Abstract
Objective:
To examine the efficacy of complementary and integrative health (CIH) approaches for reducing pain intensity (primary outcome) and depressive symptoms (secondary outcome) as well as improving physical functioning (secondary outcome) among U.S. military personnel living with chronic pain.
Method:
Studies were retrieved from bibliographic databases, databases of funded research, and reference sections of relevant manuscripts. Studies that (a) evaluated a CIH approach to promote chronic pain management among military personnel, (b) used a randomized controlled trial design, and (c) assessed pain intensity were included. Two coders extracted data from each study and calculated effect sizes. Discrepancies between coders were resolved through discussion.
Results:
Comprehensive searches identified 12 studies (k = 15 interventions) that met inclusion criteria. CIH practices included cognitive-behavioral therapies (k = 5), positive psychology (k = 3), yoga (k = 2), acupuncture (k = 2), mindfulness-based interventions (k = 2), and biofeedback (k = 1). Across these studies, participants who received the intervention reported greater reductions in pain intensity (d+ = 0.44, 95% CI = 0.21–0.67, k = 15) compared to controls. Statistically significant improvements were also observed for physical functioning (d+ = 0.36, 95% CI = 0.11–0.61, k= 11) but not for depressive symptoms (d+ = 0.21, 95% CI = −0.15−0.57, k= 8).
Conclusions:
CIH approaches reduced pain intensity and improved physical functioning. These approaches offer a nonpharmacological, nonsurgical intervention for chronic pain management for military personnel. Future studies should optimize interventions to improve depressive symptoms in military populations experiencing chronic pain.
Impact Statement:
CIH approaches provide an efficacious treatment option for veterans experiencing chronic pain. This meta-analysis provides support for the use of CIH practices to manage chronic pain symptoms with active duty and military veterans experiencing chronic pain.
Keywords: complementary and integrative health, chronic pain, military, veterans, meta-analysis
Chronic pain is most often defined as pain that is present most days or every day for a minimum of 12 weeks, typically beyond the time that would be expected for pain related to an injury to resolve (Dahlhamer et al., 2018). Chronic pain occurs frequently among adults in the U.S., with estimates ranging from 20% to 50%, depending on the frequency and severity in which individuals experience pain (Dahlhamer et al., 2018; Nahin, 2017). U.S. military members experience a higher prevalence of musculoskeletal (e.g., low back, neck, and joint pain) and neurological (e.g., migraine) pain than civilians, with nearly two-thirds reporting chronic pain in the past three months (Fontana & Rosenheck, 2008; Nahin, 2017). Active duty U.S. military members are at a higher risk for physical injuries and mental stressors during their service, despite being healthier (nomothetically) than their civilian counterparts (due to pre-enrollment health screening) (Fontana & Rosenheck, 2008; Nahin, 2017). Furthermore, veterans experience severe pain at a rate 50% greater than non-veterans (9.1% vs. 6.4%), with even higher disparities among younger veterans (i.e., 18–39 years of age) (Fontana & Rosenheck, 2008; Nahin, 2017).
The increased risk for chronic pain among military personnel is related to various experiences and exposures occurring throughout their term of service. First, military members experience high levels of musculoskeletal injury, head and brain trauma (e.g., traumatic brain injury [TBI]), and physical amputations due to combat experiences (Hoot et al., 2018). Second, service members are at heightened risk for mood disorders, trauma-related disorders due to combat and military sexual trauma, suicidality, alcohol, tobacco, or drug use disorders (Hoge et al., 2004; Hoot et al., 2018; Pizarro, Silver, & Prause, 2006; Suris & Lind, 2008). For example, the prevalence of TBI, post-traumatic stress disorder (PTSD), and chronic pain among veterans returning from deployment was 9.6%, 29.4%, and 40.2% respectively (Cifu et al., 2013). Military personnel are also more likely to have higher levels of exposure to hazardous chemicals, radiation, air pollutants, warfare agents and harmful noise or vibrations (US Department of Veteran Affairs, 2015). Toxic exposure among veterans deployed to the Iraq and Afghanistan conflicts is associated with chronic multi-symptom illness (i.e., fatigue, mood disturbances and cognitive difficulties, chronic musculoskeletal pain) after controlling for key confounders. Compared to age-matched non-veterans, veterans are also more likely to have one or more chronic health conditions such as asthma, diabetes, or heart disease (Kramarow & Pastor, 2012; Lehavot, Hoerster, Nelson, Jakupcak, & Simpson, 2012). Therefore, military members have an increased risk for combat-related and other chronic health conditions compared to their civilian counterparts. Chronic pain treatment, however, often relies on opioids which increases the risk of functional impairment among military members (Jonas & Schoomaker, 2014).
Pain management treatment approaches typically aim for a reduction in pain intensity. Chronic pain treatments involve medication (e.g., prescription opioids), therapeutic exercise (e.g., physical therapy), chiropractic, and surgery (National Institues of Health, 2011). Despite the benefits of conventional medical approaches, these interventions do not provide individuals with the skills to manage the cognitive, emotional, and physical challenges associated with living with a chronic pain condition. Furthermore, prescription opioids, once considered appropriate for the management of chronic pain conditions, is now a growing health concern in the military (Nelson, Bjarnadóttir, Wolcott, & Agarwal, 2018). This is especially salient given that the prevalence of opioid use for the treatment of chronic pain is higher among military members (15%) compared to civilians (4%) (Toblin, Quartana, Riviere, Walper, & Hoge, 2014).
In addition to the physical experience, chronic pain is often accompanied by a multitude of maladaptive cognitive and psychological processes. Persistent negative thinking and catastrophizing about one’s chronic pain leads to a cycle of physical and psychological suffering. For example, patients living with chronic pain often report fear of movement (i.e., kinesophobia), which results in decreased physical activity, muscle loss, and paradoxically exacerbates pain and risk of injury (Crombez, Eccleston, Van Damme, Vlaeyen, & Karoly, 2012). Elevated levels of stress associated with chronic pain also increases cortisol and inflammation (Edwards et al., 2008; Quartana et al., 2010). Therefore, it is critical for pain management approaches to address maladaptive stress and coping responses in the context of treatment. Examples of adaptive stress responses include skills for seeking emotional and social support and safely engaging in physical activity. CIH approaches may be helpful for military members living with chronic pain, as they can provide skills to adjust to, accept, and manage ongoing pain (McAllister, 2013) as well as to cope with life stressors associated with pain (e.g., functional limitations).
CIH practices include a wide range of psychological (e.g., cognitive restructuring, problem-solving skills-training, meditation), physical (e.g., progressive muscle relaxation, acupuncture), or blended psychological and physical (e.g., tai chi, yoga) approaches to manage or reduce stress, improve coping, and promote self-management (Lichtenthal, Simpson, & Cruess, 2005). CIH approaches, such as cognitive behavioral therapy, mindfulness, and yoga, have been shown to improve psychological and physical symptoms across multiple patient populations (e.g., patients living with cardiovascular disease, HIV) (Dunne et al., 2019; Scott-Sheldon, Balletto, et al., 2019; Scott-Sheldon, Gathright, et al., 2020; Scott-Sheldon, Kalichman, Carey, & Fielder, 2008). Additionally, small trials of military samples living with PTSD show that CIH approaches reduce PTSD symptoms (Hollifield, Sinclair-Lian, Warner, & Hammerschlag, 2007; Kearney, McDermott, Malte, Martinez, & Simpson, 2012, 2013; Rosenthal et al., 2011; Staples, Hamilton, & Uddo, 2013). Therefore, nonpharmacological, nonsurgical conventional (e.g., cognitive-behavioral therapy) or nonconventional (e.g., mindfulness, yoga) CIH approaches can help military personnel, active duty, reserves, or veterans, manage their pain symptoms as well as the distress associated with living with chronic pain (Madsen, Vaughan, & Koehlmoos, 2017). Additionally, many CIH approaches provide patients with skills to engage in self-management after the active phases of clinical interventions. Self-management is a valuable component given the chronic nature of these pain conditions and high cost of medical care associated with chronic pain.
A scoping review of CIH practices among military personnel showed that meditation and acupuncture were the most commonly evaluated practices (Elwy, Johnston, Bormann, Hull, & Taylor, 2014). While this scoping review provided a comprehensive overview of CIH approaches used to improve health in the military, the benefits of these approaches for military members remain understudied. Therefore, the purpose of this meta-analysis was to examine the efficacy of CIH practices to promote chronic pain management among military personnel. We hypothesized that CIH interventions would lead to greater reductions in pain intensity (primary outcome) as well as improving physical functioning and reducing depressive symptoms (secondary outcomes) among U.S. military personnel living with chronic pain, relative to controls. Furthermore, we also explored whether sample and intervention characteristics moderated the findings. Specifically, we expected that changes in pain intensity would be moderated by (a) sex (i.e., women would be more likely to use and benefit from alternative approaches such as yoga to manage pain symptoms) (Evans et al., 2018), (b) length of time living with chronic pain (i.e., longer durations of chronic pain would be associated with diminished physical performance and disability) (Jones, Rutledge, Jones, Matallana, & Rooks, 2008), (c) psychological distress (i.e., depressive symptoms are prevalent among patients reporting pain-related symptoms in primary care settings (L. S. Williams, Jones, Shen, Robinson, & Kroenke, 2004), and therefore may benefit more from a nonconventional approach to pain management), (d) intervention duration (i.e., improved outcomes in interventions of shorter vs. longer durations) (Van Daele, Hermans, Van Audenhove, & Van den Bergh, 2012), (e) delivery mode (i.e., individual vs. group-based interventions would be more beneficial given that individuals approaches allow for personally-tailored chronic pain management) (Hawkins, Kreuter, Resnicow, Fishbein, & Dijkstra, 2008), and (f) type of CIH approaches (i.e., cognitive-behavioral therapies would be more effective than mind-body approaches) (Skelly et al., 2018).
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed in the reporting of this meta-analysis (Moher, Liberati, Tetzlaff, & Altman, 2009). The PRISMA Checklist can be found in the Online Supplemental Material (Table S1).
Inclusion Criteria, Information Sources, and Search Strategy
Studies were included if they: (1) evaluated a CIH approach to promote chronic pain management among military personnel, including active duty and veterans; (2) used a randomized controlled trial (RCT) design; and (3) assessed pain intensity. CIH approaches included nonpharmacological, nonsurgical conventional (e.g., cognitive-behavioral therapy) or nonconventional (e.g., mindfulness, yoga) approaches to pain management. The studies sampled patients experiencing any type of chronic pain resulting from an injury or accident (e.g., musculoskeletal, traumatic brain injuries) or painful conditions (e.g., rheumatoid arthritis). Three strategies were used to identify studies: First, we searched electronic bibliographic databases (i.e., PubMed, PsycINFO, CINAHL, Embase, ProQuest Dissertations and Theses Full Text, ERIC, Cochrane Library, SocIndex, and Web of Science: Science Citation Index) using a Boolean search strategy and database-specific limiting and expanding fields and terms. For example, the PubMed search string was: [((“complementary and alternative medicine”) OR (autogenic training[MeSH]) OR (biofeedback [MeSH]) OR (“cognitive restructuring”) OR (“cognitive behavioral stress management”) OR (“deep breathing”) OR (“emotional freedom technique”) OR (guided imagery [MeSH]) OR (“mindfulness-based stress reduction”) OR (mindfulness [MeSH]) OR (meditation [MeSH]) OR (“problem-solving training”) OR (“progressive muscle relaxation”) OR (“relaxation techniques”) OR (self-disclosure) OR (self-hypnosis) OR (tai chi [MeSH]) OR (“transcendental meditation”) OR (yoga[MeSH]) OR (“stress management”)) AND ((military [MeSH]) OR (veterans [MeSH]))]. These databases were searched on three separate occasions (February 2017, April 2018, and August 2019) to ensure the retrieval of all studies available through August 2019. Second, we reviewed the reference lists of relevant reviews and other related manuscripts retrieved from our database searches. Finally, we reviewed databases of funded research (NIH RePORTER) and clinical trials (ClinicalTrials.gov).
Study Selection
All unique records (after duplicate removal) retrieved from the electronic bibliographic database searches were screened for inclusion based on title and abstract. Full-text manuscripts of any potentially relevant records were retrieved and reviewed for inclusion by two authors (MLD, JD), and verified by the principal investigator (LAJSS). Records that reported the same study and/or sample across multiple manuscripts were linked in the database and represented as a single unit. The manuscript reporting the most complete data was selected as the primary manuscript; additional papers were considered supplemental but used in the data collection process. One study author was contacted for additional data and provided the requested information (Groessl, Liu, Goodman, et al., 2017).
Data Collection Process, Data Items, and Reliability
Two of three trained independent coders (MLD, JD, BLB) extracted study information (e.g., publication year), sample characteristics (e.g., age, gender), study design (e.g., randomized controlled trial), intervention details (e.g., number of sessions), and intervention technique (e.g., cognitive-behavioral therapy, yoga) using a coding manual and form developed by the principal investigator (LAJSS) and co-investigators, then pilot tested by the research team. The methodological quality (MQ) of each study was determined using 17 items adapted from validated measures (Downs & Black, 1998; Fowkes & Fulton, 1991; Jadad et al., 1996; Miller et al., 1995). The highest possible MQ score was 25, with higher scores reflecting higher MQ. Inter-rater reliability was assessed for study, sample, design, methodological, and intervention characteristics. For categorical variables, coders agreed on 91% of the judgments (mean Cohen’s κ = 0.84). For continuous variables, reliability yielded an average intra-class correlation coefficient (ρ) of 0.85 across categories (median = 0.99). Coders discussed and resolved discrepancies; the principal investigator (LAJSS) resolved any remaining unresolved discrepancies.
Study Outcomes
Our primary outcome was pain intensity; physical functioning and depressive symptoms were secondary outcomes. Pain intensity was assessed using self-report measures that asked individuals to (a) rate their pain on a visual analog scale (VAS) or a numerical rating scale (daily diary) or (b) complete a multi-item pain assessment (e.g., Brief Pain Inventory (Cleeland & Ryan, 1994); McGill Pain Questionnaire (Melzack & Torgerson, 1971)). For the secondary outcomes, all studies used self-report measures to assess physical functioning (e.g., Brief Pain Inventory (Cleeland & Ryan, 1994); Roland-Morris Disability Questionnaire (Roland & Fairbank, 2000)) and depressive symptoms (e.g., Beck Depression Inventory (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961)). As is typical in many areas of research, a wide range of measures (or subscales) were used to assess pain. Two authors (ED, LAJSS) reviewed each pain measure in depth to determine the content. For the present meta-analysis, “pain intensity” and “pain severity” represented the pain intensity factor. Similarly, “functional limitations,” “disability,” and “pain interference” represented the physical functioning factor. Due to the limited number of studies measuring additional outcomes of interest, we were unable to assess stress processes (e.g., problem-focused coping), health behaviors (e.g., sleep quality), physiological indicators (e.g., heart rate variability), and other outcomes (e.g., cortisol) in the current meta-analysis.
Summary Measures and Synthesis of Results
Summary effect sizes (d) were calculated based on the standardized mean-change, controlling for baseline (Becker, 1988). Positive effect sizes indicated that participants who received the intervention reported lower pain intensity, improved physical functioning, or fewer depressive symptoms relative to controls. Effect sizes were weighted by sample size to correct for sample size bias (Hedges, 1981). Two independent coders calculated effect sizes. Discrepancies between coders were discussed and corrected. Weighted mean ES (d+) and 95% confidence intervals were calculated using random-effects assumptions using a maximum likelihood approach (Lipsey & Wilson, 2001). Box plots for each outcome identified outliers (Emerson & Strenio, 1983). Heterogeneity was assessed using the Q statistic; the I2 index and 95% confidence intervals were calculated to assess the proportion of heterogeneity across the studies (Higgins, Thompson, Deeks, & Altman, 2003; Huedo-Medina, Sanchez-Meca, Marin-Martinez, & Botella, 2006). Moderator analyses were performed with meta-regression and an analog to the ANOVA using a maximum likelihood random-effects model (Hedges, 1994; Lipsey & Wilson, 2001). The analyses were performed using Stata/SE 15.1 using published macros (StataCorp, 2017). Comprehensive Meta-Analysis was used to generate the forest plot for pain intensity (Borenstein, Hedges, Higgins, & Rothstein, 2013).
Risk of Bias Across Studies
Two methods (visual inspection and statistical tests of funnel plots) were used to evaluate the evidence for the presence of small-study effects (Begg & Mazumdar, 1994; Egger, Davey Smith, Schneider, & Minder, 1997; Sterne & Egger, 2001). These tests were conducted only for dependent variables with ≥10 studies (Lau, Ioannidis, Terrin, Schmid, & Olkin, 2006).
RESULTS
Study Selection
Our searches identified 2,909 unique records of which 2,108 records were excluded based on title and abstract review (559 records did not meet any inclusion criteria; 760 records were reviews, commentaries, or editorials; 108 records were protocols; 94 records were qualitative). An additional 587 records were excluded because they did not sample military personnel with chronic pain. Upon review of the full text of the remaining 801 records, an additional 774 records were excluded (see Figure 1 for details). Thus, the final sample included 12 studies and 15 supplemental manuscripts providing additional study information. An overview of the study, sample, and intervention details for the 12 included studies (k = 15 interventions) can be found in Table 1.
Figure 1.
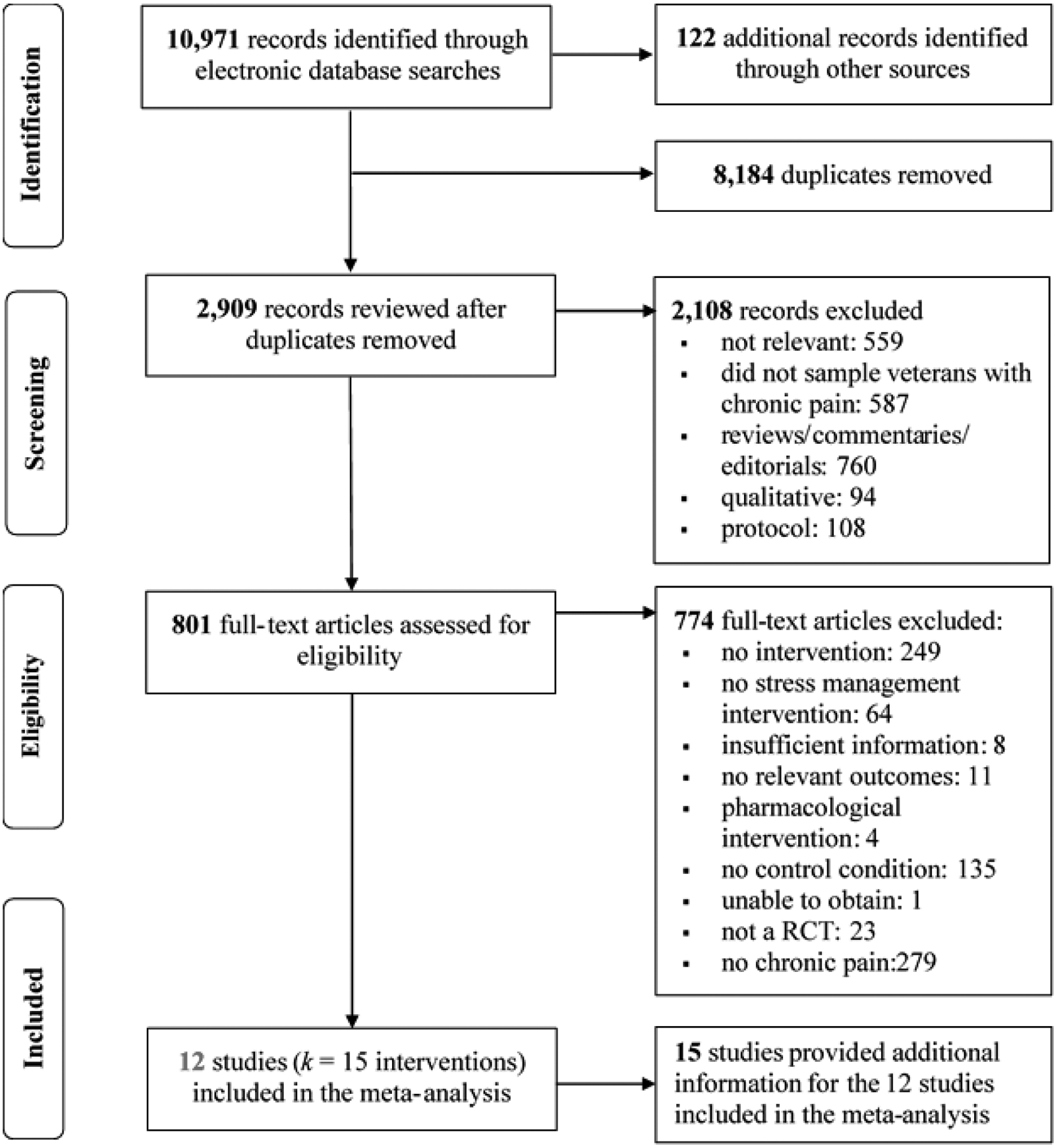
PRISMA Flow Diagram Screening and Selection Process
Table 1.
Study, Sample, and Intervention Characteristics of the 10 Studies (k = 12) included in the Meta-Analysis
| Study | Sample | Recruitment/Location | Condition | Current Treatment | Control | Intervention Details | Sessions | Dosea | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Appelbaum (1988); Linked: Appelbaum (1986) | N = 18 (39%); 11% F; Mage = 62; Myears DX = 15; 100% veterans | Outpatients referred through rheumatology or rehabilitation medicine services at the Albany VAMC; Albany, NY | RA (Stage II or III) | Pharma | WL | Cognitive Behavioral Pain Management. Theory-based cognitive behavioral pain management intervention delivered over 6 weeks that included PMR, thermal biofeedback, and cognitive pain management strategies (i.e., stress and coping, problem-solving skills). Problem-solving homework was required; HP of relaxation and biofeedback exercises was encouraged. | 10 | 630 | PI; PF; Dep |
| Berry (2014) | N = 14 (100%); 7% F; Mage = 45; 100% veterans | Patients at the Wm. Jennings Bryan Dorn VAMC; Columbia, SC | Chronic pain, injury-related | NR | SC | Biofeedback. Heart rate variability coherence biofeedback delivered over 4 weeks that included controlled breathing and self-regulation training. No HP was reported. | 4 | 160b | PI;PF |
| Carmody (2013) | N = 101 (67%); 3% F; Mage = 68; Myears dx = 18; 100% veterans | Physician referrals, flyers, and letters sent to veterans ≥55 years of age at the San Francisco VAMC or affiliated VA community-based outpatient clinics; San Francisco, CA | Chronic pain, multiple | Pharma | Phone-delivered pain education , TM | Cognitive-Behavioral Therapy (telephone-delivered). Manualized form of cognitive-behavioral therapy for pain management delivered over 20 weeks, adapted for low literacy rural populations with a primary goal to facilitate adjustment to chronic pain by teaching methods to manage negative emotions/thoughts, and improve social functioning and coping. Handouts were provided to guide home assignments. | 12 | 540 | PI; Dep |
| Groessl (2017); Linked: Groessl (2015); Groessl, Liu, and Schmalzl (2017); Groessl et al. (2016) | N = 152 (72%); 26% F; Mage = 53; Myears DX = 15; 100% veterans | VA San Diego Healthcare System; San Diego, CA | Chronic LBP | Pharma | WL | Yoga. Hatha yoga consisting of physical yoga postures, movement sequences, regulated breathing, and brief meditation delivered over 12 weeks. A HP manual was provided; participants were encouraged to practice 15–20 minutes on days without an instructor-led yoga session. | 24 | 1440 | PI; PF; Dep |
| Hausmann (2018)c; Linked: Hausmann, Ibrahim, et al. (2018) | N = 360 (86%); 24% F; Mage = 64; 100% veterans | VA Pittsburg Healthcare System, Pittsburg, PA; VA Medical Center, Philadelphia, PA | KneeOA | Pharma and Nonpharma | Neutral activities, TM | Positive Psychology. Home-based intervention that included a series of activities to build positive psychology skills (e.g., reflection, gratitude, kindness, mindfulness) introduced via a brief, 5 to 15-minute telephone call once a week for 6 weeks. A workbook was provided; participants were encouraged to practice the weekly skill each day. | 6 | 60 | PI;PF |
| Hausmann (2017) | N = 42 (100%); 17% F; Mage = 68; 100% veterans | VA Pittsburg Healthcare System, Pittsburg, PA | Knee or HipOA | Pharma and Nonpharma | Neutral activities, TM | Positive Psychology. Home-based intervention that included a series of activities to build positive psychology skills (e.g., reflection, gratitude, kindness, mindfulness) introduced via a brief, 10 to 15-minute telephone call once a week for 6 weeks. A workbook was provided; participants were encouraged to practice the weekly skill each day. | 6 | 75 | PI;PF |
| Highland (2018); Linked (2017) | N = 68 (91%); 63% F; Mage = 44; 28% veterans, 59% active duty | Patients at Walter Reed National Medical Unit; Bethesda, MD | Chronic LBP | Pharma | SC | Yoga. Weekly individual therapeutic yoga sessions that focused on postural alignment, breath work, and centering delivered over 8 weeks. This program allowed for modification in postures. An audio CD was provided for optional HP. | 12 | 720 | PI;PF |
| Kearney (2016); Linked: Martinez (2016); Stephenson, Simpson, Martinez, and Kearney (2016) | N = 55 (82%); 15% F; Mage = 50; 100% veterans (100% PG); 78% on service connection disability | Flyers posted in clinics and letters sent to GW veterans in the VA Puget Sound Healthcare System; Seattle, WA | Chronic pain, GWI | Pharma and Psych | SC | Mindfulness-based Stress Reduction. Mindfulness meditation, body scan meditation, breathing practices, gentle yoga, walking meditation and loving kindness meditation delivered over 8 weeks. Participants were provided an audio CD and assigned 30–45 minutes of HP per day, 6 days per week. Informal practices (e.g., walking or eating mindfully) were also encouraged. Participants received additional readings and a workbook for tracking progress. | 9 | 1620 | PI; Dep |
| Lathia (2009) | N=31 (90%); 10% F; Mage = 62 Myears DX = 4; 100% veterans | Physical therapy unit of Philadelphia VAMC; Philadelphia, PA | Chronic shoulder pain | Pharma | Placebo | Acupuncture (individualized). Individualized treatment points based on patients’ symptoms delivered over 6 weeks. Participants were taught a 10-minute home exercise program (consisting of range-of-motion movements) to be completed 3x/day. | 12 | 360 | PI;PF |
| Acupuncture (standard). Used fixed, standard protocol treatment points delivered over 6 weeks. Participants were taught a 10-minute home exercise program (consisting of range-of-motion movements) to be completed 3x/day. | |||||||||
| Moore and Chaney (1985); Linked: Moore (1983) | N=43 (79%); 2% F; Mage = 49; Myears dx = 17; 81% receiving disability compensati on; 100% veterans | Patients in the VA Puget Sound Healthcare System; Seattle, Washington | Chronic pain, multiple | Pharma | WL | Cognitive-Behavioral Therapy (individual). Group-based lecture/discussion on gate control concepts of pain; goal-setting exercises; and relaxation techniques (i.e., imagery, autogenic training, PMR, breathing exercises) delivered over 4 weeks. Participants were given audiotaped relaxation exercises and asked to practice relaxation 2x/day for 15 minutes. | 8 | 960 | PI; PF; Dep |
| Cognitive-Behavioral Therapy (couples). Group-based lecture/discussion on gate control concepts of pain; goal-setting exercises; and relaxation techniques (i.e., imagery, autogenic training, PMR, breathing exercises) delivered over 4 weeks. Participants were given audiotaped relaxation exercises and asked to practice relaxation 2x/day for 15 minutes. | |||||||||
| Nassif (2016); Linked: Nassif (2013); Nassif, Norris, Soltes, Blackman, etal. (2014); Nassif, Norris, Soltes, Sandbrink, etal. (2014) | (77%); 0% F; Mage = 47; 100% veterans (100% OIF/OEF), 78% army | War Related Illness and Injury Study Center, Washington DC VAMC; Washington, DC | Chronic musculosk eletal pain | NR | WL | Mindfulness Meditation. Included guided imagery, PMR, and body sensing techniques delivered over 8 weeks. Participants were provided with audio recordings for HP and a workbook to log self-practice. | 16 | 960 | PI; Dep |
| Otis (2017) | N = 23 (74%); 9% F; Mage = 50; 100% veterans | VA Boston and Connecticut Healthcare Systems; West Haven, CT; Boston, MA | Comorbid chronic pain and PTSD | NR | WL | Intensive Treatment. Outpatient therapy sessions for pain and PTSD, including cognitive-behavioral therapy and cognitive processing therapy components, delivered over 3 weeks. Both cognitive-behavioral therapy and cognitive processing therapy require HP. | 6 | 540 | PI |
Note: N (%), number of participants who consented to participate in the study (retention); F, female; Dx, diagnosis; OIF, Operation Iraqi Freedom; OEF, Operation Enduring Freedom; PG, Persian Gulf war; GW, gulf war; GWI, gulf war illness; VA, Veteran’s Administration; VAMC, Veteran’s Administration Medical Center; RA, rheumatoid arthritis; OA, osteoarthritis; LBP, lower back pain; PTSD, posttraumatic stress disorder; Pharma, pharmacological; Psych, psychological; AO, assessment only; WL, waitlist control; SC, standard of care; TM, time-matched; PMR, progressive muscle relaxation; HP, home practice; NR, not/none reported; PF, physical functioning; Dep, depressive symptoms; PI, pain intensity.
Estimated total dose (sessions × minutes) of the intervention.
Dose was estimated from Thurber et al. (2010).
Data were only reported by race (White/African American); the effect size computed for each group was treated as a separate intervention in the analyses because each effect size represented an independent subgroup.
Study and Sample Characteristics
The studies were published between 1985 and 2018 (M = 2011, SD = 12). Most studies were published in journals (92%); the remaining study included a clinical trial (Otis, 2017). Military personnel were nearly always recruited from Veterans Affairs Medical Centers (VAMC; 11 out of 12 studies); one study recruited veterans and active duty military members from a National Military Medical Center (Highland et al., 2018). Patients were recruited directly (referrals from clinic staff; 67%) or indirectly (self-referrals after seeing posted flyers or brochures, receiving a mailed letter, or telephone calls following the receipt of a mailed invitation letter; 33%). A total of 920 military members (96% veterans) living with chronic pain consented to participate in the studies; mean retention rate was 78% (SD = 0.15; range = 39–100%). Samples included 16% women (SD = 0.17) with a mean age of 55 years (SD = 9; range = 44–68 years). Participants were predominately White (M = 59%, SD = 0.23; nstudies = 9) and married (M = 56%, SD = 0.21; nstudies = 7). Six of the twelve studies sampled patients with multiple chronic pain conditions; two studies sampled patients with chronic lower back pain (Groessl, Liu, Chang, et al., 2017; Highland et al., 2018), two studies sampled patients with osteoarthritis (Hausmann, Youk, et al., 2018; Hausmann et al., 2017) one study involved patients with chronic shoulder pain (Lathia, Jung, & Chen, 2009), and one study sampled patients with Stage II/III rheumatoid arthritis (Appelbaum, Blanchard, Hickling, & Alfonso, 1988). One study enrolled patients with comorbid PTSD (Otis, 2017). Five studies reported length of time since pain diagnosis (median = 17, range = 4–21 years).
Intervention Characteristics
All CIH approaches were designed to promote chronic pain management among military personnel. One approach also targeted veterans with comorbid chronic pain and PTSD (Otis, 2017). The CIH practices included cognitive-behavioral therapies (Appelbaum et al., 1988; Carmody et al., 2013; Moore & Chaney, 1985; Otis, 2017), positive psychology program that included mindfulness (Hausmann, Youk, et al., 2018; Hausmann et al., 2017), yoga (Groessl, Liu, Goodman, et al., 2017; Highland et al., 2018), mindfulness-based interventions (Kearney et al., 2016; Nassif, 2013), acupuncture (Lathia et al., 2009), and biofeedback (Berry et al., 2014). Interventions included a median of 10 sessions (M = 10, SD = 5, range = 4–24) lasting a median of 60 minutes each (M = 66, SD = 48, range = 10–180) delivered over a median of 6 weeks (M = 7, SD = 4, range = 3–20). Home assignments and/or practice were encouraged in many interventions (93%); this included practicing new skills (e.g., coping strategies), cognitive restructuring, or mind-body exercises such as yoga or mindfulness (see Table 1 for details).
Design Characteristics
All studies used a RCT design. The control conditions reported were treatment as usual (50%), wait-list or assessment-only (17% of studies), or active comparison (i.e., sham non-penetrating acupuncture, time-matched pain education or neutral activities; 33%). The four studies that used an active comparison condition included a median of 9 sessions (M = 9, SD = 3, range = 6–12) lasting 21 minutes each (M = 24, SD = 16, range = 10–45). The median number of post-intervention follow-up assessments was two (range = 1 to 3). Assessments were conducted at immediate post-intervention through 26 weeks post-intervention. Because all studies measured the primary outcome (pain intensity) within the month following the intervention, our analyses focused on the first post-intervention assessment (Mdn = 0 weeks; range = 0 to 4.33; k = 15).1 Exploratory analyses examined the overall efficacy of CIH approaches on the primary (pain intensity) and secondary (physical functioning, depressive symptoms) outcomes observed at the last assessment for the six studies that included a delayed assessment (Mdn = 26 weeks; k = 7).2 The overall proportion of MQ criteria met across studies was 70% (SD = 10%; range = 56–88%).
Synthesis of Results
The effect sizes for the primary (pain intensity) and secondary (physical functioning, depressive symptoms) outcomes were examined for outliers prior to analyses (Emerson & Strenio, 1983). A single outlier was detected for physical functioning (Berry et al., 2014) and excluded from subsequent analyses.3 Intervention participants reported greater reductions in pain intensity (d+ = 0.44, 95% CI = 0.21–0.67, k = 15) compared to controls (see Table 2). The hypothesis of homogeneity was not supported for pain intensity (Q [14] = 30.23, p = .007); the proportion of variation due to study heterogeneity exceeded the 50% threshold (I2=54; 95% CI = 17, 74). Mixed findings were observed for the secondary outcomes, that is, intervention participants reported greater improvements in physical functioning (d+ = 0.36, 95% CI = 0.11–0.61, k= 11) but not depressive symptoms (d+ = 0.21, 95% CI = −0.15–0.57, k = 7) relative to controls. The hypothesis of homogeneity was not supported for physical functioning (Q [7] = 19.69, p = .032) and the proportion of variance due to heterogeneity exceeded the 50% threshold (I2=49; 95% CI = 0, 75). The hypothesis of homogeneity was supported for depressive symptoms (Q [6] = 5.94, p = .430; I2=0). Exploratory analyses revealed a significant improvement in physical functioning (d+ = 0.33, 95% CI = 0.03–0.63, k = 5) among participants in the intervention vs. controls at a delayed follow-up but the hypothesis of homogeneity was not supported (Q [4] = 16.10, p = .003; I2=75; 95% CI = 39, 90). There was no significant difference between interventions and controls on pain intensity (d+ = 0.24, 95% CI = −0.04–0.51, k = 7) or depressive symptoms (d+ = 0.29, 95% CI = −0.13–0.70, k = 4) at the delayed assessment. The proportion of MQ criteria met was unrelated to the overall weighted mean effect sizes for pain intensity, physical functioning, or depressive symptoms at the first (ps = .368−.492) or delayed (ps = .403–.593) assessments.
Table 2.
Summary Effect Sizes for Pain Intensity between Intervention and Controls at Postintervention.
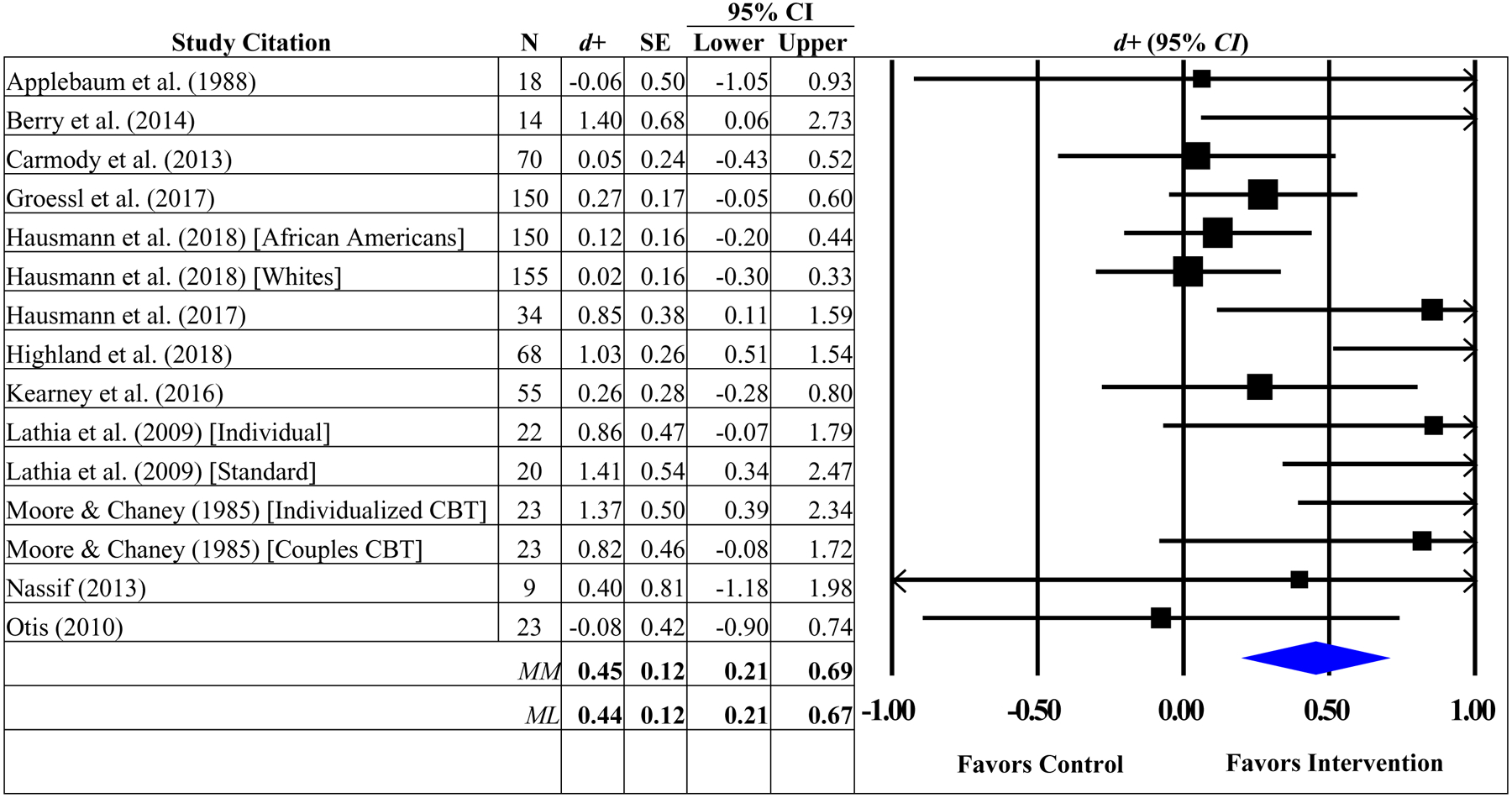
|
Note. The overall weighted mean effect sizes were calculated using random-effects models with methods of moments (MM) and full information maximum likelihood (ML) methods to estimate the between-study variance. Weighted mean effect sizes (d+) are positive for differences that favor the intervention relative to control groups. d+, weighted mean effect size; SE, standard error; CI, confidence interval; CBT, cognitive-behavioral therapy
Predictors of Pain Intensity
Moderator analyses were used to determine if the sample (proportion women, age, mean years diagnosed with chronic pain condition, depressive symptoms) or intervention characteristics such as duration (number of intervention sessions/minutes, number of home practice sessions/minutes), delivery mode (individual or group), type of CIH approach (cognitive-behavioral therapies or mind-body practices), and type of control (active vs. passive) explained the variability in the overall weighted mean effect sizes for the primary outcome (pain intensity). None of the hypothesized sample or intervention characteristics moderated the effect sizes for pain intensity or physical functioning (data not shown).
Risk of Bias Across Studies
The risk of bias was assessed only for pain intensity (k = 15) and physical functioning (k =11) due to the limited number of interventions measuring depressive symptoms (k = 7). The funnel plots for pain intensity and physical functioning appear in the Online Supplemental Materials (Figures S1 and S2). A visual inspection of the funnel plot revealed asymmetries that might be interpreted as small-study effects for pain intensity. Begg (Begg & Mazumdar, 1994) and Egger’s (Egger et al., 1997) statistical tests also indicated evidence for the presence of small study effects (Begg: Δx–y = 43, z = 2.08, p = .038; Egger: bias coefficient = 1.87, SE = 0.67, p = .016). Trim and fill procedures (Duval & Tweedie, 2000) estimated that five studies measuring pain intensity could be missing which may have changed the interpretation of our findings (i.e., estimated effect size with the imputed missing studies: d+ = 0.22, 95% CI = −0.05, 0.48) but the results of this test should be interpreted with caution given that simulation studies have consistently shown that this method performs poorly in the presence of substantial between-study heterogeneity (Peters, Sutton, Jones, Abrams, & Rushton, 2007; Terrin, Schmid, Lau, & Olkin, 2003). The visual inspection of the funnel plot and statistical tests revealed no asymmetries that might be interpreted as small-study effects for physical functioning (Begg: Δx–y = 9, z = 0.62, p = .533; Egger: bias coefficient = 1.39, SE = 0.82, p = .126).
Discussion
Chronic pain causes both physical and emotional suffering and interferes with daily functioning. Furthermore, the psychological stress associated with managing chronic pain symptoms, pain medications and their side effects, and medical follow-up care place a significant burden on patients. Previous research suggests interventions that provide cognitive and behavioral coping strategies to manage stress may be particularly valuable to patients living with chronic pain (Sturgeon, 2014). The current meta-analytic study advances the literature supporting CIH approaches for chronic health conditions (Dunne et al., 2019; Scott-Sheldon, Balletto, et al., 2019; Scott-Sheldon, Gathright, et al., 2020; Scott-Sheldon et al., 2008) by focusing on military populations experiencing chronic pain. Our meta-analysis showed that CIH practices reduced chronic pain intensity and improved physical functioning for active duty military and veterans with chronic pain.
The magnitude and direction of our findings are consistent with the Agency for Healthcare Research and Quality (AHRQ) report on nonpharmacological treatment for chronic pain conditions across multiple patient populations (Skelly et al., 2018).4 The AHRQ report found that psychological (e.g., cognitive behavioral therapy) and mind-body (e.g., yoga) interventions lessened pain severity and improved physical functioning among patients with chronic back pain, osteoarthritis, and fibromyalgia, with the overall effect sizes ranging from low to moderate in the short-term. Consistent with the AHRQ report, CIH approaches included in our meta-analysis resulted in an overall effect size of moderate strength for mind-body interventions (pain severity: d = 0.49, 95% CI = 0.21–0.77, k = 10; physical functioning: d = 0.34, 95% CI = 0.07–0.61, k = 8) but, in contrast with the AHRQ report, the effects were not significant for cognitive-behavioral therapies (pain severity: d = 0.34, 95% CI = −0.08–0.77, k = 5; physical functioning: d = 0.49, 95% CI = −0.14–1.13, k = 3). Because few studies included in this meta-analysis evaluated cognitive-behavioral therapies, we may have been underpowered to detect a significant overall between-group difference. Finally, our meta-analysis showed that mind-body interventions resulted in low to moderate improvements in physical functioning among military members at a delayed follow-up (i.e., 26 weeks following completion of the intervention). None of the studies evaluated cognitive-behavioral therapies at a delayed follow-up.
CIH approaches can provide military populations with the skills to manage their chronic pain. For instance, cognitive-behavioral therapy for chronic pain has been tailored to military veterans and includes sessions focused on restructuring maladaptive pain-related thinking, relaxation strategies, and physical activity pacing (Murphy et al., 2014). Yoga is effective at reducing pain intensity (Büssing, Ostermann, Lüdtke, & Michalsen, 2012; K. A. Williams et al., 2005) and improving mood-related outcomes (e.g., depressive symptoms, anxiety) for patients with chronic pain conditions (Lavey et al., 2005). Similarly, tai chi, considered as a gentle “moving meditation,” has been found to reduce pain (Hall, Maher, Latimer, & Ferreira, 2009) and positively influence physiological biomarkers of stress (e.g., heart rate, cortisol level) (Sandlund & Norlander, 2000). Lastly, acupuncture is frequently offered for military veterans experiencing tinnitus and symptoms of post-traumatic stress. Veterans with chronic pain may benefit from engaging in one or more of these CIH practices.
Contrary to study hypotheses, our meta-analysis did not find a significant difference between intervention participants and controls on depression, nor did depression serve as a moderator for treatment effects. These findings contrast with the prior literature (Gilpin, Keyes, Stahl, Greig, & McCracken, 2017), and may have been a result of the limited number of studies assessing depressive symptoms. In fact, depressive symptoms were reduced in six out of the seven interventions measuring this outcome, but these reductions were typically not significantly different with controls (one exception: Kearney et al. (2016)). Additionally, there may have been a restricted range of baseline depressive symptoms, as none of the included studies reported recruiting patients experiencing high levels of psychological distress. CIH approaches are likely to be more beneficial for individuals who are experiencing distress, yet few studies adequately assess samples for baseline levels of distress. Future studies should evaluate the efficacy of CIH practices among military populations with different levels of psychological distress. In addition to depressive symptoms, the present study examined several hypothesized moderators (e.g., participant sex, type of CIH approach, intervention dose) and did not find significant effects. This was surprising, given the substantial heterogeneity observed but may be most parsimoniously explained as the result of the variability in reporting of demographic and secondary factors across studies, which limited the power of the moderator tests.
Over the past decade, the U.S. military has made substantial efforts to promote chronic pain management, to reduce opioid prescribing, and to increase access to nonpharmacological and integrative medicine approaches. The Army appointed a Pain Management Task Force to coordinate treatment efforts within the Department of Defense and the Veterans Health Administration in 2009 to offer “holistic, multidisciplinary, and multimodal” treatment for veterans with chronic pain (Pain Management Task Force, 2010). The military also deployed the Sole Provider Program and the Controlled Drug Management Analysis and Reporting Tool to help identify and monitor the risk and misuse of opioid use (Sharpe Potter, Bebarta, Marino, Ramos, & Turner, 2014). These changes in chronic pain monitoring and treatment was necessitated by the significant opioid epidemic in the U.S. and the realization by the scientific and medical communities that opioid medications do not provide a long-term solution for chronic pain. Pharmacological interventions are insufficient for addressing the functional and emotional symptoms of living with chronic pain. In fact, a critical component of effective chronic pain management is motivating patients to engage in physical activity. Often patients with chronic pain develop a fear of movement, or kinesiophobia, which leads to a decline in activity and muscle deconditioning. VAMCs are increasingly using psychological, physical, and mind-body approaches to chronic pain management (Elwy et al., 2014). For example, “Whole Health” initiatives within the VA system are transforming management of chronic health conditions by utilizing “physical, mental, emotional, spiritual, and environmental elements that work together to provide the best quality of life for each Veteran” (U.S. Department of Veterans Affairs, 2017).
Limitations
The current meta-analysis is limited by several factors. First, only a small number of studies met the inclusion criteria limiting our ability to examine additional psychological outcomes often associated with chronic pain (e.g., anxiety, pain catastrophizing). In addition, the current meta-analysis is also limited by the heterogeneous group of chronic pain types given the small number of studies published using CIH approaches for pain among military populations. Future meta-analyses targeting specific chronic pain conditions among U.S. military members should be conducted as additional studies become available. Second, moderator tests were restricted by the data reported in the included studies. Potential predictors of pain severity that could not be tested included status (active-duty vs. military veterans), “theater” (i.e., where veterans served in combat), type of pain medications, and level of disability related to military service. Future studies should report these factors as they may be informative to clinical decision-making. Third, we could not detect long-term benefits because none of the studies included an extended follow-up (i.e., >6 months). Because many of the studies included in this meta-analysis included small samples, with relatively short-term improvements, we recommend that future studies recruit larger samples and follow participants longer (e.g., 12 to 18 months). Finally, study samples were primarily limited to White male veterans which limits the generalizability of the findings to all military members. Future studies should include a representative sample of military members including the active-duty force.
Conclusions
Increasingly, patients and providers are looking to nonpharmacological, nonsurgical treatment options for chronic pain management. Our results indicated that CIH approaches provide an efficacious treatment option for veterans experiencing chronic pain. This research provides support for the use of CIH practices with active duty and military veterans experiencing chronic pain. Additional randomized studies are needed with longer follow-up periods to determine the long-term benefits of CIH practices in chronic pain management. Future research should continue to evaluate CIH practices with military populations, as rates of chronic pain continue to be a major concern for returning veterans and their families.
Supplementary Material
Acknowledgements
The research reported in this paper was supported by the National Center for Complementary and Integrative Health of the National Institutes of Health under award number 5R01AT008815 to Lori A. J. Scott-Sheldon, PhD and Michael P. Carey, PhD (Multiple PIs). Eugene M. Dunne, PhD was supported by the Adolescent/Young Adult Biobehavioral HIV Training Grant (T32MH078788; Larry K. Brown, PI) from the National Institute of Mental Health and Emily C. Gathright, PhD was supported by the Cardiovascular Behavioral Medicine Training Grant (T32HL076134; Rena R. Wing, PI) from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
One study conducted two assessments within the first month following the completion of the intervention (i.e., immediate post-test and a follow-up assessment at 4 weeks); only the immediate post-test was included in the analyses (Nassif et al., 2016).
The between-group effect sizes for a single study (k = 2) could not be included because the wait-list control group received the intervention prior to the 3-month follow-up (Moore & Chaney, 1985).
The analyses including the outlier did not change the overall magnitude or the direction of the weighted mean effect size for physical functioning (d+ = 0.42, 95% CI = 0.16–0.68, k= 12; Q [11] = 27.74, p = .004; I2=60, 95% CI = 25, 79).
Our meta-analysis focused on studies sampling military populations and therefore resulted in only one overlapping study with the AHRQ report (i.e., Groessl et al., 2017).
References
- Appelbaum KA (1986). The Psychophysiological Treatment of Stage II and Stage III Rheumatoid Arthritis: A Controlled Evaluation. (8703986 Doctoral Dissertation), State University of New York at Albany, Ann Arbor. ProQuest Dissertations & Theses Global database. [Google Scholar]
- Appelbaum KA, Blanchard EB, Hickling EJ, & Alfonso M (1988). Cognitive behavioral treatment of a veteran population with moderate to severe rheumatoid arthritis. Behavior Therapy, 19(4), 489–502. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, & Erbaugh J (1961). An inventory for measuring depression. Arch Gen Psychiatry, 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Becker BJ (1988). Synthesizing standardized mean-change measures. British Journal of Mathematical and Statistical Psychology, 41(2), 257–278. [Google Scholar]
- Begg CB, & Mazumdar M (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, 50(4), 1088–1101. [PubMed] [Google Scholar]
- Berry ME, Chapple IT, Ginsberg JP, Gleichauf KJ, Meyer JA, & Nagpal ML (2014). Non-pharmacological intervention for chronic pain in veterans: A pilot study of heart rate variability biofeedback. Global Advances In Health and Medicine, 3(2), 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, & Rothstein H (2013). Comprehensive Meta-Analysis. Englewood, NJ: Biostat. [Google Scholar]
- Büssing A, Ostermann T, Lüdtke R, & Michalsen A (2012). Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. The Journal of Pain, 13(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Carmody TP, Duncan CL, Huggins J, Solkowitz SN, Lee SK, Reyes N, … Simon JA (2013). Telephone-delivered cognitive-behavioral therapy for pain management among older military veterans: a randomized trial. Psychol Serv, 10(3), 265–275. [DOI] [PubMed] [Google Scholar]
- Cifu DX, Taylor BC, Carne WF, Bidelspach D, Sayer NA, Scholten J, & Campbell EH (2013). Traumatic brain injury, posttraumatic stress disorder, and pain diagnoses in OIF/OEF/OND Veterans. J Rehabil Res Dev, 50(9), 1169–1176. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, & Ryan KM (1994). Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore, 23(2), 129–138. [PubMed] [Google Scholar]
- Crombez G, Eccleston C, Van Damme S, Vlaeyen JW, & Karoly P (2012). Fear-avoidance model of chronic pain: the next generation. Clin J Pain, 28(6), 475–483. [DOI] [PubMed] [Google Scholar]
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, … Helmick C (2018). Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. Morbidity and Mortality Weekly Report, 67(36), 1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SH, & Black N (1998). The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of Epidemiology & Community Health, 52, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne EM, Balletto BL, Donahue ML, Feulner MM, DeCosta J, Cruess DG, … Scott-Sheldon LAJ (2019). The benefits of yoga for people living with HIV/AIDS: A systematic review and meta-analysis. Complement Ther Clin Pract, 34, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S, & Tweedie R (2000). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–463. doi: 10.1111/j.0006-341x.2000.00455.x [DOI] [PubMed] [Google Scholar]
- Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, & Page GG (2008). Association of catastrophizing with interleukin-6 responses to acute pain. Pain, 140(1), 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, & Minder C (1997). Bias in meta-analysis detected by a simple, graphical test. Br Med J, 315(7109), 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwy AR, Johnston JM, Bormann JE, Hull A, & Taylor SL (2014). A systematic scoping review of complementary and alternative medicine mind and body practices to improve the health of veterans and military personnel. Med Care, 52(12 Suppl 5), S70–82. [DOI] [PubMed] [Google Scholar]
- Emerson JD, & Strenio J (1983). Boxplots and batch comparison. In Hoaglin DC, Mosteller F, & Tukey JW (Eds.), Understanding Robust and Exploratory Data Analysis (pp. 58–96). New York: John Wiley & Sons. [Google Scholar]
- Evans EA, Herman PM, Washington DL, Lorenz KA, Yuan A, Upchurch DM, … Taylor SL (2018). Gender Differences in Use of Complementary and Integrative Health by U.S. Military Veterans with Chronic Musculoskeletal Pain. Womens Health Issues, 28(5), 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A, & Rosenheck R (2008). Treatment-seeking veterans of Iraq and Afghanistan: comparison with veterans of previous wars. J Nerv Ment Dis, 196(7), 513–521. [DOI] [PubMed] [Google Scholar]
- Pain Management Task Force (2010). Providing a standardized DoD and VHA vision and approach to pain management to optimize the care for warriors and their families. In: Office of the Army Surgeon General Washington, DC Retrieved from https://www.dvcipm.org/site/assets/files/1070/pain-task-force-final-report-may-2010.pdf
- Fowkes FG, & Fulton PM (1991). Critical appraisal of published research: Introductory guidelines. Br Med J, 302(6785), 1136–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin HR, Keyes A, Stahl DR, Greig R, & McCracken LM (2017). Predictors of treatment outcome in contextual cognitive and behavioral therapies for chronic pain: a systematic review. The Journal of Pain, 18(10), 1153–1164. [DOI] [PubMed] [Google Scholar]
- Groessl EJ (2015, October 9, 2017). Yoga for Veterans with Cronic Low Back Pain. Retrieved from https://clinicaltrials.gov/ct2/show/study/NCT02524158?term=nct02524158&rank=1§=X01256
- Groessl EJ, Liu L, Goodman D, Chang DG, Wetherell JL, Bormann Jill E, … Schmalzl L (2017). Yoga for military veterans with chronic low back pain: A randomized clinical trial. American Journal of Preventive Medicine, 53, 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groessl EJ, Liu L, & Schmalzl L (2017). Yoga Therapy for Veterans with Chronic Low Back Pain. Annals of Behavioral Medicine, 51, S875–S876. [Google Scholar]
- Groessl EJ, Schmalzl L, Maiya M, Liu L, Goodman D, Chang DG, … Baxi S (2016). Yoga for veterans with chronic low back pain: Design and methods of a randomized clinical trial. Contemp Clin Trials, 48, 110–118. Retrieved from http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/277/CN-01167277/frame.html doi: 10.1016/j.cct.2016.04.006 [DOI] [PubMed] [Google Scholar]
- Hall A, Maher C, Latimer J, & Ferreira M (2009). The effectiveness of Tai Chi for chronic musculoskeletal pain conditions: A systematic review and meta-analysis. Arthritis Care & Research: Official Journal of the American College of Rheumatology, 61(6), 717–724. [DOI] [PubMed] [Google Scholar]
- Hausmann LRM, Ibrahim SA, Kwoh CK, Youk A, Obrosky DS, Weiner DK, … Parks A (2018). Rationale and design of the Staying Positive with Arthritis (SPA) Study: A randomized controlled trial testing the impact of a positive psychology intervention on racial disparities in pain. Contemp Clin Trials, 64, 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann LRM, Youk A, Kwoh CK, Gallagher RM, Weiner DK, Vina ER, … Ibrahim SA (2018). Effect of a Positive Psychological Intervention on Pain and Functional Difficulty Among Adults With Osteoarthritis A Randomized Clinical Trial. Jama Network Open, 1(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann LRM, Youk A, Kwoh CK, Ibrahim SA, Hannon MJ, Weiner DK, … Parks A (2017). Testing a Positive Psychological Intervention for Osteoarthritis. Pain Medicine, 18(10), 1908–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RP, Kreuter M, Resnicow K, Fishbein M, & Dijkstra A (2008). Understanding tailoring in communicating about health. Health education research, 23(3), 454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV (1981). Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational and Behavioral Statistics, 6, 107–128. [Google Scholar]
- Hedges LV (1994). Fixed effects models. In Cooper H & Hedges LV (Eds.), The Handbook of Research Synthesis (pp. 285–299). New York: Russell Sage Foundation. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. Br Med J, 327(7414), 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highland KB, Schoomaker A, Rojas W, Suen J, Ahmed A, Zhang Z, … Buckenmaier CC (2018). Benefits of the Restorative Exercise and Strength Training for Operational Resilience and Excellence Yoga Program for Chronic Low Back Pain in Service Members: A Pilot Randomized Controlled Trial. Archives of Physical Medicine and Rehabilitation, 99(1), 91–98. [DOI] [PubMed] [Google Scholar]
- Highland KB, Schoomaker A, Rojas W, Suen JP, Ahmed A, Zhang ZW, … Buckenmaier C (2017). Yoga Treatment for Chronic Lower Back Pain in the Military: Who Benefits the Most? Annals of Behavioral Medicine, 51, S1278–S1279. [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, & Koffman RL (2004). Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med, 351(1), 13–22. [DOI] [PubMed] [Google Scholar]
- Hollifield M, Sinclair-Lian N, Warner TD, & Hammerschlag R (2007). Acupuncture for posttraumatic stress disorder: a randomized controlled pilot trial. J Nerv Ment Dis, 195(6), 504–513. [DOI] [PubMed] [Google Scholar]
- Hoot MR, Levin HS, Smith AN, Goldberg G, Wilde EA, Walker WC, … Pugh NL (2018). Pain and chronic mild traumatic brain injury in the US military population: a Chronic Effects of Neurotrauma Consortium study. Brain Inj, 32(10), 1169–1177. [DOI] [PubMed] [Google Scholar]
- Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, & Botella J (2006). Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods, 11, 193–206. [DOI] [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, & McQuay HJ (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials, 17(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Jonas WB, & Schoomaker EB (2014). Pain and Opioids in the Military: We Must Do Better. JAMA Internal Medicine, 174(8), 1402–1403. [DOI] [PubMed] [Google Scholar]
- Jones J, Rutledge DN, Jones KD, Matallana L, & Rooks DS (2008). Self-assessed physical function levels of women with fibromyalgia: a national survey. Womens Health Issues, 18(5), 406–412. [DOI] [PubMed] [Google Scholar]
- Kearney DJ, McDermott K, Malte C, Martinez M, & Simpson TL (2012). Association of participation in a mindfulness program with measures of PTSD, depression and quality of life in a veteran sample. J Clin Psychol, 68(1), 101–116. [DOI] [PubMed] [Google Scholar]
- Kearney DJ, McDermott K, Malte C, Martinez M, & Simpson TL (2013). Effects of participation in a mindfulness program for veterans with posttraumatic stress disorder: a randomized controlled pilot study. J Clin Psychol, 69(1), 14–27. Retrieved from http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/410/CN-00906410/frame.html doi: 10.1002/jclp.21911 [DOI] [PubMed] [Google Scholar]
- Kearney DJ, Simpson TL, Malte CA, Felleman B, Martinez ME, & Hunt SC (2016). Mindfulness-based stress reduction in addition to usual care is associated with improvements in pain, fatigue, and cognitive failures among veterans with gulf war illness. American Journal of Medicine, 129(2), 204–214. [DOI] [PubMed] [Google Scholar]
- Kramarow EA, & Pastor PN (2012). The health of male veterans and nonveterans aged 25–64: United States, 2007–2010. NCHS Data Brief(101), 1–8. [PubMed] [Google Scholar]
- Lathia AT, Jung SM, & Chen LX (2009). Efficacy of acupuncture as a treatment for chronic shoulder pain. Journal of Alternative and Complementary Medicine, 15(6), 613–618. [DOI] [PubMed] [Google Scholar]
- Lau J, Ioannidis JP, Terrin N, Schmid CH, & Olkin I (2006). The case of the misleading funnel plot. BMJ, 333(7568), 597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavey R, Sherman T, Mueser KT, Osborne DD, Currier M, & Wolfe R (2005). The effects of yoga on mood in psychiatric inpatients. Psychiatr Rehabil J, 28(4), 399. [DOI] [PubMed] [Google Scholar]
- Lehavot K, Hoerster KD, Nelson KM, Jakupcak M, & Simpson TL (2012). Health indicators for military, veteran, and civilian women. Am J Prev Med, 42(5), 473–480. [DOI] [PubMed] [Google Scholar]
- Lichtenthal WG, Simpson NS, & Cruess DG (2005). Stress Management Interventions for Medical Populations. In Oxington KV (Ed.), Psychology of Stress (pp. 35–52). New York: Nova Biomedical Books. [Google Scholar]
- Lipsey MW, & Wilson DB (2001). Practical meta-analysis. Thousand Oaks, CA: Sage. [Google Scholar]
- Madsen C, Vaughan M, & Koehlmoos TP (2017). Use of integrative medicine in the United States Military Health System. Evidence-based Complementary and Alternative Medicine, 2017, Article ID 9529257-Article ID 9529257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez ME (2016). A combined analysis of two randomized controlled trials evaluating the effect of Mindfulness-Based Stress Reduction on self-reported emotional experience and physiological symptoms among veterans with Posttraumatic Stress Disorder. (10138426 Master’s), University of Washington, Ann Arbor. ProQuest Dissertations & Theses Global database. [Google Scholar]
- McAllister MJ (2013, November 4, 2013). Stress and Chronic Pain. Retrieved from https://www.instituteforchronicpain.org/blog/item/123-26stress-and-chronic-pain
- Melzack R, & Torgerson WS (1971). On the language of pain. Anesthesiology, 34(1), 50–59. [DOI] [PubMed] [Google Scholar]
- Miller WR, Brown JM, Simpson TL, Handmaker NS, Bien TH, & Luckie LF (1995). What works? A methodological analysis of the alcohol treatment outcome literature. In Hester RK & Miller WR (Eds.), Handbook of Alcohilsm Treatment Approaches: Effective Alternatives. (pp. 12–44). Boston, MA: Allyn & Bacon. [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, & Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med, 151, 264–269. [DOI] [PubMed] [Google Scholar]
- Moore JE (1983). Outpatient Cognitive-behavioral Group Treatment Of Chronic Pain: The Role Of Significant Others In Therapy (Order No. 8412795). Available from ProQuest Dissertations & Theses Global. (303164396). Retrieved from https://search.proquest.com/docview/303164396?accountid=9758
- Moore JE, & Chaney EF (1985). Outpatient group treatment of chronic pain: Effects of spouse involvement. J Consult Clin Psychol, 53(3), 326–334. [DOI] [PubMed] [Google Scholar]
- Murphy J, McKellar J, Raffa S, Clark M, Kerns R, & Karlin B (2014). Cognitive behavioral therapy for chronic pain among veterans: Therapist manual. Washington DC: US Department of Veterans Affairs. [Google Scholar]
- Nahin RL (2017). Severe Pain in Veterans: The Effect of Age and Sex, and Comparisons With the General Population. Journal of Pain, 18(3), 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassif TH (2013). Examining the effectiveness of mindfulness meditation for chronic pain management in combat Veterans with traumatic brain injury. (PhD Dissertation), American University, Washington, DC. [Google Scholar]
- Nassif TH, Chapman JC, Sandbrink F, Norris DO, Soltes KL, Reinhard MJ, & Blackman M (2016). Mindfulness meditation and chronic pain management in Iraq and Afghanistan veterans with traumatic brain injury: A pilot study. Military Behavioral Health, 4(1), 82–89. [Google Scholar]
- Nassif TH, Norris DO, Soltes K, Blackman MR, Chapman JC, & Sandbrink F (2014). Evaluating the effectiveness of mindfulness meditation for chronic musculoskeletal pain using the defense and veterans pain rating scale (DVPRS): 211. Pain Medicine, 15(3), 529–530. [Google Scholar]
- Nassif TH, Norris DO, Soltes KL, Sandbrink F, Blackman MR, & Chapman JC (2014). Using mindfulness meditation to improve pain management in combat veterans with traumatic brain injury. Annals of Behavioral Medicine, 47, S215–S215. [Google Scholar]
- National Institues of Health. (2011). Safely Managing Chronic Pain. NIH MedlinePlus, 6, 5–6. [Google Scholar]
- Nelson AD, Bjarnadóttir MV, Wolcott VL, & Agarwal R (2018). Stated Pain Levels, Opioid Prescription Volume, and Chronic Opioid Use Among United States Army Soldiers. Mil Med, 183(9–10), e322–e329. [DOI] [PubMed] [Google Scholar]
- Otis J (2017, April 12, 2017). Intensive treatment for chronic pain and posttraumatic stress disorder (PTSD). Retrieved from https://clinicaltrials.gov/ct2/show/NCT01120067?term=NCT01120067&rank=1
- Peters JL, Sutton AJ, Jones DR, Abrams KR, & Rushton L (2007). Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Statistics in Medicine, 26(25), 4544–4562. [DOI] [PubMed] [Google Scholar]
- Pizarro J, Silver RC, & Prause J (2006). Physical and Mental Health Costs of Traumatic War Experiences Among Civil War Veterans. JAMA Psychiatry, 63(2), 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartana PJ, Buenaver LF, Edwards RR, Klick B, Haythornthwaite JA, & Smith MT (2010). Pain catastrophizing and salivary cortisol responses to laboratory pain testing in temporomandibular disorder and healthy participants. J Pain, 11(2), 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland M, & Fairbank J (2000). The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine, 25(24), 3115–3124. [DOI] [PubMed] [Google Scholar]
- Rosenthal JZ, Grosswald S, Ross R, Rosenthal N, Rosenthal JZ, Grosswald S, … Rosenthal N (2011). Effects of transcendental meditation in veterans of Operation Enduring Freedom and Operation Iraqi Freedom with posttraumatic stress disorder: a pilot study. Mil Med, 176(6), 626–630. [DOI] [PubMed] [Google Scholar]
- Sandlund ES, & Norlander T (2000). The effects of Tai Chi Chuan relaxation and exercise on stress responses and well-being: an overview of research. International Journal of Stress Management, 7(2), 139–149. [Google Scholar]
- Scott-Sheldon LAJ, Balletto BL, Donahue ML, Feulner MM, Cruess DG, Salmoirago-Blotcher E, … Carey MP (2019). Mindfulness-Based Interventions for Adults Living with HIV/AIDS: A Systematic Review and Meta-analysis. AIDS Behav, 23(1), 60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Sheldon L, Gathright EC, Donahue ML, Balletto B, Feulner MM, DeCosta J, … Salmoirago-Blotcher E (2020). Mindfulness-Based Interventions for Adults with Cardiovascular Disease: A Systematic Review and Meta-Analysis. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine, 54(1), 67–73. doi: 10.1093/abm/kaz020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Sheldon LAJ, Kalichman SC, Carey MP, & Fielder RL (2008). Stress management interventions for HIV+ adults: A meta-analysis of randomized controlled trials, 1989 to 2006. Health Psychology, 27(2), 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe Potter J, Bebarta VS, Marino EN, Ramos RG, & Turner BJ (2014). Pain management and opioid risk mitigation in the military. Mil Med, 179(5), 553–558. [DOI] [PubMed] [Google Scholar]
- Skelly AC, Chou R, Dettori JR, Turner JA, Friedly JL, Rundell SD, … Winter C (2018). Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review. Comparative Effectiveness Review No. 209. Rockville, MD: Agency for Healthcare Research and Quality; Retrieved from https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/nonpharma-chronic-pain-cer-209.pdf. [PubMed] [Google Scholar]
- Staples JK, Hamilton MF, & Uddo M (2013). A yoga program for the symptoms of post-traumatic stress disorder in veterans. Mil Med, 178(8), 854–860. [DOI] [PubMed] [Google Scholar]
- StataCorp. (2017). Stata Statistical Software (Version 15.1 for Windows) College Station, TX: StataCorp LP. [Google Scholar]
- Stephenson KR, Simpson TL, Martinez MM, & Kearney DJ (2016). Changes in mindfulness and posttraumatic stress disorder symptoms among veterans emrolled in mindfulness-based stress reduction. J Clin Psychol, 73(3), 201–2017. [DOI] [PubMed] [Google Scholar]
- Sterne JA, & Egger M (2001). Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. Journal of Clinical Epidemiology, 54(10), 1046–1055. [DOI] [PubMed] [Google Scholar]
- Sturgeon JA (2014). Psychological therapies for the management of chronic pain. Psychol Res Behav Manag, 7, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suris A, & Lind L (2008). Military sexual trauma: a review of prevalence and associated health consequences in veterans. Trauma Violence Abuse, 9(4), 250–269. [DOI] [PubMed] [Google Scholar]
- Terrin N, Schmid CH, Lau J, & Olkin I (2003). Adjusting for publication bias in the presence of heterogeneity. Statistics in Medicine, 22(13), 2113–2126. [DOI] [PubMed] [Google Scholar]
- Thurber MR, Bodenhamer-Davis E, Johnson M, Chesky K, & Chandler CK (2010). Effects of Heart Rate Variability Coherence Biofeedback Training and Emotional Management Techniques to Decrease Music Performance Anxiety. Biofeedback, 38(1), 28–40. [Google Scholar]
- Toblin RL, Quartana PJ, Riviere LA, Walper KC, & Hoge CW (2014). Chronic Pain and Opioid Use in US Soldiers After Combat Deployment. JAMA Internal Medicine, 174(8), 1400–1401. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Veterans Affairs. (2017). Expanding the VA Whole Health System. Retrieved from www.va.gov/PATIENTCENTEREDCARE/features/Expanding_the_VA_Whole_Health_System.asp
- US Department of Veteran Affairs. (2015, August 20, 2015). Military exposures. Retrieved from http://www.publichealth.va.gov/exposures/
- Van Daele T, Hermans D, Van Audenhove C, & Van den Bergh O (2012). Stress reduction through psychoeducation: a meta-analytic review. Health Educ Behav, 39(4), 474–485. [DOI] [PubMed] [Google Scholar]
- Williams KA, Petronis J, Smith D, Goodrich D, Wu J, Ravi N, … Gross R (2005). Effect of Iyengar yoga therapy for chronic low back pain. Pain, 115(1–2), 107–117. [DOI] [PubMed] [Google Scholar]
- Williams LS, Jones WJ, Shen J, Robinson RL, & Kroenke K (2004). Outcomes of newly referred neurology outpatients with depression and pain. Neurology, 63(4), 674–677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


