ABSTRACT
This phase 3B, open-label, extension study (NCT01962207) evaluated long-term persistence of antibodies induced by the quadrivalent meningococcal vaccine conjugated to tetanus toxoid (MenACWY-TT) compared with the meningococcal serogroup C vaccine conjugated to CRM (MenC-CRM) and the quadrivalent meningococcal polysaccharide vaccine (MenACWY-PS) 6 to 10 y after primary vaccination in toddlers (aged 1–<2 y; MenACWY-TT and MenC-CRM) and children (aged 2–<11 y; MenACWY-TT and MenACWY-PS). Antibody responses against meningococcal serogroups A, C, W, and Y were assessed by serum bactericidal antibody assays using rabbit (rSBA) or human (hSBA) complement. A MenACWY-TT booster dose at Year 10 was given to all eligible subjects regardless of the primary vaccine received. At Year 10, the percentages of subjects with rSBA titers ≥1:8 for serogroups A, C, W, and Y were as follows: MenACWY-TT (toddlers), 65.6%, 82.8%, 31.3%, 43.8%, respectively; MenC-CRM, 88.2% for serogroup C; MenACWY-TT (children), 88.9%, 84.1%, 67.1%, 65.9%; and MenACWY-PS, 28.6%, 81.0%, 23.8%, and 23.8%. Corresponding percentages for hSBA titers ≥1:4 were as follows: MenACWY-TT (toddlers), 31.1%, 91.9%, 44.4%, 41.4%; MenC-CRM, 93.8% for serogroup C; MenACWY-TT (children), 34.8%, 91.1%, 61.2%, 72.6%; and MenACWY-PS, 33.3%, 100.0%, 26.3%, and 44.4%. One month after the MenACWY-TT booster, the percentage of subjects with vaccine response ranged from 75.7% to 100.0% across serogroups in all study groups. Postbooster vaccine responses were generally comparable between groups across serogroups. No new safety signals were identified. Antibody responses persisted 10 y after MenACWY-TT vaccination. The MenACWY-TT booster dose was well tolerated and elicited robust immune responses.
KEYWORDS: bactericidal activity, children, conjugate vaccine, persistence, quadrivalent meningococcal vaccine, toddler, booster
Introduction
Neisseria meningitidis causes meningococcal disease, including meningitis, bacteremia, and bacteremic pneumonia.1 Even with treatment, the case-fatality ratio of meningococcal disease is 10% to 15%; approximately 20% of survivors have permanent and disabling sequelae, including hearing loss, limb loss, and neurologic impairment.2 The risk of meningococcal disease is highest in infants and toddlers, with a secondary peak in late adolescence and early adulthood,3,4 making these age groups an important target for prevention.
Currently, vaccines are available targeting five meningococcal disease-causing serogroups (i.e., serogroups A, B, C, W, and Y).5 The quadrivalent meningococcal vaccine MenACWY-TT (Nimenrix®; Pfizer Ltd, Sandwich, UK), which is conjugated to tetanus toxoid, targets serogroups A, C, W, and Y.6 This vaccine is licensed in the European Union for individuals ≥6 weeks of age, with a single dose recommended for individuals ≥12 months of age.6
In a phase 2 primary vaccination study (NCT00427908), healthy toddlers (aged 1–<2 y) received either a single dose of MenACWY-TT vaccine or a monovalent meningococcal C conjugate vaccine (MenC-CRM; Meningitec®, Pfizer [formerly Wyeth Lederle], Philadelphia, PA, USA), and healthy children (aged 2–<11 y) received a single dose of MenACWY-TT vaccine or a quadrivalent meningococcal polysaccharide vaccine (MenACWY-PS; Mencevax®, GlaxoSmithKline, Rixensart, Belgium).7,8 Toddlers and children were initially assessed for antibody persistence over 5 y after primary vaccination. In toddlers vaccinated with MenACWY-TT, 73.5%, 77.6%, 34.7%, and 42.9% had detectable antibodies against serogroups A, C, W, and Y, respectively, 5 y after vaccination. Corresponding percentages in children were 90.8%, 90.8%, 78.6%, and 78.6%.9
Retrospective data indicate that circulating antibodies after primary vaccination with some MenACWY conjugate vaccines wane within 3 to 8 y.2,10 However, it is not currently established whether antibody persistence continues to decrease over longer periods or when a booster dose following vaccination with meningococcal vaccines in childhood is required. Thus, the aim of the current extension study (NCT01962207) was to further evaluate the long-term persistence of antibodies induced by MenACWY-TT versus MenC-CRM and MenACWY-PS from 6 to 10 y after vaccination in individuals 1 to <11 y of age at the time of primary vaccination. In addition, the safety and immunogenicity of a booster dose of MenACWY-TT vaccine administered to all eligible subjects 10 y after primary vaccination were evaluated.
Subjects and methods
Study design and subjects
This phase 3B, open-label, multicenter, extension study (NCT01962207) is based on a phase 2 primary study (MenACWY-TT-027 [108658]; NCT00427908), which has been described previously.7–9 Briefly, the primary study included four parallel groups of healthy subjects: toddlers (aged 1–<2 y) who received MenACWY-TT or MenC-CRM8,9 and children (aged 2–<11 y) who received MenACWY-TT or MenACWY-PS.7,9 In the primary study, subjects were to receive an extra dose of MenC-CRM by Year 5 if a suboptimal antibody response to serogroup C was documented.9 The current study uses the same four parallel groups of healthy subjects as the primary study, referred to here as MenACWY-TT (toddlers), MenC-CRM, MenACWY-TT (children), and MenACWY-PS.
The current extension study consisted of a long-term persistence phase and a booster phase (Figure 1). During the persistence phase, data were collected 6, 7, 8, 9, and 10 y after vaccination with MenACWY-TT, MenC-CRM, or MenACWY-PS in the primary study. At each study visit (Years 6, 7, 8, 9, and 10), a blood sample was collected from each subject enrolled. The booster phase started 10 y after the primary vaccination, when a MenACWY-TT booster dose was administered to all eligible subjects regardless of primary vaccination group and ended with a phone contact at 6 months postbooster.
Figure 1.
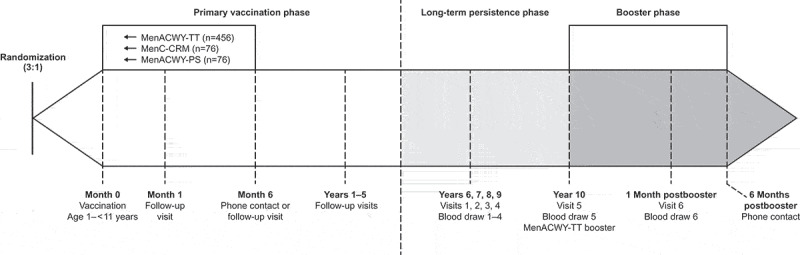
Study design.
This study was conducted in accordance with International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. The study protocol and subsequent amendments were reviewed and approved by local institutional review boards/independent ethics committees. All subjects or their parents or legal representatives provided written informed consent.
To be included in this study, subjects had to be healthy children of either sex who participated in the primary study and were vaccinated with either the MenACWY-TT, MenC-CRM, or MenACWY-PS vaccine. In addition, the subject or subject’s legal guardian had to be able to comply with the requirements of the protocol in the opinion of the investigator and provide written informed consent. Female subjects of childbearing potential could be enrolled in the study if they had a negative pregnancy test on the day of vaccination.
Exclusion criteria included being a child in care (e.g., one who is under the protection of an agency, organization, government body, or foster parent); having been previously vaccinated with a meningococcal polysaccharide or conjugate vaccine outside of the primary study (subjects who were revaccinated with a monovalent MenC conjugate vaccine because of suboptimal serogroup C response during the persistence phase of the primary study were allowed to participate); having a history of meningococcal disease due to serogroup A, C, W, or Y; having been previously vaccinated with meningococcal B vaccine; having an immunodeficient condition, a family history of congenital or hereditary immunodeficiency, or a major congenital defect or serious chronic illness; having a history of drug or alcohol abuse; or withdrawing consent to be contacted for follow-up studies.
Additional exclusion criteria for the booster phase at Year 10 included the use of any investigational product (drug or vaccine) other than the study vaccine within 30 d before the booster dose or planned use during the follow-up period, administration of a vaccine 30 d before or planned administration 30 d after the booster dose (inactivated influenza vaccine allowed), chronic administration of immunosuppressants within 6 months before the booster dose (inhaled and topical steroids allowed), administration of immunoglobulins or blood products within 3 months before the booster dose or planned use during follow-up, a history of any reaction or hypersensitivity likely to be exacerbated by vaccines, a history of neurologic disorders, acute disease and/or fever at the time of vaccination. Female subjects were excluded if they were pregnant or lactating; male and female subjects were excluded if they were planning to discontinue contraception or become pregnant.
Vaccines
In the booster phase, all eligible subjects received a single MenACWY-TT booster dose administered intramuscularly to the deltoid muscle.
Immunogenicity assessments
Functional antibody response against serogroups A, C, W, and Y was assessed by serum bactericidal assays using rabbit (rSBA) or human (hSBA) complement. As both complement assays have been conventionally used as correlate measures of protection and for vaccine licensure,11–13 with sometimes differing results,14 data for both rSBA and hSBA were evaluated in the current study. The primary immunogenicity endpoint was the percentage of subjects with rSBA titers ≥1:8 and ≥1:128 and geometric mean titers (GMTs) for serogroups A, C, W, and Y at 6, 7, 8, 9, and 10 y after primary vaccination. The ≥1:8 rSBA titer threshold is the established correlate of seroprotection for serogroup C,13,15,16 and was extended to other serogroups in the current study, as done in the primary study.7–9 The ≥1:128 cutoff was also evaluated as a more stringent measure of protection, as for the primary study.7-9 Secondary endpoints included the percentage of subjects with (1) hSBA titers ≥1:4 and ≥1:8 and GMTs for serogroups A, C, W, and Y at 6, 7, 8, 9, and 10 y after primary vaccination; (2) rSBA titers ≥1:8 and ≥1:128 and GMTs for serogroups A, C, W, and Y and rSBA booster responses 1 month after booster vaccination; and (3) hSBA titers ≥1:4 and ≥1:8 and GMTs for serogroups A, C, W, and Y and hSBA booster responses 1 month after booster vaccination. An rSBA booster response was defined as rSBA antibody titers ≥1:32 at 1 month after booster vaccination (initially seronegative subjects with prevaccination titer <1:8) or rSBA antibody titers at least four times the prevaccination antibody titers at 1 month after booster vaccination (initially seropositive subjects with prevaccination titer ≥1:8). An hSBA booster response was defined as hSBA antibody titers ≥1:8 at 1 month after booster vaccination (initially seronegative subjects with prevaccination titer <1:4) or hSBA antibody titers at least four times the prevaccination antibody titers at 1 month after booster vaccination (initially seropositive subjects with titer ≥1:4).
Subjects who received additional MenC vaccine because of a suboptimal response as described above were included in the antibody response analyses for serogroups A, C, W, and Y. However, an additional sensitivity analysis was conducted to compare antibody responses in subjects with versus without the additional MenC vaccination.
Safety assessments
In the persistence phase, the occurrence of vaccine-related serious adverse events (SAEs) and any event related to lack of vaccine efficacy (i.e., meningococcal disease) since the last persistence time point in which the subject participated (and up to each yearly visit in the current study) were assessed retrospectively. Safety assessments in the booster phase included reactogenicity events, including solicited local (pain, redness, swelling at injection site) and general (fatigue, fever, gastrointestinal symptoms, headache) symptoms on days 0 to 3 following booster vaccination as recorded on diary cards completed by parents/guardians; unsolicited events up to 31 d following booster vaccination; and SAEs and new-onset chronic illnesses (e.g., autoimmune disorders, asthma, type 1 diabetes, allergies) from administration of the booster vaccine dose until study end. Adverse events (AEs) were recorded and coded according to the Medical Dictionary for Regulatory Activities version 21.0.
Statistical analyses
All analyses were descriptive without adjustment for multiplicity.
Determination of sample size
Assuming enrollment of approximately 80% of the vaccinated population from the primary study and an annual dropout rate of 10%, it was anticipated that approximately 488 subjects would initially participate in the extension phase of this study, which started at Year 6. This included 183 subjects in each of the 2 MenACWY-TT groups and 61 subjects each in the MenC-CRM and MenACWY-PS groups.
Analysis of populations
The according-to-protocol (ATP) population (all eligible subjects who received primary vaccination, complied with blood sampling intervals and the protocol, and had assay results for ≥1 tested antigen) was used in the persistence phase analysis. The following populations were used in the booster phase: the booster total vaccinated population (used for a second immunogenicity analysis and safety analyses; included all subjects from the primary study with a documented booster vaccine administration) and the booster ATP population (used for immunogenicity analyses; included all subjects who received a booster dose of study vaccine, who met previously described inclusion criteria and had no exclusion criteria, and for whom the administration site was known).
Antibody persistence immunogenicity analyses
Descriptive statistics were compiled for Year 6 through Year 10 for rSBA and hSBA data for each antigen assessed. GMTs with 95% CIs and percentages of subjects with titers above the proposed cutoffs with exact 95% CIs were determined for each vaccine group, blood sampling time point, and antigen assessed.
Postbooster immunogenicity analyses
For each vaccine group, at each blood sampling time point (before and 1 month after booster vaccination), and for each antigen examined, the following were assessed: GMTs with 95% CIs, percentages of subjects with booster vaccine response (1 month after booster vaccination), and with rSBA and hSBA titers above the cutoffs with 95% CIs.
For both persistence and postbooster immunogenicity analyses, exploratory comparisons evaluated differences in immune responses between MenACWY-TT (toddlers) and MenC-CRM for serogroup C only and between MenACWY-TT (children) and MenACWY-PS for all serogroups (A, C, W, and Y), all with 95% CIs.
Antibody persistence safety analyses
Safety endpoints were assessed from Year 6 to Year 10 and included all SAEs, AEs, and SAEs leading to withdrawal, SAEs related to study vaccine or any event related to lack of vaccine efficacy, SAEs related to study participation, and intercurrent medical conditions.
Postbooster safety analyses
The postbooster safety analysis was conducted on all subjects receiving a booster vaccination. Safety results are presented as the percentage of subjects with ≥1 local event, with ≥1 general event, or with any AE during the 4-d follow-up period; similar results are presented by AE grade.
Results
Subjects
Of 243 subjects enrolled in the persistence phase, 197, 220, 221, 208, and 191 subjects completed the Year 6, 7, 8, 9, and 10 visits, respectively (Figure 2). The main reason for discontinuation from the persistence phase was withdrawal of consent for reasons other than an AE (12.8%). All 181 subjects enrolled in the booster phase completed the study visits. Median (range) age at entry to this study was 9.0 (7–18) y. Most subjects were white (98.9% [179/181]) and 48.6% (88/181) were boys (Table 1).
Figure 2.
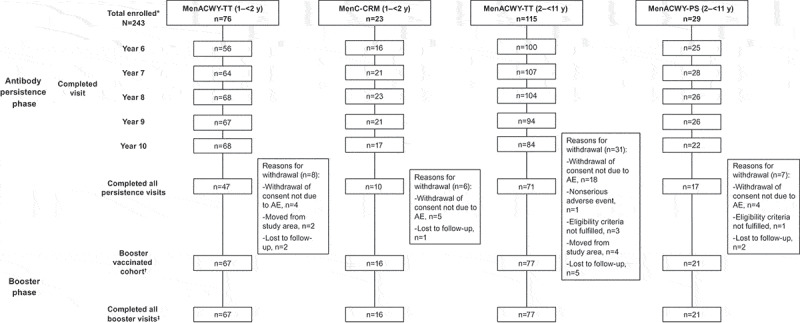
Subject flow chart.
Table 1.
Demographics in the persistence phase and booster phase.
| Characteristic | MenACWY-TT (Toddlers) n = 76 |
MenC-CRM n = 23 |
MenACWY-TT (Children) n = 115 |
MenACWY-PS n = 29 |
|---|---|---|---|---|
| Persistence phase* | ||||
| Age at enrollment (Study Year 6),† y | ||||
| Mean (SD) | 8.2 (0.7) | 8.2 (0.7) | 12.5 (2.6) | 12.1 (2.9) |
| Median (range) | 8.0 (7–10) | 8.0 (7–10) | 13.0 (8–18) | 12.0 (8–16) |
| Sex, n (%) | ||||
| Female | 40 (52.6) | 12 (52.2) | 56 (48.7) | 14 (48.3) |
| Male | 36 (47.4) | 11 (47.8) | 59 (51.3) | 15 (51.7) |
| White race, n (%) |
75 (98.7) |
22 (95.7) |
113 (98.3) |
28 (96.6) |
| Booster phase*‡ | ||||
| Age at enrollment (Year 6),§ y | ||||
| Mean (SD) | 8.2 (0.7) | 8.4 (0.8) | 12.0 (2.5) | 11.8 (2.9) |
| Median (range) | 8.0 (7–10) | 8.0 (7–10) | 12.0 (8–18) | 11.0 (8–16) |
| Sex, n (%) | ||||
| Female | 37 (55.2) | 8 (50.0) | 39 (50.6) | 9 (42.9) |
| Male | 30 (44.8) | 8 (50.0) | 38 (49.4) | 12 (57.1) |
| White race, n (%) | 66 (98.5) | 16 (100) | 77 (100) | 20 (95.2) |
*Date of birth, sex, and race were collected in the primary study.
†Age was computed based on the age at entry into the extension study.
‡All subjects received MenACWY-TT booster vaccine regardless of vaccine received in the primary study.
§Age is based on the age at entry into the extension study.
Immunogenicity in the persistence phase
rSBA responses
In all study groups (i.e., MenACWY-TT [toddlers], MenC-CRM, MenACWY-TT [children], and MenACWY-PS), percentages of participants with rSBA titers ≥1:8 for each serogroup remained stable from Year 6 through Year 10 (Figure 3; Table S1). Ten years after primary vaccination, the percentage of subjects in the MenACWY-TT (toddlers) group with rSBA antibody titers ≥1:8 was 65.6%, 82.8%, 31.3%, and 43.8% for serogroups A, C, W, and Y, respectively. For the MenC-CRM group, rSBA titers ≥1:8 were 88.2% for serogroup C at 10 y after primary vaccination. Exploratory comparisons found no difference in the percentages of subjects with rSBA titers ≥1:8 for serogroup C between the MenACWY-TT (toddlers) and MenC-CRM groups at Year 10 (Table S2).
Figure 3.
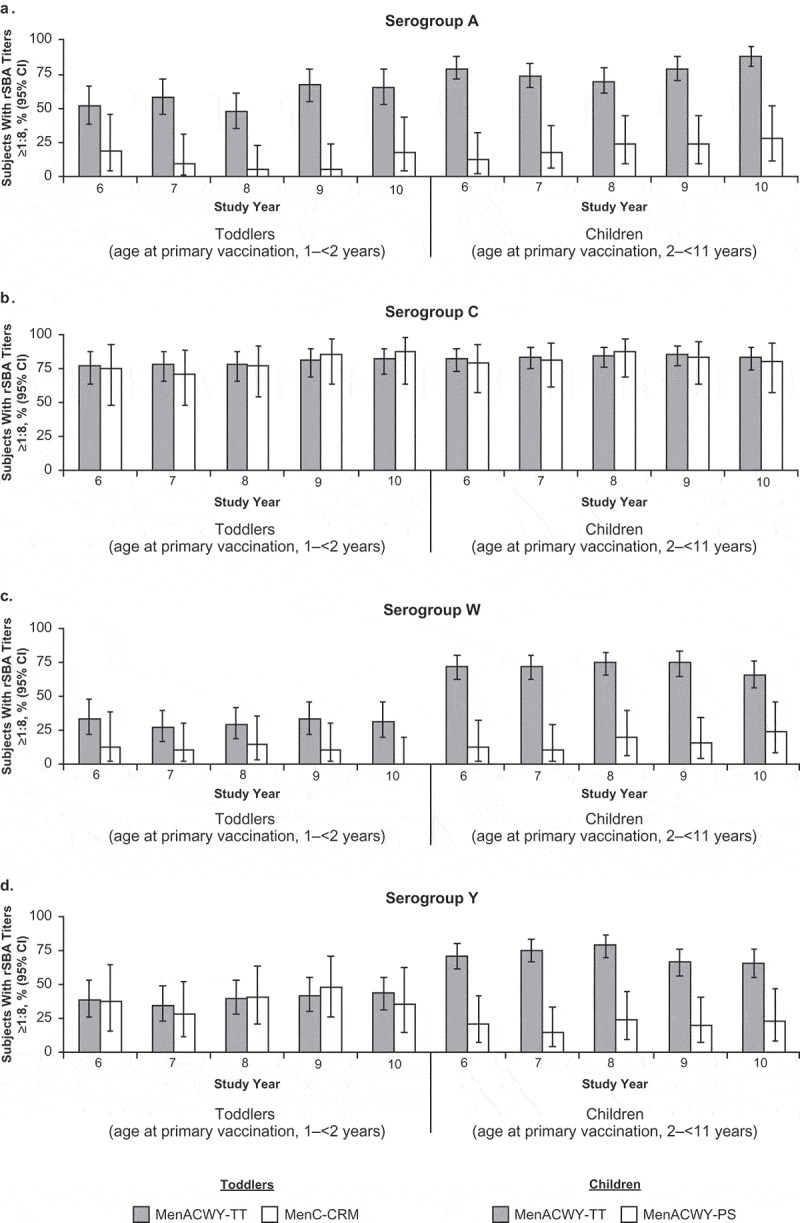
Percentages of subjects with rSBA titers ≥1:8 at 6 to 10 y after primary vaccination with MenACWY-TT, MenC-CRM, or MenACWY-PS (adapted ATP cohort) for (a) serogroup A, (b) serogroup C, (c) serogroup W, and (d) serogroup Y.
A higher percentage of subjects in the MenACWY-TT (children) group compared with the MenACWY-PS group had rSBA antibody titers ≥1:8 for serogroups A, W, and Y (Figure 3; Table S1). Ten years after primary vaccination the percentage of subjects in the MenACWY-TT (children) group with rSBA titers ≥1:8 was 88.9%, 84.1%, 67.1%, and 65.9% for serogroups A, C, W, and Y, respectively. Corresponding percentages of subjects in the MenACWY-PS group with rSBA antibody titers ≥1:8 were 28.6%, 81.0%, 23.8%, and 23.8%, respectively. Exploratory comparisons found that percentages of subjects with rSBA titers ≥1:8 at Year 10 were higher in the MenACWY-TT (children) group than the MenACWY-PS group for all serogroups except serogroup C (Table S2). rSBA antibody titers ≥1:128 and rSBA GMTs during the persistence phase are summarized in Table S1.
When subjects were stratified by whether they received an additional dose of MenC-CRM during the primary study, the percentage of subjects with rSBA titers ≥1:8 for serogroup C was higher at Years 6 through 10 among subjects who received this additional dose (113 in the MenACWY-TT [toddlers] group, 42 in the MenC-CRM group, 86 in the MenACWY-TT [children] group, and 56 in the MenACWY-PS group) compared with subjects who did not (Figure 4; Table S3). Additionally, children who were revaccinated generally showed lower responses for serogroups A, W, and Y compared with children who did not receive an additional MenC-CRM vaccination.
Figure 4.
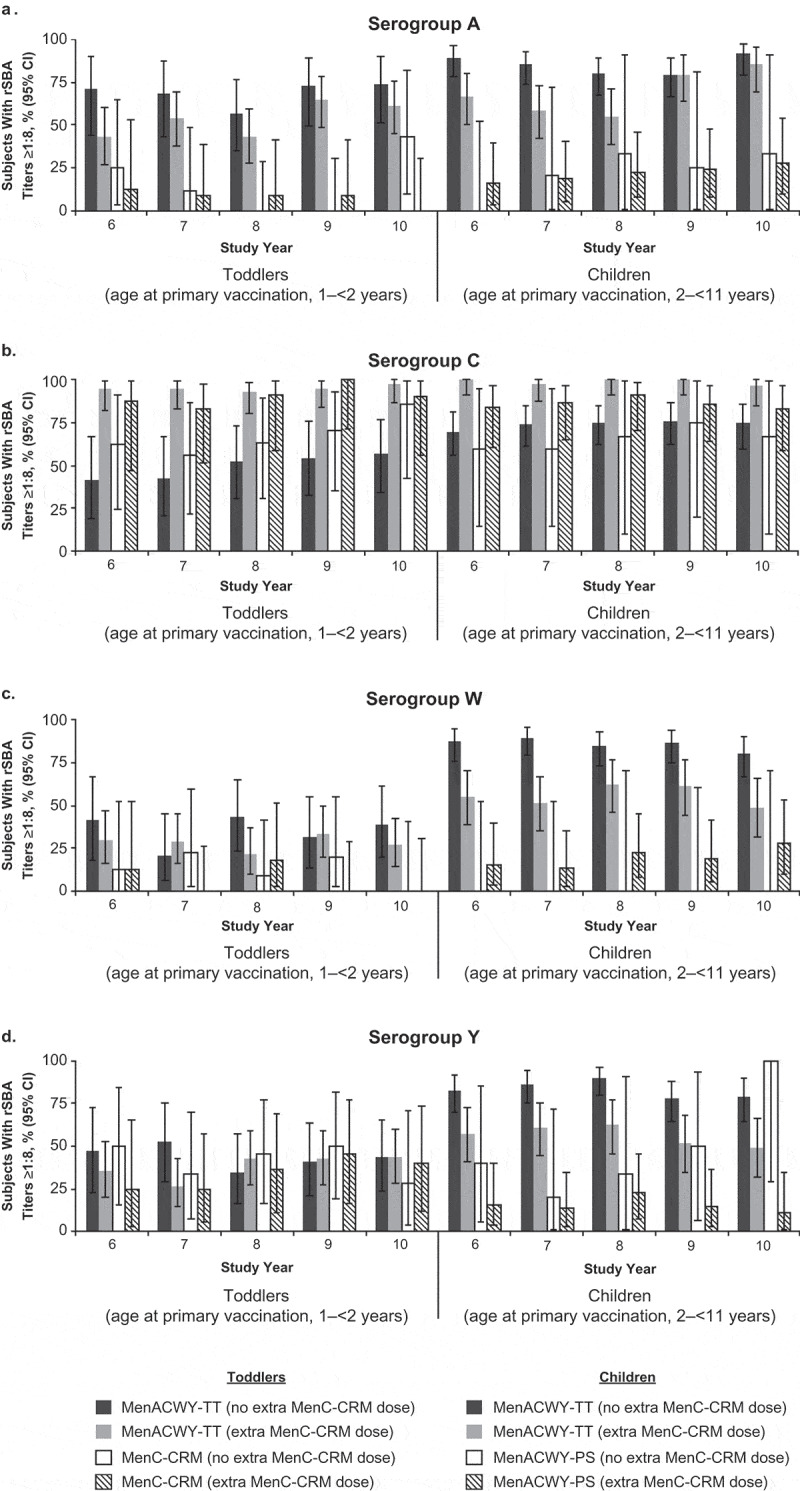
Percentages of subjects with rSBA titers ≥1:8 at 6 to 10 y after primary vaccination among those who did versus did not receive an extra dose of MenC-CRM; persistence phase (adapted ATP cohort) for (a) serogroup A, (b) serogroup C, (c) serogroup W, and (d) serogroup Y.
hSBA responses
Percentages of subjects with hSBA titers ≥1:4 for each serogroup remained stable from Year 6 through Year 10 for all study groups (Figure 5; Table S4). Ten years after primary vaccination, the percentages of subjects in the MenACWY-TT (toddlers) group with hSBA antibody titers ≥1:4 were 31.1%, 91.9%, 44.4%, and 41.4% for serogroups A, C, W, and Y, respectively. In the MenC-CRM group, 93.8% of subjects had hSBA titers ≥1:4 for serogroup C at Year 10. Exploratory comparisons found no difference in the percentages of subjects with hSBA titers ≥1:4 for serogroup C between the MenACWY-TT (toddlers) and MenC-CRM groups at Year 10 (Table S2).
Figure 5.
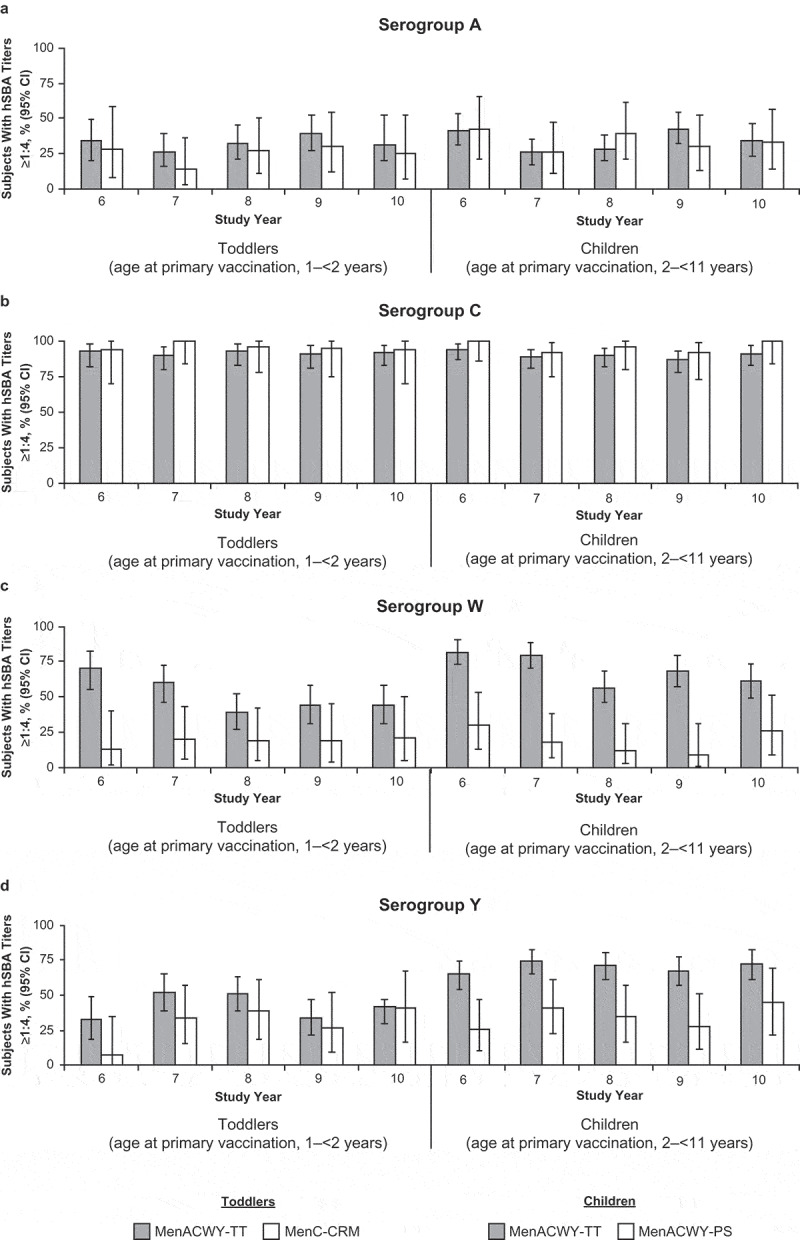
Percentages of subjects with hSBA titers ≥1:4 at 6 to 10 y after primary vaccination with MenACWY-TT, MenC-CRM, or MenACWY-PS (adapted ATP cohort) (a) serogroup A, (b) serogroup C, (c) serogroup W, and (d) serogroup Y.
Ten years after primary vaccination, the percentages of subjects in the MenACWY-TT (children) group with hSBA antibody titers ≥1:4 were 34.8%, 91.1%, 61.2%, and 72.6% for serogroups A, C, W, and Y, respectively. Corresponding percentages with hSBA antibody titers ≥1:4 in the MenACWY-PS group were 33.3%, 100.0%, 26.3%, and 44.4%, respectively. Exploratory comparisons found that percentages of subjects with hSBA titers ≥1:4 were higher in the MenACWY-TT (children) group than the MenACWY-PS group for serogroups W and Y but were similar between study groups for serogroups A and C at Year 10 (Table S2).
Antibody responses determined by hSBA titers ≥1:8 were generally consistent with those determined by hSBA titers ≥1:4 across vaccine groups (Figure S1; Table S4). The hSBA GMTs for each study group are summarized in Table S4.
When subjects were stratified by whether they received an additional dose of MenC-CRM during the primary study, it was found that all subjects in the MenACWY-TT (toddlers) and MenACWY-TT (children) groups who received an additional dose had hSBA titers ≥1:4 for serogroup C from Year 6 to Year 10 (Table S5). Among subjects who did not receive an additional MenC-CRM dose, 66.7% to 77.3% in the MenACWY-TT (toddlers) group and 76.9% to 89.3% in the MenACWY-TT (children) group had hSBA titers ≥1:4 for serogroup C. Similar to observations for rSBA responses, percentages of children with hSBA titers ≥1:4 for serogroups A, W, and Y were generally lower among those who were revaccinated.
Immunogenicity modeling
A repeated-measurement model assessing the effect of subject dropout on antibody persistence from Years 6 to 10 indicated that dropout did not affect the conclusion on the persistency results because the predicted and observed rSBA GMT values for all 4 serogroups were similar for the MenACWY-TT (children) and MenACWY-PS study groups (Figure S2).
Immunogenicity in the booster phase
The percentages of subjects meeting criteria for rSBA vaccine response at 1 month after the MenACWY-TT booster dose ranged from 82.3% to 100.0% across serogroups for primary MenACWY-TT (toddlers) groups, 87.5% to 100.0% for MenC-CRM groups, 75.7% to 100.0% for MenACWY-TT (children) groups, and 88.2% to 100.0% for MenACWY-PS groups (Table 2). Exploratory comparisons found that the percentage of subjects with vaccine responses for serogroup C was similar between the MenACWY-TT (toddlers) and MenC-CRM groups. The percentages of subjects with rSBA responses were similar between the MenACWY-TT (children) and MenACWY-PS groups for all serogroups except serogroup W, for which the percentage was higher in the MenACWY-TT (children) group (Table S6).
Table 2.
Subjects with an rSBA vaccine response and rSBA GMTs before and 1 month after MenACWY-TT booster dose (booster ATP cohort for immunogenicity).
| Vaccine responsea (95% CIb) |
GMT (95% CIc) |
|||||
|---|---|---|---|---|---|---|
| n | 1 month after MenACWY-TT booster |
n | Before MenACWY-TT booster |
n | 1 month after MenACWY-TT booster |
|
| Serogroup A | ||||||
| MenACWY-TT (toddlers) | 62 | 90.3 (80.1, 96.4) | 62 | 28.9 (16.4, 51.0) | 62 | 5122.3 (3725.6, 7042.6) |
| MenC-CRM | 16 | 100.0 (79.4, 100.0) | 16 | 5.9 (3.1, 11.3) | 16 | 4871.0 (2465.1, 9624.9) |
| MenACWY-TT (children) | 73 | 87.7 (77.9, 94.2) | 73 | 96.3 (57.1, 162.5) | 74 | 4626.4 (3040.6, 7039.4) |
| MenACWY-PS | 17 | 94.1 (71.3, 99.9) | 17 | 8.0 (3.3, 19.3) | 17 | 6414.2 (3878.5, 10,607.8) |
| Serogroup C | ||||||
| MenACWY-TT (toddlers) | 62 | 82.3 (70.5, 90.8) | 62 | 128.0 (71.1, 230.6) | 62 | 7163.5 (5478.0, 9367.7) |
| MenC-CRM | 16 | 93.8 (69.8, 99.8) | 16 | 86.7 (29.0, 259.2) | 16 | 5792.6 (3630.6, 9242.2) |
| MenACWY-TT (children) | 74 | 75.7 (64.3, 84.9) | 74 | 181.0 (105.6, 310.3) | 74 | 4020.0 (3319.0, 4869.1) |
| MenACWY-PS | 17 | 94.1 (71.3, 99.9) | 17 | 96.2 (28.9, 320.2) | 17 | 15,101.0 (7099.3, 32,121.5) |
| Serogroup W | ||||||
| MenACWY-TT (toddlers) | 62 | 100.0 (94.2, 100.0) | 62 | 15.8 (9.1, 27.6) | 62 | 25,911.2 (19,119.7, 35,115.2) |
| MenC-CRM | 15 | 100.0 (78.2, 100.0) | 16 | 4.0 (NE, NE) | 15 | 17,970.4 (11,666.4, 27,680.7) |
| MenACWY-TT (children) | 74 | 100.0 (95.1, 100.0) | 74 | 206.4 (108.6, 392.1) | 74 | 27,944.4 (22,213.8, 35,153.3) |
| MenACWY-PS | 17 | 88.2 (63.6, 98.5) | 17 | 15.4 (4.2, 56.4) | 17 | 10,462.5 (3253.5, 33,645.5) |
| Serogroup Y | ||||||
| MenACWY-TT (toddlers) | 62 | 95.2 (86.5, 99.0) | 62 | 27.4 (14.7, 51.0) | 62 | 7660.5 (5262.9, 11,150.3) |
| MenC-CRM | 16 | 87.5 (61.7, 98.4) | 16 | 24.7 (6.0, 100.8) | 16 | 6316.9 (3223.8, 12,377.5) |
| MenACWY-TT (children) | 74 | 93.2 (84.9, 97.8) | 74 | 98.5 (54.3, 178.7) | 74 | 7529.7 (5827.5, 9729.2) |
| MenACWY-PS | 17 | 100.0 (80.5, 100.0) | 17 | 10.2 (3.5, 30.2) | 17 | 6959.2 (3636.7, 13,317.1) |
ATP = according-to-protocol; GMT = geometric mean titer; n = number of subjects with available results; NE = not estimable; rSBA = serum bactericidal antibody assays using rabbit complement.
aVaccine response was defined as an rSBA titer ≥1:32 in subjects who were seronegative (rSBA titer <1:8) before booster vaccination and as a ≥4-fold increase from pre-vaccination titers in subjects who were seropositive (rSBA titer ≥1:8) before vaccination.
bExact 2-sided CIs are based on the observed proportion of subjects using the Clopper and Pearson method.
cCIs are back transformations of confidence levels based on the Student t distribution for the mean logarithm of the concentrations or the mean of the ratio.
At 1 month after the MenACWY-TT booster dose, the percentage of subjects in the MenACWY-TT (toddlers) group with rSBA titers ≥1:8 ranged from 98.4% to 100.0% across serogroups (Table S7). All subjects in the MenC-CRM group achieved rSBA titers ≥1:8 for all four serogroups at 1 month after booster dosing. The percentage of subjects with rSBA titers ≥1:8 at 1 month after the MenACWY-TT booster dose ranged from 95.9% to 100.0% across serogroups in the MenACWY-TT (children) group and 94.1% to 100.0% in the MenACWY-PS group. Robust antibody responses as measured by rSBA titers ≥1:128 at 1 month after MenACWY-TT booster dosing were observed in all study groups. Exploratory comparisons found that percentages of subjects with rSBA titers above both cutoffs for serogroup C were similar between the MenACWY-TT (toddler) and MenC-CRM groups. Percentages were comparable between the MenACWY-TT (children) and MenACWY-PS groups for all serogroups except serogroup W, for which the percentage was higher in the MenACWY-TT (children) group for both cutoffs (Table S6).
A large-fold increase in GMTs was observed 1 month after MenACWY-TT booster dosing in all groups (Table 2). Exploratory comparisons found that GMTs for serogroup C were similar between the MenACWY-TT (toddlers) and MenC-CRM groups. GMTs were lower for serogroup C but higher for serogroup W in the MenACWY-TT (children) group compared with the MenACWY-PS group, but were similar across the two groups for serogroups A and Y (Table S6).
In all study groups, the percentage of subjects with rSBA titers ≥1:8 at 1 month after the MenACWY-TT booster dose ranged from 92.9% to 100.0% across serogroups in subjects who did not receive an extra dose of MenC-CRM and 93.8% to 100.0% in subjects who received an extra dose of MenC-CRM during the primary study owing to suboptimal antibody response to serogroup C (Table S8). In the MenACWY-TT (toddlers) and MenACWY-TT (children) groups, all subjects had hSBA titers ≥1:4 at 1 month after the MenACWY-TT booster dose regardless of whether they received an extra dose of MenC-CRM (Table S9).
Safety
Persistence phase
Two (1.7%) subjects in the MenACWY-TT (children) group experienced an AE between the last study visit in the primary study and Year 10 of follow-up study (one event each of juvenile idiopathic arthritis and depression). The AE of depression resulted in withdrawal from the study. In the same period, no other AEs, including SAEs, were reported.
Booster phase
Pain was the most common local event following MenACWY-TT booster dosing in all four study groups (reported by 56.3%–61.9% of subjects across all study groups; Figure 6; Table S10); redness and swelling were reported by 23.8%–43.8% and 6.3%–23.0%, respectively. Most local events were mild to moderate in severity; severe events were reported by ≤9.5% of subjects in each study group. Fatigue, gastrointestinal events, headache, and fever were reported by 12.5%–31.3%, 0.0%–17.6%, 20.9%–31.3%, and 0.0%–1.5% across all study groups, respectively, after MenACWY-TT booster dosing. Severe general events (i.e., fatigue, gastrointestinal events, headache) were reported by ≤6.8% of subjects in each study group. No subjects experienced fever >39.5°C.
Figure 6.
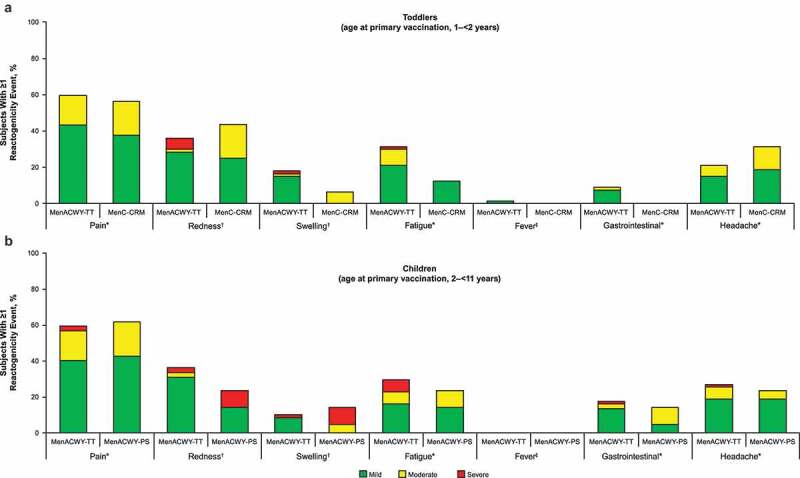
Reactogenicity events during the 4-d postbooster vaccination phase (booster total vaccinated cohort) in (a) toddlers and (b) children.
After MenACWY-TT booster dosing, AEs were reported by 23.9%, 31.3%, 35.1%, and 52.4% of subjects in the MenACWY-TT (toddlers), MenC-CRM, MenACWY-TT (children), and MenACWY-PS groups, respectively (Table 3). Corresponding percentages for related AEs were 4.5%, 6.3%, 15.6%, and 4.8%. Severe AEs occurred in 2.6% – 9.5% of MenACWY-TT booster recipients across all study groups; none was considered related to the booster vaccination. One subject experienced an SAE (abdominal pain in the MenACWY-TT [children] group), which was not considered related to vaccination. Two subjects experienced new-onset chronic illnesses (asthma in one subject in the MenACWY-TT [children] group and celiac disease in one subject in the MenACWY-PS group).
Table 3.
Adverse events during the MenACWY-TT booster phase (total booster-vaccinated cohort).
| Type of event, n (%) | MenACWY-TT (toddlers) n = 67 |
MenC-CRM n = 16 |
MenACWY-TT (children) n = 77 |
MenACWY-PS n = 21 |
|---|---|---|---|---|
| All AEs | 16 (23.9) | 5 (31.3) | 27 (35.1) | 11 (52.4) |
| AEs reported in ≥5% of subjects in any group | ||||
| Pyrexia | 2 (3.0) | 2 (12.5) | 1 (1.3) | 0 |
| Upper respiratory tract infection | 1 (1.5) | 1 (6.3) | 7 (9.1) | 2 (9.5) |
| Headache | 4 (6.0) | 1 (6.3) | 3 (3.9) | 0 |
| Injection site pruritus | 1 (1.5) | 1 (6.3) | 1 (1.3) | 0 |
| Nasopharyngitis | 0 | 1 (6.3) | 1 (1.3) | 1 (4.8) |
| Respiratory tract infection | 0 | 1 (6.3) | 0 | 1 (4.8) |
| Fatigue | 0 | 1 (6.3) | 0 | 0 |
| Foot fracture | 0 | 1 (6.3) | 0 | 0 |
| Relateda AEs | 3 (4.5) | 1 (6.3) | 12 (15.6) | 1 (4.8) |
| Lymph node pain | 0 | 0 | 1 (1.3) | 0 |
| Axillary pain | 1 (1.5) | 0 | 0 | 1 (4.8) |
| Injection site bruising | 2 (3.0) | 0 | 0 | 0 |
| Injection site hypoesthesia | 0 | 0 | 1 (1.3) | 0 |
| Injection site pruritus | 1 (1.5) | 1 (6.3) | 1 (1.3) | 0 |
| Muscle tightness | 0 | 0 | 1 (1.3) | 0 |
| Musculoskeletal pain | 0 | 0 | 1 (1.3) | 0 |
| Hypoesthesia | 0 | 0 | 1 (1.3) | 0 |
| Insomnia | 0 | 0 | 1 (1.3) | 0 |
| Cough | 0 | 0 | 1 (1.3) | 0 |
| Oropharyngeal pain | 0 | 0 | 1 (1.3) | 0 |
| All SAEs | 0 | 0 | 1 (1.3) | 0 |
| Abdominal pain | 0 | 0 | 1 (1.3) | 0 |
| Relateda SAEs | 0 | 0 | 0 | 0 |
| Fatal SAEs | 0 | 0 | 0 | 0 |
| All severe AEs | 5 (7.5) | 1 (6.3) | 2 (2.6) | 2 (9.5) |
| Relateda severe AEs | 0 | 0 | 0 | 0 |
AE = adverse event; n (%) = number (percentage) of subjects reporting the event at least once; SAE = serious adverse event.
aRelationship to MenACWY-TT as assessed by the investigator.
The classification of AEs is based on the Medical Dictionary for Regulatory Activities Version 21.0.
Discussion
Ten years after primary vaccination of toddlers (aged 1–<2 y) with MenACWY-TT, rSBA titers ≥1:8 persisted in the majority of subjects for serogroups A and C, but not for serogroups W and Y; antibody persistence for serogroup C was similar between toddlers who received a primary MenACWY-TT or MenC-CRM vaccination. However, the majority of children (aged 2–<11 y) who received primary vaccination with MenACWY-TT had rSBA titers ≥1:8 for all serogroups at Year 10. In addition, those children had higher antibody persistence at 10 y for serogroups A, W, and Y versus children who received primary vaccination with MenACWY-PS. These findings are consistent with those observed 5 y after primary vaccination.9
In subjects who received MenACWY-TT, there was a clear difference in antibody persistence observed according to the age of primary vaccination (Figures 3 and 5). Subjects vaccinated as children held protective antibodies against serogroups W and Y more commonly than those vaccinated as toddlers; the difference for serogroup A was less pronounced but visible. A MenACWY-TT booster dose administered 10 y after primary vaccination resulted in robust immunologic responses in all subjects, regardless of meningococcal vaccine used for primary immunization. Consistent with studies showing robust immunologic responses to MenACWY booster doses in adolescents primed with a MenC vaccine at preschool age,17,18 these findings suggest that a booster dose of MenACWY-TT might be a suitable option for adolescents who previously received a MenC-CRM, MenACWY-PS, or MenACWY-TT vaccine in early childhood.
Safety results with up to 10 y of follow-up after primary vaccination did not reveal any new safety signals. In addition, immunogenicity, safety, and tolerability findings following MenACWY-TT booster dosing are consistent with the established safety profile of the vaccine in other age groups, including children, adolescents, and young adults.6–8,19-22
During the primary study, subjects with suboptimal responses to serogroup C were to receive an extra dose of MenC-CRM.9 It was believed that the risk of contracting meningococcal serogroup C (MenC) disease during the follow-up was far greater than that of other meningococcal serogroups included in the vaccine.23 Moreover, MenC vaccine was licensed and part of routine immunization in many European countries whereas MenACWY vaccine was not.9 Titers for serogroup C were higher among subjects who received an additional dose of MenC-CRM compared with subjects who did not, suggesting that the addition of MenC vaccine resulted in higher antibody persistence (Figure 4). Additionally, those who had a suboptimal antibody response to serogroup C also showed a comparatively lower antibody response to the other meningococcal serogroups.
The long-term persistence of antibody responses following primary vaccination with conjugate meningococcal vaccines continues to be elucidated. Retrospective data suggest that circulating antibodies after vaccination with some MenACWY conjugate vaccines decrease within 3 to 8 y after primary vaccination.2,10 To our knowledge, this is the only study to assess antibody persistence up to 10 y after primary MenACWY vaccination in toddlers and children. In addition, this study complements antibody persistence data available up to approximately 5 y after primary MenACWY-TT vaccination reported in other age groups, including toddlers, children, adolescents, and adults.19,20,24,25
Limitations of this study include lack of a MenACWY conjugate vaccine comparator group (notably, no quadrivalent meningococcal conjugate vaccines were licensed for toddlers or children at the time of the primary study).9,26 As described previously, the rSBA assay was changed during the persistence phase, limiting comparison of persistence results to those at earlier timepoints.9 Subject dropout during the persistence phase is also a limitation; however, the modeling analysis results indicated subject dropout did not affect overall outcomes. Additionally, as all analyses conducted in this study were exploratory in nature, formal conclusions regarding between-group differences could not be made.
Conclusions
Functional antibody responses persisted 10 y after one dose of MenACWY-TT in toddlers and children, indicating long-term protection against meningococcal A, C, W, and Y disease. A booster dose of MenACWY-TT was well tolerated and elicited robust immune responses, suggesting induction of immunologic memory. These results support the administration of a MenACWY-TT booster dose in adolescents who were vaccinated with MenACWY-TT, MenC-CRM, or MenACWY-PS as toddlers or children.
Supplementary Material
Acknowledgments
Editorial/medical writing support was provided by Jill E. Kolesar, PhD, and Tricia Newell, PhD, of Complete Healthcare Communications, LLC (North Wales, PA), a CHC Group company, and was funded by Pfizer Inc.
Funding Statement
This study was funded by Pfizer Inc.
Data sharing statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the United States and/or Europe or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Disclosure of potential conflicts of interest
Drs Peyrani, Webber, Cutler, Li, and Perez are employees of Pfizer. Drs Van Der Wielen, Cheuvart, De Schrevel, and Aris and Ms Bianco are employees of GlaxoSmithKline. Dr Vesikari is an investigator for vaccine studies sponsored by Pfizer.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21645515.2020.1746110.
References
- 1.Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meer HC, Baker CJ, Messonnier NE.. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR–2):1–28. doi:rr6202a1 [pii]. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . Chapter 14: meningococcal disease. Public Health Foundation. [accessed 2019 June3] https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/mening.pdf.
- 3.European Centre for Disease Prevention and Control . Surveillance Atlas of infectious diseases. [accessed 2019 June3]. https://ecdc.europa.eu/en/surveillance-atlas-infectious-diseases.
- 4.Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, Pondo T, Arnold KE, Baumbach J, Bennett N, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998-2007: implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50(2):184–91. doi: 10.1086/649209. [DOI] [PubMed] [Google Scholar]
- 5.MacNeil J, Cohn A. Chapter 8: meningococcal disease. Manual for the surveillance of vaccine-preventable diseases. 5th ed. Atlanta (GA): Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 6.Nimenrix® (meningococcal polysaccharide serogroups A, C, W-135 and Y conjugate vaccine) . Summary of product characteristics. Sandwich (Kent, UK): Pfizer Limited; 2019. [Google Scholar]
- 7.Vesikari T, Forsten A, Boutriau D, Bianco V, Van der Wielen M, Miller JM. A randomized study to assess the immunogenicity, antibody persistence and safety of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in children aged 2-10 years. Hum Vaccin Immunother. 2012;8(12):1882–91. doi: 10.4161/hv.22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vesikari T, Forsten A, Boutriau D, Bianco V, Van der Wielen M, Miller JM. Randomized trial to assess the immunogenicity, safety and antibody persistence up to three years after a single dose of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in toddlers. Hum Vaccin Immunother. 2012;8(12):1892–903. doi: 10.4161/hv.22166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vesikari T, Forsten A, Bianco V, Van der Wielen M, Miller JM. Antibody persistence up to 5 years after vaccination of toddlers and children between 12 months and 10 years of age with a quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine. Hum Vaccin Immunother. 2016;12(1):132–39. doi: 10.1080/21645515.2015.1058457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohn AC, MacNeil JR, Harrison LH, Lynfield R, Reingold A, Schaffner W, Zell ER, Plikaytis B, Wang X, Messonnier NE, et al. Effectiveness and duration of protection of one dose of a meningococcal conjugate vaccine. Pediatrics. 2017;139(2):e20162193. doi: 10.1542/peds.2016-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . Meningococcal vaccines: WHO position paper, November 2011. Wkly Epidemiol Rec. 2011;86(47):521–39. [PubMed] [Google Scholar]
- 12.World Health Organization . WHO expert committee on biological standardization: twenty-seventh report. [accessed 2018 August3]. http://apps.who.int/iris/bitstream/handle/10665/37954/WHO_TRS_594.pdf?sequence=1&isAllowed=y.
- 13.Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine. 2005;23(17–18):2222–27. doi: 10.1016/j.vaccine.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 14.Findlow J, Balmer P, Borrow R. A review of complement sources used in serum bactericidal assays for evaluating immune responses to meningococcal ACWY conjugate vaccines. Hum Vaccin Immunother. 2019;15(10):2491–500. doi: 10.1080/21645515.2019.1593082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol. 2003;10(5):780–86. doi: 10.1128/CDLI.10.5.780-786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol. 2010;17(5):840–47. doi: 10.1128/CVI.00529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Ravenhorst MB, van der Klis FRM, van Rooijen DM, Sanders EAM, Berbers GAM. Adolescent meningococcal serogroup A, W and Y immune responses following immunization with quadrivalent meningococcal A, C, W and Y conjugate vaccine: optimal age for vaccination. Vaccine. 2017;35(36):4753–60. doi: 10.1016/j.vaccine.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Ishola DA, Andrews N, Waight P, Yung CF, Southern J, Bai X, Findlow H, Matheson M, England A, Hallis B, et al. Randomized trial to compare the immunogenicity and safety of a CRM or TT conjugated quadrivalent meningococcal vaccine in teenagers who received a CRM or TT conjugated serogroup C vaccine at preschool age. Pediatr Infect Dis J. 2015;34(8):865–74. doi: 10.1097/INF.0000000000000750. [DOI] [PubMed] [Google Scholar]
- 19.Knuf M, Helm K, Kolhe D, Van Der Wielen M, Baine Y. Antibody persistence and booster response 68 months after vaccination at 2-10 years of age with one dose of MenACWY-TT conjugate vaccine. Vaccine. 2018;36(23):3286–95. doi: 10.1016/j.vaccine.2018.04.064. [DOI] [PubMed] [Google Scholar]
- 20.Klein NP, Baine Y, Kolhe D, Baccarini CI, Miller JM, Van der Wielen M. Five-year antibody persistence and booster response after 1 or 2 doses of meningococcal A, C, W and Y tetanus toxoid conjugate vaccine in healthy children. Pediatr Infect Dis J. 2016;35(6):662–72. doi: 10.1097/INF.0000000000001123. [DOI] [PubMed] [Google Scholar]
- 21.Vesikari T, Forsten A, Bianco V, Van der Wielen M, Miller JM. Immunogenicity, safety and antibody persistence of a booster dose of quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine compared with monovalent meningococcal serogroup c vaccine administered four years after primary vaccination using the same vaccines. Pediatr Infect Dis J. 2015;34(12):e298–307. doi: 10.1097/INF.0000000000000897. [DOI] [PubMed] [Google Scholar]
- 22.Baxter R, Baine Y, Kolhe D, Baccarini CI, Miller JM, Van der Wielen M. Five-year antibody persistence and booster response to a single dose of meningococcal A, C, W and Y tetanus toxoid conjugate vaccine in adolescents and young adults: an open, randomized trial. Pediatr Infect Dis J. 2015;34(11):1236–43. doi: 10.1097/INF.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 23.European Centre for Disease Prevention and Control . Disease data from ECDC surveillance atlas for meningococcal disease. [accessed 2019 March20]. https://ecdc.europa.eu/en/meningococcal-disease/surveillance-and-disease-data/atlas.
- 24.Quiambao BP, Bavdekar A, Dubey AP, Jain H, Kolhe D, Bianco V, Miller JM, Van der Wielen M. Antibody persistence up to 5 y after vaccination with a quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine in adolescents. Hum Vaccin Immunother. 2017;13(3):636–44. doi: 10.1080/21645515.2016.1248009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borja-Tabora CF, Montalban C, Memish ZA, Boutriau D, Kolhe D, Miller JM, Van der Wielen M. Long-term immunogenicity and safety after a single dose of the quadrivalent meningococcal serogroups A, C, W, and Y tetanus toxoid conjugate vaccine in adolescents and adults: 5-year follow-up of an open, randomized trial. BMC Infect Dis. 2015;15:409. doi: 10.1186/s12879-015-1138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Memish ZA, Goubeaud A, Broker M, Malerczyk C, Shibl AM. Invasive meningococcal disease and travel. J Infect Public Health. 2010;3(4):143–51. doi: 10.1016/j.jiph.2010.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Centers for Disease Control and Prevention . Chapter 14: meningococcal disease. Public Health Foundation. [accessed 2019 June3] https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/mening.pdf.
- European Centre for Disease Prevention and Control . Surveillance Atlas of infectious diseases. [accessed 2019 June3]. https://ecdc.europa.eu/en/surveillance-atlas-infectious-diseases.
- World Health Organization . WHO expert committee on biological standardization: twenty-seventh report. [accessed 2018 August3]. http://apps.who.int/iris/bitstream/handle/10665/37954/WHO_TRS_594.pdf?sequence=1&isAllowed=y.
- European Centre for Disease Prevention and Control . Disease data from ECDC surveillance atlas for meningococcal disease. [accessed 2019 March20]. https://ecdc.europa.eu/en/meningococcal-disease/surveillance-and-disease-data/atlas.


