Abstract
Mutations in human metabolic genes can lead to rare diseases known as inborn errors of human metabolism. For instance, patients with loss-of-function mutations in either subunit of propionyl-CoA carboxylase suffer from propionic acidemia because they cannot catabolize propionate, leading to its harmful accumulation. Both the penetrance and expressivity of metabolic disorders can be modulated by genetic background. However, modifiers of these diseases are difficult to identify because of the lack of statistical power for rare diseases in human genetics. Here, we use a model of propionic acidemia in the nematode Caenorhabditis elegans to identify genetic modifiers of propionate sensitivity. Using genome-wide association (GWA) mapping across wild strains, we identify several genomic regions correlated with reduced propionate sensitivity. We find that natural variation in the putative glucuronosyltransferase GLCT-3, a homolog of human B3GAT, partly explains differences in propionate sensitivity in one of these genomic intervals. We demonstrate that loss-of-function alleles in glct-3 render the animals less sensitive to propionate. Additionally, we find that C. elegans has an expansion of the glct gene family, suggesting that the number of members of this family could influence sensitivity to excess propionate. Our findings demonstrate that natural variation in genes that are not directly associated with propionate breakdown can modulate propionate sensitivity. Our study provides a framework for using C. elegans to characterize the contributions of genetic background in models of human inborn errors in metabolism.
Introduction
Inborn errors of human metabolism are rare genetic diseases in which dietary nutrients or cellular metabolites cannot be broken down to generate energy, biomass, or remove toxic compounds. Most of these disorders are caused by loss-of-function mutations in genes encoding metabolic enzymes or metabolite transporters. Inborn errors of metabolism are often considered monogenic disorders. However, the penetrance and expressivity of these diseases can vary [1]. Therefore, it has been proposed that such diseases should be viewed as more complex traits in which not only environmental factors such as diet, but also genetic background, affect the age of onset and severity of the disease [1]. If true, modifier genes could harbor variation in different genetic backgrounds and affect the penetrance and expressivity of metabolic disorders. However, because such diseases are rare, often with incidences of 1:50,000 or fewer, identifying modifier genes in human populations has been exceedingly difficult [1, 2].
Propionic and methylmalonic acidemia are inborn errors of metabolism in which the short-chain fatty acid propionate cannot be broken down [3]. Patients with propionic acidemia carry loss-of-function mutations in both copies of either one of two genes, PCCA or PCCB, which encode the two proteins comprising propionyl-CoA carboxylase that converts propionyl-CoA to D-methylmalonyl-CoA. Methylmalonic acidemia is a bit more complicated because it can be caused by mutations in either methylmalonyl-CoA racemase, methylmalonyl-CoA mutase, or in enzymes involved in the processing of vitamin B12, which is an essential cofactor for methylmalonyl-CoA mutase [3, 4]. Propionyl-CoA is generated in the natural breakdown of the branched-chain amino acids isoleucine and valine, as well as the catabolism of methionine, threonine, and odd-chain fatty acids. It can be inter-converted with propionate, which is generated by our gut microbiota during the digestion of plant fibers. Although propionate has been found to have beneficial functions [5, 6], it is toxic when it accumulates, as exemplified by patients with propionic acidemia [3]. Propionic acidemia is a rare disorder with a worldwide live birth incidence of 1:50,000 to 1:100,000. It is diagnosed in newborn screening by the detection of elevated levels of propionylcarnitine, 3-hydroxypropionate, and other aberrant metabolites [7].
The nematode Caenorhabditis elegans is a bacterivore found around the world [8–10]. In the laboratory, C. elegans can be fed different species and strains of bacteria [11, 12], but the vast majority of studies use the Escherichia coli strain OP50. However, E. coli OP50 cannot synthesize vitamin B12 and therefore cannot support the efficient breakdown of propionate by the canonical pathway, which depends on vitamin B12 [13, 14]. Previously, we found that C. elegans transcriptionally activates an alternative propionate breakdown pathway, or shunt, when flux through the canonical pathway is low due to genetic perturbations or because of low dietary vitamin B12 [15, 16]. This beta-oxidation pathway comprises five genes and generates acetyl-CoA [15](Fig 1A). C. elegans may have evolved a dedicated pathway for alternate propionate breakdown to be able to survive eating bacteria that do not synthesize vitamin B12. It only activates the expression of propionate shunt genes when propionate accumulation is persistent, via a specific regulatory circuit known as a type-1 feed-forward loop with AND-logic gate using the nuclear hormone receptors nhr-10 and nhr-68 [16]. In propionic acidemia patients, the buildup of propionate shunt metabolites indicates the presence of the propionate shunt. However, its activity is not sufficient to mitigate propionate toxicity likely because the enzymes functioning in other metabolic pathways are repurposed [15].
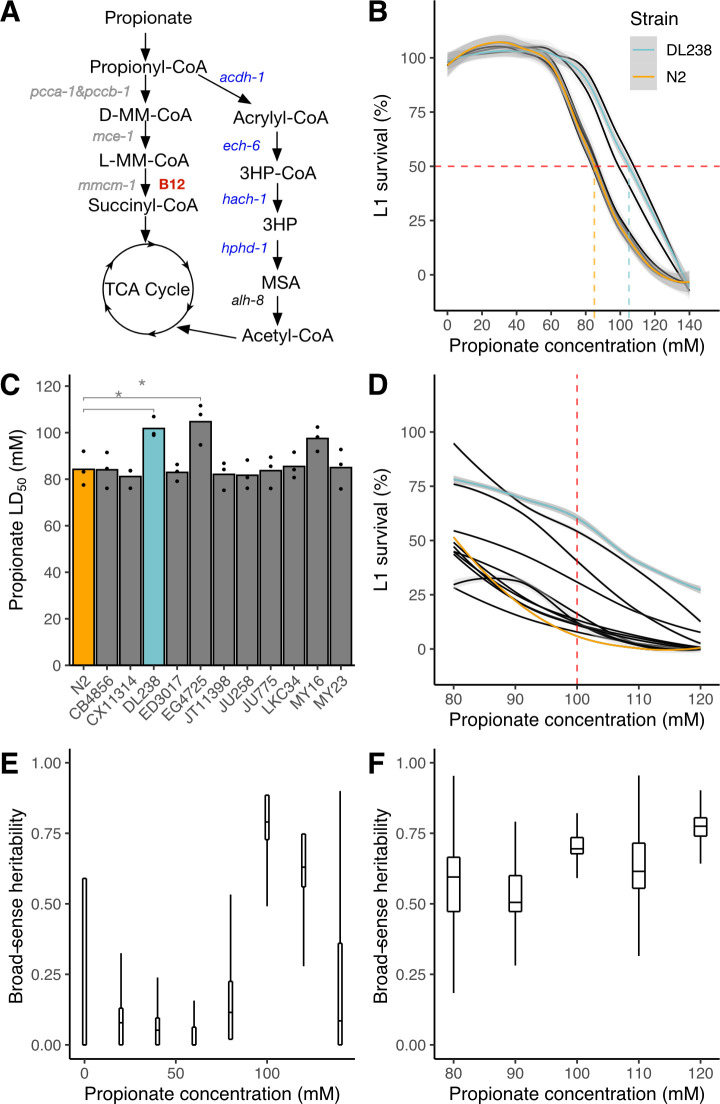
Fig 1. Natural variation in propionate sensitivity in 12 genetically diverse C. elegans strains.
(A) Propionate breakdown pathways in C. elegans. MM–methylmalonyl; TCA–tricarboxylic acid; MSA–malonic semialdehyde; HP–hydroxypropionate. (B) Propionate dose-response curves (DRCs) for 12 genetically distinct wild C. elegans strains. The Loess-smoothed fits of three biological replicates, each comprising three technical replicates, is shown by solid colored lines, and the standard error of the fit is shown in gray. For reference, the DRCs are colored for the N2 (orange, propionate-sensitive) and DL238 (blue, propionate-resistant) strains. We note that the DRCs of nine strains overlap with the N2 DRC. The horizontal dashed red line indicates 50% L1 survival and the vertical colored lines represent the LD50 concentration for N2 and DL238. (C) LD50 values of L1 survival after propionate exposure for the 12 wild C. elegans strains. Three biological replicates each with three technical replicates were performed. (* indicates Student’s t-Test p < 0.05. After adjusting for multiple testing, the p-value for the DL238—N2 comparison is 0.087 and the N2—EG4725 comparison is 0.026, using Tukey’s Honest Significant Difference method). (D) A new experiment of propionate DRCs of 12 wild C. elegans strains exposed to concentrations between 80 and 120 mM. The dashed red line indicates 100 mM propionate. For reference, the DRCs are colored for the N2 and DL238 strains as shown in (B). (E) Tukey boxplots of broad-sense heritability (H2) estimates for the dose response in panel B. Each boxplot represents 12 H2 estimates after subsampling three replicate measures. The interquartile ranges are cut off if they exceed the limits of the plotting window. (F) Tukey boxplots of broad-sense heritability (H2) estimates for the dose response in panel (D). Each boxplot represents 12 H2 estimates after subsampling three replicate measures.
The vast majority of C. elegans studies rely on the laboratory-adapted strain named N2, which was isolated from Bristol, England [17]. Over the last twenty years, hundreds of C. elegans strains have been collected worldwide from natural habitats [10, 18–23]. C. elegans is a self-fertilizing hermaphrodite and, therefore, different wild strains can be easily maintained as fully isogenic strains. These different strains have been used to identify quantitative trait loci (QTL) that contribute to a variety of phenotypes, including anthelmintic and cancer chemotherapeutic resistance, and in several cases the precise genotypic variation that is causal to phenotypic variation has been determined [24–30]. Genomic information about the different strains is organized in the C. elegans Natural Diversity Resource (CeNDR), along with different tools for genome-wide association (GWA) mappings [31].
Here, we used wild C. elegans strains to identify natural variation in loci that modify the resistance to exogenous propionate supplementation. Human propionic acidemia is caused by mutations in PCCA or PCCB. We have shown previously that C. elegans pcca-1 mutants provide a facile ‘simple’ model for this disease [14, 15]. It is impractical to generate pcca-1 loss-of-function mutants in hundreds of wild C. elegans strains. However, we previously found that an Escherichia coli OP50 diet, which is low in vitamin B12, also mimics the metabolic conditions of propionic acidemia in C. elegans because flux through the canonical pathway is hampered when this vitamin is low [14, 15]. By supplementing propionic acid to the C. elegans diet, we can mimic propionic acidemia metabolic conditions [14, 15]. GWA mapping using 133 wild strains identified several independent genomic regions or QTL associated with propionate resistance. For one of these loci, we found the causal variant in glct-3, which encodes a predicted beta-1,3-glucuronosyltransferase, and is an ortholog of human B3GAT1, 2, and 3. A human homolog, B3GAT3, catalyzes the formation of the glycosaminoglycan-protein linkage by way of a glucuronyl transfer reaction in the final step of the biosynthesis of proteoglycans [32]. Glucuronosyltransferases also catalyze reactions between metabolites, specifically the addition of glucuronic acid to toxic metabolites such as drugs [33]. Interestingly, we found that loss-of-function mutations in glct-3 confer resistance to propionate, indicating that it does not directly detoxify propionate. Our data show that quantitative toxicity phenotyping can be used to identify candidate modifier genes of traits associated with inborn errors in human metabolism.
Results
C. elegans wild strains differ in sensitivities to exogenous propionate
We previously developed a larval survival assay to test sensitivity of C. elegans to propionate supplementation [14–16]. In these assays, first larval stage (L1) animals are exposed to propionate and the proportion of animals that develop beyond that stage are quantified. Propionate dose-response curves (DRCs) showed that the laboratory-adapted strain N2 has an LD50 of approximately 80 mM [14–16]. Supplementation of vitamin B12, which supports breakdown of propionate by the canonical pathway, confers resistance to propionate (LD50 = 120 mM) and loss of the propionyl-CoA carboxylase ortholog (pcca-1) or the first gene of the propionate shunt (acdh-1) render the animals sensitive (LD50 = 50 mM) [14, 15]. Because it is technically difficult to perform DRCs for all wild strains, we first asked whether 12 wild C. elegans strains, which represent high genetic diversity [19], exhibit differences in propionate sensitivity. To mimic metabolic conditions of human propionic acidemia, we fed the animals vitamin B12-depleted E. coli OP50 bacteria, which ensures that flux through the canonical propionate breakdown pathway was low [14, 15]. We performed three biological replicate experiments, each consisting of three technical replicates, and found that the 12 strains exhibited varying degrees of propionate sensitivity (Fig 1B and 1C, S1 Table). Nine of the strains had similar propionate sensitivities as the N2 strain with an LD50 of approximately 85 mM. The other three strains were more resistant to propionate with an LD50 of 100–110 mM. Next, we performed propionate assays at concentrations between 80 and 120 mM with 10 mM increments and confirmed that most strains exhibited sensitivities similar to the N2 strain, but that the DL238 and EG4725 strains were significantly more resistant (Fig 1C and 1D, S2 Table). This result suggests that some wild strains have natural mechanisms to cope with high levels of propionate that are independent of vitamin B12 and the canonical propionate breakdown pathway.
To perform GWA mapping, we needed to test propionate sensitivity across a large set of wild C. elegans strains. Propionate sensitivity assays can be noisy, in part because of slight differences in experimental and environmental factors such as incubator and room temperature, propionate concentrations (which can change slightly due to evaporation and dilution), etc. To identify the dose with the highest reproducibility, we calculated broad-sense heritability (H2) and found 100 mM propionate to be the best dose for the GWA mapping experiment (H2 = 0.74) (Fig 1E and 1F). Additionally, we performed power analysis to determine the required number of replicate experiments prior to testing a large number of wild strains. We found that five independent experiments, each with four technical replicates, would give us 80% power to detect a 20% difference in propionate sensitivity (S1 Fig).
Four genomic loci modify sensitivity to propionate across the C. elegans population
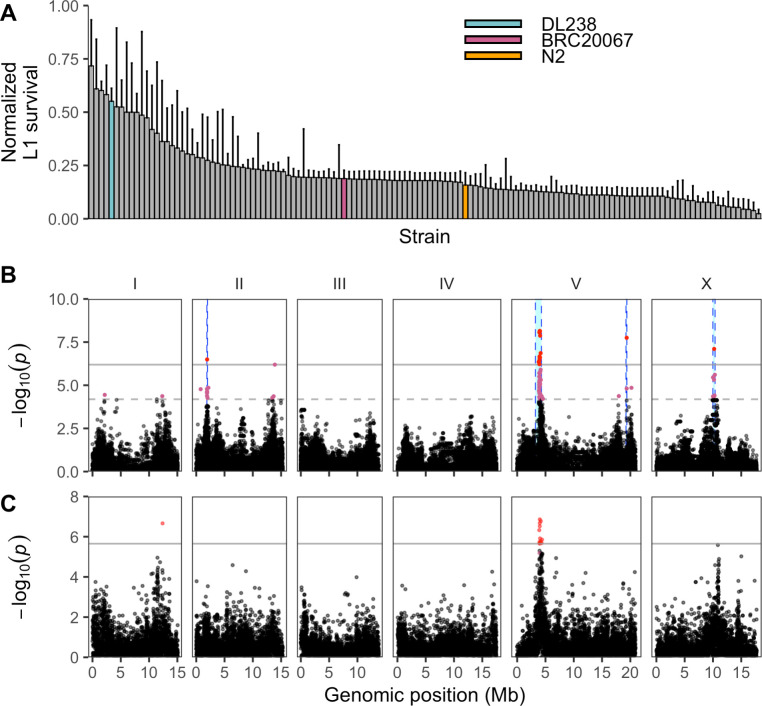
To identify the genetic basis of propionate response variation in C. elegans, we exposed 133 wild strains to 100 mM propionate and measured L1 survival (S3 Table). We tested the strains in three batches and included six strains in every batch to control for potential batch effects (S2 Fig). We observed a broad range of propionate sensitivities (Fig 2A). Using whole-genome sequence data and the L1 survival phenotype, we performed GWA mapping and identified four QTL that were above the Bonferroni-corrected significance threshold (Fig 2B, S4 and S5 Tables), one on chromosome II: (II:1880662–1993488); two on chromosome V: (V:3213649–4284434, V:19229887–19390858); and one on chromosome X: (X:9987812–10370303), coordinates are from WS245). To test the independence of these QTL, we calculated the pairwise linkage disequilibrium (LD) between each of the peak QTL markers (S3 Fig). We observed low levels of LD for the majority of QTL pairs, with the exception of the two QTL on chromosome V (r2 = 0.62, peak markers—V: 3992679 and V: 19356375), suggesting that these two QTL might not be independent. Because multiple QTL were associated with propionate sensitivity, it was difficult to decide which QTL to characterize in more detail. Therefore, we used the sequence kernel association test (SKAT), which tests an association between the phenotype of interest and the cumulative variation on a gene-by-gene basis [34]. This approach identified two QTL, one that contains 14 genes significantly associated with propionate responses and overlaps with the QTL on the left arm of chromosome V (V:3213649–4284434) identified using the single-marker mapping approach (Fig 2B). The second QTL detected by SKAT was located on chromosome I and only overlaps with the single-marker mapping approach at a lower significance threshold (I:12110679–12591430) (Fig 2C, S6 Table). This additional support for the QTL on the left arm of chromosome V motivated us to investigate this genomic region further.
Fig 2. Multiple QTL are associated with variable propionate sensitivities among C. elegans strains.
(A) Normalized L1 survival in the presence of 100 mM propionate for 133 wild C. elegans strains. L1 survival percentages were normalized by dividing each strain measurement by the maximum L1 survival percentage of all strains. Error bars show the standard deviation of replicate strain measurements. The reference strain N2 (orange) and the two strains discussed throughout this work DL238 (blue) and BRC20067 (pink) are colored. (B) Manhattan plot from marker-based GWA mapping for the normalized L1 survival percentage after propionate exposure. Each point represents an SNV that is present in at least 5% of the assayed wild population. The genomic position in Mb, separated by chromosome, is plotted on the x-axis and the -log10(p) for each SNV is plotted on the y-axis. SNVs are colored red if they pass the genome-wide Bonferroni-corrected significance (BF) threshold, which is denoted by the gray horizontal line. SNVs are colored pink if they pass the genome-wide Eigen-decomposition significance threshold, which is denoted by the dotted gray horizontal line. The genomic regions of interest surrounding the QTL that pass the BF threshold are indicated in cyan. (C) Manhattan plot from gene-based GWA mapping for L1 survival after propionate exposure. Each point represents a gene and is colored red if it passes the genome-wide BF threshold (gray line). Points are colored pink if they are significant after 1000 permutations. The genomic position in Mb, separated by chromosome, is plotted on the x-axis and the -log10(p) for each gene is plotted on the y-axis.
Chromosome V near-isogenic lines do not recapitulate propionate resistance
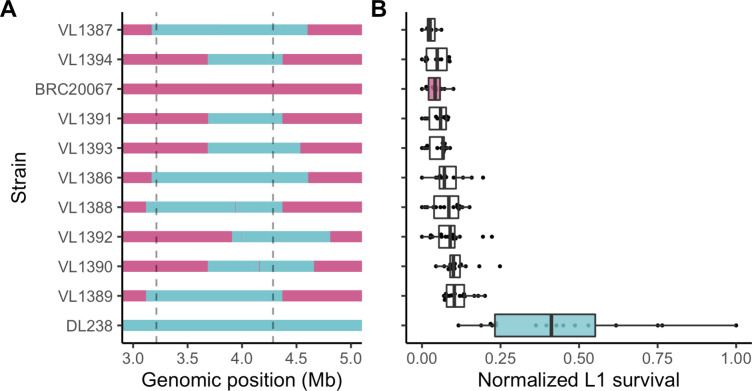
To validate the effect of the QTL on the left arm of chromosome V, we constructed near-isogenic lines (NILs) in which the region associated with propionate resistance (V:3213649–4284434) was crossed from a resistant strain into the genome of a sensitive strain. To identify candidate parental strains for NIL construction, we focused on the 12 strains that were phenotyped in the dose-response experiment (Fig 1C). Of these 12 strains, two were significantly resistant to propionate and ten were sensitive. Next, we verified that the propionate-resistant strains had the alternative genotype at the peak QTL marker identified using the single-marker mapping method and were compatible with propionate sensitive strains at the peel-1 zeel-1 [35] and sup-35 pha-1 [36] incompatibility loci. Using these criteria, we identified DL238 (propionate-resistant) and BRC20067 (propionate-sensitive) as suitable parental strains for NIL construction. We constructed nine NILs that contained the DL238 genomic region surrounding the chromosome V QTL introgressed into the BRC20067 genetic background (Fig 3A, S7 Table). When we exposed these NILs to propionate, we observed that the DL238 introgressed regions that correspond to the chromosome V QTL region of interest did not confer propionate resistance (Fig 3B, S8 Table). Because the genomic region spanned by these NILs is larger than the QTL region of interest, these results suggested that the chromosome V QTL we identified might have been the result of a spurious association with the QTL on the right of chromosome V. The LD between these two loci supports this hypothesis. Alternatively, because this genomic region has thousands of variants and we chose DL238 and BRC20067 based on their phenotype at the peak QTL marker, these two strains might not differ at the causal locus found in this QTL. Because the chromosome V QTL might have a complex relationship, we focused on the QTL identified on the right of chromosome I where the gene-based mapping overlapped the marker-based GWA mapping at the lower significance threshold (Fig 2B and 2C).
Fig 3. Chromosome V near-isogenic lines do not recapitulate the chrV right QTL effect.
(A) Chromosome V genotypes of near-isogenic lines (NILs) generated between BRC20067 (pink) and DL238 (blue). The dotted lines denote the QTL region of interest from the marker-based GWA mapping. (B) Tukey box plots of normalized L1 survival after exposure to 100 mM propionate phenotypes of each NIL and parental strain. Each dot represents a replicate L1 survival measurement.
Variation in the glct-3 gene confers propionate resistance
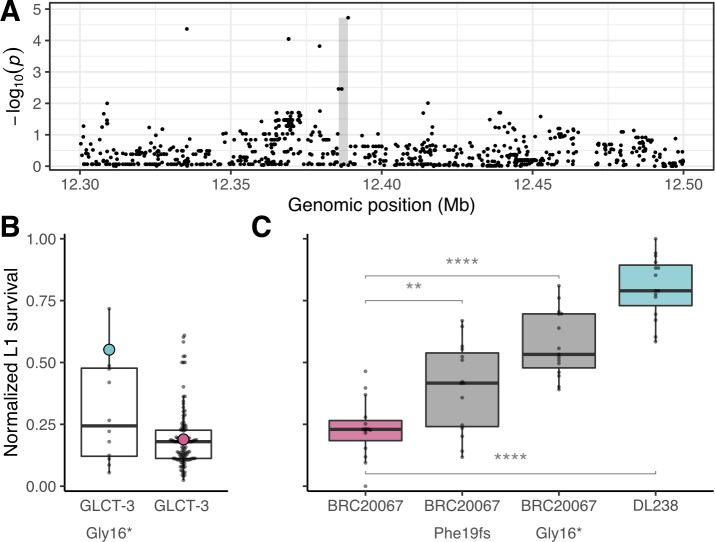
Using a gene-based GWA mapping strategy, we found that variation in the gene glct-3, which is located on the right arm of chromosome I, is associated with propionate sensitivity among the wild isolates (Fig 2C). Additionally, the most correlated marker from the marker-based GWA mapping is in close proximity to glct-3 (Fig 4A). Within the glct-3 gene, we observed eight distinct combinations of alleles (haplotypes) among the phenotyped wild strains (S4 Fig). Five of these distinct haplotypes all include the same stop-gained variant at amino acid position 16 (Gly16*), along with other variants (Gly17Arg, Ser50Ala, Ser111Thr, Tyr231Cys in the QX1793 strain; Gly17Arg Ser50Ala in the CX11276, DL238, ED3046, ED3049, and NIC252 strains; Leu184Phe in the ECA36 strain; Ile46Thr in the QX1792 strain, and only Gly16* in the MY23 and QX1791 strains). Strains with the Gly16* variant are 20% more resistant to propionate treatment than strains with no variation in glct-3 (Fig 4B). This genomic region explains 13.1% of the total genetic variation in response to exogenous propionate, indicating that, although other loci contribute to this trait, this gene is a major contributor to natural differences in resistance to propionate.
Fig 4. Variation in glct-3 underlies differential propionate sensitivity in C. elegans.
(A) Manhattan plot showing the strength of correlation between variants surrounding the glct-3 gene identified by gene-based GWA mapping of the normalized L1 survival after propionate exposure phenotype. The gray shaded rectangle represents the glct-3 gene (chrI:12385765–12388791). (B) Tukey box plots of C. elegans wild isolate’s normalized L1 survival after exposure to 100 mM propionate. Each dot represents the mean of 20 replicate measures for each strain. Strains are separated by the presence of a stop-gained variant at amino acid position 16 of GLCT-3. DL238 (blue) and BRC20067 (pink) are highlighted for reference. (C) Tukey box plots of normalized L1 survival of each CRISPR-edited and parental strain after exposure to 100 mM propionate are shown. Each dot represents a replicate L1 survival measurement. (The effect of strain on phenotype data is significant from analysis of variance [F(3,56) = 45.081, p < 0.05E-10]. For individual strain comparisons: ** represents [F(1,28) = 9.28, p = 0.005] and **** for the BRC20067 –BRC20067 (Gly16*) comparison [F(1,28) = 55.25992, p < 0.05E-6]) And for the BRC20067 –DL238 comparison [F(1,28) = 165.7699, p < 0.05E-6]).
To test whether variation in the glct-3 is causal for the difference in propionate sensitivity, we generated two independent glct-3 alleles in the propionate sensitive BRC20067 strain using CRISPR-Cas9 genome editing [37]. The ww62 allele has a one base pair deletion at position 57 in the first exon that causes a frameshift in the reading frame leading to an early stop codon, and the ww63 allele contains the same Gly16* variant found in DL238. In line with previous experiments, we found that DL238 was more resistant to propionate treatment than BRC20067 (Cohen’s F = 2.433). The strains harboring the ww62 and ww63 alleles recapitulate 23.7% and 57.7% of the difference in propionate sensitivity between DL238 and BRC20067 as measured by Cohen’s F, respectively (Fig 4C, S9 Table), demonstrating that the loss of glct-3 function confers resistance to propionate. The QTL effect size that corresponds to a loss of glct-3 function is approximately 10% of the total parental difference observed in this experiment. We note that this estimate is similar to the fraction of the total heritability explained by the GLCT-3 Gly16* allele (0.11 of broad-sense heritability). We hypothesize that the discrepancy between the QTL effect in the GWA mapping experiment and the genome-edited strains might be caused by limited epistatic interactions because we introduced the allele in a single genetic background. In agreement with this hypothesis, when we only consider the additive component of the heritability (h2) in the GWA experiment, we find that the GLCT-3 Gly16* allele accounts for approximately 40% of this additive trait variance. Finally, we note that the experimental setup for this follow-up experiment was much simpler than the GWA mapping experiment, which likely contributes to the discrepancy between explanatory power of the GLCT-3 Gly16* between the GWA mapping and genome-editing follow-up experiments.
To understand how variation in glct-3 might have arisen in the C. elegans species, we investigated the frequency of this allele across the population and the strains that harbor strongly deleterious variants. More than 330 wild C. elegans strains are currently available in CeNDR [31], and 42 of these strains contain the Gly16* variant in glct-3. The majority of strains that contain the Gly16* variant (33/42) were isolated on the Hawaiian islands (S5 Fig), which are known to harbor the most genetically divergent C. elegans individuals [10]. An additional three strains have variants that are predicted to cause a loss of glct-3 function (ECA733, JU1395, and ECA723). In agreement with the geographic distribution of the Gly16* allele, strains that harbor this allele are among the set of highly genetically divergent C. elegans strains (S6 Fig, S10 Table). However, not all of the genetically divergent strains harbor variation in glct-3.
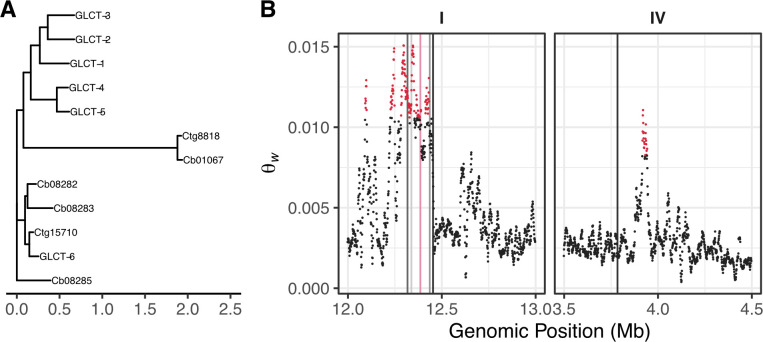
To further explore the evolutionary history of the glct-3 gene, we examined the conservation of glct-3 paralogs and their orthologs. The glct-3 gene encodes a glucuronosyl transferase-like protein that has six paralogs in C. elegans, including five closely related genes glct-1, glct-2, glct-4, glct-5, and glct-6, and one distantly related paralog sqv-8, which we will not discuss further (Fig 5A, S11 Table). Five glct genes (1–5) are located on an 80 kb region on chromosome I, and glct-6 is located on chromosome IV. The close proximity of five of the six paralogs suggests that these genes are the products of gene duplication events, as observed for other gene families in C. elegans [38–40]. We observed elevated levels of variation in the genomic region that contains glct-1 through glct-5 (Fig 5B, S12 Table), which supports the hypothesis that these sequences duplicated at some point in the C. elegans lineage and then diverged. Furthermore, the pattern of variation in the six C. elegans glct paralogs suggests that after the initial duplication event, the function of the glct-6 gene was retained, which is indicated by the absence of deleterious variants in this gene among wild isolates (Fig 5B) [31]. Similarly, glct-4 has no variation that is predicted to be deleterious. By contrast, glct-1, glct-2, glct-3, and glct-5 contain variants predicted to have large effects on gene function. Among the 330 C. elegans strains, 24 have variation that is predicted to remove the function of two or more of these four genes, with two strains that have predicted loss-of-function alleles in all four genes.
Fig 5. Expansion of the glct family.
(A) An unrooted maximum-likelihood phylogeny of the six glucuronosyl transferase-like protein sequences encoded by the C. elegans genome and homologs from C. briggsae (Cb) and C. tropicalis (Ct). (B) The genetic diversity as measured by Watterson’s theta (Θw) for the genomic regions that contain the six glucuronosyl transferase-like genes in C. elegans is shown. Each vertical line marks the position of a glct gene, from left to right on chromosome I: glct-4, glct-1, glct-3 (pink), glct-2, and glct-5; and chromosome IV: glct-6. Each dot represents a 10,000 bp genomic region and is colored red if the Θw value is greater than the 99th quantile of values across the chromosome.
Copy number of the glct gene family varies across Caenorhabditis species
Next, we explored the conservation of the glct gene family across 20 species of Caenorhabditis nematodes, including ten for which the genome assembly was recently released [41]. We found that nine species contained only one glct-3 ortholog, five contain two glct-3 orthologs, three contain three glct-3 orthologs, and one species each contain four, five, or six glct-3 orthologs (S7 Fig, S13 Table). The prevalence of low-copy numbers of glct genes among a majority of Caenorhabditis species suggests that the ancestral copy number is fewer than the six copies found in the C. elegans genome. This hypothesis is supported by the presence of one and two glct-3 orthologs in the outgroup species Heterorhabditis bacteriophora and Oscheius tipulae, respectively. Because the GLCT-6 protein and DNA sequences more closely resemble orthologous sequences among Caenorhabditis species than its paralogs in C. elegans (S8 Fig, S14 Table), this gene is likely the ancestral state of this gene family. Taken together, these results suggest that the copy number of glct genes likely affects fitness in the wild.
Discussion
In this study, we identify mutations that naturally occur in C. elegans glct-3 as modifiers of propionate sensitivity. This gene encodes a glucuronosyltransferase-like protein, which belongs to a family that includes the other GLCT proteins, as well as UGT enzymes. These enzymes generally catalyze the transfer of glucuronic acid to small molecules as part of the phase II detoxification system [42]. The addition of these adducts can make the small molecules more easily secreted or less able to interact with targets, decreasing the toxicity of these compounds. The mechanism by which mutations in glct-3 render the animal less sensitive to exogenous propionate supplementation remains unclear. The closest human homologs of glct-3 are B3GAT1, G3GAT2, and B3GAT3, but the functions of these genes are not well understood. Likewise, the family of UGT enzymes in C. elegans remain greatly understudied. These genes are likely involved in responses to the environment, so laboratory experiments often do not recapitulate the complex niches that C. elegans inhabit in the wild [8]. However, because natural variants in glct-3 are predicted to cause loss of function, and the common Gly16* allele is an early nonsense allele, enzyme function is eliminated and GLCT-3 substrates cannot be modified and detoxified. This result suggests that GLCT-3 does not directly modify propionate or any of its derviatives as a detoxifying mechanism. Instead, our data indicate that modification of a small molecule, or perhaps protein, by GLCT-3 increases the toxicity of propionate. Future studies will determine which molecules are modified by GLCT-3 and what the functional consequences of such modifications are.
In the natural environment, C. elegans likely encounters a variety of bacteria and fungi that produce a plethora of small molecules, including short-chain fatty acids such as propionate. These small molecules can accumulate and decrease fitness in the niche. When bacteria that produce vitamin B12 are also present in the niche, propionate toxicity can be reduced. For these reasons, natural strains of C. elegans might vary in their complements of glct-3 paralogs. Strains that inhabit niches with high propionate but low levels of vitamin B12 might have a more active propionate shunt [15] or fewer members of the glct-3 family to limit toxicity. Niches with less propionate and/or high levels of vitamin B12 could support strains that might have lost the propionate shunt or have more copies glct-3 family members. Microevolution of similar metabolic regulators could act through differences in copy number and not through specific changes to enzymatic function or differences in gene expression. Over the evolution of a particular pathway, individual components have the potential to expand and contract via duplication or deletion. As these changes occur, novel connections can compensate for the altered copy number of a particular pathway component. It appears that we have identified such a novel link in the C. elegans species, where a loss of glct-3 function causes propionate resistance. It is still uncertain how our findings will translate to other systems, because it is possible that the phenomenon we observed is nematode-specific. It is clear, however, that natural changes in metabolic flux are important for how organisms deal with the complex milieu of their natural environment.
Interestingly, we could not validate the most significant QTL that we detected by GWA. This QTL on chromosome V was in strong LD with another QTL on chromosome V. Long-range LD is common in selfing organisms, especially C. elegans [19], but it can confound GWA mappings. Our results emphasize that all QTL need to be validated by independent strains. NILs, as we used here, offer an effective approach to rapidly test genomic intervals for correlations with observed phenotypic differences. It is likely that the chromosome V right QTL underlies the trait difference that we tested at the chromosome V left QTL. Additional NILs, obtained from crosses between different wild isolates, need to be constructed to test this hypothesis and narrow this genomic region to a causal gene.
Our study demonstrates that natural variation can modify sensitivity to the short chain fatty acid propionate. We used a C. elegans model that mimics metabolic conditions found in patients with propionic acidemia. These data indicate that C. elegans is a fruitful model to identify genetic modifiers of inborn errors in human metabolism, which is extremely difficult with human populations as these diseases are usually rare.
Materials and methods
Strains
All the wild strains were obtained from CeNDR (S3 Table) [31] and maintained at 20°C on nematode growth medium (NGM) plates on a diet of E. coli OP50. Near-isogenic lines (NILs) were generated using a procedure described previously [43] by crossing BRC20067 and DL238. Each NIL strain harbors recombination breakpoints at different locations on chromosome V generated by crossing two single recombinant strains, followed by six times backcrossing with BRC20067 to change the other five chromosomes into the BRC20067 background.
Propionate sensitivity assays
A 2 M propionic acid stock solution was prepared in a chemical hood. For 40 ml solution, 6 ml propionic acid (sigma, #402907), 13.5 ml 5 M sodium hydroxide, and 20.5 ml water were mixed together, and the pH was adjusted to 6.0 with sodium hydroxide. The solution was filter sterilized and stored at 4°C. On day 0, arrested L1 animals were placed on seeded plates with propionate and after incubation for two days, animals that developed beyond the L1 stage were evaluated as survivors. Propionic acid survival rate was calculated as the proportion of animals that have developed beyond the L1 stage over the total number of L1 animals at day 0. We focused on L1 survival because L1 larvae are more strongly affected by propionate than later developmental stages. For example, L1s that survive 100 mM propionate treatment will continue to grow to adulthood. However, high concentrations of propionate do not affect C. elegans hatching rate, which makes using this phenotype difficult for GWA mapping. Biological triplicate experiments with three technical replicates were performed. For the panel of 133 wild isolates, 100 mM propionate was used in five biological replicates, each with four technical replicates. A biological replicate is an independent growth of a strain. For each biological replicate, strains were chunked from a starved plate, grown for two generations, bleached, synchronized as L1s in M9, and plated out on 48-well agar plates with propionate. For each technical replicate, approximately 100 arrested L1s were transferred to an individual well of a 48-well plate. Because we had a high level of replication, we were not able to complete the entire GWA phenotyping in one experiment and had to phenotype the strains in three separate sets. For each set, we included six reference strains to verify that the batch conditions did not change significantly. To process the data, we first took the mean of the four technical replicates and removed biological replicate outliers, which were defined by 1.5 times the standard deviation from the mean. This procedure eliminated one of five biological replicates for 89 strains and two of five replicates for 11 strains. Next, we corrected the strain phenotype data for biological replicate and strain set using a linear model with the formula (phenotype ~ biological replicate + strain set). Finally, we took the mean of the residual phenotypes and performed association mapping (S3 Table).
To explore the effects of replicate and strain set, we performed an analysis of variance. To perform this analysis, we focused on the six strains that were included in all of the experiments. We found that the biological replicate (p-value = 0.026, Cohen’s F = 0.119) and set (p-value = 0.012, Cohen’s F = 0.134) had moderate but significant effects on strain phenotypes. Furthermore, we found no significant effect of technical replicate (p-value = 0.83, Cohen’s F = 0.05), which is expected because technical replicates were drawn from the same strain preparation. As expected, these effects were all negligible after correction with the linear model described above.
Heritability calculations
For dose-response experiments, broad-sense heritability (H2) estimates were calculated using the lmer function in the lme4 package with the following linear mixed-model (phenotype ~ 1 + (1|strain)). H2 was then calculated as the fraction of the total variance that can be explained by the random component (strain) of the mixed model. For the complete dose-response experiment, we calculated H2 per dose. For the fine-scale dose response experiment, we subsampled three replicates twelve independent times for H2 calculations.
Power analysis
To determine the number of replicate measures we needed to collect for each wild isolate, we measured L1 survival of the DL238 strain after exposure to 100 mM propionic acid in 40 replicates. The 40 replicates consisted of eight technical replicates across five independent preparations of agar plates with propionate. For a range of mean differences (0.01 to 1, in increments of 0.01), we subsampled two to eight replicates for each of the five plate preparations 100 times and calculated the standard deviation of L1 survival for the subsamples. To calculate the power to detect a difference across a range of replicates and mean differences, we used the power.t.test function in the pwr R package with the following parameters—n = number of subsampled replicates, delta = (0.01 to 1, in increments of 0.01), sd = mean of the standard deviation subsamples, sig.level = 0.00001, alternative = “two.sided”, type = “two.sample”. With four technical replicates across five independent plate preparations, we were able to detect a 20% difference in means 80% of the time. We note that this analysis does not determine the power to detect QTL via GWA mapping, which will depend on the effect size of a given locus, the number of strains analyzed, the overall trait heritability, interactions among contributing loci, and other factors.
Marker-based genome-wide association mappings
GWA mapping was performed using phenotype data from 133 C. elegans wild strains. We performed the same mapping procedure as described previously [43]. Briefly, genotype data were acquired from the latest variant call format (VCF) release (Release 20180527) from CeNDR that was imputed using IBDseq, with the following parameters: minalleles = 5%, r2window = 1500, ibdtrim = 0, r2max = 0.8 [44]. We used BCFtools to filter variants that had any missing genotype calls and variants that were below 5% minor allele frequency [45]. We used PLINK v1.9 to LD-prune the genotypes at a threshold of r2 < 0.8, using—indep-pairwise 50 10 0.8 [46, 47]). This genotype data set consisted of 59,241 markers that were used to generate the realized additive kinship matrix using the A.mat function in the rrBLUP R package [48]. These markers were also used for genome-wide mappings. However, because these markers still have substantial LD within this genotype set, we performed eigen decomposition of the correlation matrix of the genotype matrix using eigs_sym function in Rspectra package (https://github.com/yixuan/RSpectra). The correlation matrix was generated using the cor function in the correlateR R package (https://github.com/AEBilgrau/correlateR). We set any eigenvalue greater than one from this analysis to one and summed all of the resulting eigenvalues to obtain 772 independent tests within this genotype matrix [49]. We used the GWAS function in the rrBLUP package to perform genome-wide mapping with the following command: rrBLUP::GWAS(pheno = PC1, geno = Pruned_Markers, K = KINSHIP, min.MAF = 0.05, n.core = 1, P3D = FALSE, plot = FALSE). To perform fine-mapping, we defined genomic regions of interest from the genome-wide mapping as +/- 100 single-nucleotide variants (SNVs) from the rightmost and leftmost markers above the Bonferroni significance threshold. We then generated a QTL region of interest genotype matrix that was filtered as described above, with the one exception that we did not perform LD pruning. We used PLINK v1.9 to extract the LD between the markers used for fine mapping and the peak QTL marker identified from the genome-wide scan. We used the same command as above to perform fine mapping but used the reduced variant set. The workflow for performing GWA mapping can be found at https://github.com/AndersenLab/cegwas2-nf.
Sequence Kernel Association Test (SKAT) Mapping
In parallel to marker-based GWA mappings, we performed a sequence kernel association test (SKAT), which is implemented in the RVtests software package using SKAT [34, 50].
We set the maximum allele frequency for SKAT to 50% using the—freqUpper from flag cegwas2-nf, and the minimum number of strains to share a variant to two using the—minburden flag. The model used for burden mapping was set with the following flag—kernel skat when executing rvtests. This flag implements the burden test that is represented in equation 1 of Wu et al. 2011 [34]. We used the weights suggested by Wu et al. 2011 when running the mapping (beta1 = 1, and beta2 = 25), which adds more weight to rare variants when testing a gene’s variants for association with propionate responses.
Linkage Disequilibrium
We used the LD function from the genetics package in R to calculate linkage disequilibrium and report the r2 correlation coefficient between the markers (https://cran.r-project.org/package=genetics).
Phylogenetic analysis
DNA and protein FASTA files for each species were downloaded from http://download.caenorhabditis.org/v1/sequence/ [41]. DNA and protein for each species FASTA files were combined and custom DNA and protein BLAST databases were built using makeblastdb [51]. The glct-3 coding sequence (CDS) was used to query the DNA BLAST database using the blastn command with the -evalue threshold set to 1. Homologous sequences were extracted from the database using the blastdbcmd command. Next, a multiple sequence alignment of the homolgous sequences was generated using MUSCLE [52] with default settings and output in the phylip format.
For DNA sequences, the raxmlHPC-AVX command from RAxML 8 (v 8.2.12) with the GTRGAMMA substitution model was used to generate initial phylogenies [53]. Next, we preformed bootstrapping with the following command raxmlHPC-AVX -p 12345 -x 12345 -# autoFC -m GTRGAMMA and extracted the best tree with bootstrap support.
For protein sequences, we used the bayesian information criterion model selection feature of RAxML 8 to identify VT [54] as the best substitution model with the following command: raxmlHPC-AVX -p 12345 -m PROTGAMMAAUTO—auto-prot = bic. Next, we performed bootstrapping of the phylogentic tree using the following command: raxmlHPC-AVX -p 12345 -x 12345 -# autoFC -m PROTGAMMAAUTO—auto-prot = bic. All phylogenies were visualized using the interactive tree of life software [55] or the ggtree R package [56].
Heritability estimates from genome-wide association phenotypes
We used the sommer package in R to calculate marker-based narrow-sense heritability [57]. We first calculated the marker-based heritability for the phenotype data used to perform GWA mapping using the mmer function in sommer, with the random variable in the model specified as random = ~vs(strain, Gu = A) + vs(strainE,Gu = E), where A is the additive genotype matrix (generated using the A.mat function in sommer) and E is the epistatic genotype matrix (generated using the E.mat function in sommer). To determine the effect of the GLCT-3 GLY16* allele, we performed the same heritability calculation, but we included the presence of the GLCT-3 GLY16* allele as a fixed effect in the mmer function. Narrow-sense heritability estimates were extracted from the mmer object using the sommer pin function with the formula h2 ~ (V1) / (V1+V2+V3), where V1 is the additive variance component, V2 is the epistatic variance component, and V3 is residual variation. To determine the effect of the GLCT GLY16* allele, we performed the following calculation (h2—h2_glct3)/h2, where h2 is the narrow-sense heritability of the original phenotype data, and h2_glct3 is the estimate with the GLCT GLY16* allele as a fixed effect.
Supporting information
Power analysis of L1 survival after propionate exposure is shown. We calculated power for a range of mean differences from 0 to 1, using the average standard deviation of 100 subsamples from a large-scale experiment that measured DL238 propionate survival. The solid line represents the mean of 10 replicate power calculations and the shaded area around the solid lines represent the standard deviation of the replicates. The line colors represent the sample size. The dashed red line indicates 0.8 power.
(TIFF)
(A) L1 survival in the presence of 100 mM propionate for six C. elegans strains with eight technical plus five biological replicates. (B) Experimental setup to phenotype wild isolates for GWA mapping. 133 wild C. elegans strains were divided into three batches to test their survival after exposure to 100 mM propionate. Each batch contains 48 strains, including six control strains that control for batch effects. (C) L1 survival rate in the presence of 100 mM propionate for each 48 strain batch described in B. The colored bars represent the mean L1 survival for each C. elegans strain. Tukey boxplots overlay the data. Batch-control strains are indicated by red median bars.
(TIFF)
Linkage disequilibrium (r2) of peak QTL markers identified by genome-wide association mapping is shown. The tile color represents the correlation between marker pairs.
(TIFF)
(A) The normalized L1 survival in the presence of propionate for each phenotyped C. elegans strain is shown on the x-axis. The y-axis represents unique haplotypes (numbered from 1:n) constructed from variants with moderate-to-severe predicted effects on glct-3 found to be significantly associated with propionate sensitivity. If a variant with a high predicted effect on gene function was identified, we plotted it separately. Therefore, a strain can be represented twice if it contains a variant with a high predicted effect on gene function. The red diamonds represent the median phenotype value for each unique haplotype. The blue and pink diamonds represent the DL238 and BRC20067 strains, respectively. (B) The pairwise linkage disequilibrium (r2) between the allele that encodes the Gly16* (red diamond) in GLCT-3 and all variants (black diamonds) in the surrounding genomic region is shown on the y-axis. The x-axis represents the genomic position (Mb) of each variant.
(TIFF)
Sampling locations of wild C. elegans strains. Each dot represents the location where an individual strain was sampled. Pink dots represent strains carrying the REF allele at GLCT-3, and blue dots represent strains carrying the Gly16* allele.
(TIFF)
A maximum likelihood phylogenetic tree of the C. elegans population. Branches are colored based on the GLCT-3 allele the individual strain carries, blue represents strains with the GLCT-3 Gly16* allele, and pink represents strains with the reference allele.
(TIFF)
The maximum likelihood phylogenetic relationship of glct-3 homologs is shown. Branch lengths are shown above each branch. Branch colors correspond to the bootstrap support for the split, with pink indicating higher support. If a species contains more than homolog, all homologs for that species are colored the same color. Species with only one homolog are colored black. The C. elegans glct genes are colored in black and bolded.
(TIFF)
The maximum likelihood phylogenetic relationship of glct-3 homologs is shown. Branch colors correspond to the bootstrap support for the split, with pink indicating higher support. The C. elegans GLCT protein sequences are bolded.
(TIFF)
(TSV)
(TSV)
(TSV)
(TSV)
(TSV)
(ASSOC)
(TSV)
(TSV)
(TSV)
(TXT)
(TXT)
(TSV)
(TXT)
(TXT)
Acknowledgments
We thank members of the Andersen and Walhout laboratories for critical comments on the manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
AJMW and ECA, R01-DK115690, National Institutes of Heath, NIDDK The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Argmann CA, Houten SM, Zhu J, Schadt EE. A Next Generation Multiscale View of Inborn Errors of Metabolism. Cell Metab. 2016;23(1):13–26. 10.1016/j.cmet.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saudubray JM, Garcia-Cazorla A. Inborn Errors of Metabolism Overview: Pathophysiology, Manifestations, Evaluation, and Management. Pediatr Clin North Am. 2018;65(2):179–208. Epub 2018/03/06. 10.1016/j.pcl.2017.11.002 . [DOI] [PubMed] [Google Scholar]
- 3.Deodato F, Boenzi S, Santorelli FM, Dionisi-Vici C. Methylmalonic and propionic aciduria. Am J Med Genet C Semin Med Genet. 2006;142C(2):104–12. 10.1002/ajmg.c.30090 . [DOI] [PubMed] [Google Scholar]
- 4.Banerjee R, Ragsdale SW. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu Rev Biochem. 2003;72:209–47. Epub 2003/10/07. 10.1146/annurev.biochem.72.121801.161828 . [DOI] [PubMed] [Google Scholar]
- 5.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839–49. 10.3390/nu7042839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosseini E, Grootaert C, Verstraete W, Van de Wiele T. Propionate as a health-promoting microbial metabolite in the human gut. Nutrition reviews. 2011;69(5):245–58. Epub 2011/04/28. 10.1111/j.1753-4887.2011.00388.x . [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto I, Kuhara T. A new chemical diagnostic method for inborn errors of metabolism by mass spectrometry—rapid, practical, and simultaneous urinary metabolites analysis. Mass Spectrometry Reviews. 1996;15:43–57. [DOI] [PubMed] [Google Scholar]
- 8.Frezal L, Felix MA. C. elegans outside the Petri dish. Elife. 2015;4 10.7554/eLife.05849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felix MA, Braendle C. The natural history of Caenorhabditis elegans. Curr Biol. 2010;20(22):R965–9. 10.1016/j.cub.2010.09.050 . [DOI] [PubMed] [Google Scholar]
- 10.Crombie TA, Zdraljevic S, Cook DE, Tanny RE, Brady SC, Wang Y, et al. Deep sampling of Hawaiian Caenorhabditis elegans reveals high genetic diversity and admixture with global populations. Elife. 2019;8 Epub 2019/12/04. 10.7554/eLife.50465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacNeil LT, Walhout AJM. Food, pathogen, signal: The multifaceted nature of a bacterial diet. Worm. 2013;2:e26454 10.4161/worm.26454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yilmaz LS, Walhout AJM. Worms, bacteria and micronutrients: an elegant model of our diet. Trends Genet. 2014;30:496–503. 10.1016/j.tig.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson E, MacNeil LT, Arda HE, Zhu LJ, Walhout AJM. Integration of metabolic and gene regulatory networks modulates the C. elegans dietary response. Cell. 2013;153:253–66. 10.1016/j.cell.2013.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson E, MacNeil LT, Ritter AD, Yilmaz LS, Rosebrock AP, Caudy AA, et al. Interspecies systems biology uncovers metabolites affecting C. elegans gene expression and life history traits. Cell. 2014;156:759–70. 10.1016/j.cell.2014.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson E, Olin-Sandoval V, Hoy MJ, Li C-H, Louisse T, Yao V, et al. Metabolic network rewiring of propionate flux compensates vitamin B12 deficiency in C. elegans. Elife. 2016;5:pii: e17670 10.7554/eLife.17670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bulcha JT, Giese GE, Ali MZ, Lee Y-U, Walker M, Holdorf AD, et al. A persistence detector for metabolic network rewiring in an animal. Cell Rep. 2019;26:460–8. 10.1016/j.celrep.2018.12.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterken MG, Snoek LB, Kammenga JE, Andersen EC. The laboratory domestication of Caenorhabditis elegans. Trends Genet. 2015;31(5):224–31. Epub 2015/03/26. 10.1016/j.tig.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockman MV, Kruglyak L. Recombinational landscape and population genomics of Caenorhabditis elegans. PLoS Genet. 2009;5(3):e1000419 Epub 2009/03/14. 10.1371/journal.pgen.1000419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen EC, Gerke JP, Shapiro JA, Crissman JR, Ghosh R, Bloom JS, et al. Chromosome-scale selective sweeps shape Caenorhabditis elegans genomic diversity. Nat Genet. 2012;44(3):285–90. 10.1038/ng.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barriere A, Felix MA. High local genetic diversity and low outcrossing rate in Caenorhabditis elegans natural populations. Curr Biol. 2005;15(13):1176–84. Epub 2005/07/12. 10.1016/j.cub.2005.06.022 . [DOI] [PubMed] [Google Scholar]
- 21.Barriere A, Felix MA. Temporal dynamics and linkage disequilibrium in natural Caenorhabditis elegans populations. Genetics. 2007;176(2):999–1011. Epub 2007/04/06. 10.1534/genetics.106.067223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolgin ES, Felix MA, Cutter AD. Hakuna Nematoda: genetic and phenotypic diversity in African isolates of Caenorhabditis elegans and C. briggsae. Heredity (Edinb). 2008;100(3):304–15. Epub 2007/12/13. 10.1038/sj.hdy.6801079 . [DOI] [PubMed] [Google Scholar]
- 23.Petersen C, Saebelfeld M, Barbosa C, Pees B, Hermann RJ, Schalkowski R, et al. Ten years of life in compost: temporal and spatial variation of North German Caenorhabditis elegans populations. Ecol Evol. 2015;5(16):3250–63. Epub 2015/09/19. 10.1002/ece3.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy KC, Andersen EC, Kruglyak L, Kim DH. A polymorphism in npr-1 is a behavioral determinant of pathogen susceptibility in C. elegans. Science. 2009;323(5912):382–4. 10.1126/science.1166527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh R, Andersen EC, Shapiro JA, Gerke JP, Kruglyak L. Natural variation in a chloride channel subunit confers avermectin resistance in C. elegans. Science. 2012;335(6068):574–8. 10.1126/science.1214318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zdraljevic S, Strand C, Seidel HS, Cook DE, Doench JG, Andersen EC. Natural variation in a single amino acid substitution underlies physiological responses to topoisomerase II poisons. PLoS Genet. 2017;13(7):e1006891 Epub 2017/07/13. 10.1371/journal.pgen.1006891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brady SC, Zdraljevic S, Bisaga KW, Tanny RE, Cook DE, Lee D, et al. A Novel Gene Underlies Bleomycin-Response Variation in Caenorhabditis elegans. Genetics. 2019;212(4):1453–68. Epub 2019/06/07. 10.1534/genetics.119.302286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greene JS, Brown M, Dobosiewicz M, Ishida IG, Macosko EZ, Zhang X, et al. Balancing selection shapes density-dependent foraging behaviour. Nature. 2016;539(7628):254–8. 10.1038/nature19848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burga A, Ben-David E, Lemus Vergara T, Boocock J, Kruglyak L. Fast genetic mapping of complex traits in C. elegans using millions of individuals in bulk. Nat Commun. 2019;10(1):2680 Epub 2019/06/20. 10.1038/s41467-019-10636-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao AW, Sterken MG, Uit de Bos J, van Creij J, Kamble R, Snoek BL, et al. Natural genetic variation in C. elegans identified genomic loci controlling metabolite levels. Genome Res. 2018;28(9):1296–308. Epub 2018/08/16. 10.1101/gr.232322.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook DE, Zdraljevic S, Roberts JP, Andersen EC. CeNDR, the Caenorhabditis elegans natural diversity resource. Nucleic Acids Res. 2017;45(D1):D650–D7. 10.1093/nar/gkw893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones KL, Schwarze U, Adam MP, Byers PH, Mefford HC. A homozygous B3GAT3 mutation causes a severe syndrome with multiple fractures, expanding the phenotype of linkeropathy syndromes. Am J Med Genet A. 2015;167A(11):2691–6. Epub 2015/06/19. 10.1002/ajmg.a.37209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowland A, Miners JO, Mackenzie PI. The UDP-glucuronosyltransferases: their role in drug metabolism and detoxification. Int J Biochem Cell Biol. 2013;45(6):1121–32. Epub 2013/03/19. 10.1016/j.biocel.2013.02.019 . [DOI] [PubMed] [Google Scholar]
- 34.Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X. Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet. 2011;89(1):82–93. Epub 2011/07/09. 10.1016/j.ajhg.2011.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seidel HS, Rockman MV, Kruglyak L. Widespread genetic incompatibility in C. elegans maintained by balancing selection. Science. 2008;319(5863):589–94. Epub 2008/01/12. 10.1126/science.1151107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ben-David E, Burga A, Kruglyak L. A maternal-effect selfish genetic element in Caenorhabditis elegans. Science. 2017;356(6342):1051–5. Epub 2017/05/13. 10.1126/science.aan0621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H, Ishidate T, Ghanta KS, Seth M, Conte D Jr., Shirayama M, et al. A co-CRISPR strategy for efficient genome editing in Caenorhabditis elegans. Genetics. 2014;197(4):1069–80. 10.1534/genetics.114.166389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee D, Zdraljevic S, Cook DE, Frezal L, Hsu JC, Sterken MG, et al. Selection and gene flow shape niche-associated variation in pheromone response. Nat Ecol Evol. 2019;3(10):1455–63. Epub 2019/09/25. 10.1038/s41559-019-0982-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas JH. Adaptive evolution in two large families of ubiquitin-ligase adapters in nematodes and plants. Genome Res. 2006;16(8):1017–30. Epub 2006/07/11. 10.1101/gr.5089806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas JH, Robertson HM. The Caenorhabditis chemoreceptor gene families. BMC Biol. 2008;6:42 Epub 2008/10/08. 10.1186/1741-7007-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens L, Felix MA, Beltran T, Braendle C, Caurcel C, Fausett S, et al. Comparative genomics of 10 new Caenorhabditis species. Evol Lett. 2019;3(2):217–36. Epub 2019/04/23. 10.1002/evl3.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jancova P, Anzenbacher P, Anzenbacherova E. Phase II drug metabolizing enzymes. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010;154(2):103–16. Epub 2010/07/30. 10.5507/bp.2010.017 . [DOI] [PubMed] [Google Scholar]
- 43.Zdraljevic S, Fox BW, Strand C, Panda O, Tenjo FJ, Brady SC, et al. Natural variation in C. elegans arsenic toxicity is explained by differences in branched chain amino acid metabolism. Elife. 2019;8 Epub 2019/04/09. 10.7554/eLife.40260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Browning BL, Browning SR. Detecting identity by descent and estimating genotype error rates in sequence data. Am J Hum Genet. 2013;93(5):840–51. Epub 2013/11/12. 10.1016/j.ajhg.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27(21):2987–93. Epub 2011/09/10. 10.1093/bioinformatics/btr509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7 Epub 2015/02/28. 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. Epub 2007/08/19. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Endelman JB. Ridge regression and other kernels for genomic selection with R package rrBLUP. The plant genome. 2011;4:250–5. [Google Scholar]
- 49.Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb). 2005;95(3):221–7. Epub 2005/08/04. 10.1038/sj.hdy.6800717 . [DOI] [PubMed] [Google Scholar]
- 50.Zhan X, Hu Y, Li B, Abecasis GR, Liu DJ. RVTESTS: an efficient and comprehensive tool for rare variant association analysis using sequence data. Bioinformatics. 2016;32(9):1423–6. Epub 2016/05/08. 10.1093/bioinformatics/btw079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421 Epub 2009/12/17. 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113 Epub 2004/08/21. 10.1186/1471-2105-5-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–3. Epub 2014/01/24. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muller T, Vingron M. Modeling amino acid replacement. J Comput Biol. 2000;7(6):761–76. Epub 2001/05/31. 10.1089/10665270050514918 . [DOI] [PubMed] [Google Scholar]
- 55.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 2019;47(W1):W256–W9. Epub 2019/04/02. 10.1093/nar/gkz239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu G, Lam TT, Zhu H, Guan Y. Two Methods for Mapping and Visualizing Associated Data on Phylogeny Using Ggtree. Mol Biol Evol. 2018;35(12):3041–3. Epub 2018/10/24. 10.1093/molbev/msy194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Covarrubias-Pazaran G. Genome-Assisted Prediction of Quantitative Traits Using the R Package sommer. PLoS One. 2016;11: e0156744 10.1371/journal.pone.0156744 [DOI] [PMC free article] [PubMed] [Google Scholar]