Abstract
Venous thromboembolism (VTE) whether provoked or not can be life-threatening due to an acute increase in load on the right ventricle (RV) from obstruction of the pulmonary artery (PA). Treatment for and prevention of VTE involves anti-thrombotic agents; more specifically, medications targeting the anticoagulation cascade. In spite of the widespread acceptance of anticoagulants in the treatment of VTE, there appears to be an ongoing belief that platelet reactivity contributes to thrombus burden in patients with acute pulmonary embolism (PE). This investigation of 398 patients presenting with acute PE evaluated whether anti-platelet medication use, which consisted mostly of aspirin therapy, at the time of presentation, affects PA thrombus burden, RV load, or short-term patient outcomes. We conclude that platelets may have been erroneously incriminated as direct thrombotic mediators in patients with acute PE since aspirin neither decreased PA thrombus burden, nor did aspirin improve short-term mortality following acute PE.
Keywords: Thrombus, pulmonary embolism, platelet, aspirin
Introduction
Thrombus extracted from patients with an acute venous thromboembolic (VTE) event is comprised of a complex arrangement of cells including platelets (1). While patients with pulmonary embolism (PE) are treated with anticoagulation, the contribution of platelet inhibitors to thrombus burden, right ventricular (RV) afterload, and patient outcomes remain unclear. Anti-thrombotic medications include systemic anticoagulants and anti-platelet drugs which may have a synergistic effect in patients with thrombotic disease. Taken together, anti-thrombotic medications may decrease thrombus burden, but they could potentially lower the threshold for a major bleeding event.
Platelet inhibitors including irreversible inhibitors of cyclooxygenase, P2Y12 receptor and PAR1 antagonists were reported to lower the incidence of VTE in patients with atherosclerotic diseases (2, 3). Patients with VTE were reported to display alterations in mean platelet volume which may imply changes in reactivity (4). Platelet reactivity was previously shown to affect PA thrombus burden in a murine model of PE (5) but this, to the best of our knowledge, has never been evaluated in humans. We hypothesized that patients already taking aspirin and presenting with acute PE would have a lower PA thrombus burden which would translate into less right ventricular (RV) strain, and improved short-term outcomes.
Quantification of thrombus burden in the pulmonary artery (PA) is a manual and time-consuming endeavor. One proposed strategy is the Qanadli scoring system (mQ) (Fig. 1A) which can be applied by non-imaging expert clinicians (6). This scoring system allows quantification of thrombus burden in all areas of the PA vascular bed and is associated with PE-related mortality at three months (7–9). We retrospectively evaluated the mQ score in patients presenting to a single institution with acute PE to examine the relationship between use of aspirin on thrombus burden and short-term outcomes.
Figure 1. The modified Qanadli Score for PA Thrombus Burden:
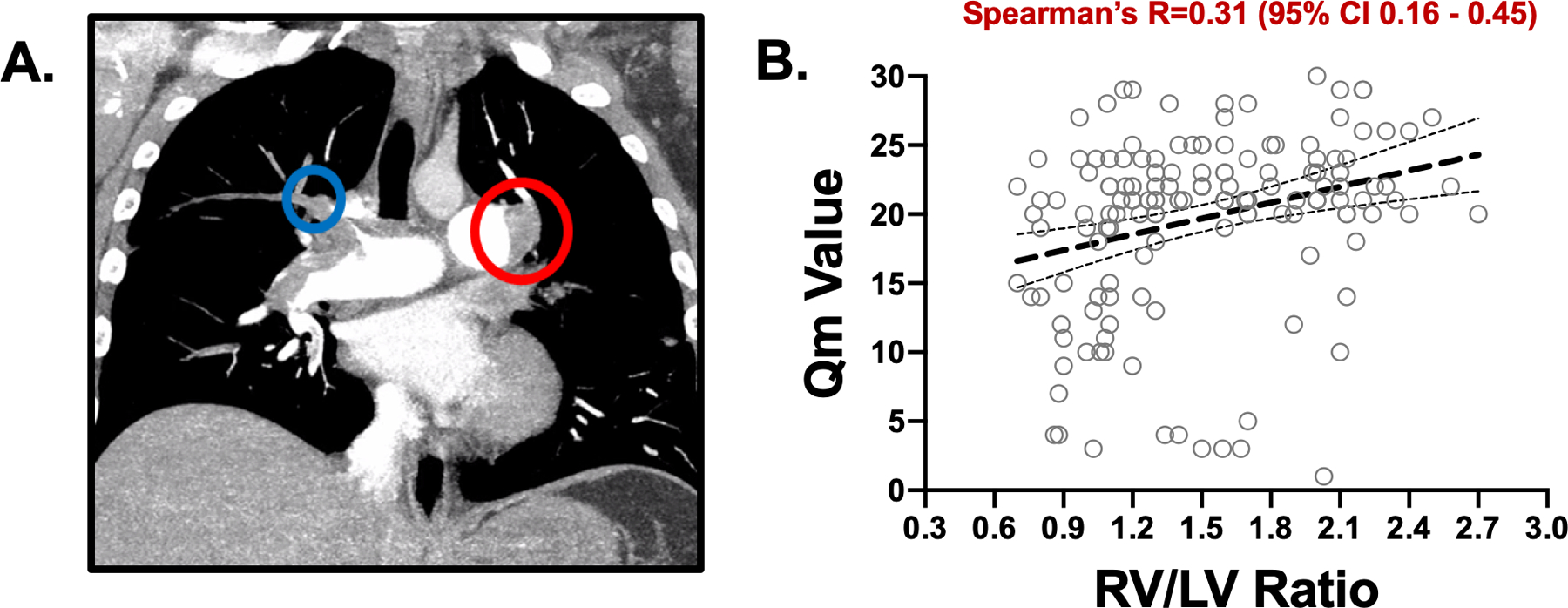
A. In this patient, the minimum score in the setting of bilateral pulmonary artery partial obstructions (red) would be 20. Embolism in the truncus anterior (blue) adds at least two more points for complete blockage of the distal right A1 and A3 segmental branches. B. Positive association of Qm (thrombus burden) and RV/LV ratio (measure of RV strain) by Spearman’s correlation, P < 0.0001 (n=153 paired data sets available).
Methods
This study was approved by the Institutional Review Board at the single center conducting the study. The International Classification of Diseases 9th Clinical Modification (ICD-9) terms for “pulmonary embolism” or “venous thromboembolism” between 11/19/2014 and 10/15/2016 identified patients with acute PE. Computed Tomography Angiography (CTA) images were used to evaluate the PA thrombus burden for each patient, and the mQ score was used to quantify PA thrombus burden. The numbers for partially occluded (weighting factor=1) and completely occluded (weighing factor=2) segmental arteries (n=20) are summed for Obstruction Index=(x/40). Proximal thrombus scores are multiplied by the number of radiating segmental branches. Subsegmental and distal thrombi were not scored (Fig 1A). A board-certified radiologist who did not have access to patient data groups adjudicated the integrity of the mQ score employed to assess PA thrombus burden. We also recorded patients with central or peripheral PA thrombus. RV/LV > 0.9 was considered to be increased and used as evidence of RV strain as described by us in a previous study (10). Death at 90 days and major bleeding within 4 months were the major outcomes reported. The predicted 30-day mortality for each patient was also calculated using the Pulmonary Embolism Severity Index (PESI) (11). Statistical evaluation of patients taking platelet inhibitors compared to those who were not at the time of acute PE diagnosis was performed in R after normality testing using the Mann-Whitney U and chi-squared tests for categorical variables. The Spearman correlation coefficient was used to assess for an association between two continuous variables. Significance was determined as a P value < 0.05.
Results
Increasing thrombus burden calculated by mQ was associated with RV strain determined by the RV/LV ratio. Of the 398 patients identified with acute PE, 99 patients (24.8%) were taking anti-platelet agents at the time of admission with PE. The majority of the patients (97; 24.3%) were taking aspirin monotherapy, 2 patients (0.5%) were taking the P2Y12 receptor antagonist clopidogrel as monotherapy, and 7 patients (1.8%) were taking dual anti-platelet therapy consisting of aspirin and clopidogrel. PA thrombus burden was significantly correlated with increased RV/LV ratio with or without antiplatelet therapy at the time of presentation (Fig. 1B and Fig. 2: Spearman’s R 0.47 [95% CI 0.20–0.67] p=0.00009 and 0.23 [95% CI 0.03–0.41] p=0.02, respectively). Anti-platelet therapy use at the time of presentation with acute PE had no effect on PA thrombus distribution, total PA thrombus burden, or RV strain. There was no correlation between PA thrombus burden quantitatively determined by mQ score and plasma N-terminal pro-BNP (NT-proBNP) concentration which has been used as an indirect surrogate of increased RV load (12–14). Anti-platelet therapy had no effect on 90-day mortality or major bleeding in patients presenting with acute PE who subsequently underwent systemic anticoagulation. The PESI score was numerically higher in the patient group presenting with acute PE taking anti-platelet medications. While there was no decrease in PA thrombus burden as anticipated with anti-platelet medication use, a sicker population could have accounted for the slightly higher mortality in patients taking anti-platelet therapy. (Fig. 2).
Figure 2. Patient Characteristics: † Thrombus in the pulmonary trunk or R or L pulmonary artery.
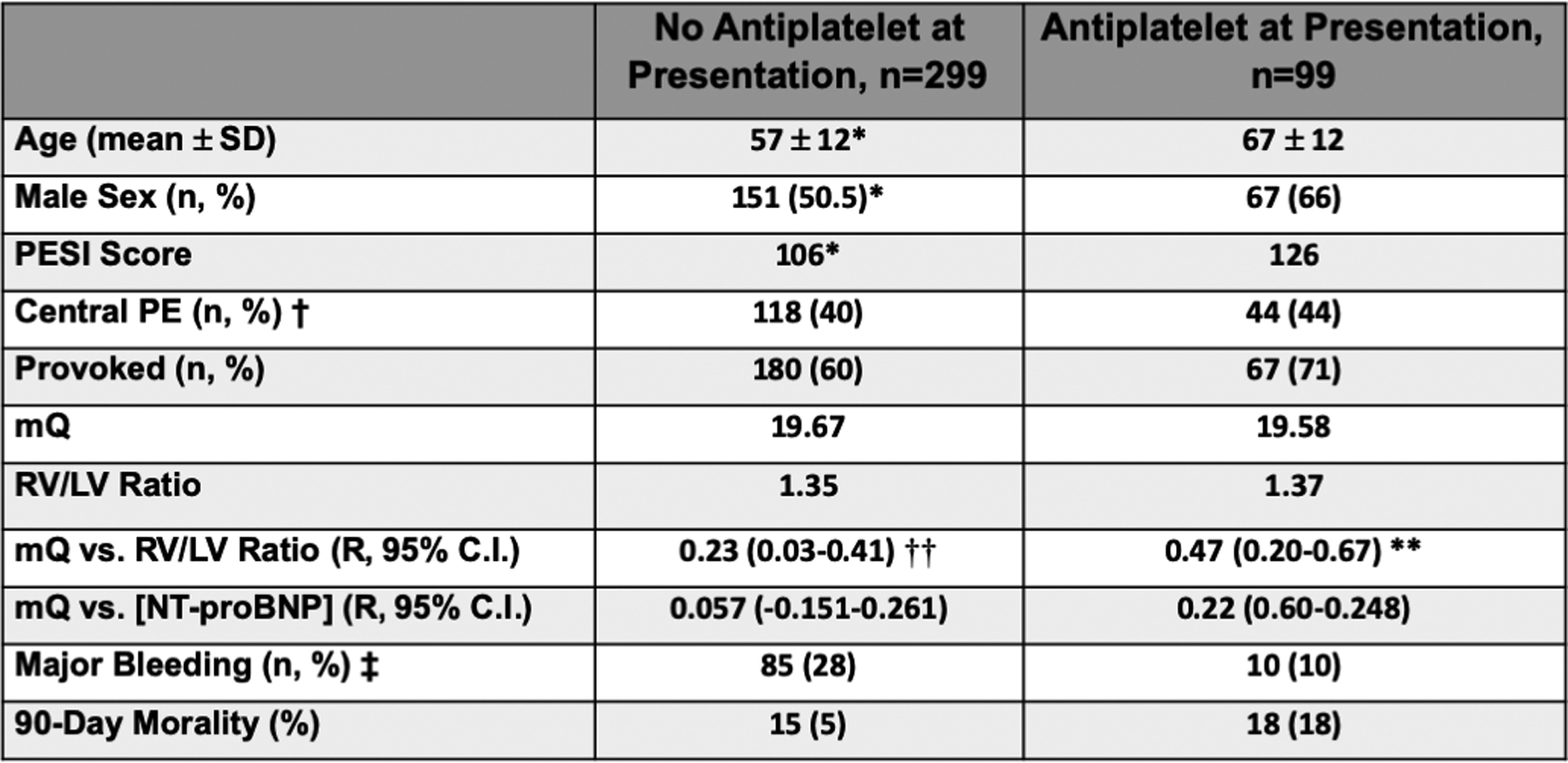
‡ Bleeding with decrease in the hemoglobin at least 2 g/dL or transfusion of at least 2 units of packed red cells, occurring at the following sites (intracranial, intraocular, intraspinal, intraarticular, intramuscular with compartment syndrome, pericardial, retroperitoneal), or death as a consequence of bleeding with 4 months. * p<0.05 anti-platelet agents on presentation vs. no anti-platelet agents on presentation. ** P < 0.0001 mQ vs. RV/LV ratio (taking anti-platelet agents) and †† P=0.02 mQ vs. RV/LV ratio (no anti-platelet agents).
Discussion
Our data suggest that aspirin therapy neither decreases PA thrombus burden nor does aspirin therapy alter short-term patient mortality or the risk for major bleeding. As previously reported, PA thrombus location does not alter short-term patient mortality following acute PE; peripheral PA thrombus is more common in patients with hypotension and central PA thrombus is more common in patients with RV strain (15). Our results appear to support and extend this study by showing quantitative assessment of increased PA thrombus burden markedly increases RV strain. This observation is noted irrespective of the patient taking aspirin, and without altering short-term mortality. A previous high-quality trial showed low dose aspirin therapy decreased the incidence of VTE following hip fracture which is a higher risk population, possibly with differences in platelet reactivity following orthopedic trauma (16). It is important to emphasize our data do not suggest every anti-platelet agent including P2Y12 receptor antagonists like clopidogrel, prasugrel, and ticagrelor and the protease-activated receptor 1 (PAR1) antagonist vorapaxar are ineffective in decreasing thrombus burden, RV strain, and mortality follow VTE. Short-term mortality and bleeding in patients already on aspirin but requiring anticoagulation following hospital discharge for PE are no different from those not taking aspirin. Future studies should evaluate whether additional anti-platelet medications used in isolation or in various permutations decrease thrombus burden in the lung as well as patient mortality following acute PE.
Acknowledgements
The authors disclose the following financial support for their research: K08HL128856 to SJC
Footnotes
Declaration of Conflicting Interest
The authors declare that there is no conflict of interest with respect to research, authorship, or publication of this article.
References
- 1.Czaplicki C, Albadawi H, Partovi S, Gandhi RT, Quencer K, Deipolyi AR, Oklu R. Can thrombus age guide thrombolytic therapy? Cardiovasc Diagn Ther. 2017;7(Suppl 3):S186–S96. doi: 10.21037/cdt.2017.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cavallari I, Morrow DA, Creager MA, Olin J, Bhatt DL, Steg PG, Storey RF, Cohen M, Scirica BS, Piazza G, Goodrich EL, Braunwald E, Sabatine MS, Bonaca MP. Frequency, Predictors, and Impact of Combined Antiplatelet Therapy on Venous Thromboembolism in Patients With Symptomatic Atherosclerosis. Circulation. 2018;137(7):684–92. doi: 10.1161/CIRCULATIONAHA.117.031062. [DOI] [PubMed] [Google Scholar]
- 3.Undas A, Brummel-Ziedins K, Mann KG. Why does aspirin decrease the risk of venous thromboembolism? On old and novel antithrombotic effects of acetyl salicylic acid. J Thromb Haemost. 2014;12(11):1776–87. doi: 10.1111/jth.12728. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs S, Csiki Z, Zsori KS, Bereczky Z, Shemirani AH. Characteristics of platelet count and size and diagnostic accuracy of mean platelet volume in patients with venous thromboembolism. A systematic review and meta-analysis. Platelets. 2019;30(2):139–47. doi: 10.1080/09537104.2017.1414175. [DOI] [PubMed] [Google Scholar]
- 5.Kim YH, Bae JU, Kim IS, Chang CL, Oh SO, Kim CD. SIRT1 prevents pulmonary thrombus formation induced by arachidonic acid via downregulation of PAF receptor expression in platelets. Platelets. 2016;27(8):735–42. doi: 10.1080/09537104.2016.1190005. [DOI] [PubMed] [Google Scholar]
- 6.Qanadli SD, El Hajjam M, Vieillard-Baron A, Joseph T, Mesurolle B, Oliva VL, Barre O, Bruckert F, Dubourg O, Lacombe P. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001;176(6):1415–20. doi: 10.2214/ajr.176.6.1761415. [DOI] [PubMed] [Google Scholar]
- 7.Vedovati MC, Germini F, Agnelli G, Becattini C. Prognostic role of embolic burden assessed at computed tomography angiography in patients with acute pulmonary embolism: systematic review and meta-analysis. J Thromb Haemost. 2013;11(12):2092–102. doi: 10.1111/jth.12429. [DOI] [PubMed] [Google Scholar]
- 8.Wu AS, Pezzullo JA, Cronan JJ, Hou DD, Mayo-Smith WW. CT pulmonary angiography: quantification of pulmonary embolus as a predictor of patient outcome--initial experience. Radiology. 2004;230(3):831–5. doi: 10.1148/radiol.2303030083. [DOI] [PubMed] [Google Scholar]
- 9.Rotzinger DC, Breault S, Knebel JF, Beigelman-Aubry C, Jouannic AM, Qanadli SD. Can a Trained Radiology Technician Do Arterial Obstruction Quantification in Patients With Acute Pulmonary Embolism? Front Cardiovasc Med. 2019;6:38. doi: 10.3389/fcvm.2019.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YL, Wright C, Pietropaoli AP, Elbadawi A, Delehanty J, Barrus B, Gosev I, Trawick D, Patel D, Cameron SJ. Right ventricular dysfunction is superior and sufficient for risk stratification by a pulmonary embolism response team. J Thromb Thrombolysis. 2019. doi: 10.1007/s11239-019-01922-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donze J, Le Gal G, Fine MJ, Roy PM, Sanchez O, Verschuren F, Cornuz J, Meyer G, Perrier A, Righini M, Aujesky D. Prospective validation of the Pulmonary Embolism Severity Index. A clinical prognostic model for pulmonary embolism. Thromb Haemost. 2008;100(5):943–8. doi: 10.1160/th08-05-0285. [DOI] [PubMed] [Google Scholar]
- 12.Coutance G, Le Page O, Lo T, Hamon M. Prognostic value of brain natriuretic peptide in acute pulmonary embolism. Crit Care. 2008;12(4):R109. doi: 10.1186/cc6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pruszczyk P, Kostrubiec M, Bochowicz A, Styczynski G, Szulc M, Kurzyna M, Fijalkowska A, Kuch-Wocial A, Chlewicka I, Torbicki A. N-terminal pro-brain natriuretic peptide in patients with acute pulmonary embolism. Eur Respir J. 2003;22(4):649–53. doi: 10.1183/09031936.03.00023303. [DOI] [PubMed] [Google Scholar]
- 14.Cameron SJ, Green GB, White CN, Laterza OF, Clarke W, Kim H, Sokoll LJ. Assessment of BNP and NT-proBNP in emergency department patients presenting with suspected acute coronary syndromes. Clin Biochem. 2006;39(1):11–8. doi: 10.1016/j.clinbiochem.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Jain CC, Chang Y, Kabrhel C, Giri J, Channick R, Rodriguez-Lopez J, Rosovsky RP, Fogerty A, Rosenfield K, Jaff MR, Weinberg I. Impact of Pulmonary Arterial Clot Location on Pulmonary Embolism Treatment and Outcomes (90 Days). Am J Cardiol. 2017;119(5):802–7. doi: 10.1016/j.amjcard.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet. 2000;355(9212):1295–302. [PubMed] [Google Scholar]


