Abstract
Objectives:
Social engagement may be an important protective resource for cognitive aging. Some evidence suggests that time spent with friends may be more beneficial for cognition than time spent with family. Because maintaining friendships has been demonstrated to require more active maintenance and engagement in shared activities, activity engagement may be one underlying pathway that explains the distinct associations between contact frequency with friends versus family and cognition.
Methods:
Using two waves of data from the national survey of Midlife in the United States (MIDUS; n= 3,707, Mage= 55.80, 51% female at baseline), we examined longitudinal associations between contact frequency with friends and family, activity engagement (cognitive activity and physical activity), and cognition (episodic memory and executive functioning) to determine whether activity engagement mediates the relationship between contact frequency and cognition.
Results:
The longitudinal mediation model revealed that more frequent contact with friends, but not family, was associated with greater concurrent engagement in physical and cognitive activities, which were both associated with better episodic memory and executive functioning.
Conclusion:
These findings suggest that time spent with friends may promote both cognitively-and physically-stimulating activities that could help to preserve not only these social relationships, but also cognitive functioning.
Keywords: friendships, social relationships, contact frequency, cognitive aging, cognitive activity, physical activity
Growing evidence suggests that social engagement may be an important protective resource for cognitive functioning in older adulthood (Barnes, Mendes de Leon, Wilson, Bienias & Evans, 2004; Crooks, Lubben, Petitti, Little & Chiu, 2008; Zahodne, Ajrouch, Sharifian & Antonucci, 2019) and may protect against brain pathology (Bennett, Schneider, Tang, Arnold & Wilson, 2006). Consistent with the cognitive reserve theory (Stern, 2002), older adults that remain actively engaged within their social networks show slower rates of cognitive decline (Béland, Zunzunegui, Alvarado, Otero, & del Ser, 2005; Zahodne et al., 2019) and have lower Alzheimer’s disease and related dementia (ADRD) incidence (Amieva et al., 2010), suggestive that contact with social network members may act as a resource that promotes cognitive efficiency and compensatory processes. Recent evidence, however, suggests that relationship type matters (Sharifian, Manly, Brickman & Zahodne, 2019; Zahodne et al., 2019). Specifically, the link between social engagement and cognition may vary dependent on whether the individual is spending time with friends or with family.
Prior cross-sectional investigations have found that having a greater portion of family within one’s social network is associated with lower cognition (Li & Dong, 2018; Sharifian et al., 2019). Further, having a higher number of close friends, but not children or neighbors, has been linked to better global cognition (Wang, He & Dong, 2015). Fewer studies have examined these associations longitudinally, but of those that did, a similar pattern of findings emerged. In a nationally representative US sample, more frequent contact averaged across relationships was prospectively associated with less decline in episodic memory. When broken down by relationship type, this finding was driven by contact frequency with friends (Zahodne et al., 2019). In another longitudinal study, having friends was associated with less cognitive decline, but only among women (Béland et al., 2005). Of note, the same longitudinal study also found a positive association between social engagement with family and cognition (i.e., Béland et al., 2005), warranting further investigation into the associations between time spent with family and cognition.
Overall, prior research suggests that contact frequency with friends may confer greater cognitive benefits than contact frequency with family members (Wang et al., 2015; Zahodne et al., 2019), however, much less is known regarding the underlying mechanisms that drive these associations. Therefore, the current study aimed to examine the underlying behavioral pathways that may partially explain the distinct influences of contact frequency with friends versus family on cognitive functioning.
The Impact of Social Contact on Activity Engagement
Activity engagement may play an important role in explaining the robust association between contact frequency with friends (as opposed to family) and cognitive functioning in later life. Specifically, the salience of friendships may be driven, in part, by relationship maintenance behaviors. As friendships are voluntary relationships, individuals must actively work to maintain them (Lee & Ishii-Kuntz, 1987). Familial ties, on the other hand, require less maintenance to retain within one’s network. For instance, in a longitudinal study following young adults transitioning from high school to college, emotional closeness with friends tended to decrease, whereas emotional closeness with familial ties remained stable. Importantly, more shared activities and communication were associated with preserved emotional closeness with friends, indicating that friendships required greater active maintenance to avoid relationship decay over time (Roberts & Dunbar, 2015).
Consistent with prior research in younger adult populations (i.e., Roberts & Dunbar, 2015), later-life friendships are a greater source of immediate joy (Larson, Mannell & Zuzanek, 1986) and provide a greater sense of companionship through informal social activities than family relationships (Huxhold, Miche & Schüz, 2013). Prior research has also linked a greater proportion of friends within one’s social network to more late-life physical activities (Shiovitz-Ezra & Litwin, 2012). When specifically asked about leisure activity engagement, older adults in a qualitative study reported that a strong motivator was to promote friendships/companionships (Ball, Corr, Knight & Lowis, 2007).
Activity Engagement and Cognitive Functioning
Extensive prior research has shown protective effects of activity engagement for cognition (Hertzog, Kramer, Wilson & Lindenberger, 2009; Jonaitis et al., 2013). In particular, both cognitive (i.e., board games, attending a play or lectures) and physical (i.e., walking, dancing, exercise) activity have been linked to better cognition (Hayes et al., 2015; Mueller, Raymond, & Yochim, 2013; Weuve et al., 2004) and lower ADRD risk (Scarmeas, Levy, Tang, Manly & Stern, 2001; Scarmeas et al., 2009). Cognitive activities may be beneficial for cognition through cognitive enrichment and the promotion of cognitive reserve (Valenzuela, Sachdev, Wen, Chen & Brodaty, 2008). Physical activities may benefit cognition through reduced cardiovascular disease risk (Warburton, Nicol & Bredin, 2006), which is a strong risk factor for later life dementia (Whitmer, Sidney, Johnston & Yaffe, 2005).
The Present Study
Despite prior research linking greater contact with friends to greater activity engagement (Ball et al., 2007; Ihle, Oris, Baeriswyl & Kliegel, 2018; Shiovitz-Ezra & Litwin, 2012) and greater activity engagement to better later-life cognition (Gill et al., 2015; Mueller et al., 2013; Reed et al., 2011; Weuve et al., 2004), less is known regarding whether the cognitive benefits accrued from time spent with friends operate through greater activity engagement. Therefore, the overall goal of the current study was to examine whether the benefits of contact frequency with friends for cognitive functioning operated, in part, through greater engagement in enriching cognitive and/or physical activities. Because prior research has shown that both cognitive and physical activity engagement independently influence cognitive aging, we considered them as separate, but related, mediators.
We hypothesized that activity engagement would significantly mediate the association between contact frequency with friends and cognition. That is, we hypothesized that more frequent contact with friends would be associated with greater engagement in cognitive activity and physical activity, and in turn, engagement in both types of activities would be independently associated with better subsequent cognitive functioning. In regards to contact frequency with family, based on prior research regarding relationship maintenance behaviors (Roberts & Dunbar, 2015), we hypothesized that activity engagement would not significantly mediate the association between contact frequency with family and cognition due to a lack of association between contact frequency with family and activity engagement. Finally, we hypothesized a positive direct effect of contact frequency with friends on cognitive functioning after accounting for covariates and activity engagement. Due to mixed literature regarding the association between contact frequency with family and cognition (Béland et al., 2005; Zahodne et al., 2019), we had no a priori hypothesis regarding this association.
Methods
Participants and Procedure
We utilized two waves of longitudinal data from the national survey of Midlife in the United States (MIDUS; Brim, Ryff & Kessler, 2004). MIDUS is a national sample of noninstitutionalized middle-aged and older adults selected by random digit dialing in the contiguous 48 states. MIDUS data collection oversampled participants who were 40 to 60 years old. Cognitive and psychosocial assessments were administered in 2004–2006 (T1) and again in 2013–2017 (T2). All participants were provided written informed consent and all study procedures were approved by the University institutional review board.
At T1, 4,198 participants were given initial tests of episodic memory and executive functioning. At T2, 2,721 of the original sample were given subsequent episodic memory and executive functioning tests. Compared to non-returnees, participants with follow-up assessments were significantly older, [F(1, 4187) = 25.20, p< .001, η2= .01], had higher education, [F(1, 4181) = 106.78, p < .001, η2= .03], had fewer chronic diseases, [F(1, 3698)= 29.43, p< .001, η2= .01], were less likely to identify as a member of a racial or ethnic minority group, [F(1, 4187)= 18.36, p < .001, η2= .004], had higher baseline episodic memory scores, [F(1, 4187)= 92.62, p< .001, η2= .02] and had higher executive functioning scores, [F(1, 4179)= 191.33, p< .001, η2= .04]. Returnees and non-returnees did not significantly differ base on gender, [F(1, 4187)= 3.22, p= .073, η2 = .001] or marital status, [F(1, 4187)= 3.55, p= .059, η2 = .001]. Importantly, missing data was managed with full information maximum likelihood with robust standard errors (Arbuckle, 1996) which handles attrition related to variables included in the model.
Our final sample included 3,707 participants that were, on average, 55.80 years old (SD= 12.31), 50.50% female and 85.60% non-Hispanic White at T1 (see Table 1). Details of the MIDUS longitudinal design, sampling, and all assessment instruments are available on the MIDUS website (http://midus.wisc.edu/).
Table 1.
Means and Standard Deviations of Variables of Interest.
| M | SD | Range | |
|---|---|---|---|
| Age (T1) | 55.80 | 12.31 | 28–84 |
| % Female (T1) | 50.50% | - | - |
| % Married (T1) | 67.10% | - | - |
| % Non-Hispanic White (T1) | 85.60% | - | - |
| Education (T1) | 7.26 | 2.52 | 1–12 |
| Chronic Illness Burden (T1) | 2.48 | 2.61 | 0–30 |
| Contact Frequency with Friends (T1) | 5.61 | 1.69 | 1–8 |
| Contact Frequency with Family (T1) | 5.97 | 1.51 | 1–8 |
| Cognitive Activity (T1) | 3.06 | 0.84 | 1–6 |
| Physical Activity (T1) | 38.20 | 21.91 | 0–70 |
| Episodic Memory (T1) | −.00 | 1.00 | −3.07–3.83 |
| Immediate Recall (T1) | 6.68 | 2.29 | 0–15 |
| Delayed Recall (T1) | 4.36 | 2.63 | 0–14 |
| Episodic Memory (T2) | −.04 | 0.99 | −2.94–3.64 |
| Immediate Recall (T2) | 6.63 | 2.40 | 0–15 |
| Delayed Recall (T2) | 4.30 | 2.71 | 0–14 |
| Executive Functioning (T1) | .00 | 1.00 | −7.37–3.39 |
| Animal Fluency (T1) | 18.58 | 6.17 | 0–42 |
| Digit Span Backwards (T1) | 4.96 | 1.53 | 0–8 |
| Number Series (T1) | 2.18 | 1.53 | 0–5 |
| Backwards Count Task (T1) | 36.67 | 11.75 | −13–100 |
| Stop & Go Switch Task (T1) | −1.10 | 0.29 | −7.36-−0.21 |
| Executive Functioning (T2) | −.19 | 0.76 | −5.63–2.02 |
| Animal Fluency (T2) | 18.41 | 6.12 | 0–42 |
| Digit Span Backwards (T2) | 4.91 | 1.52 | 0–8 |
| Number Series (T2) | 2.16 | 1.57 | 0–5 |
| Backwards Count Task (T2) | 35.25 | 11.97 | −12–90 |
| Stop & Go Switch Task (T2) | −1.41 | 0.37 | −7.67 - −0.70 |
Measures
Contact Frequency with Friends and Family.
Contact frequency with family at T1 was assessed with 1-item asking participants, “how often are you in contact with any members of your family, that is, any of your brothers, sisters, parents, or children who do not live with you, including visits, phone calls, letters or electronic mail messages?” For friends, contact frequency at T1 was assessed with 1-item asking participants, “how often are you in contact with any of your friends, including visits, phone calls, letters or electronic mail messages?” Both contact frequency with family and with friends were rated on an 8-point scale ranging from several times a day (1) to never or hardly ever (8). Scores were reverse coded so higher scores represented more frequent contact.
Cognitive Activity.
Cognitive activity was measured at T1 using 6-items assessing frequency of engagement in cognitively-stimulating leisure activities, including reading books, magazines or newspapers and attending educational lectures or courses. Engagement in each activity was rated on a 6-point scale ranging from daily (1) to never (6). Scores were reverse coded, and a composite was created by averaging responses across all 6-items. Higher scores indicated greater engagement in cognitive activities.
Physical Activity.
Physical activity was measured at T1 using 6-items assessing the frequency of light, moderate and vigorous physical leisure activities during summer and winter. Items were rated on a 6-point scale from several times a week (1) to never (6). All items were then coded such that never=0, less than once a month=1, once a month=2, several times a month=3, once a week=4, and several times a week=5 in order to facilitate conversion of scores into their metabolic equivalents (Meyer, Janke & Beaujean, 2013).
In order to calculate an overall physical activity score, first, summer and winter items were averaged for light, moderate and vigorous physical activity. Subsequently, a summary score of their total physical activity was calculated for each individual using the following formula: (Light Physical Activity × 2) + (Moderate Physical Activity × 4) + (Vigorous Physical Activity × 8). The weighted values for each level of physical activity approximate its metabolic equivalent, consistent with prior research (Meyer et al., 2013).
Cognition.
Cognition was assessed by measuring two domains, episodic memory and executive functioning, at T1 and T2 using the Brief Test of Adult Cognition by Telephone (Lachman, Agrigoroaei, Tun & Weaver, 2014). Episodic memory functioning was measured using the immediate and delayed recall trials from the Rey Auditory-Verbal Learning test (Rey, 1964). Executive functioning was assessed with category animal fluency (Borkowski, Benton & Spreen, 1967; Tombaugh, Kozak, & Rees, 1999), digit span backward (Wechsler, 1997), number series (Salthouse & Prill, 1987; Schaie, 1996), the 30 seconds backwards counting task (Lachman, Agrigoroaei, Murphy & Tun, 2010), and the Stop & Go switch task (Lachman et al., 2010). Composite scores for episodic memory and executive functioning were computed as mean z-scores within each domain using means and standard deviations from T1.
Covariates.
Analyses controlled for age, gender (1= Male, 2= Female), marital status (0= not partnered, 1= partnered), race (0= non-Hispanic White, 1= Other), education, and number of chronic illnesses at T1. Age was a self-reported continuous variable. Education was self-reported and could range from no school/some grade school (1) to Ph.D., Ed.D. M.D or other professional degree (12). Chronic illnesses was the sum of self-reported chronic conditions such as high blood pressure, diabetes, and stroke and could range from 0 (no chronic conditions reported) to 30 (all chronic conditions reported).
Analytic Strategy
Separate longitudinal mediation models were conducted to examine episodic memory and executive functioning. Within each model, cognitive activity and physical activity were regressed onto contact frequency with both friends and family. Cognitive and physical activity, as well as contact frequency with friends and family, were allowed to covary. Baseline cognition and latent change (McArdle, 2009) in cognition from T1 to T2 were both regressed onto cognitive activity, physical activity, contact frequency with friends and contact frequency with family. Covariates were regressed onto exposure, mediator and outcome variables and therefore, model fit was perfect. Descriptive statistics were calculated in SPSS (Version 25), and the mediation models were conducted in Mplus (Version 8).
To assess whether activity engagement mediated the relationship between contact frequency and cognition, indirect effects were calculated. Specifically, indirect effects were a product of the independent association between contact frequency (with friends or family) and a mediator (cognitive activity or physical activity) and the association between that mediator and an outcome (baseline cognition or latent change in cognition from T1 to T2). Direct effects were defined as the association between contact frequency (with friends or family) and an outcome (episodic memory or executive functioning), independent of all mediators and covariates.
Results
Episodic Memory
Standardized estimates for mediational pathways for this model are depicted in Figure 1A. Significant indirect effects of contact frequency with friends on both baseline episodic memory (indirect effect: β= .03, SE= .00, p< .001) and latent change in episodic memory (indirect effect: β= .01, SE= .00, p= .006) were found through cognitive activity. That is, more frequent contact with friends was associated with greater cognitive activity (β= .17, SE= .02, p< .001), and cognitive activity was, in turn, associated with higher baseline episodic memory (β= .15, SE= .02, p< .001) and less decline in episodic memory (β= .06, SE= .02, p= .005).
Figure 1.
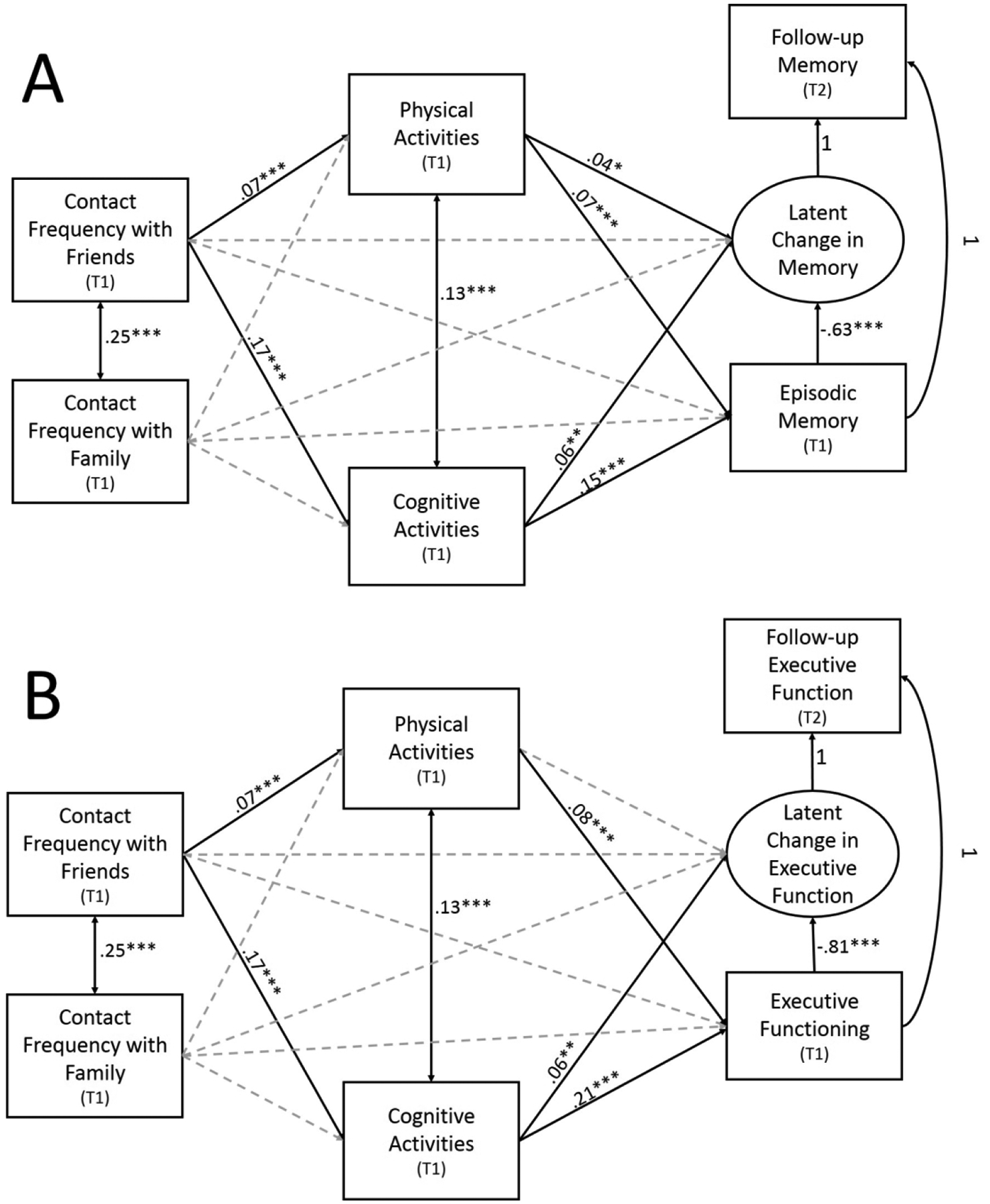
Longitudinal mediation models depicting associations between contact frequency with friends and family on (A) Episodic Memory and (B) Executive Functioning through Cognitive and Physical Leisure Activities. Nonsignificant pathways are depicted as gray, dotted lines. For simplicity, covariate associations are not depicted. * = p<.05, ** = p<.01, *** = p<.001
Significant, independent indirect effects of contact frequency with friends on both baseline memory (indirect effect: β= .01, SE= .00, p= .003) and latent change in episodic memory (indirect effect: β= .003, SE= .00, p= .042) were also found through physical activity. More frequent contact with friends was associated with greater physical activity (β= .07, SE= .02, p< .001), and physical activity was, in turn, associated with higher baseline episodic memory (β= .07, SE= .02, p< .001) and less decline in episodic memory over time (β= .04, SE= .02, p= .020). After accounting for covariates and mediators, no significant direct effect of contact frequency with friends emerged for episodic memory (baseline: β= .03, SE= .02, p= .053; memory change: β= −.01, SE= .02, p= .588).
No significant direct or indirect effects of contact frequency with family on episodic memory emerged (ps > .075). With regard to covariates, older age (β= −.28, SE= .02, p< .001), not identifying as non-Hispanic White (β= −.06, SE= .01, p< .001), and higher chronic disease burden (β= −.04, SE= .02, p= .005) were associated with lower baseline episodic memory, whereas higher education (β= .11, SE= .02, p< .001) and being female (β= .22, SE= .02, p< .001) were associated with higher baseline episodic memory. Being female (β= .16, SE= .02, p< .001) and higher education (β= .05, SE= .02, p= .011) were also associated with less decline in episodic memory over time, whereas older age (β= −.31, SE= .02, p< .001) and higher chronic disease burden (β= −.05, SE= .02, p= .010) were associated with more decline in episodic memory over time.
Executive Functioning
Standardized estimates for mediational pathways for this model are depicted in Figure 1B. Significant indirect effects of contact frequency with friends through cognitive activity were found for both baseline executive functioning (indirect effect: β= .04, SE= .00, p< .001) and latent change in executive functioning (indirect effect: β= .01, SE= .00, p< .001). More frequent contact with friends was associated with greater cognitive activity (β= .17, SE= .02, p< .001) and in turn, cognitive activity was associated with higher baseline executive functioning (β= .21, SE= .02, p< .001) and less decline in executive functioning over time (β= .06, SE= .02, p= .001).
A significant, independent indirect effect of contact frequency with friends through physical activity was found for baseline executive functioning (indirect effect: β= .01, SE= .00, p= .001), but not latent change (p= .429). More frequent contact with friends was associated with greater physical activity (β= .07, SE= .02, p< .001) and in turn, more physical activity was associated with higher baseline executive functioning (β= .08, SE= .01, p< .001). After accounting for covariates and mediators, no significant direct effect of contact frequency with friends emerged for executive functioning (baseline: β= .02, SE= .01, p= .088; executive functioning change: β= −.01, SE= .02, p= .700).
No direct or indirect effects of contact frequency with family on executive functioning emerged (ps > .16). With regard to covariates, older age (β= −.34, SE= .01, p< .001), being female (β= −.09, SE= .01, p< .001), not identifying as non-Hispanic White (β= −.10, SE= .01, p< .001), and greater chronic disease burden (β= −.08, SE= .01, p< .001) were associated with lower baseline executive functioning, whereas higher education (β= .25, SE= .02, p< .001) and being married (β= .03, SE= .01, p= .030) were associated with higher baseline executive functioning. Age (β= −.30, SE= .02, p< .001), race (β= −.04, SE= .02, p= .016), chronic disease burden (β= −.06, SE= .02, p= .003), education (β= .04, SE= .02, p= .041) and being married (β= .03, SE= .02, p= .047) were also associated with latent change in executive functioning. The direction of these covariate associations is consistent with those reported for baseline executive functioning.
Additional sensitivity analyses were conducted to (1) examine the directionality of associations involving the exposures and mediators, (2) examine an alternative physical activity coding method, (3) control for personality characteristics, (4) use an alternative method to handle missing data, and (5) exclude participants with suspected cognitive impairment. Importantly, the pattern of associations were consistent with our reported findings (see Supplementary Material).
Discussion
The goal of the current study was to investigate potential mechanisms underlying the relationship between spending time with friends versus family and cognition. Consistent with our hypotheses, we found that contact frequency with friends was associated with longitudinal changes in both episodic memory and executive functioning through cognitive activity. Contact frequency with friends was also associated with longitudinal change in episodic memory, but not executive functioning, through physical activity. Contact frequency with family was not significantly associated with activity engagement. Further, contact frequency with family was not significantly associated with cognition, coinciding with prior research (Wang et al., 2015; Zahodne et al., 2019).
Friendships and Activity Engagement
More frequent contact with friends, but not family, was associated with greater engagement in physical and cognitive activities. These findings are consistent with prior research with young adult populations which demonstrated that individuals must engage in more frequent communication and activities in order to maintain emotional closeness with friends whereas this is not the case for familial ties (Oswald & Clark, 2003; Roberts & Dunbar, 2015). Friendships have also been linked to greater activity engagement among older populations (Ihle et al., 2018; Shiovitz-Ezra & Litwin, 2012). While prior research has often shown associations between having friends and engaging in leisure activities more broadly (Huxhold et al., 2013; Ihle et al., 2018), this study extends past research by showing independent links between friendship and both cognitive and physical activities.
Friendships and family relationships may differ in their requirement of active maintenance due to the nature of these relationships. First, friends are voluntary relationships that must be actively selected. As such, ideal standards for the generation and maintenance of friendships typically exist. For instance, prior research has consistently identified shared/mutual activities as an important component of friendships (Hall, 2011; Oswald, Clark & Kelly, 2004). In contrast, individuals are not able to select who is in their family and therefore, may not carry the same standards for shared activities/mutual hobbies. Greater contact with family may also be indicative of greater dependency (i.e., requiring support) and in turn, be associated with worse outcomes due to a greater reliance on others compared to those who have greater contact with friends.
Although more frequent contact with family was not associated with activity engagement or cognition, it may still be the case that familial ties benefit cognitive health and well-being through other pathways (Rook & Ituarte, 1999). For example, in a cross-sectional study, older adults reported that family members were a greater source of social control (reducing risky health behaviors), as well as emotional and instrumental support compared to friends. In contrast, older adults’ friends were viewed as a greater source of companionship (i.e., get-togethers; Rook & Ituarte, 1999). Therefore, future research should explore other affective and behavioral pathways by which contact frequency with friends and with family members may influence cognition.
The Benefits of Cognitive and Physical Activity for Cognition
The link between cognitive activity and cognitive functioning is consistent with the ‘use it or lose it’ hypothesis, which states that engaging in cognitively-stimulating activities, such as playing board games and attending lectures, may help to preserve cognitive functioning and promote cognitive reserve, whereas disuse can lead to deterioration of cognitive skills (Hultsch, Hertzog, Small & Dixon, 1999; Reed et al., 2011). Cognitively-stimulating activities may maintain cognition through their impact on brain structure and function, such as white matter integrity (Arfanakis et al., 2016) and synaptic plasticity (Buitenweg, Murre & Riddenrinkhof, 2012). For example, in an intervention study, older adults who engaged in a strategy-based real-time video game over 7 to 8 weeks demonstrated improvements in executive functioning compared to the no-contact control group (Basak, Boot, Voss & Kramer, 2008), suggestive of the protective effects of cognitive activity for cognition.
Independent of cognitive activity, physical activity was also associated with higher baseline executive functioning and episodic memory, as well as less decline in memory functioning. Physical activity may protect cognitive health in later life by increasing cardiovascular fitness (Rogers, Meyer & Mortel, 1990). Prior longitudinal research has shown that greater cardiorespiratory fitness prospectively predicts better cognition years later (Barnes, Yaffe, Satariano & Tager, 2003). Greater cardiorespiratory fitness may benefit cognition by reducing risk of cardiovascular disease which is a known risk factor for age-related cognitive decline (Warburton et al., 2006).
The greater impact of physical activity on episodic memory change relative to executive functioning may be due to the influence of physical activity on hippocampal neurogenesis (Erickson et al., 2011). For example, in an intervention study examining exercise and brain aging, increased aerobic exercise over 1 year was associated with increased hippocampal volume, and in turn, spatial memory functioning (Erickson et al., 2011). It may be the case that physical exercise has a more pronounced effect on episodic memory due to its specific effects on the hippocampus.
Although physical activity was significantly associated with concurrently measured executive functioning, no prospective association was found. These findings, therefore, warrant caution as they may be due to reverse causation. Individuals with greater executive functioning at baseline may be more able to engage in higher levels of physical activity. Prior interventions, however, support a causal effect of aerobic physical activity on executive functioning (Erickson & Kramer, 2009). The lack of prospective association between physical activity and executive functioning in the current study may relate to its being observational rather than experimental.
After accounting for covariates and mediators, no significant direct effect of contact frequency with friends was found for either cognitive domain, suggestive of the importance of activity engagement in explaining the link between friendships and cognition. It is important to note, however, that activity engagement may not be the only pathway through which contact frequency with friends may benefit cognition. In prior longitudinal research, social activity was found to protect against age-related cognitive decline, even after controlling for the individual’s level of cognitive and physical activity (see James, Wilson, Barnes & Bennett, 2011). It may be the case that shared conversation/communication may be beneficial in and of itself. Further, friendships may also reap affective benefits that may help to alleviate stress and foster feelings of well-being (Larson et al., 1986).
Overall, we found that both cognitive activity and physical activity were independently associated with better cognition, and cognitive activity was found to be the strongest predictor of cognition, showing prospective associations with both episodic memory and executive functioning. This may indicate that cognitive activity may have more domain-general effects for improving cognition whereas physical activity may demonstrate more domain-specific effects. However, it may also be the case that self-reported physical activity has a higher rate of bias (recall bias/social desirability; Adams et al., 2005; Barnes et al., 2003), reducing its association with cognition. Additionally, it may be the case that greater variability in the types of physical activity individuals are engaging in may impact its association with cognition. Specifically, prior research has linked aerobic exercise (running/walking) but not anaerobic exercise (stretching/toning) to improved cognition (Erickson et al., 2011). Due to the self-report nature of physical activity, we do not have specific information regarding the exact types of physical activity.
Limitations and Future Directions
Although the current study illuminates behavioral pathways by which social engagement with friends might promote episodic memory and executive functioning, there are notable limitations. First, our measures of contact frequency and activity engagement are limited. Specifically, we cannot disentangle more detailed information regarding the mode or duration of contact. The effects of texting friends and family versus seeing friends and family in-person regularly may have distinct effects on cognition (Teo et al., 2015). Additionally, detailed information regarding whether cognitive and physical activities were carried out in the presence of others were not available in the current data set. The scope of the cognitive activity measure was also limited and may not account for all types of cognitively-stimulating activities an individual may engage in. This may weaken our conclusions that contact with friends increases engagement in activity engagement. Therefore, future studies should replicate these findings with more objective and detailed measures of activity engagement and more detailed information regarding contact with friends and family. Further, future research should clarify which specific cognitive activities (reading vs. board games) are most beneficial in cognitive aging, whether intensive participation in a single activity is more impactful than less intensive participations in a variety of activities and whether the benefits of particular cognitive activities differ as a function of individual characteristics.
Second, although we demonstrated a prospective association between activity engagement and cognition years later, only two time points of cognitive data were available. Therefore, the current study could only examine linear associations. Future research should examine these associations across multiple waves in order to assess whether associations between activity engagement and cognition are indeed linear. Third, contact frequency and activity engagement were measured concurrently at T1. Of note, a sensitivity analysis revealed that the relationship between activity engagement and cognition did not operate through contact frequency, consistent with our proposed directionality. Still, future research should utilized nonconcurrent assessment waves for exposures, mediators, and outcomes in order to confirm these findings (Maxwell & Cole, 2007). Fourth, the current study uses longitudinal data from MIDUS, which is a national study within the United States, but it is not nationally representative and should replicated in more heterogeneous and population-representative samples.
Additionally, although the current study utilized two distinct domains of cognitive functioning which have both previously been shown to be highly sensitive to age-related decline (Daselaar & Cabeza, 2014; Clark et al., 2012) and previous literature on the cognitive benefits of social engagement have documented effects in these domains (Seeman et al., 2011; Zahodne et al., 2019), other important cognitive domains (i.e., visuospatial functioning, language or processing speed) were not comprehensively assessed in MIDUS. Further, data regarding mild cognitive impairment or dementia status are not available in MIDUS. As our exposure and mediator variables are self-reported, this may introduce some bias in cognitively impaired participants as cognitive impairment may limit their ability to accurately respond to questions. Of note, a sensitivity analyses was conducted excluded those two standard deviations below the mean for episodic memory to exclude those with suspected cognitive impairment and this did not alter our pattern of findings. Still, future research would benefit with the inclusion of a formal screening for cognitive impairment.
Finally, although we theorized that activity engagement acts as a protective factor for cognitive aging, it may be that lower levels of activity engagement are an early indicator of neuropathology. Of note, this study shows not only cross-sectional associations between activity engagement and cognition, but also a prospective association between cognitive activity and episodic memory years later. Additionally, prior research linking cognitive activity to cognitive functioning found no link between cognitive activity and biomarkers of Alzheimer’s disease such as levels of amyloid and tau (Wilson, Scherr, Schneider, Tang & Bennett, 2007), suggestive that cognitive activity is a modifiable resource rather than a preclinical symptom of neurodegeneration.
Strengths of the current study include its longitudinal design, use of a large, national study of middle-aged and older adults, and modeling two types of activity engagement to dissect the independent effects of cognitive and physical activity on two distinct cognitive domains. An additional strength of the current study is the modeling of independent effects of contact frequency with friends versus family, which helps to illuminate the importance of considering relationship type when examining links between social engagement and cognition.
Conclusion
This study supports the view that the cognitive benefits of spending time with friends operate, in part, through cognitive and physical activity engagement. In contrast, contact frequency with family members may not consistently promote engagement in either cognitive or physical activities. These findings support previous reports that friendships require active maintenance through shared activities and that these activities provide beneficial cognitive and physical stimulation that can help to preserve cognition. These findings may help to inform interventions to promote healthy cognitive aging such that future interventions could focus on facilitating contact with friends, which may have naturally beneficial effects on cognitive aging through activity engagement.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes on Aging [grant numbers R00AG047963 and R01AG054520]. The MIDUS longitudinal study was supported by the John D. and Catherine T. MacArthur Foundation Research Network and the National Institute on Aging [grant numbers P01-AG020166 and U19-AG051426]. The sponsors had no role in the current analyses or the preparation of this paper.
Footnotes
Conflict of Interest: The authors have no conflicts.
References
- Adams SA, Matthews CE, Ebbeling CB, Moore CG, Cunningham JE, Fulton J & Hebert JR (2005). The effect of social desirability and social approval on self-reports of physical activity. American Journal of Epidemiology, 15, 389–398. 10.1093/aje/kwi054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amieva H, Stovkova R, Matharan F, Helmer C, Antonucci TC, & Dartigues JF (2010). What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosomatic Medicine, 72, 905–911. 10.1097/PSY.0b013e3181f5e121 [DOI] [PubMed] [Google Scholar]
- Arbuckle JL (1996). Full information estimation in the presence of incomplete data In Marcoulides GA & Schumacker RE (Eds.), Advanced structural equation modeling techniques (pp. 243–277). Mahwah, NJ: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Arfanakis K, Wilson RS, Barth CM, Capuano AW, Vasireddi A, Zhang S … Bennett DA (2016). Cognitive activity, cognitive function, and brain diffusion characteristics in old age. Brain Imaging and Behavior, 10, 455–463. 10.1007/s11682-015-9405-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball V, Corr S, Knight J, & Lowis MJ (2007). An investigation into the leisure occupations of older adults. British Journal of Occupational Therapy, 70, 393–400. 10.1177/030802260707000905 [DOI] [Google Scholar]
- Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, & Evans DA (2004). Social resources and cognitive decline in a population of older african americans and whites. Neurology, 28, 2322–2326. 10.1212/01.WNL.0000147473.04043.B3 [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Satariano WA, & Tager IB, (2003). A longitudinal study of cardiorespiratory fitness and cognitive function in health older adults. Journal of the American Geriatrics Society, 51, 459–465. 10.1046/j.1532-5415.2003.51153.x [DOI] [PubMed] [Google Scholar]
- Basak C, Boot WR, Voss MW & Kramer AF (2008). Can training in a real-time strategy video game attenuate cognitive decline in older adults? Psychology and Aging, 23, 765–777. 10.1037/a0013494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béland F, Zunzunegui M, Alvarado B, Otero A, & del Ser T, (2005). Trajectories of cognitive decline and social relations. Journal of Gerontology: Psychological Sciences, 60, 320–330. 10.1093/geronb/60.6.P320 [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Tang Y, Arnold SE & Wilson RS (2006). The effect of social networks on the relation between alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. The Lancet Neurology, 5, 406–412. 10.1016/S1474-4422(06)70417-3 [DOI] [PubMed] [Google Scholar]
- Borkowski JG, Benton AL, & Spreen O (1967). Word fluency and brain damage. Neuropsychologia, 5, 135–140. 10.1016/0028-3932(67)90015-2. [DOI] [Google Scholar]
- Brim OG, Ryff CD, & Kessler RC (Eds.) (2004). How healthy are we? A national study of wellbeing in midlife. Chicago, IL: University of Chicago Press. [Google Scholar]
- Buitenweg JIV, Murre JMJ & Riddenrinkhof KR (2012). Brain training in progress: A review of trainability in healthy seniors. Frontiers in Human Neuroscience, 6, 1–11. 10.3389/fnhum.2012.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LR, Schiehser DM, Weissberger GH, Salmon DP, Delis DC & Bondi MW (2012). Specific measures of executive function predict cognitive decline in older adults. Journal of the International Neuropsychological Society, 18, 118–127. 10.1017/S1355617711001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks VC, Lubben J, Petitti DB, Little D, & Chiu V (2008). Social network, cognitive function, and dementia incidence among elderly women. American Journal of Public Health, 98(7), 1221–1227. 10.2105/AJPH.2007.115923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar S & Cabeza R (2014). Age-related decline in working memory and episodic memory In Ochsner Kevin N. & Kosslyn Stephen (Eds) The Oxford Handbook of Cognitive Neuroscience. 10.1093/oxfordhb/9780199988693.013.0022 [DOI] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L … Kramer AF (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of the United States of America, 108, 317–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI & Kramer AF (2009). Aerobic exercise effects on cognitive and neural plasticity in older adults. British Journal of Sports Medicine, 43, 22–24. 10.1136/bjsm.2008.052498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SJ, Friedenreich CM, Sajobi TT, Longman RS, Drogos LL, Davenport MH … Poulin MJ (2015). Association between lifetime physical activity and cognitive functioning in middle-aged and older community dwelling adults: Results from the brain in motion study. Journal of the International Neuropsychological Society, 21, 816–830. 10.1017/S1355617715000880 [DOI] [PubMed] [Google Scholar]
- Hall JA (2011). Sex differences in friendship expectations: A meta-analysis. Journal of Social and Personal Relationships, 28, 723–747. 10.1177/0265407510386192 [DOI] [Google Scholar]
- Hayes SM, Alosco ML, Hayes JP, Cadden M, Peterson KM, Allsup K … Verfaellie M (2015). Physical activity is positively associated with episodic memory in aging. Journal of the International Neuropsychological Society, 21, 780–790. 10.1017/S1355617715000910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, & Lindenberger U (2009). Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychological Science in the Public Interest, 9, 1–65. [DOI] [PubMed] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6, 1–55. [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, & Dixon RA (1999). Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging, 14, 245–263. 10.1037/0882-7974.14.2.245 [DOI] [PubMed] [Google Scholar]
- Huxhold O, Miche M, & Shüz B (2013). Benefits of having friends in older ages: Differential effects of informal social activities on well-being in middle-aged and older adults. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 69, 366–375. 10.1093/geronb/gbt029 [DOI] [PubMed] [Google Scholar]
- Ihle A, Oris M, Baeriswyl M, & Kliegel M (2018). The relation of close friends to cognitive performance in old age: The mediating role of leisure activities. International Psychogeriatrics, 1–6. 10.1017/S1041610218000789 [DOI] [PubMed] [Google Scholar]
- James BD, Wilson RS, Barnes LL & Bennett DA (2011). Later-life social activity and cognitive decline in old age. Journal of the International Neuropsychological Society, 17, 998–1005. 10.1017/S1355617711000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonaitis E, La Rue A, Mueller KD, Koscik RL, Hermann B, & Sager MA (2013). Cognitive activities and cognitive performance in middle-aged adults at risk for alzheimer’s disease. Psychology and Aging, 28, 1004–1014. 10.1037/a0034838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman ME, Agrigoroaei S, Murphy C, & Tun PA, (2010). Frequent cognitive activity compensates for education differences in episodic memory. The American Journal of Geriatric Psychiatry, 18, 4–10. 10.1097/JGP.0b013e3181ab8b62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman ME, Agrigoroaei S, Tun PA, & Weaver SL (2014). Monitoring cognitive functioning: Psychometric properties of the brief test of adult cognition by telephone. Assessment, 21, 4040–417. 10.1177/1073191113508807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman ME & Weaver SL (1997). The midlife development inventory (MIDI) personality scales: Scale construction and scoring. Technical Report. [Google Scholar]
- Larson R, Mannell R, & Zuzanek J (1986). Daily well-being of older adults with friends and family. Psychology and Aging, 1, 117–126. 10.1037//0882-7974.1.2.117 [DOI] [PubMed] [Google Scholar]
- Lee GR, & Ishii-Kuntz M (1987). Social interaction, loneliness, and emotional well-being among the elderly. Research on Aging, 9, 459–482. 10.1177/0164027587094001 [DOI] [PubMed] [Google Scholar]
- Li M, & Dong X (2018). Is social network a protective factor for cognitive impairment in US Chinese older adults? Findings from the pine study. Gerontology. 10.1159/000485616 [DOI] [PubMed] [Google Scholar]
- Maxwell SE & Cole DA (2007). Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods, 12, 23–44. 10.1037/1082-989X.12.1.23 [DOI] [PubMed] [Google Scholar]
- McArdle JJ, (2009). Latent variable modeling of differences and changes with longitudinal data. Annual Review of Psychology, 60, 577–605. 10.1146/annurev.psych.60.110707.163612 [DOI] [PubMed] [Google Scholar]
- Meyer MRU Janke MC, & Beaujean AA (2014). Predictors of older adults’ personal and community mobility: Using a comprehensive theoretical mobility framework. The Gerontologist, 54, 398–408. 10.1093/geront/gnt054 [DOI] [PubMed] [Google Scholar]
- Mueller AE, Raymond N, & Yochim BP (2013). Cognitive activity engagement predicts future memory and executive functioning in older adults. Activities, Adaptation & Aging, 37, 251–264. 10.1080/01924788.2013.816833 [DOI] [Google Scholar]
- Oswald DL, & Clark EM (2003). Best friends forever?: High school best friendships and the transition to college. Personal Relationships, 10, 187–196. 10.1111/1475-6811.00045 [DOI] [Google Scholar]
- Oswald DL, Clark EM, & Kelly CM (2004). Friendship maintenance: An analysis of individual and dyad behaviors. Journal of Social and Clinical Psychology, 23, 413–441. 10.1521/jscp.23.3.413.35460 [DOI] [Google Scholar]
- Reed BR, Dowling M, Farias ST, Sonnen J, Strauss M, Schneider JA … Mungas D (2011). Cognitive activities during adulthood are more important than education in building reserve. Journal of the International Neuropsychological Society, 17, 615–624. 10.1017/S1355617711000014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A (1964). The clinical examination in psychology. Paris, France: Presse Universitaires de France. [Google Scholar]
- Roberts SBG, & Dunbar RIM (2015). Managing relationship decay: Network, gender, and contextual effects. Human Nature, 26, 426–450. 10.1007/s12110-015-9242-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SA & Lachman ME (2018). Perceived control and cognition in adulthood: The mediating role of physical activity. Psychology and Aging, 33, 769–781. 10.1037/pag0000273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RL, Meyer JS, & Mortel KF (1990). After reaching retirement age physical activity sustains cerebral perfusion and cognition. Journal of the American Geriatrics Society, 38, 123–128. 10.1111/j.1532-5415.1990.tb03472.x [DOI] [PubMed] [Google Scholar]
- Rook K & Ituarte PHG (1999). Social control, social support, and companionship in older adults’ family relationships and friendships. Personal Relationships, 6, 199–211. 10.1111/j.1475-6811.1999.tb00187.x [DOI] [Google Scholar]
- Salthouse TA, & Prill KA (1987). Inferences about age impairments in inferential reasoning. Psychology and Aging, 2, 43–51. 10.1037/0882-7974.2.1.43 [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Levy G, Tang MX, Manly JJ, & Stern Y (2001). Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology, 57, 2236–2242. 10.1212/WNL.57.12.2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S Tang MX, & Stern Y (2009). Physical activity, diet and risk of alzheimer disease. The Journal of the American Medical Association, 302, 627–637. 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW (1996). Intellectual development in adulthood: The seattle longitudinal study. New York, NY: Cambridge University Press. [Google Scholar]
- Seeman TE, Miller-Martinez DM, Merkin SS, Lachman ME, Tun PA & Karlamangla AS (2011). Histories of social engagement and adult cognition: Midlife in the U.S. study. The Journals of Gerontology: Series B, 66B, 141–152. 10.1093/geronb/gbq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifian N, Manly JJ, Brickman AM, & Zahodne LB (2019). Social network characteristics and cognitive functioning in ethnically diverse older adults: The role of network size and composition. Neuropsychology. EPub ahead of print: 10.1037/neu0000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiovitz-Ezra S, & Litwin H (2012). Social network type and health-related behaviors: Evidence from an American national survey. Social Science & Medicine, 75, 901–904. 10.1016/j.socsci.med.2012.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8, 448–460. https://doi.org/10.1017.S1355617701020240 [PubMed] [Google Scholar]
- Teo AR, Choi H, Andrea SB, Valenstein M, Newsom JT, Dobscha SK, & Zivin K (2015). Does mode of contact with different types of social relationships predict depression in older adults? Evidence from a nationally representative survey. Journal of the American Geriatric Society, 63, 2014–2022. 10.1111/jgs.13667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J & Rees L (1999). Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of Clinical Neuropsychology, 14, 167–177. 10.1016/S0887-6177(97)00095-4 [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P, Wen W, Chen X, & Brodaty H (2008). Lifespan mental activity predicts diminished rate of hippocampal atrophy. Plos One, 3, e2598 10.1371/journal.pone.0002598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, He P, & Dong B (2015). Associations between social networks, social contacts, and cognitive function among Chinese nonagenarians/centenarians. Archives of Gerontology and Geriatrics, 60, 522–527. 10.1016/j.archger.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Warburton DE, Nicol CW, & Bredin SS (2006). Health benefits of physical activity: The evidence. Canadian Medical Association Journal, 174, 801–809. 10.1503/cmaj.051351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1997). Wechsler adult intelligence scale – III (WAIS-III) manual. New York, NY: The Psychological Corporation. [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MMB, Ware JH, & Grodstein F (2004). Physical activity, including walking, and cognitive function in older women. JAMA, 22, 1454–1461. 10.1001/jama.292.12.1454 [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J Johnston C & Yaffe K (2005). Midlife cardiovascular risk factors and risk of dementia in late life. Neurology, 25, 277–281. 10.1212/01.WNL.0000149519.47454.F2 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Schneider JA, Tang Y & Bennett DA (2007). The relation of cognitive activity to risk of developing Alzheimer’s disease. Neurology, 69, 1911–1920. 10.1212/01.wnl.0000271087.67782.cb [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Ajrouch KJ, Sharifian N, & Antonucci TC (2019). Social relations and age-related change in memory. Psychology & Aging, 34, 751–765. 10.1037/pag0000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


