Abstract
Purpose
Patients hospitalized for infection with SARS-CoV-2 typically present with pneumonia. The respiratory failure is frequently complicated by pulmonary embolism in segmental pulmonary arteries. The distribution of pulmonary embolism in regard to lung parenchymal opacifications has not been investigated yet.
Methods
All patients with COVID-19 treated at a medical intensive care unit between March 8th and April 15th, 2020 undergoing computed tomography pulmonary angiography (CTPA) were included. All CTPA were assessed by two radiologists independently in respect to parenchymal changes and pulmonary embolism on a lung segment basis.
Results
Out of 22 patients with severe COVID-19 treated within the observed time period, 16 (age 60.4 ± 10.2 years, 6 female SAPS2 score 49.2 ± 13.9) underwent CT. A total of 288 lung segment were analyzed. Thrombi were detectable in 9/16 (56.3%) patients, with 4.4 ± 2.9 segments occluded per patient and 40/288 (13.9%) segments affected in the whole cohort. Patients with thrombi had significantly worse segmental opacifications in CT (p < 0.05) and all thrombi were located in opacitated segments. There was no correlation between d-dimer level and number of occluded segmental arteries.
Conclusions
Thrombi in segmental pulmonary arteries are common in COVID-19 and are located in opacitated lung segments. This might suggest local clot formation.
Keywords: COVID19, CT-Scan, CTPA, Pulmonary embolism, Pulmonary artery thrombosis, SARS-CoV2
Abbreviation: BMI, body mass index; CTPA, computed tomography pulmonary angiogram; ECMO, veno-venous extracorporeal membrane oxygenation; GGO, ground-glass opacification; HU, houndsfield units; ICU, intensive care unit; PAPsys, systolic pulmonary arterial pressure; PAT, pulmonary artery thrombi; ROI, region of interest; SAPS2, simplified acute physiology score 2; SD, standard deviation; TISS, therapeutic intervention scoring system
Abbreviations
- PAT
pulmonary artery thrombi
- SD
standard deviation
- BMI
body mass index
- ECMO
veno-venous extracorporeal membrane oxygenation
- PAP sys
systolic pulmonary arterial pressure
- SAPS2
Simplified Acute Physiology Score 2
- TISS
Therapeutic Intervention Scoring System
- ICU
intensive care unit
- CTPA
computed tomography pulmonary angiogram
1. Introduction
In hospitalized patients infected with SARS-CoV-2, respiratory failure is a common complication [1]. Since the beginning of the outbreak, multiple studies evaluating imaging techniques described bilateral ground-glass opacities, crazy-paving and air-space consolidations in peripheral and basal distribution in patients with COVID-19 pneumonia [2,3]. Some of these studies have investigated the changes of parenchymal opacifications during the time of infection and the assessment of severity using chest CT-scans [[4], [5], [6]].
Apart from COVID-19 pneumonia, a coagulopathy is reported in a considerable number of patients [7]. Notably, elevated d-dimer levels correlate with poor prognosis in COVID-19 [8]. Several case reports and retrospective analyses report thrombotic complications, most of which are pulmonary embolisms [9,10]. Therefore, the association between SARS-CoV-2 infection and disseminated intravascular coagulation, D-dimer levels, deep vein thrombosis, pulmonary embolisms, and microvascular thrombosis is under investigation [8,9,11]. Interestingly, during the initial SARS-CoV-2 outbreak in China, pulmonary embolisms were not described. One possible explanation is the fact that mostly chest CT scans without contrast medium were used [[2], [3], [4], [5], [6]]. The pathological mechanism causing pulmonary embolism in COVID-19 remains unclear. It has been speculated that endothelial inflammation promotes thrombosis and hypoxic pulmonary vasoconstriction might facilitate local thrombus formation mediated by activation of complement pathways and an associated procoagulant state [7,12]. This would require pulmonary artery thrombosis to be formed locally, in areas of active SARS-CoV-2 pneumonia. To our best knowledge, the distribution of pulmonary embolism in regard to lung parenchymal opacifications and normal parenchyma has not been investigated yet.
Therefore, the aim of our study was to investigate if pulmonary embolism manifestations are limited to lung segments affected by CoVID-19 pneumonia.
2. Methods
2.1. Study population
In this retrospective study, we included all patients admitted to the medical intensive care unit (ICU) of our hospital with proven SARS-CoV-2 infection between March 8th and April 15th, 2020. During this period of time a total of 22 patients with proven SARS-CoV-2 infection and severe pulmonary failure were admitted to the medical ICU; patients’ electronic medical records and, if available, computed tomography (CT) examinations were reviewed. Patients underwent a contrast enhanced CT with pulmonary angiography when likelihood of pulmonary embolism was considered high. Patients were retrospectively recruited for this study if they fulfilled the following inclusion criteria: 1) Positive test for SARS-CoV-2 infection; 2) contrast enhanced CT with pulmonary angiography.
2.2. CT examination protocol
The hypothesis of our study, that pulmonary artery thrombi would not be present in non-COVID-19 affected lung segments, was made before starting the examination and only this endpoint was evaluated. Pulmonary angiography CT scans were performed using a commercial CT scanner (SOMATOM Definition Flash; Siemens Healthineers GmbH, Forchheim, Germany) with the following scanning parameters: tube voltage, 100 kV; tube current, 90 mAs; rotation time, 0.28 s. 128 × 0.6 mm collimation with automated dose modulation (CARE dose4D, Siemens Healthineers GmbH, Forchheim, Germany).
Images were reconstructed at 1 mm slice thickness with an increment of 0,6 mm with an advanced modeled iterative reconstruction using I26f (mediastinal) and B50f (lung) kernels (Siemens Healthineers GmbH, Forchheim, Germany).
Patients without extracorporeal membrane oxygenation (ECMO) received the standard pulmonary angiography protocol with bolus-tracking method. 70 ml contrast agent (Imeron 400, Bracco Imaging, Germany) followed by a 50 ml bolus saline solution were injected at a flow rate of 4 ml/s using a jugular central venous line. The region of interest (ROI) was placed in the pulmonary trunk. The CT scan started after a threshold of 100 Houndsfield units (HU) was reached within the ROI. To give consideration to an altered blood flow in patients with ECMO device the amount of contrast agent was adjusted to 100 ml and the ROI was placed in the air. The scan was manually started when an adequate contrast was visually detected in the pulmonary trunk. If tolerated by the patient ECMO flow was reduced to 70 to 50% of the initial value after scout acquisition for the time of the contrast enhanced scan.
2.3. Imaging analysis
Two radiologists (KMP, TK) with 6 and 13 years of experience in thoracic radiology assessed the CTPA (computed tomography pulmonary angiography) scans in consensus reading for image quality, parenchymal changes and pulmonary embolism. All scans were viewed in axial and coronal 1 mm slices at standard lung and soft tissue window. Pulmonary artery contrast was documented using a ROI with a standardized size of 70 mm2, which was placed in the right and left main pulmonary artery, respectively. Average HU and standard deviation within the ROIs were documented. Overall image quality of each CTPA scan was evaluated based on imaging contrast and breathing artifacts using a five-point Likert scale (1. excellent contrast in all pulmonary vessels, no motion artifacts due to breathing; 2. good contrast in all pulmonary vessels, minimal motions artifacts due to breathing; 3. acceptable contrast in central and peripheral pulmonary vessels, sparse motions artifacts due to breathing in basal pulmonary segments; 4. Acceptable contrast in central and poor contrast in peripheral pulmonary vessels, moderate motion artifacts due to breathing in basal lung segments; 5. Poor contrast in central and unacceptable contrast in peripheral pulmonary vessels, distinct motion artifacts due to breathing in apical and basal lung segments).
Each pulmonary lung segment was separately evaluated for parenchymal abnormalities and pulmonary embolism. Parenchymal abnormalities were grouped in non-consolidating changes, including ground-glass opacities and/or crazy-paving pattern, and air space consolidation. No patient in the collective showed centrilobular nodules or parenchymal reticulation without underlying GGO (ground-glass opacification). Segmental parenchyma was defined by the dominating pattern as non-consolidating, consolidating, or, in case of an equal distribution of non-consolidating and consolidating changes, as mixed. Ground-glass opacification was defined as parenchyma with hazy increased attenuation without obscuration of underlying vessels. Crazy-paving was defined as ground-glass opacification with superimposed interlobular septal thickening and intralobular lines [13]. Parenchymal opacifications were delineated as air space consolidation when the opacifications obscured the underlying pulmonary vessels. A lung segment without any of the above-mentioned opacifications was defined as normal.
A segmental or subsegmental pulmonary embolism was defined as central filling defect within a vessel surrounded by contrast material when orthogonal or parallel to the long axis of the vessel as well as eccentric wall adherent filling defect rendering an acute angle with the vessel wall as well as complete occlusion of a dilated vessel [14].
2.4. Statistical analysis and ethics
Statistical analysis was performed using dedicated software (R 3.6.1.). The unpaired two-samples Wilcoxon test was used to analyze the association of contrast enhancement in the main pulmonary arteries and image quality. Fisher's exact test was used for contingency table analysis. Significance level was set at p < 0.05. This retrospective study was approved by the ethics committee of the Albert Ludwig University of Freiburg (file number 234–20).
3. Results
3.1. Patient selection and characteristics
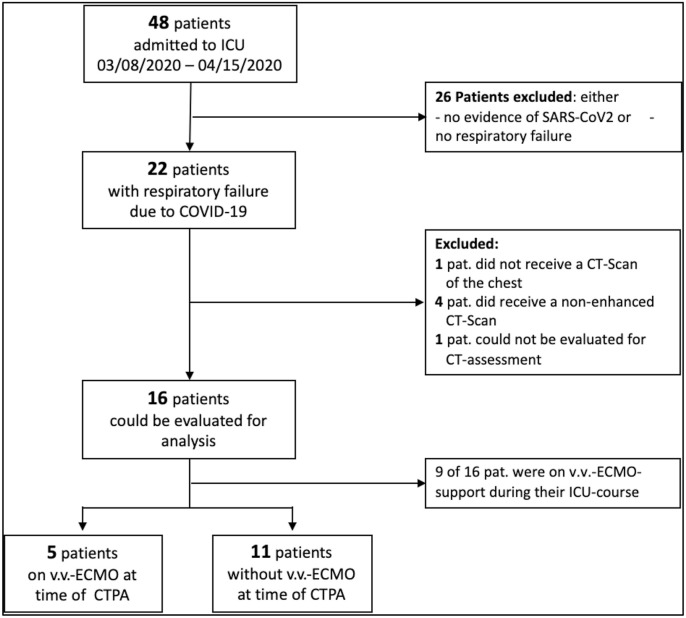
A total of 18 patients with COVID-19 pneumonia underwent CTPA within 7.5 ± 12.1 days after admission to the ICU. Two patients had to be excluded from the study, 1 for not undergoing contrast-enhanced CT and 1 for massive chronical emphysematous lung parenchyma destruction, which could not be evaluated for signs of atypical pneumonia. The process of patient selection is shown in Fig. 1 . Six female and 10 male patients were included in this analysis. The patients’ age ranged between 47 and 77 years with a mean age of 62.2 years ± 8.3 years. At the time CTPA, all patients were mechanically ventilated and 5 patients received ECMO. Mean time between positive testing for SARS-CoV-2 infection and CTPA was 10.8 ± 10.3 days. Patient characteristics are shown in Table 1 . Anticoagulation targets did not differ between patients with pulmonary artery thrombi and those without or in patients with and without extracorporeal membrane oxygenation (see additional Tables 3 and 4).
Fig. 1.
Flowchart: patient selection. CTPA computed tomography pulmonary angiography, COVID-19 Corona-Virus Disease 2019, ECMO extracorporeal membrane oxygenation.
Table 1.
Patients characteristics of all (22) patients with respiratory failure due to COVID19, which were admitted to our ICU during August 03, 2020 to 04/15/2020. The 16 evaluated patients were divided into 2 groups - with and without detection of pulmonary embolism. The percentages or standard deviations within these two groups are given in relation to this group.
| Characteristics | Whole population | PAT detected by CTPA | no PAT detected | p-value |
|---|---|---|---|---|
| number | 22 (100%) | 9 (100%) | 7 (100%) | < 0.0001 |
| age [years] | 60.4 ± 10.2 | 60.0 ± 8.7 | 65.0 ± 5.1 | 0.246 |
| female gender | 6 (27.3%) | 4 (44.0%) | 1 (14.3%) | 0.222 |
| BMI [kg/m2] | 29.6 ± 6.4 | 27.7 ± 3.8 | 27.6 ± 3.4 | 0.986 |
| ICU-mortality | 10 (45.4%) | 5 (55.5%) | 3 (42.9%) | 0.149 |
| ICU-stay (until date of submission) [days] | 23.2 ± 14.6 | 16.8 ± 12.8 | 37.3 ± 16.9 | 0.042 |
| TISS 10 - Score | 16.7 ± 7.2 | 16.0 ± 5.9 | 20.6 ± 7.4 | 0.816 |
| SAPS 2 - Score | 49.2 ± 13.9 | 51.6 ± 11.0 | 52.9 ± 10.8 | 0.190 |
| d-dimers (at time of CT-Scan) [mg/l FEU] | 14.2 ± 12.5 | 20.0 ± 15.3 | 9.0 ± 6.2 | 0.096 |
| d-dimers (at time of admission) [mg/l FEU] | 4.6 ± 4.9 | 6.5 ± 5.7 | 4.0 ± 2.6 | 0.312 |
| therapeutic anticoagulation (at time of admission) | 5 (22.7%) | 1 (4.5%) | 3 (42.9%) | 0.166 |
| fibrinogen (first value after ICU-admission) [mg/dl] | 601 ± 196 | 620 ± 190 | 558 ± 283 | 0.636 |
| blood glucose (at time of admission) [mg/dl] | 164 ± 60 | 136 ± 41 | 173 ± 38 | 0.289 |
| HbA1c [%] | 6.7 ± 0.4 | 6.2 ± 0.3 | 6.1 ± 0.5 | 0.804 |
| delay between positive SARS-CoV-2 PCR and CT-Scan [days] | 10.8 ± 10.3 | 7.4 ± 5.9 | 14.1 ± 12.9 | 0.189 |
| echocardiography: PAP sys. [mmHg] | 43.1 ± 16.1 | 48.7 ± 28.0 | 38.3 ± 8.9 | 0.188 |
| on venovenous ECMO-support | 11 (50.0%) | 5 (22.7%) | 4 (57.1%) | 0.953 |
| ECMO bloodflow (at time of CT-san) [l/min] | 3.9 ± 1.0 | 3.3 ± 0.64 | 4.1 ± 0.1 | 0.941 |
| pre-existing co-morbidities: | ||||
| lung disorder | 3 (13.6%) | 0 (0%) | 2 (28.6%) | 0.098 |
| tobacco smoke | 6 (27.3%) | 1 (11.1%) | 3 (42.9%) | 0.166 |
| diabetes mellitus | 5 (22.7%) | 1 (11.1%) | 1 (14.3%) | 0.861 |
| arterial hypertension | 9 (40.9%) | 3 (33.3%) | 3 (42.9%) | 0.719 |
| heart failure | 5 (22.7%) | 1 (11.1%) | 3 (42.9%) | 0.166 |
| chronic kidney failure | 3 (13.6%) | 1 (11.1%) | 1 (14.3%) | 0.860 |
| chronic liver failure | 1 (4.5%) | 0 (0%) | 1 (14.3%) | 0.271 |
| coagulopathy | 1 (4.5%) | 1 (11.1%) | 0 (0%) | 0.396 |
| immunodeficiency | 5 (18.2%) | 3 (33.3%) | 0 (0%) | 0.102 |
| obesity (BMI >30 kg/m2) | 8 (36.4%) | 2 (22.2%) | 2 (28.6%) | 0.789 |
3.2. Segmental analysis of lung parenchyma changes in COVID-19
Analysis of lung parenchymal changes on segmental level per patient showed a minimum of 11 and a maximum of 19 lung segments to be affected with the predominant pattern of involvement being GGO/crazy-paving and air-space consolidation in 8/16 patients, respectively. In 11/16 patients, all lung segments showed parenchymal alterations consistent with SARS-CoV-2 pneumonia. In total, 113/288 (39.2%) lung segments showed GGO/crazy-paving and 103/288 (35.8%) showed consolidations as predominant pattern. A total of 50/288 (17.4%) lung segments showed GGO/crazy-paving and consolidated areas as mixed pattern and only 22/208 (7.6%) segments showed normal parenchyma, see Figs. 3 and 4.
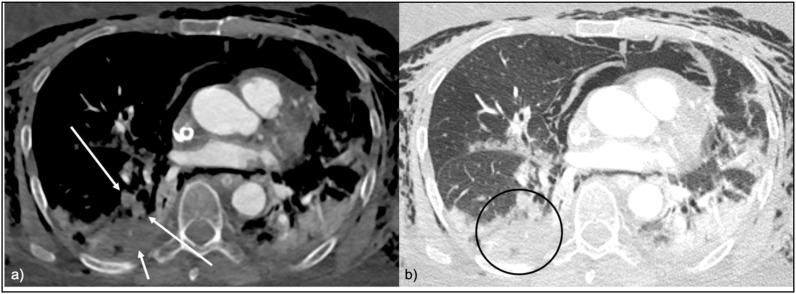
Fig. 3.
a CTPA in soft tissue window, axial view. PAT in the postero-basal lung segment of the right lower lobe with complete occlusion of the widened vessels (long red arrows). The affected lung segment shows areas of hypoenhancement consistent with embolic infarction (short red arrow).
Fig. 3b CTPA in lung window, axial view. The affected postero-basal lung segment of the right lower lobe is consolidated (red circle). Abbreviation PAT pulmonary artery thrombosis.
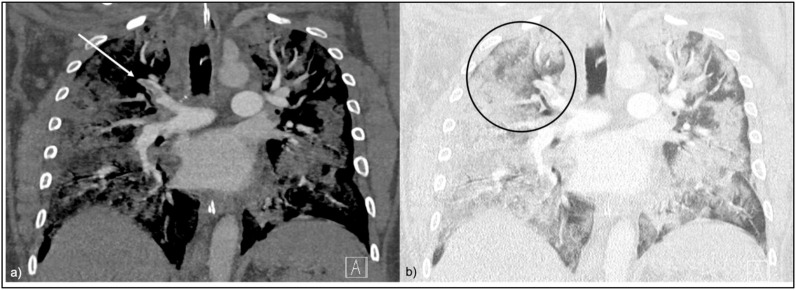
Fig. 4.
a CTPA in soft tissue window, coronal view. PAT in the apical segment of the right upper lobe with subtotal occlusion of the vessel (red arrow).
Fig. 4b CTPA in lung window, coronal view. The affected apical lung segment of the right upper lobe is opacified with a mixture of GGO and consolidations (red circle). Abbreviations: PAT pulmonary artery thrombosis; GGO ground-glass opacities.
3.3. Pulmonary artery thrombosis
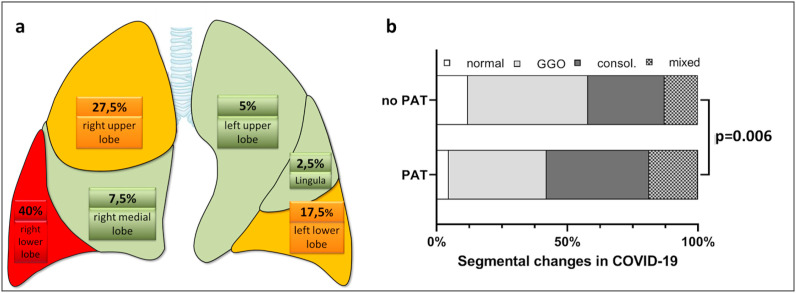
In 9 out of 16 patients, segmental and subsegmental pulmonary artery thrombi (PAT) were detected with a total of 40 segments affected in the whole cohort. In patients with PAT, an average of 4.44 ± 2.90 lung segments were affected (range 1–9). The analysis of distribution of PAT on lung lobe level, showed the right lower lobe to be affected in 7 out of 9 patients with PAT, followed by the right upper lobe which was affected in 6 out of 9 patients (see Fig. 2 a ).
Fig. 2.
a Distribution of pulmonary thrombosis on lung lobe level: most affected was the right lower lobe, followed by the right upper lobe. Colors: Red = high rate of pulmonary thrombosis, Yellow = median rate of pulmonary thrombosis, Green = low rate of pulmonary thrombosis.
b Segmental analysis of patients with COVID-19. There is a significant difference in Patients with PAT compared to those without with more.
PAT were detected in 17/113 (15.0%) segments with GGO/crazy-paving, in 16/103 (15.5%) segments with consolidations, and in 7/50 (14.0%) mixed segments. There was a significant association of pulmonary artery thrombosis with more severe parenchymal lung changes including more consolidations and less normal lung parenchyma (Chi 2 test, p = 0.006), see Fig. 2b and Supplemental Table 2.
3.4. D-dimers
We did not observe an association between d-dimer level and number of affected segments in the patients with proven PAT (Pearson correlation coefficient r = −0.05, p = 0.90). There was a trend towards higher d-dimer levels in patients with PAT compared to those without not reaching statistical significance (20.03 ± 15.33 mg/l FEU versus 9.00 ± 6.15 mg/l, unpaired two-samples Wilcoxon test P = 0.20. However, all d-dimer levels >20.00 mg/l FEU were observed in patients with at least one segment with confirmed PAT (Fisher's exact test p = 0.08).
4. Discussion
4.1. Pulmonary thrombosis
We found PAT in 9 out of 16 patients (56%) with COVID-19 undergoing contrast enhanced CTPA. This is a higher rate than previously reported, which may be biased by referral of the sickest patients to our ARDS/ECMO center. A study of 100 COVID-19 patients undergoing CTPA, pulmonary embolism was found in 25% [7]. Another study reported PAT in 18% of all patients [15]. A post mortem study of 12 autopsies however suggested PAT be the cause of death in 33% in COVID-19 [10]. The authors also reported deep vein thrombosis in 58% [15]. Reports of lower rates of VTE in patients with COVID-19 compared to our cohort might be explained by different thresholds to perform diagnostic tests such as CTPA or different disease severities. In our cohort, 31% (5/16) patients required VV-ECMO compared to 8% in other studies [7]. The severity of COVID-19 pneumonia in our collective is also reflected by the fact, that only 8% of all lung segments evaluated by CT appeared to have unaffected parenchyma.
4.2. Pulmonary embolism or pulmonary artery thrombosis?
Several findings suggest the thrombi detected by CTPA in COVID-19 could – at least in part - be locally formed by the mechanism of thromboinflammation [16] SARS-CoV-2 is known to cause coagulopathy [8,17,18]. By mediating endothelial dysfunction and systemic inflammation, the coronavirus can cause a procoagulant state [19]. In addition, any acute respiratory distress syndrome (ARDS) can cause pulmonary thrombus formation. Endothelial damage will begin in the microvasculature and might extend as local process mediated by pro-inflammatory signals [20] Autopsy studies showed microthrombi in alveolar capillaries in 5/11 COVID-19 patients [21]. Also, thrombi are found much more frequently in the lungs than in any other organ [7,15]. The fact that PAT forms under prophylactic and systemic anticoagulation might also strengthen the hypothesis of local clot formation [7,15]. There are case reports on PAT in SARS1 [22] which might suggest PAT to be a disease specific complication. We did not detect any thrombus in segments with normal parenchyma, supporting the hypothesis of pulmonary artery thrombosis. Taken together, we believe that most PAT developed locally. Additional embolism however cannot be excluded and deep vein thrombosis is also a frequent finding in severe COVID-19 [10]. Therefore, our and other available data combined support the hypothesis that pulmonary artery thrombi can originate locally. Since systemic embolism also remains a likely scenario, both mechanisms probably contribute to the full clinical picture.
4.3. Segmental changes in COVID-19 pneumonia
We found a significant heterogeneity in segmental changes in COVID-19. Our results are in line with studies describing GGO/crazy-paving and consolidations as predominant parenchyma changes and the postero-basal segment of the right lower lobe to be opacified most frequently in patients with COVID-19 pneumonia [23,24]. Long bevor the SARS-CoV-2 pandemic, studies using ventilation-perfusion-scintigraphy and autopsy as gold standard have shown PAT to be found most frequently in the right lower lobe. Our results demonstrate the same predominance of PAT for the right lower lobe. In our analysis the second most commonly involved lobe was the right upper lobe which is involved to a lesser degree in non-COVID-19 patients [25,26]. Analysis of the parenchymal changes demonstrated that the majority of patients showed involvement of all segments of the right upper lobe which is in line with the hypothesis of local vasculature damage due to COVID-19 pneumonia.
4.4. Image quality
Optimal imaging contrast in CTPA can be influenced by various factors. Sedation, artificial ventilation, altered cardiac output and ECMO systems are examples for patient factors in an ICU patient collective that challenge the ideal imaging contrast to the limit. Especially the analysis of the peripheral pulmonary vasculature can be compromised by consolidated lung parenchyma and altered circulation due to ECMO. Analyzing the association of vascular contrast intensity and subjective image quality we saw a tendency for lower subjective image quality when the measured mean HU in the main pulmonary arteries decreased. However, image quality was sufficient in all patients.
4.5. Limitations
Several limitations have to be considered when interpreting data presented here. First of all, we derived our data from a small patient collective. Since each individual segment was analyzed, a considerable number of segments could be registered. Still, data needs to be confirmed in a larger patient collective in order to prove a local clot formation. All patients were treated at a single medical intensive care unit. Decision to perform a CTPA was driven by judgement of the physicians in charge which could lead to an overestimation of rate of pulmonary artery thrombosis. Several factors, such as ventilation or ECMO, negatively influenced image quality of the CTPA examinations especially limiting the analysis of peripheral pulmonary vessels. Even though 16/22 patients underwent CTPA, a selection bias cannot be excluded. Since only ICU patients were included, we cannot extrapolate the findings to a collective of mild to moderate COVID-pneumonia. None of our patients had clinical evidence for a deep vein thrombosis. Still, we cannot exclude lower limb thromboembolism since lower limb venous duplex studies have not been routinely performed. We acknowledge the preliminary nature of these findings, including its retrospective nature and limited sample size. Data presented here has therefore be considered hypothesis generating only.
5. Conclusion
Thrombi in segmental pulmonary arteries are common in COVID-19. All Segments occluded by thrombus were opacitated and no pulmonary embolus was detectable located in a segment without opacitation. This might suggest local clot formation.
Ethics approval and consent to participate
The local ethics committee approved the study protocol (Ethik-Kommission der Albert-Ludwigs-Universität Freiburg im Breisgau 234/20). Available from the corresponding author on reasonable request.
Funding
The article processing charge was funded by the Baden-Wuerttemberg Ministry of Science, Research and Art and University of Freiburg in the funding program Open Access Publishing.
Availability of data and material
The datasets of this study are available from the corresponding author on reasonable request.
Permissions information
The authors declare that all illustrations and figures in the manuscript are entirely original and do not require reprint permission.
Consent for publication
We agree.
Code availability
Not applicable.
Declaration of competing interest
The authors declare that they have no conflicts of interests.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2020.106135.
Authors' contribution
KMP concepted and designed the study, performed data acquisition, interpretation of the data, drafted and revised the manuscript. TK contributed in interpretation of the data and revising the manuscript. MB was a major contributor in analysis, interpretation of the statistics. CNL contributed in interpretation of the data and revising the manuscript. CMB contributed in interpretation of the data and revising the manuscript. DD contributed in interpretation of the data and revising the manuscript DLS concepted and designed the study, contributed in interpretation of the data and wrote the manuscript. VZ concepted and performed data acquisition drafted the manuscript and created the artwork. All authors read and approved the final manuscript.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung M., Bernheim A., Mei X., et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu J., Zhong Z., Li H., et al. CT imaging features of 4121 patients with COVID‐19: a meta‐analysis. J Med Virol jmv. 2020;25910 doi: 10.1002/jmv.25910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding X., Xu J., Zhou J., Long Q. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur. J. Radiol. 2020;127:109009. doi: 10.1016/j.ejrad.2020.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen C., Yu N., Cai S., et al. Quantitative computed tomography analysis for stratifying the severity of Coronavirus Disease 2019. J. Pharmaceut. Biomed. Anal. 2020;10:123–129. doi: 10.1016/j.jpha.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ai T., Yang Z., Hou H., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296 doi: 10.1148/radiol.2020200642. 200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helms J., Tacquard C., et al. Crics Triggersep Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llitjos J., Leclerc M., Chochois C., et al. High incidence of venous thromboembolic events in anticoagulated severe COVID‐19 patients. J Thromb Haemost jth. 2020:14869. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wichmann D., Sperhake J.-P., Lütgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020;173 doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? European Heart Journal ehaa254. 2020;41(19):1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi S.E., Erasmus J.J., Volpacchio M., et al. “Crazy-Paving” pattern at thin-section CT of the lungs: radiologic-pathologic overview. Radiographics. 2003;23:1509–1519. doi: 10.1148/rg.236035101. [DOI] [PubMed] [Google Scholar]
- 14.Moore A.J.E., Wachsmann J., Chamarthy M.R., et al. Imaging of acute pulmonary embolism: an update. Cardiovasc. Diagn. Ther. 2018;8:225–243. doi: 10.21037/cdt.2017.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Thrombosis Research S0049384820301201; 2020. Incidence of Thrombotic Complications in Critically Ill ICU Patients with COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolson P.L.R., Welsh J.D., Chauhan A., et al. A rationale for blocking thromboinflammation in COVID-19 with Btk inhibitors. null. 2020;31:685–690. doi: 10.1080/09537104.2020.1775189. [DOI] [PubMed] [Google Scholar]
- 17.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J. Clin. Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciceri F., Beretta L., Scandroglio A.M., et al. Microvascular COVID-19 lung vessels obstructive thrombo inflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22:95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menter T., Haslbauer J.D., Nienhold R., et al. Post‐mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology his. 2020;14134 doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng K.H.L. Pulmonary artery thrombosis in a patient with severe acute respiratory syndrome. Postgrad. Med. 2005;81 doi: 10.1136/pgmj.2004.030049. e3–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worsley D.F., Kim C.K., Alavi A., Palevsky H.I. Detailed analysis of patients with matched ventilation-perfusion defects and chest radiographic opacities. J. Nucl. Med. 1993;34:1851–1853. [PubMed] [Google Scholar]
- 24.Ro A., Kageyama N., Tanifuji T., Sakuma M. Autopsy-proven untreated previous pulmonary thromboembolism: frequency and distribution in the pulmonary artery and correlation with patients' clinical characteristics: Previous untreated PE in cases of acute fatal PE. J. Thromb. Haemostasis. 2011;9:922–927. doi: 10.1111/j.1538-7836.2011.04225.x. [DOI] [PubMed] [Google Scholar]
- 25.Bernheim A., Mei X., Huang M., et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J., Wu X., Zeng W., et al. Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest. Radiol. 2020;55:257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets of this study are available from the corresponding author on reasonable request.