Abstract
Objective
The genes Bcl-2 and Bax play important roles in apoptosis. Many studies have shown that formalin has a strong deleterious effect on male fertility and can induce apoptosis. L-carnitine has been reported to potentially reverse the negative effects of formalin, leading to improved spermatogenesis. In this study, we examined the levels of expression of Bcl-2 and Bax in mice treated with formalin and L-carnitine.
Methods
Thirty adult BALB/c mice were categorized into three groups. The mice in the control group (n=10) were not injected with any substance. The mice in the second group (n=10) received 10 mg/kg of formalin daily via an intraperitoneal injection, while those in the final group (n=10) were intraperitoneally injected daily with a dose of 10 mg/kg of formalin and 100 mg/kg of L-carnitine. All mice were kept in isolated cages for 31 days.
Results
The expression of Bax was significantly higher in the formalin-treated mice than in the mice of the control group, while the expression of Bcl-2 was significantly lower in the formalin-treated mice than in the control mice. Additionally, relative to control mice, Bcl-2 expression increased and Bax expression decreased in the mice administered both formalin and L-carnitine.
Conclusion
In this study, L-carnitine was shown to augment Bcl-2 expression and to reduce Bax expression, indicating that this compound may inhibit apoptosis. Due to its positive effects, L-carnitine can be used as a prophylactic treatment for people who routinely come into direct contact with formalin as an occupational hazard.
Keywords: Apoptosis, Bax, Bcl-2, Formalin, L-carnitine
Introduction
Infertility and the personal and social problems that it may cause are extremely important issues in modern life [1,2]. Studies have demonstrated the destructive role of environmental pollutants on human fertility [3,4]. Formalin (an aqueous solution of formaldehyde) is a pollutant that is widely used in the manufacture of disinfectants and detergents as well as in hospitals and factories [5]. Formalin has been shown to affect reproduction in mammals [6]. The deleterious effects of formaldehyde on fertility in both human and laboratory animal models have been documented; these effects include alterations in spermatogenesis, apoptotic changes in the testes, and decreased testosterone secretion, all of which may decrease fertility [7-9]. The adverse impact of formalin on the male reproductive system may result from hormonal changes in the hypothalamic-pituitary-gonadal axis [10]. Formalin also causes cellular and oxidative damage in many tissues by increasing the production of reactive oxygen species (ROS) [6]. Studies have indicated that 25%–40% of infertile men exhibit increased levels of ROS. In addition, ROS generated through DNA damage are important intracellular signals in the induction of apoptosis [11-13].
L-carnitine plays a crucial role in the β-oxidation of long-chain fatty acids in the mitochondria and ultimately in the energy production of the cell [14]. L-carnitine also protects DNA and cell membranes from damage caused by oxygen free radicals [15]. Previous studies have shown that L-carnitine may act as an antioxidant to prevent H2O2-induced oxidative stress in SH-SY5Y neuroblastoma cells [16]. L-carnitine also enhances the cell’s capacity to prevent both apoptosis and the H2O2-induced accumulation of ROS in the cell. Researchers have found that L-carnitine is a therapeutic option in patients with azoospermia, as it can increase sperm count both in those patients and in those exhibiting abnormal sperm maturation [17,18]. L-carnitine has also been demonstrated to increase cytochrome oxidase activity, resulting in the increased production of adenosine triphosphate [19]. In addition, flow cytometry and caspase activity experiments have shown that L-carnitine prevents alkaline phosphatase-induced apoptosis in cardiomyocytes [20]. L-carnitine protects against cyclophosphamide-induced testicular damage by inhibiting cell apoptosis and autophagic modulation [21].
Apoptosis is a physiological and biological process that is important for normal development and the maintenance of homeostasis. However, apoptosis also occurs when cells are exposed to cytotoxic agents [22]. Additionally, severe damage to the genetic material of the cell can trigger multiple pathways that lead to apoptosis [23]. The key characteristics of apoptosis include cell wrinkling, membrane destruction, chromatin compaction, and DNA damage [24]. The control of apoptosis is very intricate and involves multiple proteins. Members of the Bcl-2 protein family, which includes apoptosis inhibitor proteins, are key regulators of this process [22]. The protein Bcl-2 acts as a suppressor of apoptosis, whereas the protein Bax promotes apoptosis. Bcl-2 helps to maintain the integrity of the mitochondrial membrane by binding to receptors on the outer mitochondrial membrane [25]. If the cell is exposed to apoptosis-inducing agents, Bax is moved from the cytoplasm to the mitochondrial membrane and acts to alter the permeability of the outer membrane [26]. These changes release cytochrome c and other apoptosis-inducing factors from the mitochondria and eventually lead to DNA fragmentation [27]. Given the positive effects of L-carnitine and the deleterious effects of formalin on male fertility, the present study was planned to examine the effect of L-carnitine on apoptotic gene expression in formalin-treated BALB/c mice.
Methods
1. Animal care
Thirty adult BALB/c mice were categorized into three groups. The mice in the control group (n=10) were not injected with any substance. The mice in the second group (n=10) received 10 mg/kg of formalin daily via intraperitoneal injection, while those in the final group were intraperitoneally injected (n=10) daily with a dose of 10 mg/kg of formalin and 100 mg/kg of L-carnitine. The mice were kept in isolated cages for 31 days under proper temperature and light conditions. At the end of that period, the animals were killed via neck dislocation, and one testis from each mouse was removed and kept in a freezer at –80°C until RNA extraction.
2. RNA isolation and complementary DNA synthesis
For RNA extraction, the frozen testis tissues were homogenized, and the total RNA was then isolated using a miRNeasy Micro Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s protocol. Then, on-column deoxyribonuclease (DNase) digestion and in-solution DNase digestion were performed to avoid DNA contamination. The purity and concentration of the RNA were determined via spectrophotometry (NanoDrop; Thermo Fisher Scientific, Waltham, MA, USA) and optical density analysis at wavelengths of 260 and 280 nm. The complementary DNA (cDNA) of Bcl-2 and Bax were synthesized using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) in accordance with the manufacturer’s protocol.
3. Real-time quantitative polymerase chain reaction
Quantitative polymerase chain reaction was accomplished on a StepOnePlus real-time polymerase chain reaction System (Applied Biosystems, Foster City, CA, USA) with 1.5 μL of produced cDNA, 9.5 μL of SYBR Green Master Mix (Applied Biosystems), 1.5 μL of each primer, and 6.0 μL of DNase-/RNase-free water for the gene expression profile. β-actin was used as the reference gene and Bcl-2 and Bax as the target genes, with designed primers listed in Table 1. The reactions consisted of an initial period of denaturation at 95°C for 12 minutes; 40 cycles of denaturation at 95°C for 12 seconds, annealing at 60°C for 32 seconds, and extension at 72°C for 32 seconds; and a final period of extension at 72°C for 12 minutes. Relative gene expression was calculated using the 2−ΔΔCt quantitative method.
Table 1.
Real-time reverse transcription-polymerase chain reaction primers applied in the experiment
| Gene | Primers (5′→3′) | Product size (bp) | TM (°C) |
|---|---|---|---|
| Bax | F: TGGAGATGAACTGGACAGCA | 201 | 60 |
| R: GATCAGCTCGGGCACTTTAG | |||
| Bcl-2 | F: CTGGCATCTTCTCCTTCCAG | 202 | 60 |
| R: ACATCTCTGCGAAGTCACGA | |||
| β-actin | F: CGACGAGGCCCAGGCAAGAGAGG | 180 | 60 |
| R: TCAGGCAGCTCATGCTCTTCTCCAGG |
bP, base pairs; TM, melting temperature; F, forward; R, reverse.
4. Data analysis
Statistical analysis of Bcl-2 and Bax gene expression in the formalintreated and L-carnitine–treated mice was conducted using the unpaired t-test, and p<0.05 was considered to indicate statistical significance. All analyses were performed using GraphPad Prism 6 software (GraphPad Software, San Diego, CA, USA).
Results
1. Relative expression of Bcl-2 and Bax in the formalin-treated group
As shown in Figures 1 and 2, the expression of Bax increased in the formalin-treated group relative to the control, whereas the expression of Bcl-2 decreased. These results indicate that exposure to formalin led to the increased expression of pre-apoptotic genes and the decreased expression of anti-apoptotic genes.
Figure 1.
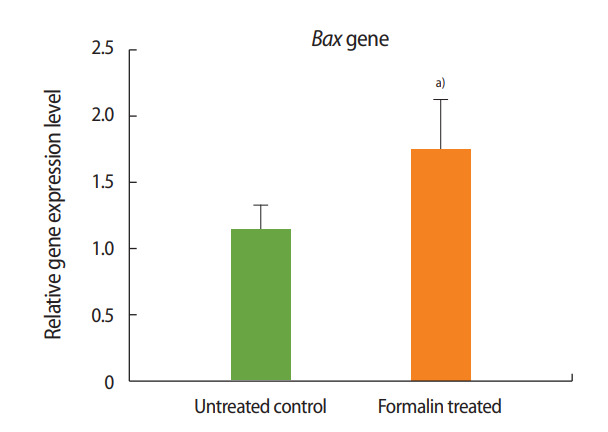
Relative expression of Bax in the formalin-treated group compared to the control group. a)p< 0.01.
Figure 2.
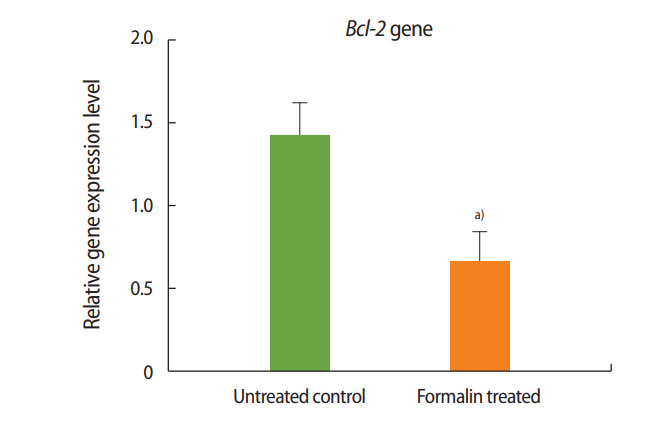
Relative expression of Bcl-2 in the formalin-treated group compared to the control group. a)p< 0.001.
2. Relative expression of Bcl-2 and Bax in the formalin- and L-carnitine–treated group
As shown in Figures 3 and 4, the expression of Bax decreased in the formalin- and L-carnitine–treated group relative to the control, whereas the expression of Bcl-2 increased. These results indicate that L-carnitine attenuated the negative effects of formalin, leading to the decreased expression of pre-apoptotic genes and the increased expression of anti-apoptotic genes.
Figure 3.
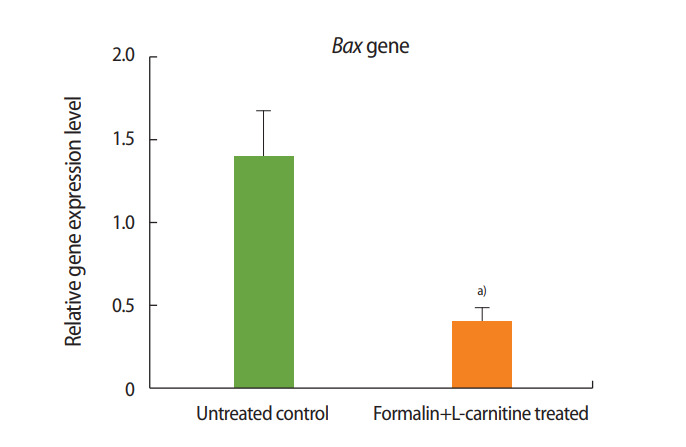
Relative expression of Bax in the group treated with formalin and L-carnitine compared to the control group. a)p< 0.01.
Figure 4.
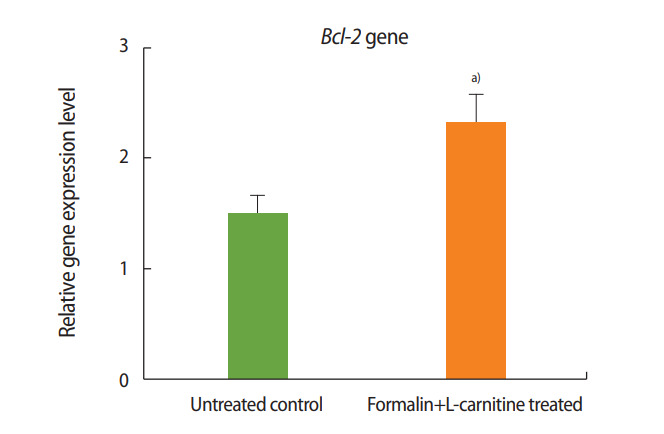
Relative expression of Bcl-2 in the group treated with formalin and L-carnitine compared to the control group. a)p< 0.001.
Discussion
Reports published in the last 2 decades have emphasized that various chemicals can affect the structure and function of the genitourinary system. Formalin is one such chemical. Formalin has a profound adverse impact on sperm characteristics, chromatin stability, and apoptosis. Formalin intake leads to impaired testicular function, reduces the efficiency of antioxidant enzymes, and exacerbates oxidative stress. Formalin also causes increased DNA damage and augments the transcription of Hsp70, p53, and genes involved in apoptosis, such as Bax [28]. Research has shown that L-carnitine can counteract the negative effects of formalin [29]. Previous studies have provided strong support for the importance of L-carnitine in improving sperm parameters, reducing oxidative stress and apoptosis, and enhancing the efficiency of adenosine triphosphate production [30,31]. Since the early 1990s, numerous studies have been conducted on the effect of L-carnitine on idiopathic infertility in men. Increased sperm motility has been observed in some studies of patients treated with L-carnitine [32,33]. The highest concentration of carnitine in the human body is in the epididymis, where the concentration is 2,000 times that found in the blood. Several studies have shown lower carnitine levels in the semen of infertile men than in the semen of fertile men [34,35]. In 2019, Micic et al. [36] revealed the beneficial impacts of carnitine derivatives on progressive motility, vitality, and sperm DNA fragmentation.
Two genes known to participate in apoptosis are Bcl-2 and Bax. Experimental results have shown that the dysfunction of these genes leads to defects in spermatogenesis and infertility in mice [37]. According to a review of the literature, the present study was the first to evaluate the impacts of formalin and L-carnitine on the expression of Bcl-2 and Bax. The outcomes of this experiment showed that the expression of Bax in the formalin-treated mice was significantly increased compared to the control group, while the expression of Bcl-2 in the formalin-treated mice was significantly decreased compared to the control mice. These results confirm that in the testis, formalin enhances the expression of apoptotic genes and accelerates the process of apoptosis, eventually leading to impaired spermatogenesis and sterility.
Bhatia [38] stated that formalin induces potent cytotoxicity due to the increased activity of caspase-9 (an initiator of apoptosis), the induction of oxidative stress, mitochondrial dysfunction, and ultimately apoptosis itself. Hegazy et al. [39] observed the destruction of the genetic content of germ cells in formalin-treated mice. Similar to the results of the present experiment, Yang et al. [40] reported that in mice administered formalin, apoptosis and testicular injury were aggravated, Bax expression was increased, and Bcl-2 expression was decreased. Cell line studies have shown that Bcl-2 protein can prevent the transfer of Bax from the cytosol to the mitochondria, thereby inhibiting apoptosis [41]. Although the lack of Bax seems to decrease apoptosis in the testis and thus to improve spermatogenesis, male Bax−/− mice exhibited defective spermatogenesis as well as infertility [42]. These results indicate that the balance between the levels of expression of genes involved in apoptosis is essential for proper spermatogenesis.
Another finding of this study was an increase in Bcl-2 expression and a decrease in Bax expression in mice administered both formalin and L-carnitine. This result suggests that L-carnitine can minimize the negative effects of formalin and reduce the expression of pro-apoptotic genes, such as Bax. In a mouse study, Cankorkmaz et al. [43] suggested that L-carnitine could facilitate the repair of testicular injury in mice by reducing apoptosis. Altun et al. [44] investigated the antiapoptotic effect of L-carnitine on the testicular tissue of mice receiving gamma radiation and reported that L-carnitine inhibited the apoptosis of germ cells during radiation therapy. In addition, several human studies have shown that L-carnitine has a positive effect on sperm parameters.
Overall, the present study showed that exposure to formalin increased Bax expression, decreased Bcl-2 expression, and could lead to increased apoptosis. L-carnitine can counteract the effects of formalin, and the results revealed that L-carnitine augmented Bcl-2 expression and reduced Bax expression, indicating that it may inhibit apoptosis. Due to the positive effects of L-carnitine, it can be used as a prophylactic treatment for people who routinely come into direct contact with formalin as an occupational hazard. Further research is needed to confirm the results of this study and to use its findings in treatment.
Acknowledgments
The authors would like to thank Dr. S. Javadi, Dr. Mehravar, and Mr. Babakhanzadeh for their valuable support.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conceptualization, Data curation, Formal analysis, Writing–original draft, review & editing: all authors.
References
- 1.Babakhanzadeh E, Khodadadian A, Rostami S, Alipourfard I, Aghaei M, Nazari M, et al. Testicular expression of TDRD1, TDRD5, TDRD9 and TDRD12 in azoospermia. BMC Med Genet. 2020;21:33. doi: 10.1186/s12881-020-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babakhanzadeh E, Nazari M, Ghasemifar S, Khodadadian A. Some of the factors involved in male infertility: a prospective review. Int J Gen Med. 2020;13:29–41. doi: 10.2147/IJGM.S241099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henriques MC, Loureiro S, Fardilha M, Herdeiro MT. Exposure to mercury and human reproductive health: a systematic review. Reprod Toxicol. 2019;85:93–103. doi: 10.1016/j.reprotox.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Schatten H, Rawe VY, Sun QY. Cytoskeletal architecture of human oocytes with a focus on centrosomes and their significant role in fertilization. In Vitro Fertilization. Berlin: Springer; 2019. [Google Scholar]
- 5.Gnanadeepam JC, Rajavel AT, Eswaran SE, Thiagarajan PR. Histological changes in testis of wistar albino rats following formaldehyde exposure by inhalation. J Clin Diagno Res. 2018;12 [Google Scholar]
- 6.Tajaddini S, Ebrahimi S, Behnam B, Bakhtiyari M, Joghataei MT, Abbasi M, et al. Antioxidant effect of manganese on the testis structure and sperm parameters of formalin-treated mice. Andrologia. 2014;46:246–53. doi: 10.1111/and.12069. [DOI] [PubMed] [Google Scholar]
- 7.El-Hak HN. The protective effects of panax ginseng extract on fertility of albino rats treated with formaldehyde vapours. Egyptian J Zoology. 2017:221–38. [Google Scholar]
- 8.Klocke S, Bundgen N, Koster F, Eichenlaub-Ritter U, Griesinger G. Slow-freezing versus vitrification for human ovarian tissue cryopreservation. Arch Gynecol Obstet. 2015;291:419–26. doi: 10.1007/s00404-014-3390-6. [DOI] [PubMed] [Google Scholar]
- 9.Naghdi M, Maghbool M, Seifalah-Zade M, Mahaldashtian M, Makoolati Z, Kouhpayeh SA, et al. Effects of common fig (Ficus carica) leaf extracts on sperm parameters and testis of mice intoxicated with formaldehyde. Evid Based Complement Alternat Med. 2016;2016:2539127. doi: 10.1155/2016/2539127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vosoughi S, Khavanin A, Salehnia M, Asilian Mahabadi H, Shahverdi A, Esmaeili V. Adverse effects of formaldehyde vapor on mouse sperm parameters and testicular tissue. Int J Fertil Steril. 2013;6:250–67. [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal A, Saleh RA. Role of oxidants in male infertility: rationale, significance, and treatment. Urol Clin North Am. 2002;29:817–27. doi: 10.1016/s0094-0143(02)00081-2. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Sharma RK, Nallella KP, Thomas AJ, Jr, Alvarez JG, Sikka SC. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006;86:878–85. doi: 10.1016/j.fertnstert.2006.02.111. [DOI] [PubMed] [Google Scholar]
- 13.Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res. 2009;129:357–67. [PubMed] [Google Scholar]
- 14.Zhou X, Liu F, Zhai S. Effect of L-carnitine and/or L-acetyl-carnitine in nutrition treatment for male infertility: a systematic review. Asia Pac J Clin Nutr. 2007;16 Suppl 1:383–90. [PubMed] [Google Scholar]
- 15.Cavallini G, Ferraretti AP, Gianaroli L, Biagiotti G, Vitali G. Cinnoxicam and L-carnitine/acetyl-L-carnitine treatment for idiopathic and varicocele-associated oligoasthenospermia. J Androl. 2004;25:761–70. doi: 10.1002/j.1939-4640.2004.tb02853.x. [DOI] [PubMed] [Google Scholar]
- 16.Yu J, Ye J, Liu X, Han Y, Wang C. Protective effect of L-carnitine against H(2)O(2)-induced neurotoxicity in neuroblastoma (SHSY5Y) cells. Neurol Res. 2011;33:708–16. doi: 10.1179/1743132810Y.0000000028. [DOI] [PubMed] [Google Scholar]
- 17.Chang B, Nishikawa M, Nishiguchi S, Inoue M. L-carnitine inhibitshepatocarcinogenesis via protection of mitochondria. Int J Cancer. 2005;113:719–29. doi: 10.1002/ijc.20636. [DOI] [PubMed] [Google Scholar]
- 18.Ye J, Li J, Yu Y, Wei Q, Deng W, Yu L. L-carnitine attenuates oxidant injury in HK-2 cells via ROS-mitochondria pathway. Regul Pept. 2010;161:58–66. doi: 10.1016/j.regpep.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Wachter S, Vogt M, Kreis R, Boesch C, Bigler P, Hoppeler H, et al. Long-term administration of L-carnitine to humans: effect on skeletal muscle carnitine content and physical performance. Clin Chim Acta. 2002;318:51–61. doi: 10.1016/s0009-8981(01)00804-x. [DOI] [PubMed] [Google Scholar]
- 20.Baghaei A, Solgi R, Jafari A, Abdolghaffari AH, Golaghaei A, Asghari MH, et al. Molecular and biochemical evidence on the protection of cardiomyocytes from phosphine-induced oxidative stress, mitochondrial dysfunction and apoptosis by acetyl-L-carnitine. Environ Toxicol Pharmacol. 2016;42:30–7. doi: 10.1016/j.etap.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Dokmeci D, Inan M, Basaran UN, Yalcin O, Aydogdu N, Turan FN, et al. Protective effect of L-carnitine on testicular ischaemia–reperfusion injury in rats: cell biochemistry and function: cellular biochemistry and its modulation by active agents or disease. Cell Biochem Funct. 2007;25:611–8. doi: 10.1002/cbf.1355. [DOI] [PubMed] [Google Scholar]
- 22.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nature Reviews Mol Cell Bio. 2019;20:175–93. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abotaleb M, Samuel SM, Varghese E, Varghese S, Kubatka P, Liskova A, et al. Flavonoids in cancer and apoptosis. Cancers (Basel) 2018;11:28. doi: 10.3390/cancers11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esmailpoor A, Ghasemian A, Dehnavi E, Peidayesh H, Teimouri M. Physalis alkekengi hydroalcoholic extract enhances the apoptosis in mouse model of breast cancer cells. Gene Rep. 2019;15:100366. [Google Scholar]
- 25.Yamaguchi R, Lartigue L, Perkins G. Targeting Mcl-1 and other Bcl-2 family member proteins in cancer therapy. Pharmacol Ther. 2019;195:13–20. doi: 10.1016/j.pharmthera.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Warren CF, Wong-Brown MW, Bowden NA. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10:177. doi: 10.1038/s41419-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knight T, Luedtke D, Edwards H, Taub JW, Ge Y. A delicate balance: the BCL-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics. Biochem Pharmacol. 2019;162:250–61. doi: 10.1016/j.bcp.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Silva AG, Lopes CF, Carvalho Junior CG, Thome RG, Dos Santos HB, Reis R, et al. WIN55,212-2 induces caspase-independent apoptosis on human glioblastoma cells by regulating HSP70, p53 and Cathepsin D. Toxicol In Vitro. 2019;57:233–43. doi: 10.1016/j.tiv.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Moretti S, Famularo G, Marcellini S, Boschini A, Santini G, Trinchieri V, et al. L-carnitine reduces lymphocyte apoptosis and oxidant stress in HIV-1-infected subjects treated with zidovudine and didanosine. Antioxid Redox Signal. 2002;4:391–403. doi: 10.1089/15230860260196191. [DOI] [PubMed] [Google Scholar]
- 30.Hosseinzadeh Colagar A, Karimi F, Jorsaraei SG. Correlation of sperm parameters with semen lipid peroxidation and total antioxidants levels in astheno- and oligoasheno- teratospermic men. Iran Red Crescent Med J. 2013;15:780–5. doi: 10.5812/ircmj.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morovvati H, Khaksar Z, Sheibani MT, Anbara H, Kafiabad MA, Moradi HR. Effect of aspartame on histological and histometrical structure of prostate gland in adult mice. Qom Univ Med Sci J. 2019;12:14–27. [Google Scholar]
- 32.Costa M, Canale D, Filicori M, D’lddio S, Lenzi A. L-carnitine in idiopathic asthenozoospermia: a multicenter study. Italian Study Group on Carnitine and Male Infertility. Andrologia. 1994;26:155–9. [PubMed] [Google Scholar]
- 33.Vitali G, Parente R, Melotti C. Carnitine supplementation in human idiopathic asthenospermia: clinical results. Drugs Exp Clin Res. 1995;21:157–9. [PubMed] [Google Scholar]
- 34.Hinton BT, Snoswell AM, Setchell BP. The concentration of carnitine in the luminal fluid of the testis and epididymis of the rat and some other mammals. J Reprod Fertil. 1979;56:105–11. doi: 10.1530/jrf.0.0560105. [DOI] [PubMed] [Google Scholar]
- 35.Sigman M, Glass S, Campagnone J, Pryor JL. Carnitine for the treatment of idiopathic asthenospermia: a randomized, doubleblind, placebo-controlled trial. Fertil Steril. 2006;85:1409–14. doi: 10.1016/j.fertnstert.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 36.Micic S, Lalic N, Djordjevic D, Bojanic N, Bogavac-Stanojevic N, Busetto GM, et al. Double-blind, randomised, placebo-controlled trial on the effect of L-carnitine and L-acetylcarnitine on sperm parameters in men with idiopathic oligoasthenozoospermia. Andrologia. 2019;51:e13267. doi: 10.1111/and.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranger AM, Malynn BA, Korsmeyer SJ. Mouse models of cell death. Nat Genet. 2001;28:113–8. doi: 10.1038/88815. [DOI] [PubMed] [Google Scholar]
- 38.Bhatia M. Understanding toxicology: mechanisms and applications. Berlin: Springer; 2017. [DOI] [PubMed] [Google Scholar]
- 39.Hegazy AA, Elsayed N, Ahmad M, Omar N. Effect of Formaldehyde on Rat Testis Structure. Acad Anat Int. 2017;3:15–23. [Google Scholar]
- 40.Yang SB, LI CY, Zhang YB. Effects of formaldehyde and benzene on the apoptosis, expression of Bcl-2 and Bax in testicle of mouse. Pract Prev Med. 2008;4 [Google Scholar]
- 41.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–32. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rucker EB 3rd, Dierisseau P, Wagner KU, Garrett L, Wynshaw-Boris A, Flaws JA, et al. Bcl-x and Bax regulate mouse primordial germ cell survival and apoptosis during embryogenesis. Mol Endocrinol. 2000;14:1038–52. doi: 10.1210/mend.14.7.0465. [DOI] [PubMed] [Google Scholar]
- 43.Cankorkmaz L, Koyluoglu G, Ozer H, Yildiz E, Sumer Z, Ozdemir O. The role of apoptosis and protective effect of carnitine in contralateral testicular injury in experimental unilateral testicular torsion. Ulus Travma Acil Cerrahi Derg. 2009;15:529–34. [PubMed] [Google Scholar]
- 44.Altun Z, Olgun Y, Ercetin P, Aktas S, Kirkim G, Serbetcioglu B, et al. Protective effect of acetyl-l-carnitine against cisplatin ototoxicity: role of apoptosis-related genes and pro-inflammatory cytokines. Cell Prolif. 2014;47:72–80. doi: 10.1111/cpr.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]


