ABSTRACT
Hepatitis E virus (HEV) is responsible for epidemic and sporadic acute hepatitis cases, especially in developing countries. Hepatitis E has become a vaccine-preventable disease in recent years with the development of a licensed vaccine. Most functional and neutralizing monoclonal antibodies (mAbs) are known to be highly sensitive to antigen conformation. In this study, a similar approach was used to characterize the conformational sensitivity of antibodies in human or mouse serum samples. Interestingly, comparative binding analysis using different antigen forms showed that the antibodies in the sera of naturally infected individuals, of human vaccinees and from mice immunized with the HEV p239 vaccine all exhibited a strong preference to particulate antigens over the monomeric form of the truncated capsid protein. The degree of discriminating the two test antigens is similar for serum samples to that for the well-characterized murine mAbs. A functional assay for assessing the inhibition of subviral particle cell entry by antibodies was used to determine the functional titers of anti-HEV antibodies in mouse sera. A good correlation was observed between the functional and binding titers in mouse sera determined using two different methods. This result supports the continued use of the enzyme-linked immunosorbent assay as the primary serological assay assuming that the coating antigen contains conformational and native-like epitopes, as in the case for HEV p239.
KEYWORDS: Conformational epitope, functional assessment, hepatitis E virus, HEV p239 vaccine, particulate antigen, neutralizing antibody, serological assay
Introduction
Hepatitis E virus (HEV) causes an annual 35 million symptomatic and asymptomatic infection case worldwide, resulting in more than 70,000 deaths.1 The average mortality rate is between 0.2% ~ 0.4%, while pregnant women infected with HEV may have a case fatality rate of 10% ~ 25%.2 HEV infection usually presents as an acute, self-limiting form of liver inflammation and extrahepatic manifestations such as neurological and renal disease.3 However, acute hepatitis can progress to chronic hepatitis, cirrhosis, liver failure and acute-on-chronic liver failure.3 HEV infection with underlying chronic liver disease may lead to a higher mortality rate of up to 75%.4 Thus, hepatitis E has become a serious threat to public health and has received significant attention.5,6
HEV is a quasi-enveloped, single-stranded, positive-sense RNA virus that is approximately 27 ~ 34 nm in diameter.7 The HEV genome consists of three discontinuous and partially overlapping open reading frames (ORFs), ORF1, ORF2, and ORF3, among which ORF2 encodes a structural protein (pORF2) constituting the viral capsid that is comprised of 660 amino acids (aa).8 Immunodominant epitopes have been found in the E2s domain (aa 459–606) and can form tight homodimers. This region was also shown to be essential for viral-host interaction.9 The 66 aa extension toward the N-terminus from the E2s domain appears to stabilize the dimeric structure of E2 (aa 394–606), rendering it a useful diagnostic agent.10 However, several pivotal amino acids (T564, V598, A599, L601 and A602) have been identified to be involved in E2 dimerization.11 The mutant protein in this study, named p213 (aa 394–606), appeared in the monomeric form, as the Thr residue was mutated to Ala (T564A) in dimer interface regions.11 Moreover, the additional 27 aa extension toward the N-terminus from the E2 domain results in the formation of multimeric p239 particles (aa 368–606), rendering this construct more suitable as a vaccine candidate with substantially enhanced immunogenicity.10,12 The HEV p239 vaccine was shown to be well tolerated and protected against HEV infection with an efficacy of 100% (95% CI: 72.1% ~ 100%) after three vaccine doses.13 The HEV p239 vaccine with the trade name Hecolin® was commercialized for human use in China in 2012.13,14 Another vaccine with p459 virus-like particles (VLPs) as an antigen showed high efficacy [95.5% (95% CI: 85.6% ~ 98.6%)] against HEV infection in a phase II trial (Figure 1(a)). However, given some obstacles, GlaxoSmithKline (GSK) halted the development of this vaccine.15
Figure 1.

Characterization of the different truncated versions of HEV pORF2. (a) Schematic representation of different truncated versions of HEV pORF2. The E2s protein (aa 459–606), the shortest version of the dimer form, harbors the major neutralizing epitopes.11 The p213 peptide (aa 394–606) existed in a monomeric form due to Thr residues being mutated to Ala (T564A).11 p239 (aa 368–606) is the vaccine antigen in Hecolin® in a particulate form.13 p495 (aa 112–608), which can form VLPs with icosahedral symmetry, is used in the other HEV vaccine that has been tested in a phase II clinical trial.15 (b) Schematic representation of p239 particle and p213 monomer evolution. p239 and p213 were used for subsequent analysis. The caveat is particulate p239 and monomeric p213 differ in size by 26-aa. (c) The retention times in SEC-HPLC for p239 and p213 were 13.7 min and 18.2 min, respectively. The mutant p213 peptide exists as a monomer with an apparent MW of ~20 kDa. The MW of p239 particles was estimated to be ~3200 kDa.18 (d) Differential scanning calorimetry (DSC) profiles of the p239 particle and p213 monomer. The melting transitions of the p239 particle and p213 monomer were estimated at peak Tm values of 73.79°C and 67.61°C, respectively. (e) SDS-PAGE analysis of p239 particles and p213 monomers. Lanes marked with “N” indicate the sample under non-reducing conditions (in the absence of β-mercaptoethanol and not heated). Lanes marked with “D” indicate the sample under reducing conditions (mixed with β-mercaptoethanol and heated at 100°C for 10 min). (f) The morphologies of the p239 particle and p213 monomer were detected by transmission electron microscopy (TEM). The scale bar was 100 nm.
The mAbs recognizing conformational epitopes against ORF2 have been raised as probes to study the critical epitopes and understand the humoral immune response to HEV ORF2-based vaccines.16 Comprehensive epitope mapping and clustering have been used to develop a tool box with representative mAbs recognizing conformational and linear epitopes.16,17 Among these mAbs, several broad neutralizing mAbs (8C11, 8G12 and 9F7) recognize conformational neutralizing epitopes in the dimerization region on ORF2 and can protect rhesus macaques from HEV challenge.16-19 Thus, these critical mAbs are usually used for the quality analysis and immune response assessment of vaccines. Previous studies showed that high immunoreactivity of convalescent sera from HEV-infected patients was demonstrated for recombinant pORF2, particularly to dimeric antigens, but not to monomeric antigens, indicating that reactive epitopes are conformational.20,21 The presence of these epitopes may be important for developing immunity to HEV infection. These findings led to the very intriguing question of whether most neutralizing antibodies elicited by the HEV p239 vaccine can recognize similar epitopes, like these critical mAbs, and answering this question would greatly increase our understanding of the protective mechanism of HEV vaccines.
In this study, we analyzed the characteristics of the human immune response, primarily assessing the behaviors of the elicited anti-HEV antibodies that were induced by the native virus and the HEV p239-based vaccine. The representative murine mAbs recognizing conformational neutralizing epitopes were analyzed quantitatively with respect to their binding preferences for particulate vs. monomeric capsid protein antigens. This index of preferential binding to a particulate antigen over the monomeric antigen was used to compare the properties of well-characterized mAbs to the binding properties of murine and human serum samples. In addition, the correlation between the binding titers and functional titers of antibodies in vaccine-immunized mouse sera was observed, confirming that practice of using direct-binding ELISA as a primary serological assay, such a simple and straightforward assay can be used to assess HEV vaccine immunogenicity.
Materials and methods
Cell lines, monoclonal antibodies (mAbs), recombinant HEV antigen and other reagents
HepG2 cells were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). HEV capsid protein-specific mAbs (8C11, 8G12, 9F7, 3A11, 12A10, 16D7) were obtained in our laboratory as previously described.12,16-19 The recombinant p239 antigen and p213 antigen were expressed in an E. coli system as previously described elsewhere.12 Allophycocyanin (APC)-conjugated streptavidin and a goat anti-mouse polyclonal antibody conjugated with horseradish peroxidase (HRP) were purchased from Invitrogen (Waltham, MA, USA). 3,3ʹ,5ʹ,5ʹ-Tetramethylbenzidine (TMB) liquid substrate was purchased from Innovax (Xiamen, Fujian, China). Biotin labeling of p239 was performed according to the protocols of Molecular Probes (Grand Island, NY, USA).
Serum samples
In each prefilled syringe, the HEV p239 vaccine Hecolin® (Innovax, Xiamen, China) contains 30 μg of the purified antigen adsorbed to 0.8 mg of aluminum hydroxide suspended in 0.5 mL of buffered saline.7 The vaccine was administered intramuscularly in 3-dose regimens at months 0, 1 and 6. Vaccine-immunized human sera were collected one month after the last immunization.7,13 The convalescent patient sera were obtained from Huashan Sub-Hospital of Fudan University in Shanghai.
Balb/C female mice, aged 6 weeks, were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). The 32 Balb/C mice were divided randomly into two groups. The mice in the two groups were injected with three doses intramuscularly in the thigh at interval of 2 weeks (Week 0, 2, 4) with 0.5 μg and 1.0 μg doses of the vaccine in 0.1 mL, respectively. Serum samples were collected prior to injection and then collected every week after the first immunization.
Differential scanning calorimetry (DSC)
DSC analyses of p239 and p213 were performed using a MicroCal VP-DSC instrument (GE Healthcare, Northampton, MA, USA). Protein samples (0.2 mg/mL) were measured at a heating rate of 1.5°C/min using a scanning temperature that ranged from 10°C to 90°C. The software program MicroCal Origin 7.0 (Origin-Lab Corp, Northampton, MA, USA) was used to calculate the melting temperatures (Tm) based on the melting curves.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Polyacrylamide gels comprising 12% acrylamide in the separating gel and 5% acrylamide in the stacking gel were used. Protein samples were mixed with equal volumes of loading buffer (100 mmol/L Tris-HCl pH 6.8, 200 mmol/L β-mercaptoethanol, 4% sodium dodecyl sulfate, 0.2% bromophenol blue and 20% glycerol). Sample mixtures were heated at 100°C for 10 min and subsequently loaded onto the separating gel. For the non-reducing SDS gel, the buffer contained only 0.1% SDS and no β-mercaptoethanol, and the sample was not boiled.
Size exclusion chromatography-high-performance liquid chromatography (SEC-HPLC)
p239 and p213 were subjected to size analysis under native conditions at a neutral pH of 7.4. These proteins were separated by chromatography using a Agilent 1200 HPLC system (Agilent Technologies, Santa Clara, CA, USA) with an analytical TSK Gel PW5000xl 7.8 mm × 300 mm column (TOSOH, Tokyo, Japan) after column equilibration and conditioning in phosphate buffer saline (PBS). The flow rate was maintained at 0.5 mL/min, and the protein signals in the eluents were detected at 280 nm.
Enzyme-linked immunosorbent assay (ELISA)
Briefly, p239 and denatured p213 (100 ng/well) were coated on 96-well microplates. For denatured p213, the antigen was treated with dithiothreitol in carbonate buffer (pH = 9.6) and dried overnight at 37°C to achieve denaturation on the solid surface in 96-well microtiter plates. The plates were blocked with assay dilution buffer (PBS containing 0.5% casein, 2% gelatin and 0.1% proclin) for 2 h. The wells were then incubated with two-fold serial dilutions of sera or mAbs for 1 h at 37°C. After five washes, an HRP-conjugated goat anti-mouse IgG antibody was added for 1 h at 37°C. After five washes, TMB (100 µL/well) was added for 15 min at 37°C. The reaction was stopped by the addition of 50 μL of 2 mol/L H2SO4, and the absorbance was read at 450 nm (reference, 620 nm) using an automated ELISA reader (TECAN, Männedorf, Switzerland).
The cutoff value was defined as the average of that of the negative control mice plus three times the standard deviation.22 The binding titer of vaccine-immunized mouse sera was expressed as the endpoint titer, which was defined as the highest dilution reaching an absorbance value equal to or higher than the cutoff value. The ratio of absorbance values at 450 nm (particle/monomer) at a serum dilution of 1:160 or 1:20 represents the fold change in the immunoreactivity of serum against particles and monomers. The binding activities of mAbs to particles and monomers are expressed as the median effective concentration (EC50, ng/mL). EC50 is defined as the antibody concentration for 50% maximal binding. The ratio of EC50 values (EC50, monomer/EC50, particle) represents the fold change in the binding activity of a mAb to particles and monomers. Data analysis was performed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA).
In vitro functional titers assay of mouse sera
The functional titers of pooled mouse sera from multiple mice for blocking the cellular entry of subviral particles (p239) were assessed using a previously described flow cytometry neutralization assay.23 Briefly, mixtures of biotin-conjugated p239 (2.0 μg/mL) and two-fold serial dilutions (beginning with a dilution of 1:5) of sera were added to a 96-well culture plate seeded with HepG2 cells (5 × 104 per well) and then incubated at 37°C for 30 min. After washing three times, the cells were reincubated with a 1:100 dilution of allophycocyanin-conjugated streptavidin for 30 min. The cells were then washed with PBS and subjected to flow cytometry (Beckman Coulter, Cary, NC, USA). The mock-adsorbed cells were treated equally and used for background. The percentage of positive cells was measured.
The functional titers of mouse sera are expressed as the neutralization titer (NT50), defined as the dilution required to inhibit 50% of p239 particles from cellular entry. According to the binding titers obtained in Week 11, the sera of two different dose groups (0.5 μg and 1.0 μg) were divided into three grades (high, medium and low levels). Sera at the same level were mixed in equal volumes.
Statistical analysis
Statistical analyses of correlations in the data obtained from the ELISA-based assay and cell-based assay were performed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA). Four-parameter logistic fit curve-fitting was performed for the direct-binding ELISA to derive EC50 values.
Ethics statement
All animal studies were conducted in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Research Council of the National Academies. The experimental procedures and animal use and care protocols were carried out in accordance with the guidelines of the Xiamen University Institutional Committee for the Care and Use of Laboratory Animals and were approved by the Committee on Ethical Use of Animals of Xiamen University. The study with samples from individuals was carried out in accordance with Chinese rules and regulations for the protection of human subjects. Written informed consent was obtained from each subject.
Results
Characterization of recombinant HEV capsid proteins
The different truncated versions of HEV pORF2 were overexpressed, and they were found to spontaneously form various types of protein assemblies, with one specific truncation and the mutation construct, the truncated viral capsid protein remains as the monomeric form in aqueous solution. The particulate and monomeric antigens were used to assess the preferential binding behaviors of antibodies in naturally infected patient sera and HEV p239 vaccine-immunized sera from humans and mice. The assembled forms of different truncated versions of HEV pORF2 were characterized for their assembly status, and their expected assembly forms were also confirmed prior to experiments for antigenicity assays (Figure 1). The p239 peptide (HEV p239), the antigen in the licensed vaccine Hecolin®, can self-assemble into a particulate form with a total of 239 amino acids (aa 368–606) (Figures 1(a,b)). To assess the binding properties of different antibodies, a monomeric form of the capsid protein was designed and expressed. p213, a shorter truncated version of pORF2 with a total of 213 amino acids (aa 394–606), was demonstrated to exist as a monomer by mutating Thr residues to Ala (T564A) (Figure 1(b)).11 The retention times in SEC-HPLC for p239 and p213 were 13.7 min and 18.2 min, respectively (Figure 1(c)); this reflects the much smaller molecular size of p213 compared with that of p239 in solution. In DSC thermograms, a 6.8°C change in the melting temperature (Tm) was discernible between p239 and p213 (Figure 1(d)), supporting the notion that the monomeric p213 has a much less ordered structure than p239. Aside from the high-order structures, the molecular weights of the protein monomers were not that different. Under non-reducing SDS-PAGE conditions (Figure 1(e)), p239 proteins presented predominantly as homodimers with a molecular mass of ~45 kDa, whereas p213 proteins presented as monomers with a molecular mass of ~20 kDa. Both proteins became monomeric after denaturation under more stringent denaturing conditions (Figure 1(e) Lane D). Finally, in negative-stain transmission electron microscopy, p239 presented as particles with a range of diameters of 20 ~ 30 nm. No particles were observed in the transmission electron microscopy (TEM) images for p213 (Figure 1(f)), confirming that p213 existed in the monomeric form in aqueous solution.
Strong preferential binding to conformational epitopes of human anti-HEV antibodies
To determine the characteristics of immune responses induced by natural virions and the HEV p239 vaccine, sera from naturally infected patients and vaccine-immunized humans were tested against particulate and monomeric antigens, respectively. The comparative binding assays yielded a quantitative index of binding to particulate vs. monomeric antigens for a given test sample. The fold change in the immunoreactivity of sera against particles and monomers was expressed as the ratio of absorbance values at 450 nm (particle/monomer) in parallel experiments run under otherwise identical conditions. The immunoreactivities of the naturally infected patient sera at dilutions of 1:160 and 1:20 were more than ~10- to 20-fold and ~5- to 10-fold higher than the immunoreactivity against the monomeric antigen, respectively (Figures 2(a,c)). Similarly, the immunoreactivities of sera from vaccinees at dilutions of 1:160 and 1:20 against the particulate antigen were more than ~10- to 20-fold and ~5- to 10-fold higher than the immunoreactivity against the monomeric antigen, respectively (Figures 2(b,c)). Furthermore, the endpoint titers were derived for all the test samples. The binding titers for particulate antigen were approximately ~15- to 25-fold higher than those for binding to the monomeric antigen in parallel experiments (Table S1). Therefore, naturally infected patient sera and vaccine-immunized human sera showed comparable preference for binding the particulate antigen over the monomeric antigen, supporting the notion that the p239 antigen mimics the conformational epitopes on virions with high fidelity.
Figure 2.
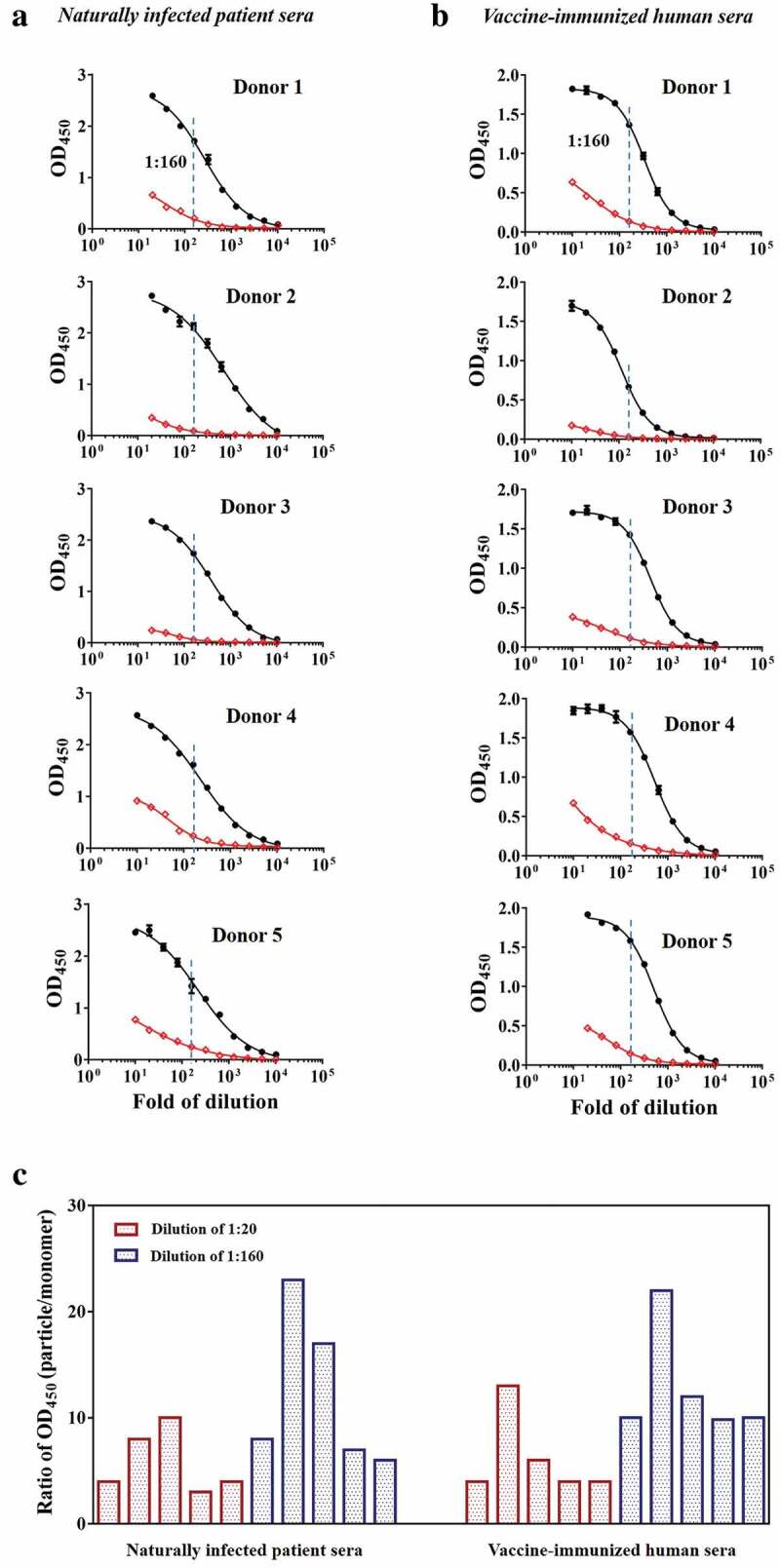
The immunoreactivities of naturally infected patient sera and vaccine-immunized human sera against particles and monomers. The sera showed different immunoreactivities against the particle (black curve) and monomer (red curve) forms. The fold change in the immunoreactivity of sera against particles and monomers was expressed as the ratio of absorbance values at 450 nm (particle/monomer) at a serum dilution of 1:160. (a) Naturally infected patient sera. (b) Vaccine-immunized human sera. (c) Fold changes in the immunoreactivities of naturally infected patient sera and vaccine-immunized human sera at 1:20 and 1:160 dilutions against particles and monomers.
Conformational sensitivity of murine mAbs
A panel of mAbs with non-overlapping recognizing epitopes is a critical asset in the epitope characterization of vaccine antigens and in the characterization of elicited antibodies in vaccine-immunized human or animal sera. Six murine mAbs were analyzed with respect to their conformational sensitivity using the previously mentioned particulate and monomeric antigens in parallel as plate-coating antigens. Three of the mAbs (8C11, 8G12 and 9F7) are functional, neutralizing antibodies that recognize three independent conformational antigenic sites on the HEV capsid (Table S2). In addition, 3A11 and 16D7 recognize linear non-neutralizing epitopes, whereas 12A10 recognizes a linear neutralizing epitope. The binding activities are expressed as EC50 values (ng/mL; a smaller EC50 value is indicative of higher binding activity) (Table S1 and S2). The fold change in the binding activities of mAbs to particles and monomers was expressed as the ratio of EC50 values (EC50, monomer/EC50, particle) or relative binding activity. The mAbs (12A10, 3A11, and 16D7) targeting surface linear epitopes presented comparable binding activities to particulate and monomeric forms (Figure 3(a)). By contrast, the mAbs (8G12, 8C11 and 9F7) targeting conformational epitopes demonstrated a ~20- to 90-fold higher preferential binding to the particulate over the monomeric antigen (Figure 3(b)). The degree of discrimination for 8C11 was higher than that of 8G12 and 9F7, coupled to the fact that 8C11 can capture the authentic HEV virions in solution efficiently,18 suggesting that the structural requirement for the 8C11 epitope on the viral capsid is highly stringent.
Figure 3.

The binding activities of six representative murine mAbs to particles and monomers. (a) mAbs recognizing linear epitopes (3A11, 12A10 and 16D7) to particles (black curve) and monomers (red curve). (b) mAbs recognizing conformational epitopes (8C11, 8G12, and 9F7) to particles (black curve) and monomers (red curve). The neutralizing mAb 8C11 showed more pronounced (>50-fold higher) preferential binding to the particulate over the monomeric antigen than the other two mAbs (8G12 and 9F7) tested in the same comparative study.
Conformation-sensitivity of antibodies elicited by 3-dose immunization in mice
Although the mouse model is widely used in vaccine potency evaluation, existing discrepancies in the immune response between mice and humans could not be overlooked. To further study whether the HEV p239 vaccine is capable of eliciting antibodies targeting conformational and neutralizing epitopes in mice, 32 mice were immunized with a 3-dose regimen using the HEV p239 vaccine to mimic vaccination procedures in humans (Figure 4(a)). The anti-HEV binding titers of mouse serum samples were monitored using HEV p239 as coating antigen (Fig. S1). The mouse sera in Week 9 and 10 were used to evaluate the immunoreactivity against particulate and monomeric antigens (Figures 4(b,c)). Similar to the results above, notably different binding activities of mouse sera to particulate antigen over monomeric antigen were observed. A strong preference of immunoreactivity to particulate antigen over monomeric antigen was demonstrated in the vaccine-immunized mouse sera. Specifically, the immunoreactivity of mouse sera at a dilution of 1:160 against particle was ~5- to 15-fold higher than that against monomer based on absorbance values in ELISA. Using endpoint titers, the mouse sera showed a ~ 40- to 130-fold difference with particulate antigen vs. monomeric antigen as coating antigen (Figure 4(c) and Table S1). The extent of prominent preference for binding conformational epitopes of mouse sera is comparable to those of the functional mAbs 8C11 and 9F7 (> 50- and ~90-fold), whereas the relatively moderate fold change of ~10- to 20-fold in the binding titers of human sera is similar to that of the mAb 8G12 (~20-fold) (Figure 4(c)).
Figure 4.

The immunoreactivities of vaccine-immunized mouse sera against particles and monomers. (a) The 32 Balb/C mice were divided into two groups and immunized intramuscularly at three doses at an interval of two weeks (Week 0, 2, 4) with 0.5 µg and 1.0 µg of the p239 protein, respectively. Serum samples were collected once a week (weeks 0–12). (b) The immunoreactivities of sera pooled from multiple mice against particles (black curve) and monomers (red curve). Sera from mice in the same group were mixed at equal volumes. S-a and S-b represent the serum samples from mice at Week 9 administered doses of 0.5 µg and 1.0 µg, respectively; S-c and S-d represent the serum samples from mice at Week 10 administered doses of 0.5 µg and 1.0 µg, respectively. The fold change in the immunoreactivity was expressed as the ratio of absorbance values at 450 nm (particle/monomer) at a mouse serum dilution of 1:160. (c) The fold changes in the reductions of binding activities of vaccine-immunized human sera, mouse sera and murine mAbs to particulate vs. monomeric antigens. The left y-axis depicts the ratio of the endpoint titer (particle/monomer), which represents the fold changes in the reductions of the activities of vaccine-immunized human sera and mouse sera. The ratio of EC50 values (EC50, monomer/EC50, particle) represents the fold change in the reduction of mAb activity. The right y-axis depicts the ratio of absorbance values at 450 nm (particle/monomer) at a serum dilution of 1:160.
Correlation between binding titers and functional titers
Recently, a cell-based assay was developed for a surrogate assessment of the functionality of anti-HEV antibodies by measuring the efficiency of viral neutralization.23 The assay is based on fluorescence-labeled p239 for the measurement of cell entry (Figure 5(a)). The binding titers (endpoint titers) and functional titers (NT50) of mouse sera are summarized in Table S3. A good correlation was observed between the binding titers and functional titers, with a Pearson’s correlation coefficient of 0.9117 (Figure 5(b) and Fig. S2). Therefore, the binding activities of antibodies in mouse sera, as reflected to the binding to epitopes on recombinant HEV p239, made a measurable contribution to the serum functional activity in response to HEV.
Figure 5.

The correlation between binding titers and functional titers. (a) The flow cytometry neutralization assay was used to evaluate the functional titers of vaccine-immunized mouse sera based on fluorescence-labeled p239 for the measurement of cell entry.23 The functional titer was expressed as NT50, which was defined as the dilution required to inhibit 50% of p239 particles from cellular entry. (b) The binding titer was plotted against the corresponding functional titer for the same set of pooled sera. The sera were divided into three grades (high, medium and low levels) based on the binding titers of mouse sera at Week 11; sera at the same level were mixed at equal volumes. All data listed in Table S3 were used for Pearson correlation assay between the binding titers and functional titers. The correlations between binding titers and functional titers were assessed by Pearson correlation analysis. The correlation coefficient was calculated using GraphPad Prism 7.0.
Discussion
The recombinant HEV capsid protein, or pORF2, can spontaneously assemble into virus-like or subviral particles. These particulate antigens were shown to have antigenic properties similar to those of the capsid of the infectious virus based on immunochemical and structural studies.21,24 The native virus capsid and p239 particle share certain common antigenic determinants, especially the conformational determinants associated with its particulate form. Regarding the two truncated HEV recombinant proteins of ORF2 used in this study, particulate p239 and monomeric p213 share a common region (aa 394–606) that encompasses linear epitopes (aa 394–495) and immunodominant conformational epitopes (aa 495–606).11 The antigenic activity of p239 particles is related to their tertiary and quaternary structures. The conformational epitopes are comprised of multi-peptide segments folded in proximity to form a given epitope. In contrast, a linear epitope is based on a linear peptide with a stretch of a continuously arranged series of amino acids comprising the primary structure of the monomeric peptide, as demonstrated by p213.
The strong preference for binding to the conformational epitopes in p239 (over monomeric p213) is indicative that in hepatitis E human convalescent serum samples are predominantly the functional and conformation-sensitive antibodies induced by persistent and chronic infection. This observation is consistent with a previously reported phenomenon in which convalescent patient sera reacted well with predominantly pORF2 dimers, not monomers, in dot blot or Western blot assays, whereas the serum samples from the acute phase were less discriminating.20,21,25 Since acute patient sera should contain both IgM and IgG molecules capable of binding to dimers and monomers, the convalescent serum immune response post maturation should consist of primary IgGs with further affinity maturation.21,25,26 The IgGs should be more focused on the more immunodominant epitopes on the pORF2 capsid protein after an affinity maturation process. These clinically relevant epitopes, mapped to the minimal unit of dimers on the viral capsid, are the structural features that diagnostic agents (for measuring meaningful titers in serum) and vaccine antigens (for eliciting functional titers via immunization) should mimic faithfully for the native virions.
Using the vaccine antigen HEV p239 (in a licensed vaccine), we showed that the vaccine-immunized human sera tested strongly preferred the particulate antigen over the monomeric antigen, highly similar to the convalescent serum samples regarding the degree of discrimination between conformational vs. linear epitopes. The data strongly suggest that the overall conformation and fine-epitope structures in the recombinant vaccine antigen are largely the same as those of the native virion capsid. Sera from both naturally infected and vaccine-immunized humans failed to show significant binding activity to the monomeric antigen p213, indicating that only a very small portion of antibodies were directed toward the unfolded monomeric capsid protein. This result is indicative of a successful antigen design and bioprocessing during vaccine development using HEV p239 as the antigen in this HEV prophylactic vaccine.
In general, the neutralizing and immunodominant epitopes on viral antigens are generally conformational epitopes. Thus, the immunogenic structure of a viral protein, mimicking that of the native virions, is essential for the antigen design and development of prophylactic vaccines.24,27 Related immunochemical tools, such as mAbs with non-overlapping recognizing epitopes, are critical for the epitope characterization of vaccine antigens and the characterization of antibodies elicited by vaccines.24,28,29 More importantly, because of the importance of the 8C11 and 8G12 epitopes (Figure 6(a)), these functional and neutralizing mAbs were used in the assays to assess vaccine antigen integrity and stability.17-19 The broad neutralizing antibodies (8C11, 8G12, 9F7) showed strong preferential binding to the particulate over the monomeric antigen. The degree of discrimination for these murine mAbs was also similar to that of the human sera (from naturally infected or vaccinated individuals) or mouse sera (with 3-dose immunization). Specifically, 8G12 could significantly block the binding of HEV convalescent sera as well as HEV p239 vaccine-immunized human sera to the vaccine antigen.19 These data suggest that the neutralizing antibodies in sera elicited by the HEV p239 vaccine, showing comparable the degree of conformational preference on viral capsid antigen with mAbs (8C11, 8G12, 9F7), should play key roles in fending off HEV infection.
Figure 6.

Epitopes of HEV ORF2 recognized by mAbs and the different methods of HEV virus neutralization by functional antibodies. (a) HEV pORF2 and a schematic representation of mAb binding sites localized within the HEV capsid protein. 12A10, 16D7, and 3A11 are the mAbs recognizing the linear epitopes located in aa 423–438, aa 428–442, and aa 443–457, respectively. 8C11, 8G12 and 9F7 are the mAbs recognizing the conformational epitopes located in aa 459–606. (b) The different methods of HEV virus neutralization by functional antibodies.
While it is critically important to measure the virus neutralization efficiency in sera samples from vaccinees, there is no convenient way to measure the functional activity of HEV neutralizing antibody. The conventional methods are not only laborious but are also restricted by the limited HEV infection efficiency in vitro.30 The pseudovirus system is also infeasible for assessing the functionality of anti-HEV antibodies.24 As a surrogate functional assay, a cell-based assay was developed for assessment of the functionality of anti-HEV antibodies based on fluorescence-labeled p239 to detect the antibody blocking of virus/subviral particle entry into cells.23 This p239 blocking assay was shown to be highly specific and sufficiently sensitive to evaluate the functional antibodies in sera.23,31 Good correlation was observed between the binding titers and functional titers of antibodies in mouse sera, indicating that the convenient ELISA-based assay with HEV p239 as coating antigen might be used as a primary serological assay for assessing functional antibodies after immunization with a vaccine. Interestedly, Thönes et al.22 also showed a good correlation between antibody results obtained from enzyme-linked immunosorbent assays and neutralization assays for sera from human papillomavirus (HPV) VLP-immunized mice.
Prophylactic vaccines confer protection against viral infection by inducing effective functional antibody titers. There are several potential mechanisms by which functional antibodies could prevent or suppress viral infection (Figure 6(b)).32 They can directly block virus attachment to target cells by interfering with virus-receptor interactions (attachment). They could also block conformational changes and the requisite interactions between the viral and endosomal membranes required for fusion (cellular entry). Moreover, the antibodies can also prohibit virus uncoating, leading to the inhibition of RNA release. Protection from virus infection was conferred by such an efficacious prophylactic vaccine,13 based on the recombinant version of the sole capsid protein of the virions.
Recently, Yin et al.33 reported that a secreted form of the ORF2 protein (ORF2S) was observed and characterized in HEV-infected rhesus macaque or patient serum. ORF2S exhibits substantial conformational epitopes overlapping with the capsid protein and could induce immune response generated conformational neutralizing antibodies.33 Importantly, both HEV antigen (ORF2S) and nucleic acids (HEV RNA) showed long-term persistence (5 ~ 7 weeks) in the serum or urine samples from HEV-infected monkeys and humans.34,35 ORF2S antigen remained in HEV-infected patient sera, raising the possibility of decoying, leading to partial or full depletion of neutralizing antibodies in the convalescent and acute sera.33-36 Thus, hepatitis E patients may be re-infected at a later time. ORF2S antigen in HEV-infected patient sera could reduce the protective efficiency after vaccination with HEV vaccine. Therefore, further characterization of ORF2S, particularly on the kinetics of ORF2S clearance, should be of clinical importance.
In summary, vaccination with the HEV p239 vaccine can confer protection from HEV infection. This protection mainly depends on the elicitation of functional antibodies recognizing conformational neutralizing epitopes. The generation of functional antibodies is dependent on the correct antigen conformation and native-like epitope presentation on the VLP surface in the vaccines. Similar to the conformation-sensitive nature of some functional murine monoclonal antibodies, a strong preference for binding to a well-assembled particulate capsid antigen over a monomeric form was observed for viral infection- or vaccination-induced antibodies in humans. This is another line of evidence that recombinant p239 in a licensed vaccine harbors clinically relevant and highly conformation-sensitive epitopes. Using a series of mouse sera, a good correlation was observed between ELISA-based binding titers and cell-based functional titers, confirming that the convenient direct-binding ELISA with properly folded proteins as coating antigens can be used as the primary serological assay for assessing vaccine immunogenicity. Future efforts should be invested to analyze correlation between the functional and binding titers in human serum samples from vaccines to further support the use of ELISA as high throughput assays for clinical studies.
Acknowledgments
The authors would like to acknowledge Ms. Min Li and Ms. Wei Cai from National Institute of Diagnostics and Vaccine Development in Infectious Diseases at Xiamen University for their technical assistance.
Funding Statement
This work was supported by National Natural Science Foundation of China [81871247 & 31670939], and National Key Project of China on Major Infectious Diseases [2018ZX10101001-002].
Abbreviations
- aa
Amino acid
- APC
Allophycocyanin
- DSC
Differential scanning calorimetry
- ELISA
Enzyme-linked immunosorbent assay
- GSK
GlaxoSmithKline
- HEV
Hepatitis E virus
- HPV
Human papillomavirus
- HRP
Horseradish peroxidase
- mAbs
Monoclonal antibodies
- ORFs
Open reading frames
- PBS
Phosphate buffer saline
- SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SEC-HPLC
Size exclusion chromatography-high-performance liquid chromatography
- TMB
Tetramethylbenzidine
- TEM
Transmission electron microscopy
- VLPs
Virus like-particles
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Wu X, Chen P, Lin H, Hao X, Liang Z.. Hepatitis E virus: current epidemiology and vaccine. Hum Vaccin Immunother. 2016;12:2603–10. PMID:27184971. doi: 10.1080/21645515.2016.1184806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988–97. PMID:22121109. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 3.Nan Y, Zhang YJ. Molecular biology and infection of hepatitis E virus. Front Microbiol. 2016;7:1419. PMID:27656178. doi: 10.3389/fmicb.2016.01419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navaneethan U, Al Mohajer M, Shata MT. Hepatitis E and pregnancy: understanding the pathogenesis. Liver Int. 2008;28:1190–99. PMID:18662274. doi: 10.1111/j.1478-3231.2008.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalton HR, Hazeldine S, Banks M, Ijaz S, Bendall R. Locally acquired hepatitis E in chronic liver disease. Lancet. 2007;369:1260. PMID:17434400. doi: 10.1016/S0140-6736(07)60595-9. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Gracia MT, Suay-Garcia B, Mateos-Lindemann ML. Hepatitis E and pregnancy: current state. Rev Med Virol. 2017;27:e1929. PMID:28318080. doi: 10.1002/rmv.1929. [DOI] [PubMed] [Google Scholar]
- 7.Li SW, Zhao Q, Wu T, Chen S, Zhang J, Xia NS. The development of a recombinant hepatitis E vaccine HEV 239. Hum Vaccin Immunother. 2015;11:908–14. PMID:25714510. doi: 10.1080/21645515.2015.1008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Lázaro D, Hernandez M, Cook N. Hepatitis E virus: a new foodborne zoonotic concern. Adv Food Nutr Res. 2018;86:55–70. PMID:30077224. doi: 10.1016/bs.afnr.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Emerson SU, Nguyen HT, Torian U, Burke D, Engle R, Purcell RH. Release of genotype 1 hepatitis E virus from cultured hepatoma and polarized intestinal cells depends on open reading frame 3 protein and requires an intact PXXP motif. J Virol. 2010;84:9059–69. PMID:20610720. doi: 10.1128/JVI.00593-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Q, Zhang J, Wu T, Li SW, Ng MH, Xia NS, Shih JW. Antigenic determinants of hepatitis E virus and vaccine-induced immunogenicity and efficacy. J Gastroenterol. 2013;48:159–68. PMID:23149436. doi: 10.1007/s00535-012-0701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Tang X, Seetharaman J, Yang C, Gu Y, Zhang J, Du H, Shih JW, Hew CL, Sivaraman J, et al. Dimerization of hepatitis E virus capsid protein E2s domain is essential for virus-host interaction. PLoS Pathog. 2009;5:e1000537. PMID:19662165;. doi: 10.1371/journal.ppat.1000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li SW, Zhang J, Li YM, Ou SH, Huang GY, He ZQ, Ge SX, Xian YL, Pang SQ, Ng MH, et al. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine. 2005;23:2893–901. PMID:15780738. doi: 10.1016/j.vaccine.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 13.Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895–902. PMID:20728932. doi: 10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Shih JW, Xia NS. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:2265–66. PMID:26039604. doi: 10.1056/NEJMoa1406011. [DOI] [PubMed] [Google Scholar]
- 15.Innis BL, Lynch JA. Immunization against Hepatitis E. Cold Spring Harb Perspect Med. 2018;8:a032573. PMID:29530951. doi: 10.1101/cshperspect.a032573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao M, Li XJ, Tang ZM, Yang F, Wang SL, Cai W, Zhang K, Xia NS, Zheng ZZ. A comprehensive study of neutralizing antigenic sites on the Hepatitis E Virus (HEV) capsid by constructing, clustering, and characterizing a tool box. J Biol Chem. 2015;290:19910–22. PMID:26085097. doi: 10.1074/jbc.M115.649764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang X, Yang C, Gu Y, Song C, Zhang X, Wang Y, Zhang J, Hew CL, Li S, Xia N, et al. Structural basis for the neutralization and genotype specificity of hepatitis E virus. Proc Natl Acad Sci USA. 2011;108:10266–71. PMID:21642534. doi: 10.1073/pnas.1101309108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei M, Zhang X, Yu H, Tang ZM, Wang K, Li Z, Zheng Z, Li S, Zhang J, Xia N, et al. Bacteria expressed hepatitis E virus capsid proteins maintain virion-like epitopes. Vaccine. 2014;32:2859–65. PMID:24662711. doi: 10.1016/j.vaccine.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Gu Y, Tang X, Zhang X, Song C, Zheng M, Wang K, Zhang J, Ng MH, Hew CL, Li S, et al. Structural basis for the neutralization of hepatitis E virus by a cross-genotype antibody. Cell Res. 2015;25:604–20. PMID:25793314. doi: 10.1038/cr.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Zhang J, Xia N. Lessons from hepatitis E vaccine design. Curr Opin Virol. 2015;11:130–36. PMID:25913817. doi: 10.1016/j.coviro.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Riddell MA, Li F, Anderson DA. Identification of immunodominant and conformational epitopes in the capsid protein of hepatitis E virus by using monoclonal antibodies. J Virol. 2000;74:8011–17. PMID:10933710. doi: 10.1128/jvi.74.17.8011-8017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thönes N, Herreiner A, Schädlich L, Piuko K, Müller M. A direct comparison of human papillomavirus type 16 L1 particles reveals a lower immunogenicity of capsomeres than viruslike particles with respect to the induced antibody response. J Virol. 2008;82:5472–85. PMID:18385253. doi: 10.1128/JVI.02482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai W, Tang ZM, Wen GP, Wang SL, Ji WF, Yang M, Ying D, Zheng ZZ, Xia NS. A high-throughput neutralizing assay for antibodies and sera against hepatitis E virus. Sci Rep. 2016;6:25141. PMID:27122081. doi: 10.1038/srep25141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Xin L, Li S, Fang M, Zhang J, Xia N, Zhao Q. Lessons learned from successful human vaccines: delineating key epitopes by dissecting the capsid proteins. Hum Vaccin Immunother. 2015;11:1277–92. PMID:25751641. doi: 10.1080/21645515.2015.1016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang JZ, Im SW, Lau SH, Chau TN, Lai ST, Ng SP, Peiris M, Tse C, Ng TK, Ng MH. Occurrence of hepatitis E virus IgM, low avidity IgG serum antibodies, and viremia in sporadic cases of non-A, -B, and -C acute hepatitis. J Med Virol. 2002;66:40–48. PMID:11748657. doi: 10.1002/jmv.2109. [DOI] [PubMed] [Google Scholar]
- 26.Khudyakov Y, Kamili S. Serological diagnostics of hepatitis E virus infection. Virus Res. 2011;161:84–92. PMID:21704091. doi: 10.1016/j.virusres.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Yin XC, Wang X, Zhang ZG, Li Y, Lin Z, Pan H, Gu Y, Li S, Zhang J, Xia N, et al. Demonstration of real-time and accelerated stability of hepatitis E vaccine with a combination of different physicochemical and immunochemical methods. J Pharm Biomed Anal. 2019;177:112880. PMID:31546137. doi: 10.1016/j.jpba.2019.112880. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Q, Modis Y, High K, Towne V, Meng Y, Wang Y, Alexandroff J, Brown M, Carragher B, Potter CS, et al. Disassembly and reassembly of human papillomavirus virus-like particles produces more virion-like antibody reactivity. Virol J. 2012;9:52. PMID:22356831. doi: 10.1186/1743-422X-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Li M, Lin Z, Pan H, Tang Z, Zheng Z, Li S, Zhang J, Xia N, Zhao Q, et al. Multifaceted characterization of recombinant protein-based vaccines: an immunochemical toolbox for epitope-specific analyses of the hepatitis E vaccine. Vaccine. 2018;36:7650–58. PMID:30396752. doi: 10.1016/j.vaccine.2018.10.089. [DOI] [PubMed] [Google Scholar]
- 30.Wu X, Chen P, Lin H, Su Y, Hao X, Cao Y, Li L, Zhu F, Liang Z. Dynamics of 8G12 competitive antibody in “prime-boost” vaccination of Hepatitis E vaccine. Hum Vaccin Immunother. 2017;13:1–6. PMID:28272983. doi: 10.1080/21645515.2017.1291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu C, Cai W, Yin X, Tang Z, Wen G, Ambardekar C, Li X, Ying D, Feng Z, Zheng Z, et al. An optimized high-throughput neutralization assay for Hepatitis E Virus (HEV) involving detection of secreted porf2. Viruses. 2019;11:64. PMID: 30650547. doi: 10.3390/v11010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marasco WA, Sui J. The growth and potential of human antiviral monoclonal antibody therapeutics. Nat Biotechnol. 2007;25:1421–34. PMID:18066039. doi: 10.1038/nbt1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin X, Ying D, Lhomme S, Tang Z, Walker CM, Xia N, Zheng Z, Feng Z. Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection. Proc Natl Acad Sci USA. 2018;115:4773–78. PMID:29669922. doi: 10.1073/pnas.1721345115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geng Y, Zhao C, Huang W, Harrison TJ, Zhang H, Geng K, Wang Y. Detection and assessment of infectivity of hepatitis E virus in urine. J Hepatol. 2016;64:37–43. PMID:26362822. doi: 10.1016/j.jhep.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 35.Wen GP, Tang ZM, Yang F, Zhang K, Ji WF, Cai W, Huang SJ, Wu T, Zhang J, Zheng ZZ, et al. A valuable antigen detection method for diagnosis of acute hepatitis E. J Clin Microbiol. 2015;53:782–88. PMID:25540394 https://jcm.asm.org/content/53/3/782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montpellier C, Wychowski C, Sayed IM, Meunier JC, Saliou JM, Ankavay M, Bull A, Pillez A, Abravanel F, Helle F, et al. Hepatitis E virus lifecycle and identification of 3 forms of the ORF2 capsid protein. Gastroenterology. 2018;154:211–23. PMID:28958858. doi: 10.1053/j.gastro.2017.09.020. [DOI] [PubMed] [Google Scholar]


