Abstract
Molecular and behavioral timekeeping is regulated by the circadian system which includes the brain’s suprachiasmatic nucleus (SCN) that translates environmental light information into neuronal and endocrine signals aligning peripheral tissue rhythms to the time of day. Despite the critical role of circadian rhythms in fertility, it remains unexplored how circadian rhythms change within reproductive tissues during pregnancy. To determine how estrous cycle and pregnancy impact phase-relationships of reproductive tissues, we used PER2::Luciferase (PER2::LUC) circadian reporter mice and determined the time of day of PER2::LUC peak (phase) in the SCN, pituitary, uterus, and ovary. The relationships between reproductive tissue PER2::LUC phases changed throughout the estrous cycle and late pregnancy and were accompanied by changes to PER2::LUC period in the SCN, uterus, and ovary. To determine if the phase relationship adaptations were driven by sex steroids, we asked if progesterone, a hormone involved in estrous cyclicity and pregnancy, could regulate Per2-luciferase expression. Using an in vitro transfection assay, we found that progesterone increased Per2-luciferase expression in immortalized SCN (SCN2.2) and arcuate nucleus (KTAR) cells. In addition, progesterone shortened PER2::LUC period in ex vivo uterine tissue recordings collected during pregnancy. As progesterone dramatically increases during pregnancy, we evaluated wheel-running patterns in PER2::LUC mice. We confirmed that activity levels decrease during pregnancy and found that activity onset was delayed. Although SCN, but not arcuate nucleus, PER2::LUC period changed during late pregnancy, onset of locomotor activity did not correlate with SCN or arcuate nucleus PER2::LUC period.
Keywords: Per2::luciferase, pregnancy, circadian rhythms, estrous cycle, wheel-running, suprachiasmatic nucleus, uterus, ovary, pituitary, arcuate nucleus
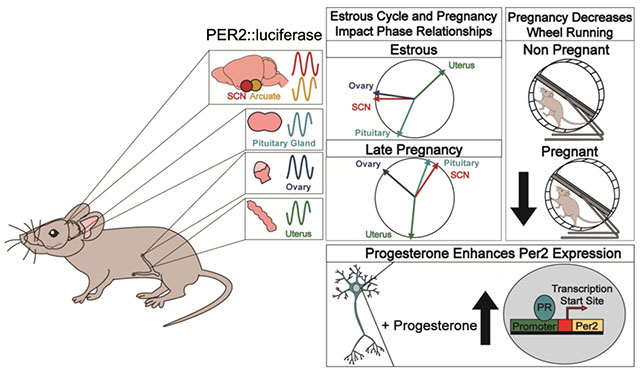
Graphical Abstract
Functional circadian rhythms are essential for fertility and successful reproduction. PER2::LUC circadian reporter mice determined the relationships between circadian rhythms during the estrous cycle and pregnancy and how these rhythms were regulated by progesterone, a sex steroid regulating reproductive function.
Introduction
Circadian timekeeping plays an essential role in successful pregnancy, which requires the precise coordination of a number of essential processes to occur, including ovarian follicular development and maturation (Sellix and Menaker, 2010), ovulation (Hellier et al., 2018; Simonneaux et al., 2017), mating behavior initiation (Hellier et al., 2018; Simonneaux et al., 2017) and mature oocyte release (Sellix and Menaker, 2010; Simonneaux et al., 2017). Each of these processes are controlled by a fine-tuned feedback mechanism in which the timing of hormone release is synchronized with receptor expression throughout the female reproductive system to ensure pregnancy success (Boden et al., 2013; Sen and Hoffmann, 2020). On a cellular level, circadian rhythms are generated by an autoregulated transcription-translation feedback loop of molecular clock transcription factors, of which Period 1/2 (Per1/2), Brain and Muscle ARNT-Like 1 (Bmal1), Clock and Cryptochrome1/2/3, comprise the core mechanism, reviewed in (Ko and Takahashi, 2006). To synchronize these cellular circadian rhythms to environmental conditions, a combined mechanism encompassing both neural and hormonal signals is coordinated by the brain’s primary circadian pacemaker, the suprachiasmatic nucleus (SCN) (Sellix and Menaker, 2010; Simonneaux et al., 2017; Zhang et al., 2016). The SCN’s principal role is to translate environmental lighting information into neuronal and hormonal signals, allowing synchronization of behavioral activity, hormonal release, and tissue sensitivity (Paul and Brown, 2019). Despite the well-established role of day-length and circadian rhythms in regulating reproductive status in seasonal breeders (Dardente et al., 2019; Robinson and Follett, 1982; Robinson and Karsch, 1984; Wang et al., 2019), the luteinizing hormone surge promoting ovulation (Kriegsfeld and Williams III, 2012; Mosko and Moore, 1979; Smarr et al., 2013; Williams III et al., 2010), and the circadian timing of labor onset (Backe, 1991; Cagnacci et al., 1998; Olcese, 2012; Reppert, 1983), little is known about how pregnancy impacts circadian rhythms and daily changes in behavior (Martin-Fairey et al., 2019, 2016). A significant step towards understanding behavioral and circadian changes in pregnancy was published by Martin-Fairey et al., 2019, who showed that pregnancy in both humans and mice was associated with a reduction in locomotor activity and a shift in the timing of activity onset (Martin-Fairey et al., 2019), two behaviors known to be regulated by the SCN (LeSauter and Silver, 1999; Schwartz and Zimmerman, 1991; Stephan and Zucker, 1972).
In addition to understanding the role of circadian changes in behavior during pregnancy, metabolic and hormonal changes during pregnancy may be influenced by circadian rhythms, although this remains largely unexplored. Pregnancy is associated with dramatic changes in metabolism (Bell and Bauman, 1997) and hormone release patterns (Kumar and Magon, 2012), both of which maintain strong ties to circadian rhythmicity (Albers et al., 1981; Froy, 2009; Huang et al., 2011; Morin et al., 1977; Rutter et al., 2002; Takahashi and Menaker, 1980). Progesterone is a sex steroid which increases towards late pregnancy, and peaks around gestation day (GD) 15-17 in mice (Barkley et al., 1977; Barkley and Geschwind, 1979; Virgo and Bellward, 1974). The primary role of progesterone during pregnancy is to allow implantation (Bhurke et al., 2016; Bindon, 1971; Halasz and Szekeres-Bartho, 2013; Moriyama and Sugawa, 1972) and to silence uterine contractions prior to labor onset. Progesterone performs these functions through activation of both nuclear receptors, progesterone receptor A and B (PRA and PRB, respectively), as well as membrane bound progesterone receptors (Cabral et al., 1994; Garg et al., 2017; Grimm et al., 2016; Karteris et al., 2006). During late pregnancy (beginning approximately at GD 17 in the mouse), a reduction in progesterone in combination with PRA and PRB function allows for the progression of parturition (Bhurke et al., 2016; Wu and DeMayo, 2017), initiation of lactation (Cowie and Lyons, 1959; Hartmann et al., 1973; Lyons, 1958; Meites, 1954), and maternal behaviors (Bridges, 1984; Bridges et al., 1978). It should be noted that the role of progesterone in labor initiation varies between species (Nielsen et al., 2016). In addition to the aforementioned roles, progesterone also acts as a regulator of metabolic function (Kalkhoff, 1982). Recent work indicates this metabolic action of progesterone might be regulated by the arcuate nucleus, a hypothalamic structure which express high levels of progesterone receptors (Marraudino et al., 2018; Padilla et al., 2019; Stephens et al., 2015).
While it is known that disrupted circadian rhythms can be detrimental to reproductive success (Dolatshad et al., 2005; Mahoney, 2010), it remains largely unknown how they change from the non-pregnant state to pregnancy. Understanding the role of circadian rhythms in regulating both behavior and tissue specific circadian function during the estrous cycle and in pregnancy is an essential first step towards elucidating the underlying mechanisms associated with infertility and pregnancy complications, which are more prevalent in women with disrupted circadian rhythms (Goldberg et al., 2012; Nisa et al., 2018; Pappa et al., 2013; Zornoza-Moreno et al., 2013). We hypothesize that circadian timekeeping during late pregnancy influences mouse behavior and reproductive tissue circadian function in preparation for labor. Here, we confirm locomotor activity changes during pregnancy and describe the changes in molecular circadian time-keeping between the estrous cycle and pregnancy using the circadian knock-in reporter mouse, PER2::Luciferase (PER2::LUC). Finally, we explore the potential role of PRA/B in driving circadian rhythm changes.
Methods and Materials
Mice
All methods described here have been approved by the Institutional Animal Care and Use Committee of Michigan State University and conducted in accordance with the Guide for the Care and Use of Laboratory Animals. Period2::Luciferase (PER2::LUC) mice were purchased from JAX (strain B6.129S6-Per2tm1Jt/J, #006852, https://www.jax.org/strain/006852). Mice were housed under a 12 h light-dark cycle, lights on at 6AM (Zeitgeber time 0, ZT 0), with food and water ad libitum. Mice were sacrificed by isoflurane or CO2 overdose, followed by cervical dislocation. Experimental mice were 6-14 weeks of age at the start of experiments.
Timed mating
Two females and one male were housed together at ZT 10-11 and vaginal plug formation was checked at ZT 3-4 during the mating assay. On the day of vaginal plug identification, the female was separated from the male. If no vaginal plug was found, mating pairs remained co-housed for up to 5 days, and daily checks for vaginal plugs were continued. Following vaginal plug identification, pregnancy was confirmed by a significant increase in body weight, where a weight gain of >2 g from gestational day (GD) 1 to 10 was indicative of pregnancy (Rugh, 1968). Gestational stage was further confirmed the day of tissue collection, where embryo development was established using Theiler Stage (https://www.emouseatlas.org/emap/ema/theiler_stages/StageDefinition/stagedefinition.html; accessed January 2020). For wheel running behavior, timed mating was conducted as described above, except that one female was mated with one male.
Wheel-running behavior
During timed mating, female and male mice were housed in light and temperature controlled circadian cabinets (standard mouse circadian cabinet, Actimetrics, Wilmette, IL) within polypropylene cages (33.2 × 15 × 13cm) containing a metal running wheel (11 cm diameter). Males utilized for timed mating were individually housed in the same behavioral cabinet as the females. Females were allowed 2-5 days acclimatization to running wheels prior to experimental start. Female locomotor activity rhythms were monitored with a ClockLab data collection system (Version 3.603, Actimetrics, Wilmette, IL) through the number of electrical closures triggered by wheel rotations. Light intensity varied between 268-369 Lux inside the mouse cage with wheel. Cage changes were scheduled at 3-week intervals. Wheel-running activity was analyzed using ClockLab Analysis (Actimetrics Software) and complied into 5-minute bins by persons blind to experimental group. Activity data collected during timed mating were not included in statistical analyses. Daily onset of activity was defined as the first time when activity was counted for at least 1 h after at least 4 h of inactivity. Pregnant females were euthanized and gestation day assigned according to timed mating and Theiler Stage as described in “Timed Mating”. Following euthanasia, ex vivo tissue explants were monitored for PER2::LUC bioluminescence as described in “Monitoring of PER2::LUC bioluminescence”. To correlate locomotor activity onset with SCN and PER2::LUC period, only PER2::LUC females used in the running wheel locomotor activity set-up were used.
Determination of estrous stage
To assess estrous stage, vaginal smears were performed at the time of euthanasia between ZT 3-6 on female mice (3-6 months) by vaginal lavage (Hoffmann, 2018). Smears were collected on glass slides and counterstained with 0.1% methylene blue (Spectrum, Gardena, CA). Cell type was observed through bright field microscopy to determine the corresponding stage of the estrous cycle, by persons blind to experimental group.
Monitoring of PER2::LUC bioluminescence
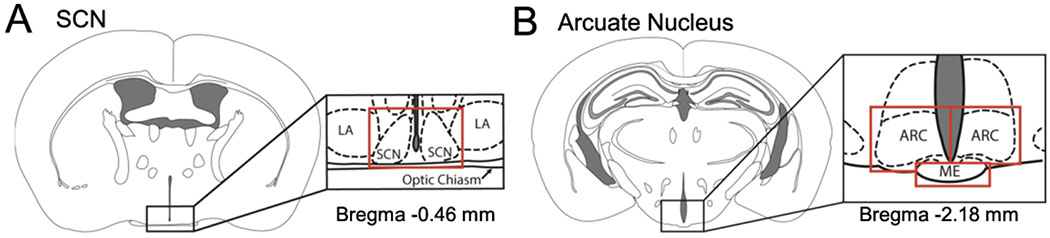
Mice were euthanized at ZT 3-6 or ZT 15-17. Data from mice euthanized at ZT15-17 were not used in phase analyses. Following euthanasia, the uterus, ovary, pituitary and brain were removed and placed in a semi-frozen 1x Hank’s buffered salt solution (HBSS, 14065-056, Gibco). Using a dissection scope the ovary and pituitary were isolated and the uterus dissected into pieces of ≈ 4 mm2 (~2 mm x 2 mm). To prepare the uterine pieces the whole uterus was cleaned from fat, and the uterine segment surrounding the fetuses cut in the longitudinal direction of the uterine horn. The uterine horn was opened into a sheet and pinned down to a dissection dish. Using a ruler, 2 x 2 mm uterine strips were collected midway between the cervix and the ovary near the placental attachment and placed with the endometrium side down onto the MiliCell membrane (MilliCell, PICM0RG50; MilliporeSigma, Burlington, MA). Ice-cold brains were sliced coronally on a vibratome (Leica VT 1200S) at 300 μm. After sectioning on the vibratome, a dissection scope was used to identify the appropriate brain regions. The SCN and arcuate nucleus were identified through anatomical identification (Franklin and Paxinos, 2008). The SCN was dissected as previously described Figure 1A (Landgraf et al., 2016; Welsh and Noguchi, 2012). For the arcuate nucleus, the median eminence was removed, where after two bilateral scalpel incisions allowed to isolate the arcuate nuclei Figure 1B. Both arcuate nuclei were placed onto a MilliCell membrane. MiliCell membranes were placed in 35 mm dishes (Nunc, Thermo Fisher Scientific, Rochester, NY) containing 1.5 ml of 35.5 °C recording medium (Neurobasal, 1964475, Gibco) supplemented with 20 mM HEPES (pH 7.2), B27 supplement (2%; 12349-015, Gibco), 1 mM luciferin (luciferin sodium salt; 1-360242-200, Regis, Grove, IL), and antibiotics (8 U/ml penicillin, 0.2mg/ml streptomycin, 4mM L-glutamine; Sigma-Aldrich). Dishes were sealed using vacuum grease and placed into a LumiCycle (Actimetrics, Wilmette, IL) inside a light-tight 35.5 °C, 5% CO2, non-humidified environmental chamber. Uterine tissue was treated with vehicle (1/50 dilution of diH2O) or water-soluble progesterone (P4, Sigma Aldrich #P7556, 50 nM and 100 nM, data was pooled as no difference in effect was observed). The bioluminescence signal was counted every ten minutes for 1.11 min for 6 days (day 1- day 6 of recording time). Data were normalized by subtraction of the 24 h running average from the raw data and then smoothed with a 1 h running average (Luminometer Analysis, Actimetrics) and analyzed blind to experimental group. During the initial ~24 h (day 0) in the LumiCycle, the PER2::LUC signal tends to decrease significantly prior to achieving a stable waveform. In our analysis of PER2::LUC period, we exclude the first 24 h of recording to account for this. Incomplete data sets, as caused by loss of data points, other technical problems, or explants failing to show two PER2::LUC peaks (deemed arrhythmic as per (Landgraf et al., 2016)) were not included in the analyses (Total of 6 SCN, 2 arcuate nucleus, 5 pituitary, 2 ovary and 1 uterine explants). PER2::LUC phase was determined as the time-of-day of first PER2::LUC peak. PER2::LUC period was analyzed by the Luminometer Analysis software (Actimetrics) as the time difference in hours between the two peaks, with LM fit (damped sin) as the mathematical model. To determine the phase of the tissue, we utilized the time peak activity on day 1 of recording (time of first peak). All phase data are reported in degrees and reference to zeitgeber time (ZT) with time of lights on at ZT 0 from the day of euthanasia.
Figure 1. Diagram of SCN and arcuate nucleus dissections.
Representative diagram of dissected A.) suprachiasmatic nucleus (SCN) and B.) arcuate nucleus (ARC) for monitoring of Per2::luciferase bioluminescence. Red lines indicate where individual cuts were made in the tissue slices. Abbreviations are as follows: LA- lateroanterior hypothalamic nucleus, SCN-suprachiasmatic nucleus, ME- median eminence, ARC-arcuate nucleus.
Cell culture, transfections, luciferase assays and hormone treatment.
NIH3T3 (mouse embryonic fibroblasts, American Type Culture Collection), KTAR (female arcuate nucleus neurons, mouse) (Jacobs et al., 2016); and SCN2.2 cells (immortalized rat suprachiasmatic nucleus neurons) (Earnest et al., 1999), were cultured in a humidified 5% CO2 environment in DMEM (Corning/Mediatech), with 1% penicillin-streptomycin (Sigma Aldrich) and 10% heat inactivated fetal bovine serum (Sigma Aldrich) or 10% charcoal stripped fetal bovine serum (Gibco Cat#A33821), as indicated in figures. NIH3T3, KTAR, and SCN2.2 cells were seeded into 24-well plates (Thermo Scientific) at 0.05x106 (NIH3T3 and KTAR) and 0.3x106 (SCN2.2) cells per well. Transfection of cells was performed 24 h after the cells were plated. Transient transfections were performed using PolyJet™ (SignaGen Laboratories, Rockville, MD), following a previously published protocol (Hoffmann et al., 2018, 2016). For luciferase assays, cells were transfected with 200 ng/well mouse −1128 to +2129 bp Per2-luciferase reporter plasmid (pGL6 plasmid, Addgene.org) (Yoo et al., 2004) and 100 ng progesterone receptor A, B or pcDNA empty vector (Lee et al., 2013). Five ng/well pGL4.74 Renilla luciferase reporter plasmid (hRluc, Promega) was added and served as an internal control. To equalize the amount of DNA transfected into cells, we systematically equalized plasmid concentrations by adding the corresponding plasmid empty vector. Twenty-four hours after transfection, culture medium was replaced with DMEM containing 0.1% Bovine Serum Albumin (BSA, Fisher Scientific) and progesterone (water soluble P4, 100 nM, Sigma Aldrich #P7556) or vehicle control (water 1/50 dilution). For luciferase assays, cells were harvested 48 h after transfection in 1x Passive lysis buffer (Promega Dual-Luciferase Reporter Assay System Kit). Dual-Luciferase assays were performed following manufacturers recommendations. Luciferase values are normalized to hRluc values to control for transfection efficiency. Values are normalized to pGL3 and are expressed as fold change as compared to control plasmid as indicated in the figure legends. Data represent the mean ± SEM of at least four independent experiments done in replicate.
Statistical analysis
Data were analysed using GraphPad Prism 8 (Graph Pad Software, La Jolla, CA). Significant differences were designated as P < 0.05. Wheel running activity (total wheel rotations) was analysed via a repeated measures mixed effects model. Correlation analyses were completed using Pearson r. Wheel running onset, PER2::LUC period, and transient transfection data were analyzed via one-way ANOVA. Post-hoc tests were completed using Tukey’s multiple comparison test. PER2::LUC timing of first peak phase relationships were analyzed via a One Criterion Analysis of Variance for Circular Data, followed by pairwise comparisons, Watson’s Two Sample Test of Homogeneity using Bonferroni’s correction to accommodate familywise error rate, where appropriate. Phase data were reported in degrees and standard error (SE). All data passed normality testing. Statistical analyses for outliers (Grubbs’ test) were conducted on all data sets, and no outliers were identified. Using G*Power software (Faul et al., 2007), we estimated the number of animals required for each experiment. Based on the literature, we expected that pregnancy would dramatically reduce locomotor activity (Martin-Fairey et al., 2019). Using the ANOVA repeated measures with an effect size of 0.5, alfa-probability of 0.05, with a power at 0.95, with 6 measurements (repeated measure of activity level) the experiment requires a total sample size of n = 8.
Results
Late pregnancy impacts activity levels and activity onset independent of litter size
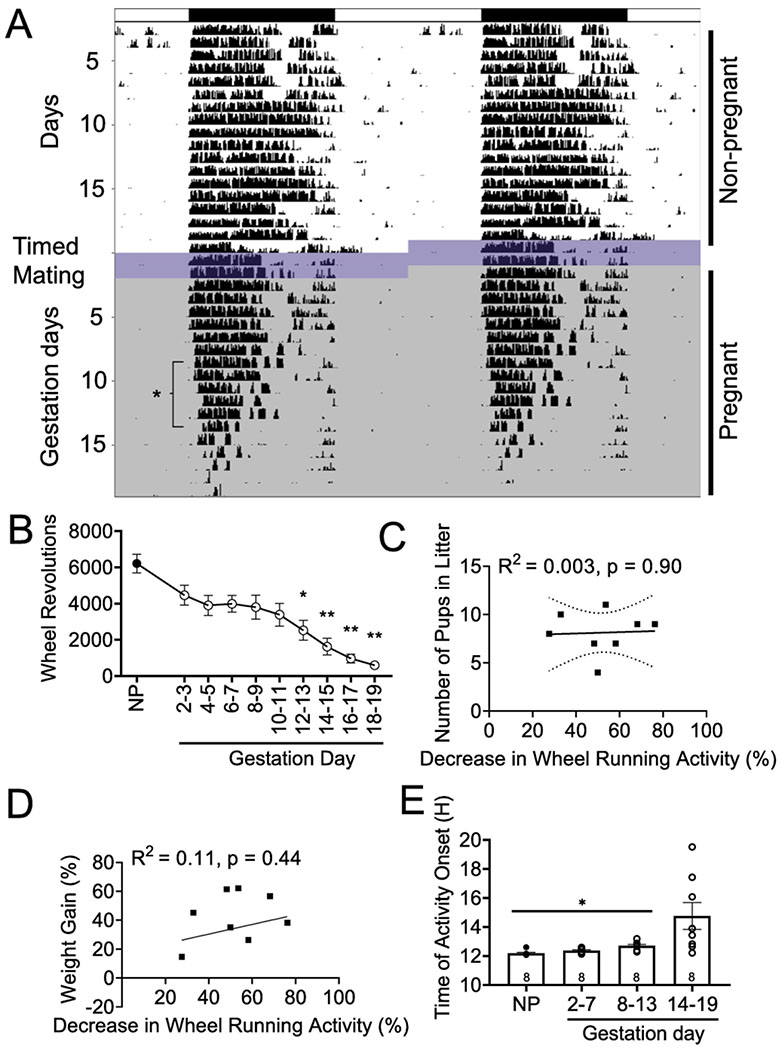
Pregnancy is associated with dramatic physiological changes, including great weight gain (Abrams et al., 1995; School et al., 1995). Recent work showed that pregnancy caused a reduction in activity levels of both humans and mice (Martin-Fairey et al., 2019). To determine if the reduction in activity levels was directly associated with litter size, we placed virgin female mice on running wheels for 15-20 days with light 12h:dark 12h (LD12:12) to establish their baseline activity level Figure 2A, B (non-pregnant; NP). A male was introduced to the chamber to allow pregnancy (timed mating). After positive identification of male mounting (as evidenced by a vaginal plug), the male was removed and female running-wheel activity monitored till late gestation (Gestation day 18-19; GD 18-19) Figure 2A, B. In agreement with the previous study (Martin-Fairey et al., 2019), wheel running activity decreased significantly during pregnancy, specifically during late gestation [GD 12-19, F (2.20, 13.46) = 19.99, n = 6-8/time point p < 0.0001], Figure 2B. This decrease in wheel running activity was not significantly correlated with the number of pups in each litter [r(8) = 0.052, n = 8, p = 0.902; R2 = 0.003], Figure 2C, or significantly correlated with percentage of weight gain during pregnancy [r(8) = 0.321, n = 8, p = 0.44; R2 = 0.11], Figure 2D. Interestingly, females displayed a delayed onset in activity during mid pregnancy [GD 8-13, F (1.026, 7.182) = 6.182, n = 8/time point, p = 0.037], Figure 2E. The changes to total activity and onset timing were not accompanied with changes in wheel running period, for non-pregnant (24.00 h ± 0.02), early (GD 2-7; 24.06 h ± 0.02), mid (GD 8-15; 24.06 h ± 0.06), or late (GD 16-19; 24.06 h ± 0.2) pregnancy [F(1.09, 7.27) = 0.149, n = 7-8/time point, p = 0.15]. Given the high variability in onset times during GD 14-19, we compared onset times from GD 14-15, GD 16-17, and GD 18-19; however there were no differences [F(1.604, 9.625) = 0.766, n = 7/time point, p = 0.46].
Figure 2. Pregnancy impacts activity levels and activity onset independent of litter size.
A.) Representative double plotted actogram of wheel running in a female mouse before pregnancy (Day 1-20), during timed mating (Day 21-22; dark blue-purple shading), and during pregnancy [Gestation day (GD) 1-19; light gray shading]. B.) Total number of wheel revolutions decrease beginning GD 12-13 and continued to decrease through late pregnancy. Pearson correlation examining the relationship between the percentage decrease in wheel running activity from non-pregnant to late pregnant levels (GD 14-19) compared to the number of pups in each litter (C., n = 8, individual mice are shown as squares) and the percentage of weight gain during pregnancy (D., n = 8). R2 and p values are indicated next to linear regression lines. Dashed curves indicate 95% confidence intervals. E.) Timing of activity onset is significantly delayed during mid pregnancy (GD 8-13), compared to non-pregnant (NP), early (GD 2-7), and late pregnancy (GD 14-19). Individual mice are shown as circles. Data were analyzed via repeated-measures one-way ANOVA followed by a Tukey post hoc. *, p<.05; **, p<.01.
PER2::LUC period in the SCN and arcuate nucleus does not correlate with locomotor activity onset in late pregnancy
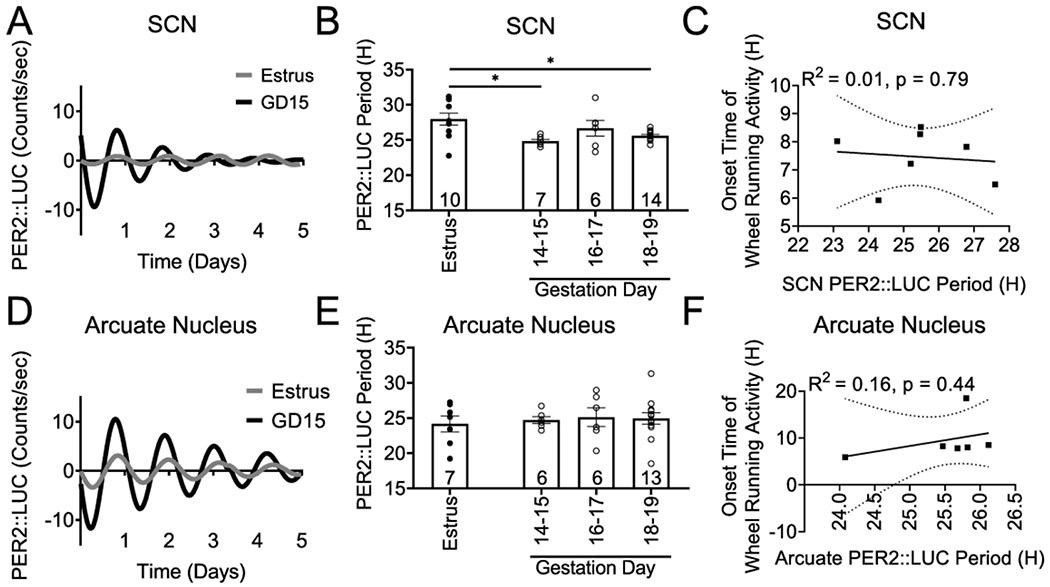
To further understand the molecular mechanisms driving the changes in activity onset and locomotor activity patterns in late pregnancy Figure 2, we used the validated circadian reporter mouse, PER2::LUC to establish ex vivo tissue circadian rhythms in non-pregnant and pregnant females. It is well-established that PER2::LUC rhythms reliably recapitulate SCN function and reflect on behavioral wheel-running patterns (Yoo et al., 2004). We compared PER2::LUC period in the SCN from estrus (non-pregnant females), GD 14-15, GD 16-17 and GD 18-19 females. There was a significant decrease in PER2::LUC period between estrus and early (GD 14-15) and late (GD 18-19) pregnancy [F(3, 33) = 5.61, n = 6-14/group, p = 0.003], Figure 3A, B. This suggests that changes to SCN period may be involved in the delayed locomotor onset in pregnancy. However, due to the large behavioral variation in activity onset during late pregnancy Figure 2E, we established the correlation between SCN PER2::LUC period and time of day of wheel running onset Figure 3C. These correlation data [r(7) = 0.072, n = 7, p = 0.80; R2 = 0.014], suggest the SCN probably does not contribute to the delayed activity onset in pregnancy. Recent work identified the arcuate nucleus as important in modulating metabolic status-driven locomotor activity changes (Padilla et al., 2019). To determine if the molecular clock in the arcuate nucleus was involved in changing locomotor onset during pregnancy, we recorded arcuate nucleus circadian rhythms in our newly established arcuate nucleus slice preparation. The arcuate nucleus presents a circadian expression of PER2::LUC Figure 3D, E. There were no significant changes in PER2::LUC period between estrus and any of the studied gestation days [F(3, 28) = 1.104, n = 6-13/group, p = 0.36], nor a significant correlation between arcuate PER2::LUC period and time of day of wheel running onset [r(6) = 0.739, n = 6, p = 0.44; R2 = 0.16], Figure 3F.
Figure 3. SCN and arcuate nucleus PER2::LUC period do not correlate with locomotor activity onset during late pregnancy.
A.) Example traces of representative SCN PER2::LUC recordings. B.) Histogram of PER2::LUC period in the SCN during pregnancy (n = 6-14/group). C.) Pearson correlation examining the relationship between the SCN PER2::LUC period and onset of wheel running activity on the day of euthanasia (GD 16-19), n = 7. D.) Example traces of representative arcuate nucleus PER2::LUC recordings, and E.) histogram of PER2::LUC period in the arcuate nucleus during pregnancy (n = 6-13/group). F.) Pearson correlation examining the relationship between the arcuate nucleus PER2::LUC period and onset of wheel running the day of euthanasia (GD 16-19), n = 6. Period data (B., E.) were analyzed via one-way ANOVA followed by a Tukey post hoc. *, p<.05. Individual values are indicated by circles or squares, and n is indicated on the bars. R2 values and p-values are indicated next to linear regression lines. Dashed curves indicate 95% confidence intervals.
Pregnancy alters reproductive tissue phase relationships
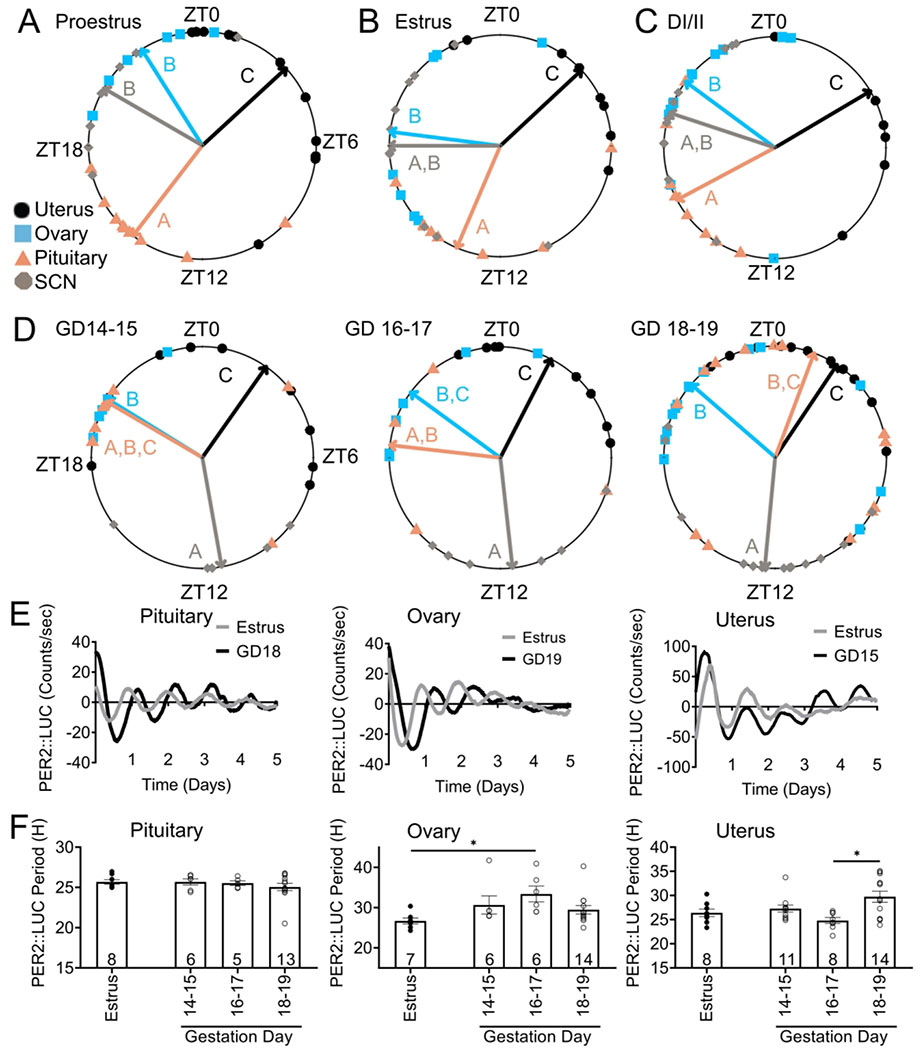
Changes in SCN output (as evidenced by changes in PER2::LUC period and behavioral changes, Figures 2 and 3), might impact phase relationships between peripheral tissues. No studies have, to our knowledge, addressed how phase relationships and circadian period changes in normal pregnancy. We compared phase-relationships between reproductive tissues during each stage of the estrous cycle and late pregnancy (GD 14-19) Figure 4A–C. There were significant differences in the timing of PER2::LUC peak expression between the different tissues during each stage of the estrous cycle [estrus: F(3, 29) = 21.7, n = 33, p = 1.4e−07; proestrus: F(3, 26) = 49.58, n = 34, p = 1.7e−10; diestrus I/II: F(3, 36) = 20.17, n = 40, p = 7.7e−08]. Specifically, during proestrus, pituitary peak PER2::LUC expression (expressed in mean degrees ± standard deviation; 217.51 degrees ± 0.55, n = 10) was significantly different from peak timing in the ovary (327.77 degrees ± 0.48, n = 6; Watson’s Two Sample Test of Homogeneity (referred to as Watson’s) = 0.32, 0.001 < p < 0.01), uterus (47.30 degrees ± 0.89, n = 11; Watson’s = 0.41, p < 0.001), and SCN (300.16 degrees ± 0.67, n = 7; Watson’s = 0.21, 0.01 < p < 0.05). The ovary and SCN both exhibited a phase difference from the uterus (ovary: Watson’s = 0.23, 0.01 < p < 0.05, and SCN: Watson’s = 0.24, 0.01 < p < 0.05), but did not differ from each other (Watson’s = 0.09, p > 0.10). During estrus, SCN (270.02 degrees ± 0.89, n = 10 ; Watson’s = 0.34, 0.001 < p < 0.01) and pituitary (203.11 degrees ± 0.80, n = 8; Watson’s = 0.30, 0.001 < p < 0.01), peak expression occurred ~2-8 h later than in the uterus (46.97 degrees ± 0.77, n = 8). The pituitary phase also significantly differed from the ovary (277.30 degrees ± 1.01, n = 7; Watson’s = 0.22, 0.01 < p < 0.05) and uterus (Watson’s = 0.24, 0.01 < p < 0.05), with no differences between ovarian peak expression and the SCN (Watson’s = 0.11, p > 0.10). Diestrus I/II peak phase analyses revealed that SCN and pituitary time of peak expression did not differ (SCN,169.95 degrees ± 0.64, n = 11 vs pituitary, 300.29 degrees ± 1.29, n = 9; Watson’s = 0.19, 0.01 < p < 0.05), and both the pituitary (Watson’s = 0.36, 0.001 < p < 0.01) and SCN (Watson’s = 0.39, p < 0.001) exhibited peaks later in the day than peak expression in the uterus (35.10 degrees ± 1.27, n = 8). DI/II ovary phase (301.69 degrees ± 0.37, n = 12) differed from the pituitary (Watson’s = 0.26, 0.01 < p < 0.05) and uterus (Watson’s = 0.26, 0.01 < p < 0.05) with no differences between the ovary and SCN (Watson’s = 0.09, p > 0.10) Figure 4A–C. To understand phase relationships during late pregnancy, we next analyzed phase relationships across two-day periods from GD 14-19, Figure 4D. There were significant differences in the timing of PER2::LUC peak expression between the different tissues during each of the 2-day periods [GD 14-15: F(3, 22) = 16.86, n = 26, p = 6.5e−06; GD 16-17: F(3, 20) = 25.6, n = 24, p = 4.7e−07; GD 18-19: F(3, 51) = 50.8, n = 55, p = 2.4e−15]. Specifically, during GD 14-15, SCN PER2::LUC peaked (169.95 degrees ± 0.64, n = 5) after peak timing in the uterus (35.10 degrees ± 1.27, n = 10; Watson’s = 0.29, 0.001 < p < 0.01), and ovary (301.69 degrees ± 0.37, n = 5; Watson’s = 0.23, 0.01 < p < 0.05), with no differences between the SCN and pituitary (pituitary: 300.29 degrees ± 1.29; n = 6; Watson’s = 0.18, 0.5 < p < 0.10). GD 14-15 pituitary phase did not differ from peak phase in the uterus (Watson’s = 0.13, p > 0.10) or ovary (Watson’s = 0.07, p > 0.10), however, the ovary and uterus exhibited different phases (Watson’s = 0.20, 0.01 < p < 0.05). For GD 16-17, the SCN (173.68 degrees ± 0.69, n = 6), again differed from the uterus (27.18 degrees ± 0.68, n = 8; Watson’s = 0.30, 0.001 < p < 0.01) and ovary (306.16 degrees ± 0.70, n = 6; Watson’s = 0.26, 0.01 < p < 0.05), with no differences between the SCN and pituitary (pituitary: 276.54 degrees ± 1.47, n = 4; Watson’s = 0.15, 0.5 < p < 0.10). GD 16-17 pituitary phase differed from the phase in the uterus (Watson’s = 0.24, 0.01< p < 0.05), but not the ovary (Watson’s = 0.05, p > 0.10). During this time point, the ovary and uterus phases did not differ (Watson’s = 0.14, p > 0.10). GD 18-19 peak phase analyses revealed that SCN time of peak expression differed from all other tissues (185.36 degrees ± 0.79, n = 14; ovary: Watson’s = p < 0.001, uterus: Watson’s = 0.53, p < 0.001, pituitary: Watson’s = 0.37, 0.001< p < 0.01 ). The ovary (310.97 degrees ± 1.08 n = 14) and uterus (33.73 degrees ± 0.89, n = 14) differed, Watson’s = 0.30, 0.001 < p < 0.01, and there were no differences between the pituitary (20.49 degrees ± 1.85, n = 13), compared to the ovary (Watson’s = 0.14, p < 0.10) or uterus (Watson’s = 0.15, 0.05 < p < 0.10). There were no significant changes in PER2::LUC period in the pituitary [F (3, 28) = 0.82 n = 5-13/group, p = 0.49], Figure 4E, F. However the PER2::LUC period in the ovary was significantly different between estrus and GD 16-17 [F (3, 28) = 3.10, n = 6-13/group, p = 0.042]. Uterine PER2::LUC period revealed a significant change [F(3, 36) = 4.97, p = 0.006], with a lengthening of period from GD 16-17 to GD 18-19, Figure 4E, F. Correlation analyses between time of first peak and period revealed a significant correlation in the ovary [r(50) = −0.324, p = 0.02; R2 = 0.105], whereas no significant correlations were seen in the pituitary [r(50) = 0.062, p = 0.67; R2 = 0.004], or uterus [r(59) = 0.073, p = 0.58; R2 = 0.005].
Figure 4. Reproductive tissue phase-relationships during estrous cycle and pregnancy.
Time of day of first PER2::LUC peak was used to establish the phase of the studied tissues during the estrous cycle and during pregnancy. Phase-relationships during A.) estrus, n= 7-10/group, B.) proestrus, n= 6-11/group, C.) diestrus I and II (DI/II). n=8-12/group, and D.) late pregnancy [gestation day (GD) 14-19], n =5-15/group, except GD 16-17 pituitary, n=4. Mean time of first peak is indicated by the vector lines and symbols indicate individual data points. Data were analyzed via circular ANOVA where different letters indicate significantly different phases. E.) Representative PER2::LUC traces during estrus and GD 15-19 in the pituitary, ovary and uterus and F.) histogram of PER2::LUC period was compared between the indicated days in the pituitary, ovary, and uterus. Data were analyzed via one-way ANOVA followed by a Tukey post hoc. *, p<.05. For periods, individual values are shown as circles and n is as indicated.
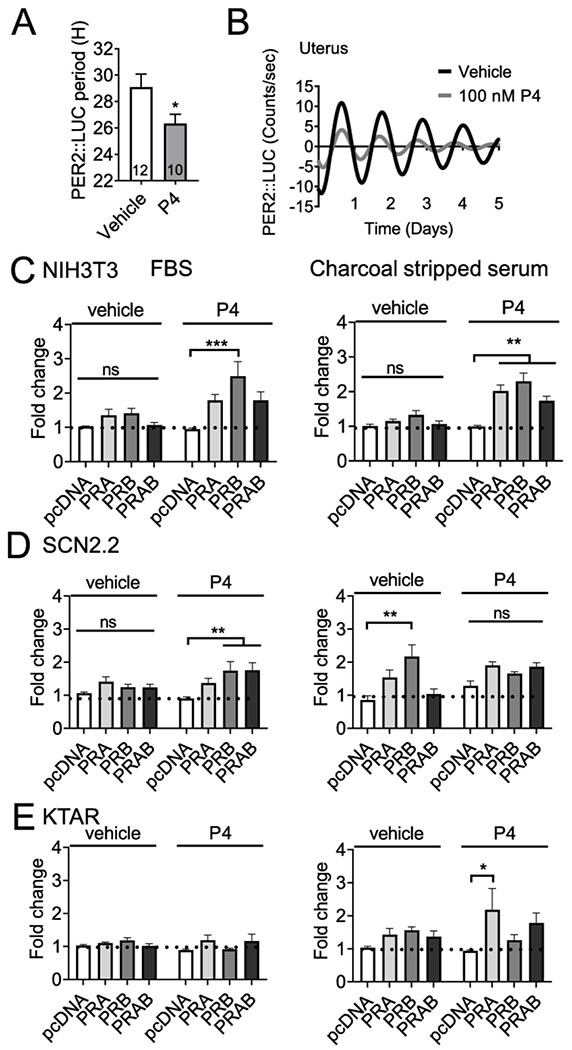
Progesterone regulates PER2::LUC period in uterine tissue in late pregnancy
Given the important role of progesterone in uterine function in preparation for parturition (Brown et al., 2004; Zakar and Hertelendy, 2007), we examined the effect of progesterone on PER2::LUC period of uterine tissue by treating ex vivo GD 18-19 uterine tissue with either vehicle (water) or progesterone (50 and 100 nM) and following recorded the PER2::LUC period. We found that progesterone significantly shortened PER2::LUC period [Student’s t-test, t = 2.195, df = 20, n = 10-12, p = 0.040], Figure 5A, B.
Figure 5. Progesterone regulates Per2-luciferase expression.
A.) Histogram and B.) example trace of PER2::LUC period in the GD 18-19 uterus in response to progesterone (P4 50-100 nM) or veichle control. “PER2::LUC period was analyzed with student’s t-test, N = 10-12, *p < 0.05”. C,-E.) Transient transfections of NIH3T3 (mouse fibroblasts), SCN2.2 (rat SCN cells), and KTAR (mouse arcuate nucleus kisspeptin neurons) with Per2-luciferase, PRA, PRB or empty vector (pcDNA), cultured in in heat inactive heat inactivated FBS (FBS, left side) or charcoal stripped serum (right side). The capacity of progesterone (100 nM) or vehicle to drive Per2-luciferase expression was evaluated. Data is expressed as fold change as compared to control (pcDNA, vehicle). N=3-6 in duplicate. Statistical analysis by two-way ANOVA, followed by a Tukey post hoc. *, p<0.05; **, p<.01; ***, p<.001, ns: non-significant.
Progesterone receptors regulate Per2-luciferase expression in vitro
The potential role of PRs in the SCN remains elusive (Kruijver and Swaab, 2002; Murphy et al., 2013), whereas PRs in the arcuate nucleus are known to be important in regulating the negative feedback controlling the LH surge (Goodman et al., 2011). To determine if PRA and/or PRB can regulate the mouse Per2-luciferase promoter in vitro, we transiently transfected NIH3T3 cells (control cell line), SCN2.2 (rat SCN cell line) and KTAR cells (mouse arcuate nucleus cell line) with Per2-luciferase with and without PRA/B. As progesterone levels change dramatically during the estrous cycle and pregnancy, we compared the capacity of exogenous progesterone to regulate Per2-luciferase in NIH3T3, SCN2.2 and KTAR cells cultured in heat-inactivated FBS (contains progesterone), and charcoal stripped serum (depleted of progesterone) Figure 5C–E. In NIH3T3 cells, progesterone (100 nM), enhanced Per2-luciferase expression through PRA/B, independent of the type of media Figure 5C (NIH3T3). Interestingly, Per2-luciferase expression in SCN2.2 cells responded differently to progesterone depending on the type of culture media, where progesterone significantly enhanced Per2-luciferase expression in heat inactivated FBS (Figure 5D, left), which was not the case in charcoal stripped media (Figure 5D, right). In addition, in SCN2.2 cells Per2-luciferase expression increased in cells transfected with PRB in absence of progesterone, suggesting this receptor might acquire constitutive activity in this cell line Figure 5D. In the arcuate nucleus kisspeptin cell line (KTAR), progesterone enhanced Per2-lucefase expression through PRA in charcoal stripped buffer (Figure 5E, right), but had no effect in heat-inactivated FBS (Figure 5E, left).
Discussion
The precise timing of hormone release and downstream signaling is central to optimal functioning of the reproductive axis (Miller and Takahashi, 2014; Sen and Hoffmann, 2020). The SCN, pituitary, ovary, and uterus are all important components of female reproductive function, where each of these tissues exhibit circadian rhythms which are necessary for female fertility, including, but not limited to, ovulation (Loh et al., 2014; Mereness et al., 2016) and embryo implantation (Liu et al., 2014; Ratajczak et al., 2009; Sellix, 2013). Measures of circadian-alignment between reproductive tissues can be obtained using the validated circadian reporter mouse, PER2::LUC, PCR, or western blot studies. Such experiments have determined that phase of reproductive tissues changes throughout the estrous cycle (Karman and Tischkau, 2006; Nakamura et al., 2010). During the estrous cycle, the changes in circadian rhythms and tissue phase, are in part driven by steroid hormone signaling, including progesterone (Murphy et al., 2013; Nakamura et al., 2010), a central hormone in estrous cycling and pregnancy (Brown et al., 2004; Nadeem et al., 2016; Wharfe et al., 2016). To further these studies, we assessed PER2::LUC period and phase relationships between the SCN, pituitary, ovary, and uterus during the estrous cycle and GD 14-19. Throughout all stages of the estrous cycle, the uterus and pituitary displayed different phases, ~10-12h apart, of peak timing in PER2::LUC expression. Interestingly, the SCN exhibited phase relationships during proestrus that were different from estrus or DI/II, which suggests a possible role of progesterone on the phase of the SCN, due to the peak of progesterone in proestrus, the estrous stage preceding estrus (Miller and Takahashi, 2014). Specifically, during proestrus, the SCN exhibited a similar PER2::LUC peak to the uterus and ovary. During both estrus and DI/II, the SCN was in phase with the pituitary and ovary, while the uterine PER2::LUC peak occurred earlier in the day than the SCN and pituitary. The fact that the SCN displays different phase relationships within estrous cycle stages supports its sensitivity to estrogen and/or progesterone, a mechanism supported in the work presented here, where we found progesterone can regulate Per2-luciferase expression in vitro in SCN cells. However, more work, including studies directly examining hormone application to tissue explants, is needed to further understand the impact of estrogen and progesterone on the circadian timing of these tissues. In pregnancy, progesterone and estrogen levels also change dramatically, and, as such, these hormones could also change circadian rhythms throughout the reproductive axis at this time of life. Indeed, we found that progesterone can shorten PER2::LUC period in the ex vivo uterus during late gestation. Further, during late gestation (GD 14-15, GD 16-17, and GD 18-19), we observed significant differences between the phases of reproductive tissues at each studied timepoint. Perhaps the most striking changes involve the relationships surrounding the pituitary. During both GD 14-15 and GD 16-17, the pituitary is in phase with the SCN, however, during GD 18-19, the pituitary exhibits an approximate 13 h delay following SCN peak phase. It is possible that these different relationships could be occurring in preparation for parturition, potentially driven by the hormonal changes involved in the transition to labor, including changes in oxytocin release, however more work is necessary to investigate this hypothesis. It is important to note that while we examined phase relationships within each stage of estrous and late pregnancy, we did not perform any statistical analysis of the phase relationships of individual tissues between stages of estrous and pregnancy. We chose not to run such statistical tests, as doing so would have greatly reduced our statistical power, given the necessary additional p-value corrections required to examine the tissue phase across the six time points. Nonetheless, given the dramatic changes in phase relationships within each stage of estrous cycle and during late pregnancy, we believe these adaptations reflect upon a combined effect of changes in hormone release and tissue sensitivity. This hypothesis is supported by our finding that progesterone, a central sex steroid released from the corpus luteum after ovulation, increases Per2-luciferase expression in SCN and kisspeptin cells in vitro, and shortens PER2::LUC period in uterine tissue in late gestation. Others have explored similar ideas and demonstrated that individual changes in tissue phase occur between proestrus and diestrus, and that such circadian changes can be regulated by sex steroids and gonadotropins (He et al., 2007; Karman and Tischkau, 2006; Nakamura et al., 2010; Yoshikawa et al., 2009). Examining the contribution of hormones involved in reproductive function and metabolism in adapting these relationships through the estrous cycle and pregnancy will be of interest.
With the multitude of hormonal and physiological changes occurring during late pregnancy, it is no surprise that behavioral activity patterns are altered during this time period. Others have indicated a decrease in locomotor activity during pregnancy in a number of species, including humans (Martin-Fairey et al., 2019), non-human primates (Honnebier and Nathanielsz, 1994), and rodents (Albers et al., 1981; Martin-Fairey et al., 2019, 2016; Rosenwasser et al., 1987). The work presented here confirms this decrease in locomotor activity during pregnancy and suggests that this decline in activity occurs independently from litter size and may be only modestly caused by weight gain. Importantly, others have found that pseudopregnancy, which is associated with increased progesterone (Welschen et al., 1975), causes decreased locomotor activity (Albers et al., 1981). This suggests that a hormonal mechanism, and not metabolic changes or fetal signaling, is a plausible candidate driving the decrease in activity observed in pregnancy. In addition to decreases in locomotor activity, the onset timing of locomotor activity is altered during pregnancy (Martin-Fairey et al., 2019, 2016). Both our study and work by Martin-Fairey et al (2019) observed a change in activity onset time during mid-pregnancy; however, their results differ from ours in direction, where we observed a significant delay in onset of wheel running activity, while they observed an advanced onset. These differences could be attributed to several factors, including mouse strain, which has recently been shown to influence circadian wheel running activity between C57BL6/N and C57BL6/J males under constant light (Capri et al., 2019), as well as differences in locomotor activity analysis (5-min bins in our work versus 6-min bins), and/or rodent housing, where we maintained males within the behavioral chamber where the females were housed throughout the experiment. Even given these differences, it is evident that circadian locomotor rhythms are highly influenced by pregnancy. As several aspects of circadian rhythms, including period, timing onset, and length of activity combine to produce specific rhythmic behavior patterns, it is important to consider how circadian variables influence each other. One such relationship is an established correlation between increased locomotor activity with advanced activity onset, whereas reduced locomotor activity delayed activity onset in constant conditions (Edgar et al., 1991). This is supported by our findings, where pregnancy dramatically decreased overall locomotor activity and delayed activity onset. To further our understanding of the underlying mechanisms driving the changes in onset timing and overall locomotor activity during pregnancy, we focused on the known role of the SCN in regulating wheel running behaviors (LeSauter and Silver, 1999; Schwartz and Zimmerman, 1991; Stephan and Zucker, 1972). Using ex vivo SCN explants, we found that PER2::LUC period was significant shortened during two timepoints during late gestation, as compared to estrus. However, this change in PER2::LUC period did not correlate with activity onset, suggesting the SCN is not a strong driver of this behavioral change. That said, light can mask the effects of SCN function on locomotor activity (Morin and Studholme, 2009), and, as such, future studies done in constant darkness are required to understand if the changes in locomotor activity are driven by the SCN. A second limitation of our study is the caveat induced by evaluating locomotor activity using running wheels. Although wheel running is a standard measure of circadian locomotor activity, it does possess limitations, such as its rewarding properties and the potential difficulty for a late-pregnant mouse to climb onto the elevated wheel, due to the significant weight gain and change in body proportions. Thus, it is possible that wheel running decreases, but overall activity may not. To determine if this is the case, in house activity could be evaluated.
Aside from the SCN’s role in driving locomotor activity, recent work has implicated kisspeptin neurons from the arcuate nucleus in modulating behavioral rhythms in addition to the timing of food intake, sleep, and body temperature in female mice (Padilla et al., 2019). Given the considerable metabolic changes occurring in the mother during pregnancy to support the developing fetus (Lain and Catalano, 2007) and the strong ties to circadian rhythmicity and metabolic state (Froy, 2009; Huang et al., 2011), is likely that such metabolic changes play into the circadian time-keeping system. This made us examine how PER2::LUC rhythms in the arcuate nucleus adapted to pregnancy. Our data did not reveal changes to PER2::LUC period during late pregnancy, nor was there a positive correlation between PER2::LUC period in the arcuate nucleus and activity onset. Despite these negative results, to our knowledge, this is the first record of PER2::LUC rhythms evaluated in the arcuate nucleus, and shows this structure possess a molecular clock, which can easily be studied using the PER2::LUC reporter mouse.
In conclusion, this work describes both behavioral and tissue-specific changes in circadian rhythms that occur during the estrous cycle and pregnancy. Our findings suggest that progesterone is involved in coordinating late pregnancy circadian rhythm function in the SCN, arcuate nucleus, and uterus. These studies are a first step towards understanding how the circadian time-keeping system adapts during pregnancy and will be instrumental in elucidating the molecular pathways involved in pregnancy loss and pregnancy associated complications.
Significance Statement.
Biological timekeeping is essential for the coordination of physiological processes and behavior, including reproduction. To date, the influence of estrous cycle and pregnancy on the circadian (~24 h) time-keeping system is not fully understood. To elucidate circadian changes during the estrous cycle and pregnancy in reproductive tissues and the brain, we examined the influence of circadian rhythms on behavioral and cellular molecular function. This work provides a necessary step towards understanding the temporal coordination of physiological functions essential for successful pregnancy and lays the foundation for future studies examining the influence of circadian disfunction on pregnancy-associated disease pathologies.
Acknowledgements
We thank Dr. Earnest, Texas A&M for sharing the SCN2.2 cells, Dr. Patrick E. Chappell at Oregon State University for sharing the KTAR cells and Dr. Jaewook Jeong at Michigan State University for the PRA and PRB overexpression plasmids. We thank Jeffery Doser from the Michigan State University College of Agriculture and Natural Resources (CANR) Biometry Group – Statistical Consulting Center for assistance with circular statistics. We thank Brooke M. Devries, Tulasi Talluri and Asad Muhammed for technical assistance and feedback on the manuscript.
H.M.H. was in part supported by Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R00HD084759, the USDA National Institute of Food and Agriculture Hatch project MICL1018024 and by the March of Dimes Grant no 5-FY19-111. A.M.Y was partially supported by Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number T32HD087166.
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Abrams B, Carmichael S, Selvin S, 1995. Factors associated with the pattern of maternal weight gain during pregnancy. Obstet. Gynecol 86, 170–176. [DOI] [PubMed] [Google Scholar]
- Albers EE, Gerall AA, Axelson JF, 1981. Effect of reproductive state on circadian periodicity in the rat. Physiol. Behav 26, 21–25. [DOI] [PubMed] [Google Scholar]
- Backe B, 1991. A circadian variation in the observed duration of labor. Acta Obstet. Gynecol. Scand 70, 465–468. [DOI] [PubMed] [Google Scholar]
- Barkley MS, Geschwind II, 1979. The Gestational and Progesterone Pattern Secretion of Estradiol, in Selected Testosterone Strains of Mice surges on. Biol. Reprod 733–738. [DOI] [PubMed] [Google Scholar]
- Barkley MS, Michael SD, Geschwind II, Bradford GE, 1977. Plasma testosterone during pregnancy in the mouse. Endocrinology 100, 1472–1475. 10.1210/endo-100-5-1472 [DOI] [PubMed] [Google Scholar]
- Bell AW, Bauman DE, 1997. Adaptations of glucose metabolism during pregnancy and lactation. J. Mammary Gland Biol. Neoplasia 2, 265–278. [DOI] [PubMed] [Google Scholar]
- Bhurke AS, Bagchi IC, Bagchi MK, 2016. Progesterone-regulated endometrial factors controlling implantation. Am. J. Reprod. Immunol 75, 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindon BM, 1971. The role of progesterone in implantation in the sheep. Aust. J. Biol. Sci 24, 149–158. [DOI] [PubMed] [Google Scholar]
- Boden MJ, Varcoe TJ, Kennaway DJ, 2013. Circadian regulation of reproduction: From gamete to offspring. Prog. Biophys. Mol. Biol 10.1016/j.pbiomolbio.2013.01.003 [DOI] [PubMed] [Google Scholar]
- Bridges RS, 1984. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology 114, 930–940. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Rosenblatt JS, Feder HH, 1978. Serum progesterone concentrations and maternal behavior in rats after pregnancy termination: behavioral stimulation after progesterone withdrawal and inhibition by progesterone maintenance. Endocrinology 102, 258–267. [DOI] [PubMed] [Google Scholar]
- Brown AG, Leite RS, Strauss JF, 2004. Mechanisms underlying “functional” progesterone withdrawal at parturition. Ann. N. Y. Acad. Sci 1034, 36–49. 10.1196/annals.1335.004 [DOI] [PubMed] [Google Scholar]
- Cabral R, Gutiérrez M, Fernández AI, Cantabrana B, Hidalgo A, 1994. Progesterone and pregnanolone derivatives relaxing effect on smooth muscle. Gen. Pharmacol 25, 173–178. 10.1016/0306-3623(94)90029-9 [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Soldani R, Melis GB, Volpe A, 1998. Diurnal rhythms of labor and delivery in women: modulation by parity and seasons. Am. J. Obstet. Gynecol 178, 140–145. [DOI] [PubMed] [Google Scholar]
- Capri KM, Maroni MJ, Deane HV, Concepcion HA, Decoursey H, Logan RW, Seggio JA, 2019. Male C57BL6/N and C57BL6/J mice respond differently to constant light and running-wheel access. Front. Behav. Neurosci 13, 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie AT, Lyons WR, 1959. Mammogenesis and lactogenesis in hypophysectomized, ovariectomized, adrenalectomized rats. J. Endocrinol 19, 29-NP. [DOI] [PubMed] [Google Scholar]
- Dardente H, Wood S, Ebling F, Sáenz de Miera C, 2019. An integrative view of mammalian seasonal neuroendocrinology. J. Neuroendocrinol 31, e12729. [DOI] [PubMed] [Google Scholar]
- Dolatshad H, Campbell EA, O’hara L, Maywood ES, Hastings MH, Johnson MH, 2005. Developmental and reproductive performance in circadian mutant mice. Hum. Reprod 21, 68–79. [DOI] [PubMed] [Google Scholar]
- Earnest DJ, Liang FQ, DiGiorgio S, Gallagher M, Harvey B, Earnest B, Seigel G, 1999. Establishment and characterization of adenoviral E1A immortalized cell lines derived from the rat suprachiasmatic nucleus. J. Neurobiol 39, 1–13. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Kilduff TS, Martin CE, Dement WC, 1991. Influence of running wheel activity on free-running sleep/wake and drinking circadian rhythms in mice. Physiol. Behav 50, 373–378. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A, 2007. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences, in: Behavior Research Methods. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G, 2008. The mouse brain in stereotaxic coordinates. Academic press; New York: [Google Scholar]
- Froy O, 2009. Metabolism and circadian rhythms—implications for obesity. Endocr. Rev 31, 1–24. [DOI] [PubMed] [Google Scholar]
- Garg D, Ng SSM, Baig KM, Driggers P, Segars J, 2017. Progesterone-Mediated Non-Classical Signaling. Trends Endocrinol. Metab 28, 656–668. 10.1016/j.tem.2017.05.006 [DOI] [PubMed] [Google Scholar]
- Goldberg RJ, Ye C, Sermer M, Connelly PW, Hanley AJG, Zinman B, Retnakaran R, 2012. Circadian variation in the response to the glucose challenge test in pregnancy: implications for screening for gestational diabetes mellitus. Diabetes Care 35, 1578–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL, Holaskova I, Nestor CC, Connors JM, Billings HJ, Valent M, Lehman MN, Hileman SM, 2011. Evidence that the arcuate nucleus is an important site of progesterone negative feedback in the ewe. Endocrinology 152, 3451–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm SL, Hartig SM, Edwards DP, 2016. Progesterone Receptor Signaling Mechanisms. J. Mol. Biol 428, 3831–3849. 10.1016/j.jmb.2016.06.020 [DOI] [PubMed] [Google Scholar]
- Halasz M, Szekeres-Bartho J, 2013. The role of progesterone in implantation and trophoblast invasion. J. Reprod. Immunol 97, 43–50. [DOI] [PubMed] [Google Scholar]
- Hartmann PE, Trevethan P, Shelton JN, 1973. Progesterone and oestrogen and the initiation of lactation in ewes. J. Endocrinol 59, 249–259. [DOI] [PubMed] [Google Scholar]
- He PJ, Hirata M, Yamauchi N, Hashimoto S, Hattori MA, 2007. Gonadotropic regulation of circadian clockwork in rat granulosa cells. Mol. Cell. Biochem 10.1007/s11010-007-9432-7 [DOI] [PubMed] [Google Scholar]
- Hellier V, Brock O, Candlish M, Desroziers E, Aoki M, Mayer C, Piet R, Herbison A, Colledge WH, Prévot V, Boehm U, Bakker J, 2018. Female sexual behavior in mice is controlled by kisspeptin neurons. Nat. Commun 9, 400 10.1038/s41467-017-02797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann HM, 2018. Determination of Reproductive Competence by Confirming Pubertal Onset and Performing a Fertility Assay in Mice and Rats. JoVE (Journal Vis. Exp e58352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann HM, Gong P, Tamrazian A, Mellon PL, 2018. Transcriptional interaction between cFOS and the homeodomain-binding transcription factor VAX1 on the GnRH promoter controls Gnrh1 expression levels in a GnRH neuron maturation specific manner. Mol. Cell. Endocrinol 461, 143–154. 10.1016/j.mce.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann HM, Trang C, Gong P, Kimura I, Pandolfi EC, Mellon PL, 2016. Deletion of Vax1 from Gonadotropin-Releasing Hormone (GnRH) Neurons Abolishes GnRH Expression and Leads to Hypogonadism and Infertility. J. Neurosci 36, 3506–3518. 10.1523/JNEUROSCI.2723-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnebier M, Nathanielsz PW, 1994. Primate parturition and the role of the maternal circadian system. Eur. J. Obstet. Gynecol. Reprod. Biol 55, 193–203. [DOI] [PubMed] [Google Scholar]
- Huang W, Ramsey KM, Marcheva B, Bass J, 2011. Circadian rhythms, sleep, and metabolism. J. Clin. Invest 121, 2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DC, Veitch RE, Chappell PE, 2016. Evaluation of immortalized AVPV-And Arcuate-Specific neuronal kisspeptin cell lines to elucidate potential mechanisms of estrogen responsiveness and temporal gene expression in females. Endocrinology 157, 3410–3419. 10.1210/en.2016-1294 [DOI] [PubMed] [Google Scholar]
- Kalkhoff RK, 1982. Metabolic effects of progesterone. Am. J. Obstet. Gynecol 142, 735–738. 10.1016/S0002-9378(16)32480-2 [DOI] [PubMed] [Google Scholar]
- Karman BN, Tischkau SA, 2006. Circadian clock gene expression in the ovary: effects of luteinizing hormone. Biol. Reprod 75, 624–632. [DOI] [PubMed] [Google Scholar]
- Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P, 2006. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: Potential role in functional progesterone withdrawal at term. Mol. Endocrinol 20, 1519–1534. 10.1210/me.2005-0243 [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS, 2006. Molecular components of the mammalian circadian clock. Hum. Mol. Genet 15, R271–R277. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Williams WP III, 2012. Circadian control of neuroendocrine circuits regulating female reproductive function. Front. Endocrinol. (Lausanne). 3, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijver FPM, Swaab DF, 2002. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology. 10.1159/000057339 [DOI] [PubMed] [Google Scholar]
- Kumar P, Magon N, 2012. Hormones in pregnancy. Niger. Med. J. J. Niger. Med. Assoc 53, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lain KY, Catalano PM, 2007. Metabolic changes in pregnancy. Clin. Obstet. Gynecol 50, 938–948. [DOI] [PubMed] [Google Scholar]
- Landgraf D, Long JE, Welsh DK, 2016. Depression-like behaviour in mice is associated with disrupted circadian rhythms in nucleus accumbens and periaqueductal grey. Eur. J. Neurosci 43, 1309–1320. 10.1111/ejn.13085 [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim TH, Oh SJ, Yoo JY, Akira S, Ku BJ, Lydon JP, Jeong JW, 2013. Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus. FASEB J. 27, 2553–2563. 10.1096/fj.12-225664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSauter J, Silver R, 1999. Localization of a suprachiasmatic nucleus subregion regulating locomotor rhythmicity. J. Neurosci 19, 5574–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Johnson BP, Shen AL, Wallisser JA, Krentz KJ, Moran SM, Sullivan R, Glover E, Parlow AF, Drinkwater NR, Schuler LA, Bradfield CA, 2014. Loss of BMAL1 in ovarian steroidogenic cells results in implantation failure in female mice. Proc. Natl. Acad. Sci 111 10.1073/pnas.1209249111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh DH, Kuljis DA, Azuma L, Wu Y, Truong D, Wang HB, Colwell CS, 2014. Disrupted reproduction, estrous cycle, and circadian rhythms in female mice deficient in vasoactive intestinal peptide. J. Biol. Rhythms 29, 355–369. 10.1177/0748730414549767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons WR, 1958. Hormonal synergism in mammary growth. Proc. R. Soc. London. Ser. B-Biological Sci 149, 303–325. [DOI] [PubMed] [Google Scholar]
- Mahoney MM, 2010. Shift work, jet lag, and female reproduction. Int. J. Endocrinol 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraudino M, Martini M, Trova S, Farinetti A, Ponti G, Gotti S, Panzica G, 2018. Kisspeptin system in ovariectomized mice: estradiol and progesterone regulation. Brain Res. 1688, 8–14. [DOI] [PubMed] [Google Scholar]
- Martin-Fairey CA, McCarthy R, Ma X, England S, Herzog E, 2016. Chronotype changes during pregnancy. Reprod. Sci 23, 291A. [Google Scholar]
- Martin-Fairey CA, Zhao P, Wan L, Roenneberg T, Fay J, Ma X, McCarthy R, Jungheim ES, England SK, Herzog ED, 2019. Pregnancy Induces an Earlier Chronotype in Both Mice and Women. J. Biol. Rhythms 34, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites J, 1954. Recent studies on the mechanisms controlling the initiation of lactation. Rev. Can. Biol 13, 359–370. [PubMed] [Google Scholar]
- Mereness AL, Murphy ZC, Forrestel AC, Butler S, Ko CM, Richards JAS, Sellix MT, 2016. Conditional deletion of Bmal1 in ovarian theca cells disrupts ovulation in female mice. Endocrinology 157, 913–927. 10.1210/en.2015-1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Takahashi JS, 2014. Central circadian control of female reproductive function. Front. Endocrinol. (Lausanne). 4, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin L, Studholme K, 2009. Millisecond light stimuli evoke cessation of locomotion followed by sleep-like behavior that persists in the absence of light. J. Biol. Rhythms 24, 497 10.1177/0748730409349059.Millisecond [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I, 1977. Estradiol shortens the period of hamster circadian rhythms. Science (80-.). 196, 305–307. [DOI] [PubMed] [Google Scholar]
- Moriyama Ii., Sugawa T, 1972. Progesterone facilitates implantation of xenogenic cultured cells in hamster uterus. Nat. New Biol 236, 150–152. [DOI] [PubMed] [Google Scholar]
- Mosko SS, Moore RY, 1979. Neonatal ablation of the suprachiasmatic nucleus. Effects on the development of the pituitary-gonadal axis in the female rat. Neuroendocrinology 29, 350–361. [DOI] [PubMed] [Google Scholar]
- Murphy ZC, Pezuk P, Menaker M, Sellix MT, York N, 2013. Effects of Ovarian Hormones on Internal Circadian Organization in Rats 1 89, 1–9. 10.1095/biolreprod.113.109322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeem L, Shynlova O, Matysiak-Zablocki E, Mesiano S, Dong X, Lye S, 2016. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat. Commun 10.1038/ncomms11565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TJ, Sellix MT, Kudo T, Nakao N, Yoshimura T, Ebihara S, Colwell CS, Block GD, 2010. Influence of the estrous cycle on clock gene expression in reproductive tissues: Effects of fluctuating ovarian steroid hormone levels 75, 203–212. 10.1016/j.steroids.2010.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen BW, Bonney EA, Pearce BD, Donahue LR, Sarkar IN, (PREBIC), P.B.I.C., 2016. A cross-species analysis of animal models for the investigation of preterm birth mechanisms. Reprod. Sci 23, 482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisa H, Qi KHT, Leng J, Zhou T, Liu H, Li W, Wang L, Li N, Hu G, Qi L, 2018. The Circadian Rhythm-Related MTNR1B Genotype, Gestational Weight Gain, and Postpartum Glycemic Changes. J. Clin. Endocrinol. Metab 103, 2284–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcese J, 2012. Circadian aspects of mammalian parturition: a review. Mol. Cell. Endocrinol 349, 62–67. [DOI] [PubMed] [Google Scholar]
- Padilla SL, Perez JG, Ben-Hamo M, Johnson CW, Sanchez REA, Bussi IL, Palmiter RD, Horacio O, 2019. Kisspeptin neurons in the arcuate nucleus of the hypothalamus orchestrate circadian rhythms and metabolism. Curr. Biol 29, 592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa KI, Gazouli M, Anastasiou E, Iliodromiti Z, Antsaklis A, Anagnou NP, 2013. Circadian clock gene expression is impaired in gestational diabetes mellitus. Gynecol. Endocrinol 29, 331–335. [DOI] [PubMed] [Google Scholar]
- Paul S, Brown T, 2019. Direct effects of the light environment on daily neuroendocrine control. J. Endocrinol 1. [DOI] [PubMed] [Google Scholar]
- Ratajczak CK, Boehle KL, Muglia LJ, 2009. Impaired steroidogenesis and implantation failure in Bmal1 −/−mice. Endocrinology 150, 1879–1885. 10.1210/en.2008-1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, 1983. Time of birth in the rat is gated to the daily light cycle by a circadian mechanism. Pediatr Res 17, 154A. [Google Scholar]
- Robinson JE, Follett BK, 1982. Photoperiodism in Japanese quail: the termination of seasonal breeding by photorefractoriness. Proc. R. Soc. London. Ser. B. Biol. Sci 215, 95–116. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Karsch FJ, 1984. Refractoriness to inductive day lengths terminates the breeding season of the Suffolk ewe. Biol. Reprod 31, 656–663. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Hollander SJ, Adler NT, 1987. Effects of pregnancy and parturition on free-running circadian activity rhythms in the rat. Chronobiol. Int 4, 183–187. [DOI] [PubMed] [Google Scholar]
- Rugh R, 1968. The mouse. Its reproduction and development, mouse. Its Reprod. Dev
- Rutter J, Reick M, McKnight SL, 2002. Metabolism and the control of circadian rhythms. Annu. Rev. Biochem 71, 307–331. [DOI] [PubMed] [Google Scholar]
- School TO, Hediger ML, Schall JI, Ances IG, Smith WK, 1995. Gestational weight gain, pregnancy outcome, and postpartum weight retention. Obstet. Gynecol 86, 423–427. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Zimmerman P, 1991. Lesions of the suprachiasmatic nucleus disrupt circadian locomotor rhythms in the mouse. Physiol. Behav 49, 1283–1287. [DOI] [PubMed] [Google Scholar]
- Sellix MT, 2013. Clocks underneath : the role of peripheral clocks in the timing of female reproductive physiology 4, 1–6. 10.3389/fendo.2013.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellix MT, Menaker M, 2010. Circadian clocks in the ovary. Trends Endocrinol. Metab 21, 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Hoffmann HM, 2020. Role of core circadian clock genes in hormone release and target tissue sensitivity in the reproductive axis. Mol. Cell. Endocrinol 10.1016/j.mce.2019.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneaux V, Bahougne T, Angelopoulou E, 2017. Daily rhythms count for female fertility. Best Pract. Res. Clin. Endocrinol. Metab 31, 505–519. [DOI] [PubMed] [Google Scholar]
- Smarr BL, Gile JJ, De La Iglesia HO, 2013. Oestrogen-independent circadian clock gene expression in the anteroventral periventricular nucleus in female rats: Possible role as an integrator for circadian and ovarian signals timing the luteinising hormone surge. J. Neuroendocrinol 25, 1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK, Zucker I, 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci 69, 1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens SBZ, Tolson KP, Rouse ML Jr, Poling MC, Hashimoto-Partyka MK, Mellon PL, Kauffman AS, 2015. Absent progesterone signaling in kisspeptin neurons disrupts the LH surge and impairs fertility in female mice. Endocrinology 156, 3091–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, Menaker M, 1980. Interaction of estradiol and progesterone: effects on circadian locomotor rhythm of female golden hamsters. Am. J. Physiol. Integr. Comp. Physiol 239, R497–R504. [DOI] [PubMed] [Google Scholar]
- Virgo BB, Bellward GD, 1974. Serum Progesterone Levels in the Pregnant and Postpartum Laboratory Mouse. Endocrinology 95, 1486–1490. 10.1210/endo-95-5-1486 [DOI] [PubMed] [Google Scholar]
- Wang D, Li N, Tian L, Ren F, Li Z, Chen Y, Liu L, Hu X, Zhang X, Song Y, 2019. Dynamic expressions of hypothalamic genes regulate seasonal breeding in a natural rodent population. Mol. Ecol [DOI] [PubMed] [Google Scholar]
- Welschen R, Osman P, Dullaart J, De Greef WJ, Uilenbroek JTJ, De Jong FH, 1975. Levels of follicle-stimulating hormone, luteinizing hormone, oestradiol-17β and progesterone, and follicular growth in the pseudopregnant rat. J. Endocrinol 64, 37–47. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Noguchi T, 2012. Cellular bioluminescence imaging. Cold Spring Harb. Protoc 7, 852–866. 10.1101/pdb.top070607 [DOI] [PubMed] [Google Scholar]
- Wharfe MD, Mark PJ, Wyrwoll CS, Smith JT, Yap C, Clarke MW, Waddell BJ, 2016. Pregnancy-induced adaptations of the central circadian clock and maternal glucocorticoids. J. Endocrinol 228, 135–147. 10.1530/JOE-15-0405 [DOI] [PubMed] [Google Scholar]
- Williams WP III, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ, 2010. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology 152, 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-P, DeMayo FJ, 2017. Progesterone receptor signaling in uterine myometrial physiology and preterm birth, in: Current Topics in Developmental Biology. Elsevier, pp. 171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-H, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong H-K, Oh WJ, Yoo OJ, 2004. PERIOD2:: LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci 101, 5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Sellix M, Pezuk P, Menaker M, 2009. Timing of the ovarian circadian clock is regulated by gonadotropins. Endocrinology. 10.1210/en.2008-1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakar T, Hertelendy F, 2007. Progesterone withdrawal: key to parturition. Am. J. Obstet. Gynecol 196, 289–296. 10.1016/j.ajog.2006.09.005 [DOI] [PubMed] [Google Scholar]
- Zhang WX, Chen SY, Liu C, 2016. Regulation of reproduction by the circadian rhythms. Sheng li xue bao[Acta Physiol. Sin 68, 799–808. [PubMed] [Google Scholar]
- Zornoza-Moreno M, Fuentes-Hernández S, Prieto-Sánchez MT, Blanco JE, Pagán A, Rol M, Parrilla JJ, Madrid JA, Sánchez-Solis M, Larqué E, 2013. Influence of gestational diabetes on circadian rhythms of children and their association with fetal adiposity. Diabetes. Metab. Res. Rev 29, 483–491. [DOI] [PubMed] [Google Scholar]