ABSTRACT
A previous phase 3, randomized, multicenter study showed the immunogenicity of a primary vaccination of subjects aged 11 to 17 years with the quadrivalent meningococcal vaccine conjugated to tetanus toxoid (MenACWY-TT) or the quadrivalent meningococcal polysaccharide vaccine (MenACWY-PS). This extension study evaluated the safety and immunogenicity of a MenACWY-TT booster 10 years after receiving a primary dose of either MenACWY-TT or MenACWY-PS. The primary immunogenicity endpoint was booster response, evaluated using serum bactericidal antibody assays with rabbit complement (rSBA), 1 month postbooster. Safety endpoints included the percentage of subjects experiencing local and general adverse events (AEs) ≤4 days after MenACWY-TT booster. Of 229 subjects enrolled, 169 and 58 in the MenACWY-TT and MenACWY-PS groups, respectively, completed the booster phase. The 1 month postbooster response for each serogroup ranged from 81.5% to 95.7% for MenACWY-TT and 66.7% to 94.1% for MenACWY-PS. Similar percentages of MenACWY-TT and MenACWY-PS recipients had a booster response to serogroups A, W, and Y, whereas more MenACWY-TT recipients than MenACWY-PS recipients had a booster response to serogroup C. For the MenACWY-TT and MenACWY-PS groups, respectively, the MenACWY-TT booster elicited rSBA titers ≥1:8 in 100% and ≥98.0% of subjects across all serogroups; 100% and ≥96.1% of all subjects had titers ≥1:128. No new safety signals were observed during the booster phase. In conclusion, a MenACWY-TT booster dose after receiving either a primary dose of MenACWY-TT or MenACWY-PS elicited robust immune responses and was well tolerated. Functional antibody responses last up to 10 years after primary MenACWY-TT vaccination.
KEYWORDS: Antibody, booster, immunogenicity, MenACWY-TT, persistence, safety
Introduction
Neisseria meningitidis causes invasive meningococcal disease (IMD), a serious health threat globally.1 Case-fatality rates are approximately 15% and up to 20% of patients develop long-term sequelae.2
Quadrivalent meningococcal vaccines target 4 of the 5 most common disease-causing N meningitidis serogroups, A, C, W, and Y (MenACWY),1,3,4 and include the meningococcal conjugate vaccine MenACWY-TT (capsular polysaccharides from meningococcal serogroups A, C, W, and Y each conjugated to tetanus toxoid; Nimenrix®, Pfizer Ltd, Sandwich, UK)5 and the meningococcal polysaccharide vaccine MenACWY-PS (Mencevax®, GlaxoSmithKline, Rixensart, Belgium).6
Meningococcal vaccinations often are administered during childhood.7 However, waning immune responses to meningococcal conjugate vaccination in early childhood likely pose a challenge to protection during peak vulnerability at later adolescent ages without booster doses.8,9 In addition, individuals who receive the vaccine in early adolescence (aged 11–12 years) may require a booster dose at age 16 years to improve long-term vaccination protection.8 Previous meningococcal polysaccharide vaccination may also influence the immune response of a meningococcal conjugate vaccine when administered within the past 10 years.10,11 As polysaccharide vaccines do not induce anamnestic immune responses, they do not provide long-term protection against disease, whereas conjugate vaccines elicit complete maturation of B cells to produce immunologic memory.11
Given these nuances, a better understanding of the long-term impact of polysaccharide or conjugate primary vaccination on booster efficacy is important to effectively provide protection against IMD during age-related peaks in vulnerability. Therefore, an extension study was performed in subjects who had received 1 primary dose of either the conjugate vaccine MenACWY-TT or the polysaccharide vaccine MenACWY-PS as adolescents (aged 11–17 years). The objectives were to evaluate the safety and immunogenicity of a booster dose of MenACWY-TT administered approximately 10 years after the primary vaccination and to assess the long-term antibody persistence of this primary dose administered to subjects aged 11 to 17 years.
Materials and methods
Study design and participants
This phase 3b, open-label study (EudraCT number 2013-001512-29) is an extension of the primary study (NCT00464815), which was previously described.12 Briefly, the primary study was a phase 3, open-label, randomized, multicenter study conducted in 3 countries (India, the Philippines, and Taiwan) during 2007 to 2008; subjects 11 to 17 years of age received a primary dose of either MenACWY-TT or MenACWY-PS. Subjects from India and the Philippines were examined in a separate follow-up study (NCT00974363) at 2 years13 and then annually through 5 years14 after primary vaccination. Healthy subjects who completed the primary study were eligible to enroll in the current extension study, conducted only in the Philippines, according to their primary study vaccination group.
In the current study, a booster dose of MenACWY-TT was administered intramuscularly at Visit 1 (10 years postprimary vaccination) to all subjects in both study groups. Blood samples were taken from each subject before and 1 month after booster vaccination. Key inclusion criteria were for subjects to be considered healthy based on medical history and physical examination and to have completed the vaccination per protocol in the primary study. Key exclusion criteria included (i) use of any investigational or nonregistered drug or vaccine other than the study vaccine within 30 days before the study dose or planned use during the study period, (ii) chronic administration (>14 days total) of immunosuppressants or immune-modifying drugs within 6 months before vaccine dose, (iii) administration of immunoglobulins and/or blood products within 3 months before study vaccination or during the booster vaccination phase, (iv) confirmed or suspected immunosuppressive or immunodeficiency condition, (v) history of reaction or hypersensitivity to any component of the vaccine, (vi) acute disease and/or fever at the time of vaccination, and (vii) being pregnant, lactating, or planning to get pregnant or father a child.
This study was conducted according to good clinical practice and in accordance with the Declaration of Helsinki. The protocol and associated documents were reviewed and approved by local institutional review boards/independent ethics committees and approved by the Research Institute for Tropical Medicine Institutional Review Board, which has a level 3 accreditation from the Philippine Health Research Ethics Board. Written informed consent was obtained from all subjects prior to study entry.
Immunogenicity
The primary immunogenicity endpoint was booster response 1 month after booster vaccination, assessed with serum bactericidal antibody assays using rabbit complement (rSBA), to the meningococcal antigens (rSBA-MenA, rSBA-MenC, rSBA-MenW, and rSBA-MenY). Booster response to meningococcal serogroups A, C, W, and Y was defined as rSBA antibody titers ≥1:32 at 1 month after vaccination for initially seronegative subjects (prevaccination rSBA titer <1:8) or a ≥ 4-fold increase in rSBA titers from prevaccination to 1 month after vaccination in initially seropositive subjects (prevaccination rSBA titer ≥1:8).
The secondary immunogenicity endpoints included rSBA-MenA, rSBA-MenC, rSBA-MenW, and rSBA-MenY titers ≥1:8 and ≥1:128 and geometric mean titers (GMTs) at 1 month after booster vaccination. Anti-TT concentrations ≥0.1 IU/mL, ≥1.0 IU/mL, and geometric mean concentrations (GMCs) were also evaluated before and 1 month after booster vaccination. For long-term antibody persistence, rSBA-MenA, rSBA-MenC, rSBA-MenW, and rSBA-MenY titers ≥1:8 and ≥1:128 and GMTs were assessed at Year 10 before booster vaccination.
Safety
The safety endpoints were the percentage of subjects experiencing solicited local and general adverse events (AEs) ≤4 days (Days 0–3) after MenACWY-TT booster vaccination as follows: (i) percentage of subjects experiencing unsolicited AEs ≤31 days (Days 0–30) after MenACWY-TT booster vaccination, (ii) percentage of subjects experiencing serious AEs (SAEs), new onset of chronic illness (NOCI; asthma, autoimmune disorders, type 1 diabetes mellitus, allergies) or Guillain-Barré syndrome (GBS) ≤31 days (Days 0–30) after MenACWY-TT booster vaccination, and (iii) percentage of subjects experiencing SAEs associated with primary vaccination and events due to lack of vaccine efficacy between the subjects’ last primary study visit and the beginning of the current study.
Statistical analyses
Determination of sample size
This extension study was designed to enroll approximately 200 subjects. For the analysis at Visit 2 (ie, 1 month after booster vaccination given at Year 10), it was estimated that approximately 10% of subjects would not be evaluable. Approximately 180 subjects were expected to be included in the according-to-protocol (ATP) cohort for assessing immunogenicity of the booster dose (135 in the MenACWY-TT group and 45 in the MenACWY-PS group).
The booster total vaccinated cohort for safety comprised all subjects who were vaccinated in the primary study and had received a documented MenACWY-TT booster vaccine. The booster ATP cohort for immunogenicity included all subjects who (i) received a dose of MenACWY-TT or MenACWY-PS in the primary study and a MenACWY-TT booster dose, (ii) had not received a vaccine not specified or forbidden by protocol through the persistence phase, (iii) had blood sample assay results that were available for antibodies against ≥1 tested antigen at 1 month postbooster, and (iv) were not administered an unanticipated vaccine before the postbooster blood sample was taken.
The ATP cohort for antibody persistence at Year 10 included subjects who (i) received the primary vaccination, (ii) had available assay results for ≥1 antigen tested at Year 10, (iii) did not receive a meningococcal vaccine not specified by the protocol before Year 10, (iv) did not have a history of meningococcal disease before Year 10, (v) complied with the defined blood sampling intervals, (vi) did not have immunocompromising medical conditions, (vii) did not receive immune-modifying drugs or investigational vaccines during the study period, and (viii) were not excluded from the ATP cohort for immunogenicity in the primary study or the preceding ATP persistence cohorts unless for noncompliance with sampling timeframes or lack of available immunogenicity results at previous time points.
Postbooster immunogenicity analysis
Analysis of postbooster immunogenicity was based on the booster ATP cohort. At each blood sampling time point (ie, before and 1 month after booster vaccination), the percentages of subjects with an rSBA response or with titers/concentrations above cutoffs were calculated for each serogroup with 95% CIs. Exact 2-sided CIs were based on the observed percentage of subjects using the Clopper-Pearson method. For GMTs/GMCs, 95% CIs were calculated as back transformations of CIs based on the Student t distributions for the mean logarithm of the titers. No adjustments were made for multiplicity. Exploratory comparisons for differences between study groups in booster response 1 month after vaccination were also performed, with 2-sided 95% CIs calculated using the Miettinen-Nurminen method.
Safety analysis
The primary safety analysis was performed after booster dosing using the booster total vaccinated cohort. Safety results are presented as the percentage of subjects with ≥1 reported AE during the 4-day follow-up period. AEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) for adverse reaction terminology.
Results
Subject disposition
Of 229 subjects enrolled in this extension study, 169 and 58 subjects in the MenACWY-TT and MenACWY-PS groups, respectively, completed the booster phase (1 subject withdrew from each group; Table 1). The mean (SD) ages of subjects enrolled in the MenACWY-TT and MenACWY-PS groups were 24.2 (1.9) and 24.0 (2.0) years, respectively. Overall, 52.9% of subjects enrolled in the MenACWY-TT group and 61.0% in the MenACWY-PS group were male. All subjects were of Asian or Southeast Asian race.
Table 1.
Subject demographics in the MenACWY-TT booster phase (booster total vaccinated cohort).
| Demographic | Primary MenACWY-TT | Primary MenACWY-PS |
|---|---|---|
| Total enrolled, n | 170 | 59 |
| Total completed visits, n | 169 | 58 |
| Sex, n (%) | ||
| Male | 90 (52.9) | 36 (61.0) |
| Female | 80 (47.1) | 23 (39.0) |
| Age at enrollment, y | ||
| Mean (SD) | 24.2 (1.9) | 24.0 (2.0) |
| Median (range) | 24.0 (21–27) | 24.0 (21–28) |
| Race, n (%) | ||
| Asian/Southeast Asian | 170 (100) | 59 (100) |
| Time since last vaccination at each visit | ||
| Primary (Visit 1), weeks, mean (SD) | 530.2 (8.3) | 531.5 (9.2) |
| Booster (Visit 2), weeks, mean (SD) | 4.8 (0.6) | 4.7 (0.4) |
MenACWY-PS = quadrivalent meningococcal polysaccharide vaccine; MenACWY-TT = quadrivalent meningococcal tetanus toxoid conjugate vaccine; SD = standard deviation.
Booster response
Among MenACWY-TT recipients, 81.5% to 95.7% had rSBA booster responses against each serogroup 1 month after vaccination (Figure 1). Among MenACWY-PS recipients, rSBA responses were 66.7% to 94.1% across serogroups. Exploratory analyses revealed similar percentages of MenACWY-TT and MenACWY-PS recipients had a booster response to sero-groups A, W, and Y, whereas more MenACWY-TT than MenACWY-PS recipients had a booster response to serogroup C (88.3% vs 66.7%).
Figure 1.
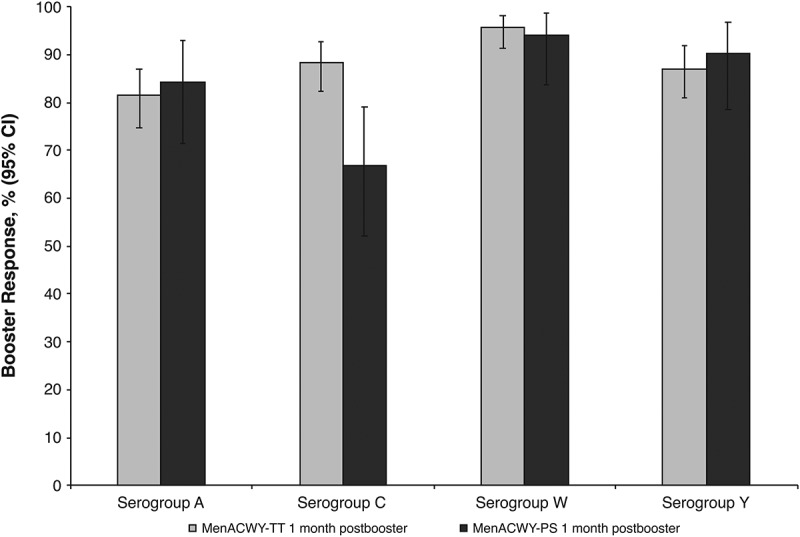
Percentages of subjects with booster responses for serogroups A, C, W, and Y at 1 month after booster dose of MenACWY-TT (booster ATP cohort). ATP = according-to-protocol; MenACWY-PS = quadrivalent meningococcal polysaccharide vaccine; MenACWY-TT = quadrivalent meningococcal tetanus toxoid conjugate vaccine.
For the MenACWY-TT and MenACWY-PS groups, the MenACWY-TT booster dose respectively elicited rSBA titers ≥1:8 in 100% and ≥98.0% of subjects across all serogroups (Figure 2a); 100% and ≥96.1% of all subjects had titers ≥1:128 (Figure 2b). For all serogroups, rSBA GMTs at 1 month after the booster dose were higher than before the booster dose (Table 2). For the MenACWY-TT and MenACWY-PS groups, 98.8% and 96.1% of subjects, respectively, had anti-TT concentrations ≥0.1 IU/mL at 1 month after the booster dose (Table 3).
Figure 2.
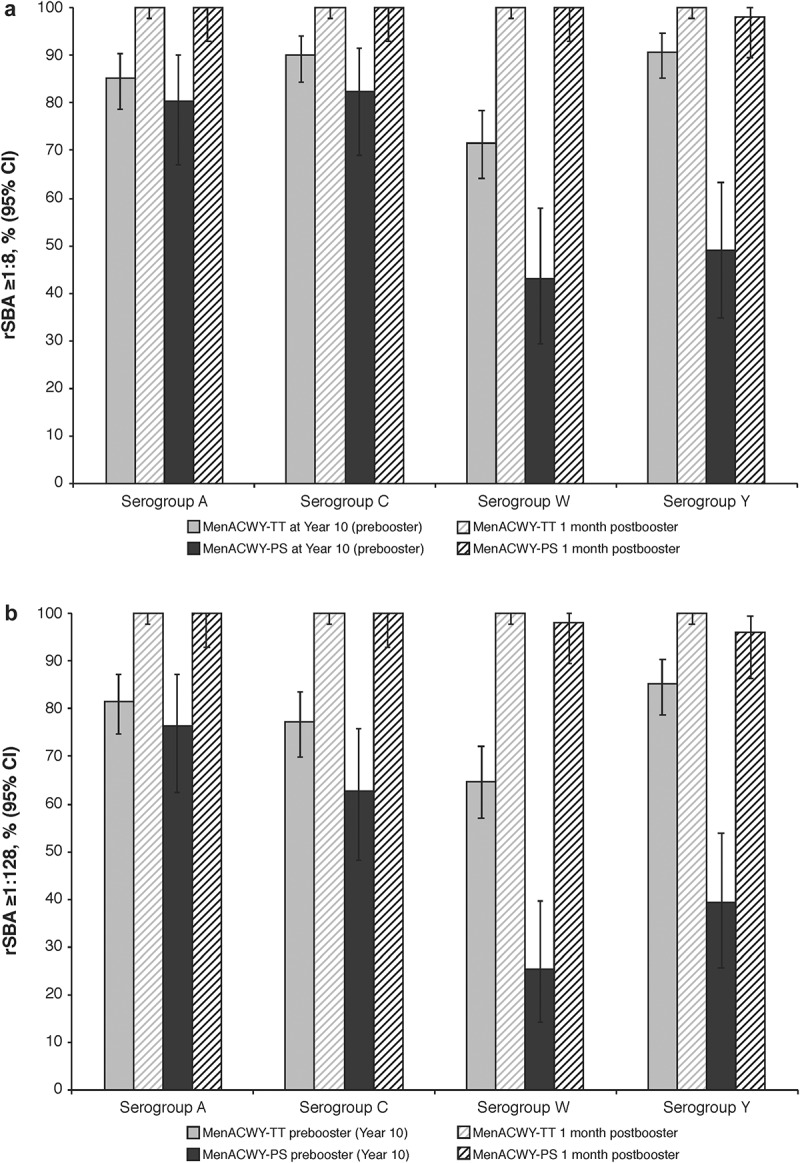
Percentages of subjects with rSBA titers (A) ≥1:8 and (B) ≥1:128 at Year 10 (before booster) and at 1 month after a booster dose of MenACWY-TT (booster ATP cohort). ATP = according-to-protocol; MenACWY-PS = quadrivalent meningococcal polysaccharide vaccine; MenACWY-TT = quadrivalent meningococcal tetanus toxoid conjugate vaccine; rSBA = serum bactericidal antibody assay using baby rabbit complement.
Table 2.
GMTs for serogroups A, C, W, and Y before and 1 month after a booster dose of MenACWY-TT (booster ATP cohort).
| N | Prebooster GMT (95% CI) | 1 Month Postbooster GMT (95% CI) | |
|---|---|---|---|
| Serogroup A | |||
| MenACWY-TT | 162 | 248.4 (181.4–340.2) | 3760.1 (3268.3–4325.9) |
| MenACWY-PS | 51 | 142.7 (80.5–252.9) | 2956.0 (2040.5–4282.1) |
| Serogroup C | |||
| MenACWY-TT | 162 | 244.2 (181.6–328.5) | 8697.7 (7391.2–10,235.1) |
| MenACWY-PS | 51 | 177.4 (86.1–365.3) | 3879.3 (2714.6–5543.7) |
| Serogroup W | |||
| MenACWY-TT | 162 | 145.5 (97.6–217.1) | 11,243.4 (9366.8–13,496.0) |
| MenACWY-PS | 51 | 16.4 (9.2–29.4) | 3674.0 (2353.9–5734.4) |
| Serogroup Y | |||
| MenACWY-TT | 162 | 446.5 (332.7–599.1) | 7584.8 (6748.4–8524.7) |
| MenACWY-PS | 51 | 32.9 (17.1–63.3) | 3295.5 (1998.7–5433.7) |
ATP = according to protocol; GMT = geometric mean titer; MenACWY-PS = quadrivalent meningococcal polysaccharide vaccine; MenACWY-TT = quadrivalent meningococcal tetanus toxoid conjugate vaccine.
Table 3.
Percentage of subjects with anti-tetanus toxoid concentrations ≥0.1 IU/mL and ≥1.0 IU/mL and GMCs before and 1 month after booster dose of MenACWY-TT (booster ATP cohort).
| Prebooster | 1 Month Postbooster | |
|---|---|---|
| MenACWY-TT | ||
| ≥0.1 IU/mL, n/N (%; 95% CI) | 140/162 (86.4; 80.2–91.3) | 160/162 (98.8; 95.6–99.9) |
| ≥1.0 IU/mL, n/N (%; 95% CI) | 62/162 (38.3; 30.8–46.2) | 149/162 (92.0; 86.7–95.7) |
| GMC (95% CI) | 0.608 (0.476–0.775) | 5.057 (4.274–5.984) |
| MenACWY-PS | ||
| ≥0.1 IU/mL, n/N (%; 95% CI) | 28/51 (54.9; 40.3–68.9) | 49/51 (96.1; 86.5–99.5) |
| ≥1.0 IU/mL, n/N (%; 95% CI) | 14/51 (27.5; 15.9–41.7) | 42/51 (82.4; 69.1–91.6) |
| GMC (95% CI) | 0.252 (0.154–0.411) | 5.115 (3.073–8.514) |
ATP = according to protocol; GMC = geometric mean concentration; MenACWY-PS = quadrivalent meningococcal polysaccharide vaccine; MenACWY-TT = quadrivalent meningococcal tetanus toxoid conjugate vaccine; N = number of subjects; n = number of subjects in a given category.
Long-term persistence of primary dose (year 10)
At Visit 1 (Year 10), responses in the ATP cohort for persistence ranged from 71.8% to 90.8% and 65.0% to 85.3% for rSBA titers ≥1:8 and ≥1:128, respectively, across all serogroups among subjects who had received MenACWY-TT primary vaccination, whereas corresponding percentages ranged from 41.5% to 83.0% and 24.5% to 77.4% for MenACWY-PS recipients. These percentages were similar for prebooster rSBA titers ≥1:8 and ≥1:128 at Year 10 in the booster ATP cohort, which ranged from 71.6% to 90.7% and 64.8% to 85.2% (MenACWY-TT) for all serogroups, respectively, and 43.1% to 82.4% and 25.5% to 76.5% (MenACWY-PS; Figure 2a,b).
The rSBA GMTs across serogroups in the ATP cohort for persistence were higher in the MenACWY-TT group than in the MenACWY-PS group at Year 10, with GMTs ranging from 146.0 to 446.9 in the MenACWY-TT group and 15.6 to 182.2 for the MenACWY-PS group. Similar results were observed in the booster ATP cohort (Table 2). Furthermore, both the GMC and the percentage of subjects in the booster ATP cohort with anti-TT concentrations ≥0.1 IU/mL or ≥1.0 IU/mL were higher in the MenACWY-TT group than in the MenACWY-PS group at Year 10 (Table 3).
Safety
No new safety signals were observed during the booster phase. Solicited and unsolicited AEs were reported by 80 subjects (47.1%) in the MenACWY-TT group and 27 subjects (45.8%) in the MenACWY-PS group in the 4-day period after booster vaccination. Grade 3 (ie, severe) solicited and unsolicited AEs were reported by 3 subjects (1.8%) in the MenACWY-TT group and in 0 subjects in the MenACWY-PS group in the 4-day period after booster vaccination (Figure 3). Pain and headache were the most reported solicited local and general AEs, respectively, in the 4-day period after booster vaccination. No SAEs, NOCIs, or cases of GBS were reported after booster vaccination during the study period. No subjects reported SAEs, including meningococcal disease, between the last primary study visit and the beginning of the current study.
Figure 3.
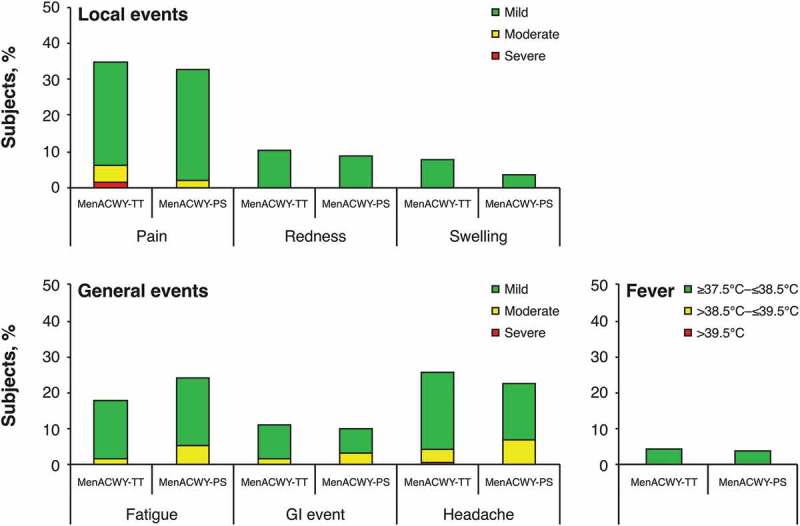
Percentages of subjects reporting solicited local and general events during 4-day follow-up to booster vaccination (booster total vaccinated cohort). Intensity scale (mild, moderate, or severe) was classified by grade 1, 2, or 3, respectively, for pain, fatigue, GI event, and headache; and 0–≤20 mm, >20–≤50 mm, or >50 mm for redness and swelling. GI = gastrointestinal; MenACWY-PS = quadrivalent meningococcal polysaccharide vaccine; MenACWY-TT = quadrivalent meningococcal tetanus toxoid conjugate vaccine.
Discussion
In this study, MenACWY-TT booster vaccination 10 years after primary MenACWY-TT or MenACWY-PS vaccination in adolescents elicited a robust immune response in all subjects across all serogroups. Exploratory comparisons showed a similar percentage of MenACWY-TT and MenACWY-PS recipients had a booster response to serogroups A, W, and Y, whereas a larger percentage of MenACWY-TT subjects had a booster response to serogroup C. This finding is in contrast to that from the primary vaccination study, which found significantly higher postvaccination rSBA GMTs against all serogroups after MenACWY-TT versus MenACWY-PS vaccination.12 Reasons underlying this difference remain unclear, but one possibility is that the differences may reflect subjects’ exposure to serogroup C over the 10 years after the primary dose, although the current study did not specifically investigate this possibility.
Some retrospective studies suggest that persistence of antibody responses diminishes within 3 to 5 years after vaccination with the meningococcal conjugate vaccines MenACWY-D (Menactra®, Sanofi Pasteur Inc, Swiftwater, PA, USA) and MenACWY-CRM (Menveo®, GlaxoSmithKline Vaccines, Sovicille, Italy).2,8 Other long-term follow-up studies of primary vaccination with MenACWY conjugate vaccines suggest that the antibody responses persist up to 5 years after the first dose in adolescents and young adults.14-19 However, few studies examined the antibody responses to primary vaccination with meningococcal conjugate vaccines over a longer period. In this study, we found that functional antibody responses persisted up to 10 years after primary MenACWY-TT and MenACWY-PS vaccinations. In general, a larger percentage of subjects who received MenACWY-TT for primary vaccination showed long-term persistence compared with those who received MenACWY-PS. The current study results are consistent with those of other reports regarding long-term antibody persistence for MenACWY-TT up to 10 years from primary vaccination in adolescents and adults.20
Evidence suggests that the type of meningococcal vaccine used for primary vaccination may influence the subsequent immune response to meningococcal conjugate vaccine boosters if administered within 10 years of each other.10,11 Individuals primed with polysaccharide meningococcal vaccines seem to have less robust immune responses to booster vaccination with meningococcal conjugate vaccines compared with those who are initially primed with the conjugate vaccine.10,11 In our study, no clinically relevant differences in antibody responses were apparent after the booster vaccination between subjects who initially received MenACWY-TT or MenACWY-PS. Thus, whether the booster response potentially benefits from priming with a conjugate vaccine remains unclear. Additional data are needed to answer this question.
No new safety signals with MenACWY-TT vaccination were observed in this study. The most commonly reported local and general solicited AEs after booster vaccination were pain and headache, respectively, and no SAEs were reported through Year 10. These results are consistent with those of the primary study, which also found pain at the injection site to be the most common local reaction, occurring at similar rates between MenACWY-TT and MenACWY-PS recipients.
This study has important implications for improving long-term vaccine protection in adolescents and young adults. Because adolescents and young adults have the highest meningococcal carriage rates among age-based populations, they are an important target group for meningococcal vaccination to reduce transmission; IMD also peaks in this age group.21,22 Our study results showed long-term antibody persistence up to 10 years after the primary MenACWY-TT vaccination in this age group, suggesting that vaccination in adolescents and young adults may offer protection against this peak in disease incidence and ultimately reduce carriage rates among these individuals. Further, our study demonstrates most participants will be protected against infection after a booster MenACWY-TT vaccination across all serogroups, regardless of primary meningococcal vaccination received (Figure 2; 98%–100% of subjects had rSBA titers ≥1:8 postbooster vs 43.1%–90.7% prebooster), suggesting a booster vaccination program could further lower the burden of IMD.
Strengths of our study are that it assessed long-term immunogenicity for up to 10 years after primary vaccination with either MenACWY-TT or MenACWY-PS and that a high percentage of subjects returned for the long-term persistence evaluation. However, a limitation of the study was the small sample size and generalizability of the results to other populations may be limited because a single study population from the Philippines was used. In addition, as previously described,14 changes to the rSBA assay methodology 3 years after the primary study limit direct comparisons between the immunogenicity results presented here and those of earlier time points.
Conclusions
A booster dose of MenACWY-TT elicited robust immune responses and was well tolerated. Functional antibody responses persisted after primary MenACWY-TT vaccination up to 10 years. These results in adolescents and young adults provide important insights for optimizing the long-term protection from primary meningococcal vaccination and recognizing the benefits of booster dosing for those who are at high risk of IMD.
Acknowledgments
The authors wish to thank all study participants from the Philippines. The authors also acknowledge the study site staff, particularly those who were involved with the study since the primary vaccination, for their excellent tracking of the study participants over 10 years. Editorial/medical writing support was provided by Kim Kridsada, PhD, at Complete Healthcare Communications, LLC (North Wales, PA, USA), a CHC Group Company, and was funded by Pfizer Inc.
Funding Statement
This study was sponsored by Pfizer Inc.
Disclosure of potential conflicts of interest
Drs Peyrani, Li, Cutler, Perez, and Webber are employees of Pfizer Inc. Dr Van Der Wielen is an employee of GlaxoSmithKline. Dr Quiambao has no conflict of interest to disclose.
Data sharing statement
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
- 1.Harrison LH. Epidemiological profile of meningococcal disease in the United States. Clin Infect Dis. 2010;50(suppl 2):S37–44. doi: 10.1086/648963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . Chapter 14: meningococcal disease. Public Health Foundation; [accessed 2020 January28] https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/mening.pdf.
- 3.MacNeil J, Cohn A. Chapter 8: meningococcal disease. Manual for the surveillance of vaccine-preventable diseases. 5th ed. Atlanta (GA): Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 4.Borrow R, Alarcon P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echaniz-Aviles G, Findlow J, Head C, et al. The global meningococcal initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16(4):313–28. doi: 10.1080/14760584.2017.1258308. [DOI] [PubMed] [Google Scholar]
- 5.Nimenrix®: EPAR - product information . Summary of product characteristics. Sandwich (UK): Pfizer Ltd; 2019. [Google Scholar]
- 6.Mencevax® ACWY (meningococcal ACWY vaccine) . Full prescribing information. Rixensart (Belgium): GlaxoSmithKline Biologicals; 2015. [Google Scholar]
- 7.Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meer HC, Baker CJ, Messonnier NE. . Prevention and control of meningococcal disease: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep; [accessed 2020 February3] https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6202a1.htm. [PubMed]
- 8.Cohn AC, MacNeil JR, Harrison LH, Lynfield R, Reingold A, Schaffner W, Zell ER, Plikaytis B, Wang X, Messonnier NE, et al. Effectiveness and duration of protection of one dose of a meningococcal conjugate vaccine. Pediatrics. 2017;139(2):e20162193. doi: 10.1542/peds.2016-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vesikari T, Forsten A, Bianco V, Van der Wielen M, Miller JM. Immunogenicity, safety and antibody persistence of a booster dose of quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine compared with monovalent meningococcal serogroup C vaccine administered four years after primary vaccination using the same vaccines. Pediatr Infect Dis J. 2015;34(12):e298–307. doi: 10.1097/INF.0000000000000897. [DOI] [PubMed] [Google Scholar]
- 10.Keyserling H, Papa T, Koranyi K, Ryall R, Bassily E, Bybel MJ, Sullivan K, Gilmet G, Reinhardt A. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adolesc Med. 2005;159(10):907–13. doi: 10.1001/archpedi.159.10.907. [DOI] [PubMed] [Google Scholar]
- 11.Dbaibo G, Van der Wielen M, Reda M, Medlej F, Tabet C, Boutriau D, Sumbul A, Anis S, Miller JM. The tetravalent meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine is immunogenic with a clinically acceptable safety profile in subjects previously vaccinated with a tetravalent polysaccharide vaccine. Int J Infect Dis. 2012;16(8):e608–15. doi: 10.1016/j.ijid.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Bermal N, Huang LM, Dubey A, Jain H, Bavdekar A, Lin TY, Bianco V, Baine Y, Miller JM. Safety and immunogenicity of a tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine in adolescents and adults. Hum Vaccin. 2011;7(2):239–47. doi: 10.4161/hv.7.2.14068. [DOI] [PubMed] [Google Scholar]
- 13.Quiambao BP, Jain H, Bavdekar A, Dubey AP, Kolhe D, Bianco V, Van der Wielen M, Miller JM. Persistence of the immune response two years after vaccination with quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine (MenACWY-TT) in Asian adolescents. Hum Vaccin Immunother. 2016;12(8):2162–68. doi: 10.1080/21645515.2016.1163455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quiambao BP, Bavdekar A, Dubey AP, Jain H, Kolhe D, Bianco V, Miller JM, Van der Wielen M. Antibody persistence up to 5 y after vaccination with a quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine in adolescents. Hum Vaccin Immunother. 2017;13(3):636–44. doi: 10.1080/21645515.2016.1248009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baxter R, Keshavan P, Welsch JA, Han L, Smolenov I. Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants. Hum Vaccin Immunother. 2016;12(5):1300–10. doi: 10.1080/21645515.2015.1136040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baxter R, Baine Y, Kolhe D, Baccarini CI, Miller JM, Van der Wielen M. Five-year antibody persistence and booster response to a single dose of meningococcal A, C, W and Y tetanus toxoid conjugate vaccine in adolescents and young adults: an open, randomized trial. Pediatr Infect Dis J. 2015;34(11):1236–43. doi: 10.1097/INF.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 17.Baxter R, Reisinger K, Block SL, Izu A, Odrljin T, Dull P. Antibody persistence and booster response of a quadrivalent meningococcal conjugate vaccine in adolescents. J Pediatr. 2014;164(6):1409–1415.e1404. doi: 10.1016/j.jpeds.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Ostergaard L, Van der Wielen M, Bianco V, Miller JM. Persistence of antibodies for 42 months following vaccination of adolescents with a meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine (MenACWY-TT). Int J Infect Dis. 2013;17(3):e173–76. doi: 10.1016/j.ijid.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson RM, Jackson LA, Reisinger K, Izu A, Odrljin T, Dull PM. Antibody persistence and response to a booster dose of a quadrivalent conjugate vaccine for meningococcal disease in adolescents. Pediatr Infect Dis J. 2013;32(4):e170–77. doi: 10.1097/INF.0b013e318279ac38. [DOI] [PubMed] [Google Scholar]
- 20.Peyrani P, Webber C, Van Der Wielen M, Cheuvart B, De Schrevel N, Bianco V, Aris E, Cutler M, Li P, Perez JL.. Long-term antibody persistence after primary vaccination with MenACWY-TT and immunogenicity of a booster dose in individuals aged 11–55 years. Paper presented at: 37th Annual Meeting of the European Society of Paediatric Infectious Diseases; 2019. May 6–11; Ljubljana (Slovenia). [Google Scholar]
- 21.National Center for Immunization and Respiratory Diseases Office of Infectious Diseases. Enhanced meningococcal disease surveillance report, 2017 (CS283195). Atlanta (GA): Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 22.Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–61. doi: 10.1016/S1473-3099(10)70251-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Centers for Disease Control and Prevention . Chapter 14: meningococcal disease. Public Health Foundation; [accessed 2020 January28] https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/mening.pdf.


