S. cerevisiae,24 and Pichia pastoris23 was low and thus may impede further product development. In addition, the dose–response relationship of VLPs or adjuvant, and the immunogenicity difference between VLPs based vaccine and inactivated vaccine remain elusive.
In the present work, highly efficient expression system of EV71-VLPs in Pichia pastoris has been successfully established, the immunogenicity of VLPs based vaccine and inactivated EV71 virus-based vaccine was analyzed, and the dose–response relationship of EV71-VLPs and adjuvant for vaccine development was investigated.
Materials and methods
Cells and viruses
Rhabdomyosarcoma cells (RD cells, ATCC No. CCL-136) were cultured in MEM solution (Invitrogen) with 10% fetal bovine serum (GIBCO) at 37°C. The virus was added to RD cells with 80% confluence. After 2 days of growth in MEM/2% FBS, the supernatant was collected for the titer determination through the Reed and Muench method. The titer assay based on the cytopathic effect (CPE) of RD cells was used, the titer represented tissue culture infective dose (TCID50). ZR-14 strain (C4 genotype, Shanghai Zerun Biotechnology) was used as a general neutralization assay with serum sample. EU812515 strain provided by Institute of Medical Biology, Chinese Academy of Medicine Science, was used to evaluate the neutralizing antibody response induced by EV71-VLPs vaccine.25 Strain of EU812515 was also used as the challenge virus in the animal model.
Generation of recombinant Pichia pastoris
Codon-optimized DNA sequences of EV71 (GenBank #FJ606449.1) P1 and 3C proteins were synthesized and inserted into pPICZαB(Invitrogen) plasmid using BstBI and KpnI restriction enzymes to generate P1-pPICZαB and 3C-pPICZαB. The AOXI promoter of 3C-pPICZαB was replaced by PEX8 promoter that was amplified from the genome of Pichia pastoris GS115 (Invitrogen) to generate 3C-pPEXZ. The P1 gene expression cassette, obtained from P1-pPICZαB digested with BglII and BamHI restriction enzymes, was inserted into 3C-pPEXZ digested with BamH I restriction enzyme to generate P13C-pPEXZ. The primer sequences included: P1 (F, CCAAGCTCTTCGAAACGATGGGTTCTCAAGTCT. R, AGCGGTACCCTATTATAAAGTAGTA), 3C (F, TTTAGTTCTTCGAAGCTAGCATGGGTCCATCTCTGG. R, GGCGGTACCCTATTATTGTTCTGAA) and PEX8 (F, GCCGAGATCTTATATCTCTATGTAGT.R,CCATGCTAGCTAACAGGCACCTGAAG). Then, the P13C-pPEXZ was linearized by SacI enzyme and transformed into Pichia pastoris SMD1168H (Invitrogen) by electroporation. The recombinant Pichia pastoris clones were selected by 200 µg/ml Zeocin in the yeast extract peptone dextrose (YPD) medium containing 1 M sorbitol at 30°C for 72 h. The positive clones were inoculated in the YPD medium at 30°C for 24 h and then inoculated in the buffered methanol-complex medium (BMMY) at 30°C for another 72 h. EV71-VLPs production by the positive clones was confirmed by sucrose density gradient ultracentrifugation, western blot, and electron microscopy. Thereafter, the recombinant Pichia pastoris P13C-pPEXZ-SMD1168H was fermented in BIOENGINEERING F22 fermenter according to the Invitrogen Pichia fermentation process guidelines. The final EV71-VLPs antigen was purified with column chromatography.
SDS-PAGE, western blot, and ELISA assay
The purified EV71-VLPs were prepared with loading buffer (100mM Tris-HCl, pH 6.8, 20% glycerol, 4% SDS (w/v), 0.2% bromophenol blue, 100mM DTT) and boiled for 10 min. Target protein compositions of VLPs were detected by 12% SDS-PAGE and western blot assay using polyclonal antibodies (anti-VP1, anti-VP2, anti-VP3, and anti-VP4, purified by protein A affinity chromatography from rabbit serum, which is immunized by Ni-NTA affinity chromatography purified VP1/VP2/VP3/VP4 protein with his-tag expressed in Escherichia coli, respectively, Shanghai Zerun Biotechnology). The purified EV71-VLPs specific activity was measured with sandwich ELISA (AbMax Biotechnology, China) according to the manufacture’s protocols with China’s national standards of EV71 antigen content provided by Chinese National Institutes for Food and Drug Control.26,27
Sandwich ELISA was performed to quantify EV71-VLPs expression level in yeast lysate with purified EV71-VLPs as the reference standard. Briefly, ELISA plates were coated with rabbit anti-EV71-VLPs serum (Shanghai Zerun Biotechnology), supernatant of yeast lysate or EV71-VLP reference standard serially diluted was added and followed by anti-EV71-VLPs polyclonal antibody (Shanghai Zerun Biotechnology), then HRP-Conjugated goat anti-mouse IgG (Abcam) was added. The absorbance at 450nm of each well was measured after the termination of color development.
The antigen-specific IgG titers were determined with endpoint titer assay.28 Briefly, ELISA plates were coated with 0.1µg/ml purified EV71-VLPs. PBST-diluted serum was added and followed by HRP-conjugated goat anti-mouse IgG (Abcam). The absorbance at 450nm of each well was measured after the termination of color development.
Sucrose gradient ultracentrifugation
The EV71-VLPs were purified on 20% sucrose cushion by ultracentrifugation at 40,000 rpm for 30 h from the yeast lysate. The purified pellets were resuspended in PBS solution and analyzed by electron microscopy.
Characterization of EV71-VLPs
The purified EV71-VLPs samples were negatively stained with 0.5% aqueous uranyl acetate and analyzed with the transmission electron microscope (TEM, CM-12S, Philips). Besides, the particles were also analyzed with dynamic light scattering (DLS, Zetasizer Nano ZS, Malvern), analytical ultracentrifuge (AUC, ProteomeLab XL-1, Beckman) and SEC-HPLC (TSK-GEL PW4000XL column, TOSOH) with a mobile phase of 20 mM PB, 0.3 M NaCl (pH 7.4). Pichia pastoris host cell proteins were analyzed by ELISA Kit (Cat. #F140, Cygnus Technologies). Other analyses, like residual host DNA, endotoxin and sterility testing of the bulk, were performed according to the Chinese Pharmacopoeia (2010).
Preparation of EV71-VLPs vaccine and inactivated EV71 vaccine for immunization
The purified EV71-VLPs and inactivated EV71 (made in our lab by column chromatography, data not shown) with a different concentration in formulation buffer were emulsified with 450 µg Al/ml adjuvant (Alhydrogel, Brenntag) to form the final vaccines (0.5 ml/dose).
Mouse immunization
Female BALB/c mice (6 ~ 8 weeks old, supplied by Shanghai Laboratory Animal Research Center) were divided into seven groups (8 ~ 10 each) and injected intraperitoneally (i.p.) two times at a 2-week interval with 0.5 ml of vaccines. Blood was collected from the post-orbital vein 2 weeks post the last vaccination to prepare serum samples for measurement of total IgG and neutralization antibodies.
Neutralization assay
The neutralization assay was carried out by a TCID50-reduction assay using RD cells.27 Serum was inactivated for 30 min at 56°C. Samples were twofold serially diluted from 1:8, mixing with equal volumes of 100 TCID50 EV71 viruses. After incubation at 37°C for 2 h in 96-wellplates, 0.1 ml RD cell suspension (2 × 105 cells/ml) was added. Cell control, serum control, and virus control were set on each plate, and virus backdrops were set for each test. Tests were considered successful if backdrop results were 32 ~ 320 TCID50/well. They were then placed in a CO2 incubator at 35°C for 6 ~ 7 days. CPE was observed by microscopy. Neutralizing antibody titers were defined as the highest dilution capable of inhibiting 50% of the CPEs. Neutralization titers greater than 1:8 were considered positive for neutralizing antibodies. Results were considered negative if neutralizing antibody titer less than 1:8 and were calculated as 1:4.
Evaluation of protective efficacy in suckling mice
Female ICR mice (9 ~ 10 weeks old, Vital River Laboratories, Ltd., Beijing, China) were divided into six groups and immunized i.p. at five different doses of EV71-VLPs antigen with 225 µg Al adjuvant: 5 µg VLPs/225 µg Al/0.5 ml, 1.25 µg VLPs/225 µg Al/0.5 ml, 0.312 µg VLPs/225 µg Al/0.5 ml, 0.104 µg VLPs/225 µg Al/0.5 ml, 0.026 µg VLPs/225 µg Al/0.5 ml, Al (OH) 3 adjuvant alone were used as a negative control. One hour after immunization, the female mice were caged and mated with naive males. Pregnant dams delivered pups 21 ~ 28 days post first immunization. On the first postnatal day, EU812515 was administered i.p. to all newborn suckling mice at 2 times the median lethal dose (LD50). The suckling mice were then observed for 14 days, recording their health, disease onset, and death rate. Results were only considered valid if the death rate in the negative control group reached 90% within 14 days. Two independent experiments were performed; due to good repeatability, the results were combined for statistical analysis.
Serum samples collected from adult female mice after immunization with 5 µg VLPs/225 µg Al/0.5 ml were pooled, heat inactivated at 56°C for 30 min, and the presence of neutralizing antibody was determined and diluted to 1:150, 1:30, and 1:15 titers. 2xLD50 of EU812515 was mixed with an equal volume of diluted serum at 37°C for 1 h, then the mixture was injected i.p. into 1-day-old sucking mice. The suckling mice were then observed as described above.
Statistical analysis
All data were analyzed with GraphPad Prism 6.00. Neutralizing antibody titers <1:8 were assumed to be 1:4 and >1:4096 were assigned a value of 1:4096. Significance between data values was assessed with two-tailed Mann Whitney unpaired test and assumed when p < .05.
Results
Expression, purification, and characterization of EV71-VLPs
Codon-optimized sequences of EV71 P1 and 3C genes were cloned into pPICZB vector and transformed into Pichia pastoris. The EV71-VLPs were purified through a 3-step of column chromatographic process. The quality attributes of three batches of purification processes in a small scale are shown in Table 1. The purity over 99% was achieved. Besides, the residual host proteins and residual DNA were lower than 0.1% and 10 ng/20 μg (internal enterprise standard, 20 µg EV71-VLPs antigen containing 10 ng residual host DNA), respectively.
Table 1.
EV71-VLPs preparation and process-achieved quality parameters.
| Harvest wet cell (g/L) | Expression level (mg/L) | Wet cell disrupted (g) | Purification yield(mg/g wet cell) | DLS (nm) | HPLC Purity (%) | Residual host DNA | Residual host cell proteins (%) | Specific activity (U/µg) | |
|---|---|---|---|---|---|---|---|---|---|
| Batch #1 | 242 | 253 | 300 | 0.13 | 35.3 | 99.6 | <10ng/20μg | 0.004 | 1049 |
| Batch #2 | 213 | 270 | 250 | 0.15 | 35.9 | 99.7 | <10ng/20μg | 0.012 | 885 |
| Batch #3 | 240 | 246 | 280 | 0.11 | 35.7 | 99.4 | <10ng/20μg | 0.019 | 847 |
The purified EV71-VLPs were also identified and characterized by several different methods. The expression and cleavage of target proteins were identified by SDS-PAGE and western blot (Figure 1(a)). The bands of EV71-VLPs were almost identical to those of inactivated EV71 virus. Western blot with anti-VP2 polyclonal antibodies showed two bands at around 39 and 28 kD representing VP0 and VP2, which indicated a small amount of VP0 was cleaved into VP2 and VP4. The diameter of the particles detected by electron microscope (Figure 1(b)) and DSL (Figure 1(d)) was about 35 nm and the sedimentation coefficient detected by AUC was about 80S (Figure 1(c)). HLPC analysis (Figure 1(e)) demonstrated the ability of the process to generate homogeneous and pure EV71-VLPs particle samples. Altogether, these results indicate that EV71-VLPs could be correctly self-assembled and purified with similar morphology to authentic virus particles.
Figure 1.
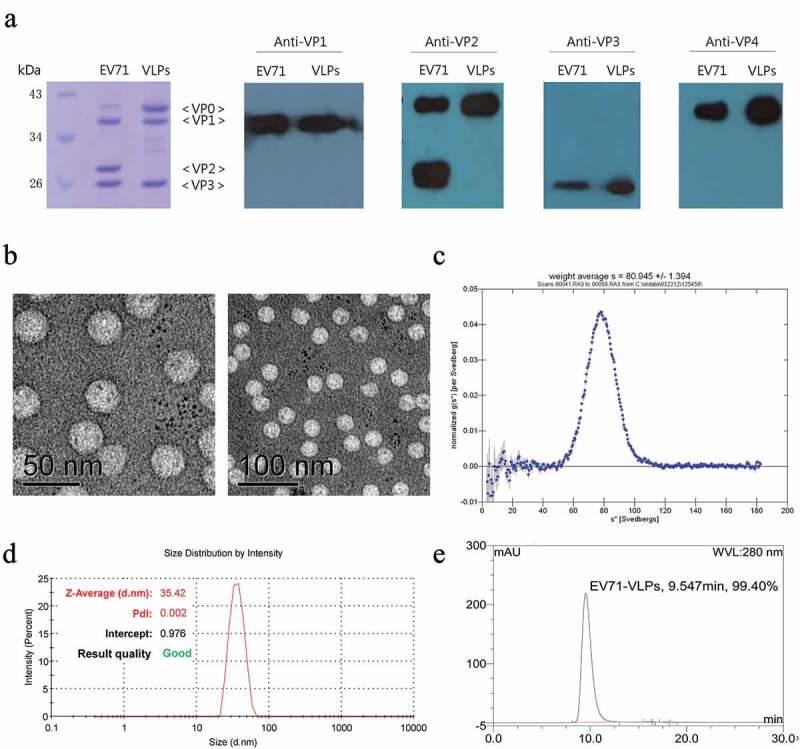
Characterization of purified EV71-VLPs.
EV71-VLPs elicits good specific humoral responses as compared with EV71 inactivated vaccine
To evaluate the immunogenicity of purified EV71-VLPs, female BALB/c mice were immunized with three different doses of VLPs adjuvanted with aluminum hydroxide. The inactivated EV71 vaccine with the equivalent amount of protein was used as a positive control. Two weeks after a prime-boost vaccination, the specific total IgG titers and neutralizing titers were measured. All antigens induced significant humoral responses in mice with the highest total IgG titer stimulated by 0.3 μg of EV71-VLPs (Figure 2(a)). EV71-VLPs also induced a likely higher titer of neutralizing antibody titer than inactivated vaccine, although it was not significant (Figure 2(b)). The lowest amount of EV71-VLPs (0.08 μg) induced significant lower neutralizing antibody responses even though a good total IgG titer was achieved. Therefore, our EV71-VLPs could elicit equivalent or even stronger antigen-specific antibody responses to EV71 inactivated vaccine in mice.
Figure 2.
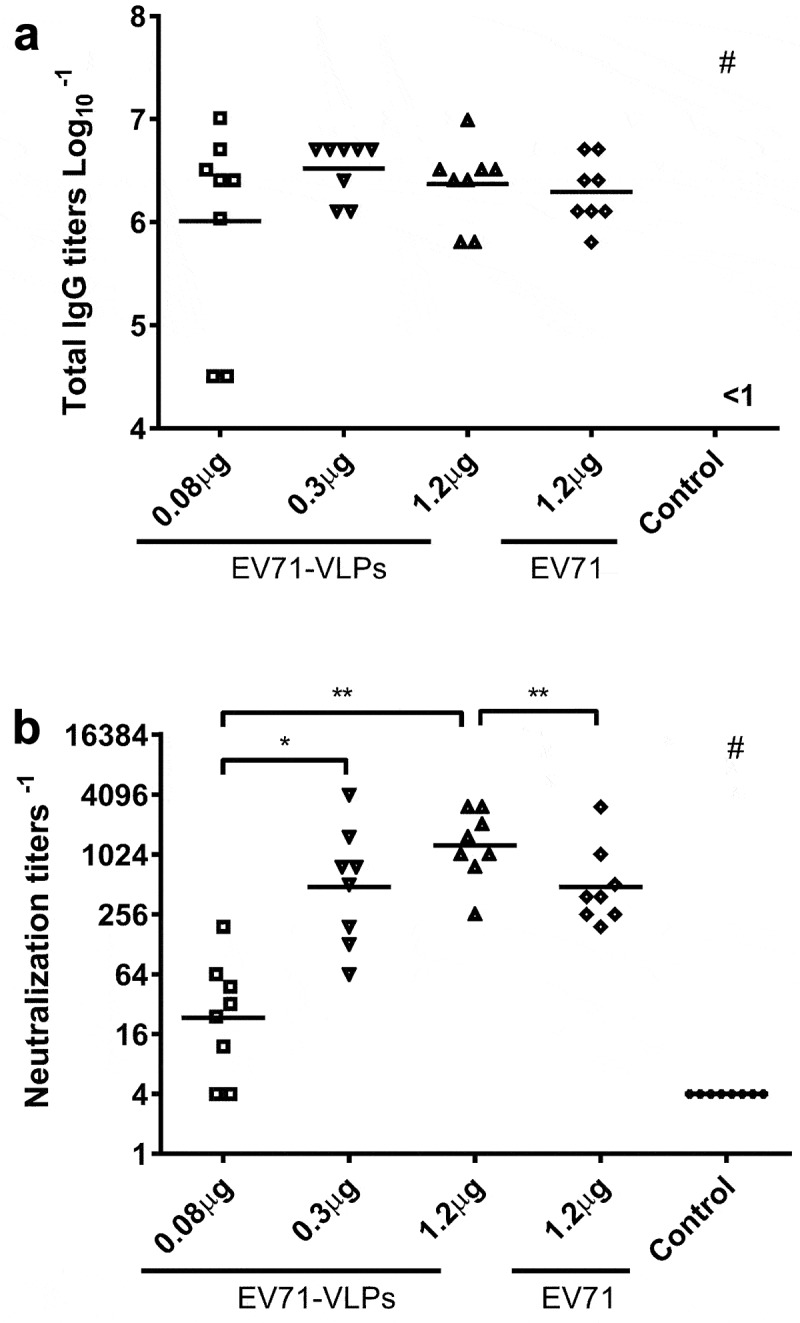
Serum antibody titers of mice immunized with EV71-VLPs and inactive EV71 virus.
Antibody titers induced by various doses of adjuvant
To further illuminate the effect of aluminum adjuvant on immune response, we evaluated the EV71-VLPs induced specific mouse antibody responses with different dose combinations of EV71-VLPs and the adjuvant. BALB/c mice were immunized as described previously. Total IgG and neutralizing titers were detected from mouse serum. Although EV71-VLPs antigen alone could induce dose-dependent total antibody responses, the adjuvant could elevate at least 10-fold higher titer (Figure 3(a)). The highest dose of aluminum adjuvant seems to induce the highest total IgG response. We seem observed a “maturation” of total IgG and neutralizing antibody titers in the presence of the adjuvant, since there was no significant difference between 1 μg and 5 μg doses of VLPs antigens although the dose of adjuvants had been increased from 56 μg to 450 μg (Figure 3(b)). Thus, the aluminum adjuvant allows the lower amount of antigens to induce significant high antibody titers.
Figure 3.
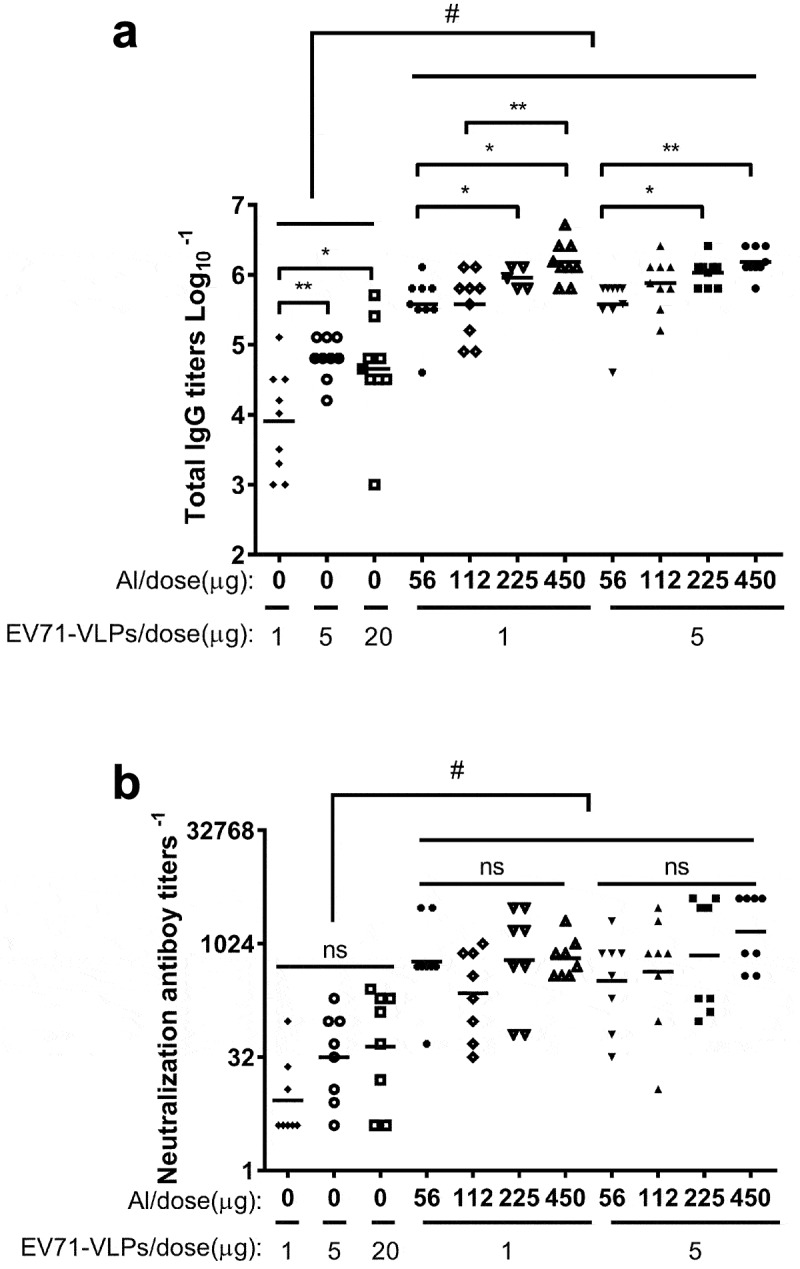
Serum antibody titers of mice immunized with EV71-VLPs combined with different doses of aluminum adjuvant.
Anti-EV71-VLPs serum could protect against lethal EV71 challenge in neonate mice
To evaluate the protection of maternal anti-EV71-VLPs antibody, maternal female mice were immunized as before and the pups were challenged with lethal EV71 virus. It has been observed that the survival rate is vaccine dose dependent (Figure 4(a)). Two weeks post viral challenge, all pups in the adjuvant alone or negative control group, and the lowest EV71-VLPs dose group were dead. The highest dose of EV71-VLPs antigen (5 μg) showed full protection. Furthermore, to assess the protective effect of antiserum in vivo, neonate mice were passively transferred with 2xLD50 of live EV71 virus pre-mixed with different titer of antiserum. Mice that were given the control serum were all dead 10 days after the challenge; the anti-EV71-VLPs immune serum could completely protect mice from EV71 infection (Figure 4(b)).
Figure 4.
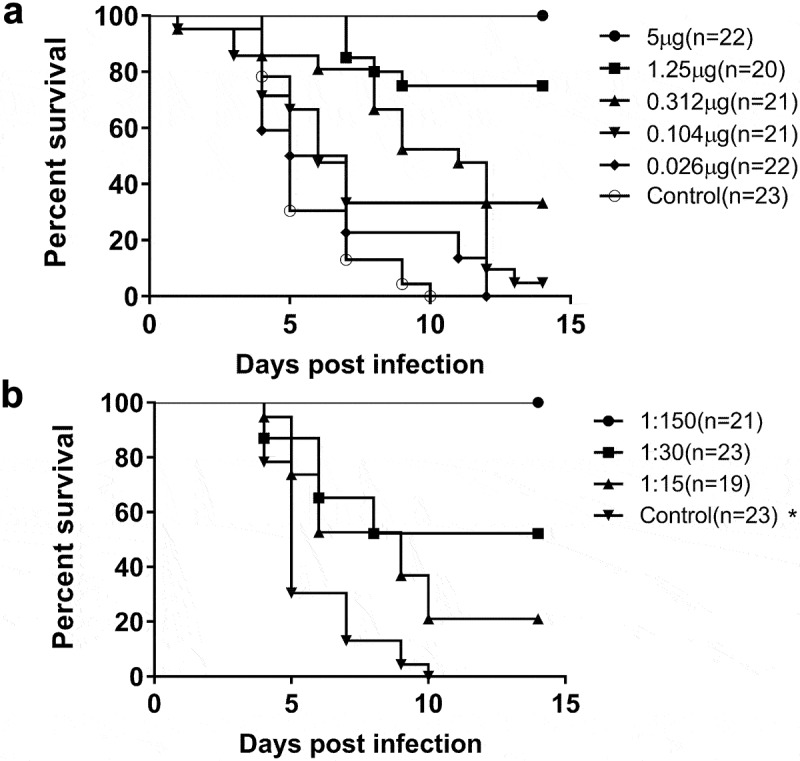
Survival curves of pups born to dams after lethal viral challenge.
Discussion
In the past two decades, outbreaks of HFMD have been documented in Asia-Pacific countries, including China, Malaysia, Japan, Singapore, Vietnam, and Cambodia. From 2008 to 2015, about 13 million HFMD cases were reported, including 123,261 severe cases and 3,322 deaths in mainland China.2 EV71 and CVA16 are responsible for more than 90% of HFMD cases, and EV71 is responsible for most severe cases and deaths.29 Three inactivated EV71 vaccines have shown protective efficacy in phase 3 clinical trials and been approved for commercialization in mainland China,8–10,29 but the low production of inactivated EV71 means it is difficult to supply enough inactivated EV71 vaccines for nationwide or worldwide mass immunization of the at-risk populations at the outbreaks of EV71 HFMD. Pichia pastoris is a commonly used yeast expression system for recombinant proteins of interest and has many advantages in vaccine development, including low fermentation cost, high expression levels, and high-density growth in fermentation bioreactors. In light of the potential of EV71-VLPs as a vaccine candidate and the efficient and cost-effectiveness of Pichia pastoris system, the main object of this work is to produce EV71-VLPs using the Pichia pastoris expression system. In order to increase the expression level of EV71-VLPs, the nucleic acid sequences of the P1 gene and 3C gene are codon-optimized with the Pichia pastoris biased codons. In the study, the expression level of EV71-VLPs reached 270 mg per liter yeast culture, which is much higher than the yield achieved in Vero cells (1.5 mg/L),1 S. cerevisiae yeast (0.25 mg/L),24 insect cell (64.3 mg/L).22 The yield of purified VLPs expressed in Pichia pastoris was over 0.1 mg/g cell weight, which is higher than that of EV71 virus cultivated in Vero cells30 or VLPs expressed in the baculovirus-insect expression system.17,31 After process improvement, the purification yield by chromatography reached up to 0.11 ~ 0.15 mg EV71-VLPs per g wet yeast cell (equal to 27 ~ 40 mg/L fermentation volume calculated from Table 1), a very promising commercial yield. Interestingly, EV71-VLPs expression level is also higher than that expressed in Pichia pastoris by Zhang (150 mg/L).23 Several factors may attribute to this difference, including the construction method (different promoters vs. same promoters), the host stain (SMD1168H vs. PichiaPinkTM) and the fermentation condition.
EV71-VLPs were fully characterized for vaccine development. There are two types of EV71 viral particles, native full particles (F-particles), and native empty particles (E-particles), they have similar icosahedral structures, but their sizes are slightly different, 31 ~ 33 nm for the F-particles and 33 ~ 35 nm for the E-particles, respectively. For F-particles, the VP0 protein is cleaved into VP2 and VP4 by autocatalytic action that involves the viral RNA resulting in smaller particles, so the buoyancy density of F-particles is larger than that of E-particles, the sedimentation coefficient of F-particles (150S) was greater than that of E-particles (82S).30,32 In this study, EV71-VLPs were purified by column chromatography and almost all of the host cell proteins and DNA were effectively removed and the purity of the final EV71-VLPs bulk was over 99% (Table 1). The purified EV71-VLPs were verified by SDS-PAGE and western blot, and characterized by EM, DLS, and AUC (Figure 1). The diameter (35nm) and the sedimentation coefficient (80S) were characterized to be similar to those of E-particles as measured by EM, DSL, and AUC (Figure 1). SDS-PAGE and western blot showed that the EV71-VLPs contain three major protein bands with molecular weights of 39 kDa, 36 kDa, 27 kDa corresponding to enterovirus capsid component proteins VP0 (39 kDa), VP1 (36 kDa), VP3 (27 kDa), indicating that the EV71-VLPs are similar to E-particles in protein components. A light band at 39kd representing VP0 in inactivated EV71 suggests that the natural EV71 virions contain some E-particles.
The data in this work demonstrated that the EV71-VLPs produced in Pichia pastoris is a promising vaccine antigen candidate for inducing neutralizing antibody responses. In mainland China, three inactivated EV71 C4 genotype strain vaccines have been shown to prevent over 90% of EV71 HFMD.12–14 The neutralizing antibodies raised by immunization with the inactivated EV71 vaccines are thought to play the major role in the protection and considered as the standard of the vaccine evaluation. In this study, both the higher dose (1.2 µg) and lower dose (0.08 µg) of EV71-VLPs vaccine produced high total IgG response. The higher dose from 0.3 µg to 1.2 µg elicited stronger neutralizing antibody responses while the lower dose such as 0.08 µg of EV71-VLPs exhibited significantly lower response. Lower dose (0.08 µg) elicited high total antibody response but insufficient neutralizing antibody titers, indicating that neutralizing antibody titer is not proportional to total antibody level. At lower dose, there are enough epitopes to stimulate the body to produce total antibodies, but there are not enough epitopes to produce sufficient neutralizing antibodies. At higher dose (over 0.3 µg), the neutralizing epitopes were sufficient, so there was no significant difference in neutralizing antibody titers between the two groups of 0.3 and 1.2 µg. As neutralizing antibodies are critical contributors to the protection against HFMD, an appropriate amount of antigen is necessary to ensure that the vaccine can induce high titers of neutralizing antibodies. The corresponding neutralizing antibody titer of 0.3 ~ 1.2 µg/dose in mice was 480 ~ 1250, which is equivalent to that of the vaccine already on the market.33 China’s national standards of EV71 antigen content had been used for testing EV71-VLPs specific activity by ELISA,29,30 if calculated by antigen content (about 850 U/µg, Table 1), the antigen content corresponding to this dose range is 255 ~ 1,020 U/dose. The antigen content level is also equivalent to that of the vaccines already on the market (Sinovac, 400 U, two-dose; Chinese Academy Medical Sciences, 100 U, two-dose and Beijing Vigoo, 320 U, two-dose).8,29,34,35 Interestingly, our EV71-VLPs induced higher total IgG and neutralizing antibody responses than inactivated EV71 vaccine, this is in consistence with a previous report by Chung et al.22 The lower antibody response associated with the inactivated virus-based vaccine might be due to loss of epitopes during virus inactivation process.18 In this work, 1 µg EV71-VLPs was equal to 850 U antigen content, this is obviously higher than 246 U/µg of EV71-VLPs expressed in insect cell16 and 289 U/µg of inactivated EV71 in this study. The high specific antigenic activity in this study is not clear although it may be related to the amino acid sequence of P1 polyprotein.
The dose–response relationship of aluminum adjuvant was fully evaluated for vaccine development. Several inactivated EV71 virus or EV71-VLPs vaccines based on Al adjuvant (Alhydrogel from Brenntag,15 Imject Alum from Thermo Scientific,24,36 Alhydrogel from Invivogen,23 other Al adjuvant not clarified33,37,38) have shown good immunogenicity in mice or monkeys in terms of neutralizing antibody titer. However, these studies were based on a single adjuvant dose and did not systematically study the immune effects of different adjuvant doses. The vaccine is designed to be used in young children, an excessive aluminum adjuvant may be harmful to the human body and the maximum amount of aluminum per dose in human vaccines is restricted by regulations (0.85 mg for the USA, 1.25 mg for Europe and WHO).39–41 The isoelectric point (PI) of P1 polyprotein of this study was 5.86 using the ExPASy Compute pI/Mw tool (http://web.expasy.org/compute pi/), which suggested that EV71-VLPs carry negative charges at neutral pH. The point of zero charge for aluminum hydroxide and aluminum phosphate are approximately 11 and 5, respectively,42 based on the formulation pH (6.3) and the PI of EV71-VLPs, aluminum hydroxide was chosen to maximize adjuvant-antigen electrostatic interactions by having oppositely charged antigen and adjuvant. The data shown in this work demonstrated that 225 µg and 450 µg doses of adjuvant could all produce stronger total IgG response, and showed no significant increase in neutralizing antibody level with the aluminum adjuvant dose increased to 450 µg. According to the related reference information, test results and guidelines, we recommend that the best aluminum adjuvant for EV71-VLPs vaccine would be aluminum hydroxide with a dose of 225 µg, which is also the regular dose used in most commercially available vaccines.39
The protective efficiency of EV71-VLPs vaccine was evaluated in both maternal-transferred antibody mouse model and passive transfer protection model. Both models showed that the EV71-VLPs-based vaccine provided good protection against lethal challenge with EV71 strain EU812515 and demonstrated a humoral mechanism of protection, most likely via neutralizing antibodies.18,37 The survival rate showed a significant dose-dependence. When the dose was lower than 0.312 µg, the survival rate was lower than 35%. When the dose reached 5 µg, the vaccine provided 100% protection from lethal challenge, and it demonstrated that EV71-VLPs immunization conferred protection that was passed from the mother mice to the neonatal mice. There are significant differences in the degree of protection of the challenged animals between groups of 1 and 5 µg adjuvanted EV71-VLPs vaccine, but there are no differences in total IgG and neutralizing antibodies, this might be related to the different levels of antibody affinity maturation.43 The affinity between groups of 1 and 5 µg was different, consequently, the protection level was not the same. The differences between in vivo and in vitro studies indicated that the mechanism of protection in animals should be further characterized and the importance of in vivo test should not be neglected in evaluating the protective effect of EV71-VLPs vaccine. Considering the good protection against lethal EV71 virus challenge provided by EV71-VLPs with a dose range of 1–5 µg in Figure 4; the high neutralizing antibody level produced by EV71-VLPs with a dose range greater than 1 µg in Figure 3, we recommend the appropriate dose of EV71-VLPs of the final vaccine should be 1 ~ 5 µg/dose, equal to 850–3,400 U/dose. The antigen content in U is higher than that of the three inactivated vaccines available on the market (100 ~ 400 U/dose),8,29,34,35 which suggests that EV71-VLPs based vaccine can induce a good immune response to provide protection effect.
However, it should be noted that our study has some limitations. First, in order to evaluate the humoral responses of EV71-VLPs vaccine, blood was collected 2 weeks post the last vaccination, the VLP-specific total IgG titers and neutralizing titers were based on a single point of data, further study of persistence of the specific total IgG and neutralizing antibodies would be helpful to analyze the immunogenicity of the vaccine. Second, the evaluation of the protective efficiency of EV71-VLPs vaccine in maternal-transferred antibody mouse model was based on the protection data, further determination of the neutralizing antibody titers and the viral titers in tissues in pups surviving lethal challenge might help to further elucidate the protective mechanisms of the model and further explain the protection differences of different doses of vaccines. Third, it was reported that the inactivated EV71 vaccine was shown to affect the balance of the Th1/Th2 immune response;44 for the EV71-VLPs-based vaccine, the Th1/Th2 immune response after vaccination will be further studied.
In summary, EV71-VLPs were efficiently produced in yeast Pichia pastoris, reaching expression yield 270 mg/L. The vaccine with EV71-VLPs of 1 ~ 5 µg/dose and adjuvant of 225 µg/dose could elicit robust protective neutralizing antibody responses and provide protection against lethal challenge in mice. Therefore, EV71-VLPs produced in Pichia pastoris is considered a promising vaccine antigen candidate to protect against HFMD.
Funding Statement
This work was supported by the Shanghai Science and Technology Talent Project [19QB1406600].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Chong P, Hsieh SY, Liu CC, Chou AH, Chang JY, Wu SC, Liu SJ, Chow YH, Su IJ, Klein M.. Production of EV71 vaccine candidates. Hum Vaccin Immunother. 2012;8(12):1775–83. doi: 10.4161/hv.21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang J, Liao QH, Ooi MH, Cowling BJ, Chang ZR, Wu P, Liu FF, Li Y, Luo L, Yu SB, et al. Epidemiology of recurrent hand, foot and mouth disease, China, 2008–2015. Emerg Infect Dis. 2018;24(3):432–42. doi: 10.3201/eid2403.171303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koh WM, Bogich T, Siegel K, Jin J, Chong EY, Tan CY, Chen MIC, Horby P, Cook AR. The epidemiology of hand, foot and mouth disease in Asia a systematic review and analysis. Pediatr Infect Dis J. 2016;35(10):E285–E300. doi: 10.1097/inf.0000000000001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang PC, Chen SC, Chen KT. The current status of the disease caused by enterovirus 71 infections: epidemiology, pathogenesis, molecular epidemiology, and vaccine development. Int J Environ Res Public Health. 2016;13(9):890. doi: 10.3390/ijerph13090890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jang S, Suh SI, Ha SM, Byeon JH, Eun BL, Lee YH, Seo HS, Eun SH, Seol HY. Enterovirus 71-related encephalomyelitis: usual and unusual magnetic resonance imaging findings. Neuroradiology. 2012;54(3):239–45. doi: 10.1007/s00234-011-0921-8. [DOI] [PubMed] [Google Scholar]
- 6.Weng KF, Chen LL, Huang PN, Shih SR. Neural pathogenesis of enterovirus 71 infection. Microbes and infection/Institut Pasteur. 2010;12(7):505–10. doi: 10.1016/j.micinf.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Gan Z-K, Jin H, Li J-X, Yao X-J, Zhou Y, Zhang X-F, Zhu F-C. Disease burden of enterovirus 71 in rural central China: a community-based survey. Hum Vaccin Immunother. 2015;11(10):2400–05. doi: 10.1080/21645515.2015.1059980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y, Zeng G, Chu K, Zhang J, Han W, Zhang Y, Li J, Zhu F. Five-year immunity persistence following immunization with inactivated enterovirus 71 type (EV71) vaccine in healthy children: a further observation. Hum Vaccin Immunother. 2018;14(6):1517–23. doi: 10.1080/21645515.2018.1442997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei M, Meng F, Wang S, Li J, Zhang Y, Mao Q, Hu Y, Liu P, Shi N, Tao H, et al. 2-year efficacy, immunogenicity, and safety of vigoo enterovirus 71 vaccine in healthy Chinese children: a randomized open-label study. J Infect Dis. 2017;215(1):56–63. doi: 10.1093/infdis/jiw502. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Kong Y, Jiang Z, Li C, Wang L, Xia J. Comprehensive safety assessment of a human inactivated diploid enterovirus 71 vaccine based on a phase III clinical trial. Hum Vaccin Immunother. 2016;12(4):922–30. doi: 10.1080/21645515.2015.1115934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrell CJ, Howard CR, Murphy FA, editors. Chapter 11 - vaccines and vaccination. In: Burrell CJ, Howard CR, Murphy FA, editors. Fenner and White’s medical virology. 5th ed. London (UK): Academic Press; 2017. p. 155–67. doi: 10.1016/B978-0-12-375156-0.00011-4 [DOI] [Google Scholar]
- 12.Kushnir N, Streatfield SJ, Yusibov V. Virus-like particles as a highly efficient vaccine platform: diversity of targets and production systems and advances in clinical development. Vaccine. 2012;31(1):58–83. doi: 10.1016/j.vaccine.2012.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad M, Hirz M, Pichler H, Schwab H. Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl Microbiol Biotechnol. 2014;98(12):5301–17. doi: 10.1007/s00253-014-5732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Dai WL, Zhang C, Zhou Y, Xiong P, Wang SX, Ye XH, Liu QW, Zhou DM, Huang Z. A virus-like particle-based tetravalent vaccine for hand, foot, and mouth disease elicits broad and balanced protective immunity. Emerg Microbes Infect. 2018;7:94. doi: 10.1038/s41426-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmons B, Lim PY, Djurup R, Cardosa J. Non-clinical safety assessment of repeated intramuscular administration of an EV-A71 VLP vaccine in rabbits. Vaccine. 2018;36(45):6623–30. doi: 10.1016/j.vaccine.2018.09.062. [DOI] [PubMed] [Google Scholar]
- 16.Sun SY, Gao F, Mao QY, Shao J, Jiang LP, Liu DW, Wang YP, Yao X, Wu X, Sun B, et al. Immunogenicity and protective efficacy of an EV71 virus-like particle vaccine against lethal challenge in newborn mice. Hum Vaccin Immunother. 2015;11(10):2406–13. doi: 10.1080/21645515.2015.1053675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung YC, Huang JH, Lai CW, Sheng HC, Shih SR, Ho MS, Hu YC. Expression, purification and characterization of enterovirus-71 virus-like particles. World J Gastroenterol. 2006;12(6):921–27. doi: 10.3748/wjg.v12.i6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung YC, Ho MS, Wu JC, Chen WJ, Huang JH, Chou ST, Hu YC. Immunization with virus-like particles of enterovirus 71 elicits potent immune responses and protects mice against lethal, challenge. Vaccine. 2008;26(15):1855–62. doi: 10.1016/j.vaccine.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 19.Ku ZQ, Liu QW, Ye XH, Cai YC, Wang XL, Shi JP, Li DP, Jin X, An WQ, Huang Z. A virus-like particle based bivalent vaccine confers dual protection against enterovirus 71 and coxsackievirus A16 infections in mice. Vaccine. 2014;32(34):4296–303. doi: 10.1016/j.vaccine.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Lim PY, Hickey AC, Jamiluddin MF, Hamid S, Kramer J, Santos R, Bossart KN, Cardosa MJ. Immunogenicity and performance of an enterovirus 71 virus-like-particle vaccine in nonhuman primates. Vaccine. 2015;33(44):6017–24. doi: 10.1016/j.vaccine.2015.05.108. [DOI] [PubMed] [Google Scholar]
- 21.Somasundaram B, Chang C, Fan YY, Lim PY, Cardosa J, Lua L. Characterizing Enterovirus 71 and Coxsackievirus A16 virus-like particles production in insect cells. Methods. 2016;95:38–45. doi: 10.1016/j.ymeth.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Chung CY, Chen CY, Lin SY, Chung YC, Chiu HY, Chi WK, Lin YL, Chiang BL, Chen WJ, Hu YC. Enterovirus 71 virus-like particle vaccine: improved production conditions for enhanced yield. Vaccine. 2010;28(43):6951–57. doi: 10.1016/j.vaccine.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, Ku ZQ, Liu QW, Wang XL, Chen T, Ye XH, Li DP, Jin X, Huang Z. High-yield production of recombinant virus-like particles of enterovirus 71 in Pichia pastoris and their protective efficacy against oral viral challenge in mice. Vaccine. 2015;33(20):2335–41. doi: 10.1016/j.vaccine.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 24.Li HY, Han JF, Qin CF, Chen R. Virus-like particles for enterovirus 71 produced from Saccharomyces cerevisiae potently elicits protective immune responses in mice. Vaccine. 2013;31(32):3281–87. doi: 10.1016/j.vaccine.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Mao Q, Li N, Yu X, Yao X, Li F, Lu F, Zhuang H, Liang Z, Wang J. Antigenicity, animal protective effect and genetic characteristics of candidate vaccine strains of enterovirus 71. Arch Virol. 2012;157(1):37–41. doi: 10.1007/s00705-011-1136-3. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Mao C, Ma S, Wang X, Sun Z, Yi Y, Guo M, Shen X, Sun L, Bi S. Generation of neutralizing monoclonal antibodies against Enterovirus 71 using synthetic peptides. Biochem Biophys Res Commun. 2009;390(4):1126–28. doi: 10.1016/j.bbrc.2009.09.103. [DOI] [PubMed] [Google Scholar]
- 27.Liang Z, Mao Q, Gao Q, Li X, Dong C, Yu X, Yao X, Li F, Yin W, Li Q, et al. Establishing China’s national standards of antigen content and neutralizing antibody responses for evaluation of enterovirus 71 (EV71) vaccines. Vaccine. 2011;29(52):9668–74. doi: 10.1016/j.vaccine.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Liu Q, Yan K, Feng Y, Huang X, Ku Z, Cai Y, Liu F, Shi J, Huang Z. A virus-like particle vaccine for coxsackievirus A16 potently elicits neutralizing antibodies that protect mice against lethal challenge. Vaccine. 2012;30(47):6642–48. doi: 10.1016/j.vaccine.2012.08.071. [DOI] [PubMed] [Google Scholar]
- 29.Mao QY, Wang YP, Bian LL, Xu M, Liang ZL. EV-A71 vaccine licensure: a first step for multivalent enterovirus vaccine to control HFMD and other severe diseases. Emerg Microbes Infect. 2016;5(7):e75. doi: 10.1038/emi.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu CC, Guo MS, Lin FH, Hsiao KN, Chang KH, Chou AH, Wang YC, Chen YC, Yang CS, Chong PC. Purification and characterization of enterovirus 71 viral particles produced from vero cells grown in a serum-free microcarrier bioreactor system. PLoS One. 2011;6(5):e20005. doi: 10.1371/journal.pone.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin SY, Chiu HY, Chiang BL, Hu YC. Development of EV71 virus-like particle purification processes. Vaccine. 2015;33(44):5966–73. doi: 10.1016/j.vaccine.2015.04.077. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Peng W, Ren J, Hu Z, Xu J, Lou Z, Li X, Yin W, Shen X, Porta C, et al. A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71. Nat Struct Mol Biol. 2012;19(4):424–29. doi: 10.1038/nsmb.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong CH, Liu LD, Zhao HL, Wang JJ, Liao Y, Zhang XM, Na RX, Liang Y, Wang LC, Li QH. Immunoprotection elicited by an enterovirus type 71 experimental inactivated vaccine in mice and rhesus monkeys. Vaccine. 2011;29(37):6269–75. doi: 10.1016/j.vaccine.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 34.Liu LD, Mo ZJ, Liang ZL, Zhang Y, Li RC, Ong KC, Wong KT, Yang EX, Che YC, Wang JJ, et al. Immunity and clinical efficacy of an inactivated enterovirus 71 vaccine in healthy Chinese children: a report of further observations. BMC Med. 2015;13:226. doi: 10.1186/s12916-015-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen YJ, Meng FY, Mao QY, Li JX, Wang H, Liang ZL, Zhang YT, Gao F, Chen QH, Hu YM, et al. Clinical evaluation for batch consistency of an inactivated enterovirus 71 vaccine in a large-scale phase 3 clinical trial. Hum Vaccin Immunother. 2014;10(5):1366–72. doi: 10.4161/hv.28397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ku Z, Ye X, Huang X, Cai Y, Liu Q, Li Y, Su Z, Huang Z. Neutralizing antibodies induced by recombinant virus-like particles of enterovirus 71 genotype C4 inhibit infection at pre- and post-attachment steps. PLoS One. 2013;8(2):e57601. doi: 10.1371/journal.pone.0057601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bek EJ, Hussain KM, Phuektes P, Kok CC, Gao Q, Cai F, Gao Z, McMinn PC. Formalin-inactivated vaccine provokes cross-protective immunity in a mouse model of human enterovirus 71 infection. Vaccine. 2011;29(29–30):4829–38. doi: 10.1016/j.vaccine.2011.04.070. [DOI] [PubMed] [Google Scholar]
- 38.Lin Y-L, Yu C-I, Hu Y-C, Tsai T-J, Kuo Y-C, Chi W-K, Lin A-N, Chiang B-L. Enterovirus type 71 neutralizing antibodies in the serum of macaque monkeys immunized with EV71 virus-like particles. Vaccine. 2012;30(7):1305–12. doi: 10.1016/j.vaccine.2011.12.081. [DOI] [PubMed] [Google Scholar]
- 39.Vecchi S, Bufali S, Skibinski DA, O’Hagan DT, Singh M. Aluminum adjuvant dose guidelines in vaccine formulation for preclinical evaluations. J Pharm Sci. 2012;101(1):17–20. doi: 10.1002/jps.22759. [DOI] [PubMed] [Google Scholar]
- 40.Di, Pasquale A, Preiss S, Tavares Da Silva F, Garcon N. Vaccine adjuvants: from 1920 to 2015 and Beyond. Vaccines (Basel). 2015;3(2):320–43. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He P, Zou Y, Hu Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccin Immunother. 2015;11(2):477–88. doi: 10.1080/21645515.2014.1004026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.HogenEsch H, O’Hagan DT, Fox CB. Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want. NPJ Vaccines. 2018;3:51. doi: 10.1038/s41541-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doria-Rose NA, Joyce MG. Strategies to guide the antibody affinity maturation process. Curr Opin Virol. 2015;11:137–47. doi: 10.1016/j.coviro.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu LD, Zhang Y, Wang JJ, Zhao HL, Jiang L, Che YC, Shi HJ, Li RC, Mo ZJ, Huang T, et al. Study of the integrated immune response induced by an inactivated EV71 vaccine. PLoS One. 2013;8(1):e54451. doi: 10.1371/journal.pone.0054451. [DOI] [PMC free article] [PubMed] [Google Scholar]


