Abstract
Sex differences in response to neurotoxicant exposure that initiate epileptogenesis are understudied. We used telemetry implanted male and female adult rats exposed to an organophosphate (OP) neurotoxicant, diisopropylflourophosphate (DFP), to test sex differences in the severity of status epilepticus (SE) and the development of spontaneous recurrent seizures (SRS). Females had significantly less severe SE and decreased epileptiform spikes compared to males, although females received a higher dose of DFP than males. The estrous stages had no impact on seizure susceptibility, but rats with severe SE had a significantly prolonged diestrus. A previously demonstrated disease-modifying agent, an inducible nitric oxide synthase inhibitor, 1400W, was tested in both sexes. None of the eight males treated with 1400W developed convulsive SRS during four weeks post-DFP exposure, while two of seven females developed convulsive SRS. Concerning gliosis and neurodegeneration, there were region-specific differences in the interaction between sex and SE severity. As SE severity influences epileptogenesis and females had significantly less severe SE, sex as a biological variable should be factored in the design of future OP nerve agent experiments while evaluating neurotoxicity and optimizing potential disease-modifying agents.
Introduction
Females in experimental research are underrepresented1–3. Drugs and dosing regimens that are effective in males are sometimes proven to be ineffective in females or cause unexpected side effects4–6. To avoid these biases, the NIH Health Revitalization Act established guidelines to include females in clinical trials in 19937. Although the inclusion of females in studies has increased, many preclinical studies still do not include females or analyze sex as a biological variable (SABV)2,8–11. The exclusion of females from research is largely due to the impact of hormonal changes during various stages of the estrous cycle, which could result in high variability12–14. Levels of estrogen, progesterone, luteinizing hormone, and follicle-stimulating hormone fluctuate similarly to humans though the rat estrous cycle is shorter, typically lasting 4-5 days15,16. As hormone levels are influential in many biological processes, consideration of estrous staging is appropriate when analyzing data from female animals.
Females are understudied in epilepsy and organophosphate (OP) neurotoxicity research. Epilepsy is a neurological disorder characterized by spontaneous recurrent seizures (SRS). Most studies report that approximately 40% of females with epilepsy have catamenial epilepsy, likely due to a reduction in seizure threshold as a result of fluctuating hormones17,18. Animal models of epilepsy, induced by chemoconvulsants, showed resistance of females to the induction of status epilepticus (SE) compared to males for the same dose of pilocarpine (a muscarinic agonist) but not with kainic acid (KA, a glutamatergic agonist)19. In an OP nerve agent (soman) model, female rats in proestrus required higher doses to induce SE compared to females in estrus, and males; a similar pattern was shown in a study using picrotoxin14,20. Diisopropylflourophosphate (DFP) is also an OP neurotoxicant often used to model nerve agent neurotoxicity in rodents21–23. Following DFP administration, animals typically develop seizures within 10 minutes and seize for a prolonged period. We and others have demonstrated that DFP-induced SE also initiates epileptogenesis in the majority of animals, which is manifested by SRS24–28. Animals subjected to chemoconvulsants, including DFP and other nerve agents, develop neuroinflammation and neurodegeneration29–33. Sex differences, however, are under-investigated in DFP-induced long-term neurotoxicity studies. The goal of this study is to evaluate sex and estrous stage-dependent differences in initial SE severity induced by DFP and its’ long-term effects measured by electroencephalography (EEG), gliosis, and neurodegeneration.
OP nerve agents pose a significant threat to both civilian and military populations; thus, the need to identify effective countermeasures is imperative. Benzodiazepines are currently used following OP intoxication but delayed administration, as is often the case in an “after-field evacuation and in-hospital” scenario, is ineffective in mitigating the long-term effects of poisoning34–37. The DFP model of neurotoxicity is useful to evaluate potential disease-modifying agents to reduce the overall occurrence of SRS and associated co-morbidities as well as reactive gliosis and neurodegeneation32,33,38. These studies, including ours, used only male animals. We had demonstrated a significant neuroprotective effect using a novel disease-modifying agent, 1400W, an inducible nitric oxide synthase (iNOS) inhibitor, in adult male rats using the DFP model of neurotoxicity33. 1400W is highly selective for iNOS over both eNOS and nNOS, and it has been shown to reduce SRS or prevent epileptogenesis, reactive gliosis, and neurodegeneration in the rat KA and DFP models33,39,40. In addition to evaluating sex differences in DFP intoxication, we also tested the long-term effects of 1400W in females and compared with males to understand SABV.
Methodology
2.1. Animal source, care, and ethics
Both male and female adult Sprague Dawley rats (7-8 weeks at the time of experimentation) were purchased from Charles River (MA, USA). Animal groups used for each analysis are outlined in Table S1. The procedures used in this study were approved by the Institutional Animal Care and Use Committee at Iowa State University (IACUC-18-160). Animals were housed and cared for by the Laboratory of Animal Resources and given ab libitum access to food and water in a controlled environment (19°C-22°C) with alternating 12-hour light and dark cycles. Cages had Alpha Dri bedding and plastic bones for enrichment; all animals were single housed throughout the experiment. Animals were given at least 72 hours of acclimation before beginning testing and were given appropriate care following surgical procedures, outlined in the Supplementary Methods. Animals were randomized, grouped, and coded for naïve (nothing administered), or DFP treatment. At the end of the experiment, animals were euthanized with 100 mg/kg pentobarbital sodium (i.p.) as per the American Veterinary Medical Associations Guidelines for the Euthanasia of Animals. All procedures complied with ARRIVE guidelines41.
2.2. Chemicals and reagents
DFP (Sigma Aldrich, USA) was diluted in cold 0.1M phosphate-buffered saline (PBS) before administration. Notably, DFP is unstable at room temperature, which could influence the animals’ response to its exposure; therefore, DFP was stored at −200C for stability and to minimize variability42. Atropine sulfate (ATS, Acros Organics, USA) and pralidoxime (2-PAM, Sigma Aldrich, USA) were dissolved in sterile saline while 1400W (99.6% pure, Tocris Biosciences, USA) was prepared in sterile distilled water. GC-MS and HPLC-MS were used for authentication of DFP and 1400W, as described in our recent publication33. Diazepam (DZP), buprenorphine, and pentobarbital sodium were purchased from the Iowa State University Lloyd Veterinary Medical Center Hospital Pharmacy. 4% paraformaldehyde was prepared in PBS for perfusion. Gelatin for tissue embedding consisted of 15% type A porcine gelatin, 7.5% sucrose, and 0.1% sodium azide. Citric acid buffer for antigen retrieval consisted of 10 mM citric acid and 0.05% tween-20 at pH 6.0.
For immunohistochemical analysis, primary antibodies were purchased from Abcam (MA, USA) including ionized calcium-binding adaptor molecule 1 (IBA1, goat polyclonal, 1:500); cluster of differentiation 68 (CD68, rabbit polyclonal, 1:400), and glial fibrillary acidic protein (GFAP, mouse monoclonal, 1:400). NeuN (rabbit polyclonal, 1:400) was purchased from EMD Millipore (MA, USA). Fluoro-Jade B (FJB) was purchased from Histochem Inc (AR, USA) and was diluted (0.01%) in 0.1% acetic acid before use. Secondary antibodies, FITC conjugated (anti-mouse, anti-rabbit, 1:80); biotin-conjugated (anti-goat, anti-rabbit, 1:400) and Streptavidin (1:300) were purchased from Jackson ImmunoResearch Laboratories (PA, USA).
2.3. Surgical implantation of electrodes and transmitter device
Radio-wireless telemetry devices were purchased from Data Sciences International (DSI, Minneapolis, USA) and implanted into 20 female rats and 27 male rats as previously described33,40. Twenty-three of the male animals were previously reported, and the raw data was used to compare with females to reduce the number of animals used. An additional four male animals were implanted with electrodes and used solely for histological analysis to match the time-points. These four animals were not considered for EEG or mortality analysis as they were tested at a different time than the rest of the male rats33. Procedures, except vaginal cytology, were carried out identically in both sexes. Detailed surgical procedures are described in the Supplementary Methods.
2.4. Evaluation of the estrous cycle- vaginal cytology
Vaginal lavage and sampling were done daily between 10 AM and 11 AM both before and after DFP administration, as described in the published literature43. Sample collection and stage identification are detailed in the Supplementary Methods. Representative images for each stage of the estrous cycle are presented in figure 1B.
Figure 1.
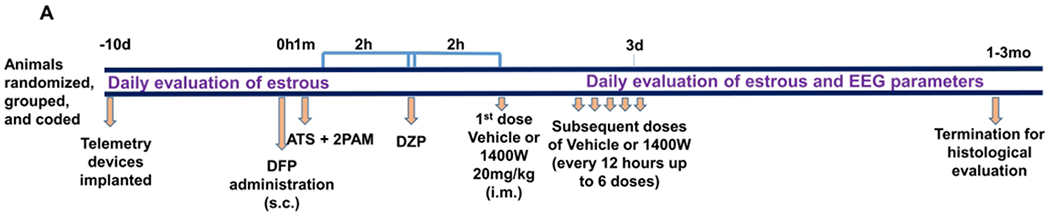
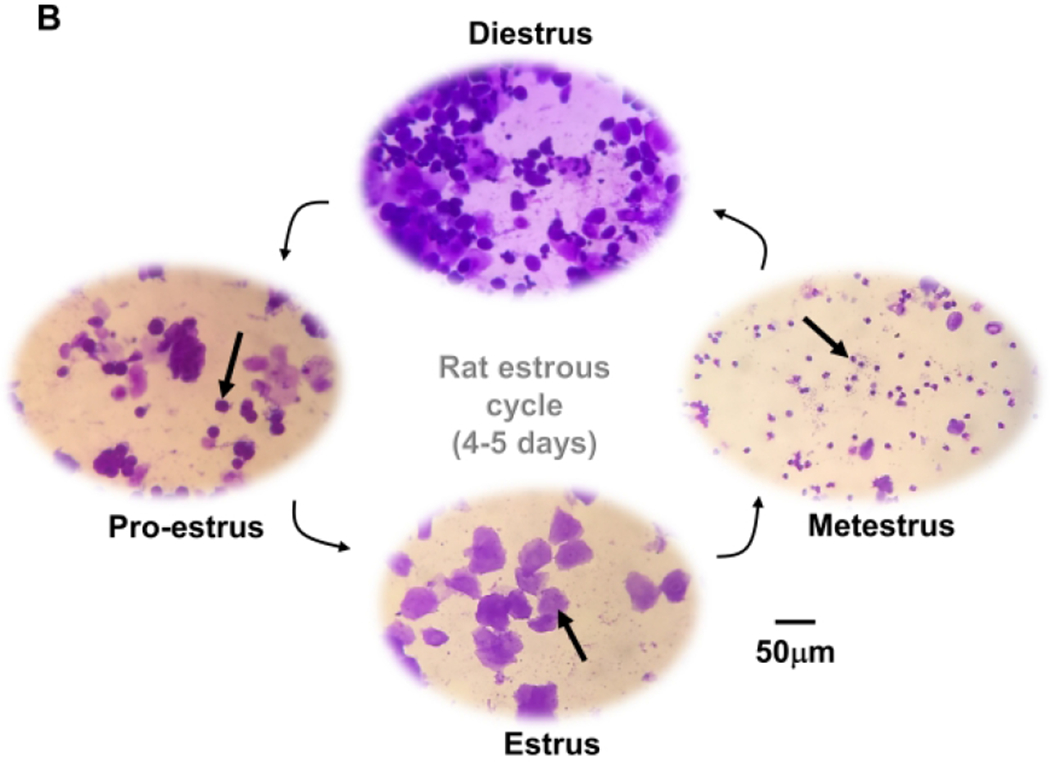
Experimental timeline (A) and evaluation of estrous cycle stages (B). (A) Male and female Sprague Dawley rats were implanted with radiotelemetric devices 10 days prior to DFP exposure (4mg/kg, females; 3mg/kg males) followed immediately by atropine sulfate (2mg/kg, i.m.) and 2-PAM (25mg/kg, i.m.), 2h later DZP (diazepam, 5mg/kg i.m.), and a further 2h later 1400W (20mg/kg, i.m. every 12h) or vehicle were administered. EEG and estrous stages were monitored throughout the experiment. (B) Estrous stages were evaluated once a day in the morning via vaginal smears stained with cresyl violet. Proestrus and estrus had predominantly nucleated epithelial cells or anucleated epithelial cells, respectively. Metestrus and diestrus were characterized by massive infiltration of neutrophils. Examples of corresponding cell types are indicated with arrows. D= days, h=hours, m=minutes, mo=months. Scale, 50um
2.4. DFP administration and seizure severity evaluation
The experimental timeline is illustrated in figure 1A. Following 10 days of recovery from surgical implantation of electrodes, males and females received 3 and 4mg/kg DFP (s.c.) respectively, followed by 2mg/kg ATS (i.m) and 25mg/kg 2-PAM (i.m) to reduce mortality. Although some models of DFP utilize pyridostigmine bromide as a pretreatment to model military scenarios, it was excluded from this model as it might more accurately reflect a civilian exposure scenario. Additionally, pyridostigmine bromide was found to have no impact on the survival of animals in the DFP model44. Animals developed seizures within 10 minutes of DFP administration. Animals displayed seizures of various stages for two hours before behavioral SE was controlled with DZP (5 mg/kg, i.m.). Seizures were ranked on a staging scale of 1–5 with increasing severity according to behavior as described previously33 and summarized in Table S2. Stages one and two are classified as non-convulsive seizures (NCS), and stages three through five are classified as convulsive seizures (CS). Representative images from each stage for male and female animals are shown in figure 2A. The number of minutes each animal spent in a CS during SE allowed us to randomly assign animals for either vehicle or 1400W treatment to maintain equal SE severity between groups.
Figure 2.
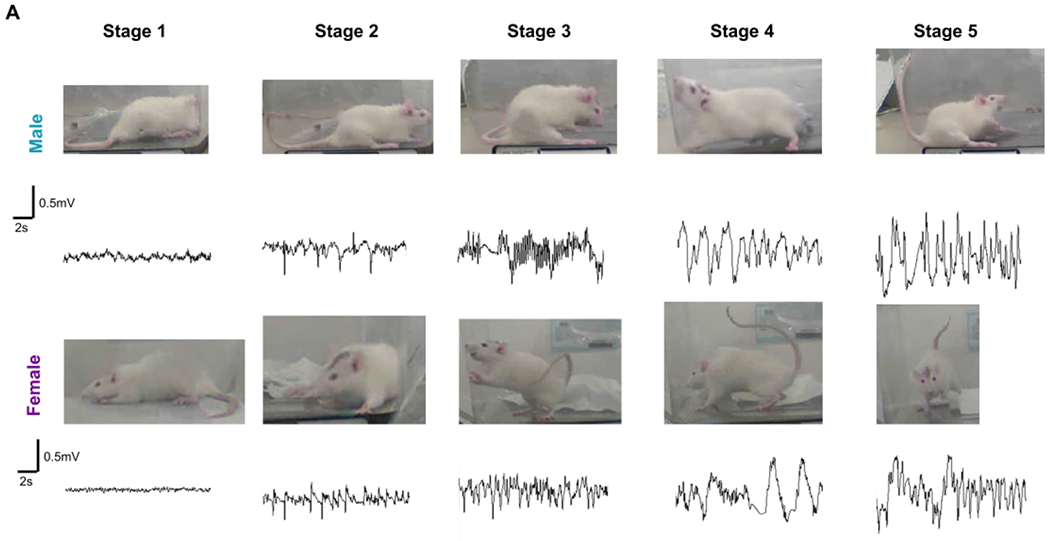
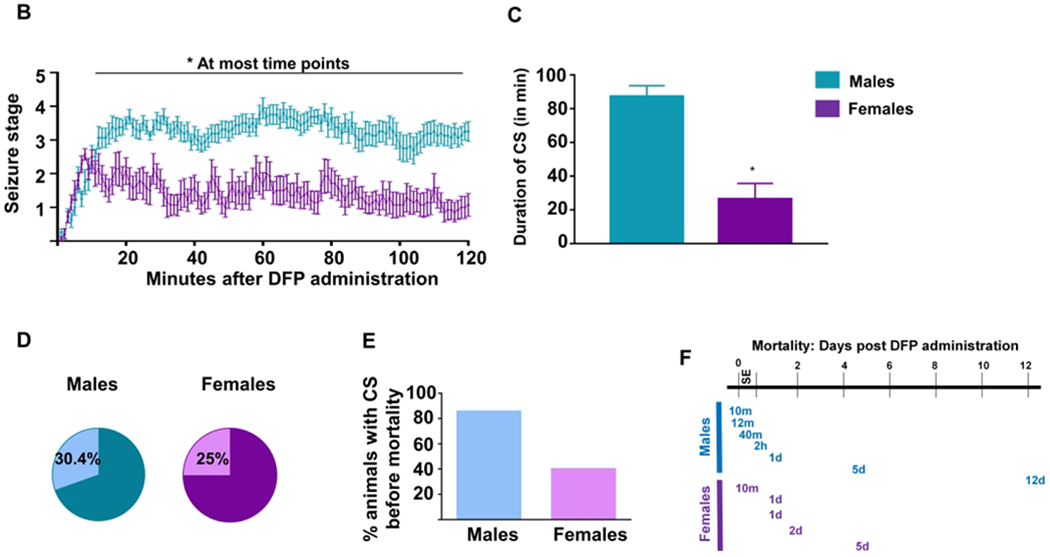
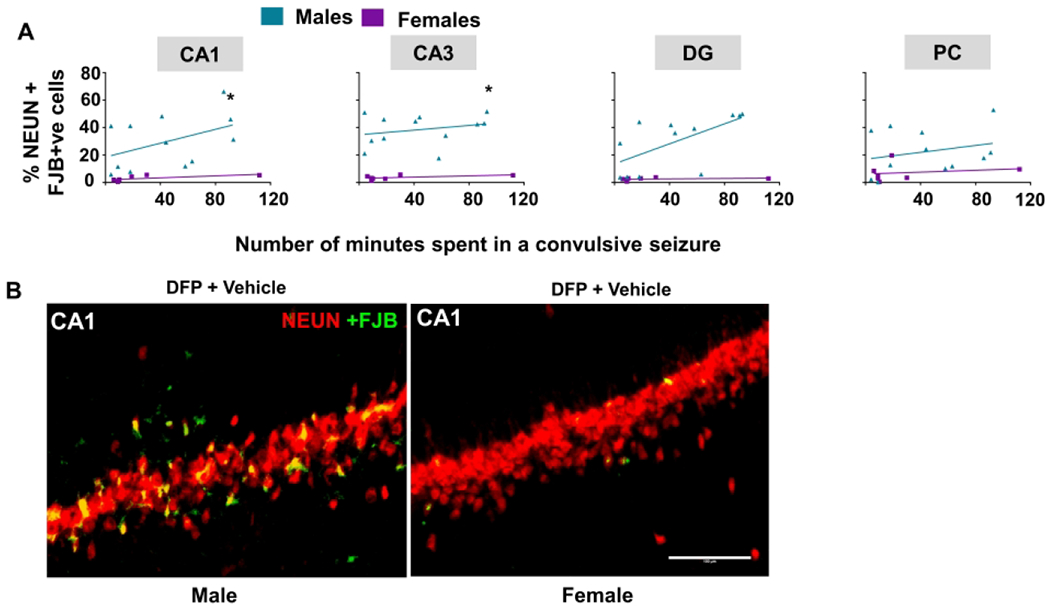
Female animals showed increased resistance to DFP induced status epilepticus (SE) compared to male animals. (A) Representative images of behavioral seizures in a male and female rat and corresponding EEG traces from each seizure stage during SE are presented. Stages 1-2 were considered nonconvulsive, while stages 3-5 were considered convulsive seizures (CS). (B) Average seizure stages graphed over time for the two hours following DFP administration. Two-way mixed measures ANOVA with Sidak post-hoc revealed significant sex differences in SE severity after 15 minutes of DFP exposure (n=14-16). (C) The total number of minutes each animal spent in a CS during SE was calculated, and unpaired t-test showed that males spent significantly longer time in CS. (D) Seven of twenty-three males and five of twenty females died after DFP exposure. Fisher’s Exact test showed no significant difference between sexes. (E) Of the animals that died, 85.7% of males and only 40% of females had >10mins CS during SE. (F) Times at which all animals died following DFP administration. *p<0.05. Data points indicate means and standard error of the mean.
2.5. Assessment of spontaneous recurrent seizures (SRS), epileptiform spiking, and powerbands
Animals were continuously video-EEG recorded 24 hours a day for one month following DFP injection. DataQuest software acquired integrated raw video-EEG data, and NeuroScore 3.2.0 software was used for offline analyses. Baseline EEG acquired before DFP injection was used to determine a spike threshold (~160 to 300mV) for each animal to automatically count spikes within the threshold during and following exposure, as described for mice in the kainate model previously45,46; these were considered epileptiform spikes. The spike rate (number of spikes per minute) was quantified to evaluate the effect of SAVB on DFP-induced brain dysfunction as well as 1400W treatment in both sexes. Spike characteristics on EEG and integrated video allowed for verification of spikes generated from exploratory behavior or electrical noise and these spikes were discounted from the analysis as previously described45,46. SRS were identified as large spiking clusters with behavioral accompaniment (stage 3-5) verified by the integrated video. SRS were generally 30-60s duration, but we did not quantify the duration of each SRS. Although SRS can refer to both convulsive and nonconvulsive seizures, from now on, we will use SRS to describe only spontaneous convulsive seizures. Convulsive seizures (spontaneous or during SE) were accompanied by the prevalence of gamma bands, which NeuroScore software generated via the Fast Fourier Transformation. Powerbands were classified as described in Table S3. Nonconvulsive SRS were not quantified; however, the epileptiform spike counts include spikes from both nonconvulsive and convulsive SRS.
To further assess electrical activity, the magnitude of powerbands was graphed over time to determine the effect of the estrous cycle. Analysis of all animals did not yield any specific patterns; therefore, the seizing animals were considered in the analysis. Three females had SRS during the period of regular estrous cycling, and one had unacceptable levels of electrical noise, thus leaving two animals for analysis. Electrical noise and baseline activity allowed for the generation of a threshold to determine periods of elevated powerband frequencies; these are outlined in Table S3. Each day of the data acquisition was divided into 1-minute epochs, and the percent of epochs within the threshold was graphed over time to determine periods of elevated activity. Delta and theta powers were graphed separately to visualize cyclical activity.
2.6. Euthanasia and immunohistochemical evaluation of brain sections
Animals were euthanized with pentobarbital sodium one to three months post-DFP administration. Timepoints across groups were balanced. Animals were then perfused (60mL/min at 80Hg) with PBS, followed by 4% paraformaldehyde (PFA). Brains were further incubated in PFA for 24 hours before transferring to a 25% sucrose solution for 3-4 days at 4°C. Brains were then embedded in gelatin and stored at 4oC overnight. The tissue blocks were snap-frozen in isopentane-cooled by liquid nitrogen and stored at −80°C. Brains were then sectioned coronally at 16 μm and collected onto CAG (chrome alum gelatin) coated slides as described previously47. Each slide contained 4-5 sections that were 480 μm apart to assay the rostral to caudal parts of the hippocampus and piriform cortex. Slides were then stored at −20°C until used for immunohistochemical evaluation. Detailed methodology for immunohistochemical evaluation is described in Supplementary Methods.
2.7. Analysis of gliosis and neurodegeneration
Immunostained brain sections were visualized using the Axiovert 200 Zeiss inverted fluorescence microscope, imaged via the Hamamatsu camera (Deutschland, Germany) and captured by HCImage Live software (Hamamatsu, PA, USA). 20X images of the cornu ammonis 1 and 3 (CA1 and CA3), the dentate gyrus (DG), and piriform cortex (PC) were captured at consistent locations from rostral to caudal parts of the brain. Groups were blinded throughout analysis. Following imaging, ImageJ software was used for cell quantification. Total number of microglia and percent reactive microglia were counted. CD68 is considered a marker of phagocytosis, and therefore the percentage of IBA1 cells co-stained with CD68 was also quantified to obtain an additional measure of reactive microglia48–50. GFAP staining was evaluated similarly to determine the extent of astrogliosis. Total number of astrocytes was counted as well as evaluated for their morphology. The total number of neurons (stained with NeuN) co-immunolabeled with FJB was calculated to estimate degenerating neurons47. More details concerning cell counting are provided in the Supplementary Methods.
2.8. Statistical analysis
The statistical analyses used in this study are briefly outlined here and in the figure legends and Results section. Two-way mixed measures ANOVAs were used to evaluate sex and treatment differences in seizure severity over time and immunohistochemical parameters. One-way ANOVAs or Kruskal Wallis (repeated measures when appropriate) with Tukey post-hocs were used for the data from more than two groups. Normality was tested using the Shapiro Wilk test. Student’s t-tests were used when only two groups required analysis. A linear model was used to compare sexes while controlling for SE severity in the histological evaluation. We used GraphPad Prism 7.0 as well as R-Studio version 3.5.1 to graph and analyze results. All graphical representations show means and standard errors of the means, and a p-value of less than 0.05 was considered statistically significant.
Results
3.1. The impact of sex on response to DFP exposure
Our previous KA studies51 and DFP pilot studies in telemetry male rats suggested a decreased seizure threshold to acute exposure to chemoconvulsants at ten days post-surgery. There was >50% mortality at 4mg/kg DFP (without pyridostigmine pre-treatment) in telemetered male rats, which prompted to reduce the dose to 3mg/kg to achieve a similar SE severity with 4mg/kg in non-telemetered males animals32. Therefore, in this study, males were treated with 3mg/kg DFP while females were exposed to a higher dose of DFP (4mg/kg) as we suspected there might be some seizure resistivity based on the studies in the rat pilocarpine model18. Images from a male and female rat displaying the behavioral characteristics and corresponding EEG traces that were used for staging SE are illustrated in Figure 2A. The spike frequency and amplitude corresponded with the severity of seizure stage. Average stages at each minute for males (n=16) and females (n=14) were calculated for the 2h SE and compared to understand the effects of SABV. One female rat that did not show NCS or CS during the 2h post-exposure was considered a DFP non-responder and excluded from the study. Two-way ANOVA with mixed measures and Sidak post-hoc was used to compare SE severity between the groups at each time point. Despite being treated with a lower dose of DFP, males achieved significantly higher SE severity after 15 minutes post-exposure (Fig. 2B). Males spent significantly greater time in CS than the females during the 2h period following DFP administration (Fig. 2C, unpaired t-test; p<0.001). Interestingly, despite a significant difference in SE severity between males and females, the differences in mortality were not significant (males 30.4%, females 25%; Fig. 2D, Fisher’s Exact test). Further analysis of the animals that died during the experiment revealed 85% of the male animals, and only 40% of female animals had greater than 10 minutes of CS; however, the difference was not significant (Fig 2E, Fisher’s Exact test). The timeline of death of these animals is presented in Figure 2F. The majority of the animals died in less than 24h of DFP exposure.
3.2. The relationship between the estrous cycle and seizure susceptibility to DFP exposure
The stages of the estrous cycle were determined from vaginal cytology using the standard criteria52–54. The female animals in this study had estrous cycles between 4 and 5 days. Representative images for each stage of the estrous in this study are presented in figure 1B.
Vaginal samples were collected from females before DFP exposure to determine the estrous stage at the time of DFP exposure. Experimenters were blind to the stage of estrous while evaluating the animals for SE severity. The number of minutes spent in CS during the 2h period of SE was averaged for animals in each stage (metestrus, diestrus, proestrus, and estrus), and the animals that died during SE were excluded (Fig. 3A). The number of spikes per minute during SE was also calculated for each animal to investigate the impact of stages of estrous on epileptiform spike rate (Fig 3B). There were no significant differences in the number of minutes in CS or spike rate during SE between the stages of the estrous cycle (Fig. 3A–B Kruskal Wallis).
Figure 3.
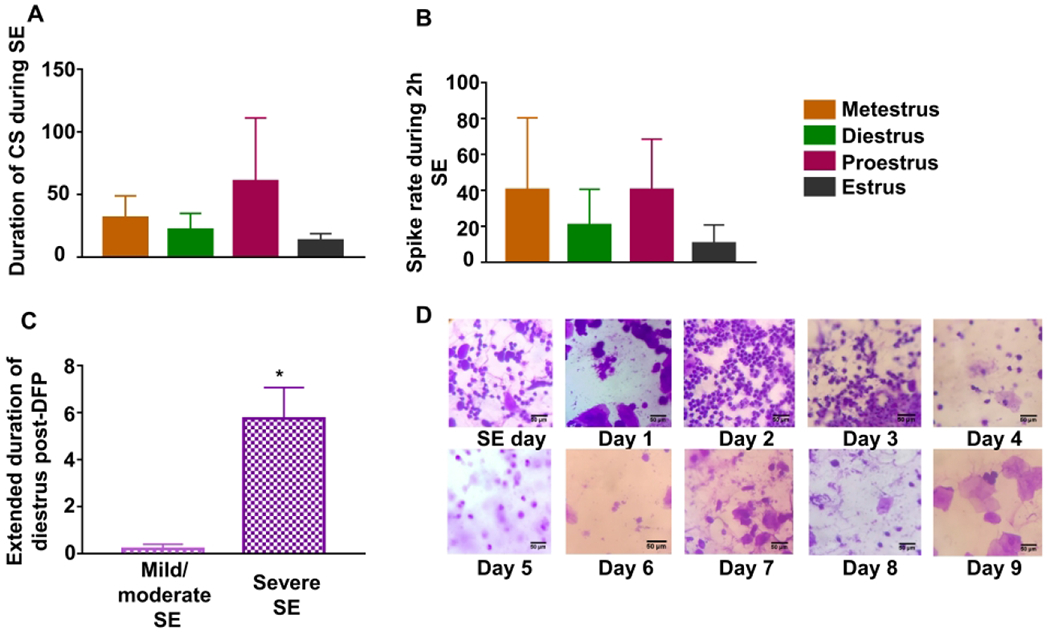
The relationship between estrous stages and susceptibility to DFP exposure. Vaginal cytology revealed the stage of estrous at the time of DFP administration. The number of minutes in a CS (A) and the spikes per minute (B) were averaged for each of the stages, and no significant differences were found (Kruskal Wallis, n=2-8). Females were characterized as having severe (>30 mins of CS) or mild/moderate (<30 mins of CS) SE. Animals with severe SE spent significantly more time in diestrus than those with mild/moderate SE (n=4-10). Extended diestrus was calculated by counting the number of days in addition to 2 days spent in diestrus (unpaired t-test). (D) Representative vaginal stains images from an animal with extended diestrus are presented. *p<0.05. SE = status epilepticus, data points represent means and standard error of the mean.
As there was a high variability in the SE severity amongst the females, the animals were categorized as having mild (<15 min CS) and moderate (15-30 min CS) or severe (>30 min CS) SE. Interestingly, the animals that had severe SE had a disrupted estrous cycle. These animals had a significantly extended diestrus (an average of 5.75 days) compared to the rats with mild and moderate SE, which only spent an average of 0.16 days before returning to estrus (Fig. 3C, unpaired t-test). Diestrus typically ends 2-3 days after estrus, so the number of days in addition to 2 days in diestrus was considered in the analysis. Figure 3D illustrates representative images of an extended diestrus following DFP-induced SE, where the animal returned to estrus nine days after SE.
3.3. The impact of sex on epileptiform spikes and SRS occurrence
Representative EEG traces from both male (Fig. 4A) and female rats (Fig. 4B), before and after DFP exposure, are presented. Spikes per minute were calculated for the first 28 days following DFP exposure. The females had significantly less spiking activity at 2, 4, 7 and 9-19 days post-exposure (Fig. 4C, two-way ANOVA with mixed measures) and over the 28 days when compared with males (Fig. 4D, unpaired t-test). EEG traces for convulsive SRS matched behavioral seizure stages 3-5, and increased gamma power in a male and female rat are shown in figure 4E and 4F. Four of eight males and two of seven females showed SRS in four weeks post-exposure (not significant following Fisher’s Exact test). The distribution of seizures by week is represented in Figure 4G. There were no significant differences between male and female SRS numbers over time during the four weeks study (Fig. 4H, two-way ANOVA with mixed measures) or pooled SRS over time (Fig 4I, unpaired t-test).
Figure 4.
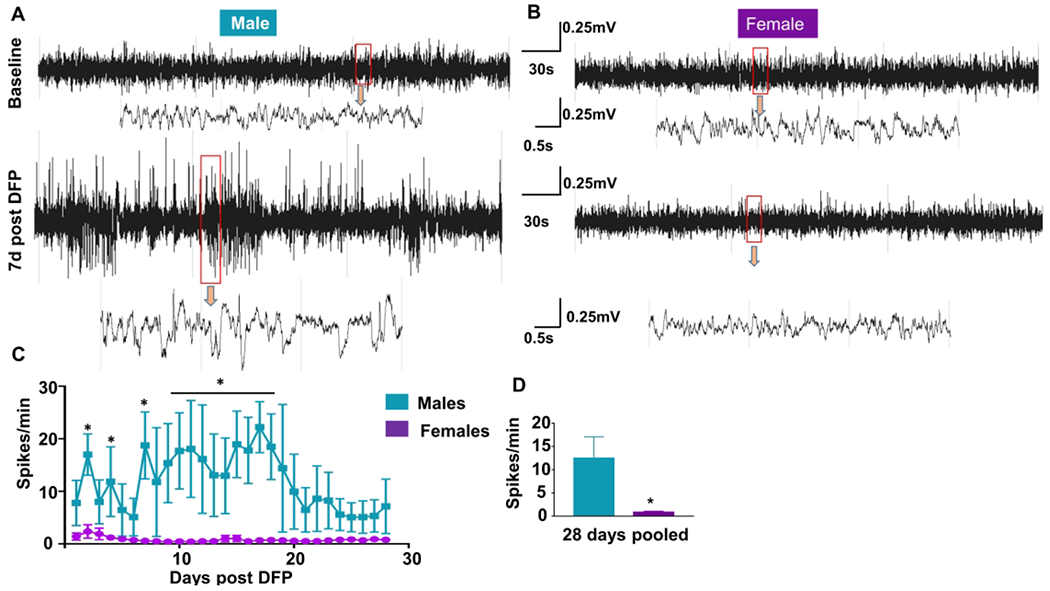
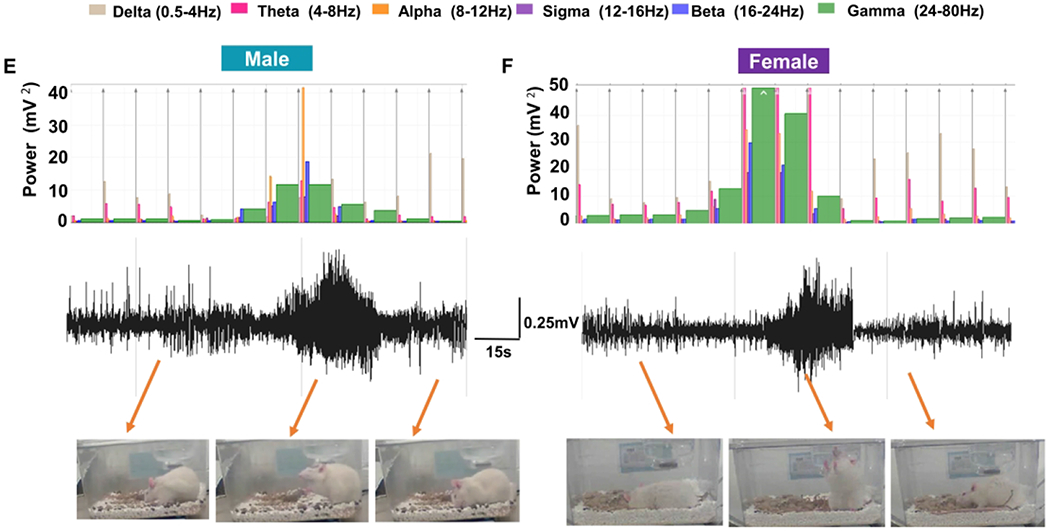
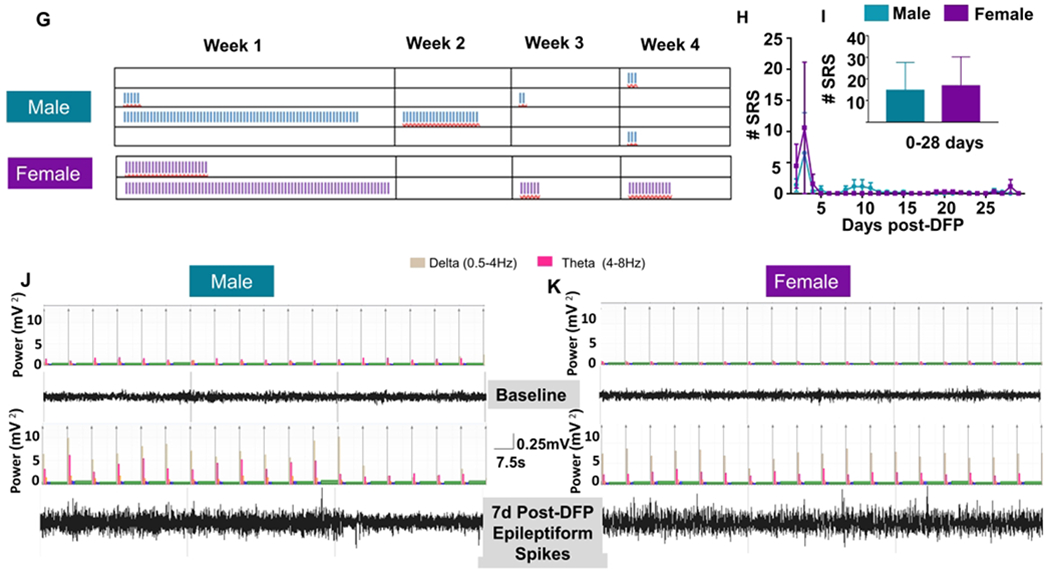
The influence of sex on epileptiform spike rate and spontaneous recurrent seizures (SRS). Representative EEG traces from male (A) and female (B) animals with epileptiform spikes. (B) NeuroScore software was used to determine the number of spikes per minute, and two-way mixed measures ANOVA revealed females had significantly less epileptiform spikes at several time points (C, Sidak post-hoc test, n=6-7). Pooled spikes per minute showed females had overall less epileptiform spikes (D, unpaired t-test, n=6-7). Representative SRS from a male (E) and female animals (F). (G) The distribution of seizures by week; each vertical line in each box represents a CS. The number of seizures was graphed over time (H) and pooled (I); there were no significant differences between males and females (Two-way mixed measures ANOVA with Sidak post-hoc test, n=7-8). Representative EEG traces showing epileptiform spiking and theta and delta powers during post-DFP from both male (J) and female (K) animals are presented. Data points represent means and standard error of the mean.
In nonconvulsive SRS or interictal spike trains, no overt behavioral changes were visible on video, but the theta and delta powers were increased in both male and female animals while the gamma power was consistently low in both sexes (Fig. 4J–K). It is important to note that all male rats had several hundreds of spontaneous non-convulsive seizures; thus, all eight male rats were considered to have developed epilepsy during the four weeks. In females, however, only four animals had NCS, including, those that exhibited CS. Instead of counting all NCS, which is tedious, we quantified the epileptiform spikes in NCS episodes, the spike trains, and clusters, and compared between the groups (Fig. 4C, 4D).
3.4. The impact of a disease-modifying agent, 1400W on epileptiform spikes and SRS in females
1400W, an iNOS inhibitor, has been previously found to have disease-modifying effects in the male rat DFP and KA models33,40. In male rats, 1400W significantly suppressed epileptiform spikes and SRS33. Interestingly, in this study, 1400W treated females showed significantly more spiking activity on the second day after DFP challenge when compared with the vehicle-treated female animals. However, no significant differences were found on subsequent days (Fig. 5A, two-way ANOVA with repeated measures). The pooled data for the first three days (i.e., during the time of 1400W treatment) as well as for the remaining days of the study also did not show statistically significant differences between groups in females (Fig. 5B–C, unpaired t-test). We further compared epileptiform spiking in males and females treated similarly with 1400W after DFP exposure and found males had significantly more spiking activity at various time-points than females (Fig. 5D, two-way ANOVA with repeated measures). There were no significant differences when the data was pooled for the first three days or the remaining days of study (Fig 5E–F, unpaired t-test)
Figure 5.
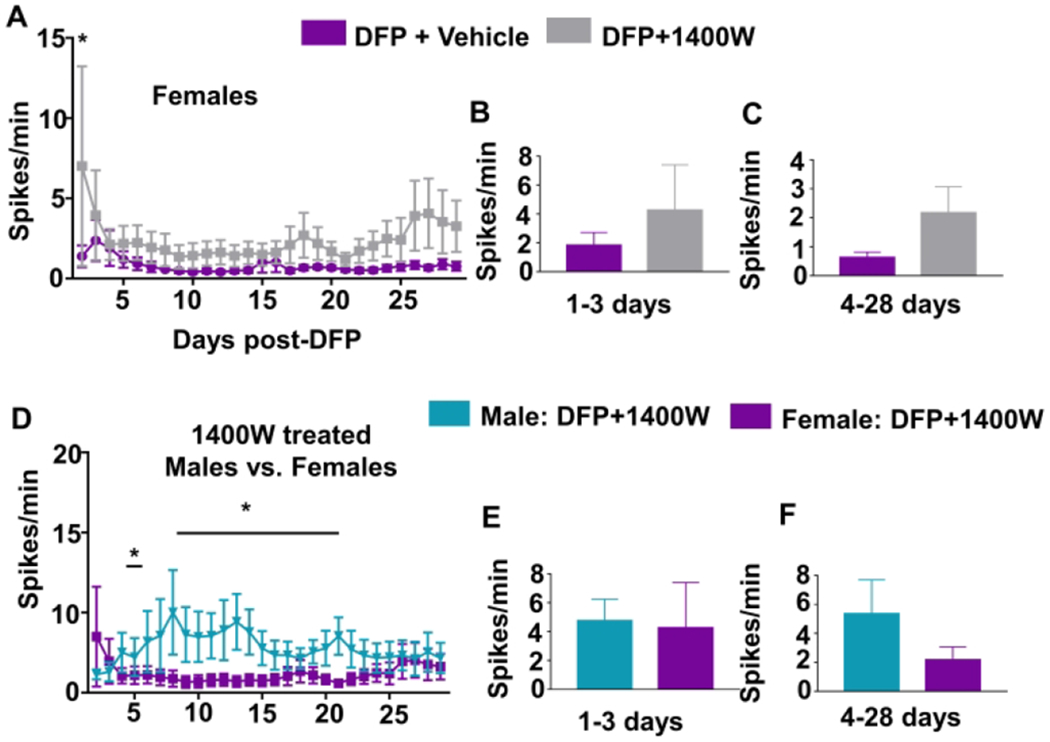
The effect of 1400W on EEG parameters. Number of epileptiform spikes per minute was calculated for each day (A) and pooled for the first three days (B, two-way mixed measures ANOVA, n=6-7) and the remainder of the experiment (C, unpaired t-test). Males and females treated with 1400W are also presented similarly (D-F, n=6-8). Two-way mixed measures ANOVA showed only an increase in epileptiform spikes in 1400W treated females compared to vehicle-treated females on the first day and at various time points compared to the males. Average number of seizures over time (G) and pooled for the first three days (H) and the duration of the study (I). Two-way mixed measures ANOVA showed only an increase in seizures on the second day, n=6-7. (J) One-way repeated measures ANOVA revealed no differences in the percentage of seizures during any stage of an estrous (K-L, n=3). The percent of 1-minute epochs with a threshold (Table S3) were graphed over time for each power to reveal trends in power bands patterns at different stages of the estrous cycle. Data points represent means and standard error of the mean.
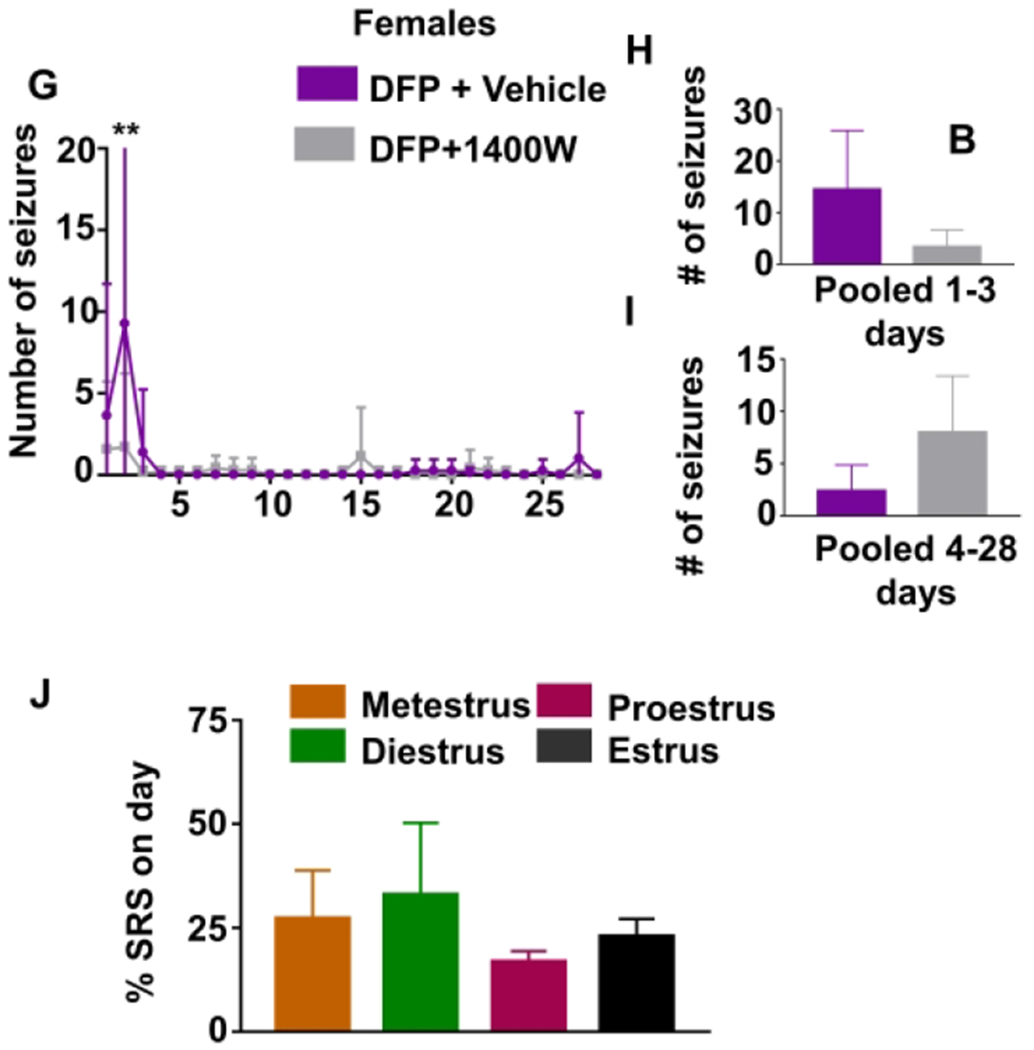
It is important to note that even though males had higher epileptiform spiking during the first four weeks post-DFP exposure, none of the eight males treated with 1400W developed SRS, while two of seven females did not respond to 1400W during this period. Regardless of significantly higher epileptiform spiking during the second-day post-DFP in 1400W treated females, they had significantly less seizures on the same day compared to vehicle-treated female rats (Fig. 5G, two-way ANOVA with repeated measures). However, there were no statistically significant differences on other days, pooled over the treatment period, or for the remainder of the experiment (Fig. 5H–I, unpaired t-test).
3.5. The impact of the stages of the estrous cycle on SRS and the power oscillations
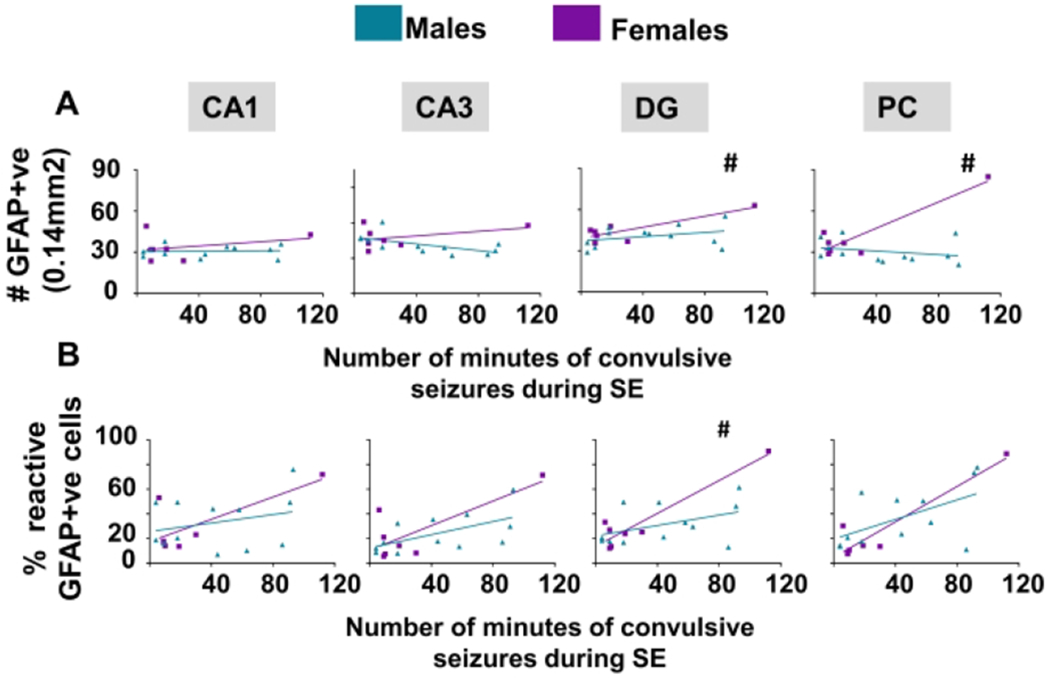
The estrous cycle was monitored for all animals throughout the course of the experiment. All animals experiencing SRS were investigated to determine the effect of estrous on SRS. As all the animals with severe initial SE experienced extended diestrus, only SRS that occurred after the first week were considered to determine if there was a relationship between estrous stages and SRS onset. Notably, an average of 69% of SRS occurred during the period of extended diestrus. However, there were no significant differences between the stages of the estrous cycle and the percent of SRS occurring during each stage (Fig. 5J, repeated measures ANOVA).
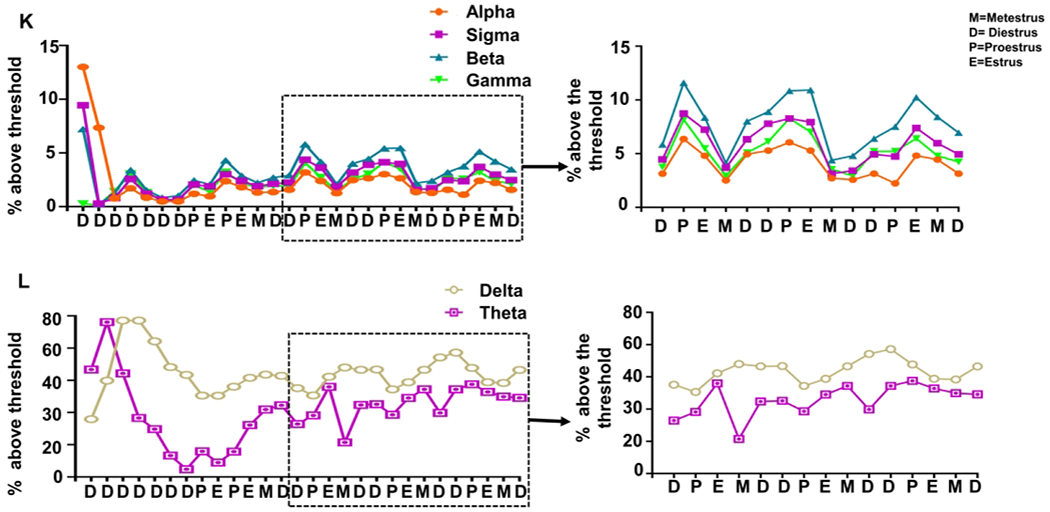
To further evaluate the impact of the estrous cycle on brain electrical activity, we quantified the distribution of powerbands over time. Beginning around the third week of data collection after the animals returned to estrus and began a regular cycle every 4-5 days, severely affected animals most consistently had a peak in alpha, sigma, beta, and gamma powers around the time of proestrus and estrus (Fig. 5K–L). Interestingly, this pattern was not observed for delta and theta bands.
3.6. The effect of sex and 1400W on gliosis and neurodegeneration
It has been well established that SE induction by chemoconvulsants, including DFP, leads to the upregulation of inflammatory factors, which can, in turn, lower the threshold for SRS33,55,56. The hippocampus and piriform cortex have been most commonly studied in the DFP model as these regions are highly susceptible to SE-induced changes57,58. As SE severity is likely a highly influential factor in generating histological changes, SE severity was included in the model to determine the effects of sex on gliosis and neurodegeneration. A linear model with the main effects of sex and SE severity and their interaction was used in the vehicle-treated animals to provide information about how SE severity influences the response variables concerning each sex. This model was used in CA1, CA3, and DG of the hippocampus as well as the piriform cortex for each measure of gliosis and neurodegeneration. Notably, a female rat with extreme severe SE (continuous for 2h) was an outlier in region-specific analyses. As this was the only female experiencing extreme severe SE, we decided to keep it in the analysis. However, a large number of females with severe SE are needed to determine these effects completely. Correlation values are detailed in Supplementary Tables S4–S6.
To determine the effects of 1400W in females, two-way mixed measures ANOVA was used since the severity of initial SE was not significantly different between the two groups (Fig. 6E). Naive female rats (no treatment or surgery) were used as control to determine baseline gliosis and neurodegeneration. Main effects, as well as simple effects between treatments, were analyzed. Animals used for histology were euthanized at either one or three months, and the timepoints were balanced across groups.
Figure 6.
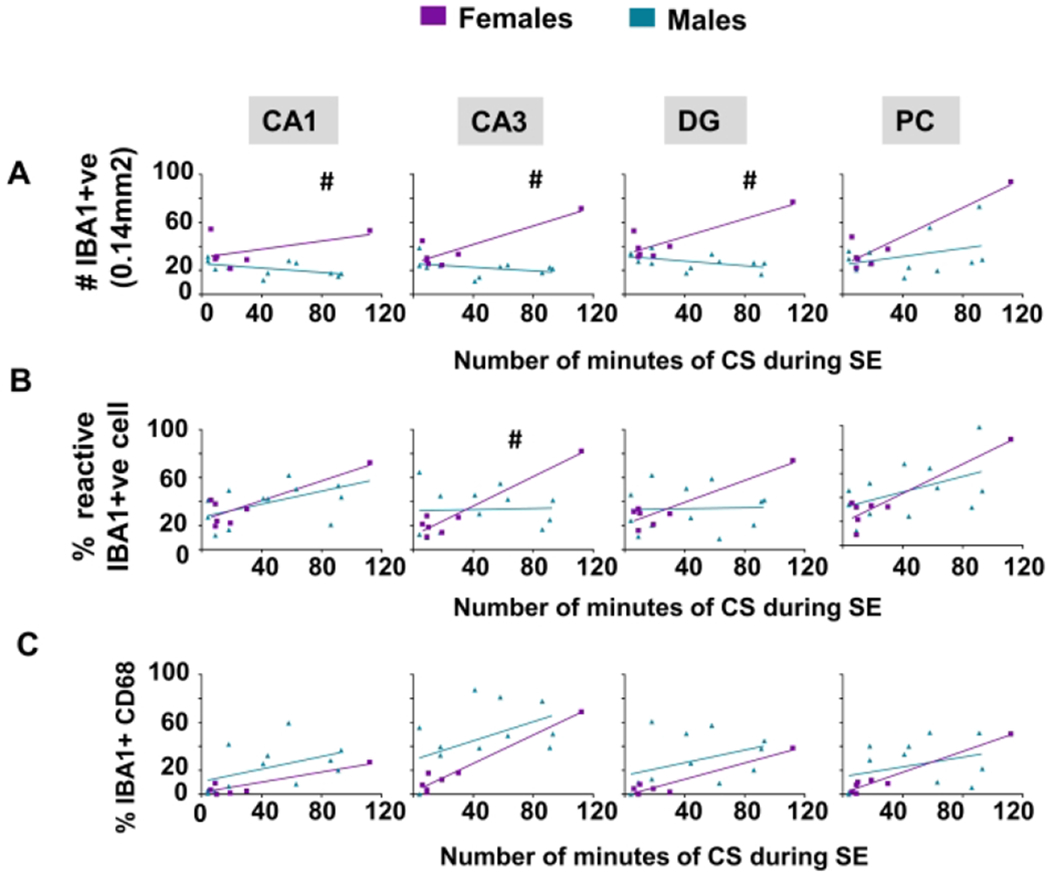
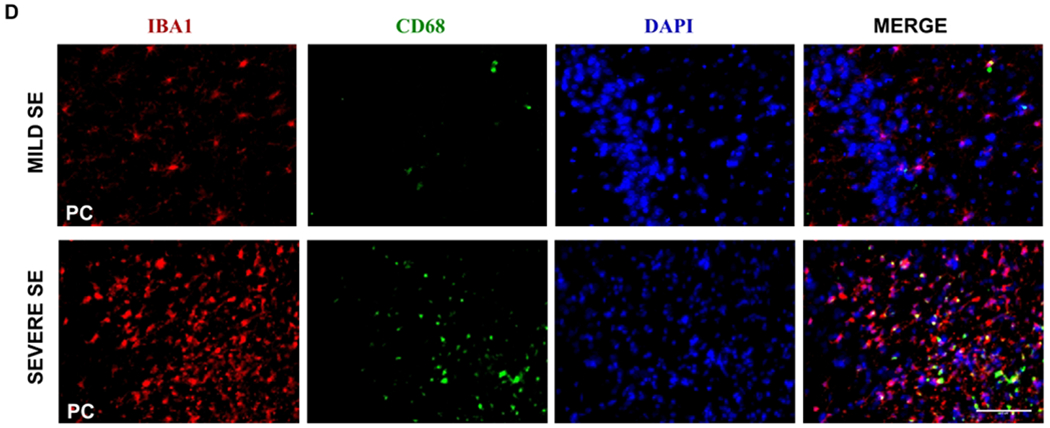
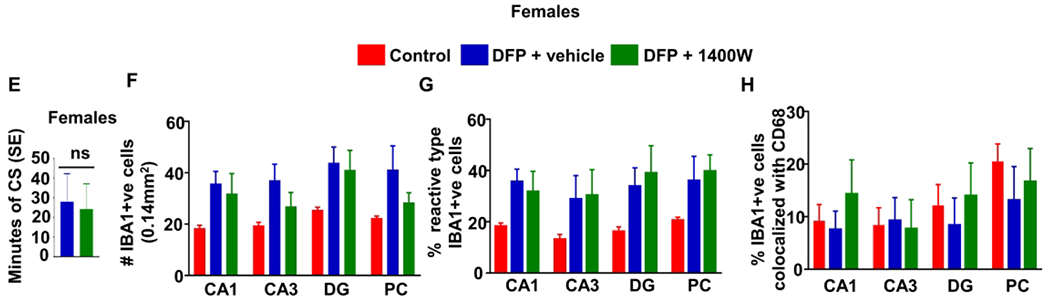
The impact of sex and 1400W on microgliosis at one to three months in vehicle-treated animals. Number of minutes each animal spent in a CS during SE are graphed against the total number of microglia (IBA1 positive, A), percent of microglia displaying reactive-type morphology (B), and the number of microglia colocalized with CD68 (C). A linear model evaluated sex differences and the interaction between sex and SE severity, n=7-12, #p<0.05 for the interaction. (D) Representative images (PC) from a female rat at 3m with mild and severe SE showing mostly non-reactive and reactive microglia, respectively. (E) Vehicle and 1400W treated animals showed no significant difference in the amount of time spent in CS during SE. Quantification for total number of microglia (F), percent reactive microglia (G), and percent of microglia colocalized with CD68 (H); analyzed via two-way mixed measures ANOVA for the treatment groups (control, DFP+vehicle, and DFP+1400W females), n=5-7. CA= cornu ammonis, DG= dentate gyrus, PC= piriform cortex. Scale 100um. Data points represent means and standard error of the mean.
3.6.1. The impact of sex and 1400W on microgliosis
Brain sections from animals in each group were co-stained with IBA1 and CD68 and counterstained with DAPI to examine the effects of sex and 1400W treatment on microgliosis. We counted the total number of IBA1 positive cells (Fig. 6A, 6F), the percent of IBA1 positive cells with reactive morphology (Fig. 6B, 6G), and the percent of IBA1 positive cells colocalized with CD68 (Fig. 6C, 6H). The detailed cell counting procedure is described in the Supplementary Methods. Representative images from a female with severe SE showing mostly reactive-type microglia and a female with mild/moderate SE showing mostly nonreactive microglia are presented (Fig. 6D) to demonstrate the effects of SE severity on cell number and morphology. All areas of the hippocampus (CA1, CA3, DG) showed significant interaction between sex and SE severity for the total number of microglia when compared to males (Fig. 6A). The analysis also showed a similar significant trend for the percentage of reactive microglia in CA3 (Fig. 6B). However, there were no significant differences between sexes in CD68 positive IBA1 cells in any regions analyzed (Fig. 6C).
Two-way mixed measures ANOVA was used to evaluate the effects of 1400W on DFP-induced microgliosis in females. Unlike the male rats in our recent publication33, females rats had no significant main effects or simple effects of treatment for any analyses of microgliosis (Fig. 6F–H). This is likely due to the compromised initial SE severity in female rats, in contrast to male rats, which may have confounded the real effect of 1400W on countering the DFP-induced microgliosis in females.
3.6.2. Impact of sex and 1400W on astrogliosis
Brain sections were stained with GFAP to locate astrocytes; the total number of GFAP positive cells were counted (Fig. 7A, 7D) and evaluated for morphology (Fig 7B, 7E). An example of images from a brain section from a female with mild/moderate SE showing nonreactive-type astrocytes and from severe SE female with mostly reactive-type astrocytes is presented in figure 7C. The percentage of cells that were considered reactive was calculated from the total GFAP positive cells for each animal. Using the linear model, females showed significantly increased astrogliosis in the DG and piriform cortex with increasing SE severity compared to males (Fig. 7A). A similar trend occurred in the DG for reactive astrogliosis (percent reactive astrocytes) (Fig. 7B)
Figure 7.
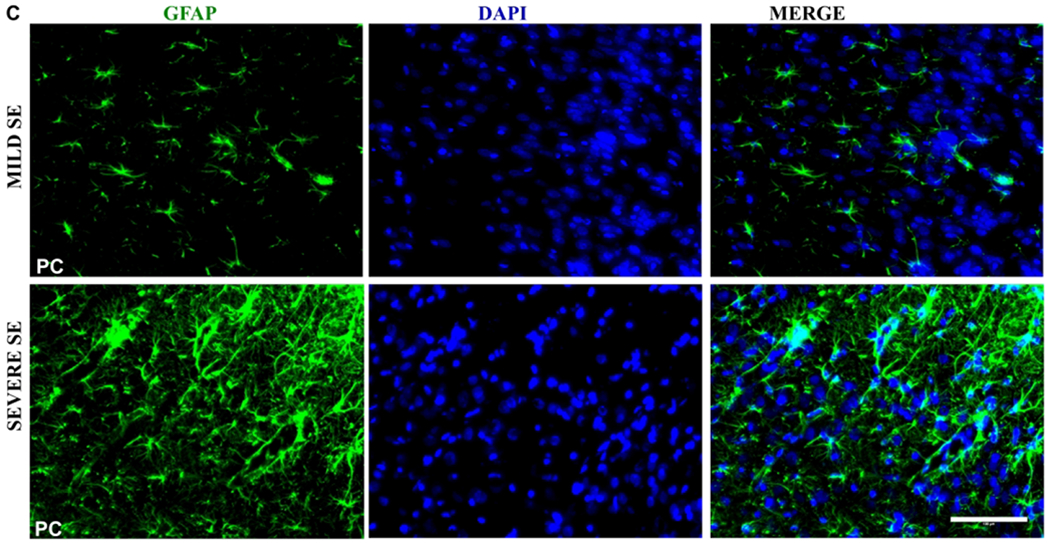
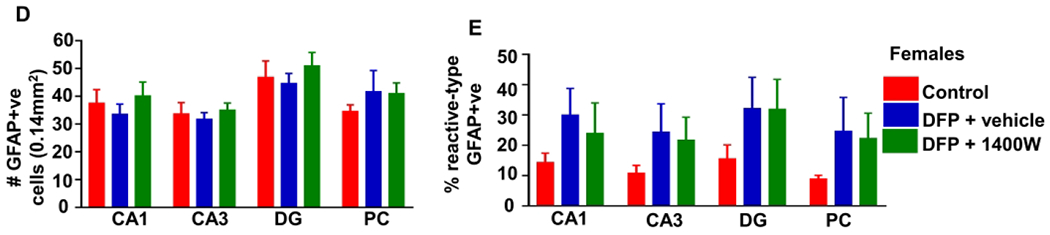
The impact of sex and 1400W on astrogliosis at one to three months in vehicle-treated animals. Number of minutes spent in a convulsive seizure during SE was graphed for the total number of GFAP positive cells (A) and percent of astrocytes with reactive type morphology (B). A linear model determined sex differences and the interaction between sex and SE severity, n=7-12, #p<0.05 with respect to the interaction. (C) Representative images (PC) from a female animal at 3m with mild and severe SE with mostly non-reactive and reactive astrocytes, respectively. Quantification for total number of astrocytes (D) and percentage of reactive astrocytes (E). Two-way mixed measures ANOVA showed no differences between the groups(control, DFP+vehicle and, DFP+1400W females), n=5-7. CA= cornu ammonis, DG= dentate gyrus, PC= piriform cortex, GFAP=glial fibrillary acidic protein. Scale 100um. Data points represent means and standard error of the mean.
A two-way mixed measures model was used to determine the effects of DFP and 1400W in female animals. Unlike in male rats33, there were no significant main effects or simple effects in any brain region concerning the total number of astrocytes or the percentage of reactive astrocytes in female rats (Fig. 7D–E).
3.6.3. Impact of Sex and 1400W on neurodegeneration
Brains were stained with NeuN as a marker for neurons and FJB as a marker for degeneration; their colocalization indicates degenerating neurons59. Linear model analysis between vehicle-treated males and females, after exposure to DFP, showed an increase in colocalization in males while controlling for SE severity in CA1 and CA3 of the hippocampus (Fig. 8A). Representative images of a brain section from a male and female demonstrating differences in neurodegeneration are shown in Figure 8B.
Figure 8.
The impact of sex on neurodegeneration at one to three months for vehicle-treated animals. (A) The number of minutes spent in a convulsive seizure during SE was graphed for the total number of NeuN cells colocalized with FJB. A linear model showed sex differences in CA1 and CA3, n=7-12, *p<0.05 with respect to sex while controlling for SE severity. (B) Representative images from CA1 in a male and female at 3m. CA= cornu ammonis, DG= dentate gyrus, PC= piriform cortex, NeuN= Neuronal Nuclear Antigen and Neuron Differentiation Marker, FJB= Fluoro-Jade B. Scale 100um. Data points represent means and standard error of the mean.
Discussion
The purpose of this study was to determine the sex-dependent responses of female rats to DFP exposure and then further evaluate other long-term EEG and histological changes in the brain. Females had a decreased response to DFP exposure in terms of the severity and total time spent in SE despite being treated with a higher dose. Males were given a lower dose of DFP than nontelemetry animals that we have used in prior experiments as we knew surgical implantation of electrodes can lower the seizure threshold60. In a pilot study in females, we observed less SE severity, and as we knew there was some resistivity in the pilocarpine model18, we decided to use an increased dose in females61. In female rats, the basal levels of commonly known esterases such as AChE, butyryl cholinesterase, and carboxylesterase alter throughout the estrous cycle14. DFP binds to AChE in the plasma and reduces the concentration of free DFP62. AChE levels were not evaluated in this study but it is plausible that hormone levels could impact the interaction between DFP and AChE in the blood62,63. The sex difference observed in this study was similar to the finding in the pilocarpine model, as sex differences might exist in cholinergic chemoconvulsant models, with females being more resistant to SE induction61. In contrast, a study using sarin, an OP nerve agent, found that only female rats in proestrus had a significantly higher LD50 than in other female rats14. Only two of the female rats in our study were in proestrus at the time of DFP exposure, and thus predominance of this stage of estrus does not fully explain the reduced sensitivity in females as compared to males. It is also notable that the sarin study14 measured cholinesterase activity across the cycle and found no correlation between proestrus AchE levels and SE susceptibility indicating that DFP susceptibility might be more complex than cholinesterase levels and estrous stages. Another study that used pyridostigmine treatment before DFP (4.0 to 6.25mg/kg) reported that there was no difference in seizure susceptibility between male and female rats64. The use of pyridostigmine pretreatment and range of DFP doses may explain the differences between the previous study64 and ours.
Mortality occurs in both males and females in response to an optimum dose of chemoconvulsants that induce SE in most of the tested populations33,40,45,65. Even with increased SE severity in the males, we did not find significant differences in mortality between sexes, and there was no correlation between mortality, SE severity, and the stages of the estrous cycle. It is also worth noting the three of five females and one of seven males that died in this study did not show frequent convulsive seizures during SE. DFP-induced mortality in both sexes may be due to the peripheral effects. For example, human case studies using OP nerve agent exposure have confirmed the occurrence of respiratory failure66,67. The rat pilocarpine study found higher mortality rate in females compared to males, and no impact of the estrous cycle reported18. Although we found significant sex differences in DFP-induced SE severity, there were no differences in SE severity between the stages of the estrous cycle. It is notable, however, that there was high variability between animals in each stage of estrous, thus increased power is needed to validate the observation further.
Animals that experienced a high degree of SE severity (>30 minutes of CS), compared to those that had mild/moderate seizures (<30 min), had disrupted estrous cycle with an (extended period of diestrus). This suggests that the SE severity disrupted the estrous cycle, and the peripheral effects of DFP itself did not cause dysregulation of the estrous cycle. Previous studies in the pilocarpine model also demonstrated irregular estrus cycle with an increase in diestrus following seizure induction68. In the pilocarpine model, decreased levels of progesterone, luteinizing hormone, and follicle stimulating hormone were reported, which might explain the prolonged diestrus observed in our study. Human females with epilepsy have also been reported to have had increased menstrual disturbances, which provides further evidence for the role of seizures in effecting the estrous cycle69.
Although there are some investigations of sex differences in response to chemoconvulsants and initial SE severity, there are fewer studies on the long-term impact of DFP intoxication with respect to sex19. In our study, females displayed overall fewer epileptiform spikes in comparison to males throughout the one-month study. This difference was most likely due to the overall decreased SE severity in females, compared to males, as the development of epileptiform spikes, electrographic NCS, and SRS are dependent on initial SE severity. This difference in severity, though not significant as per the Fisher’s exact test (p=0.608), was also supported by the number of animals that developed convulsive SRS (4 of 8 males and 2 of 7 females) in four weeks. All male animals and only four female animals showed epileptiform spikes and NCS irrespective of whether they had SRS. This highlights the importance of considering SE severity when analyzing the long-term effects in both sex-related studies as well as evaluation of disease-modifying agents. Both of the females that showed SRS experienced > 30 minutes of SE, demonstrating that high SE severity is needed to initiate epileptogenesis.
Animals with disrupted estrous cycle returned to normal cycling pattern around one to two weeks post-DFP. Although other patterns have been observed, seizures in women with catamenial epilepsy tend to occur periovulatory likely due to the decline in progesterone, along with an increase in estrogen17. Therefore, it was reasonable to predict that a similar pattern might occur in female rats exhibiting SRS. There was, however, no correlation between SRS occurrence and the stages of the estrous cycle in this study. We did observe trends of increasing frequency of alpha, sigma, beta, and gamma power around the time of proestrus and estrus for seizing animals during a period of regular cycling. However, hormonal assays might reveal the role of sex hormones in electrical excitability. Notably, there was no clear fluctuation of delta and theta powers. In a mouse study, the GABAA receptors expression in parvalbumin interneurons seems to be inversely related to the amplitude of gamma oscillations70. As the expression of GABAA was lowest around the period of estrus, this would support the peaks we saw around proestrus and estrus in our study. It is important to note that the transition between each stage of the cycle is gradual. Proestrus, for example, typically begins in the morning and does not last an entire 24 hours53; in our study, we determined the estrous stages based on vaginal cytology performed at 10-11 am each day. All rats were administered DFP around 2-3 PM, and it may be interesting to test DFP toxicity at various times in the day. To investigate the role of the estrous cycle in seizure susceptibility, other models that induce consistent SE severity or models that modulate estrogen and progesterone levels would be useful71.
The secondary goal of this study was to evaluate a disease-modifying drug, an iNOS inhibitor, 1400W in female animals. Markers of oxidative stress, such as iNOS, are modulated by chemoconvulsants, including DFP28,33,72,73. As fluctuating hormones and pharmacodynamic sex differences might dictate response to disease-modifying agents, we tested 1400W in female rats exposed to DFP. On the first day of treatment, 1400W suppressed seizures but not the epileptiform spikes in those females that had severe SE (Fig. 5A, 5G). Although this may seem an interesting observation, a large sample size with severe SE would be useful to determine whether these effects are due to the impact of iNOS inhibition by 1400W per se or an idiosyncratic reaction. 1400W administration in male animals caused a significant reduction in epileptiform spike rate at several days compared to vehicle-treated animals. It is important to note that none of the 1400W treated males had SRS in the first month compared to the two 1400W treated females that developed SRS. Notably, one male treated with 1400W did develop SRS at three months, but females were not studied on EEG past one month, and thus we cannot make comparisons between these animals at longer time points. Therefore, a further long-term study with a large sample size is needed to test the efficacy of 1400W as a disease-modifying agent in females.
The limitation in testing the disease-modifying agent in females for a prolonged period in this study was the lack of initial SE severity. Although five of seven females did not develop SRS, all of these animals had less than 30 minutes of convulsive seizures during SE. SRS and epileptiform spiking suppression during post-SE was likely due to compromised initial SE severity rather than the efficacy of the drug itself. Future studies will consider using increased doses of DFP and 1400W in females with large samples size to achieve SE severity similar to the males to evaluate the efficacy of the disease-modifying drug.
Despite the lack of similar SE severity in females, in contrast to males, changes in neuroinflammation and neurodegeneration can serve as a secondary means of evaluating the effects of DFP and 1400W. Neuroinflammation is a hallmark of SE-induced epilepsy74–77. Interestingly, a recent study in humans exposed to low levels of sarin and cyclosarin (OP nerve agents), diffusor tensor imaging revealed significant changes in the hippocampus, including changes in the mean diffusivity of those exposed to a munitions dump78. This suggests that even a low-level exposure and less severe SE could cause structural changes in an experimental model. Our previous study in male rats showed increased neuroinflammation and neurodegeneration in response to DFP, which was significantly mitigated by 1400W treatment33. When analyzing the females, there were neither significant main effects nor simple effects for any of the parameters suggesting that SE severity indeed determines an inflammatory state in the DFP model at the time point studied. Notably, the females with severe SE, despite treating with 1400W, showed increases in gliosis and neurodegeneration, suggesting that 1400W was ineffective at 20mg/kg dose. Further analysis, comparing the degree of inflammatory markers in male and female vehicle-treated animals, revealed that there were region-specific significant interactions between SE severity and sex. These results suggest that females respond with increased gliosis as SE severity increases compared to males. As the gliosis was increased in females with severe SE, this might explain the lack of response to 1400W at the same dosing regimen as for the male rats tested previously (20mg/kg, twice daily for the first three days). Many of these region-wise interaction effects seem to be driven by one female subject. More females with similar levels of SE severity would be necessary to verify these findings. The significant increase in spikes during the first 24h and suppression of CS on day two in 1400W treated animals in comparison to vehicle-treated animals might suggest an increased dose and duration of 1400W treatment could be explored for females to treat DFP-induced toxicity.
In summary, unlike in male rats, to fully evaluate the efficacy of 1400W in female animals, further investigation with increased SE severity is required. A large sample size to accommodate different stages of estrous and a dose-response for DFP would be useful. This study, however, clearly demonstrates the importance of SE severity as a variable when considering SABV in testing disease-modifying investigational new drugs.
Supplementary Material
Acknowledgments
This study supported by NIH-NINDS/CounterACT grants 1R21NS110648-01 and 1R21NS099007-01A1 (1400W related data)- Principal Investigator, T. Thippeswamy. We also acknowledge Dr. Chong Wang Ph.D. (Iowa State Department of Statistics) for providing council concerning statistical analyses.
Footnotes
Competing Interests
Authors have no competing interests.
References
- 1.Beery AK & Zucker I Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev 35, 565–72 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zucker I & Beery AK Males still dominate animal studies. Nat. 2010 4657299 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Clayton JA Studying both sexes: a guiding principle for biomedicine. FASEB J. 30, 519–524 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holdcroft A Gender bias in research: how does it affect evidence based medicine? J. R. Soc. Med 100, 2–3 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tingen CM, Kim AM, Wu P-H & Woodruff TK Sex and Sensitivity: The Continued Need for Sex-Based Biomedical Research and Implementation. Women’s Heal. 6, 511–516 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Franconi F, Brunelleschi S, Steardo L & Cuomo V Gender differences in drug responses. Pharmacol. Res 55, 81–95 (2007). [DOI] [PubMed] [Google Scholar]
- 7.NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research | grants.nih.gov. https://grants.nih.gov/grants/funding/women_min/guidelines.htm.
- 8.Birmingham K The NIH improves its record on women in trials. Nat. Med. 2000 66 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Carnes SGKRFH The More Things Change, the More They Stay the Same. Acad. Med 93, 630–635 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SK Sex as an important biological variable in biomedical research. BMB Rep. 51, 167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu KA & Mager NAD Women’s involvement in clinical trials: historical perspective and future implications. Pharm. Pract. (Granada) 14, 708 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon DY et al. Sex bias exists in basic science and translational surgical research. Surgery 156, 508–516 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Beery AK Inclusion of females does not increase variability in rodent research studies. Curr. Opin. Behav. Sci 23, 143–149 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith CD, Wright LKM, Garcia GE, Lee RB & Lumley LA Hormone-dependence of sarin lethality in rats: Sex differences and stage of the estrous cycle. Toxicol. Appl. Pharmacol 287, 253–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staley K & Scharfman H A woman’s prerogative. Nat. Neurosci 8, 697–699 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Plant TM (Tony M ., Zeleznik A & Knobil E Knobil and Neill’s physiology of reproduction.
- 17.Penovich PE & Helmers S Chapter 4 Catamenial Epilepsy. in International review of neurobiology vol. 83 79–90 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Herzog AG Catamenial epilepsy: Update on prevalence, pathophysiology and treatment from the findings of the NIH Progesterone Treatment Trial. Seizure 28, 18–25 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Scharfman HE & MacLusky NJ Sex differences in the neurobiology of epilepsy: a preclinical perspective. Neurobiol. Dis 72 Pt B, 180–92 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan M & Tan U Sex difference in susceptibility to epileptic seizures in rats: importance of estrous cycle. Int. J. Neurosci 108, 175–91 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Sisó S et al. Editor’s Highlight: Spatiotemporal Progression and Remission of Lesions in the Rat Brain Following Acute Intoxication With Diisopropylfluorophosphate. Toxicol. Sci 157, 330–341 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim DK, Hoskins B & Ho IK Assessment of diisopropylfluorophosphate (DFP) toxicity and tolerance in rats. Res. Commun. Chem. Pathol. Pharmacol 39, 399–418 (1983). [PubMed] [Google Scholar]
- 23.Todorovic MS, Cowan ML, Balint CA, Sun C & Kapur J Characterization of status epilepticus induced by two organophosphates in rats. Epilepsy Res. 101, 268–276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitkänen A, Engel J Jr. & Past and Present Definitions of Epileptogenesis and Its Biomarkers. Neurotherapeutics 11, 231 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Todorovic MS, Cowan ML, Balint CA, Sun C & Kapur J Characterization of status epilepticus induced by two organophosphates in rats. Epilepsy Res. 101, 268–76 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putra M et al. Inducible nitric oxide synthase inhibitor, 1400W, mitigates DFP-induced long-term neurotoxicity in the rat model. Neurobiol. Dis (2019) doi: 10.1016/J.NBD.2019.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deshpande LS, Carter DS, Blair RE & DeLorenzo RJ Development of a Prolonged Calcium Plateau in Hippocampal Neurons in Rats Surviving Status Epilepticus Induced by the Organophosphate Diisopropylfluorophosphate. Toxicol. Sci 116, 623–631 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guignet M et al. Persistent behavior deficits, neuroinflammation, and oxidative stress in a rat model of acute organophosphate intoxication. Neurobiol. Dis (2019) doi: 10.1016/j.nbd.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flannery BM et al. Persistent neuroinflammation and cognitive impairment in a rat model of acute diisopropylfluorophosphate intoxication. J. Neuroinflammation 13, 267 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y-B et al. Organophosphate-induced brain injuries: delayed apoptosis mediated by nitric oxide. Environ. Toxicol. Pharmacol 7, 147–152 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Li Y et al. Spatiotemporal pattern of neuronal injury induced by DFP in rats: A model for delayed neuronal cell death following acute OP intoxication. Toxicol. Appl. Pharmacol 253, 261–269 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rojas A, Ganesh T, Wang W, Wang J & Dingledine R A rat model of organophosphate-induced status epilepticus and the beneficial effects of EP2 receptor inhibition. Neurobiol. Dis (2019) doi: 10.1016/j.nbd.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putra M et al. Inducible nitric oxide synthase inhibitor, 1400W, mitigates DFP-induced long-term neurotoxicity in the rat model. Neurobiol. Dis (2019) doi: 10.1016/j.nbd.2019.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X, Kuruba R & Reddy DS Midazolam-Resistant Seizures and Brain Injury after Acute Intoxication of Diisopropylfluorophosphate, an Organophosphate Pesticide and Surrogate for Nerve Agents. J. Pharmacol. Exp. Ther 367, 302–321 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuruba R, Wu X & Reddy DS Benzodiazepine-refractory status epilepticus, neuroinflammation, and interneuron neurodegeneration after acute organophosphate intoxication. Biochim. Biophys. Acta - Mol. Basis Dis 1864, 2845–2858 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shrot S et al. Prevention of organophosphate-induced chronic epilepsy by early benzodiazepine treatment. Toxicology 323, 19–25 (2014). [DOI] [PubMed] [Google Scholar]
- 37.McDonough JH, Dochterman LW, Smith CD & Shih TM Protection against nerve agent-induced neuropathology, but not cardiac pathology, is associated with the anticonvulsant action of drug treatment. Neurotoxicology 16, 123–32 (1995). [PubMed] [Google Scholar]
- 38.Ferchmin PA et al. 4R-cembranoid protects against diisopropylfluorophosphate-mediated neurodegeneration. Neurotoxicology 44, 80–90 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garvey EP et al. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J. Biol. Chem 272, 4959–63 (1997). [DOI] [PubMed] [Google Scholar]
- 40.Puttachary S et al. 1400W, a highly selective inducible nitric oxide synthase inhibitor is a potential disease modifier in the rat kainate model of temporal lobe epilepsy. Neurobiol. Dis 93, 184–200 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Kilkenny C, Browne WJ, Cuthill IC, Emerson M & Altman DG Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 8, e1000412 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heiss DR et al. Synthesis and Storage Stability of Diisopropylfluorophosphate. J. Chem 2016, 1–5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLean AC, Valenzuela N, Fai S & Bennett SAL Performing Vaginal Lavage, Crystal Violet Staining, and Vaginal Cytological Evaluation for Mouse Estrous Cycle Staging Identification. J. Vis. Exp e4389 (2012) doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruun DA, Guignet M, Harvey DJ & Lein PJ Pretreatment with pyridostigmine bromide has no effect on seizure behavior or 24 hour survival in the rat model of acute diisopropylfluorophosphate intoxication. Neurotoxicology 73, 81–84 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tse K, Puttachary S, Beamer E, Sills GJ & Thippeswamy T Advantages of Repeated Low Dose against Single High Dose of Kainate in C57BL/6J Mouse Model of Status Epilepticus: Behavioral and Electroencephalographic Studies. PLoS One 9, e96622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puttachary S et al. Immediate Epileptogenesis after Kainate-Induced Status Epilepticus in C57BL/6J Mice: Evidence from Long Term Continuous Video-EEG Telemetry. PLoS One 10, e0131705 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puttachary S, Sharma S, Thippeswamy A & Thippeswamy T Immediate epileptogenesis: Impact on brain in C57BL/6J mouse kainate model. Front. Biosci. (Elite Ed) 8, 390–411 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Fu R, Shen Q, Xu P, Luo JJ & Tang Y Phagocytosis of microglia in the central nervous system diseases. Mol. Neurobiol 49, 1422–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fumagalli S et al. The phagocytic state of brain myeloid cells after ischemia revealed by superresolution structured illumination microscopy. J. Neuroinflammation 16, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiragi T, Ikegaya Y & Koyama R Microglia after Seizures and in Epilepsy. Cells 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma S, Puttachary S, Thippeswamy A, Kanthasamy AG & Thippeswamy T Status Epilepticus: Behavioral and Electroencephalography Seizure Correlates in Kainate Experimental Models. Front. Neurol 9, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hubscher C, Brooks D & Johnson J A quantitative method for assessing stages of the rat estrous cycle. Biotech. Histochem 80, 79–87 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Marcondes FK, Bianchi FJ & Tanno AP Determination of the estrous cycle phases of rats: some helpful considerations. Brazilian J. Biol 62, 609–614 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Westwood FR The Female Rat Reproductive Cycle: A Practical Histological Guide to Staging. Toxicol. Pathol 36, 375–384 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Choi J & Koh S Role of Brain Inflammation in Epileptogenesis. Yonsei Med. J 49, 1 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vezzani A, French J, Bartfai T & Baram TZ The role of inflammation in epilepsy. Nat. Rev. Neurol 7, 31–40 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zimmer LA, Ennis M & Shipley MT Soman-induced seizures rapidly activate astrocytes and microglia in discrete brain regions. J. Comp. Neurol 378, 482–492 (1997). [DOI] [PubMed] [Google Scholar]
- 58.Myhrer T Neuronal structures involved in the induction and propagation of seizures caused by nerve agents: Implications for medical treatment. Toxicology 239, 1–14 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Schmued LC, Albertson C & Slikker W Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 751, 37–46 (1997). [DOI] [PubMed] [Google Scholar]
- 60.Sharma S, Puttachary S, Thippeswamy A, Kanthasamy AG & Thippeswamy T Status Epilepticus: Behavioral and Electroencephalography Seizure Correlates in Kainate Experimental Models. Front. Neurol 9, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scharfman HE & MacLusky NJ The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia 47, 1423–40 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Overstreet DH, Russell RW, Kerni W & Netherton RA The influence of ovariectomy on the sex-dependent effects of the anticholinesterase diisopropyl fluorophosphate. Psychopharmacology (Berl). 74, 391–392 (1981). [DOI] [PubMed] [Google Scholar]
- 63.Overstreet DH, Russell RW, Helps SC, Runge P & Prescott AM Sex differences following pharmacological manipulation of the cholinergic system by DFP and philocarpine. Psychopharmacology (Berl). 61, 49–58 (1979). [DOI] [PubMed] [Google Scholar]
- 64.Scholl EA, Miller-Smith SM, Bealer SL, Lehmkuhle MK, Eskstrand JJ, Dudek FE, McDonough JH. Age-dependent behaviors, seizure severity and neuronal damage in response to nerve agents or the organophosphate DFP in immature and adult rats. Neurotoxicology 66, 10–21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scharfman HE, Kim M, Hintz TM & MacLusky NJ Seizures and reproductive function: insights from female rats with epilepsy. Ann. Neurol 64, 687–97 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hulse EJ, Davies JOJ, Simpson AJ, Sciuto AM & Eddleston M Respiratory Complications of Organophosphorus Nerve Agent and Insecticide Poisoning. Implications for Respiratory and Critical Care. Am. J. Respir. Crit. Care Med 190, 1342–1354 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LoMauro A & Aliverti A Sex differences in respiratory function. Breathe (Sheffield, England) 14, 131–140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amado D & Cavalheiro EA Hormonal and gestational parameters in female rats submitted to the pilocarpine model of epilepsy. Epilepsy Res. 32, 266–74 (1998). [DOI] [PubMed] [Google Scholar]
- 69.Svalheim S et al. Do women with epilepsy have increased frequency of menstrual disturbances? Seizure 12, 529–33 (2003). [DOI] [PubMed] [Google Scholar]
- 70.Barth AMI, Ferando I & Mody I Ovarian cycle-linked plasticity of Î′-GABAA receptor subunits in hippocampal interneurons affects Î3 oscillations in vivo. Front. Cell. Neurosci 8, 222 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reddy DS Perimenstrual Catamenial Epilepsy. Women’s Heal. 3, 195–206 (2007). [DOI] [PubMed] [Google Scholar]
- 72.Pearson-Smith JN, Liang L-P, Rowley SD, Day BJ & Patel M Oxidative Stress Contributes to Status Epilepticus Associated Mortality. Neurochem. Res 42, 2024–2032 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puttachary S, Sharma S, Stark S & Thippeswamy T Seizure-induced oxidative stress in temporal lobe epilepsy. Biomed Res. Int 2015, 745613 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vezzani A, French J, Bartfai T & Baram TZ The role of inflammation in epilepsy. Nat. Rev. Neurol 7, 31–40 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kadar T, Cohen G, Sahar R, Alkalai D & Shapira S Long-Term Study of Brain Lesions Following Soman, in Comparison to DFP and Metrazol Poisoning. Hum. Exp. Toxicol 11, 517–523 (1992). [DOI] [PubMed] [Google Scholar]
- 76.Pessah IN et al. Models to identify treatments for the acute and persistent effects of seizure-inducing chemical threat agents. Ann. N. Y. Acad. Sci 1378, 124 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vezzani A, Dingledine R & Rossetti AO Immunity and inflammation in status epilepticus and its sequelae: possibilities for therapeutic application. Expert Rev. Neurother 15, 1081–92 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chao LL & Zhang Y Effects of low-level sarin and cyclosarin exposure on hippocampal microstructure in Gulf War Veterans. Neurotoxicol. Teratol 68, 36–46 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


