Abstract
Background: Emergence, prevalence and widely spread of plasmid-mediated colistin resistance in Enterobacteriaceae strongly impairs the clinical efficacy of colistin against life-threatening bacterial infections. Combinations of antibiotics and FDA-approved non-antibiotic agents represent a promising means to address the widespread emergence of antibiotic-resistant pathogens.
Methods: Herein, we investigated the synergistic activity between melatonin and antibiotics against MCR (mobilized colistin resistance)-positive Gram-negative pathogens through checkerboard assay and time-killing curve. Molecular mechanisms underlying its mode of action were elucidated. Finally, we assessed the in vivo efficacy of melatonin in combination with colistin against drug-resistant Gram-negative bacteria.
Results: Melatonin, which has been approved for treating sleep disturbances and circadian disorders, substantially potentiates the activity of three antibiotics, particularly colistin, against MCR-expressing pathogens without enhancing its toxicity. This is evidence that the combination of colistin with melatonin enhances bacterial outer membrane permeability, promotes oxidative damage and inhibits the effect of efflux pumps. In three animal models infected by mcr-1-carrying E. coli, melatonin dramatically rescues colistin efficacy.
Conclusion: Our findings revealed that melatonin serves as a promising colistin adjuvant against MCR-positive Gram-negative pathogens.
Keywords: antibiotic adjuvant, bacterial infections, colistin, gram-negative pathogen, melatonin
Introduction
Antibiotic resistance is a growing problem that threatens the conventional regimens to treat bacterial infectious diseases 1, 2. It has been predicted that the antibiotic resistant bacteria would kill 10 million lives per year and lead to 100 trillion USD of economic loss worldwide by 2050 3. Colistin, a nonribosomal peptide antibiotic, is one of last-resort antibiotics against multidrug-resistant Gram-negative pathogens, particularly for carbapenem-resistant Enterobacteriaceae 4, 5. The bactericidal activity of colistin is mainly dependent on the disruption of membrane permeability and leakage of bacterial components through the electrostatic interaction between positively-charged residues of colistin and negatively charged lipid A moieties of lipopolysaccharides (LPS) anchored to the bacterial outer membrane 6. However, the mobilized colistin resistance gene (mcr-1) and its variants that encode phosphoethanolamine transferases reduce the negative charge of lipid A and confer a substantial resistance to colistin 7, 8. More problematically, the mcr-1 has already spread to over 40 countries/regions covering five of seven continents 9. Therefore, there is an urgent need to identify novel strategies to overcome MCR-mediated acquired colistin resistance in Gram-negative pathogens.
Compared with the time and money-consuming development of novel antibiotics, antibiotic adjuvant strategy offers a more cost-effective approach by preventing bacterial resistance or enhancing antibiotic modes of action 10-12. For example, inhibitors of β-lactamases such as clavulanic acid is the most clinically successful adjuvant to date 13. In addition, a fungus-derived product aspergillomarasmine A rescues meropenem activity by suppressing metallo-β-lactamase (MBLs) activity 14. Cholesterol lowering drugs such as statins disassemble bacterial membrane microdomains, and restore MRSA susceptibility to penicillin 15. The anti-protozoal drug pentamidine potentiates hydrophobic antibiotics activity against Gram-negative pathogens through disrupting the bacterial outer membrane 16. These examples inspired us to look for colistin adjuvants from the FDA-approved compounds. Thus far, several strategies have been reported to partially restore colistin activity 17-21. For instance, pterostilbene is a natural product obtained from fresh leaves or fruits that enhances the therapeutic effect of polymyxins 17, but the underlying mechanism is unclear. In addition, the combination of colistin with Gram-positive antibiotics such as rifampicin also contribute to overcoming mcr-1 mediated colistin resistance 18. However, this combination efficacy is highly dependent on the antibacterial effect of Gram-positive antibiotics on membrane-disrupted Gram-negative bacteria, and may be ineffective against multidrug-resistant pathogens. Despite these ongoing efforts, no colistin adjuvants have been tested in human clinical trials so far due to practical and technical limitations. Thus, safer and more effective colistin adjuvants for resistant bacteria are urgently required.
Melatonin (N-acetyl-5-methoxytryptamine) is a neurohormone that transmits the information “darkness” and contributes to the synchronization of circadian oscillators 22. Moreover, it is involved in numerous other physiological processes 23 such as regulation of blood pressure 24 and core body temperature 25, suppression of oncogenesis 26, and immune function 27. Melatonin is produced by the pineal gland and possibly all extra-pineal organs including the skin, retina, cerebellum, kidneys, liver, pancreas, and ovaries, as well as in plants and other phototrophic organisms 28. Although multiple beneficial effects of melatonin have been uncovered, its potential application in treatment of pathogenic bacteria has not been fully explored. Herein, we focused on the potency of melatonin as a novel antibiotic adjuvant. Interestingly, we found that melatonin effectively reverses MCR-mediated colistin resistance both in vivo and in vitro through multiple modes of action. The discovery of melatonin as a new and safe colistin adjuvant provides a therapeutic regimen for combating Gram-negative bacteria infections.
Materials and Methods
Bacteria and reagents
Strains used in this study are listed in Table S1. Escherichia coli MG1655 deleted mutants were obtained through homologous recombination mediated by suicide plasmid pLP12 and confirmed by PCR analysis. Isolation and detection of clinical isolates were performed based on previous study 29. Broth suspension of samples were inoculated onto MacConkey agar plates containing 2 μg/mL colistin. Positive colonies were confirmed for the mcr-1 gene by PCR analysis. Unless otherwise noted, strains were grown in Mueller-Hinton Broth (MHB) or on MH agar (MHA) plates. CHO and HEK293T cells were grown in DMEM (Gibco, MA, USA) supplemented with 10% heat-inactivated FBS (Invitrogen, CA, USA). Melatonin was purchased from Sigma-Aldrich (MO, USA). Antibiotics were obtained from China Institute of Veterinary Drug Control and other chemical reagents were purchased from Aladdin (Shanghai, China).
Antibacterial test
MICs of compounds were measured using the standard broth micro-dilution method, according to the CLSI 2016 guideline 30 and previous report 31. All drugs were two-fold diluted in MHB and equally mixed with bacterial suspensions in a 96-well microtiter plate (Corning, New York, USA). MIC values were defined as the lowest concentrations of drugs with no visible growth of bacteria after 18 h incubation at 37 °C.
Checkerboard assays
Synergistic activity of antibiotics and melatonin was evaluated by checkerboard assays with two-fold serially dilution of drugs (8 × 8 matrix). After 18 h co-incubation with bacterial suspension (1.5 × 106 CFUs/well), the absorbance of bacterial culture at 600 nm was measured by Microplate reader. Two biological replicates were performed for each combination and the means were used for FIC index (FICI) calculation according to the formula as follows 32:
| FIC index = FICIa + FICIb = MICab / MICa + MICba / MICb. |
MICa is the MIC of compound A alone; MICab is the MIC of compound A in combination with compound B; MICb is the MIC of compound B alone; MICba is the MIC of compound B in combination with compound A. Synergy is defined as an FIC index of ≤ 0.5.
Time-dependent killing
Overnight E. coli B2 were diluted 1/1,000 in MHB, and incubated for 4 h (exponential phase) or 8 h (stationary phase) at 37 °C. Bacteria were then treated with melatonin (1 mg/mL) and colistin (2 μg/mL) alone, or their combination for 24 h. At intervals, 100 μL aliquots were removed, centrifuged and resuspended in 100 μL sterile PBS. Subsequently, ten-fold serially diluted suspensions were plated on MHA plates and incubated overnight at 37 °C. Bacterial colonies were counted and the primary CFUs/mL was calculated.
E. coli MG1655 and its four deletion mutants at stationary phase were challenged with the combination of melatonin (1 mg/mL) and (0.0625 μg/mL) for 8 h. At intervals, the colonies (CFUs/mL) were counted and calculated. All experiments were performed with at least three biological replicates.
Safety assessment
Hemolytic activity of colistin in the presence of melatonin was evaluated based on previous reports 33, 34. 8% sheep blood cells were treated with colistin (16 to 128 μg/mL) and/or melatonin (0 to 1,000 μg/mL) for 1 h. Triton X-100 (0.2%) was used as a positive control. After incubation, the absorbance of supernatant at 576 nm was measured and hemolysis rate was calculated by comparing with positive control.
Cytotoxicity on CHO and HEK293T cells was determined using the water-soluble tetrazolium salt-1 (WST-1, Roche, Switzerland) assay 35. Colistin (0 to 128 μg/mL) with melatonin (0 to 1,000 μg/mL) and 1 × 104 cells were simultaneously added into 96-well plates and cultured at 37 °C for 24 h. Then, the absorbance of cell culture at 450 nm was measured and corresponding cytotoxicity was calculated.
Transcriptomic analysis
E. coli B2 were grown in MHB to the early-exponential phase, and treated with colistin (40 μg/mL) alone or in combination of melatonin (1 mg/mL) for 4 h. Total RNA of samples was extracted by an EASYspin Plus kit (Aidlab, Beijing, China) and quantified by using a Nanodrop spectrophotometer (Thermo Scientific, MA, USA), and sequenced by using the Illumina Hiseq 2000 system (Majorbio, Shanghai, China). Library construction of purified mRNA was conducted with Illumina Truseq RNA sample prep Kit according to the manufacturer's protocol. After amplification by bridge PCR with Illumina Truseq PE Cluster Kit v3-cBot-HS on cBot (Illumina), samples were sequenced by using Hiseq2000 Truseq SBS Kit v3-HS (200 cycles) (Illumina) with the read length as 2 × 100 (PE100). Raw sequencing reads were filtrated and mapped against the reference genome of E. coli K-12. Differentially-expressed genes were identified by using the FPKM (Fragments Per Kilobase of transcript per Million mapped reads) method with p-values ≤ 0.05 and fold change (FC) values ≥ 2 (log2 FC ≥ 1 or log2 FC ≤ -1). Differences between these two treatments were analyzed by Cuffdiff program (http://cufflinks.cbcb.umd.edu/).
RT-PCR analysis
E. coli B2 were grown to early-exponential phase, and incubated with colistin (40 μg/mL) alone or in combination of melatonin (1 mg/mL) for 4 h. Then, total RNA was extracted and quantified by the ratio of absorbance (260 nm/280 nm). Reverse transcription of 1 μg extracted RNA was performed using the PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Dalian, China) following the manufacturer's protocol. RT-PCR analysis was performed by 7500 Fast Real-Time PCR System (Applied Biosystem, CA, USA) using the TB Green qPCR Kit (Takara) with the optimized primers (Table S4). Relative quantitative method was applied to calculate the fold changes of mRNA expression relative to the reference genes (16S rRNA) in E. coli.
Biochemical factors analysis
Pretreatments of biochemical assays were performed using similar protocols as follows. Overnight E. coli B2 were washed, suspended in 5 mM HEPES (pH 7.0, plus 5 mM glucose) with an OD600 of 0.5, and incubated with fluorescent dyes for 30 min. Then, 190 μL of probed-cells were mixed with 10 μL of melatonin alone (0 to 1,000 μg/mL), or colistin (0 to 64 μg/mL) without or with melatonin (500 μg/mL) in a 96-well plate. After incubation at 37 °C for 1 hour, fluorescence intensity or absorbance or luminescence was measured by an Infinite M200 Microplate reader (Tecan).
Outer membrane permeability
1-N-phenylnaphthylamine (NPN) (10 μm) 36 with the excitation wavelength of 350 nm and emission wavelength of 420 nm was used to evaluate the outer membrane permeability.
Cell membrane integrity
Fluorescence intensity of 10 nM propidium iodide (PI)-labeled cells in the presence of increasing drugs was measured with the excitation wavelength of 535 nm and emission wavelength of 615 nm.
Membrane depolarization
3, 3-dipropylthiadicarbocyanine iodide (DiSC3(5), 0.5 μM) was applied to determine the membrane potential 37. Dissipated membrane potential of E. coli B2 was measured with the excitation wavelength of 622 nm and emission wavelength of 670 nm.
Total ROS and H2O2
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA, 10 μM) was applied to monitor levels of ROS in E. coli B2 38, following the manufacturer's instruction (Beyotime). Fluorescence intensity was measured with the excitation wavelength of 488 nm and emission wavelength of 525 nm. In addition, production of H2O2 in E. coli B2, induced by melatonin in the absence and presence of colistin, was determined by a Hydrogen Peroxide Assay Kit (Beyotime, China). After incubation for one hour, the absorbance of lysis buffer at 570 nm was measured.
Intracellular ATP
Intracellular ATP levels of E. coli B2 were determined using an Enhanced ATP Assay Kit (Beyotime, China). Overnight E. coli B2 were washed and resuspended to obtain an OD600 of 0.5. After treating with various concentrations of colistin alone or in combination with melatonin (500 μg/mL) for 1 h, bacterial cultures were centrifuged and the supernatant was removed. Bacterial precipitates were lysed by lysozyme, and the supernatant was prepared for intracellular ATP levels measurement. Detecting solution was added to a 96-well plate and incubated at room temperature for 5 min. Subsequently, the luminescence of supernatants was monitored by Infinite M200 Microplate reader (Tecan). Intracellular ATP levels in E. coli were calculated from the luminescence signals.
SOD activity
Intracellular superoxide dismutase (SOD) activity of E. coli B2 treated with melatonin, colistin or their combination was measured using the Total Superoxide Dismutase Assay Kit with WST-8 (S0101, Beyotime, China).
Animal studies
6-8-week-old female BALB/c mice were obtained from Comparative Medicine Centre of Yangzhou University (Jiangsu, China). Mice were adapted for one week prior to infection. Mouse studies were performed in accordance with the relevant guidelines and regulations (ID: SCXK-2017-0007). The laboratory animal usage license number is SCXK-2017-0044, certified by Jiangsu Association for Science and Technology.
Pharmacokinetic analysis
BALB/c female mice were intraperitoneally injected with a single dose of colistin (10 mg/kg) and melatonin (50 mg/kg). Plasma samples were taken from three mice at each time point (5 min, 10 min, 15 min, 30 min, 1 h, 2 h, 4 h, 8 h, 12 h and 24 h). Plasma (100 μL) was mixed with acetonitrile (300 μL), vigorously vortexed and centrifuged at 12,000 rpm for 10 min. The precipitate was re-extracted with acetonitrile (100 μL). Combined supernatants were filtered through a 0.22 μm filter membrane before LC-MS/MS analysis. Colistin and melatonin concentrations in supernatants were determined by AB SCIEX 6500 QTRAP™ mass spectrometer (Applied Biosystems, CA, USA) with positive ionization multiple reaction monitoring (MRM) mode (Table S5). 0.1% formic acid in water and 0.1% formic acid in acetonitrile were used as mobile phase. Limit of detection (LOD), limit of quantitation (LOQ), recoveries and intra-day relative standard deviation (RSD) of detection method are presented in Tables S6 and S7. Pharmacokinetic parameters were performed using a non-compartmental analysis model by WinNonlin 6.4 software.
Galleria mellonella infection model
Galleria mellonella larvae (Huiyude Biotech Company, Tianjin, China) were randomly divided into four groups (n = 10 per group) and infected with E. coli B2 suspension (10 µL, 1.0 × 105 CFUs per larvae) at the right posterior gastropoda. At one-hour post-infection, Galleria mellonella larvae were injected with PBS, colistin (10 mg/kg), melatonin (50 mg/kg), or the combination of colistin with melatonin (10 + 50 mg/kg) at left posterior gastropoda. Survival rates of Galleria mellonella larvae were recorded for 5 days.
Mouse peritonitis-sepsis infection model
Female BALB/c mice (n = 8 or 9 per group) were intraperitoneally infected with a dose of 3.0 × 108 CFUs E. coli B2 suspension. At one-hour post-infection, mice were treated with a single dose of colistin (10 mg/kg), melatonin (50 mg/kg), or combinations of colistin plus melatonin (5 + 50 mg/kg, 10 + 20 mg/kg or 10 + 50 mg/kg) via intraperitoneal injection. Survival rates of mice were recorded for 7 days.
Neutropenic mouse thigh infection model
Female BALB/c mice (n = 8 per group) were rendered neutropenic by two consecutive doses of cyclophosphamide (150 and 100 mg/kg delivered on 4 and 1 days before infection). E. coli B2 suspension (100 μL, 1.0 × 105 CFUs per mouse) was injected into the right thighs of mice. At one-hour post-infection, mice were intraperitoneally injected with PBS, colistin (10 mg/kg), melatonin (50 mg/kg), or combinations of the two agents (5 + 50 mg/kg, 10 + 20 mg/kg or 10 + 50 mg/kg). At 48 h post-infection, mice were euthanized by cervical dislocation. The right thigh muscle was aseptically removed, homogenized, serially diluted and plated on MHA for CFUs titres.
Statistical analyses
Statistical analysis was performed using GraphPad Prism 7 and SPSS software. All data are presented as mean ± SD. For the in vitro studies, unpaired t-test (normally distributed data) between two groups or one-way ANOVA among multiple groups were used to calculate P-values. For the in vivo studies, n represents the number of animals per group and statistical significance was determined by log-rank (Mantel-Cox) test or the Mann-Whitney U test. Differences with P < 0.05 were considered significant. Significance levels are indicated by numbers of asterisks: *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Synergistic activity of melatonin with antibiotics
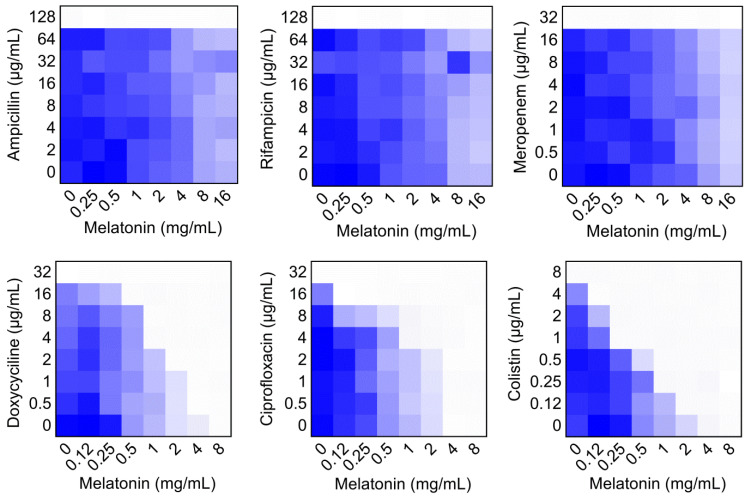
To evaluate the potential efficacy of melatonin, checkerboard dilution assays between melatonin and six classes of antibiotics were performed, including ampicillin (target cell wall), rifampicin (RNA synthesis), meropenem (cell wall), doxycycline (protein synthesis), ciprofloxacin (DNA synthesis) and colistin (membrane damage). Interestingly, we found that melatonin potentiated doxycycline, ciprofloxacin and colistin activities against mcr-1 carrying E. coli B2, but not other three antibiotics (Figure 1). Notably, melatonin displayed the highest synergistic activity with colistin (FICI = 0.063), accompanied by a 32-fold decrease in MIC values from 8 μg/mL to 0.25 μg/mL, which is below the clinical breakpoint (2 μg/mL, according to EUCAST 2017 and CLSI 2016) (Table S2). We next tested this synergistic effect in other mcr variants or notorious Gram-negative pathogens. As expected, we observed a significant synergy in another bacterium and mcr-3/mcr-8 carrying Enterobacteriaceae, suggesting a robust potentiation of melatonin with colistin against mcr-carrying Gram-negative pathogens (Figure S1 and Table S3). In addition, this synergistic activity was also evidenced in thirteen clinical colistin-resistant E. coli from elk (Figure S2).
Figure 1.
Checkerboard broth microdilution assays between different classes of antibiotics and melatonin against MCR-1 positive E. coli B2, related to Table S1. Dark blue regions represent higher bacterial cell density. The mean OD at 600 nm of two biological replicates is shown.
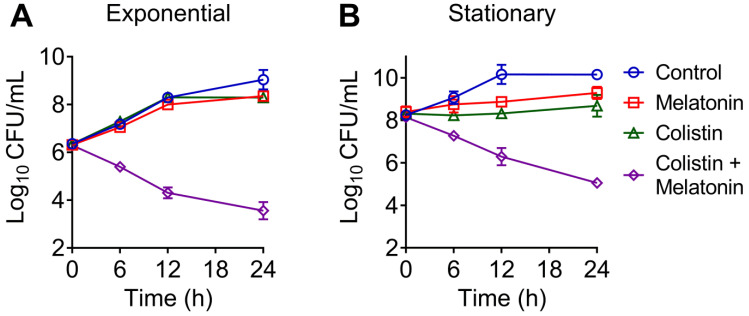
To further investigate their synergistic bactericidal activity, time-dependent killing curves for different growth phases of bacteria, including exponential and stationary E. coli, were determined. It has been demonstrated that many severe human and animal infections are caused by quiescent or slow-growing bacteria, which are difficult to treat by traditional regimens 39. We found that neither melatonin nor colistin monotreatment killed exponentially growing or stationary E. coli B2. In contrast, the combination led to a reduction of bacterial load approximate by 4-log10 in a growth phase-independent manner (Figure 2A and 2B). These results suggested that melatonin indeed drastically enhances colistin bactericidal activity against MCR-positive pathogens. In addition, we found a weak synergistic effect in MCR-negative pathogens such as E. coli MG1655 and E. coli ATCC 25922 (FICI = 0.375), and S. enterica ATCC 13076 (FICI = 0.14) (Table S3), implying that its mechanism is not limited to the inhibition of bacterial resistance.
Figure 2.
Time-dependent killing curve of E. coli (mcr-1) by the combination of melatonin and colistin. E. coli B2 were grown to exponential (A) and stationary (B) phase, and challenged with melatonin (1 mg/mL) and colistin (2 µg/mL) alone or their combination for 24 h. Data are representative of three independent experiments and shown as mean ± SD.
Safety and stability evaluation of colistin and melatonin combination
A critical factor that restricts the colistin application in the clinic is the potential toxicity effects including nephrotoxicity and neurotoxicity 40. To assess whether melatonin influences the toxicity of colistin, we analyzed hemolysis and cytotoxicity of colistin in the presence of melatonin. Encouragingly, we did not observe enhanced toxicity in the combination treatment. Instead we found that melatonin decreased the hemolytic activity of colistin at 128 μg/mL to RBCs by approximately 20% (Figure S3A), and slightly reduced the cytotoxicity of colistin in CHO and HEK293T cells (Figure S3B). We next evaluated the stability of this combination in the presence of serum, DMEM or different salt ions. It has been suggested that divalent cations (Mg2+ and Ca2+) are essential to bridge negative-charged phosphate groups between the LPS molecules, which helps to avoid the accumulation of repulsive forces and maintain the stability of the bacterial outer membrane 41. Melatonin retained its synergistic activity with colistin in the presence of 10% serum or DMEM (Table S2). In agreement with the assumed effects of divalent cations, we found that EDTA enhanced their synergistic activity against both E. coli B2 (mcr-1) and E. coli ATCC 25922, whereas Mg2+ and Ca2+ suppressed it, suggesting that the synergistic mechanisms of melatonin may be relevant to the disruption of the bacterial outer membrane (Figure S4 and Table S2). These data also imply that the joint use of colistin and melatonin is safe and stable.
Structure-activity relationship of melatonin
To gain further insights into the specific moieties of melatonin in enhancing colistin activity against resistant pathogens, we performed structure-activity relationship studies with melatonin and structurally similar molecules (Table 1). Specifically, we found that the indole moiety alone (7) has some synergistic activity, and the replacement of the indolic structure by an imidazole ring such as in histamine (8) drastically abolished any colistin potentiation, indicating that indole moiety is the basic chemical structure for synergistic activity of melatonin. In addition, the substitution of the ethyl-acetamido residue by 2-aminopropionic acid such as in 5-hydroxy-L-tryptophan (3) and L-tryptophan (4) strongly diminished the potentiation toward colistin. Deletion of the N-acetyl group as in 5-methoxytryptamine (6) retained its activity to colistin. Moreover, serotonin (1), tryptamine (2) and N-acetyl-5-hydrotryptamine (5) displayed synergistic activities similar to that of melatonin, indicating that a 5-methoxy group at ring atom 5 is not required for a synergistic effect on colistin. Consistent with this observation, several tryptamine derivatives were confirmed to sensitize colistin-resistant bacteria to colistin killing 42, 43.
Table 1.
Structure-activity relationship of melatonin with colistin against E. coli (mcr-1)
| Analogues | Chemical structure | MICa (μg/mL) | FIC index | MICb (μg/mL) | Potentiation (fold)c |
|---|---|---|---|---|---|
| Melatonin | 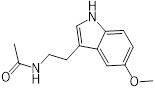 |
8 | 0.063 | 0.25 | 32 |
| 1 (Serotonin) |
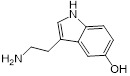 |
8 | 0.188 | 1 | 8 |
| 2 (Tryptamine) |
 |
8 | 0.156 | 0.25 | 32 |
| 3 (5-Hydroxy-L-tryptophan) |
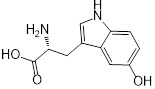 |
8 | 1 | 4 | 2 |
| 4 (L-tryptophan) |
 |
8 | 2 | 8 | 1 |
| 5 (N-acetyl-5-hydroxytryptamine) |
 |
8 | 0.094 | 0.25 | 32 |
| 6 (5-Methoxytryptamine) |
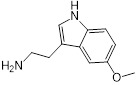 |
8 | 0.156 | 0.25 | 32 |
| 7 (Indole) |
 |
8 | 0.154 | 1 | 8 |
| 8 (Histamine) |
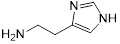 |
8 | >4 | >32 | - |
a/b MICs of colistin in the absence or presence of sub-MIC of melatonin and its analogues;
c Degree of colistin potentiation in the presence of sub-MIC of melatonin and its analogues.
Melatonin enhances the membrane-damaging ability of colistin
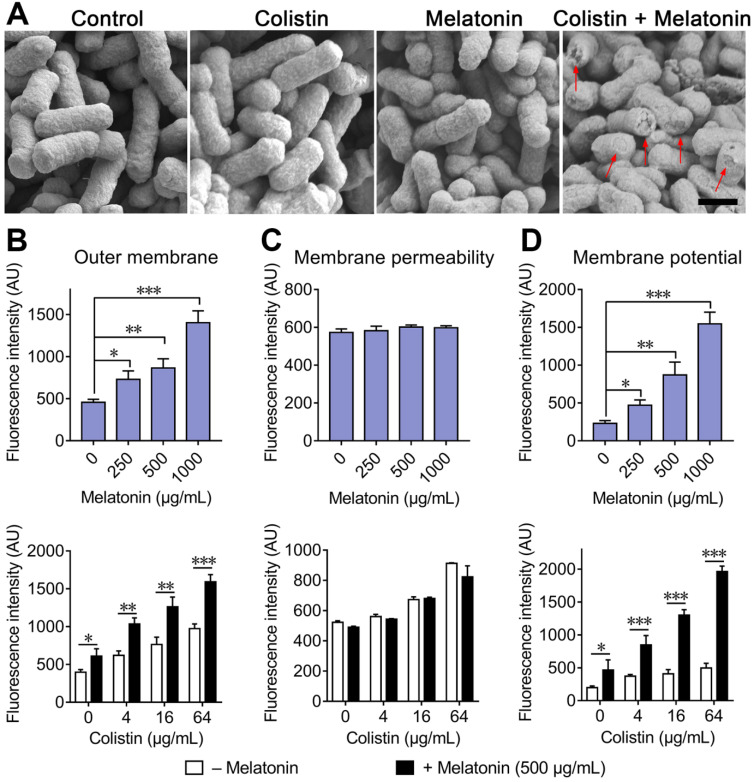
Colistin exhibits bactericidal activity against Gram-negative bacteria through specifically interacting with LPS in the bacterial outer membrane. Accordingly, colistin resistance is primarily related to modified LPS and decreased affinity between colistin and components of the bacterial outer membrane. Thus, we first speculated as to whether the addition of melatonin restores colistin ability on disruption of bacterial membrane. To validate our hypothesis, we investigated the morphological changes of E. coli treated by sub-MIC of colistin or melatonin and their combination by SEM analysis. Compared with the monotreatment, we observed a significant damage of the outer membrane in the combination group (Figure 3A). To further confirm this, we investigated the outer membrane permeability by means of 1-N-phenylnaphthylamine (NPN) (Figure 3B), membrane permeability by propidium iodide (PI) (Figure 3C) and the membrane potential using 3,3-dipropylthiadicarbocyanine iodide (DiSC3(5)) (Figure 3D) in E. coli B2 (mcr-1). Consistently, we found that the addition of melatonin significantly increased outer membrane permeability and caused dissipation of the cytoplasmic membrane potential, but had no effect on whole membrane permeability, indicating that the structural integrity of the inner membrane was largely maintained, although its functionality was affected. Taken together, these results demonstrated that melatonin potentiates colistin activity through enhancing the membrane-damaging ability of colistin.
Figure 3.
Melatonin potentiates the damage of colistin to bacterial membrane. (A) Morphological changes of E. coli B2 treated with sub-MIC of colistin or melatonin or their combination visualized with SEM. Scar bar, 0.5 µm. Destroyed outer membrane was marked by red arrows. (B) Melatonin permeabilizes the outer membrane, and enhances outer membrane disruption of colistin. Permeability was evaluated by measuring the fluorescence intensity of 1-N-phenylnaphthylamine (NPN) after 1 h exposure to either increasing concentrations of melatonin, colistin or colistin plus melatonin (500 µg/mL). (C) No effect on membrane permeability for propidium iodide (PI) in E. coli after treatment with melatonin. (D) Melatonin causes dissipation of membrane potential and drastically enhances colistin effects on membrane potential. Fluorescence dye DiSC3(5) was used to assess membrane potential changes induced by melatonin, colistin or combination. All experiments were performed with biological replicates and presented as mean ± SD. Unpaired t-test between two groups or one-way ANOVA among multiple groups were used to calculate P-values (*P < 0.05, **P < 0.01, ***P < 0.001).
Combination of colistin and melatonin promotes oxidative damage, prevents LPS modification and inactivates efflux pump
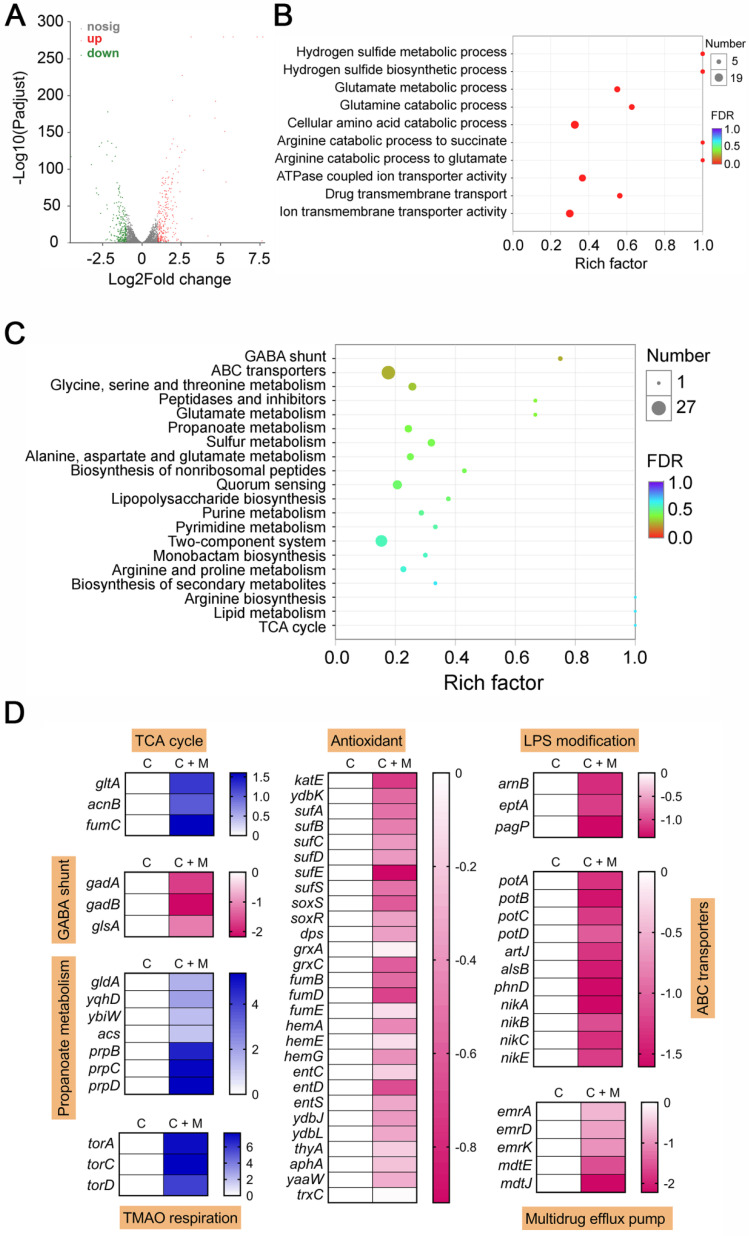
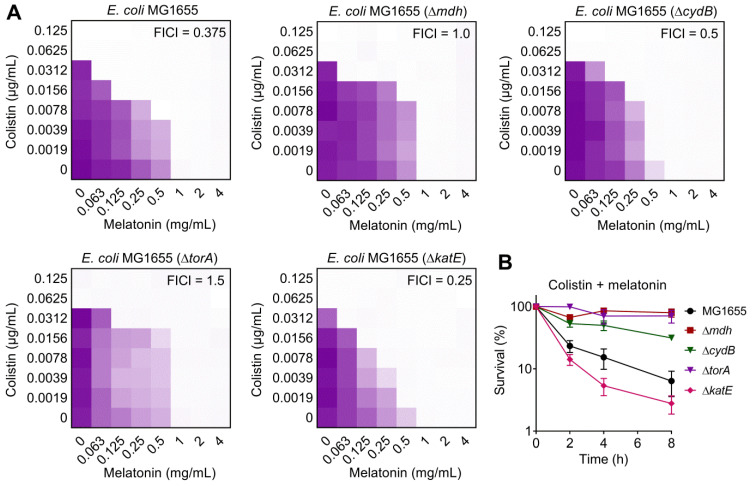
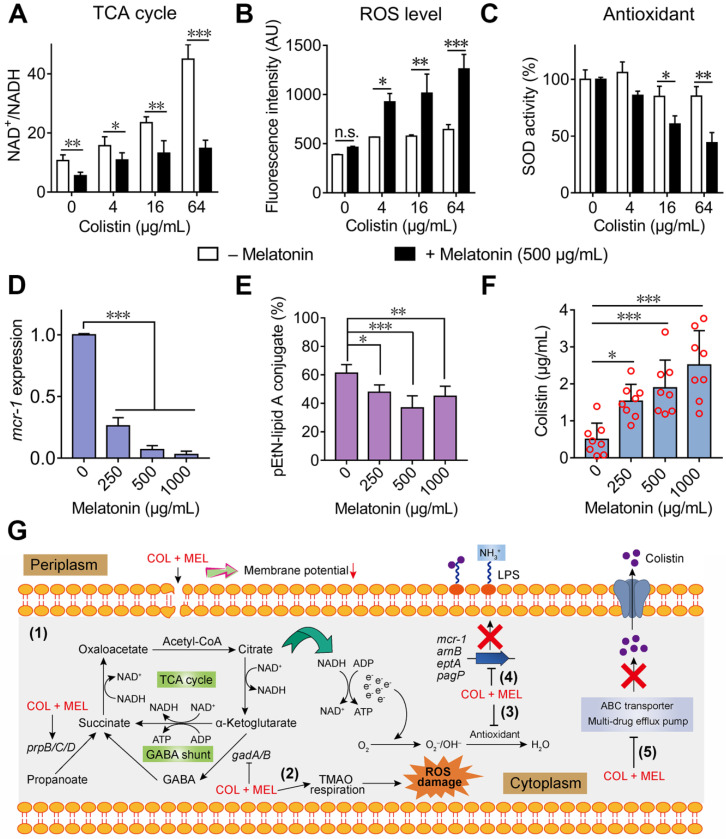
After having demonstrated that melatonin enhances membrane disruption by colistin, the clarification of specific molecular mechanisms is still required. Moreover, the reasons for their weak synergistic activity in mcr-negative pathogens remained unclear. To address these issues, we performed transcription analyses of E. coli (mcr-1) under treatment with colistin or colistin plus melatonin for 4 h. The comparison of treatment with combination to colistin alone revealed an up-regulation of 266 genes and down-regulation of 217 genes (>two-fold) (Figure 4A). Go and KEGG enrichment analysis showed that these differentially expressed genes (DEGs) were involved in GABA shunt, ABC transporters, two-component system, and bacterial metabolism related pathways (Figure 4B and 4C). Specifically, we found that the genes with increased expression were involved in TCA cycle, propanoate metabolism and TMAO respiration, and repressed gene expression in GABA shunt, antioxidant function, LPS modification, ABC transporters and multidrug efflux pumps (Figure 4D). Notably, these multidrug efflux pumps encoded by emr or mdt genes correlate with colistin resistance in Gram-negative bacteria 44. Expression profiling of representative genes by RT-PCR analysis was consistent with the transcription results (Figure S5). To verify the transcriptome results, we performed gene knockout experiments on related pathways using a reference strain E. coli MG1655 that is easily genetically manipulated. TCA cycle knockout strain (Δmdh), electron transport chain (ETC) knockout strain (ΔcydB), TMAO respiration knockout strain (ΔtorA) and antioxidant knockout strain (ΔkatE) were constructed through homologous recombination. Then, checkerboard broth microdilution assays (Figure 5A) and killing curves (Figure 5B) were performed. As a result, impaired synergistic activity of melatonin and colistin (FICI ≥ 0.5) were observed on Δmdh, ΔcydB and ΔtorA compared with wild type E. coli MG1655. However, ΔkatE was more sensitive to the combination treatment than wild type. These results suggested that TCA cycle, ETC, TMAO respiration and antioxidant in E. coli play a role in the synergistic activity of melatonin and colistin.
Figure 4.
Transcriptomic analysis of E. coli B2 treated by colistin or the combination of colistin plus melatonin. Volcano plot (A), GO (B) and KEGG enrichment analysis (C) of the differential expression genes (DEGs) in E. coli B2 after exposure to colistin or the combination of colistin plus melatonin. The x and y axis in A represent the expression changes and corresponding statistically significant degree, respectively. (D) Selected DEGs involved in TCA cycle, GABA shunt, propanoate metabolism, TMAO respiration, antioxidant response, LPS modification, ABC transporters and multidrug efflux pump. C, colistin alone; C + M, the combination of colistin and melatonin.
Figure 5.
Effect of deficiency in TCA cycle, electron transport chain, TMAO respiration and antioxidant on synergistic activity of melatonin and colistin against E. coli. (A) Checkerboard broth microdilution assays between melatonin and colistin against E. coli MG1655 and its gene knockout mutants (Δmdh, ΔcydB, ΔtorA and ΔkatE), related to Table S3. The mean OD at 600 nm of two biological replicates is presented. (B) Survival of E. coli MG1655 and its gene knockout mutants (Δmdh, ΔcydB, ΔtorA and ΔkatE) after the combination treatment of melatonin (1 mg/mL) and colistin (0.0625 µg/mL).
To further validate whether the colistin and melatonin combination accelerates the TCA cycle compared with colistin alone, the NAD+/NADH ratio in E. coli B2 was determined. Consistently, we found that the addition of melatonin significantly decreased the NAD+/NADH ratio, indicating an enhanced TCA cycle under combination treatment (Figure 6A). In bacteria, an accelerated TCA cycle is always accompanied by enhanced bacterial respiration and generation of ROS 45. In addition, the transcription analysis revealed that multiple pathways were involved in oxidant damage of E. coli. Therefore, we hypothesized that the combination of melatonin and colistin may result in enhanced oxidative damage. To that end, we first determined the generation of ROS and SOD activity by colistin in the absence and presence of sub-MIC of melatonin (500 μg/mL). Consequently, we found that the combination of colistin and melatonin drastically promoted the generation of total ROS (Figure 6B) and decreased SOD activity compared with colistin alone (Figure 6C). However, melatonin alone had no direct effect on the total ROS level and SOD activity (Figure S6A and S6B). In cells, ROS include superoxide (O2•-), hydrogen peroxide (H2O2) and hydroxyl radical (OH•). Interestingly, we also found that melatonin significantly promoted production of H2O2 in a dose-dependent manner, in both single or combination treatments (Figure S6C and S6D). Additionally, melatonin resulted in enhanced TMAO respiration, which also correlates with the generation of ROS 46. Taken together, we conclude that the combination of colistin and melatonin leads to increased ROS damage through promoting TCA cycle and TMAO respiration, and inhibiting the bacterial antioxidant system. Consistently, addition of ROS scavengers including N-acetylcysteine (NAC) 47 and thiourea 48 partially abolished the potentiation of melatonin to colistin (Table S2), indicating that ROS are involved in their synergistic activity.
Figure 6.
Synergistic molecular mechanisms of melatonin with colistin against E. coli. (A) Accelerated TCA cycle was observed under combination treatment of colistin and melatonin. (B) Melatonin supplementation significantly increases the production of ROS level induced by colistin, whereas it alone does not affect ROS levels. E. coli B2 was probed by 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) and exposed to colistin in the absence or presence of melatonin (500 µg/mL). After 1 h incubation, the fluorescence of DCF was measured. (C) Melatonin supplementation impairs the bacterial oxidative defenses when in combination with colistin. SOD activity in cells was measured by biochemical assay. (D and E) Melatonin inhibits expression of resistance gene mcr-1 (D) and thereby decreases the modification of lipid A by phosphoethanolamine (PE) through MCR-1 (E). The percent of pEtN-lipid A conjugate was determined based on LC-MS/MS analysis. (F) Melatonin enhances colistin intracellular accumulation, measured by LC-MS/MS analysis. (G) Scheme summarizing the synergistic mechanisms of colistin and melatonin. Combination treatment of colistin and melatonin enhances membrane damage and oxidative damage, inhibits the modification of lipid A and multidrug efflux pump. P values (*P < 0.05, **P < 0.01, ***P < 0.001) in (A-C) and (D-F) were determined by unpaired t-test between two groups or one-way ANOVA among multiple groups, respectively. All data are presented as mean ± SD.
Considering that the addition of melatonin inhibited the LPS modification related gene expression, as revealed by RNA sequencing (Figure 4D), we determined the mcr-1 expression upon treatment with melatonin with or without colistin. As expected, the mcr-1 expression in E. coli B2 was down-regulated in the presence of melatonin (Figure 6D). To investigate whether this inhibition could eventually prevent the LPS modification by phosphoethanolamine (PE), the proportion of modified-LPS in E. coli (mcr-1) under different concentrations of melatonin was measured by LC-MS/MS. Consistently, we observed a decreased pEtN-lipid A conjugate (1920.3 Da) in E. coli after melatonin treatment (Figure 6E). Since supplementation with melatonin down-regulated the ABC transporter and multidrug efflux pump, we hypothesized that melatonin may enhance intracellular colistin accumulation. To test this, we evaluated colistin concentrations in E. coli upon co-incubation with varying doses of melatonin. We found that melatonin indeed enhanced colistin in cells in a dose-dependent manner (Figure 6F). Collectively, these data demonstrated that melatonin enhances colistin activity by promoting oxidative damage, preventing LPS modification and efflux pump function (Figure 6G).
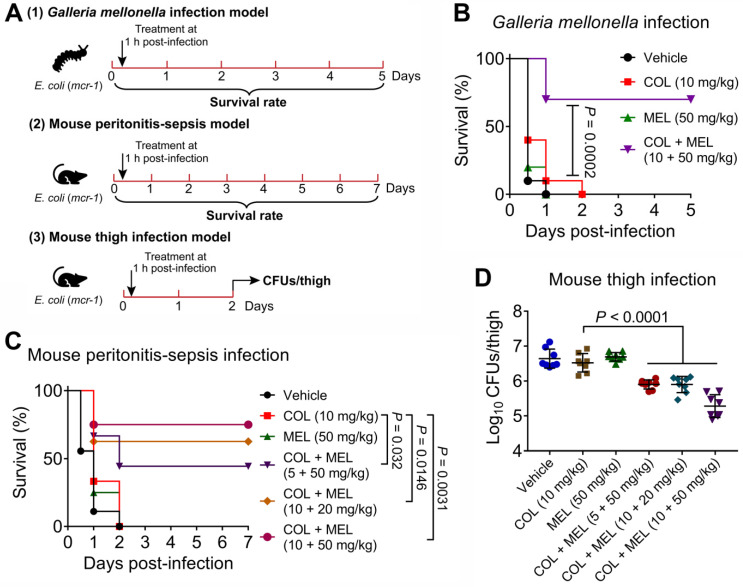
Melatonin restores colistin activity in vivo
Given that the combination of colistin and melatonin displayed excellent synergistic bactericidal activity against active and dormant pathogens in vitro, we reasoned that melatonin would reverse MCR-mediated colistin resistance in vivo and thereby restore its clinically efficacy. To that end, we first explored their pharmacokinetic characters after a single i.p. injection in mice. Consequently, we found that these two drugs exhibited similar serum drug concentration-time curves and pharmacokinetic parameters, e.g., Tmax and MRT, implying that they could make full use of their synergistic activity in vivo (Figure S7A and 7B). Then, we tested in vivo efficacy of this combination in three animal infection models (Figure 7A). In a Galleria mellonella infection model, insect larvae after infection by E. coli B2 (mcr-1) with PBS or colistin treatment all died within 48 hours. However, the combination therapy resulted in 70% survival, which was significantly higher than that obtained by the monotherapy (P = 0.0002) (Figure 7B). This survival advantage was also validated in a mouse peritonitis-sepsis model using E. coli B2 (mcr-1). Remarkably, although colistin or melatonin alone did not prevent a lethal infection by MCR-1-positive E. coli, a single dose of the combination treatments led to increased survival of mice at 7 days following infection (Figure 7C). Finally, this combination was tested in a neutropenic mouse thigh infection model. Similarly, three combinations of colistin and melatonin significantly reduced the bacterial load in mouse thigh muscle (P < 0.0001) compared with colistin monotherapy (Figure 7D). These data confirmed that melatonin dramatically rescues colistin activity in vivo.
Figure 7.
Melatonin rescues colistin activity in three animal infection models. (A) Scheme of the experimental protocols for three animal infection models. In three infection models, animals were infected by colistin-resistant E. coli B2 (mcr-1), and treated with a single dose of colistin, melatonin or their combination at one-hour post-infection. In the Galleria mellonella infection model (1), survival rate of larvae (n = 10 per group) was monitored during 5 days, related to Figure 7B. In the mouse peritonitis-sepsis infection model (2), the percentage of surviving mice (n = 8 or 9 per group) was recorded during 7 days, related to Figure 7C. In neutropenic mouse thigh infection model (3), thigh muscle bacterial loads in single and combination therapy (n = 8 per group) were counted at 2 days post-infection, related to Figure 7D. (B) Combination of colistin (10 mg/kg) and melatonin (50 mg/kg) significantly improved survival rate of G. mellonella larvae (n = 10 per group) infected by colistin-resistant E. coli (mcr-1) compared with colistin monotherapy (10 mg/kg). P values were determined by log-rank (Mantel-Cox) test. (C) Combination of colistin and melatonin profoundly increased survival rate of mice infected by colistin-resistant E. coli (mcr-1) during 7 days post-infection in a dose-dependent manner. BALB/c mice (n = 8 or 9 per group) were given a lethal dose of E. coli (3.0 × 108 CFUs), and treated with a single dose of colistin (10 mg/kg), melatonin (50 mg/kg), a combination of colistin plus melatonin (5 + 50 mg/kg, 10 + 20 mg/kg and 10 + 50 mg/kg), or PBS by intraperitoneal injection. P values were determined by log-rank (Mantel-Cox) test. (D) Decreased bacterial load in the neutropenic mouse thigh infection model by combination therapy. Neutropenic BALB/c mice (n = 8 per group) were intramuscularly given a non-lethal dose of E. coli (1.0 × 105 CFUs), and treated with a single dose of colistin (10 mg/kg), melatonin (50 mg/kg), a combination of colistin plus melatonin (5 + 50 mg/kg, 10 + 20 mg/kg and 10 + 50 mg/kg), or PBS by intraperitoneal injection. P values were determined by Mann-Whitney U test. Data are presented as mean ± SD.
Discussion
Infectious diseases caused by Gram-negative bacteria are a matter of global concern due to limited and ineffective treatments in the clinic 49. Despite the notion that colistin has been widely recognized as one of critical clinically relevant antibiotics against Gram-negative bacteria, MCR-mediated acquired colistin resistance severely diminishes its clinical efficacy. Therefore, the identification of potent adjuvants to rescue colistin activity is of great importance. Although multiple biofunctions of melatonin in prevention and treatment of diseases such as cardiac and brain ischemia-reperfusion injury 50, retinal neovascularization and neuroglial dysfunction 51, obesity 52, and breast cancer 53 have been demonstrated, its potential in bacterial diseases has not been fully explored. In this study, despite the weak antibacterial effect of melatonin at low dosage on bacteria 54, we unexpectedly found that melatonin exhibits the highest potentiation (8 to 32-fold) with colistin in resistant bacteria. Additionally, this activity is independent of bacterial species and resistance gene types. To our knowledge, this study is the first to employ the co-application of melatonin and colistin to treat infectious diseases caused by resistant bacteria.
Importantly, we found that the addition of melatonin slightly reduces the in vitro toxicity of colistin. An important reason that limits the clinical use of colistin is its neurotoxicity and nephrotoxicity in mammals 40. The discovery of novel detoxification agents for colistin is meaningful. For example, minocycline 55 and rapamycin 56 were found to attenuate colistin-induced neurotoxicity via suppression of oxidative stress and mitochondrial dysfunction. In particular, one study has indicated that melatonin (5 mg/kg) effectively attenuated colistin-induced nephrotoxicity in rats 57. However, the detailed detoxification mechanism of melatonin is still unknown.
In the experiments on their synergistic mechanisms, an intriguing phenotype is the discovery of the restored affinity between colistin and bacterial modified-LPS in the presence of melatonin, which may be an indispensable change that accounts for their synergistic activity. In-depth mechanistic analysis showed that melatonin potentiates colistin activity through multiple pathways, including the promotion of oxidative damage, inhibition of LPS modification by PE and deprivation of multidrug efflux pump functions. Notably, the promotional effect on ROS generation in bacteria appears to be inconsistent with the previous notion that melatonin alone possesses antioxidant activity in normal mammalian cells 58. There are several reasons that may account for this seemingly controversial phenomenon. First, melatonin alone would not stimulate the increase of total ROS level in E. coli. Increased ROS levels are only observed in combination treatment of the two drugs. Secondly, the antioxidant activity of melatonin strongly depends on the possible electron transfer reactions with the respective ROS. Thus, melatonin is an efficacious scavenger of OH• 59, and other free radicals that are capable of undergoing single-electron transfer reactions 60, but has less effect on O2•- and H2O2. By contrast, we found that melatonin significantly facilitates the production of H2O2. Of additional interest is that Aghdam et al. also found that melatonin treatment triggers H2O2 accumulation in strawberry fruits 61. Moreover, melatonin is known to exert prooxidant effects in mammalian tumor cells, in the context of its pro-apoptotic actions on transformed cells 62, 63. In both tumor and nontumor cells, melatonin turned to prooxidant behavior under conditions of apoptosis induction at highly elevated concentrations 64, 65. Collectively, these findings underline melatonin's potential of acting in a prooxidant manner. In the case of Gram-negative bacteria, we speculate that melatonin, when combined with colistin, enhances the antibiotic-induced oxidative damage by accelerating the TCA cycle and TMAO respiration, regardless of the partial clearance of total ROS. This mode of action partially explains why this synergistic activity is also applicable in mcr-negative bacteria. In addition, melatonin reduces the expression of LPS modification and multidrug efflux pump associated genes. Consistently, decreased pEtN-lipid A conjugate in E. coli (mcr-1) by melatonin is observed. This also explains our earlier observation that melatonin restored the membrane-damaging ability of colistin in resistant pathogens. Meanwhile, increased antibiotic accumulation in E. coli is found, which are necessary for antibiotic killing of Gram-negative bacteria 66. These findings on molecular mechanisms of melatonin undoubtedly provide a basis allowing screening for other, perhaps more effective antibiotic adjuvants. Nevertheless, in the future work, more in-depth studies on each synergistic pathway of melatonin are still required to provide a better understanding of its modes of action. In addition to these in vitro synergistic mechanisms, the beneficial effects of melatonin in immunomodulation 67 and anti-inflammation 68 that have been widely reported in mammals may contribute to improving the in vivo efficacy of this combination.
In conclusion, our data have shown that the FDA-approved melatonin exhibits a potent synergistic activity with several antibiotics, in particular, colistin against resistant pathogens both in vitro and in vivo. The discovery of melatonin as a novel colistin adjuvant highlights the huge potential of non-antibiotic agents against bacterial infectious diseases. We posit that melatonin and, perhaps, analogues thereof represent attractive lead compounds for antibiotic adjuvants to address the increasing threat by infections with colistin-resistant Gram-negative bacteria.
Supplementary Material
Supplementary figures and tables.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2018YFA0903400), National Natural Science Foundation of China (31872526, 31922079, 31872365 and 31790411), Natural Science Foundation of Jiangsu Province of China (BK20190893), China Postdoctoral Science Foundation funded project (2019M651984), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Lift Engineering of Young Talents of Jiangsu Association for Science and Technology.
Author contributions
YL and ZW designed the experiments; YL, YJ, KY, ZT and JS conducted the experiments; YL, YJ, KY, RL and XX analyzed the data, prepared the figures and tables, and drafted the manuscript; YL, WR, RH, RR and ZW revised and approved the final manuscript.
Abbreviations
- MCR
mobilized colistin resistance
- FDA
Food and Drug Administration
- MBLs
metallo-β-lactamase
- PBP2a
penicillin-binding protein 2a
- LPS
lipopolysaccharides
- MRSA
methicillin-resistant Staphylococcus aureus
- MHB
Mueller-Hinton Broth
- RBCs
red blood cells
- CHO
Chinese Hamster Ovary cell
- HEK293T
Human Embryonic Kidney cell
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- MIC
minimum inhibitory concentration
- FICI
fractional inhibitory concentration index
- RT-PCR
real-time reverse transcriptase-polymerase chain reaction
- GABA
γ-aminobutyric acid
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- OD
optical density
- MIC
minimum inhibitory concentration
- COL
colistin
- MEL
melatonin
References
- 1.Yelin I, Kishony R. Antibiotic Resistance. Cell. 2018;172:1136–1136. doi: 10.1016/j.cell.2018.02.018. e1131. [DOI] [PubMed] [Google Scholar]
- 2.Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 3. O'NEILL J. Tackling drug-resistant infections globally: final report and recommendations (Review on Antimicrobial Resistance, 2016)
- 4.Yahav D, Farbman L, Leibovici L, Paul M. Colistin: new lessons on an old antibiotic. Clin Microbiol Infect. 2012;18:18–29. doi: 10.1111/j.1469-0691.2011.03734.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Ding S, Shen J, Zhu K. Nonribosomal antibacterial peptides that target multidrug-resistant bacteria. Nat Prod Rep. 2019;36:573–592. doi: 10.1039/c8np00031j. [DOI] [PubMed] [Google Scholar]
- 6.Biswas S, Brunel J-M, Dubus J-C, Reynaud-Gaubert M, Rolain J-M. Colistin: an update on the antibiotic of the 21st century. Expert Rev Anti Infect Ther. 2012;10:917–934. doi: 10.1586/eri.12.78. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J. et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Zhang H, Liu Y-H, Feng Y. Towards understanding MCR-like colistin resistance. Trends Microbiol. 2018;26:794–808. doi: 10.1016/j.tim.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Wang R, Dorp LV, Shaw LP, Bradley P, Balloux F. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat Commun. 2018;9:1179. doi: 10.1038/s41467-018-03205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Li R, Xiao X, Wang Z. Antibiotic adjuvants: an alternative approach to overcome multi-drug resistant Gram-negative bacteria. Crit Rev Microbiol. 2019;45:301–314. doi: 10.1080/1040841X.2019.1599813. [DOI] [PubMed] [Google Scholar]
- 11.Tyers M, Wright GD. Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat Rev Microbiol. 2019;17:141–155. doi: 10.1038/s41579-018-0141-x. [DOI] [PubMed] [Google Scholar]
- 12.Douafer H, Andrieu V, Phanstiel Ot, Brunel JM. Antibiotic Adjuvants: make antibiotics great again! J Med Chem. 2019;62:8665–8681. doi: 10.1021/acs.jmedchem.8b01781. [DOI] [PubMed] [Google Scholar]
- 13.Wright GD. Antibiotic adjuvants: rescuing antibiotics from resistance. Trends Microbiol. 2016;24:862–871. doi: 10.1016/j.tim.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 14.King AM, Reid-Yu SA, Wang W, King DT, De Pascale G, Strynadka NC. et al. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature. 2014;510:503–506. doi: 10.1038/nature13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Fernandez E, Koch G, Wagner RM, Fekete A, Stengel ST, Schneider J. et al. Membrane microdomain disassembly inhibits MRSA antibiotic resistance. Cell. 2017;171:1354–1367. doi: 10.1016/j.cell.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stokes JM, MacNair CR, Ilyas B, French S, Cote JP, Bouwman C. et al. Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat Microbiol. 2017;2:17028. doi: 10.1038/nmicrobiol.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Liu S, Wang T, Li H, Tang S, Wang J. et al. Pterostilbene, a potential MCR-1 inhibitor that enhances the efficacy of polymyxin B. Antimicrob Agents Chemother. 2018;62:AAC.02146–02117. doi: 10.1128/AAC.02146-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macnair CR, Stokes JM, Carfrae LA, Fiebigcomyn AA, Coombes BK, Mulvey MR. et al. Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat Commun. 2018;9:458. doi: 10.1038/s41467-018-02875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daly SM, Sturge CR, Felder-Scott CF, Geller BL, Greenberg DE. MCR-1 inhibition with peptide-conjugated phosphorodiamidate morpholino oligomers restores sensitivity to polymyxin in Escherichia coli. mBio. 2017. 8. [DOI] [PMC free article] [PubMed]
- 20.Zhou Y, Wang J, Guo Y, Liu X, Liu S, Niu X. et al. Discovery of a potential MCR-1 inhibitor that reverses polymyxin activity against clinical mcr-1-positive Enterobacteriaceae. J Infect. 2019;78:364–372. doi: 10.1016/j.jinf.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Son SJ, Huang R, Squire CJ, Leung IKH. MCR-1: a promising target for structure-based design of inhibitors to tackle polymyxin resistance. Drug Discov Today. 2019;24:206–216. doi: 10.1016/j.drudis.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Hardeland R, Madrid JA, Tan DX, Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res. 2012;52:139–166. doi: 10.1111/j.1600-079X.2011.00934.x. [DOI] [PubMed] [Google Scholar]
- 23.Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93:350–384. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43:192–197. doi: 10.1161/01.HYP.0000113293.15186.3b. [DOI] [PubMed] [Google Scholar]
- 25.Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci USA. 1994;91:1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reiter RJ, Rosales-Corral SA, Tan D-X, Acuna-Castroviejo D, Qin L, Yang S-F. et al. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int J Mol Sci. 2017;18:843. doi: 10.3390/ijms18040843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia Y, Chen S, Zeng S, Zhao Y, Zhu C, Deng B. et al. Melatonin in macrophage biology: Current understanding and future perspectives. J Pineal Res. 2019;66:e12547. doi: 10.1111/jpi.12547. [DOI] [PubMed] [Google Scholar]
- 28.Hardeland R. Melatonin in plants-diversity of levels and multiplicity of functions. Front Plant Sci. 2016;7:198. doi: 10.3389/fpls.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen Y, Zhou H, Xu J, Wang Y, Zhang Q, Walsh TR. et al. Anthropogenic and environmental factors associated with high incidence of mcr-1 carriage in humans across China. Nat Microbiol. 2018;3:1054–1062. doi: 10.1038/s41564-018-0205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clinical Lab Standards Institute. Performance standards for antimicrobial susceptibility testing. CLSI. 2016.
- 31.Syal K, Mo M, Yu H, Iriya R, Jing W, Guodong S. et al. Current and emerging techniques for antibiotic susceptibility tests. Theranostics. 2017;7:1795–1805. doi: 10.7150/thno.19217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Jia Y, Yang K, Li R, Xiao X, Wang Z. Anti-HIV agent azidothymidine decreases Tet(X)-mediated bacterial resistance to tigecycline in Escherichia coli. Commun Biol. 2020;3:162. doi: 10.1038/s42003-020-0877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Ding S, Dietrich R, Märtlbauer E, Zhu K. A biosurfactant-inspired heptapeptide with improved specificity to kill MRSA. Angew Chem Int Ed. 2017;56:1486–1490. doi: 10.1002/anie.201609277. [DOI] [PubMed] [Google Scholar]
- 34.Ma L, Xie X, Liu H, Huang Y, Wu H, Jiang M. et al. Potent antibacterial activity of MSI-1 derived from the magainin 2 peptide against drug-resistant bacteria. Theranostics. 2020;10:1373–1390. doi: 10.7150/thno.39157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Song M, Ding S, Zhu K. Discovery of linear low-cationic peptides to target methicillin-resistant Staphylococcus aureus in vivo. ACS Infect Dis. 2019;5:123–130. doi: 10.1021/acsinfecdis.8b00230. [DOI] [PubMed] [Google Scholar]
- 36.Ejim L, Farha MA, Falconer SB, Wildenhain J, Coombes BK, Tyers M. et al. Combinations of antibiotics and nonantibiotic drugs enhance antimicrobial efficacy. Nat Chem Biol. 2011;7:348. doi: 10.1038/nchembio.559. [DOI] [PubMed] [Google Scholar]
- 37.Hamamoto H, Urai M, Ishii K, Yasukawa J, Paudel A, Murai M. et al. Lysocin E is a new antibiotic that targets menaquinone in the bacterial membrane. Nat Chem Biol. 2015;11:127–133. doi: 10.1038/nchembio.1710. [DOI] [PubMed] [Google Scholar]
- 38.WO C, PK N, JP R. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol. 1994;55:253–258. doi: 10.1002/jlb.55.2.253. [DOI] [PubMed] [Google Scholar]
- 39.Hurdle JG, O'neill AJ, Chopra I, Lee RE. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol. 2011;9:62. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velkov T, Dai C, Ciccotosto GD, Cappai R, Hoyer D, Li J. Polymyxins for Cns infections: Pharmacology and neurotoxicity. Pharmacol Ther. 2018;181:85–90. doi: 10.1016/j.pharmthera.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Clifton LA, Skoda MWA, Le Brun AP, Ciesielski F, Kuzmenko I, Holt SA. et al. Effect of divalent cation removal on the structure of Gram-negative bacterial outer membrane Models. Langmuir. 2015;31:404–412. doi: 10.1021/la504407v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephens MD, Hubble VB, Ernst RK, van Hoek ML, Melander RJ, Cavanagh J. et al. Potentiation of Francisella resistance to conventional antibiotics through small molecule adjuvants. MedChemComm. 2016;7:128–131. doi: 10.1039/C5MD00353A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minrovic BM, Hubble VB, Barker WT, Jania LA, Melander RJ, Koller BH. et al. Second-generation tryptamine derivatives potently sensitize colistin resistant bacteria to colistin. Acs Med Chem Lett. 2019;10:828–833. doi: 10.1021/acsmedchemlett.9b00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X-Z, Plésiat P, Nikaido H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev. 2015;28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meylan S, Porter CBM, Yang JH, Belenky P, Gutierrez A, Lobritz MA. et al. Carbon sources tune antibiotic susceptibility in Pseudomonas aeruginosa via tricarboxylic acid cycle control. Cell Chem Biol. 2017;24:195–206. doi: 10.1016/j.chembiol.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee K-M, Park Y, Bari W, Yoon MY, Go J, Kim SC. et al. Activation of cholera toxin production by anaerobic respiration of trimethylamine N-oxide in Vibrio cholerae. J Biol Chem. 2012;287:39742–39752. doi: 10.1074/jbc.M112.394932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang C F. Antibiotic and ROS linkage questioned. Nat Biotechnol. 2013;31:415–416. doi: 10.1038/nbt.2574. [DOI] [PubMed] [Google Scholar]
- 49.Peleg AY, Hooper DC. Hospital-acquired infections due to Gram-negative bacteria. N Engl J Med. 2010;362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou H, Ma Q, Zhu PJ, Ren J, Reiter RJ, Chen YD. Protective role of melatonin in cardiac ischemia-reperfusion injury: From pathogenesis to targeted therapy. J Pineal Res. 2018. 64. [DOI] [PubMed]
- 51.Xu Y, Lu X, Hu YG, Yang BY, Tsui CK, Yu SS, Melatonin attenuated retinal neovascularization and neuroglial dysfunction by inhibition of HIF-1 alpha-VEGF pathway in oxygen-induced retinopathy mice. J Pineal Res. 2018. 64. [DOI] [PubMed]
- 52.Cipolla-Neto J, Amaral F, Afeche S, Tan D, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014;56:371–381. doi: 10.1111/jpi.12137. [DOI] [PubMed] [Google Scholar]
- 53.Sánchez-Barceló EJ, Cos S, Mediavilla D, Martínez-Campa C, González A, Alonso-González C. Melatonin-estrogen interactions in breast cancer. J Pineal Res. 2005;38:217–222. doi: 10.1111/j.1600-079X.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 54.Tekbas OF, Ogur R, Korkmaz A, Kilic A, Reiter RJ. Melatonin as an antibiotic: new insights into the actions of this ubiquitous molecule. J Pineal Res. 2008;44:222–226. doi: 10.1111/j.1600-079X.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- 55.Dai CS, Ciccotosto GD, Cappai R, Wang Y, Tang SS, Xiao XL. et al. Minocycline attenuates colistin-induced neurotoxicity via suppression of apoptosis, mitochondrial dysfunction and oxidative stress. J Antimicrob Chemother. 2017;72:1635–1645. doi: 10.1093/jac/dkx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dai C, Ciccotosto GD, Cappai R, Wang Y, Tang S, Hoyer D. et al. Rapamycin confers neuroprotection against colistin-induced oxidative stress, mitochondria dysfunction, and apoptosis through the activation of autophagy and mTOR/Akt/CREB signaling pathways. ACS Chem Neurosci. 2018;9:824–837. doi: 10.1021/acschemneuro.7b00323. [DOI] [PubMed] [Google Scholar]
- 57.Yousef JM, Chen G, Hill PA, Nation RL, Li J. Melatonin attenuates colistin-induced nephrotoxicity in rats. Antimicrob Agents Chemother. 2011;55:4044–4049. doi: 10.1128/AAC.00328-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 59.Tan D-X. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr J. 1993;1:57–60. [Google Scholar]
- 60.Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27:119–130. doi: 10.1385/endo:27:2:119. [DOI] [PubMed] [Google Scholar]
- 61.Aghdam MS, Fard JR. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria× anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017;221:1650–1657. doi: 10.1016/j.foodchem.2016.10.123. [DOI] [PubMed] [Google Scholar]
- 62.Bejarano I, Espino J, Barriga C, Reiter RJ, Pariente JA, Rodríguez AB. Pro-oxidant effect of melatonin in tumour leucocytes: relation with its cytotoxic and pro-apoptotic effects. Basic Clin Pharmacol Toxicol. 2011;108:14–20. doi: 10.1111/j.1742-7843.2010.00619.x. [DOI] [PubMed] [Google Scholar]
- 63.Chen L, Liu L, Li Y, Gao J. Melatonin increases human cervical cancer HeLa cells apoptosis induced by cisplatin via inhibition of JNK/Parkin/mitophagy axis. In vitro Cell Dev-An. 2018;54:1–10. doi: 10.1007/s11626-017-0200-z. [DOI] [PubMed] [Google Scholar]
- 64.Bonmati-Carrion M, Álvarez-Sánchez N, Hardeland R, Madrid J, Rol M. A comparison of B16 melanoma cells and 3T3 fibroblasts concerning cell viability and ROS production in the presence of melatonin, tested over a wide range of concentrations. Int J Mol Sci. 2013;14:3901–3920. doi: 10.3390/ijms14023901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian T, Li J, Li Y, Lu YX, Tang YL, Wang H. et al. Melatonin enhances sorafenib-induced cytotoxicity in FLT3-ITD acute myeloid leukemia cells by redox modification. Theranostics. 2019;9:3768–3779. doi: 10.7150/thno.34327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Richter MF, Drown BS, Riley AP, Garcia A, Shirai T, Svec RL. et al. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature. 2017;545:299–304. doi: 10.1038/nature22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calvo JR, Rafii-EI-ldrissi M, Pozo D, Guerrero JM. Immunomodulatory role of melatonin: specific binding sites in human and rodent lymphoid cells. J Pineal Res. 1995;18:119–126. doi: 10.1111/j.1600-079x.1995.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 68.Najafi M, Shirazi A, Motevaseli E, Rezaeyan A, Salajegheh A, Rezapoor S. Melatonin as an anti-inflammatory agent in radiotherapy. Inflammopharmacology. 2017;25:403–413. doi: 10.1007/s10787-017-0332-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and tables.