Abstract
Objectives:
To describe the pharmaceutical management of sedation, analgesia, and neuromuscular blockade medications administered to children in Intensive Care Units (ICUs).
Design:
A retrospective analysis using data extracted from the national database Health Facts® (Cerner Corporation, Kansas City, MO).
Setting:
161 ICUs in the United States with pediatric admissions.
Patients:
Children in ICUs receiving medications from 2009 to 2016.
Exposure/Intervention:
Frequency and duration of administration of sedation, analgesia, and neuromuscular blockade medications.
Measurements and Main Results:
Of 66,443 patients with a median age of 1.3 years (IQR 0-14.5); 63.3% (n = 42,070) received non-opioid analgesic, opioid analgesic, sedative and/or neuromuscular blockade medications consisting of 83 different agents. Opioid and non-opioid analgesics were dispensed to 58.4% (n = 38,776) of which non-opioid analgesics were prescribed to 67.4% (n = 26149). Median duration of opioid analgesic administration was 32 hours (IQR 7-92). Sedatives were dispensed to 39.8%% (n = 26,441) for a median duration of 23 hours (IQR 3-84), of which benzodiazepines were most common (73.4%, n =19,426). Neuromuscular blocking agents were dispensed to 17.3% (n =11,517) for a median duration of 2 hours (IQR 1-15).Younger age was associated with longer durations in all medication classes. A greater proportion of operative patients received these medication classes for a longer duration than non-operative patients. A greater proportion of patients with musculoskeletal and hematologic/oncologic diseases received these medication classes.
Conclusion:
Analgesic, sedative and neuromuscular blocking medications were prescribed to 63.3% of children in ICUs. The durations of opioid analgesic and sedative medication administration found in this study can be associated with known complications, including tolerance and withdrawal. Several medications dispensed to pediatric patients in this analysis are in conflict with FDA warnings, suggesting that that there is potential risk in current sedation and analgesia practice that could be reduced with practice changes to improve efficacy and minimize risks.
Keywords: Sedation, Analgesia, Neuromuscular Blockade, Pediatrics, Pediatric Critical Care
Introduction:
Numerous factors determine the practice of sedation, analgesia, and neuromuscular blockade. In pediatric intensive care, age-related differences in perception and expression of pain and anxiety, individual needs, therapeutic goals and the interaction of critical illness physiology with medications result in diverse practice patterns that vary according to physician preferences, training experience, and geography.1,2 Additionally, the treatment of pain and alleviation of anxiety continue to undergo an evolution. For example, historical perspectives from the 1960’s and 1970’s suggested that young children could not localize or remember painful stimuli, and therefore did not require analgesia to the same degree as adults.3,4,5 Current practices focus on maximizing pain relief and treating anxiety consistent with a universal recognition that children experience pain and anxiety.6
Medications used for pain, sedation and neuromuscular blockade may alter risk profiles for hemodynamic and respiratory instability affecting the course of critical illness, including the duration of mechanical ventilation, unconsciousness, and dependency. These medications are associated with serious critical care complications, including withdrawal, delirium and immobility that can contribute to post-intensive care myopathy.7–9 Inadequate treatment of pain can lead to a heightened inflammatory response as well as an exaggerated response to future painful stimuli. 6,10–15 Pain and sedation medications can be useful to pre inadvertent removal of invasive devices such as endotracheal tubes, and central venous catheters, and to maintain ventilator synchrony.
Data regarding the practice of administering sedation, analgesia and neuromuscular blockade to pediatric patients during the course of critical illness has been primarily derived from surveys, small trials, and two multi-institutional trials designed to determine the efficacy and safety of protocol-driven, nurse-directed sedation practice.1,16–20 Evidence-based guidelines for sedation, analgesia and neuromuscular blockade administration in pediatrics are lacking.21,22 Our aim was to describe the practice patterns and pharmaceutical management of sedation, analgesia, and neuromuscular blockade medications administered to children in ICUs utilizing an electronic health record (EHR)-derived national database.
Methods:
Database
The dataset was derived from the Health Facts® (Cerner Corporation, Kansas City, MO) database that collects comprehensive clinical data on patient encounters from hospitals in the United States with a Cerner data use agreement. Health Facts® is a voluntary program to facilitate the capture of patient data from the EHR since 2000 from more than 500 hospitals. Health Facts® provides episodic and longitudinal, date and time-stamped data from affiliated patient care locations including admission and demographic data, care-setting characteristics, laboratory results, medication data derived from pharmacy records, microbiology results, diagnostic and procedure codes, vital signs, respiratory data, and hospital outcome, providing a temporal relationship between treatment patterns and clinical information. Cerner Corporation has established Health Insurance Portability and Accountability Act compliance operating policies to establish de-identification of Health Facts®.
The Health Fact’s® database is representative of the nation, inclusive of academic and non-academic hospitals of varied sizes and locations.23,24,25,26 The varied patient population in the Health Fact’s® database has been used to develop major advances in clinical medicine23,24,25,26, including the APACHE score.27 Data is captured directly from the EHR, and therefore required cleaning prior to analysis. Data cleaning included eliminating data inconsistent with valid entries, including but not limited to duplicate or null values, admission times of zero, spurious or absent age values, and/or lab or vital sign values outside of the range of physiologic possibility. Notably, not all data were available for all patients. Further details about data processing are in Appendix 1.
All children in ICUs who received medications during their ICU admission were included in this analysis. Pediatric patients were defined as < 22 years of age at time of admission, consistent with the American Academy of Pediatrics’ definition of a pediatric patient and patient’s routinely cared for in pediatric ICUs.28 Pediatric age cohorts used for age-related analyses were based on the World Health Organization position paper on age categories for the administration of medications.29 Children cared for in ICUs were identified by associating the ICU care setting with laboratory, medication or discharge orders. This cohort included patients from161 hospitals. If a patient had more than 1 ICU admission, medication information was combined and analyzed at the patient level. Neonatal intensive care unit patients were excluded. Details of the definitions, identification of ICU patients, and cleansing and reliability checks are in Appendix 1. The dates of the dataset used for this analysis were from January 2009 to June 2016.
Data Elements
Descriptive data consisted of age, gender, diagnostic information, and procedures. The primary diagnosis for each patient was categorized into 17 diagnostic groups from the ICD9-10 classifications.30,31 When the primary diagnostic group was Diseases Originating in Childbirth, the Perinatal Period, or Congenital Diseases, the secondary and tertiary diagnostic codes were evaluated to classify the primary system of physiological dysfunction/reason for admission (Table 1). Positive pressure ventilation and operations organized by organ system were identified from procedure codes (ICD-9, ICD-10-PCS, HCPCS, and CPT-4), detailed in Table 1.
Table 1.
Characteristics for Children in ICUs Receiving Medications (n = 66,443)
| Characteristics | Patients who Received Non-Opioid Analgesic, Opioid Analgesic, Sedative and NMB (n=42,070) | Patients who did not receive Non-Opioid Analgesic, Opioid Analgesic, Sedative and NMB (n = 24,373) | Significance level (1) |
|---|---|---|---|
| Age in Years (Median (IQR) | 5.3 (0.2-16.9) | 0.003 (0.001-2.4) | p < 0.005 |
| Age Groups (N (%)) | |||
| < 1 months | 8887 (21.1) | 16158 (66.3) | p < 0.005 |
| >= 1mo- < 2 years | 7868 (18.7) | 1923 (7.9) | p < 0.005 |
| >= 2 - <6 years | 4738 (11.3) | 1167 (4.8) | p < 0.005 |
| >= 6 - <13 years | 5301 (12.6) | 1562 (6.4) | p < 0.005 |
| >= 13 - <22years | 15276 (36.3) | 3563 (14.6) | p < 0.005 |
| Female (N (%)) | 19195 (45.6) | 11628 (47.7) | p < 0.005 |
| Race (N, (%)) | |||
| Caucasian | 22216 (52.8) | 11885 (48.8) | p < 0.005 |
| African American | 12191 (29.0) | 7248 (29.7) | p < 0.005 |
| Unknown/Other | 7663 (19.4) | 5240 (21.5) | p < 0.005 |
| ICU Length of Stay (median (IQR) | 2.8 (1.3-7.5) | 2.9 (1.2-7.1) | p < 0.005 |
| Hospital Mortality (N (%)) | 1335 (3.2) | 185 (0.8) | p < 0.005 |
| Diagnostic Categories (N (%)) (2) | |||
| Respiratory System | 4808 (19.3) | 1798 (12.3) | p < 0.005 |
| Injury and Poisoning | 3845 (15.5) | 932 (6.4) | p < 0.005 |
| Endocrine, Nutrition, and Immunity | 2617 (10.5) | 2130 (14.6) | p < 0.005 |
| Central Nervous System | 2640 (10.6) | 578 (3.4) | p < 0.005 |
| Circulatory System | 1722 (6.9) | 764 (5.2) | p < 0.005 |
| Gastrointestinal System | 1585 (6.4) | 539 (3.7) | p < 0.005 |
| Infection and Parasitic Diseases | 1775 (7.1) | 597 (4.1) | p < 0.005 |
| Hematology and Oncology | 1505 (6.1) | 380 (2.6) | p < 0.005 |
| Genitourinary System | 643 (2.6) | 259 (1.8) | p < 0.005 |
| Musculoskeletal System | 740 (2.9) | 74 (0.5) | p < 0.005 |
| Dermatological System | 335 (1.3) | 444 (3.0) | p < 0.005 |
| Psychiatric | 498 (2.0) | 218 (1.5) | p < 0.005 |
| Diseases Not Otherwise Specified | 2172 (8.7) | 5916 (40.4) | p < 0.005 |
| Operations by Organ System (N (%)) (3) | |||
| Cardiovascular Operations | 3604 (8.6) | 449 (1.8) | p < 0.005 |
| Digestive System Operations | 1482 (3.5) | 57 (0.2) | p < 0.005 |
| Respiratory Operations | 1139 (2.7) | 51 (0.2) | p < 0.005 |
| Musculoskeletal Operations | 826 (2.0) | 21 (0.1) | p < 0.005 |
| Nervous System Operations | 692 (1.6) | 15 (0.1) | p < 0.005 |
| Integumentary Operations | 346 (0.8) | 16 (0.1) | p < 0.005 |
| Otolaryngology Operations | 293 (0.7) | 15 (0.1) | p < 0.005 |
| Genitourinary Operations | 253 (0.6) | 26 (0.1) | p < 0.005 |
| Lymphatic Operations | 143 (0.3) | 9 (0.0) | p < 0.005 |
| Ophthalmology Operations | 95 (0.2) | 5 (0.0) | p < 0.005 |
| Endocrine Operations | 83 (0.2) | 7 (0.0) | p < 0.005 |
| Positive Pressure Ventilation (N, (%))(3) | 7182 (17.1) | 2452 (10.1) | p < 0.005 |
Abbreviations: ICU =Intensive Care Unit; NMB = Neuro Muscular Blockade; IQR = Interquartile range.
Univariate comparisons.
Diagnostic Categories were available for 39,514 patients, and missing for 26,929. The primary diagnostic code was used for categorization (see methods). The percentages are shown for the sample with diagnostic information.
Procedures were available from 23,935 patients, and unavailable in 42,508 patients. The percentages are shown are from the total sample. Procedure codes indicating positive pressure ventilation including: Continuous Invasive Mechanical Ventilation, Non-Invasive Mechanical Ventilation, Continuous positive airway pressure ventilation, Assistance with Respiratory Ventilation, Intermittent Positive Airway Pressure
Medication data included generic and brand names, and the national drug code (NDC). The times a medication was ordered and discontinued were determined from pharmacy records. If multiple doses of a medication or medication class occurred concomitantly, the overall length of administration was determined by the start and end time. If one dose of medication or medication class was ordered in an hour period of time, the patient was considered to have received one hour of medication. Reliable per kilogram dosing information was not consistently available. Therefore dosing was not evaluated. Each medication was linked to Cerner Multum™, an industry standard for medication classification, using the NDC.32,33 A total of 96% of the NDC codes were classified into Multum™ classes, and the remaining medications were classified by the authors (AP, MP, JC) into the existing Multum™ classes. The classes analyzed for this assessment included non-opioid analgesics, opioid analgesics, sedatives, general intravenous (IV) anesthetics, and neuromuscular blockers. Propofol and ketamine, which are classified as anesthetic agents, were analyzed as sedative medications because of their predominant clinical use in the ICU.
Statistical Analysis:
Patient, medication, diagnostic and procedure data were summarized using descriptive statistics. Continuous variables are expressed as medians with 25-75th percentile interquartile ranges (IQR). Data for medication classes and generic medications included the number of patients, age distributions and durations of administration during their ICU admission. The Wilcoxon rank sum test was used to compare continuous measures and the chi-squared test was used to compare categorical measures. The Kruskal-Wallis test was used for comparing multiple groups. When the Kruskal-Wallis test was significant, the analysis was followed with paired comparisons using the Wilcoxon rank sum test. Durations of medication administration were controlled for ICU length of stay by determining the proportion of time each patient received each medication class during their ICU admission. The software utilized was R version 3.5.1.34,35,36,37,38,39 All statistical analyses were performed by E.T.R.
Results
At total of 66,443 children utilizing 518,711 days of ICU care had medications dispensed to them while in the ICU. Table 1 describes the patient characteristics between patients who received sedation, analgesia and neuromuscular blockade and those who did not. Overall, the median age was 1.3 years (IQR 0 – 14.5 years) and the predominant age group was < 1 month (37.7%) with 12.5% of patients (n = 8,279) between 18 and <22 years old. Median ICU length of stay was 2.8 days (IQR 1.2 – 7.3 days). Overall hospital mortality rate was 2.3%. A total of 59.5% of patients (n = 39,551) had diagnostic information with the predominant primary diagnoses involving the respiratory system and injury and poisonings. Of the 9,693 patients with operative data, cardiovascular, digestive, and respiratory system operations were the most common. Positive pressure ventilation was received by 14.5% (n=9,634).
A total of 63.3% (n=42,070) of ICU patients received non-opioid analgesic, opioid analgesic, sedative and/or neuromuscular blockade medications consisting of 83 different generic medications (Table 2). Figure 1 is a Venn diagram of the overlap of usage among the medication classes. A total of 34.1% of patients received medications in only one medication class, with non-opioid analgesic medications being the most common. The second and third largest groups of patients receiving combinations of medication classes were patients receiving all four classes (18.6%) and three classes (16.2%).
Table 2.
Frequency and Duration of the Most Common Non-Opioid Analgesic, Opioid Analgesic, Sedative and Neuromuscular Blockade Medications (N = 66,443)
| Medication Class | Overall N (%) Duration (hours, (IQR)) | Age <1mo N (%) Duration (hours, (IQR)) | Age 1mo - <2 yrs N (%) Duration (hours, (IQR)) | Age 2 yrs - <6 yrs N (%) Duration (hours, (IQR)) | Age 6 yrs - <13 yrs N (%) Duration (hours, (IQR)) | Age 13 yrs - <22 yrs N (%) Duration (hours, (IQR)) |
|---|---|---|---|---|---|---|
| Non-Opioid Analgesic1 | 38776 (58.4) | 3783 (15.1) | 5909 (60.4) | 3466 (58.7) | 3741 (54.5) | 9876 (52.4) |
| 26 (7-69) | 36 (10-98) | 31 (8-86) | 23 (5-61) | 24 (6-60) | 23 (6-52) | |
| Acetaminophen | 27397 (41.2) | 4399 (17.6) | 6258 (63.9) | 3589 (60.8) | 3843 (56.0) | 9308 (49.4) |
| 27 (10-73) | 16 (0-73.5) | 25 (5-76) | 19 (2-54) | 19 (2-53) | 20 (3-49) | |
| Ibuprofen | 5436 (8.2) | 340 (1.4) | 1551 (15.8) | 1313 (22.2) | 1254 (18.3) | 2374 (12.6) |
| 30 (10-76)) | 29 (7-50) | 16 (1-56) | 11 (1-45) | 11 (1-42) | 7 (1-33) | |
| Ketorolac | 2728 (4.1) | 49 (0.2) | 353 (3.6) | 429 (7.3) | 602 (8.8) | 2000 (10.6) |
| 23 (7-45) | 1 (0-2) | 11 (1-27) | 8 (1-26) | 9 (1-28) | 2 (1-22) | |
| Aspirin | 1553 (2.3) | 215 (0.9) | 406 (3.6) | 274 (4.6) | 260 (23.5) | 798 (42.4) |
| 45 (15-101) | 74 (14-346) | 34 (6-92) | 26 (3-70) | 23.5 (3.8-57) | 18.5 (2-48) | |
| Opioid Analgesic2 | 26149 (39.4) | 5295 (21.1) | 4047 (41.3) | 2192 (37.1) | 2872 (41.8) | 9931 (52.7) |
| 32 (7-92) | 62 (10-183) | 42 (9-119 | 24 (5-68) | 25 (5.8-65.3) | 23 (5-57) | |
| Fentanyl | 17428 (26.2) | 4560 (18.2) | 3508 (44.6) | 1613 (27.3) | 2032 (29.6) | 5715 (30.3) |
| 15 (2-81) | 19 (1-124) | 6 (1-74.3) | 2 (1-28) | 2 (0-19) | 2 (1-16) | |
| Morphine | 17142 (25.8) | 3553 (14.2) | 2901 (36.9) | 1717 (29.1) | 2413 (35.2) | 6558 (34.8) |
| 26 (6-69) | 38 (3-119) | 21 (2-61) | 14 (1-43) | 17 (2-46) | 15 (2-43) | |
| Hydromorphone | 3731 (5.6) | 92 (0.4) | 127 (1.6) | 129 (2.2) | 312 (4.5) | 3071 (16.3) |
| 19 (2-51) | 47 (1-345) | 19 (1-79.5) | 4 (1-31) | 3.5 (1-27) | 7 (1-35) | |
| Acetaminophen-Hydrocodone | 3159 (4.8) | 40 (0.2) | 295 (3.7) | 314 (5.3) | 468 (6.8) | 2502 (13.3) |
| 32 (16-58) | 16 (0-62.3) | 24 (6-44.5) | 12 (1-32) | 9 (0-30.3) | 9 (1-29) | |
| Oxycodone | 2722 (4.1) | 35 (0.1) | 234 (3.0) | 204 (3.5) | 414 (6.0) | 1835 (9.7) |
| 26 (6-69) | 30 (1-56.5) | 21.5 (1-64) | 10 (1-40.3) | 13 (1-41) | 15 (1-44.5) | |
| Acetaminophen-Oxycodone | 1652 (2.5) | 32 (0.2) | 51 (0.7) | 57 (1.0) | 166 (2.4) | 1695 (9.0) |
| 27 (9-62) | 1 (0-10) | 1 (0-11.5) | 4 (1-15) | 4 (1-25) | 9 (1-29) | |
| Acetaminophen-Codeine | 1383 (2.1) | 56 (0.2) | 449 (5.7) | 448 (7.6) | 399 (5.8) | 392 (2.1) |
| 24 (8-51) | 26 (7-71.5) | 15 (1-41) | 8 (1-28.3) | 6 (1-28) | 6 (0-26) | |
| Meperidine | 1230 (1.9) | 11 (0.0) | 35 (0.4) | 51 (0.9) | 97 (1.4) | 1405 (7.5) |
| 11 (2-40) | 1 (0-14) | 1 (0-6) | 1 (0-2) | 1 (0-4) | 1 (0-6) | |
| Methadone | 1148 (1.7) | 305 (1.2) | 472 (6.0) | 145 (2.5) | 90 (1.3) | 136 (0.7) |
| 73 (27-182) | 92 (30-236) | 57 (17-154) | 47 (12-98) | 57 (16-140) | 42 (6.3-143) | |
| Sedatives3 | 26441 (39.8) | 3923 (15.7) | 4026 (41.1) | 2328 (39.4) | 2723 (39.7) | 8225 (43.7) |
| 23 (3-84) | 57 (5-202) | 30 (5-120) | 16 (2-55) | 15 (3-44) | 15 (3-44) | |
| Midazolam | 14395 (21.7) | 3070 (12.3) | 3107 (31.7) | 1702 (28.8) | 1901 (27.7) | 4615 (24.5) |
| 7 (2-69) | 10 (1-114) | 7 (1-77) | 2 (1-22) | 1 (0-12) | 1 (0-6) | |
| Lorazepam | 9362 (14.1)) | 1984 (7.9) | 2222 (22.7) | 1095 (18.5) | 1197 (17.4) | 3749 (19.9) |
| 23 (3-91) | 28 (1-150) | 22 (2-100) | 11 (1-45) | 9 (1-45) | 10 (1-41) | |
| Propofol | 8792 (13.2) | 1083 (4.3) | 1478 (15.1) | 1042 (17.6) | `1422 (20.7) | 3767 (20.0) |
| 2 (1-15) | 1 (0-1) | 1 (0-2) | 1 (0-5) | 1 (0-4.8) | 2 (1-17) | |
| Diphenhydramine | 6896 (10.4) | 176 (0.1) | 760 (7.8) | 816 (13.8) | 1208 (17.6) | 3936 (20.9) |
| 15 (3-46) | 7 (0.8-66) | 5 (1-36) | 5 (1-40.5) | 9 (1-42) | 7 (1-30) | |
| Ketamine | 2800 (4.2) | 381 (1.5) | 754 (7.7) | 474 (8.0) | 490 (7.1) | 701 (3.7) |
| 2 (1-6) | 1 (0-2) | 1 (0-4) | 1 (1-4) | 1 (0-4) | 1 (0-3) | |
| Phenobarbital | 2622 (3.9) | 1179 (4.7) | 743 (7.6) | 265 (4.5) | 237 (3.5) | 198 (1.1) |
| 57 (16-188) | 74 (9-233) | 31 (3-123) | 17 (2-52) | 34 (9-84) | 26.5 (2-90) | |
| Diazepam | 2188 (3.3) | 37 (0.1) | 235 (2.4) | 293 (5.0) | 542 (7.9) | 1081 (5.7) |
| 37 (11-90) | 6 (1-72) | 4 (1-39) | 6 (1-36) | 18 (2-45) | 17 9(1-42) | |
| Dexmedetomidine | 2003 (3.0) | 213 (0.9) | 607 (6.2) | 313 (5.3) | 329 (4.8) | 541 (2.9) |
| 24 (7-52) | 5 (0-44) | 23 (1-50) | 14 (1-33) | 6 (1-30) | 10 (1-32) | |
| Neuromuscular Blocking Agents | 11517 (17.3) | 2189 (8.7) | 2287 (23.4) | 909 (15.4) | 1005 (14.6) | 2440 (13.0) |
| 2 (1-15) | 3 (1-27) | 4 (1-34) | 2 (1-18) | 2 (1-7) | 2 (1-4) | |
| Rocuronium | 6209 (9.3) | 1270 (5.1) | 1555 (15.9) | 600 (10.2) | 790 (1.2) | 1994 (10.6) |
| 1 (1-2) | 1 (0-1) | 1 (1-2) | 1 (0-2) | 2 (0-3) | 1 (0-2) | |
| Vecuronium | 5897 (8.9) | 1757 (7.0) | 1675 (17.1) | 642 (10.9) | 650 (9.5) | 1173 (6.2) |
| 3 (1-28) | 3 (1-27) | 3 (1-30) | 2 (1-20) | 4 (1-49.5) | 1 (0-4) | |
| Succinylcholine | 2099 (3.2) | 209 (0.8) | 321 (3.3) | 19 (3.2) | 239 (3.5) | 1139 (6.0) |
| 1 (1-2) | 1 (0-1) | 1 (0-1) | 1 (1-1) | 1 (1-7.3) | 1 (0-1) | |
| Pancuronium | 758 (1.1) | 243 (1.0) | 240 (2.5) | 90 (1.5) | 76 (1.1) | 109 (0.6) |
| 1 (1-16) | 1 (1-18) | 1 (1-20) | 1 (1-3) | 1 (0-1) | 1 (1-2) | |
| Cisatracurium | 730 (1.1) | 151 (0.6) | 211 (2.1) | 79 (1.3) | 95 (1.4) | 194 (1.0) |
| 18.5 (1-68) | 1 (0-3) | 16 (2-59) | 10 (1-73.5) | 1 (0-2) | 1 (1-10.3) | |
| Atracurium | 182 (0.3) | 9 (0.0) | 40 (0.4) | 10 (0.2) | 21 (0.3) | 102 (0.5) |
| 3 (1-45) | 1 (1-2) | 31 (1-60.3) | 2 (0.3-3.8) | 1 (1-7) | 2 (1-5) |
Other medications included naproxen (n=452), Indomethacin (n=56), sumatriptan (n=30), meloxicam (n=9), apap/dichloralphenazone/isometheptene (3), caffeine-ergotamine (n=2), caffeine-ergotamine (n=2), acetaminophen/butalbital/caffeine (1), choline salicylate-magnesium salicylate (1), diclofenac (n=1), nabumetone (n=1), naratriptan (n=1), piroxicam (n=1), rizatriptan (n=1), zolmitriptan (n=1), and dihydroergotamine (1).
Other medications included nalbuphine (n=1047), butorphanol (n=593), remifentanil (n=550), alfentanil (n=406), sufentanil (n=309), tramadol (n=270), codeine (n=109), belladonna-opium (33), bupivacaine-fentanyl (30), tapentadol (n=25), opium (n=11), buprenorphine-naloxone (11), fentanyl-ropivicaine (7), buprenorphine (n=6), acetaminophen-tramadol (4), hydrocodone-ibuprofen (2), and meperidine-promethazine (2), aspirin-oxycodone (1), and bupivacaine-hydromorphone (1).
Other medications included zolpidem (n=1229), chloral hydrate (n=1195), clonazepam, (n=715), pentobarbital, (n=470) Hydroxyzine (n=438), alprazolam (n=369), temazepam (n=207), and clobazam (n=151).
Figure 1:
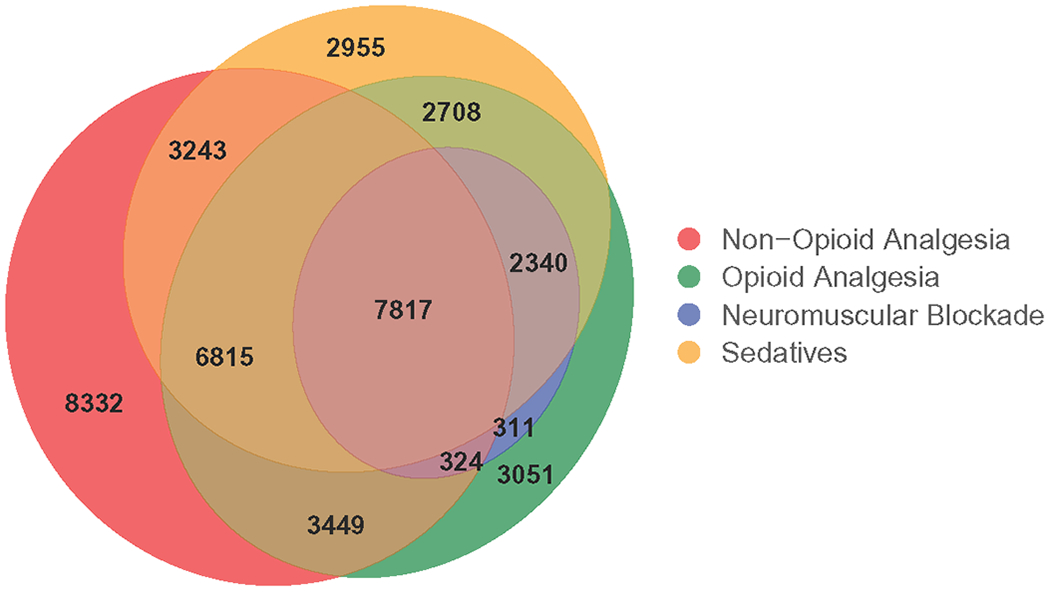
Venn Diagram of Patients Receiving Non-Opioid Analgesic, Opioid Analgesic, Sedative and Neuromuscular Blockade Medications (N = 42,070)
Non-opioid and opioid analgesic medications were dispensed to 58.4% (n=38,776) of patients with 70.7% receiving non-opioid acetaminophen preparations, 67.4% receiving opioid-based medications, and 25.7% receiving non-steroidal anti-inflammatory agents (Table 2). Patients less than 1 month received the smallest proportion of opioid (21.1%) and non-opioid (15.1%) analgesic medications. Patients >13 years received the highest percentage of opioid analgesics (52.7%). Of the patients receiving opioid analgesic medications, fentanyl was most commonly dispensed (63.0%, n=17,428) followed by morphine (60.7%, n=17,142) and hydromorphone (12.1%, n=3731). Median durations of administration were less than 2 days for all opioid analgesics except methadone. However, a substantial number of patients received opioid analgesics for relatively long periods (75th percentile = 92 hours). For example, the median duration of administration of fentanyl was 15 hours but the IQR was 2 hours to 81 hours. Methadone was prescribed for a longer duration of administration than the shorter acting opioid analgesics (median 73 hours; IQR 27 −182 hours).
Sedatives (Table 2) were dispensed to 39.8% (n=26,441) of ICU patients. Benzodiazepines were the most commonly administered sedative class (73.5% of patients receiving sedatives, n=19,426), followed by ketamine and propofol (39.6%, n =10,477), and miscellaneous sedatives inclusive of antihistamines and dexmedetomidine (28.1%, n =7,455). Patients less than 1 month received the lowest proportion of sedative medications (15.7%), whereas those13 years to 21 years received the highest proportion (43.7%). Midazolam was the most commonly dispensed sedative (52.3% of patients receiving sedatives, n = 14,395), followed by lorazepam (35.4%, n=9,362) and propofol (29.1%, n=8,792). Similar to opioid analgesic medications, the median duration of administration for sedatives was less than 2 days with wide variability among the specific agents. For example, the median duration of use of lorazepam was 23 hours (IQR 3- 91 hours) compared to propofol with a median of 2 hours (IQR 1-15 hours).
Neuromuscular blocking medications were dispensed to 17.3% (n =11,517) of ICU patients consisting of six generic agents. Rocuronium was the most common agent (53.9% of patients receiving neuromuscular blocking medications, n=6,209), followed by vecuronium (51.2%, n=5,897) and succinylcholine (18.2%, n=2099). The smallest proportion of neuromuscular blockade medications were dispensed to patients less than 1 month (8.7%) and the highest proportion were dispensed to patients between the ages of 1month to 2 years. Unlike analgesics and sedatives, neuromuscular blocking medications were administered for short periods of time with less variability. Most agents were used for a median of 3 hours or less. Cisatracurium had the longest duration of administration (median 18.5 hours) and the most variability of the neuromuscular blocking medications (IQR 1-68 hours).
Of the 14.5% (n = 9,634) of patients treated with positive pressure ventilation, all received an opioid analgesic for a median duration of 41 hours (IQR 9-111 hours) with fentanyl and morphine being the most common. A total of 95% (9,152) received a sedative medication for a median duration of 29 hours (IQR 4-107 hours), with midazolam and lorazepam being the most common. Neuromuscular blocking agents were dispensed for 53.3% (n = 5,134) with a median duration of 2 hours (IQR 1-15 hours) (Table 4).
Table 4:
Differences in the Duration of Administration of Non-Opioid Analgesic, Opioid Analgesic, Sedative and Neuromuscular Blockade Medications to Children in ICUs by Age.
| Variable | Age <1mo | Age 1mo - <2 yrs | Age 2 yrs - <6 yrs | Age 6 yrs - <13 yrs | Age 13 yrs - <22 yrs | Significance Level1 |
|---|---|---|---|---|---|---|
| Median ICU LOS (IQR days) | 4.8 (2.0 – 11.8) | 1.6 (0.3 – 4.9) | 0.9 (0.2 – 2.3) | 1.1 (0.3 – 2.5) | 1.2 (0.4-2.4) | p < .005 |
| Median Hospital LOS (IQR) days) | 8.1 (3.8 - 21.3) | 2.8 (1.50 - 5.9) | 5.0 (2.3-13.0) | 3 (1.5 - 6.4) | 3.3 (1.7-6.8) | p < .005 |
| Death Rate (95% CI) | 2.6% (2.6, 2.7) | 2.7% (2.6, 2.9) | 2.2% (1.9, 2.4) | 1.6% (1.4, 1.8) | 1.9% (1.9, 2.0) | NS |
| Median Sedative Duration (IQR, hours) | 57 (5-202) | 30 (5-120) | 16 (2-55) | 19 (3-59) | 15 (3-44) | p < .005 |
| Median Opioid Analgesic Duration (IQR, hours) | 62 (10-183) | 42 (9-119) | 24 (5-68) | 25 (5.8-65.3) | 23 (5-57) | p < .005 |
| Median Neuromuscular Blockade Duration (IQR, hours) | 3 (1-27) | 4 (1-34) | 2 (1-18) | 2 (1-7) | 2 (1-4) | p < .005 |
Kruskal-Wallis test
Pairwise comparisons of durations of administration are in Supplemental Table 3.
Operative patients (n=9692) received proportionally more non-opioid analgesic, opioid analgesic, sedative and neuromuscular blockade medications with longer durations of administration than non-operative patients (Figure 2, Table 3). Operative patients received a greater proportion of opioid analgesic medications (60.0%, n=5,411) than non-opioid analgesic medications (48.0%, n=4,657). This pattern was not observed in the non-operative patients where non-opioid analgesic medications (60.1%, n=34,119) were prescribed more frequently than opioid analgesic medications (36.5%, n=20,738) (Table 3).
Figure 2.
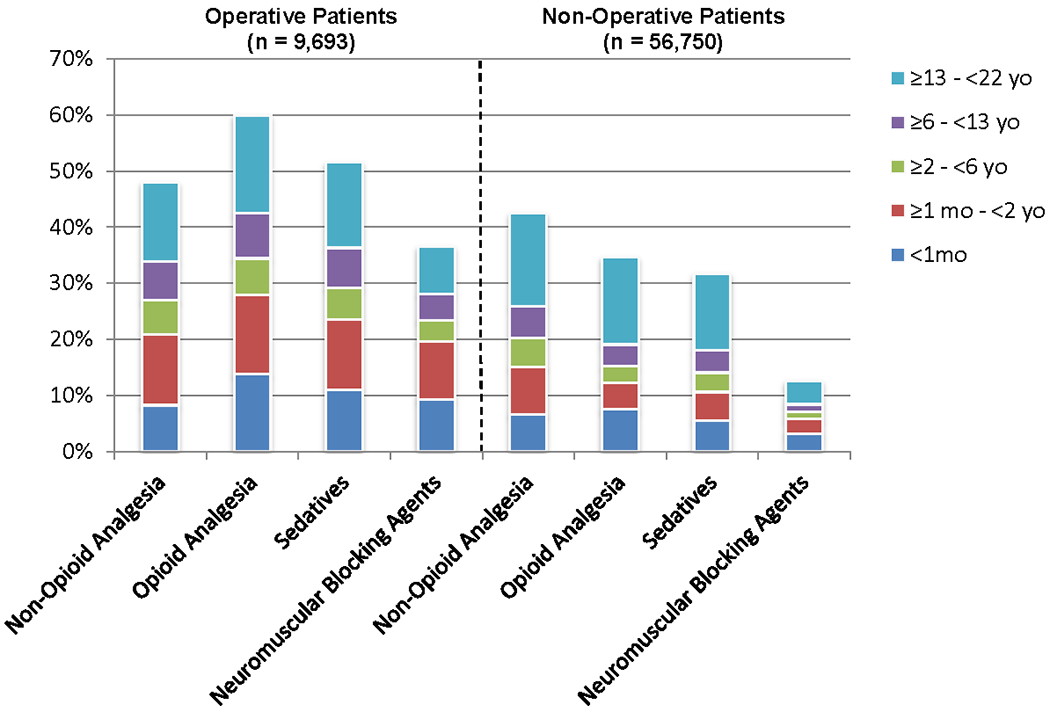
Non-Opioid Analgesic, Opioid Analgesic, Sedative and Neuromuscular Blockade Medications by Age
Table 3.
Frequency and Duration of Non-Opioid Analgesic, Opioid Analgesic, Sedative and Neuromuscular Blockade Medications in Operative/Non-Operative, and Ventilated/Non-Ventilated Patients.
| Medication Class | Operative(1) (n = 9693) | Non-Operative(1) (n = 56750) | Positive Pressure Ventilation(2) (n = 9634) | No Positive Pressure Ventilation(2) (n = 56,809) | |
|---|---|---|---|---|---|
| Non-Opioid Analgesic | N% | 4657 (48.0) | 34119 (60.1) | 9634 (100) | 29142 (51.3) |
| (hours, IQR) | 52 (20-132) | 23 (5-57) | 38 (11-97) | 22 (5-52) | |
| Opioid Analgesic | N% | 5411 (60.0) | 20738 (36.5) | 9634(100) | 16515 (29.1) |
| (hours, IQR) | 50 (17-127) | 25 (5-74) | 41 (9-111) | 23 (5-66) | |
| Sedatives | N% | 4526 (51.6) | 21915 (38.6) | 9152 (95.0) | 17289 (30.4) |
| (hours, IQR) | 40 (6-134) | 18 (3-64) | 29 (4-107) | 16 (3-55) | |
| Neuromuscular Blocking Agents | N% | 2926 (36.6) | 8591 (15.1) | 5134 (53.3) | 6383 (11.2) |
| (hours, IQR) | 2 (1-22.8) | 2 (1-10) | 2 (1-15) | 2 (1-12) |
P< 0.005 for both proportions and durations for each medication class.
P<0.005 for both proportions and durations for each medication class.
Younger age was significantly (p<0.005) associated with longer durations of administration, even when controlling for ICU length of stay (Table 4). Patients <1 month of age had the longest (p<.05) opioid analgesic (median 62 hours, IQR 10 −183 hours) and sedative (median 57 hours, IQR 5-202 hours) durations of medication administration. Patients 1 month-2 years had the longest neuromuscular blocking agent use (median 3 hours, IQR 1-27 hours, p<.05), with the <1 month group having the second longest duration of administration. The oldest age group (13-22 years) had the shortest duration of opioid analgesic, sedative and neuromuscular blocking agent durations of administration (p < 0.005).
The highest proportion of patients receiving analgesic, opioid analgesic, sedative, and neuromuscular blocking agents had primary diagnoses involving musculoskeletal dysfunction or hematology/oncology diagnoses (Figure 3 and Supplemental Table 3). Patients with dermatologic and not otherwise specified diagnoses were least likely to receive any of the studied medication classes. The highest proportion of all medication classes were used in the 13 to < 22 year old patients across all diagnostic groups.
Figure 3.
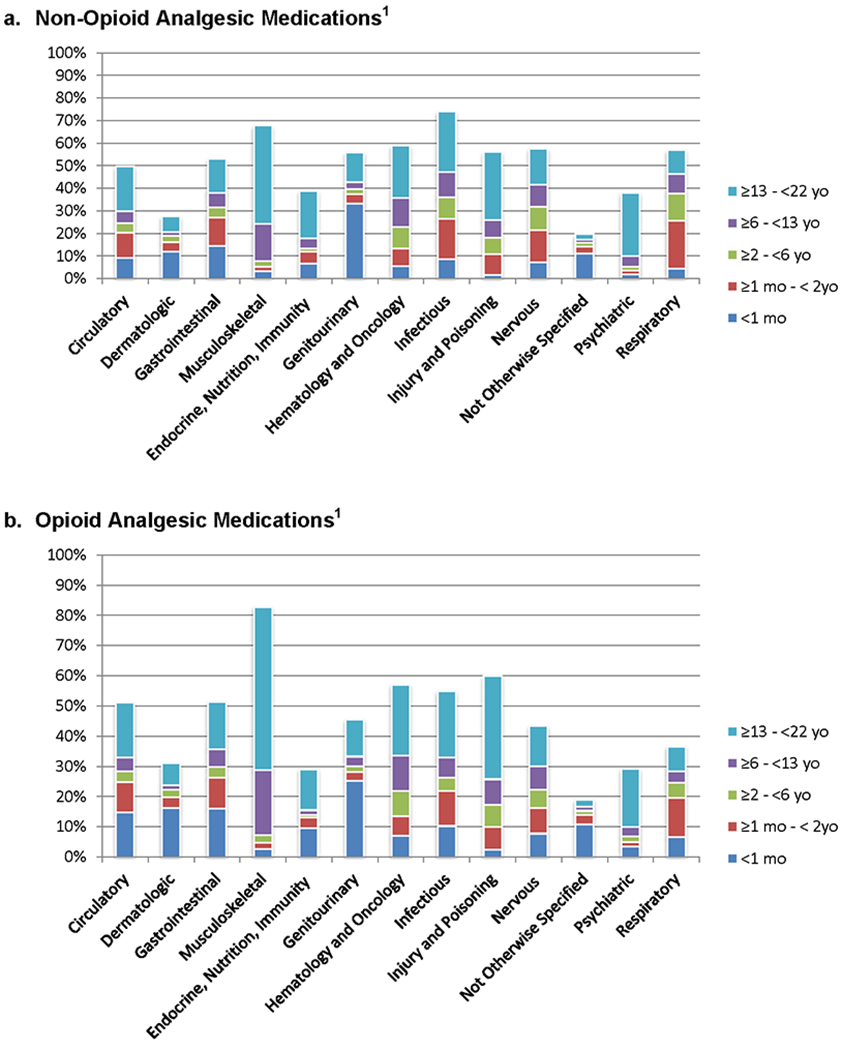
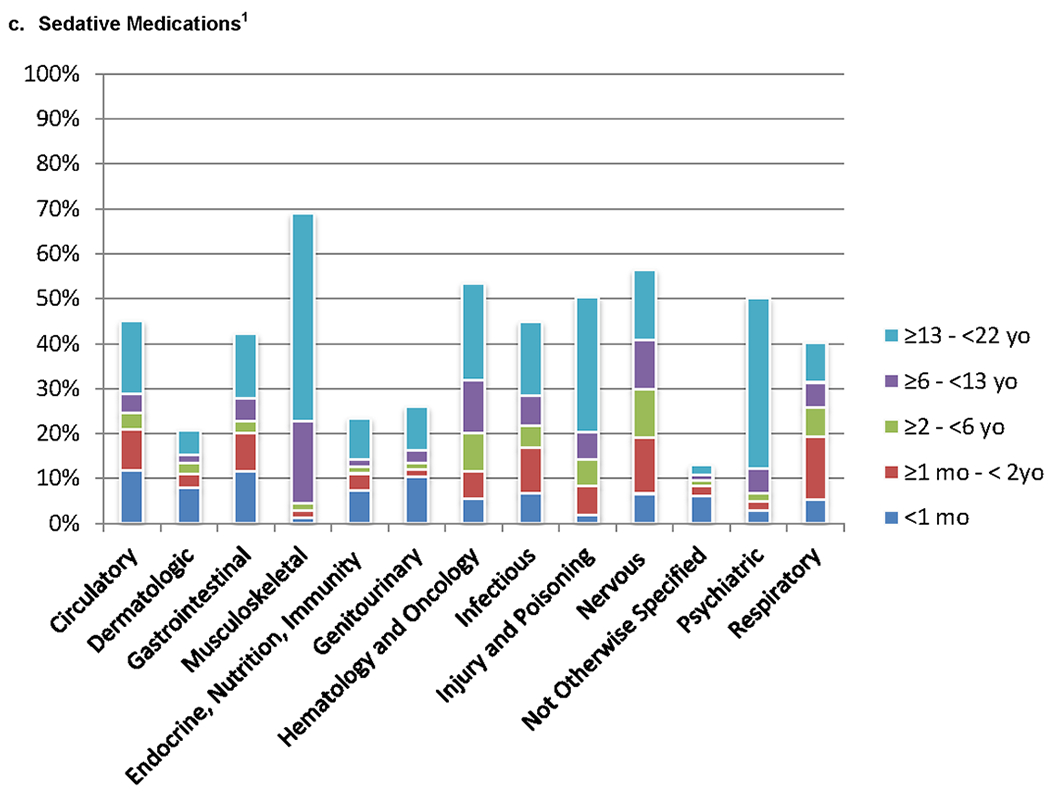
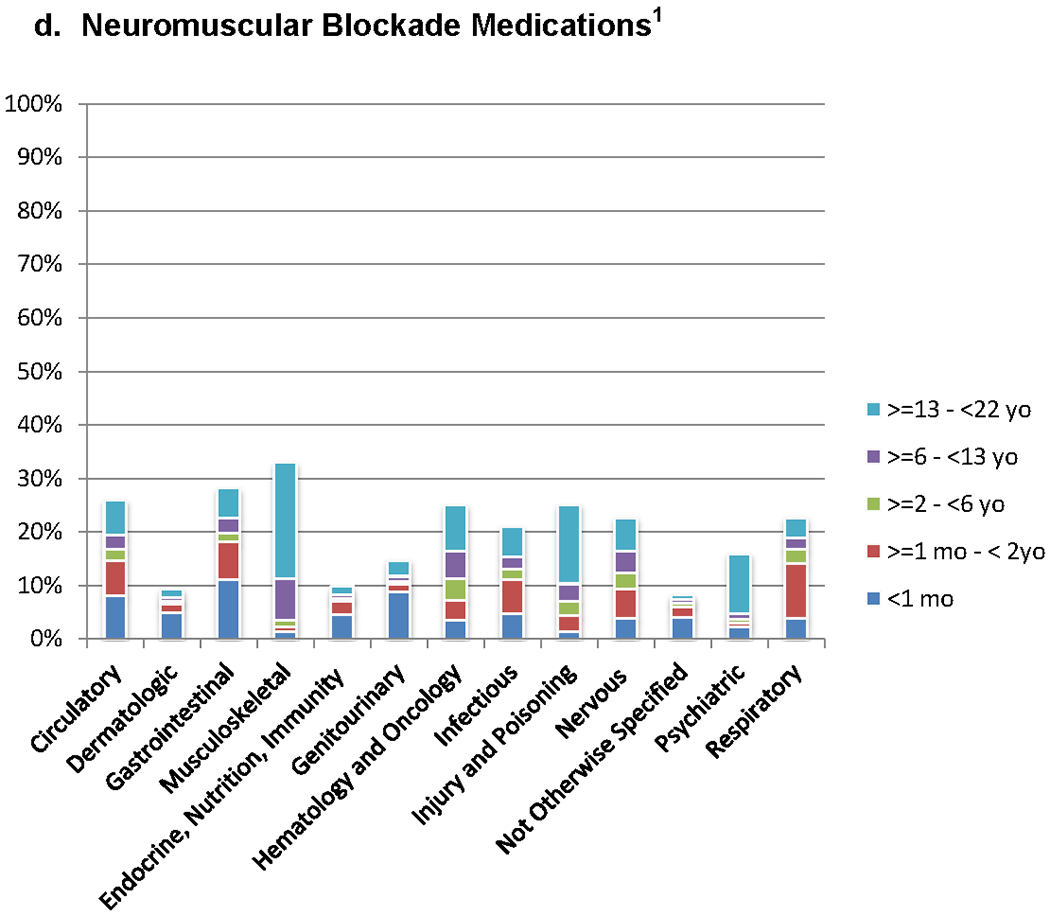
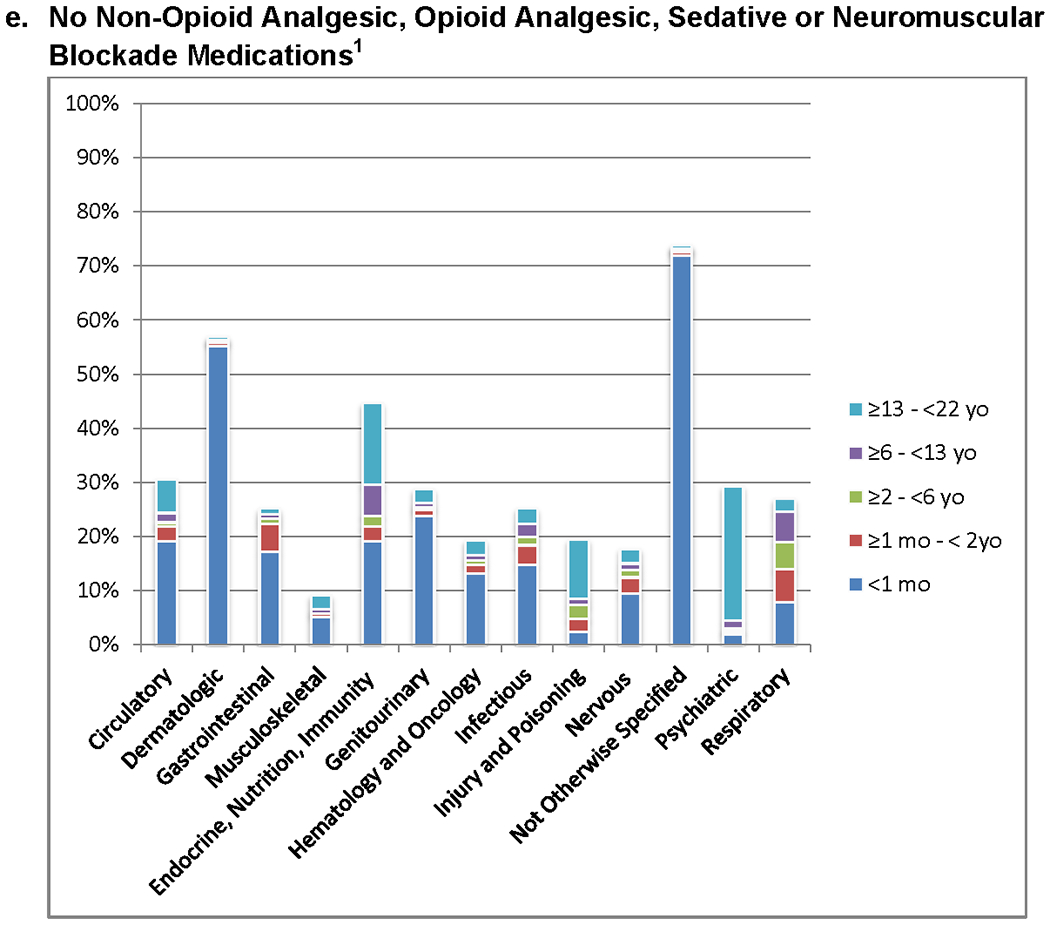
a – e. Patients Receiving Non-Opioid Analgesic, Opioid Analgesic, Sedative and Neuromuscular Blockade Medications for Diagnostic Categories (n = 39,514). Pairwise comparisons of the proportion of medication classes administered between each diagnostic group are significantly different (p<0.05). Pairwise comparisons are detailed in Supplemental Appendix 3.
1. P<0.05 for comparison of proportions in different diagnostic groups.
Discussion
This was the first application of a large, national database to determine the spectrum of sedative, analgesic, and neuromuscular blockade medications used in pediatric patients cared for in the ICU. Prior national and international observational studies had either small populations, examined a protocol intervention, or relied on physician and nurse surveys on medication preferences and practices. 1,17–20,40,41,42
Analgesic and sedative medication use in children in ICUs is common. Analgesic and sedative agents were dispensed to 58.4% and 39.8% of patients receiving medications, respectively, with 64.9% of these patients receiving both medication classes. Opioid analgesics were prescribed to 67.4% of those receiving analgesics, with fentanyl and morphine being the most common agents. Benzodiazepines were the most frequently dispensed sedative medications, with midazolam being the most common. In a recent pediatric ICU survey, 72% of respondents reported that opioid and benzodiazepine medications were the most commonly administered with fentanyl and midazolam as the preferred agents.18 Neuromuscular blocking agents were dispensed to 17.3% of the cohort, all of whom also received either analgesics and/or sedatives. A European study of neonatal ICUs reported a 5.3% rate of neuromuscular blockade, similar to the 8.7% rate found in the less than 30 day old patients in this analysis.43
A substantial proportion of patients received medications for prolonged time periods. For example, 25% of patients less than 2 years of age received opioid analgesics and sedatives for greater than 5 days, durations associated with complications including tolerance, withdrawal, and delirium.7,22,44–46 Similarly, neuromuscular blocking agents were generally used for less than 2 hours , but 25% of children < 2 year of age received neuromuscular blockade for greater than 27 hours, durations that increase the risk for myopathy and are associated with prolonged mechanical ventilation.47,48
There was a significant diversity of pharmacologic practice regarding specific medications. Overall, 49 different opioid and non-opioid analgesic medications, 28 different sedative medications, and 6 different neuromuscular blocking medications were dispensed. Children in ICUs often have unique pharmacodynamics and individualized pain and sedation requirements, so the diversity of practice is expected. However, some of the agent choices is notable. For example, ketorolac was used in only 8.2% of patients older than 6 months although it has a pain relief profile comparable to opioids and can reduce overall opioid consumption in multi-modal pain regimens .49,50 Ketorolac and other NSAIDs have limitations including contraindication in children less than 6 months and a risk of kidney injury. Fentanyl, which has a similar analgesic profile as morphine, was used with a similar frequency as morphine despite an increased risk of tolerance and withdrawal.51,52,53 A possible benefit to fentanyl use is its favorable hemodynamic profile including a reduced risk of histamine release.54
The FDA has warnings concerning some common pharmacologic practices found in this study. A recent warning was issued against use of general anesthetics (propofol, ketamine) and sedatives (benzodiazepines) in children under 3 years old due to their possible negative effect on the developing brain.55 Benzodiazepines, propofol and ketamine were used in 17.5%, 19.4% and 8.2% respectively in children under 2 years old. Additionally, propofol was the third most common sedative medication used in this pediatric population (administered to 13.2% of patients), despite the FDA “black box” warning concerning the potentially lethal propofol related infusion syndrome (PRIS).56,57,58
Studies utilizing large data repositories such as the Health Facts® database have limitations. First, medication use was implied from pharmacy records, not assessed through individual patient records. Second, weight-based dosing was not available which may have added further practice variability. Third, the database did not explicitly identify pediatric ICUs, requiring the identification of children in ICUs using age and general ICU labels. Fourth, the data analyzed are from 2009 to June 2016, and therefore may affect generalizability to current medication practices. Lastly, since 40.5% of patients did not have diagnostic data, there could be a bias in the assessments based on diagnoses.
Conclusion
This study of analgesics, sedatives, and neuromuscular blockade practices demonstrates both substantial diversity as well as commonalities in prescribing patterns. These medications are often necessary adjuncts to critical care management to maintain physiologic stability. While most medications were dispensed for relatively short time periods, a substantial proportion of patients received durations of medications that can be associated with serious complications. Some medication practices were in conflict with FDA warnings. These data suggest there are risks in current sedation and analgesia practices that could be reduced with practice changes to improve efficacy and minimize risks.
Supplementary Material
Acknowledgments
Financial Support
Funding was provided, in part, by Mallinckrodt LLC, and by Awards Ul1TR001876 and KL2TR001877 from the National Institutes of Health, National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
“Copyright form disclosure: Drs. Patel, Workman, Chamberlain, and Morizono’s institutions received funding from Awards Ul1TR001876 and KL2TR001877 from the National Institutes of Health (NIH) National Center for Advancing Translational Sciences. Drs. Patel, Trujillo-Rivera, Zeng-Treitler, Chamberlain, Morizono, Bost, and Pollack’s institutions received funding from Mallinckrodt Pharmaceuticals. Drs. Patel, Workman, Zeng-Treitler, Chamberlain, Morizono, Kim, and Pollack received support for article research from the NIH. Drs. Morizono and Bost received support for article research from and Mallinckrodt Pharmaceuticals. The remaining authors have disclosed that they do not have any potential conflicts of interest.”
Footnotes
Conflict of Interest: There are no conflicts of interest to report for any authors of this manuscript.
Contributor Information
Anita K Patel, Department of Pediatrics, Division of Critical Care Medicine, Children’s National Health System and George Washington University School of Medicine and Health Sciences.
Eduardo Trujillo-Rivera, George Washington University School of Medicine and Health Sciences.
Farhana Faruqe, Children’s National Health System.
Julia Heneghan, Department of Pediatrics, Division of Critical Care Medicine, Children’s National Health System and George Washington University School of Medicine and Health Sciences.
T. Elizabeth Workman, George Washington University School of Medicine and Health Sciences.
Qing Zeng-Treitler, George Washington University School of Medicine and Health Sciences.
James Chamberlain, Department of Pediatrics, Division of Emergency Medicine, Children’s National Health System and George Washington University School of Medicine and Health Sciences.
Hiroki Morizono, Department of Genomics and Precision Medicine, GWU School of Medicine and Health Sciences
Dongkyu Kim, Department of Pediatrics, Children’s National Health System and George Washington University School of Medicine and Health Sciences
James E. Bost, Children’s National Health System and George Washington University School of Medicine and Health Sciences.
Murray M. Pollack, Department of Pediatrics, Division of Critical Care Medicine, Children’s National Health System and George Washington University School of Medicine and Health Sciences.
References
- 1.Zeilmaker-Roest GA et al. An international survey of management of pain and sedation after paediatric cardiac surgery. BMJ Paediatr. Open 1, e000046 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gozal D & Mason KP Pediatric sedation: a global challenge. Int. J. Pediatr 2010, 701257 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy DM The Infant’s Earliest Memory of Inoculation: A Contribution to Public Health Procedures. J. Genet. Psychol 96, 3–46 (1960). [DOI] [PubMed] [Google Scholar]
- 4.Merskey H On the development of pain. Headache 10, 116–23 (1970). [DOI] [PubMed] [Google Scholar]
- 5.Anand KJS & Hickey PR Pain and Its Effects in the Human Neonate and Fetus. N. Engl. J. Med 317, 1321–1329 (1987). [DOI] [PubMed] [Google Scholar]
- 6.Fletcher AB Pain in the Neonate. N. Engl. J. Med 317, 1347–1348 (1987). [DOI] [PubMed] [Google Scholar]
- 7.Tobias JD Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit. Care Med 28, 2122–32 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Tobias JD Sedation and analgesia in paediatric intensive care units: a guide to drug selection and use. Paediatr. Drugs 1, 109–26 [DOI] [PubMed] [Google Scholar]
- 9.Tobias JD Sedation and analgesia in the pediatric intensive care unit. Pediatr. Ann 34, 636–45 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Taddio A, Katz J, Ilersich AL & Koren G Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet 349, 599–603 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Weisman SJ, Bernstein B & Schechter NL Consequences of inadequate analgesia during painful procedures in children. Arch. Pediatr. Adolesc. Med 152, 147–9 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Anand KJ & Ward-Platt MP Neonatal and pediatric stress responses to anesthesia and operation. Int. Anesthesiol. Clin 26, 218–25 (1988). [DOI] [PubMed] [Google Scholar]
- 13.Crellin DJ, Harrison D, Santamaria N & Babl FE Systematic review of the Face, Legs, Activity, Cry and Consolability scale for assessing pain in infants and children. Pain 156, 2132–2151 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Sigurdsson GH, Lindahl S & Nordén N Influence of premedication on the sympathetic and endocrine responses and cardiac arrhythmias during halothane anaesthesia in children undergoing adenoidectomy. Br. J. Anaesth 55, 961–8 (1983). [DOI] [PubMed] [Google Scholar]
- 15.Anand KS Relationships between stress responses and clinical outcome in newborns, infants, and children. Crit. Care Med 21, S358–9 (1993). [DOI] [PubMed] [Google Scholar]
- 16.Curley MAQ et al. Protocolized Sedation vs Usual Care in Pediatric Patients Mechanically Ventilated for Acute Respiratory Failure. JAMA 313, 379 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia Guerra G et al. Survey of Sedation and Analgesia Practice Among Canadian Pediatric Critical Care Physicians. Pediatr. Crit. Care Med 17, 823–30 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Kudchadkar SR, Yaster M & Punjabi NM Sedation, Sleep Promotion, and Delirium Screening Practices in the Care of Mechanically Ventilated Children: A Wake-up Call for the Pediatric Critical Care Community. Crit Care Med 42, 1592–1600 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vet NJ, Kleiber N, Ista E, de Hoog M & de Wildt SN Sedation in Critically Ill Children with Respiratory Failure. Front. Pediatr 4, 89 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vet NJ et al. A randomized controlled trial of daily sedation interruption in critically ill children. Intensive Care Med. 42, 233–244 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Playfor S et al. Consensus guidelines on sedation and analgesia in critically ill children. Intensive Care Med. 32, 1125–1136 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Harris J et al. Clinical recommendations for pain, sedation, withdrawal and delirium assessment in critically ill infants and children: an ESPNIC position statement for healthcare professionals. Intensive Care Medicine (2016). doi: 10.1007/s00134-016-4344-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan WW, Waltman Johnson K, Friedman HS & Navaratnam P Association between cardiac, renal, and hepatic biomarkers and outcomes in patients with acute heart failure. Hosp. Pract 44, 138–145 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Petrick JL, Nguyen T & Cook MB Temporal trends of esophageal disorders by age in the Cerner Health Facts database. Ann. Epidemiol 26, 151–154.e4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grodzinsky A et al. Prevalence and Prognosis of Hyperkalemia in Patients with Acute Myocardial Infarction. Am. J. Med 129, 858–865 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer JB, Friedman HS, Waltman Johnson K, Navaratnam P & Gottlieb SS Association of persistent and transient worsening renal function with mortality risk, readmissions risk, length of stay, and costs in patients hospitalized with acute heart failure. Clinicoecon. Outcomes Res 7, 357–67 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryant C et al. Apache Outcomes Acriss Venues Predicing Inpatient Mortality Using Electronic Medical Record Data. Crit. Care Med 46, 8 (2018). [Google Scholar]
- 28.Hardin AP & Hackell JM Age Limit of Pediatrics. Pediatrics 140, e20172151 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Position Paper Paediatric Age Categories to be Used in Differentiating Between Listing on a Model Essential Medicines List for Children.
- 30.Center for Health Statisticsl, N. ICD-9-CM Official Guidelines for Coding and Reporting. (2011).
- 31.Center for Health Statistics, N. ICD-10-CM Official Guidelines for Coding and Reporting. (2018).
- 32.Fung KW, Kapusnik-Uner J, Cunningham J, Higby-Baker S & Bodenreider O Comparison of three commercial knowledge bases for detection of drug-drug interactions in clinical decision support. J. Am. Med. Inform. Assoc 24, 806–812 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.UMLS Metathesaurus - MMSL (Multum) - Synopsis. Available at: https://www.nlm.nih.gov/research/umls/sourcereleasedocs/current/MMSL/. (Accessed: 25th July 2018)
- 34.R Core Team. R Version 3.5.1: A Language and Environment for Statistical Copmuting. (2017).
- 35.R Studio Team. R Studio Version 1.2.1186: Integrated Development for R. (2015).
- 36.R Core Team. MASS. (2018).
- 37.R Core Team. STATS. (2018).
- 38.Wickham H Tidyverse: Easily Install and Load the ‘Tidyverse’. (2017).
- 39.Chen H VennDiagram: Generate High-Resolution Venn and Euler Plots. (2018).
- 40.Curley MAQ et al. Protocolized Sedation vs Usual Care in Pediatric Patients Mechanically Ventilated for Acute Respiratory Failure. JAMA 313, 379 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia Guerra G et al. Survey of Sedation and Analgesia Practice Among Canadian Pediatric Critical Care Physicians*. Pediatr. Crit. Care Med 17, 823–830 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Jenkins IA, Playfor SD, Bevan EC, Gerald D & R. WA Current United Kingdom sedation practice in pediatric intensive care. Pediatr. Anesth 17, 675–683 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Carbajal R et al. Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. Lancet Respir. Med 3, 796–812 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Smith HAB et al. Delirium and Benzodiazepines Associated With Prolonged ICU Stay in Critically Ill Infants and Young Children*. Crit. Care Med 45, 1427–1435 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Patel AK et al. Delirium in Children After Cardiac Bypass Surgery*. Pediatr. Crit. Care Med 18, 165–171 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Best KM, Boullata JI & Curley MA Q. Risk Factors Associated With Iatrogenic Opioid and Benzodiazepine Withdrawal in Critically Ill Pediatric Patients. Pediatr. Crit. Care Med 16, 175–183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cocoros NM et al. Factors Associated With Pediatric Ventilator-Associated Conditions in Six U.S. Hospitals. Pediatr. Crit. Care Med 18, e536–e545 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Arroliga A et al. Use of sedatives and neuromuscular blockers in a cohort of patients receiving mechanical ventilation. Chest 128, 496–506 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Cepeda MS et al. Comparison of morphine, ketorolac, and their combination for postoperative pain: results from a large, randomized, double-blind trial. Anesthesiology 103, 1225–32 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Jelinek GA Ketorolac versus morphine for severe pain. Ketorolac is more effective, cheaper, and has fewer side effects. BMJ 321, 1236–7 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anand KJS et al. Opioid analgesia in mechanically ventilated children: results from the multicenter Measuring Opioid Tolerance Induced by Fentanyl study. Pediatr. Crit. Care Med 14, 27–36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anand KJS et al. Tolerance and Withdrawal From Prolonged Opioid Use in Critically Ill Children. Pediatrics 125, e1208–e1225 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katz R, Kelly HW & Hsi A Prospective study on the occurrence of withdrawal in critically ill children who receive fentanyl by continuous infusion. Crit. Care Med 22, 763–7 (1994). [DOI] [PubMed] [Google Scholar]
- 54.Kart T, Christrup LL & Rasmussen M Recommended use of morphine in neonates, infants and children based on a literature review: Part 2-Clinical use. Pediatr. Anesth 7, 93–101 (2008). [DOI] [PubMed] [Google Scholar]
- 55.FDA Drug Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-review-results-new-warnings-about-using-general-anesthetics-and. (Accessed: 15th November 2019)
- 56.Bray RJ Propofol infusion syndrome in children. Paediatr. Anaesth 8, 491–9 (1998). [DOI] [PubMed] [Google Scholar]
- 57.Parke TJ et al. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports. BMJ 305, 613–6 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar MA, Urrutia VC, Thomas CE, Abou-Khaled KJ & Schwartzman RJ The Syndrome of Irreversible Acidosis After Prolonged Propofol Infusion. Neurocrit. Care 3, 257–259 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


