ABSTRACT
Background
We aimed to describe the episodes and trends of admissions for community-acquired Respiratory Infections (RI) over a 12-year period and to assess the impact of Haemophilus influenza type b (Hib) vaccine on RI admissions in children aged up to 3 years.
Methods
We conducted a twelve-year retrospective observational study on all community-acquired RI admitted to Fattouma Bourguiba Hospital in Monastir Governorate (Tunisia) from 1 January 2002 to 31 December 2013. RI cases were selected from the Regional Registry of Hospital Morbidity. Data were coded according to ICD-10. To assess the impact of the Hib vaccine, three cohorts were defined based on vaccine status (unvaccinated cohort, first vaccinated cohort (VC) by monovalent form and second VC by pentavalent combination).
Results
Admissions for RI represented 17.6% (CI95%: 17.3–18.1) of all communicable diseases hospitalizations (n = 6 061/34 289). The crude incidence rates (CIR) per 100,000 inh were 24.2 for upper RI (URI) and 77.5 and for Lower RI (LRI) (p < 0.0001). Pneumonias represented 53.9% of LRI. Sex-ratio (male/female) was 1.12 for URI and 1.64 for LRI (p < 0.0001). At admission, the median age was 22 years (IQR: 3–52). Admission for Pneumonia increased significantly during study period (slope ‘b’ = 5.16; p < 0.0001) especially in children up to 5 years old (slope ‘b’ = 5.53) and in elderly (slope ‘b’ = 2.13). Among children up to 3 years old, the CIRs per 100,000 for Hib pneumonia admission were 11.6 in Non-Vaccinated Cohort (NVC), 10.6 in Vaccinated Cohort (VC) by protocol 1 (Hib Vaccine monovalent) and 0.80 in VC by protocol 2 (pentavalent vaccine combination).The relative risk reduction was 99% for protocol 2 (p < 0.001).
Conclusion
Admissions for RI in a tertiary level hospital were common with an increasing trend. The Hib immunization program, in particular the pentavalent combination, has had a positive impact on the reduction of related acute diseases.
KEYWORDS: Respiratory Infection, inpatients, trends, burden, pneumonia, Hib vaccine, Tunisia
1. Introduction
Community-acquired Respiratory Infections (RI) is a frequent, severe and costly public health problem. RIs are particularly common in the two extreme age groups (children and the elderly) because of their fragility and comorbidities [1]. RI admission criteria in university hospital are related to the severity of clinical and radiological signs. Trends of RI had an important fluctuation over time. In last decades the decrease of communicable diseases was spectacular. Several factors, however, have reversed these trends, including increased resistance to antibiotics, irregular vaccination programs and drastic climatic changes [2]. In 2015, upper respiratory infections (URI) had globally acute incidence in excess of 1 billion (17.2 billion, 95% CI: 15.4–19.2 billion) [3]. Lower respiratory infections (LRI) are a major cause of morbidity and mortality worldwide especially among children under 5 years (1.4 million deaths) [4,5]. Community-acquired pneumonia (CAP) represents 5% to 12% of all adult LRI managed in general practice [6]. Streptococcus pneumonia (SP) and Haemophilus influenza type b (Hib) are the main pathogens that caused RI [7,8]. Many efforts are ongoing worldwide to improve awareness of health-care professionals about antibiotic resistance and to standardize vaccination programs [9].
In Tunisia, few studies have focused on the epidemiology of RI and evaluated the impact of the strategies followed by health professionals to manage this public health problem. The Tunisian Expanded Program of Immunization (TEPI) was introduced in 1979 targeting world common bacterial diseases. As for vaccines for respiratory diseases in this program, Hib vaccine was provided as liquid Hib conjugate vaccine (monovalent) between 2002 and 2005 and then reintroduced in 2011 as Hib conjugate in combination with Hepatitis B, diphtheria, Poliomyelitis and Tetanus (DPT) called the pentavalent vaccine [10]. Currently, the TEPI includes the Hib vaccine and the pneumococcal vaccine. The latter has just been included in April 2019.
This study aimed to describe the frequency, trends and health burden of admissions for RI during a period of 12 years in a low-middle income country. We sought also to assess the impact of both forms of Hib vaccine on admissions among hospitalized children aged up to 3 years.
2. Methods
2.1. Study design
We carried out a twelve-year retrospective observational study using the regional register morbidity data of hospitalized people in a university hospital in Tunisia.
3. Study setting
Monastir is a coastal city in Tunisia. The population of Monastir represented 4.99% of the Tunisian population. This study was conducted in the main university hospital of this city. According to the Tunisian health card 2015, the total capacity of beds in public and private hospital structures in Monastir was in total 1441 beds of which 82% belonged to the public sectors. University Hospital Fattouma Bourguiba is the unique third line hospital in the region of Monastir and it has a capacity of 858 beds (73% of total public beds) and a workforce of 248 doctors and pharmacists [11]. It included 1177 health professionals. Emergency, Pediatric, Pulmonary, Otorhinolaryngology and Infectious departments managed the quasi totality of community-acquired RI cases.
3.1. Participants
We included inpatients residents in Monastir Governorate and admitted for community-acquired RI (URI and LRI) from 1 January 2002 to 31 December 2013.
Admission criteria for all pathologies and ages were related to the severity of the clinical presentation or to an associated comorbidity.
Concerning pneumonia in pediatric age, the admission was systematic for children under 3 years of age, children with fever >39 and those with respiratory distress or associated comorbidities.
Health-care associated RIs were not included.
3.2. Variables
Register data involved socio-demographic variables such as age and gender. Variables related to hospitalization characteristics were admission dates, discharge diagnosis, entry mode, Length of Hospital Stay (LHS) and in-hospital deaths. Patients’ diagnosis was provided at the hospital discharge.
Infectious or communicable diseases were defined as an illness caused by another living agent, or its products, that can be spread from one person to another [12].
In adults, the diagnosis of pneumococcal pneumonia (PP) was done on the identification of SP in blood cultures.
For pediatric age, the diagnosis of pneumonia was based on the clinical symptomatic triad (cough, fever and breathing difficulties) and chest x-ray. An etiological approach was adopted to differentiate between pneumonia with viral agent, atypical agent or bacterial agent (SP or Hib). To identify the bacterial etiology of pneumonia, blood cultures were an accurate way to determine whether the SP or Hib was the cause of pneumonia. Given that Hib culture requires special transport and growth conditions and that these cultures are only positive for a small proportion, the diagnosis of Hib pneumonia was retained in front of a compatible clinical presentation and negative blood culture for SP.
For immunization, vaccination against Hib was introduced in TEPI in October 2002 for children born after 1 July 2002. The Hib vaccine was provided as liquid Hib conjugate vaccine (monovalent form) and given at 3, 4, 6 months of life. Then, Hib vaccination was officially discontinued in November 2005 and reintroduced in April 2011 as Hib conjugate vaccine in the pentavalent vaccine combination (Hib, Hepatitis B, and DPT) [10]. Thus, three cohorts were defined as follows:
The not vaccinated cohort (NVC): hospitalized children born before July 2002 or those born after November 2005 to March 2011 were considered unvaccinated against Hib.
The first vaccinated cohort (VC) with monovalent form at age of 3, 4 and 6 months: hospitalized children born between July 2002 or those born before November 2005 were considered vaccinated with Hib monovalent vaccine.
The second VC with the pentavalent vaccine at age of 2, 3 and 6 months: all hospitalized children born after April 2011 were considered vaccinated with pentavalent vaccine.
3.3. Data sources
Data collected from the regional register of hospital morbidity were used. We selected RI from hospital morbidity data encoded according to ICD-10. Each patient had a unique identifier. Patients’ diagnosis was provided at the hospital discharge. More details are available elsewhere [13–15].
URI included otitis externa and acute otitis media (AOM) (H60-H62; H66-H67), mastoiditis and labyrinthitis (H70; H75; H83); acute nasopharyngitis, acute sinusitis, amygdalitis and flu (J00-J06, J09-J11) and phlegmonous angina, parapharyngeal abscess (J36; J39).
LRI included pneumococcal pneumonia (J13), pneumonia with Hib (J14), bacterial pneumonia, not elsewhere classified (J15), Pneumonia due to other infectious organisms, not elsewhere classified (J16), pneumonia during an illness classified elsewhere (J17), or with unspecified microorganism (J18), acute bronchitis (J20), acute bronchiolitis (J21), unspecified bronchitis (J40) and lung and mediastinum abscess, pleural empyema (J85, J86).
3.4. Statistical methods
3.4.1. Descriptive analysis
Data were verified, and analyzed using IBM SPSS Statistics version 22.0 software. Categorical variables (age group, sex, and mortality) were presented as effectives and percentages, quantitative variables by median and interquartile Range (IQR). Pearson chi2 was used.
3.4.2. Trends
Linear regression using the slope ‘b’ of the least-squares line and ‘r’ spearman coefficients were determined to estimate URI and LRI trends according to gender and age group. A p-value of <0.05 was considered statistically significant.
3.4.3. Standardization
Crude incidence rates (CIRs) of RI were calculated based on the Tunisian National Institute of Statistics and expressed in 100,000 inh [16]. The age-standardized incidence rate (SIR) per 100,000 person-years (PY) was calculated among the world standard population according to the WHO 2013 report [17]. Death parameters were fatality and age-standardized mortality rates (SMR).
3.4.4. Health burden
Disability Adjusted Life Years (DALYs) were calculated by the sum of years of life lost (YLLs) and years lived with disability (YLDs). YLD estimates are calculated by the equation (YLDs = Prevalent cases × Disability weight). Disability weights for GBD 2013 were derived from a pooled analysis of data from the GBD 2010 Disability Weights Measurement Study [18].
3.4.5. Impact of Hib immunization
To compare the unvaccinated cohort and the vaccinated one, we calculated absolute risk/100,000 inh, absolute risk reduction (ARR)/100,000 inh; relative risk (RR), relative risk reduction and number needed to treat (NNT) using Excel.
3.4.6. Ethical considerations
The data register was collected at hospital discharge, in accordance with international guidelines, national laws and local regulations, including data protection.
4. Results
4.1. Admission characteristics
During the study period, 6891 hospitalized RI were notified. Of them, 6061 were inhabitants of Monastir (87.9%) representing 17.6% (CI95%: 17.3–18.1) of all hospitalizations for communicable diseases. Hospitalization for LRI and URI represented, respectively, 76.3% and 23.7% of all hospitalized RI.
Thirty-one patients had three hospitalizations for RI and 400 patients had 2 hospitalizations. Two-thirds of RI inpatients were males. Sex-ratio was 1.12 for URI and 1.64 for LRI (p < 0.0001). At admission, the median age was 22 years (IQR: 3–52). It was 24 (IQR: 3–55) for males and 20 years (IQR: 3–47) for females (p = 0.004). The median age was 1.25 years (IQR: 1–1.50) for acute bronchiolitis and 31 years (IQR: 17–45) for otitis (p < 0.0001) (Figure 1).
Figure 1.
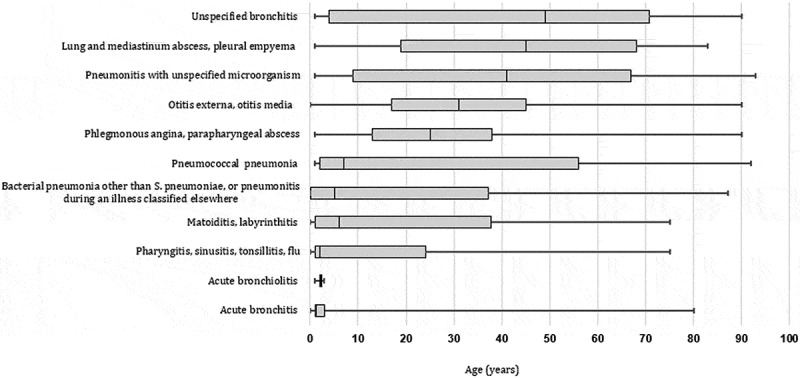
Age distribution of patients admitted for acute Respiratory Infections (Monastir; 2002–2013).
Legend: The half of pneumococcal pneumonia occurred in childhood (<18 years old)
The median length hospital stay (LHS) was 5 days (IQR 4–8 days) for URI and 6 days (IQR 4–9 days) for LRI. Emergency Department (ED) was the major entry mode of LRI. Most of patients with URI were admitted in otorhinolaryngology department (n = 1251,87.1%) and pediatrics department (n = 141,10%). As for patients with LRI, pediatrics and pulmonary departments took charge of 66.4% (n = 3072) and 23.7% (n = 1097) of admissions, respectively. Fifty-eight patients were admitted in intensive care during the period study.
4.2. Hospitalized RI incidence and mortality rates
For hospitalized URI, 1436 admissions were recorded (120 hospitalizations per year). The CIR was 24.19 per 100,000 inh. It was 31.9 for children aged up to 5 years and 21.9 for inpatients aged more than 60 years old. The age-SIR was 21.95 per 100,000 PY, 23.09 for men and 20.89 for women, respectively (Table 1).
Table 1.
Crude and standardized incidence rates and death cases of hospitalization for upper and lower respiratory infection (Monastir; 2002 − 2013).
| Upper RI |
Lower RI |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | CIR/105inh | ASR/105 PY | Death cases$ (Fatality): n (%) | Cases | CIR/105inh | ASR/105 PY | Death cases (Fatality): n (%) | A-SMR/105inh | |
| (2002–2013) | 1436 | 1 (0.07) | 4625 | 65 (1.41) | 0.64 | ||||
| Age | |||||||||
| [0–5[ | 167 | 31.9 | 1 (0.6) | 2653 | 506.6 | 32 (1.21) | |||
| [5–24[ | 485 | 21.4 | 547 | 24.1 | 9 (1.65) | ||||
| [25–40[ | 342 | 23.8 | 213 | 14.8 | 1 (0.47) | ||||
| [40–60[ | 321 | 27.7 | 391 | 33.7 | 5 (1.28) | ||||
| ≥ 60 | 109** | 21.9 | 760** | 152.7 | 18 (2.37) | ||||
| Valid | 1424 | 24.19 | 21.9 | 4563 | 77.5 | 71.63 | |||
| Gender | |||||||||
| Males | 761 | 25.2 | 23.09 | 1 (0.13) | 2871 | 95,0 | 90.2 | 33 (1.15) | 0.68 |
| Females | 675* | 23.1 | 20.89 | 1753** | 59,5 | 53.6 | 32 (1.83) | 0.60 | |
Missing values (age) = 73 (1.2%); $: only one case of death have been unregistered among upper RI; RI: Respiratory infections, CIR: Crude incidence rate; ASR: Age-standardized incidence rates, inh: inhabitants; PY: person year, A-SMR: Age-standardized mortality rates.* p < 0.05; ** p < 0.001; fatality (%) = (number of deaths/number of cases)*100.
For hospitalized LRI, 4624 admissions were recorded (385 hospitalizations per year). The CIR was 77.5 per 100,000 inh. It was 506.6 for children aged up to 5 years. The in-hospital fatality was 1.41%. The age-SIR was 71.63 per 100,000 PY, 90.2 for men and 53.6 for women, respectively. The SMR was 0.64 per 100,000 PY, 0.68 for men and 0.60 for women, respectively (Table 1).
4.3. Distribution and trends of admissions for RI
URI were mainly otitis in 66.2% of cases, of which 98% were AOM. Among inpatients aged up to 5 years, admissions for pharyngitis, sinusitis, tonsillitis and influenza were predominant. URI decreased significantly over time (b = −4.90, p < 0.000). It declined for both sexes and for all age groups (Tables 2 and 3). No season distribution was recorded for AOM (p = 0.328).
Table 2.
Distribution and trend of admissions for respiratory infections according to sex (2002 to 2013 Monastir Tunisia).
| All (n = 6061) |
Males (n = 3631) |
Females (n = 2428) |
|||||
|---|---|---|---|---|---|---|---|
| N (%) | Slope (SE) | N (%) | Slope (SE) | N (%) | Slope (SE) | Sex-ratio | |
| Upper Respiratory Infections: Overall | 1436 (100) | −4.90 (0.14) ** | 761 (100) | −2.50 (0.10) | 675 (100) | −2.08 (0.13) ** | 1.12 |
| Otitis externa, Otitis media | 950 (66.2) | −2.67 (0.12) ** | 481 (63.2) | −1.43 (0.07) ** | 469 (69.48) | −1.14 (0.11) ** | 1.02 |
| Mastoiditis, Labyrinthitis | 60 (4.2) | −0.26 (0.01) ** | 30 (3.9) | −0.002(0.01) | 30 (4.44) | − 0.21 (0.01) ** | 1 |
| Pharyngitis, sinusitis, tonsillitis, flu | 111 (7.7) | −0.32 (0.04) ** | 67 (8.8) | −0.14 (0.04) ** | 44 (6.51) | −0.11 (0.03) ** | 1.52** |
| Phlegmonous Angina, Parapharyngeal abscess | 315 (21.9) | −1.65 (0.07) ** | 183 [24] | −0.93 (0.05) ** | 132 (19.55) | −0.62 (0.07) ** | 1.39 |
| Lower Respiratory Infections: Overall | 4625 (100) | 13.81 (0.61) ** | 2870 (100) | 9.92 (0.48) ** | 1753 (100) | 4.04 (0.38) ** | 1.64** |
| Pneumococcal Pneumonia (J13) | 823(17.8) | 17.32 (0.14) ** | 542 (18.9) | 12.1 (0.09) ** | 280 (16.0) | 6.5 (0.5) ** | 1.93** |
| Other Pneumonias (J14, J15, J16, J17,J18) | 1670(36.1) | −11.16 (0.11) ** | 1107(38.6) | −6.4 (0.06) ** | 653(37.2) | −5.78 (0.05) ** | 1.69** |
| Acute bronchitis (J20) | 137 (2.9) | 0.80 (0.07) ** | 72 (2.56) | 0.47 (0.05) ** | 65 (3.7) | 0.49 (0.06) ** | 1.11 |
| Acute bronchiolitis (J21) | 1652 (35.7) | 8.62(0.35) ** | 1022 (35.6) | 3.85 (0.27) ** | 630 (35.9) | 4.32 (0.33) ** | 1.62** |
| Unspecified Bronchitis (J40) | 301(6.5) | −0.59 (0.13) ** | 191(6.65) | −0.11 (0.11) | 110 (6.) | −0.08 (0.15) | 1.74** |
| Lung and Mediastinum Abscess (J85), pleural empyema (J86) | 42 (0.9) | 0.06 (0.01) ** | 26 (0.9) | −0.003 (0.010) | 15 (0.85) | −0.013 (0.014) | 1.73** |
*: p < 0.05; **: P ≤ 0.001, SE: Standard Error *; slope = b; p values calculated using the test of linear regression.
Table 3.
Distribution and trend of admissions for respiratory infections according to age groups (Monastir; 2002–2013).
| [0–5[ |
[5–25[ |
[25–60[ |
≥ 60 |
|||||
|---|---|---|---|---|---|---|---|---|
| Age (years) | N (%) | Slope (SE) | N (%) | Slope (SE) | N (%) | Slope (SE) | N (%) | Slope (SE) |
| Overall: Upper Respiratory Infectious | 167 | −0.46 (0.19) * | 485 | −2.45 (0.14) ** | 663 | −1.93 (0.11) ** | 109 | −0.11 (0.08) |
| Otitis externa, otitis media | 47 (28.14) | 0.25 (0.08) * | 325 (67.01) | −1.99 (0.11) ** | 492 (74.20) | −0.89 (0.10) ** | 80 (73.39) | −0.09 (0.06) |
| Mastoiditis, labyrinthitis | 25 (14.97) | −0.13 (0.04) ** | 16 (3.29) | 0.02 (0.02) | 16 (2.41) | −0.06 (0.01) ** | 3 (2.75) | - |
| Pharyngitis, sinusitis, tonsillitis, flu | 66 (39.52) | −0.54 (0.12) ** | 16 (3.29) | 0.09 (0.02) ** | 23 (3.46) | 0.039 (0.02) | 3 (2.75) | - |
| Phlegmonous angina, parapharyngeal abscess | 29 (17.36) | −0.03 (0.04) | 128 (26.39) | −0.56 (0.07) ** | 132 (19.9) | −1.02 (0.05) ** | 23 (21.1) | 0.00 (0.05) |
| Overall: Lower Respiratory Infectious | 2676 | 18.52 (0.46) ** | 547 | 0.81 (0.08) ** | 604 | 0.26 (0.07) | 759 | 2.31 (0.10) ** |
| Pneumococcal Pneumonia (J13) | 371(13.9) | 12.8 (0.09) ** | 161(29.4) | 3.3 (0.02) ** | 103(17.1) | 3.3 (0.02) | 188(24.7) | 4.5 (0.03) ** |
| Other Pneumonias (J14, J15, J17, J18) | 487(18.2) | −7.33 (0.06) ** | 304(55.6) | −2.9 (0.02) ** | 408(67.5) | −2.9 (0.2) | 571(75.2) | −2.37 (0.03) |
| Acute bronchitis (J20) | 105 (3.92) | 0.72 (0.05) | 20 (3.55) | −0.12 (0.01) ** | 4 (0.66) | NA | 6 (0.79) | NA |
| Acute bronchiolitis (J21) | 1622 (60.61) | 11.09 (0.30) ** | NA | NA | NA | NA | NA | NA |
| Unspecified Bronchitis acute or chronic (J40) | 86 (3.21) | −0.31 (0.05) ** | 32 (5.85) | 0.21 (0.02) ** | 76 (12.58) | 1.03 (0.04) | 107 (14.09) | 0.35 (0.04) ** |
| Lung and Mediastinum Abscess (J85), pleural empyema (J86) | 6 (0.22) | 0.04 (0.01) ** | 7 (1.27) | 0.02 (0.004) ** | 13 (2.15) | −0.01 (0.01) ** | 15 (1.97) | 0.02 (0.04) |
*: p < 0.05; **: P ≤ 0.001, SE: Standard Error, NA = non applicable
LRI represented 76.3% of all RI admissions (Figure 2). Acute bronchiolitis and pneumonia represented, respectively, 35.7% and 53.9% of LRI. Admissions for LRI increased greatly and significantly (b = 13.81, p < 0.0001) (Figure 3). Admission for pneumonia increased significantly during study period (b = 5.16; p < 0.0001) especially in children up to 5 years old (b = 5.53). Admission for PP increased significantly during study period (b = 25.9; p < 0.0001) especially in children under five (b = 10.6) and among inpatients aged more than 60 years (b = 6.0) (Tables 2 and 3). It was largely predominant in males (sex-ratio = 5.06; p < 0.0001) and had no seasonal variation (p = 0.765). In children under 3 years, winter predominance was noted (p < 0.0001) for PP admissions (38.7%) and for bronchiolitis (53%). Admissions for Hib pneumonia were decreasing for both sexes and for all age groups. However, admissions for acute bronchitis (J20) increased in children aged under 5 years and decreased in the next age group [5–25 years old].
Figure 2.
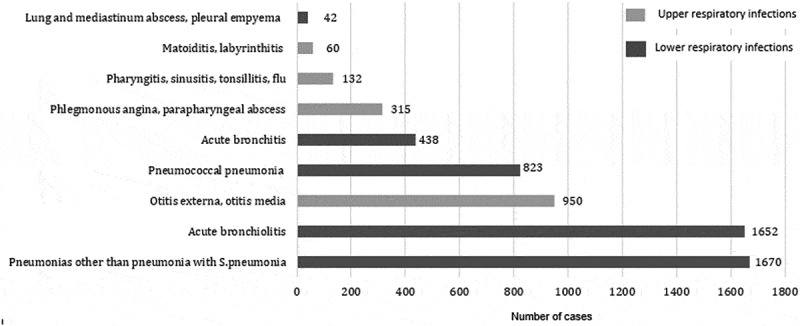
Distribution of admissions for acute respiratory infections (Monastir; 2002–2013).
Legend: Hospitalization for LRI was dominated by pneumonia while otitis represented the major causes of URI in the university hospital of Monastir (2002–2013).
Figure 3.
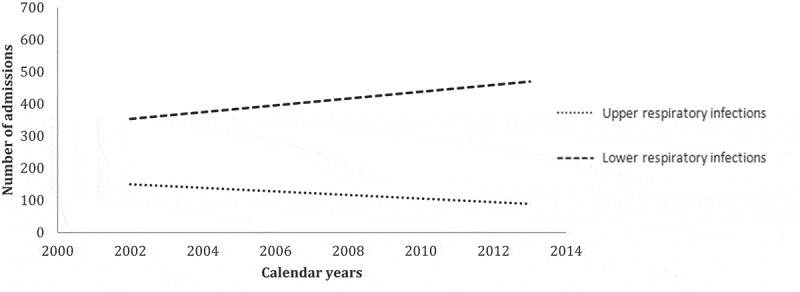
Trends of hospitalized upper and lower respiratory infections (Monastir, 2002–2013).
Legend: URI decreased and LRI increased significantly during study period.
4.4. Burden of severe RI
We recorded 3250 YLLs and 4032 DALYs during study period representing 54.14 YLL and 69.71 DALYs per 100,000 inh per year. Bacterial pneumonia was responsible for 2190 DALYs corresponding to 38.93/100,000 inh every year, composed by 88% of YLLs and 12% of YLDs (Table 4).
Table 4.
Burden of admissions for severe respiratory infections (Monastir; 2002–2013).
| YLL (%) | YLDs (%) | DALYs (%) | YLLs per 100,000 inh | YLDs per 100,000 inh | DALYs per 100,000 inh | |
|---|---|---|---|---|---|---|
| All | 3260.9 | 781.91 | 4032.31 | 54.14 | 3.51 | 69.71 |
| Age group | ||||||
| [0–5[ | 2599.0 (80.0) | 375.1 (48) | 2974.1 (73.8) | 44.15 | 6.95 | 51.10 |
| [5–25[ | 373.6 (11.5) | 137.3 (17.6) | 510.9 (12.7) | 6.35 | 2.54 | 8.89 |
| [25–40[ | 86.8 (2.7) | 73.8 (9.4) | 160.6 (4.0) | 1.47 | 1.37 | 2.84 |
| [40–59[ | 116.1 (3.6) | 94.7 (12.1) | 210.8 (5.2) | 1.47 | 1.75 | 3.73 |
| ≥ 60 | 74.9 (2.3) | 101.1 (12.9) | 176.0 (4.4) | 1.44 | 1.87 | 3.15 |
| Gender | ||||||
| Males | 1541.9 (47.3) | 483.0(59.9) | 2025.0 (49.8) | 26.19 | 8.95 | 35.15 |
| Females | 1719.0 (52.7) | 322.92 (40.1) | 2041.9 (50.2) | 29.20 | 5.98 | 35.19 |
| Main RI categories | ||||||
| Acute bronchiolitis | 879.0 (26.9) | 219.72 (27.3) | 1098 | 14.93 | 2.77 | 17.7 |
| Bacterial Pneumonia | 1932(59.3) | 259.09(32.2) | 2190 | 32.83 | 6.10 | 38.93 |
| URI and Other LRI | 449.8 (13.8) | 327.31 (40.6) | 776 | 6.37 | 6.07 | 12.44 |
YLLs: years of life lost; YLDs: years lived with disability; DALYs: Disability Adjusted Life Years.
4.4.1. Impact of Hib vaccine on admissions for RI in children aged less than 3years old
In children aged up to 3 years, Hib pneumonia admission CIRs per 100,000 were 11.6 in NVC, 10.6 in VC by protocol 1 (Hib Vaccine monovalent) and 0.80 in VC by protocol 2 (Hib vaccine pentavalent combination), the relative risk reduction was 99% for protocol 2 (p < 0.001). AOM admission CIRs per 100,000 were 1.47 in NVC, 0.98 in VC by protocol 1 and 0.44 in VC by protocol 2, the relative risk reduction was 69% for protocol 2 (p < 0.001). The relative risk reduction, for all in-hospital deaths related to RI in infants under 3, was 57% and 89% for protocol 1 and protocol 2, respectively (Table 5). For Hib pneumonia admissions sex-ratio was 1.27 for protocol one (p = 0.124), 1.40 for NVC (p = 0.006) and 1.25 for protocol 2 (p = 0.739) (Table 5).
Table 5.
Impact of Hib vaccine on admissions and in-hospital deaths related to respiratory infections among children aged under 3 years old.
| Cases | Population | Absolute risk * (95% CI) | ARR* | Relative risk (95% CI) | RRR (95% CI) | NNT | P | ||
|---|---|---|---|---|---|---|---|---|---|
| Hib Pneumonia | |||||||||
| Protocol 1 | Vaccinated | 121 | 1121344 | 10.7 (8.86; 12.71) | 0.84 | 0.92 (0.74;1.15) | 0.492 | ||
| Unvaccinated | 261 | 2242688 | 11.6 (10.2; 13.0) | ||||||
| Protocol 2 | Vaccinated | 9 | 1121344 | 0.80 (0.027; 1.30) | 10.83 | 0.068 (0.035;0.13) | 0.99 (0.94;0.99) | 9230 (7773;11359) | < 0.0001 |
| Unvaccinated | 261 | 2242688 | 8.17 (7.31;9.03) | ||||||
| Acute otitis media | |||||||||
| Protocol 1 | Vaccinated | 11 | 1121344 | 0.98 (0.40;1.56) | 0.49 | 0.66 (0.33;1.32) | 0.24 | ||
| Unvaccinated | 33 | 2242688 | 1.47 (0.96;1.97) | ||||||
| Protocol 2 | Vaccinated | 5 | 1121344 | 0.44 (0.05;0.84) | 1.02 | 0.30 (0.11;0.77) | 0.69 (0.22;0.88) | 9751 (55946;379283) | 0.012 |
| Unvaccinated | 33 | 2242688 | 1.47 (0.96;1.97) | ||||||
| Deaths all causes aged <3 years | |||||||||
| Protocol 1 | Vaccinated | 2 | 1121344 | 0.17 (0.06;0.43) | 0.83 | 0.16 (0.04,0.70) | 0.83 (0.29;0.96) | 112135 (65703;382310) | 0.0149 |
| Unvaccinated | 24 | 2242688 | 1.07 (0.64; 1.49) | ||||||
| Protocol 2 | Vaccinated | 0 | 1121344 | 0 | 1.07 | 0.0408 (0.002;0.67) | 0.99 | 95434 (60030;232629) | 0.0252 |
| Unvaccinated | 24 | 2242688 | 1.07 (0.64; 1.49) | ||||||
*: per 100,000 inh; unvaccinated cohort: patients born between 2005 and 2011; vaccinated by protocol 1: patients born between 2002 and 2005, with Hib monovalent vaccine at 3, 4, 6 months; vaccinated by protocol 2: patients born after 2011, with a Hib pentavalent combination vaccine at 2, 3 and 6 months; ARR: Absolute Risk Reduction; RRR: relative risk reduction; NNT: Number Needed to Treat
5. Discussion
This study had as objectives to assess the trends and the health burden of hospitalized RI and their trends during a period of 12 years. The impact of Hib vaccine was also evaluated by comparing the VC and the NVC.
RI admissions accounted for approximately the fifth of all communicable disease hospitalizations, dominated by LRI. Children aged up to 5 years were the most affected. Otitis and bronchiolitis were the main diseases causing admission to university hospitals. Bacterial Pneumonia was the leading cause of YLLs.
In addition, we found that Hib vaccine had a significant impact on the reduction of RI-related hospital admissions and deaths in children up to 3 years.
Admission for LRI was higher than for URI, consistent with other studies conducted in hospital setting, qualifying LRI as the second cause of morbidity and the fourth leading cause of mortality in all age groups in 2013 with a high burden related to pneumonia [19–21]. However, URI has long been regarded as the most frequent RI in community [22].
According to the literature, AOM represented the most common admissions for URI and were frequent mainly in young people [23,24]. Hospital admission for AOM is a useful marker for otitis media severity [25]. AOM had a decreasing trend in our studies. However, it was increasing in other studies conducted in developed countries [25,26]. The homogeneous seasonal distribution of AOM admissions contrasted with the results of a study conducted in Taiwan that showed a peak from March to May, which could be related to climate differences [23]. Pharyngitis hospitalization rates were decreasing also in our sample due to the efficacy of Tunisian national program in preventing acute articular rheumatism and to the medical practice performance in primary care [27].
Similarly to literature, admissions for LRIs are predominant among men, children aged up to 5 years and the elderly [28]. This great difference in sex and age can be explained by the high prevalence of male smokers in Tunisia [29] and by chronic comorbidities, in particular COPD, proving the need to generalize the pneumococcal and influenza vaccines for this population [30].
A systematic analysis showed a consistently higher incidence of admissions for severe LRI in boys than in girls for all age groups [31]. The hypothesis to explain could be the evidence of smaller airway size in young boys than in young girls [32].
The seasonal variation was not observed in the rate of LRI admissions to the hospital. In other studies, the highest number of pneumonia cases occurred in winter and autumn [33,34]. Another data on the seasonality of influenza pneumonia in Thailand showed a sharp increase during the months of January through April [35].
Pneumonia was the most frequent cause of hospitalizations for LRI. The higher rates were seen in older adults and children <5 years old. The increasing trend of LRI was related in particular to PP which increased significantly during study period. A nationwide retrospective database analysis conducted from 2009 to 2016 in Poland showed the same results with an increasing trend of SP pneumonia and decreasing one for Hib pneumonia [36]. These trends could be explained by the vaccination status for both pathogens. In fact, in that period of time in Poland, pneumococcal conjugate vaccine (PCV) was not yet introduced in their national immunization program to protect children against invasive pneumococcal disease.
Our results showed that RIs increased mortality rather than disability especially for children. A systematic analysis for the Global Burden of Disease Study 2013 showed that for children, two causes each accounted for more than half a million deaths and collectively accounted for more than a third of deaths: lower respiratory infections, and malaria [37].
When comparing the health burden of RI by gender, we found that DALY were very close (2025.0 in males vs 2041.9 in females). It may be explained by YLL which was higher in females than in males given that the life expectancy at birth in females is higher than in males as well. However, males had more YLD than females.
Our study showed that immunization against Hib by the pentavalent form was more effective than the monovalent form. We found that admissions for Hib pneumonia in infants under 3 decreased significantly among the cohort vaccinated by the combined form of vaccine. This leads to the belief that the pentavalent form is more immunizing.
However, there is no conclusive evidence of differences in the immune response to monovalent or combined Hib conjugate vaccines. In fact, a review about the successes and the contribution of hexavalent combination Hib vaccines in Europe from 2000 to 2014; showed a higher acceptance. On the other hand, there are some concerns that this combined form may reduce Hib immunogenicity [38]. Our results may be related to the high Hib vaccine coverage among children born since 2011. This high coverage vaccine provided by combined vaccines is explained by the reduction of injections number for babies and the reduction of visits to health-care centres.
In addition, the effect of Hib Conjugate Vaccine on the prevention of pneumonia in hospitalized infants for bronchiolitis was shown by a case-control study [39]. In NVC, male children dominated Hib pneumonia admissions whereas no sex-difference was notified in VC, this result was concordant with conclusions in literature [40].
Haemophilus influenzae serotype b is responsible for approximately 95% of all invasive disease (pneumonia or meningitis) due to Haemophilus influenzae. Other serotypes and non-encapsulated strains can cause otitis media in children and disease in the elderly and in immunocompromised populations [41,42]. As shown in our study, the introduction of Hib conjugate vaccines led to a major decline in levels of nasopharyngeal infections such as AOM [43]. This has led to a substantially greater reduction in disease incidence than can be directly attributed to the effects of the vaccine, suggesting that widespread use of the vaccine has resulted in the induction of herd protection [44].
The burden of RI may be more important than the result found. We recognize that many children could not receive hospital care, such as in other developing countries. In addition, our study did not include the secondary level health-care institutions which had weak capacity compared to the study area. Therefore, our regional estimates may underestimate the true burden of hospitalized RI [31].
6. Conclusion
Admissions for community-acquired RI were pronounced especially for the two extreme age groups. Our findings suggested that the introduction of Hib vaccine contributed greatly in reducing admissions for pneumonia and AOM. Immunization against Hib and more especially pentavalent combination vaccines had an impact on the admission and severity of RI in children. The recent introduction of anti-pneumococcal vaccine in the TEPI will improve this decrease and give a great contribution to alleviate the burden of RI [45,46]. In fact, a substantial reduction in hospitalization for pneumonia was observed after the introduction of PCV in many countries [28,45]. A national surveillance data must be more rigorous to determine immunization outcomes.
Disclosure statement
The authors declare that they have no competing interests.
Ethics approval
The study was approved by the Regional Scientific Ethical Committee (Monastir) since 1996 and re-approved in 2006.
References
- [1].Lieberman D, Lieberman D.. Management of respiratory infections in the elderly. Expert Rev Anti Infect Ther. 2003. October;1(3):505–9. [DOI] [PubMed] [Google Scholar]
- [2].Korbel L, Spencer JD. Diabetes mellitus and infection: an evaluation of hospital utilization and management costs in the USA. J Diabetes Complications. 2015. March;29(2):192–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].GBD 2015 . Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Lond Engl. 2016. 08;388(10053):1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Niederman MS, Krilov LR. Acute lower respiratory infections in developing countries. Lancet Lond Engl. 2013. April 20;381(9875):1341–1342. [DOI] [PubMed] [Google Scholar]
- [5].Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet Lond Engl. 2012. June;379(9832):2151–2161. [DOI] [PubMed] [Google Scholar]
- [6].Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009. October 1;64(Suppl 3):iii1–55. [DOI] [PubMed] [Google Scholar]
- [7].O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009. September 12;374(9693):893–902. [DOI] [PubMed] [Google Scholar]
- [8].Watt JP, Wolfson LJ, O’Brien KL, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009. September;374(9693):903–911. [DOI] [PubMed] [Google Scholar]
- [9].Hansen MP, Hoffmann TC, McCullough AR, et al. Antibiotic resistance: what are the opportunities for primary care in alleviating the crisis? Front Public Health. [Internet]. 2015. February 24 [cited 2019 October20];3. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4338603/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kacem M, Dhouib W, Bennasrallah C, et al. Expanded program of immunization in the Maghreb. Case study of Tunisia. Systematic review of the literature. Tunis Med. 2018. November;96(10–11):696–705. [PubMed] [Google Scholar]
- [11].CARTE SANITAIRE ANNEE 2015.:159.
- [12].Edemekong PF, Huang B. Epidemiology of prevention of communicable diseases. in: statPearls [Internet]. Treasure Island (FL):StatPearls Publishing. 2020. cited 2020 April18. Available at: http://www.ncbi.nlm.nih.gov/books/NBK470303/ [PubMed] [Google Scholar]
- [13].Ben Fredj M, Sriha Belguith A, Abroug H, et al. Hospitalizations for communicable diseases in a developing country: prevalence and trends-Monastir, Tunisia, 2002-2013. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2017;55:102–108. [DOI] [PubMed] [Google Scholar]
- [14].El Mhamdi S, Herizi C, Sriha A, et al. [Profile and trends of pediatric hospital morbidity in the region of Monastir (Tunisia) for a decade]. Rev Med Brux. 2015. October;36(5):410–414. [PubMed] [Google Scholar]
- [15].El Mhamdi S, Chaieb R, Bouanene I, et al. Trends in hospital morbidity among adults in the region of Monastir (Tunisia) between 1996 and 2007. Tunis Médicale. 2011. December 1;89:905–909. [PubMed] [Google Scholar]
- [16].Recencement [Internet]. INS. [cited 2019 October20]. Available from: http://census.ins.tn/fr/recensement
- [17].World (WHO 2000-2025) Standard-Standard Populations-SEER Datasets [Internet]. SEER. [cited 2019 October20]. Available from: https://seer.cancer.gov/stdpopulations/world.who.html
- [18].Salomon JA, Haagsma JA, Davis A, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015. November 1;3(11):e712–23. [DOI] [PubMed] [Google Scholar]
- [19].Global, regional, and national age–sex specific all-cause and cause-specific mortality for . 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015. January 10;385(9963): 117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nair H, Watts AT, Williams LJ, et al. Pneumonia hospitalisations in Scotland following the introduction of pneumococcal conjugate vaccination in young children. BMC Infect Dis. 2016. August;9(16):390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Driver C. Pneumonia part 1: pathology, presentation and prevention. Br J Nurs Mark Allen Publ. 2012. February 26; 21(2):103–106.. [DOI] [PubMed] [Google Scholar]
- [22].Simoes EAF, Cherian T, Chow J, et al. Acute Respiratory Infections in Children. In: Jamison DT, Breman JG, Measham AR, et al., editors. Disease control priorities in developing countries [Internet], 2nd. Washington (DC): World Bank; 2006. cited 2018 January2. Available at http://www.ncbi.nlm.nih.gov/books/NBK11786/ [Google Scholar]
- [23].Ting PJ, Lin CH, Huang FL, Lin MC, Hwang KP, Huang YC, et al . Epidemiology of acute otitis media among young children: A multiple database study in Taiwan. J Microbiol Immunol Infect. 2012. December;45(6):453–458.. [DOI] [PubMed] [Google Scholar]
- [24].Mustafa G, Aidaroos AYA, Abaidani ISA, et al. Incidence and economic burden of acute otitis media in children aged up to 5 years in three Middle Eastern countries and Pakistan: A multinational, retrospective, observational study. J Epidemiol Glob Health. 2017. February 8;7(2):123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kvaerner KJ, Austeng ME, Abdelnoor M. Hospitalization for acute otitis media as a useful marker for disease severity. Pediatr Infect Dis J. 2013. September;32(9):946–949. [DOI] [PubMed] [Google Scholar]
- [26].Finnbogadóttir AF, Petersen H, Laxdal T, et al. An increasing incidence of mastoiditis in children in Iceland. Scand J Infect Dis. 2009;41(2):95–98. [DOI] [PubMed] [Google Scholar]
- [27].Sriha Belguith A, Koubaa Abdelkafi A, El Mhamdi S, et al. Rheumatic heart disease in a developing country: incidence and trend (Monastir; Tunisia: 2000–2013). Int J Cardiol. 2017. February 1;228:628–632.. [DOI] [PubMed] [Google Scholar]
- [28].De Miguel-díez J, Jiménez-García R, Hernández-Barrera V, et al. Trends in hospitalizations for community-acquired pneumonia in Spain: 2004 to 2013. Eur J Intern Med. 2017. May;40:64–71. [DOI] [PubMed] [Google Scholar]
- [29].Matsuse H. [Pneumococcal vaccine and influenza vaccine for prevention of exacerbation in patients with COPD]. Nihon Rinsho Jpn J Clin Med. 2016. May;74(5):839–842. [PubMed] [Google Scholar]
- [30].Daldoul H, Denguezli M, Jithoo A, et al. Prevalence of COPD and Tobacco Smoking in Tunisia—results from the BOLD Study. Int J Environ Res Public Health. 2013. December;10(12):7257–7271.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nair H, Simões EA, Rudan I, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013. April 20;381(9875):1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hoo A-F, Dezateux C, Hanrahan JP, et al. Sex-specific prediction equations for Vmax (FRC) in infancy: a multicenter collaborative study. Am J Respir Crit Care Med. 2002. April 15;165(8):1084–1092. [DOI] [PubMed] [Google Scholar]
- [33].Williams NP, Coombs NA, Johnson MJ, et al. Seasonality, risk factors and burden of community-acquired pneumonia in COPD patients: a population database study using linked health care records. Int J Chron Obstruct Pulmon Dis. 2017;12:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Saha S, Hasan M, Kim L, et al. Epidemiology and risk factors for pneumonia severity and mortality in Bangladeshi children <5 years of age before 10-valent pneumococcal conjugate vaccine introduction. BMC Public Health [Internet]. 2016. December 7 [cited 2018 January2];16. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5142317/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Simmerman JM, Chittaganpitch M, Levy J, et al. Incidence, Seasonality and Mortality Associated with Influenza Pneumonia in Thailand: 2005–2008. Plos One. 2009. November 11;4(11):e7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gajewska M, Goryński P, Paradowska-Stankiewicz I, et al. Monitoring of community-acquired pneumonia hospitalisations before the introduction of pneumococcal conjugate vaccine into Polish National Immunisation Programme (2009-2016): A nationwide retrospective database analysis. Vaccine. 2020 Jan 10;38(2):194–201.. [DOI] [PubMed] [Google Scholar]
- [37].GBD 2013 . Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Lond Engl. 2015. January 10;385(9963): 117–171.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang S, Tafalla M, Hanssens L, et al. A review of Haemophilus influenzae disease in Europe from 2000-2014: challenges, successes and the contribution of hexavalent combination vaccines. Expert Rev Vaccines. 2017;16(11):1095–1105. [DOI] [PubMed] [Google Scholar]
- [39].Salas AA, Salazar HJ, Velasco VH. Haemophilus influenza type b conjugate vaccine for preventing pneumonia in infants hospitalized for bronchiolitis: a case-control study. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2010. January;14(1):e68–72. [DOI] [PubMed] [Google Scholar]
- [40].Boef AGC, Van der Klis FRM, Berbers GAM, et al. Differences by sex in IgG levels following infant and childhood vaccinations: an individual participant data meta-analysis of vaccination studies. Vaccine. 2018. 08; 36(3): 400–407.. [DOI] [PubMed] [Google Scholar]
- [41].Liese JG, Silfverdal SA, Giaquinto C, et al. Incidence and clinical presentation of acute otitis media in children aged <6 years in European medical practices. Epidemiol Infect. 2014. August;142(8):1778–1788.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhou F, Shefer A, Kong Y, et al. Trends in acute otitis media-related health care utilization by privately insured young children in the USA, 1997-2004. Pediatrics. 2008. February;121(2):253–260. [DOI] [PubMed] [Google Scholar]
- [43].Norhayati MN, Ho JJ, Azman MY. Influenza vaccines for preventing acute otitis media in infants and children. Cochrane Database Syst Rev Internet]. 2017. October 17 [cited 2019 November5];2017(10). Available from. ;(). : https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6485791/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gil A, Kenney LL, Mishra R, et al. Vaccination and heterologous immunity: educating the immune system. Trans R Soc Trop Med Hyg. 2015. January;109(1):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nair H, Watts AT, Williams LJ, et al. Pneumonia hospitalisations in Scotland following the introduction of pneumococcal conjugate vaccination in young children. BMC Infect Dis. 2016. 09; 16(1): 390.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ben Salah A; El Mhamdi S; Ben Fredj M; Ben Meriem C; Bouguila J; Ben Helel K; et al. Coût hospitalier des pathologies invasives à pneumocoque chez les enfants âgés de moins de 15 ans en Tunisie. East Mediterr Health J. 2018;25(12):861–871. . [DOI] [PubMed] [Google Scholar]


