Abstract
Background
Fatty acids are crucial in embryologic development, including cardiogenesis. The impact of maternal periconceptional dietary fat intake on the risk of congenital heart defects (CHDs) has not been clearly elucidated. We hypothesized that maternal dietary fat intake during pregnancy is associated with risk of CHDs in offspring.
Methods
We analyzed CHD cases and non-malformed controls from the National Birth Defects Prevention Study, a case-control, multicenter population-based study of birth defects. We used multivariable logistic regression to analyze the association between maternal periconceptional dietary fat intake and occurrence of CHDs.
Results
We examined 11,393 infants with CHDs (cases) and 11,029 infants without birth defects (controls). Multivariable analysis of maternal dietary fat intake adjusted for maternal energy intake demonstrated modest change in risk for 2 of the 25 CHDs analyzed; otherwise there was no association. Maternal dietary fat intake unadjusted for total energy was associated with increased risk for several CHDs.
Conclusion
After adjusting for total energy intake, maternal periconceptional dietary fat intake has a modest association with risk of a few specific CHDs. If maternal dietary fat intake does impact CHD risk, the effect is minimal.
Introduction
Congenital heart defects (CHDs) are the most common structurally-related group of birth defects in humans, affecting at least 9/1,000 live births.(1) CHDs also represent the leading cause of neonatal and infant death due to congenital causes.(2) Medical and surgical therapies have advanced, and survival to adulthood is now expected in the large majority of children born with even the most severe forms of CHDs.(3) In spite of improved survival and the growing population living with CHDs, a thorough understanding of prenatal risk factors for CHDs remains elusive.
Maternal dietary contributors to the risk of birth defects have been studied, though data on the risk for CHDs limited. Some of our group have used data from the National Birth Defects Prevention Study (NBDPS) to assess impact of overall diet quality on various CHDs.(4) Worsening diet quality index, characterized by a higher percent of dietary calories from fat and lower intake of other nutrients, was associated with higher odds of tetralogy of Fallot, d-transposition of the great arteries, and secundum atrial septal defect. Other investigators have shown a diet high in fish and seafood intake is associated with lower risk of various CHD phenotypes.(5)
The role of maternal dietary fat intake in the risk of CHDs is uncertain. Arachidonic acid, a long-chain polyunsaturated fatty acid obtained by the embryo through maternal dietary intake, is an essential component of all cells, including cardiac myocytes.(6,7) Arachidonic acid levels also regulate the expression of vascular endothelial growth factor (VEGF), which plays a critical role in cardiac development.(8)
In light of the role fatty acids play in fetal cardiac development, some epidemiologists have investigated whether an association exists between maternal fat intake and the risk of CHDs in offspring. Using data from the NBDPS, Sotres-Alvarez and colleagues found that, when compared to a “prudent diet” (characterized by higher intakes of fruits and vegetables and healthy foods such as yogurt, reduced-fat milk, whole-wheat bread, fortified cereal, and fish) with a higher fat intake, a typical Western diet was associated with increased odds of conotruncal and septal defects, and a low-calorie Western diet (characterized by higher intakes of processed foods, starches, sweetened beverages, and lower consumption of fruits and vegetables) was associated with increased odds of septal defects.(9) After adjusting for total energy intake, Smedts and colleagues have shown a maternal diet lower in total and monounsaturated fat was associated with reduced risk of CHDs. Additionally, those investigators demonstrated low maternal intakes of riboflavin and nicotinamide were associated with ventricular outflow tract defects.(10) Recently, we used data from the NBDPS and found that increased maternal fat intake, not adjusted for total energy intake, was associated with decreased odds of double-inlet ventricle.(11) Given these limited and seemingly discordant findings in prior studies, the impact, if any, of maternal dietary fat (and the various subtypes of fat) intake on CHDs remains uncertain. Therefore, we sought to determine if lower or higher dietary fat intake during pregnancy is associated with risk of CHDs in offspring.
Methods
For this study, we analyzed data from the NBDPS. The NBDPS was a population-based, case-control study that recruited structural birth defect cases and non-malformed controls from centers in Arkansas, California, Georgia, Iowa, Massachusetts, New Jersey, North Carolina, Utah, and Texas. Details of the NBDPS design and methodology have been previously reported.(12) Each center enrolled eligible cases from livebirths, stillbirths, and elective terminations. Case eligibility was determined by a clinical geneticist. Cases with chromosomal or single-gene abnormalities were excluded. Contemporaneous, unaffected livebirth controls were identified from birth certificates or birth hospital records from the same geographic area. Approximately 6 weeks to 24 months after the estimated date of delivery, mothers completed a standardized, computer-assisted, telephone interview conducted by trained interviewers.
For this study, we included cases of CHDs and controls with estimated date of delivery from October 1997 to December 2011. Participation in the interview was 67% among case mothers of infants with CHD and 64% among control mothers. Interviews with 12,584 case mothers and 11,829 control mothers were completed within an average of 11 months from the date of delivery for cases, and 9 months for controls. Study participants who had type 1 or 2 pregestational diabetes (466 cases and 83 controls) were excluded.
A shortened version of the Willett food frequency questionnaire was used to assess frequency of intake of 58 food items during the year before pregnancy.(13) Separate, more detailed questions were used to assess intakes of breakfast cereals and sweetened beverages during the 3 months before pregnancy. The USDA version 27 nutrient database served as the source of nutrient values.(14) Dietary data were considered missing for the 1,055 women who had more than one missing food item and for an additional 387 women (all cases and controls combined) whose average daily kilocalorie (kcal) consumption was improbably high or low, i.e., <500 or ≥5,000 kcal. These selected thresholds of improbable caloric intake (i.e. <500 and ≥5,000) are consistent with prior studies using NBDPS.(15)
In our analyses, we included total fat, saturated fat, monounsaturated fat, polyunsaturated fat, and cholesterol. To measure the effect of fat intake independent of total energy intake, we also created energy-adjusted fat intake variables computed as the residuals from regression models with total energy intake as the independent variable and absolute fat intake as the dependent variable. Since residuals have a mean of zero and include negative values, we added a constant (the expected fat intake for the mean total energy intake determined among controls).(16) Throughout the manuscript, these variables are referred to as “energy-adjusted” fats. We also included percent of calories from fat. Analyses estimated odds ratios (ORs) and 95% confidence intervals (CIs) using logistic regression with SAS 9.4 (SAS Institute, Cary, NC). All fat intake variables were divided into quartiles based on the intake among controls. Odds ratios and 95% CIs were calculated comparing women with intake below 25th percentile, and above 75th percentile, relative to women with intake between the 25th and 75th percentiles. These quartile analyses were performed with unadjusted data and subsequently the multivariable model adjusted for maternal energy intake (kcal), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other), body mass index (calculated as pre-pregnancy weight in kilograms divided by height in meters squared, kg/m2), study center (Arkansas, California, Georgia, Iowa, Massachusetts, North Carolina, New Jersey, New York, Texas, and Utah), maternal periconceptional alcohol intake (any versus none), and maternal cigarette smoking (any versus none) during the month before pregnancy or the first two months of pregnancy (the periconceptional period). Finally, all the analyses were repeated by disaggregating on whether women did or did not report periconceptional intake of folate-containing multivitamin supplements.
We performed an additional multivariable analysis after disaggregating total energy intake into the following 10 categories: total fat (without energy-adjustment), saturated fat (without energy-adjustment), monounsaturated fat (without energy-adjustment), polyunsaturated fat (without energy-adjustment), cholesterol (without energy-adjustment), percent of calories from fat, energy-adjusted total fat, energy-adjusted saturated fat, energy-adjusted monounsaturated fat, and energy-adjusted polyunsaturated fat. These queries included performing analyses with different methods of adjusting for total energy intake. For the first six fat-related variables, we adjusted for total energy intake in the multiple logistic regression model, and for the remaining four energy-adjusted fat components, we did not adjust for total energy intake within the multivariable model.
For individual CHD lesion groups, we analyzed those groups with numbers totaling ≥1% (150 cases) of the total cases, which included nearly all groups in NBDPS.
Results
We included 11,393 infants with a CHD and 11,029 control infants enrolled in the NBDPS. As shown in Table 1, no substantive differences in demographic characteristics, vitamin use, smoking, or intake of alcohol were observed between the overall group of CHD cases and controls.
Table 1.
Characteristics of infants with congenital heart defects, and control infants without major birth defects delivered in selected areas of the United States from 1997–2011, National Birth Defects Prevention Study.
| Cases (n=11393) | Controls (n=11029) | |||
| No. | %* | No. | %* | |
| Maternal Race/Ethnicity | ||||
| Non-Hispanic White | 6778 | 59.5 | 6515 | 59.1 |
| Non-Hispanic Black | 1220 | 10.7 | 1179 | 10.7 |
| Hispanic | 2605 | 22.9 | 2623 | 23.8 |
| Other | 789 | 6.9 | 708 | 6.4 |
| Missing | 1 | <0.1 | 4 | <0.1 |
| Child sex | ||||
| Male | 6107 | 53.6 | 5614 | 50.9 |
| Female | 5276 | 46.3 | 5405 | 49.0 |
| Missing | 10 | 0.1 | 10 | 0.1 |
| Maternal age at delivery | ||||
| <20 years | 989 | 8.7 | 1068 | 9.7 |
| 20–24 years | 2613 | 22.9 | 2474 | 22.4 |
| 25–29 years | 3139 | 27.6 | 3076 | 27.9 |
| 30–34 years | 2915 | 25.6 | 2865 | 26.0 |
| ≥35 years | 1737 | 15.3 | 1546 | 14.0 |
| Parity | ||||
| 0 | 4539 | 39.8 | 4371 | 39.6 |
| 1 | 3608 | 31.7 | 3611 | 32.7 |
| 2 | 1955 | 17.2 | 1897 | 17.2 |
| >2 | 1285 | 11.3 | 1147 | 10.4 |
| Missing | 6 | 0.1 | 3 | <0.1 |
| Maternal education | ||||
| <High school | 1886 | 16.6 | 1781 | 16.2 |
| High school grad | 2855 | 25.1 | 2592 | 23.5 |
| 1–3 years of college | 3181 | 27.9 | 2961 | 26.9 |
| 4 or more years of college | 3384 | 29.7 | 3614 | 32.8 |
| Missing | 87 | 0.8 | 81 | 0.7 |
| Maternal multivitamin Use† | ||||
| No | 2532 | 22.2 | 2415 | 21.9 |
| Yes | 8695 | 76.3 | 8481 | 76.9 |
| Missing | 166 | 1.5 | 133 | 1.2 |
| Maternal cigarette smoking† | ||||
| No | 9103 | 79.9 | 9007 | 81.7 |
| Yes | 2239 | 19.7 | 1984 | 18.0 |
| Missing | 51 | 0.5 | 38 | 0.3 |
| Maternal alcohol intake † | ||||
| No | 7323 | 64.3 | 6920 | 62.7 |
| Yes | 3974 | 34.9 | 4035 | 36.6 |
| Missing | 96 | 0.8 | 74 | 0.7 |
| Study center | ||||
| Arkansas | 1769 | 15.5 | 1415 | 12.8 |
| California | 1172 | 10.3 | 1200 | 10.9 |
| Iowa | 1068 | 9.4 | 1259 | 11.4 |
| Massachusetts | 1431 | 12.6 | 1294 | 11.7 |
| New Jersey | 520 | 4.6 | 569 | 5.2 |
| New York | 774 | 6.8 | 947 | 8.6 |
| Texas | 1398 | 12.3 | 1237 | 11.2 |
| CDC/Atlanta | 1231 | 10.8 | 1081 | 9.8 |
| North Carolina | 773 | 6.8 | 953 | 8.6 |
| Utah | 1257 | 11.0 | 1074 | 9.7 |
| mean | SD | mean | SD | |
| Body Mass Index (kg/m2) | 25.7 | 6.3 | 25.3 | 6.0 |
| Daily Energy Intake (Kcal) | 1584.1 | 693.2 | 1603.0 | 685.7 |
Percentages may not equal 100 owing to rounding.
Refers to use in the period 1 months before through 2 months after conception.
When adjusting for multiple covariates, analyses identified only two statistically precise associations of maternal dietary energy-adjusted total fat intake with increased odds of a specific CHD (Table 2). Specifically, high dietary total fat intake was associated with a decreased risk of pulmonary valve stenosis (OR: 0.86, 95% CI: 0.75, 0.99) and an increased risk of the combined lesion of coarctation of the aorta with ventricular septal defect (OR: 1.35, 95% CI: 1.02, 1.78). Periconceptional vitamin supplements (whether use or non-use) did not substantially impact the results shown in Table 2 (data not shown).
Table 2.
Odds ratios of congenital heart defects by energy-adjusted, maternal total fat intake in the 1-year prior to conception, National Birth Defects Prevention Study, 1997–2011
| Congenital Heart Defects | Energy-adjusted total fat 1 | Cases 2 | Adjusted OR (95% CI) 3 |
|---|---|---|---|
| Heterotaxia with congenital heart defect | ≤44.73 | 94 | 1.06 (0.80,1.40) |
| 44.74–57.88 | 151 | Reference | |
| >57.88 | 55 | 0.77 (0.56,1.05) | |
| Conotruncal defects | ≤44.73 | 580 | 0.96 (0.85,1.08) |
| 44.74–57.88 | 1,234 | Reference | |
| >57.88 | 585 | 0.98 (0.87,1.09) | |
| Tetralogy of Fallot | ≤44.73 | 256 | 0.88 (0.75,1.04) |
| 44.74–57.88 | 582 | Reference | |
| >57.88 | 277 | 0.99 (0.85,1.15) | |
| D-Transposition of the great arteries | ≤44.73 | 168 | 0.99 (0.82,1.21) |
| 44.74–57.88 | 370 | Reference | |
| >57.88 | 187 | 1.05 (0.87,1.27) | |
| Double-outlet right ventricle | ≤44.73 | 80 | 1.04 (0.77,1.40) |
| 44.74–57.88 | 137 | Reference | |
| >57.88 | 66 | 0.98 (0.72,1.34) | |
| Atrioventricular canal defect | ≤44.73 | 72 | 0.91 (0.68,1.22) |
| 44.74–57.88 | 167 | Reference | |
| >57.88 | 93 | 1.05 (0.80,1.36) | |
| Anomalous pulmonary venous return | ≤44.73 | 99 | 1.13 (0.86,1.48) |
| 44.74–57.88 | 167 | Reference | |
| >57.88 | 88 | 1.12 (0.85,1.46) | |
| Total anomalous pulmonary venous return | ≤44.73 | 83 | 1.12 (0.83,1.52) |
| 44.74–57.88 | 131 | Reference | |
| >57.88 | 70 | 1.17 (0.86,1.58) | |
| Left ventricular outflow tract defects | ≤44.73 | 464 | 0.96 (0.84,1.08) |
| 44.74–57.88 | 1,056 | Reference | |
| >57.88 | 551 | 1.02 (0.91,1.15) | |
| Hypoplastic left heart syndrome | ≤44.73 | 134 | 0.87 (0.69,1.08) |
| 44.74–57.88 | 310 | Reference | |
| >57.88 | 165 | 1.01 (0.83,1.24) | |
| Coarctation of the aorta | ≤44.73 | 244 | 1.00 (0.84,1.18) |
| 44.74–57.88 | 539 | Reference | |
| >57.88 | 293 | 1.10 (0.94,1.28) | |
| Aortic valve stenosis | ≤44.73 | 120 | 1.10 (0.87,1.39) |
| 44.74–57.88 | 253 | Reference | |
| >57.88 | 112 | 0.83 (0.66,1.05) | |
| Right ventricular outflow tract defects | ≤44.73 | 489 | 0.99 (0.87,1.12) |
| 44.74–57.88 | 1,012 | Reference | |
| >57.88 | 476 | 0.91 (0.80,1.03) | |
| Pulmonary atresia | ≤44.73 | 63 | 0.90 (0.64,1.26) |
| 44.74–57.88 | 123 | Reference | |
| >57.88 | 58 | 1.01 (0.73,1.39) | |
| Pulmonary valve stenosis | ≤44.73 | 352 | 0.97 (0.84,1.12) |
| 44.74–57.88 | 762 | Reference | |
| >57.88 | 350 | 0.86 (0.75,0.99) | |
| Ebstein anomaly | ≤44.73 | 42 | 0.98 (0.66,1.46) |
| 44.74–57.88 | 84 | Reference | |
| >57.88 | 46 | 1.10 (0.75,1.59) | |
| Tricuspid atresia | ≤44.73 | 53 | 1.25 (0.84,1.85) |
| 44.74–57.88 | 71 | Reference | |
| >57.88 | 39 | 1.14 (0.76,1.71) | |
| Any septal defect | ≤44.73 | 1,143 | 1.06 (0.96,1.16) |
| 44.74–57.88 | 2,151 | Reference | |
| >57.88 | 1,088 | 0.98 (0.90,1.07) | |
| Perimembranous/conoventricular VSD | ≤44.73 | 427 | 0.96 (0.84,1.10) |
| 44.74–57.88 | 879 | Reference | |
| >57.88 | 383 | 0.88 (0.77,1.01) | |
| Muscular ventricular septal defect | ≤44.73 | 200 | 1.10 (0.91,1.33) |
| 44.74–57.88 | 371 | Reference | |
| >57.88 | 189 | 1.04 (0.86,1.25) | |
| Secundum atrial septal defect | ≤44.73 | 582 | 1.09 (0.96,1.22) |
| 44.74–57.88 | 1,062 | Reference | |
| >57.88 | 580 | 1.01 (0.90,1.13) | |
| Single ventricle/complex | ≤44.73 | 88 | 1.19 (0.89,1.60) |
| 44.74–57.88 | 139 | Reference | |
| >57.88 | 60 | 0.90 (0.65,1.23) | |
| coarctation + VSD | ≤44.73 | 57 | 0.88 (0.63,1.22) |
| 44.74–57.88 | 135 | Reference | |
| >57.88 | 86 | 1.35 (1.02,1.78) | |
| VSD + secundum ASD | ≤44.73 | 190 | 1.02 (0.84,1.24) |
| 44.74–57.88 | 360 | Reference | |
| >57.88 | 155 | 0.87 (0.71,1.06) | |
| Pulmonary valve stenosis + ASD | ≤44.73 | 67 | 1.22 (0.88,1.70) |
| 44.74–57.88 | 109 | Reference | |
| >57.88 | 61 | 0.99 (0.71,1.38) |
Abbreviations: OR indicates odds ratio; VSD, ventricular septal defect; ASD, atrial septal defect
Bold type indicates statistical significance.
Three percentile categories were constructed corresponding to percentile categories ≤25, 25 – 75 (as reference group), and >75. These categories were determined from nutrient intake levels among control mothers.
For controls, there were 2,757 per quartile. As a result, there were 5,515 controls in the composite group of the 2nd and 3rd quartiles. Counts for cases and controls are based on total counts regardless of any that might have been missing covariates (e.g. maternal parity, education, cigarette smoking, multivitamin use, and/or alcohol intake).
Adjusted for race/ethnicity (white, black, Hispanic, or other), body mass index, study center (Arkansas, California, Georgia, Iowa, Massachusetts, North Carolina, New Jersey, New York, Texas, and Utah), any drinking and smoking during the month before pregnancy or the first 2 months of pregnancy.
Additional multivariable analyses of the 10 dietary components demonstrated several slightly elevated odds of some CHDs, some of which were statistically significant (Supplemental Figure). As was the case in Table 2, additional analysis confirmed that mothers in the highest quartile of total fat intake had lower odds of having a child with pulmonary valve stenosis and increased odds of having a child with the combination lesion of coarctation of the aorta and ventricular septal defect. Additionally, those mothers in the highest quartile of energy-adjusted monounsaturated fat intake had decreased odds of having a child with heterotaxy with an associated CHD. Also, those in the lowest quartile of energy-adjusted polyunsaturated fat intake had increased odds of having a child with double-outlet right ventricle.
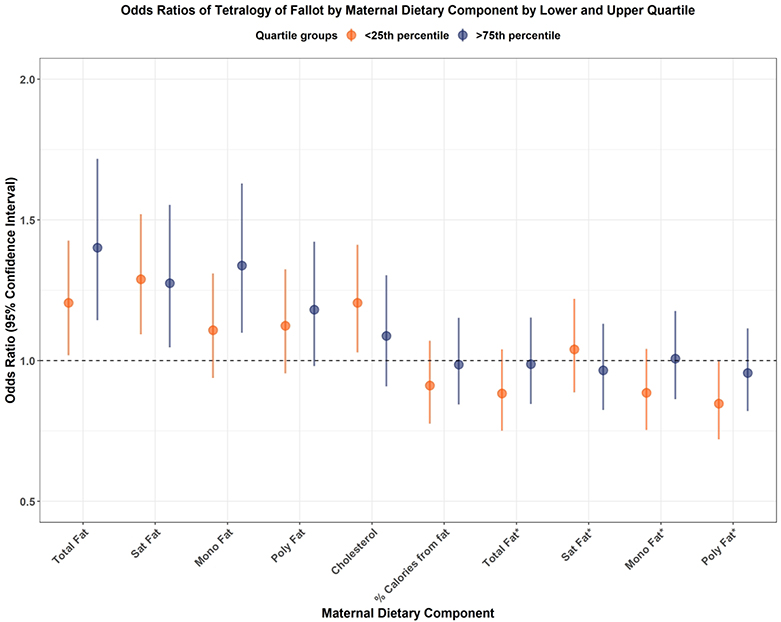
Sub-analysis of the 10 maternal dietary components and the risk of tetralogy of Fallot are demonstrated in Figure 1. Lower and higher energy-unadjusted total fat, saturated fat, and cholesterol intake were associated with increased odds of tetralogy of Fallot adjusting for total energy intake in the multivariate regression model. However, these associations disappeared among energy-adjusted fat components. This pattern of small increased odds in energy-unadjusted fats was demonstrated in a number of the CHDs analyzed (Supplemental Figures S1–S24).
Figure 1.
Forest plot demonstrating odds ratios of tetralogy of Fallot by lower and upper quartiles of maternal dietary fat nutrient intake relative to those in the interquartile range (25–75%). Lower unadjusted total fat, saturated fat, and cholesterol intake were associated with increased odds ratios of tetralogy of Fallot. However, when fat nutrient takes were adjusted for total maternal energy intake, there were no increased odds ratios of tetralogy of Fallot. Similar to the lower quartile, higher unadjusted total fat, saturated fat, and monounsaturated fat intake were associated with increased odds ratios of tetralogy of Fallot. However, comparable to the lowest quartile, when fat nutrient takes were adjusted for total maternal energy intake, there were no increased odds ratios of tetralogy of Fallot. Abbreviations: Sat fat indicates saturated fat; mono fat, monounsaturated fat; poly fat, polyunsaturated fat; *, intake of selected fat nutrient adjusted for total maternal energy intake.
Discussion
Using data from a large population-based, case-control study of birth defects, we did not observe statistically significant increased ORs for the association between energy-adjusted maternal periconceptional dietary fat intake and CHD in offspring. Data on the impact of maternal dietary fat intake on the risk of birth defects, especially CHDs, are limited. Our results contribute to an important unanswered question, one that has been previously represented by disparate results in smaller studies.
Our initial hypothesis that maternal fat intake could affect the risk of CHDs is reasonable from a developmental biology perspective. Essential and long-chain polyunsaturated fatty acids, both vital for fetal development, cannot be synthesized de novo and must be obtained from maternal circulation.(6) Long-chain polyunsaturated fatty acids, with arachidonic acid being the most abundant, are important structural components of cell membrane phospholipids.(7) Increases in arachidonic acid upregulate the expression of vascular endothelial growth factor (VEGF),(8) a key regulator of vascular and cardiac development.(17) Precisely timed expression of normal levels of VEGF is critically important for normal cardiovascular development.(18) Alterations in VEGF expression, whether increased or decreased, have been associated with multiple CHDs including defects in atrial and ventricular septation, disturbance in cardiac outflow tract formation,(17) pulmonary valve stenosis, and tetralogy of Fallot,(19) some of the lesions we identified in our present study.
In the present study, we were surprised to find essentially no association between increased total energy-adjusted maternal dietary fat intake and risk of CHDs, especially given the crucial importance of VEGF on fetal cardiovascular development. Maternal high-fat diet upregulates the expression of placental mRNA in the arachidonic acid metabolism pathway.(20) The increase in maternal arachidonic acid that accompanies a high-fat diet would be expected to increase the fetal arachidonic acid level, which would thus upregulate the fetal expression of VEGF. Overexpression of VEGF in the myocardium inhibits the process of epithelial-mesenchymal transformation responsible for endocardial cushion formation.(17) Impairment of endocardial cushion formation precipitates defects in septation and in outflow tract formation.(17) To this end, Miquerol et al have shown that overexpression of VEGF-A in embryonic mice results in embryonic lethality due to defects in ventricular septation and outflow tract formation.(21) Similarly, Smedts et al have shown high maternal saturated fat intake is associated with increased risk of outflow tract defects.(22) Those same authors subsequently demonstrated a two-fold increased risk of CHD in infants born to mothers with abnormal lipid profiles.(23) Previously, we have shown no association among maternal intake of total fat, linoleic acid, or oleic acid with either tetralogy of Fallot or d-transposition of the great arteries.(24) Similar to that work, in the present study, we did not find an association of increased maternal dietary intake of total fat or specific fat subgroups with any form of CHD. The lack of an association when controlled for total maternal caloric intake may be related to our methodology, but it also is likely related to the complex biochemical milieu at play between maternal and fetal circulations.
In the setting of a plausible biochemical system and experimental results that give credence to a maternal high-fat diet being associated with increased risk of CHD, the inverse would seem likely as well; that is, a maternal low-fat diet might be expected to result in decreased risk of CHDs. There are limited published data regarding this question. Smedts et al have previously demonstrated that decreased maternal dietary monounsaturated fat intake was associated with a reduced risk of ventricular outflow tract defects.(22) Conversely, we have previously demonstrated an association between decreased maternal dietary fat intake and increased risk of double-inlet ventricle.(11) The finding in that study of increased risk of specific CHDs in association with decreased maternal dietary fat intake could be construed as unexpected vis-à-vis the prior studies outlined. However, when the exquisite balance of VEGF signaling is considered, the potential for increased risk of CHDs in the setting of decreased maternal dietary fat intake is more conceivable. With decreased maternal dietary fat intake, lower levels of circulating essential and long-chain polyunsaturated fatty acids would be available for placental transfer to the fetus. The decreased fetal availability of arachidonic acid would enact lower expression of VEGF. Decreased VEGF expression impairs vascular development.(25) In fact, a 50% reduction in VEGF-A impairs vascular development and leads to embryonic lethality at mid-gestation.(26) Decreased myocardial VEGF-A expression results in impaired endocardial cushion morphogenesis,(27) the same issue seen in the setting of overexpression of VEGF-A.(21) In light of the potential for maternal low-fat diet to either increase or decrease the risk of CHDs, in the present study we found no association of calorie-adjusted, maternal fat intake with any form of CHD.
The divergence of our results between total energy-unadjusted and -adjusted dietary fat intake is worthy of further investigation, particularly given that it may be indicative of measurement error. That is, food frequency questionnaires, such as the one used in the NBDPS, have poor correlation between questionnaire-estimated caloric intake and true energy intake (0.1 to 0.3) assessed by more rigorous methodologies.(28,29) Measurement error associated with food frequency questionnaires may explain some of the inconsistencies observed in other studies and may have attenuated estimated risks in our study. This issue of reliability of the total calorie estimates based on standard food frequency questionnaires has led some investigators to suggest adjusting for total energy intake may result in erroneous estimation. Jakes et al. suggested routine adjustment for estimated energy intake is not the best methodological approach, as they have shown the estimate of energy intake derived from a food frequency questionnaire “is almost independent of total energy expenditure.”(28) With these concerns in mind, the differences between our total energy-adjusted and unadjusted analyses may not be surprising. In the presence of potentially significant measurement error in dietary intakes, small influences of specific dietary fat intakes on ORs of specific CHDs may be obscured. This could be at play in the current study. As such, whether the adjusted or unadjusted analyses are more indicative of the true risks, we cannot say. Nevertheless, even based on the unadjusted analyses which showed an increased number of statistically significant associations, if there is a true association of maternal dietary fat intake with the risk of CHDs (as measured by food frequency intakes), the effect size was quite small irrespective of low or high intake of fats.
Because our observations did not clearly identify associations that require further comment, we did not employ techniques to correct for multiple comparisons. However, while our study provides important data on maternal dietary fat intake and CHDs risk from a large, case-control cohort, there are limitations that must be considered. First, the food frequency questionnaire used to assess maternal dietary intake patterns was completed at a mean of 11 months after delivery. There is a potential for inaccurate recall of dietary intake by the mothers, and there are certainly measurement limitations to this type of instrument.(30) We did not internally validate the instrument, but validation studies have shown it provides reliable estimates of dietary intake, even for past dietary patterns.(30) The study estimated risk for CHDs based on maternal dietary fat intake levels. We did not assess maternal circulating fat levels. Given variations in intestinal fat absorption and fat metabolism, it is unknown how well maternal fat intake approximates circulating and transplacental fat levels. Similarly, we did not assess maternal lipid metabolism, and it is unknown as to what degree that metabolism may mediate epigenetic influences on fundamental, developmental gene expressions thereby potentially impacting the development of CHDs. This is a vital question that heretofore remains unexplored and unanswered.
Supplementary Material
Impact Statement.
The key message of the article
In this large, case-control study, after adjusting for total caloric intake, maternal periconceptional dietary fat intake was not associated with increased odds of congenital heart defects.
What does the study add to the literature
This study investigates the hypothesis that women’s periconceptional fat intake alters the risk of congenital heart defects in offspring.
What is the impact of the study
Our results raise questions about the role maternal fat intake may play in cardiogenesis and risk of congenital heart defects. Additionally, they raise the question about whether maternal lipid metabolism, as opposed to fat intake, may influence cardiac development.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the California Department of Public Health. We thank the California Department of Public Health, Maternal Child and Adolescent Division, for providing data for these analyses. This work was supported by the Centers for Disease Control and Prevention, Centers of Excellence No. U01DD001033 and grant No. DK56350 from the University of North Carolina, Department of Nutrition Clinical Research Center, Nutrition Epidemiology Core.
Statement of financial support: This work was supported by the Centers for Disease Control and Prevention Centers of Excellence No. U01DD001033 and grant No. DK56350 from the University of North Carolina Department of Nutrition Clinical Research Center, Nutrition Epidemiology Core.
Footnotes
Disclosure statement: None of the authors have any financial ties to any products in the study, and they have no potential or perceived conflicts of interest.
Category of study: Clinical science
References
- 1.van der Linde D, Konings EEM, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58(21):2241–7. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman JIE, Kaplan S, Liberthson RR. Prevalence of congenital heart disease. Am Heart J 2004;147(3):425–39. [DOI] [PubMed] [Google Scholar]
- 3.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease. Circulation 2019;139(14):e637–e697. [DOI] [PubMed] [Google Scholar]
- 4.Botto LD, Krikov S, Carmichael SL, et al. Lower rate of selected congenital heart defects with better maternal diet quality: a population-based study. Arch Dis Child 2016;101(1):F43–9. [DOI] [PubMed] [Google Scholar]
- 5.Obermann-Borst SA, Vujkovic M, de Vries JH, et al. A maternal dietary pattern characterised by fish and seafood in association with the risk of congenital heart defects in the offspring. Br J Obstet Gyn 2011;118(10):1205–15. [DOI] [PubMed] [Google Scholar]
- 6.Woollett LA. The origins and roles of cholesterol and fatty acids in the fetus. Curr Opin Lipidol 2001;12(3):305–12. [DOI] [PubMed] [Google Scholar]
- 7.Leaf AA, Leighfield MJ, Costeloe KL, Crawford MA. Long chain polyunsaturated fatty acids and fetal growth. Early Hum Develop 1992;30(3):183–91. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, Kim MH, Han HJ. Arachidonic acid potentiates hypoxia-induced VEGF expression in mouse embryonic stem cells: involvement of Notch, Wnt, and HIF-1alpha. Am J Physiol, Cell Physiol 2009;297(1):C207–16. [DOI] [PubMed] [Google Scholar]
- 9.Sotres-Alvarez D, Siega-Riz AM, Herring AH, et al. Maternal dietary patterns are associated with risk of neural tube and congenital heart defects. Am J Epidemiol 2013;177(11):1279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smedts HPM, Rakhshandehroo M, Verkleij-Hagoort AC, et al. Maternal intake of fat, riboflavin and nicotinamide and the risk of having offspring with congenital heart defects. Eur J Nutr 2008;47(7):357–65. [DOI] [PubMed] [Google Scholar]
- 11.Paige SL, Yang W, Priest JR, et al. Risk factors associated with the development of double-inlet ventricle congenital heart disease. Birth Defects Res 2019;111(11):640–8. [DOI] [PubMed] [Google Scholar]
- 12.Reefhuis J, Gilboa SM, Anderka M, et al. The National Birth Defects Prevention Study: A review of the methods. Birth Defect Res A 2015;103(8):656–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willett WC, Reynolds RD, Cottrell-Hoehner S, Sampson L, Browne ML. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc 1987;87(1):43–7. [PubMed] [Google Scholar]
- 14.US Department of Agriculture ARS. USDA National Nutrient Database for Standard Reference, Release 27 [Internet]. 2014. [cited 2019 Jun 19]:1–154. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl
- 15.Gilboa SM, Lee KA, Cogswell ME, et al. Maternal intake of vitamin E and birth defects, national birth defects prevention study, 1997 to 2005. Birth Defect Res A 2014;100(9):647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149(6):531–40. [DOI] [PubMed] [Google Scholar]
- 17.Lambrechts D, Carmeliet P. Genetics in zebrafish, mice, and humans to dissect congenital heart disease: insights in the role of VEGF. Curr Top Dev Biol 2004;62:189–224. [DOI] [PubMed] [Google Scholar]
- 18.Dor Y, Camenisch TD, Itin A, et al. A novel role for VEGF in endocardial cushion formation and its potential contribution to congenital heart defects. Development 2001;128(9):1531–8. [DOI] [PubMed] [Google Scholar]
- 19.van den Akker NMS, Molin DGM, Peters PPWM, et al. Tetralogy of Fallot and alterations in vascular endothelial growth factor-A signaling and notch signaling in mouse embryos solely expressing the VEGF120 isoform. Circ Res 2007;100(6):842–9. [DOI] [PubMed] [Google Scholar]
- 20.Dekker Nitert M, Vaswani K, Hum M, et al. Maternal high-fat diet alters expression of pathways of growth, blood supply and arachidonic acid in rat placenta. J Nutr Sci 2013;2:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development 2000;127(18):3941–6. [DOI] [PubMed] [Google Scholar]
- 22.Smedts HPM, Rakhshandehroo M, Verkleij-Hagoort AC, et al. Maternal intake of fat, riboflavin and nicotinamide and the risk of having offspring with congenital heart defects. Eur J Nutr 2008;47(7):357–65. [DOI] [PubMed] [Google Scholar]
- 23.Smedts HPM, van Uitert EM, Valkenburg O, et al. A derangement of the maternal lipid profile is associated with an elevated risk of congenital heart disease in the offspring. Nutr Metab Cardiovasc Dis 2012;22(6):477–85. [DOI] [PubMed] [Google Scholar]
- 24.Shaw GM, Carmichael SL, Yang W, Lammer EJ. Periconceptional nutrient intakes and risks of conotruncal heart defects. Birth Defect Res A 2010;88(3):144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996;380(6573):435–9. [DOI] [PubMed] [Google Scholar]
- 26.Damert A, Miquerol L, Gertsenstein M, Risau W, Nagy A. Insufficient VEGFA activity in yolk sac endoderm compromises haematopoietic and endothelial differentiation. Development 2002;129(8):1881–92. [DOI] [PubMed] [Google Scholar]
- 27.Enciso JM, Gratzinger D, Camenisch TD, Canosa S, Pinter E, Madri JA. Elevated glucose inhibits VEGF-A-mediated endocardial cushion formation: modulation by PECAM-1 and MMP-2. J Cell Biol 2003;160(4):605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakes RW, Day NE, Luben R, et al. Adjusting for energy intake--what measure to use in nutritional epidemiological studies? Int J Epidemiol 2004;33(6):1382–6. [DOI] [PubMed] [Google Scholar]
- 29.Kipnis V, Subar AF, Midthune D, et al. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol 2003;158(1):14–21. [DOI] [PubMed] [Google Scholar]
- 30.Willett WC. Implications of total energy intake for epidemiologic analyses In: Kelsey JL, Marmot MG, Stolley PD, Vessey MP, eds. Nutritional Epidemiology, 2nd ed. New York, Oxford University Press, 1998:273–301. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.