Abstract
Purpose of Review
Lung tissues are highly susceptible to airway inflammation as they are inevitably exposed to inhaled pathogens and allergens. In the lungs, clearance of infectious agents and regulation of inflammatory responses are important for the first-line defense, where surfactants play a role in host defense mechanisms. In this review, clinical significance of pulmonary surfactants in asthma has been highlighted.
Recent Findings
Surfactants, such as surfactant protein A (SP-A) and SP-D released from alveolar epithelium, reduce pathogen infection and control immune-cell activation. Especially, SP-D directly binds to eosinophil surface, leading to inhibition of extracellular trap formation and reduction in airway inflammation. Production of surfactants is commonly determined by both genetic (single nucleotide polymorphisms) and environmental factors influencing processes involved in the development of asthma. In addition, nintedanib (an intracellular inhibitor of tyrosine kinases) could increase SP-D levels and is used in patients with idiopathic pulmonary fibrosis. These findings may provide a possible application of SP-D in asthma.
Summary
Surfactants are key players contributing to host defense through maintaining the immune system. As clinical implications of surfactants involved in asthma have been suggested, further translational studies are needed to apply surfactants as an effective therapeutic target in patients with asthma.
Keywords: Asthma, Eosinophil, Epithelium, Surfactant, Airway inflammation, Therapy
Introduction
Pulmonary surfactants, a unique mixture of lipids and proteins, form a layer between the aqueous airway liquid and the inspired air throughout the lungs. To date, they have been intensively studied to characterize their synthesis, secretion, metabolism, and function [1]. Initially, surfactants were regarded as a biophysical factor, but recent work has suggested an important role in innate and adaptive immunity of the lungs due to their immunomodulatory properties [2•]. In addition, the pathogenetic relevance of surfactants in various lung diseases, such as acute respiratory distress syndrome, idiopathic pulmonary fibrosis, and pneumonia, has been revealed [3]. The possible involvement of surfactants in the pathophysiology of asthma with a predominant disturbance in the airways has also been demonstrated [4]. The following sections review characteristics, functions, and potential therapeutic applications of surfactants in asthma.
Association Between Surfactants and Asthma
Asthma is a major health problem in society, and it is estimated that more than 300 million people worldwide suffer from the disease. This number contributes to the high health care expenditure associated with this disease [5]. This chronic airway disease seriously affects children as well as adults and is considerably increased in urban areas and high-income countries [6]. Most patients with asthma have mild to moderate symptoms; however, some patients (approximately 5–10% of adult asthmatic patients) show more severe symptoms, high comorbid burden, and frequent asthma exacerbations. In addition, patients with severe asthma are in poorly controlled status despite daily uses of high doses of inhaled corticosteroids and additional treatments [7–9]. Because asthma treatment remains a constant clinical challenge, further studies about asthma pathophysiology and therapeutics are needed to improve health, reduce societal costs, and to improve individual quality of life.
Asthma is commonly characterized by type 2 airway inflammation with typical symptoms such as coughing, wheezing, and dyspnea [10]. However, asthma is likely to be not a single disease, but a heterogeneous disease as multiple phenotypes or endotypes have been reported, depending on the combination of clinical, demographic, and pathological characteristics of asthma [11, 12]. Moreover, several immune cells (mast cells, eosinophils, neutrophils, and innate lymphoid cells) as well as structural cells (epithelial cells, vessels, nerves) and released cytokines/mediators have been shown to contribute to the pathogenesis of asthma [13, 14]. Such a complexity in pathogenesis and heterogeneity in treatment responses require further investigations of pathophysiologic mechanisms and future targets.
Many risk factors, such as genetic predisposition, viral infection, exposure to allergens or pollutants, and changes in microbiome, are important determinants in various steps of asthma pathogenesis [15–18]. When environmental factors are introduced, the airway epithelium is considered a central regulator (initiation and maintaining) of immune responses [19]. This first-line barrier not only expresses pattern recognition receptors but also secretes several components, including enzymes, mucins, and surfactants as well as cytokines upon damage to the epithelium [20]. Previously, altered levels of surfactants in bronchoalveolar lavage fluid or serum samples were demonstrated to be associated with multiple lung diseases including asthma [21•] (Table 1). In addition, recent studies have revealed that surfactants play an essential role in the development of asthma with eosinophilia [22, 23]. To date, application of surfactants in asthma is still lacking, but recent studies suggest that surfactants may act beneficially by supporting pulmonary host defense in some conditions.
Table 1.
Altered levels of pulmonary surfactants in patients with asthma
| Phenotype | Surfactant | Sample | Observation | Reference |
|---|---|---|---|---|
| Bronchial asthma | SP-A/SP-D | BALF | Increase | [76] |
| SP-D | Salivary | Increase | [77] | |
| SP-D | Serum | Stable/increase | [78], [79] | |
| SP-D | Tissue | Increase | [80] | |
| Severe asthma | SP-D | BALF | Decrease/increase | [81•], [82] |
| SP-D | Serum | Increase | [81•], [83] | |
| SP-D | Sputum | Increase | [82] | |
| Obese asthma | SP-A | BALF | Decrease | [84] |
| AERD | SP-D | Serum | Decrease | [59] |
AERD, aspirin-exacerbated respiratory disease; BALF, bronchoalveolar lavage fluid; SP, surfactant protein
Basic Characteristics of Surfactants
Surfactants are composed of approximately 90% lipids and 10% proteins synthesized by alveolar epithelial cells (also called pneumocytes) enriched in the endoplasmic reticulum and lamellar bodies (specialized surfactant-storing organelles). In addition, these lipid and protein mixtures are assembled, transported, secreted, and recycled in the alveolar space [24]. Surfactants are highly dynamic molecules in the context of a surface exposed to constant compression–expansion dynamics (stress and stretch forces) [25, 26]. Although lipid homeostasis is well regulated under normal physiological conditions, abnormal surfactant metabolism due to oxidation, proteolytic degradation, and inhibition of surfactants leads to respiratory distress with attendant morbidity and mortality [27].
Surfactant-specific proteins comprise 2 types: hydrophilic surfactants (surfactant protein A (SP-A) and SP-D) and hydrophobic surfactants (SP-B, SP-C) [28]. The hydrophilic surfactants play an important physical role in lowering the alveolar surface-tension, whereas the hydrophobic surfactants are associated with immune defense mechanisms in the alveolar space [29]. Particularly, SP-A and SP-D are a subgroup of mammalian lectins called collectins or C-type lectins which are composed of oligomers with C-terminal carbohydrate recognition domains in association with N-terminal collagen-like domains [30]. In addition to 4 surfactants, 2 novel surfactant proteins, including SP-G and SP-H, have also been identified in the lungs [31]; nevertheless, the significance of SP-A and SP-D in asthma has mostly been highlighted.
SP-B and SP-C are small proteins encoded by single genes on chromosome 2 and on chromosome 8, respectively [32]. However, both SP-A and SP-D are structurally related multimeric proteins encoded by a multigene family on chromosome 10 located near other members of the collectin family [33]. The secreted SP-A is an octadecamer comprising 6 trimeric subunits, but the released SP-D is a dodecamer consisting of 4 trimeric subunits [34]. Although the degree of multimerization is different between species and even between individuals, all collectins form multimers to increase their affinity to pathogens and immune cells [35]. Among the collectins, SP-D has the largest and most flexible collagen domain interacting with various bound organisms or cells (Table 2).
Table 2.
Summary of the domains presented within each surfactant protein
| Type | Structure | Function |
|---|---|---|
| SP-A/SP-D | N-terminal domain | Stabilization of the oligomeric structure through cysteine-rich region (disulfide bond) |
| Collagen-like domain | Maintenance of molecule shape | |
| Neck domain | Nucleation point for refolding | |
| Carbohydrate recognition domain | Binding to lipopolysaccharide or carbohydrates at the surface of microorganisms | |
| SP-B/SP-C | N-terminal domain |
Dimerization through Cys residues (SP-B) Formation of an amphipathic β-hairpin (SP-C) |
| C-terminal domain |
Additional saposin-like domains in proSP-B (SP-B) Stabilization of the proper folding of extremely hydrophobic transmembrane (SP-C) |
Immunological Aspects of Surfactants
The immune system needs to properly respond to harmful, but not to harmless molecules to avoid an inappropriate immune response. Especially, the innate immune system is available for host defense against initial infection linked to the adaptive immune system [36]. In the lungs, emerging evidence has revealed that SP-A and SP-D play an important role in the maintenance of immune balance [37]. Increased expressions of SP-A and SP-D are associated with reduced allergic immune responses [38]; however, surfactant deficiency contributes to enhanced allergic immune responses [39], indicating these molecules are a potential negative regulator in asthma. Functions of SP-A and SP-D in the pathogenesis of asthma are summarized in Table 3.
Table 3.
Several functions of pulmonary surfactants in asthma
| Surfactant | Function | Target | |
|---|---|---|---|
| SP-A/SP-D | Host defense | Binding to pathogen | Virus, bacteria, and fungi |
| Induction of phagocytosis | Pathogens and apoptotic cells | ||
| Immune regulation | Suppression of cell maturation (SP-A) | Dendritic cells | |
| Enhancement of antigen presentation (SP-D) | Dendritic cells | ||
| Reduction of cell activation/proliferation | Lymphocytes (T cells) | ||
| Modulation of cell migration/recruitment | Monocytes and neutrophils | ||
| Inhibition of extracellular trap formation (SP-D) | Eosinophils | ||
| SP-B/SP-C | Surface film formation | Reduction of surface tension | Air–liquid interface |
Function of Surfactants in Host Defense
SP-A and SP-D act as a pattern recognition receptor facilitating phagocytosis by binding to viruses, bacteria, and fungi. The association between viral infections and asthma exacerbations is well defined; human rhinovirus, respiratory syncytial virus, and influenza A virus are predominantly associated with development or exacerbation of asthma [40, 41]. The severity of asthma exacerbations is commonly due to the lack of specific antiviral agents. However, surfactants enhance clearance of viruses via a carbohydrate recognition domain (CRD)–dependent manner [42••]. More recently, certain strains of coronavirus have also been revealed to be involved in asthma exacerbations [43]. A glycoprotein on coronavirus was specifically recognized by SP-D, but not by mannan-binding lectin [44].
In addition to viral infection, bacterial infection has been shown to increase the probability of asthma exacerbation. Especially, Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis enhance the risk for more severe respiratory illnesses and asthma exacerbations [45]. For S. pneumoniae, SP-A and SP-D bind to the bacterial cell wall components, such as lipoteichoic acid and peptidoglycan, via a CRD region [46]. Moreover, these surfactants have been demonstrated to play an important role in innate immune responses to H. influenzae [47]. Although SP-A and SP-D recognize most species of gram-negative bacteria composed of lipopolysaccharide, studies about the effect of surfactants against M. catarrhalis are still limited. Nevertheless, surfactants have been revealed to respond to other pathogenic bacteria including Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus aureus.
A close association between fungal sensitization and asthma severity was well established by skin-test reactivity to one or more fungi such as Alternaria tenuis, Cladosporium cladosporoides, Helminthosorium maydis, and Epicoccum nigrum [48]. In addition, allergic bronchopulmonary aspergillosis occurs in susceptible patients with asthma due to colonization of Aspergillus fumigatus [49]. SP-A and SP-D bind to A. fumigatus, leading to phagocytosis by alveolar macrophages and neutrophils [50, 51]. Furthermore, a recent study has shown that SP-D inhibits adhesion of A. fumigatus to the epithelium surface [52]. These suggest that surfactants are a key player of pulmonary defense against infections.
Function of Surfactants in Immune Modulation
The role of surfactants in the modulation of immune responses is becoming increasingly clear. Eosinophilia in blood or sputum is commonly found in asthmatics with more severe symptoms, worse management, and worse prognosis [53]. Previously, SP-A has been demonstrated to suppress the production of interleukin (IL)-8 by eosinophils [54]. Although multiple functions of eosinophils were suggested, recent studies have highlighted the role of eosinophil extracellular traps (EETs) in the type 2 inflammation of severe eosinophilic asthma [55, 56]. Especially, eosinophil granule proteins (eosinophil-derived neurotoxin) localized in EETs may be related to asthma severity and lung function decline [57]. However, SP-D directly binds to the eosinophil membrane and inhibits extracellular trap formation in concentration- and carbohydrate-dependent manners [58]. A recent study demonstrated a critical role of SP-D (a negative regulatory feedback) in asthma; SP-D deficiency could enhance eosinophil-mediated airway inflammation/remodeling in patients with aspirin-exacerbated respiratory disease [59•].
Proliferation and activation of lymphocytes are critical for the induction of the adaptive immune system in asthma. For example, T lymphocytes release IL-5 which leads to the differentiation, recruitment, activation, and survival of eosinophils [60], but lymphocyte activity was downregulated by SP-A and SP-D [61]. Moreover, surfactant treatment inhibited the ability of lymphocytes to produce IL-2 [62, 63] which is a key cytokine for the induction of allergic immune responses [64]. However, dramatically augmented IL-13 concentration was found in SP-A or SP-D deficiency [65, 66], leading to goblet cell hyperplasia, airway hyperactivity, and tissue remodeling [67]. Furthermore, SP-A and SP-D decrease proliferation of lymphocytes in response to house dust mite allergens in a dose-dependent manner [68]. Taken together, surfactants have a potential benefit in 2 aspects of asthma pathogenesis: reduction of asthma exacerbation and attenuation of type 2 airway inflammation (Fig. 1).
Fig. 1.
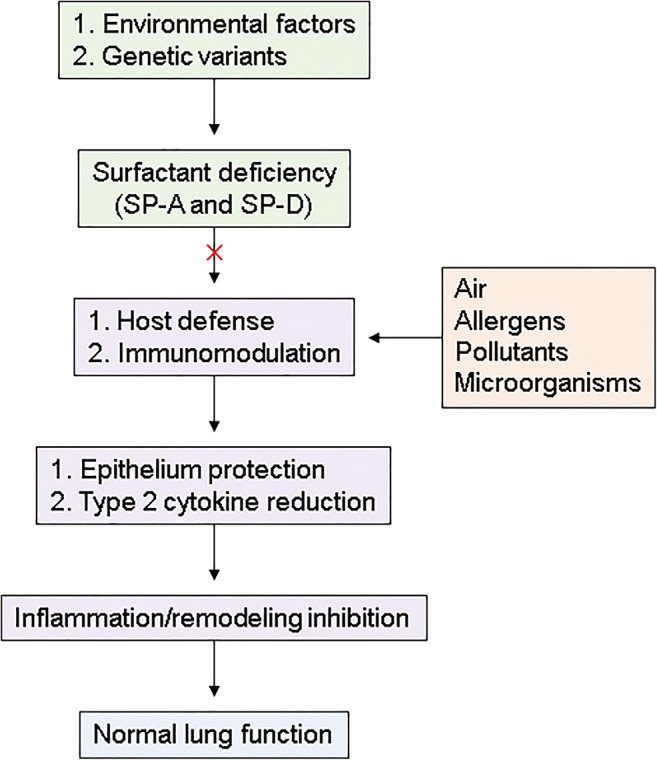
Roles of pulmonary surfactants in maintenance of normal lung function. In asthmatic patients, environmental or genetic factors cause deficiency of surfactants, which are important for host defense and immunomodulation. In the absence of surfactants, inhaled exposures such as allergens, pollutants, and microorganisms enhance epithelium damage or cytokine production, leading to airway inflammation/remodeling associated with lung dysfunction. SP, surfactant protein
Potential Therapeutic Applications of Surfactants
Surfactant replacement was established as a novel therapeutic strategy in patients with surfactant deficiency. In the 1960s, the first attempt of exogenous surfactant administration was made in respiratory distress syndrome (RDS) in preterm infants, leading to reduction in mortality, the incidence of pulmonary air leak, and the risk of chronic lung disease [69]. In addition, surfactant therapy was beneficial in infants with pneumonia and in children with acute lung injury (ALI) or acute respiratory distress syndrome (ARDS); however, extension of surfactant therapy to adults with ALI or ARDS failed [70, 71]. This failure may be associated with an inability of the surfactants to substantially impact the underlying pathophysiology of ALI and ARDS in adults. Although numerous subsequent trials were performed in neonatal RDS, clinical applications have not been conducted in asthma. Nevertheless, the development of pharmaceutical surfactants may provide a promising therapeutic approach to asthma treatment in children, but not in adults.
To date, animal-derived (from bovine or porcine origin) and synthetic surfactants (protein-free) are available. However, natural surfactants have some limitations such as costs, biological risk, and inconsistent production. Therefore, a need of synthetic surfactants, which improve immunological concerns and give consistent response, has emerged. Recently, a third-generation surfactant (CHF5633) yielded promising results for RDS therapy [72, 73], suggesting synthetic surfactants have better efficacy. In addition to commercially available surfactants, other factors affecting endogenous or exogenous surfactant production have been identified. In particular, antenatal corticosteroid treatment showed beneficial effects on inducing surfactant production by maturation of alveolar epithelial cells [74]. Moreover, nintedanib treatment has been revealed to modulate surfactant production [75]. Although nintedanib was neither first developed to enhance surfactant production nor intended to be used for asthma management, this medication may extend further approaches to surfactant replacement therapy, especially in patients with severe eosinophilic asthma who suffer from frequent respiratory infections and asthma exacerbations. Further investigations are required to find safe and effective ways for applying surfactants as correct targets in patients with asthma.
Surfactant treatment has some clinical issues and limitations. First, surfactants are commonly administered by an endotracheal/oropharyngeal tube or a nebulizer; however, the tube for using surfactants can damage of patient airways [74]. Positive pressure by a ventilator can also induce interstitial lung injury. Secondly, there remains a risk of immune responses to animal-derived proteins or treatment-related infections [67]. Thirdly, harvest of natural surfactants from bovine or porcine lungs is difficult to scale. Nevertheless, surfactant therapy may be expected as an advanced treatment for various lung diseases when such limitations are solved.
Conclusions
Pulmonary surfactants play an important role in the first defense mechanism of the lungs constantly exposed to environmental factors, as they act as a barrier by removing pathogens and by modulating inflammatory responses. Surfactant (especially SP-D) imbalance is responsible for enhancing type 2 airway inflammation in asthma as immune cell activation (especially, eosinophils, and lymphocytes) and impaired regulation of immune cell–epithelium interactions. Therapeutic interventions improve surfactant homeostasis by stimulating the endogenous surfactant production or by exogenous surfactant supplementation, which may have a potential benefit in asthmatic patients with eosinophilia, although further studies are needed to explore the possibility of surfactants as a targeted therapy.
Funding
This study was financially supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI16C0992).
Compliance with Ethical Standards
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Basic and Applied Science
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Nayak A, Dodagatta-Marri E, Tsolaki AG, Kishore U. An insight into the diverse roles of surfactant proteins, SP-A and SP-D in innate and adaptive immunity. Front Immunol. 2012;3:131. doi: 10.3389/fimmu.2012.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev. Immunol. 2005;5(1):58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 3.Han S, Mallampalli RK. The role of surfactant in lung disease and host defense against pulmonary infections. Ann Am Thorac Soc. 2015;12(5):765–774. doi: 10.1513/AnnalsATS.201411-507FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hohlfeld JM. The role of surfactant in asthma. Respir Res. 2002;3:4. doi: 10.1186/rr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson RK, Bush A, Stokes J, Nair P, Akuthota P. Eosinophilic asthma. J Allergy Clin Immunol Pract. 2020;8(2):465–473. doi: 10.1016/j.jaip.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Jansson SA, Backman H, Andersson M, Telg G, Lindberg A, Stridsman C, et al. Severe asthma is related to high societal costs and decreased health related quality of life. Respir Med. 2020;162:105860. doi: 10.1016/j.rmed.2019.105860. [DOI] [PubMed] [Google Scholar]
- 7.Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55(1). 10.1183/13993003.00588-2019. [DOI] [PubMed]
- 8.Song WJ, Lee JH, Kang Y, Joung WJ, Chung KF. Future risks in patients with severe asthma. Allergy Asthma Immunol Res. 2019;11(6):763–778. doi: 10.4168/aair.2019.11.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agache I, Cojanu C, Laculiceanu A, Rogozea L. Critical points on the use of biologicals in allergic diseases and asthma. Allergy Asthma Immunol Res. 2020;12(1):24–41. doi: 10.4168/aair.2020.12.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi Y, Sim S, Park HS. Distinct functions of eosinophils in severe asthma with type 2 phenotype: clinical implications. Korean J Intern Med. 2020. 10.3904/kjim.2020.022. [DOI] [PMC free article] [PubMed]
- 11.McDowell PJ, Heaney LG. Different endotypes and phenotypes drive the heterogeneity in severe asthma. Allergy. 2020;75(2):302–310. doi: 10.1111/all.13966. [DOI] [PubMed] [Google Scholar]
- 12.Pembrey L, Barreto ML, Douwes J, Cooper P, Henderson J, Mpairwe H, et al. Understanding asthma phenotypes: the World Asthma Phenotypes (WASP) international collaboration. ERJ Open Res. 2018;4(3). 10.1183/23120541.00013-2018. [DOI] [PMC free article] [PubMed]
- 13.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18(5):673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 14.Hirahara K, Shinoda K, Morimoto Y, Kiuchi M, Aoki A, Kumagai J, et al. Immune cell-epithelial/mesenchymal interaction contributing to allergic airway inflammation associated pathology. Front Immunol. 2019;10:570. doi: 10.3389/fimmu.2019.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siroux V, Bouzigon E. Asthma heterogeneity: the increasing genetic evidence. Lancet Respir Med. 2019;7(6):469–471. doi: 10.1016/S2213-2600(19)30047-5. [DOI] [PubMed] [Google Scholar]
- 16.Altman MC, Beigelman A, Ciaccio C, Gern JE, Heymann PW, Jackson DJ, et al. Evolving concepts in how viruses impact asthma: a work group report of the Microbes in Allergy Committee of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2020;145(5):1332–1344. doi: 10.1016/j.jaci.2019.12.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukacs NW, Huang YJ. Microbiota-immune interactions in asthma pathogenesis and phenotype. Curr Opin Immunol. 2020;66:22–26. doi: 10.1016/j.coi.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi Y, Park H, Park HS, Kim YK. Extracellular vesicles, a key mediator to link environmental microbiota to airway immunity. Allergy Asthma Immunol Res. 2017;9(2):101–106. doi: 10.4168/aair.2017.9.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18(5):684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 20.Eisele NA, Anderson DM. Host defense and the airway epithelium: frontline responses that protect against bacterial invasion and pneumonia. J Pathog. 2011;2011:249802. doi: 10.4061/2011/249802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartl D, Griese M. Surfactant protein D in human lung diseases. Eur J Clin Invest. 2006;36(6):423–435. doi: 10.1111/j.1365-2362.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 22.Choi Y, Lee DH, Lee JH, Shin YS, Kim SH, Park HS. Immunomodulatory function of surfactant protein D in eosinophilic asthma. Allergy. 2019;74(1):192–195. doi: 10.1111/all.13588. [DOI] [PubMed] [Google Scholar]
- 23.Choi Y, Lee Y, Park HS. Which factors associated with activated eosinophils contribute to the pathogenesis of aspirin-exacerbated respiratory disease? Allergy Asthma Immunol Res. 2019;11(3):320–329. doi: 10.4168/aair.2019.11.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Rodriguez E, Gay-Jordi G, Mucci A, Lachmann N, Serrano-Mollar A. Lung surfactant metabolism: early in life, early in disease and target in cell therapy. Cell Tissue Res. 2017;367(3):721–735. doi: 10.1007/s00441-016-2520-9. [DOI] [PubMed] [Google Scholar]
- 25.Haller T, Ortmayr J, Friedrich F, Volkl H, Dietl P. Dynamics of surfactant release in alveolar type II cells. Proc Natl Acad Sci U S A. 1998;95(4):1579–1584. doi: 10.1073/pnas.95.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haller T, Dietl P, Pfaller K, Frick M, Mair N, Paulmichl M, et al. Fusion pore expansion is a slow, discontinuous, and Ca2 +-dependent process regulating secretion from alveolar type II cells. J Cell Biol. 2001;155(2):279–289. doi: 10.1083/jcb.200102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nkadi PO, Merritt TA, Pillers DA. An overview of pulmonary surfactant in the neonate: genetics, metabolism, and the role of surfactant in health and disease. Mol Genet Metab. 2009;97(2):95–101. doi: 10.1016/j.ymgme.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haagsman HP, Diemel RV. Surfactant-associated proteins: functions and structural variation. Comp Biochem Physiol A Mol Integr Physiol. 2001;129(1):91–108. doi: 10.1016/s1095-6433(01)00308-7. [DOI] [PubMed] [Google Scholar]
- 29.Crouch EC. Collectins and pulmonary host defense. Am J Respir Cell Mol Biol. 1998;19(2):177–201. doi: 10.1165/ajrcmb.19.2.140. [DOI] [PubMed] [Google Scholar]
- 30.Vieira F, Kung JW, Bhatti F. Structure, genetics and function of the pulmonary associated surfactant proteins A and D: the extra-pulmonary role of these C type lectins. Ann Anat. 2017;211:184–201. doi: 10.1016/j.aanat.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schicht M, Rausch F, Finotto S, Mathews M, Mattil A, Schubert M, et al. SFTA3, a novel protein of the lung: three-dimensional structure, characterisation and immune activation. Eur Respir J. 2014;44(2):447–456. doi: 10.1183/09031936.00179813. [DOI] [PubMed] [Google Scholar]
- 32.Weaver TE. Synthesis, processing and secretion of surfactant proteins B and C. Biochim Biophys Acta. 1998;1408(2–3):173–179. doi: 10.1016/s0925-4439(98)00066-0. [DOI] [PubMed] [Google Scholar]
- 33.Nogee LM. Genetic causes of surfactant protein abnormalities. Curr Opin Pediatr. 2019;31(3):330–339. doi: 10.1097/MOP.0000000000000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crouch E, Persson A, Chang D, Heuser J. Molecular structure of pulmonary surfactant protein D (SP-D) J Biol Chem. 1994;269(25):17311–17319. [PubMed] [Google Scholar]
- 35.Crouch E, Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev. Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 36.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16(4):343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finn PW, Bigby TD. Innate immunity and asthma. Proc Am Thorac Soc. 2009;6(3):260–265. doi: 10.1513/pats.200807-064RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brinker KG, Garner H, Wright JR. Surfactant protein A modulates the differentiation of murine bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol. 2003;284(1):L232–L241. doi: 10.1152/ajplung.00187.2002. [DOI] [PubMed] [Google Scholar]
- 39.Brandt EB, Mingler MK, Stevenson MD, Wang N, Khurana Hershey GK, Whitsett JA, et al. Surfactant protein D alters allergic lung responses in mice and human subjects. J Allergy Clin Immunol. 2008;121(5):1140–1147. doi: 10.1016/j.jaci.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliver BG, Robinson P, Peters M, Black J. Viral infections and asthma: an inflammatory interface? Eur Respir J. 2014;44(6):1666–1681. doi: 10.1183/09031936.00047714. [DOI] [PubMed] [Google Scholar]
- 41.Kim CK, Callaway Z, Gern JE. Viral infections and associated factors that promote acute exacerbations of asthma. Allergy Asthma Immunol Res. 2018;10(1):12–17. doi: 10.4168/aair.2018.10.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.•• Wang J, Li P, Yu Y, Fu Y, Jiang H, Lu M, et al. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science. 2020;367(6480). 10.1126/science.aau0810 This article highlighted the development of pulmonary surfactant-biomimetic liposomes to effectively avert exaggerated inflammation in the lung. [DOI] [PMC free article] [PubMed]
- 43.Tan KS, Lim RL, Liu J, Ong HH, Tan VJ, Lim HF, et al. Respiratory viral infections in exacerbation of chronic airway inflammatory diseases: novel mechanisms and insights from the upper airway epithelium. Front Cell Dev Biol. 2020;8:99. doi: 10.3389/fcell.2020.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leth-Larsen R, Zhong F, Chow VT, Holmskov U, Lu J. The SARS coronavirus spike glycoprotein is selectively recognized by lung surfactant protein D and activates macrophages. Immunobiology. 2007;212(3):201–211. doi: 10.1016/j.imbio.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133(5):1301–1307, 7 e1–3. doi:10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed]
- 46.van de Wetering JK, van Eijk M, van Golde LM, Hartung T, van Strijp JA, Batenburg JJ. Characteristics of surfactant protein A and D binding to lipoteichoic acid and peptidoglycan, 2 major cell wall components of gram-positive bacteria. J Infect Dis. 2001;184(9):1143–1151. doi: 10.1086/323746. [DOI] [PubMed] [Google Scholar]
- 47.Ujma S, Horsnell WG, Katz AA, Clark HW, Schafer G. Non-pulmonary immune functions of surfactant proteins A and D. J Innate Immun. 2017;9(1):3–11. doi: 10.1159/000451026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006;27(3):615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 49.Knutsen AP, Slavin RG. Allergic bronchopulmonary aspergillosis in asthma and cystic fibrosis. Clin Dev Immunol. 2011;2011:843763. doi: 10.1155/2011/843763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madan T, Reid KB, Singh M, Sarma PU, Kishore U. Susceptibility of mice genetically deficient in the surfactant protein (SP)-A or SP-D gene to pulmonary hypersensitivity induced by antigens and allergens of Aspergillus fumigatus. J Immunol. 2005;174(11):6943–6954. doi: 10.4049/jimmunol.174.11.6943. [DOI] [PubMed] [Google Scholar]
- 51.Madan T, Reid KB, Clark H, Singh M, Nayak A, Sarma PU, et al. Susceptibility of mice genetically deficient in SP-A or SP-D gene to invasive pulmonary aspergillosis. Mol Immunol. 2010;47(10):1923–1930. doi: 10.1016/j.molimm.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 52.Ordonez SR, van Eijk M, Escobar Salazar N, de Cock H, Veldhuizen EJA, Haagsman HP. Antifungal activities of surfactant protein D in an environment closely mimicking the lung lining. Mol Immunol. 2019;105:260–269. doi: 10.1016/j.molimm.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Schleich FN, Chevremont A, Paulus V, Henket M, Manise M, Seidel L, et al. Importance of concomitant local and systemic eosinophilia in uncontrolled asthma. Eur Respir J. 2014;44(1):97–108. doi: 10.1183/09031936.00201813. [DOI] [PubMed] [Google Scholar]
- 54.Cheng G, Ueda T, Nakajima H, Nakajima A, Kinjyo S, Motojima S, et al. Suppressive effects of SP-A on ionomycin-induced IL-8 production and release by eosinophils. Int Arch Allergy Immunol. 1998;117(Suppl 1):59–62. doi: 10.1159/000053574. [DOI] [PubMed] [Google Scholar]
- 55.Choi Y, Le Pham D, Lee DH, Lee SH, Kim SH, Park HS. Biological function of eosinophil extracellular traps in patients with severe eosinophilic asthma. Exp Mol Med. 2018;50(8):104. doi: 10.1038/s12276-018-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi Y, Kim YM, Lee HR, Mun J, Sim S, Lee DH, et al. Eosinophil extracellular traps activate type 2 innate lymphoid cells through stimulating airway epithelium in severe asthma. Allergy. 2020;75(1):95–103. doi: 10.1111/all.13997. [DOI] [PubMed] [Google Scholar]
- 57.Lee Y, Lee JH, Yang EM, Kwon E, Jung CG, Kim SC, et al. Serum levels of eosinophil-derived neurotoxin: a biomarker for asthma severity in adult asthmatics. Allergy Asthma Immunol Res. 2019;11(3):394–405. doi: 10.4168/aair.2019.11.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yousefi S, Sharma SK, Stojkov D, Germic N, Aeschlimann S, Ge MQ, et al. Oxidative damage of SP-D abolishes control of eosinophil extracellular DNA trap formation. J Leukoc Biol. 2018;104(1):205–214. doi: 10.1002/JLB.3AB1117-455R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi Y, Lee DH, Trinh HKT, Ban GY, Park HK, Shin YS, et al. Surfactant protein D alleviates eosinophil-mediated airway inflammation and remodeling in patients with aspirin-exacerbated respiratory disease. Allergy. 2019;74(1):78–88. doi: 10.1111/all.13458. [DOI] [PubMed] [Google Scholar]
- 60.Pelaia C, Paoletti G, Puggioni F, Racca F, Pelaia G, Canonica GW, et al. Interleukin-5 in the pathophysiology of severe asthma. Front Physiol. 2019;10:1514. doi: 10.3389/fphys.2019.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc. 2007;4(3):252–257. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borron P, Veldhuizen RA, Lewis JF, Possmayer F, Caveney A, Inchley K, et al. Surfactant associated protein-A inhibits human lymphocyte proliferation and IL-2 production. Am J Respir Cell Mol Biol. 1996;15(1):115–121. doi: 10.1165/ajrcmb.15.1.8679215. [DOI] [PubMed] [Google Scholar]
- 63.Borron PJ, Crouch EC, Lewis JF, Wright JR, Possmayer F, Fraher LJ. Recombinant rat surfactant-associated protein D inhibits human T lymphocyte proliferation and IL-2 production. J Immunol. 1998;161(9):4599–4603. [PubMed] [Google Scholar]
- 64.Nag S, Lamkhioued B, Renzi PM. Interleukin-2-induced increased airway responsiveness and lung Th2 cytokine expression occur after antigen challenge through the leukotriene pathway. Am J Respir Crit Care Med. 2002;165(11):1540–1545. doi: 10.1164/rccm.2109012. [DOI] [PubMed] [Google Scholar]
- 65.Francisco D, Wang Y, Conway M, Hurbon AN, Dy ABC, Addison KJ, et al. Surfactant protein-A protects against IL-13-induced inflammation in asthma. J Immunol. 2020;204(10):2829–2839. doi: 10.4049/jimmunol.1901227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schaub B, Westlake RM, He H, Arestides R, Haley KJ, Campo M, et al. Surfactant protein D deficiency influences allergic immune responses. Clin Exp Allergy. 2004;34(12):1819–1826. doi: 10.1111/j.1365-2222.2004.02068.x. [DOI] [PubMed] [Google Scholar]
- 67.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282(5397):2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 68.Wang JY, Kishore U, Lim BL, Strong P, Reid KB. Interaction of human lung surfactant proteins A and D with mite (Dermatophagoides pteronyssinus) allergens. Clin Exp Immunol. 1996;106(2):367–373. doi: 10.1046/j.1365-2249.1996.d01-838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hentschel R, Bohlin K, van Kaam A, Fuchs H, Danhaive O. Surfactant replacement therapy: from biological basis to current clinical practice. Pediatr Res. 2020. 10.1038/s41390-020-0750-8. [DOI] [PMC free article] [PubMed]
- 70.Raghavendran K, Willson D, Notter RH. Surfactant therapy for acute lung injury and acute respiratory distress syndrome. Crit Care Clin. 2011;27(3):525–559. doi: 10.1016/j.ccc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Willson DF, Notter RH. The future of exogenous surfactant therapy. Respir Care. 2011;56(9):1369–1386. doi: 10.4187/respcare.01306. [DOI] [PubMed] [Google Scholar]
- 72.Sweet DG, Turner MA, Stranak Z, Plavka R, Clarke P, Stenson BJ, et al. A first-in-human clinical study of a new SP-B and SP-C enriched synthetic surfactant (CHF5633) in preterm babies with respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 2017;102(6):F497–F503. doi: 10.1136/archdischild-2017-312,722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Madsen J, Panchal MH, Mackay RA, Echaide M, Koster G, Aquino G, et al. Metabolism of a synthetic compared with a natural therapeutic pulmonary surfactant in adult mice. J Lipid Res. 2018;59(10):1880–1892. doi: 10.1194/jlr.M085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gladstone IM, Mercurio MR, Devenny SG, Jacobs HC. Antenatal steroids, postnatal surfactant, and pulmonary function in premature rabbits. J Appl Physiol (1985) 1989;67(4):1377–1382. doi: 10.1152/jappl.1989.67.4.1377. [DOI] [PubMed] [Google Scholar]
- 75.Kamio K, Usuki J, Azuma A, Matsuda K, Ishii T, Inomata M, et al. Nintedanib modulates surfactant protein-D expression in A549 human lung epithelial cells via the c-Jun N-terminal kinase-activator protein-1 pathway. Pulm Pharmacol Ther. 2015;32:29–36. doi: 10.1016/j.pupt.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Cheng G, Ueda T, Numao T, Kuroki Y, Nakajima H, Fukushima Y, et al. Increased levels of surfactant protein A and D in bronchoalveolar lavage fluids in patients with bronchial asthma. Eur Respir J. 2000;16(5):831–835. doi: 10.1183/09031936.00.16583100. [DOI] [PubMed] [Google Scholar]
- 77.Okazaki S, Murai H, Kidoguchi S, Nomura E, Itoh N, Hashimoto N, et al. The biomarker salivary SP-D may indicate small airway inflammation and asthma exacerbation. J Investig Allergol Clin Immunol. 2017;27(5):305–312. doi: 10.18176/jiaci.0174. [DOI] [PubMed] [Google Scholar]
- 78.Fakih D, Akiki Z, Junker K, Medlej-Hashim M, Waked M, Salameh P, et al. Surfactant protein D multimerization and gene polymorphism in COPD and asthma. Respirology. 2018;23(3):298–305. doi: 10.1111/resp.13193. [DOI] [PubMed] [Google Scholar]
- 79.Koopmans JG, van der Zee JS, Krop EJ, Lopuhaa CE, Jansen HM, Batenburg JJ. Serum surfactant protein D is elevated in allergic patients. Clin Exp Allergy. 2004;34(12):1827–1833. doi: 10.1111/j.1365-2222.2004.02083.x. [DOI] [PubMed] [Google Scholar]
- 80.Xu J, Singhera GK, Dorscheid DR. Expression of surfactant protein D in airways of asthmatics and interleukin-13 modulation of surfactant protein D in human models of airway epithelium. Respir Res. 2015;16:26. doi: 10.1186/s12931-015-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mackay RM, Grainge CL, Lau LC, Barber C, Clark HW, Howarth PH. Airway surfactant protein D deficiency in adults with severe asthma. Chest. 2016;149(5):1165–1172. doi: 10.1016/j.chest.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Emmanouil P, Loukides S, Kostikas K, Papatheodorou G, Papaporfyriou A, Hillas G, et al. Sputum and BAL Clara cell secretory protein and surfactant protein D levels in asthma. Allergy. 2015;70(6):711–714. doi: 10.1111/all.12603. [DOI] [PubMed] [Google Scholar]
- 83.Benfante A, Battaglia S, Principe S, Di Mitri C, Paterno A, Spatafora M, et al. Asthmatics with high levels of serum surfactant protein D have more severe disease. Eur Respir J. 2016;47(6):1864–1867. doi: 10.1183/13993003.02142-2015. [DOI] [PubMed] [Google Scholar]
- 84.Lugogo N, Francisco D, Addison KJ, Manne A, Pederson W, Ingram JL, et al. Obese asthmatic patients have decreased surfactant protein A levels: mechanisms and implications. J Allergy Clin Immunol. 2018;141(3):918–926. doi: 10.1016/j.jaci.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]


