Abstract
Background & Aims:
It is not clear whether a healthy lifestyle affects mortality of patients with inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC).
Methods:
We collected data form the Nurses’ Health Study (1986–2014), Nurses’ Health Study II (1991–2015), and Health Professionals Follow-up Study (1986–2014), which assess lifestyles with serial questionnaires. We estimated joint and individual associations between 5 healthy lifestyle factors after IBD diagnosis (never smoking, body mass index 18.5–24.9 kg/m2, vigorous physical activity in the highest 50% with non-zero value, alternate Mediterranean diet score ≥4, and light drinking [0.1–5.0 g/d]) and mortality using Cox proportional hazards models.
Results:
We documented 83 deaths in 363 patients with CD during 4741 person-years and 80 deaths in 465 patients with UC during 6061 person-years. The median age of IBD diagnosis was 55 y. Compared to patients with IBD with no healthy lifestyle factors, patients with IBD with 3–5 healthy lifestyle factors had a significant reduction in all-cause mortality (hazard ratio [HR], 0.29; 95% CI, 0.16–0.52; Ptrend<.0001). This reduction was significant in patients with CD (Ptrend =.003) as well as in patients with UC (Ptrend =.0003). Individual associations were more than 25 pack-years (HR, 1.92; 95% CI, 1.24–2.97; Ptrend<.0001), physical activity (HR according to quintiles, 0.55–0.31; Ptrend=.001), Mediterranean diet (HR, 0.69; 95% CI, 0.49–0.98), and alcohol consumption (HR0.1–5 g/d 0.61; 95% CI, 0.39–0.95 vs HR>15 g/d 1.84; 95% CI, 1.02–3.32). The findings did not change when we adjusted for family history of IBD, immunomodulator use, and IBD-related surgery.
Conclusions:
In an analysis of data from 3 large cohort studies, we associated adherence to a healthy lifestyle with reduced mortality in patients with CD or UC.
Keywords: exercise, risk, survival, obesity, food
INTRODUCTION
Inflammatory bowel diseases (IBD) (Crohn’s disease [CD], ulcerative colitis [UC]) have emerged as global diseases1. While the incidence was traditionally highest in northern Europe and North America, there has been a recent emergence in regions undergoing westernization including Asia, Latin and South America2. This emergence has been postulated to be due to population-wide shifts in diet and lifestyle toward a western diet and sedentary lifestyle3. In support of this is evidence from large cohorts that have defined several environmental risk factors for these diseases4. However, apart from the impact of smoking, whether factors associated with risk of IBD exert a continued impact after disease diagnosis has not been well established.
Despite onset in predominantly young population, patients with IBD have higher age-specific mortality when compared to the general population5–8. In the general population, studies have demonstrated reduced mortality associated with adherence to certain lifestyle measures, including moderate physical activity9, adherence to a Mediterranean diet10, light alcohol consumption11, maintenance of healthy weight12, and avoidance of smoking13. However, some of these recommendations are contrary to what is often advised or practiced by IBD patients. For example, many patients are advised to lower fiber intake, particularly in the setting of strictures or prior abdominal surgery, while cessation of smoking is associated with worsening disease activity in patients with UC14. Active inflammation and gastrointestinal symptoms often reduce the ability to practice regular physical activity while many patients avoid alcohol due to gastrointestinal symptoms15. Further, there are competing influences on mortality risk in patients with IBD including penetrating complications from persistent systemic inflammation16 and treatment related morbidity and mortality17. Consequently, the degree to which such lifestyle measures is beneficial in patients with IBD is unknown. Defining the impact of these factors on mortality is particularly important given that a growing fraction of IBD patients consist of older individuals18. This population may be particularly vulnerable to both the complications of IBD and competing co-morbidity that influences mortality.
In this study, we aimed to (1) determine the association of adherence to an overall healthy lifestyle with mortality in older patients with established CD and UC; and (2) to define the potential mortality benefit conferred by each individual lifestyle factor.
METHODS
Study population
We used data from three ongoing prospective cohorts for our analysis. Established in 1976, the Nurses’ Health Study (NHS) is a prospective cohort of 121,700 female registered nurses in the United States who were 30 to 55 years old at baseline19. The NHS II, established in 1989, enrolled 116,429 female nurses between the ages of 25 and 42 years. The Health Professionals Follow-up Study (HPFS) enrolled 51,529 male health professionals between the ages of 40 and 75 in 198620. In all three cohorts, questionnaires were mailed to participants at enrollment and every two years thereafter to obtain information on various lifestyle factors and medical history. Diet was assessed using validated semi-quantitative food frequency questionnaires (FFQs) beginning in 1980, 1991, and 1986 in the NHS, NHS II, and HPFS, respectively and updated every four years. The study protocol was approved by the Institutional Review Boards of the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Ascertainment of inflammatory bowel disease diagnosis
We have previously detailed our methods for defining cases of CD and UC21. Briefly, with each questionnaire, participants self-reported a physician diagnosis of CD or UC. We then obtained permission from such participants for review of medical records. Participants who self-reported CD or UC were also invited to complete a detailed supplemental questionnaire detailing type of IBD, date of diagnosis, disease complications, and treatment. These records were reviewed by two board-certified gastroenterologists (H.K., P.L., K.E.B., J.M.R., A.N.A.) independently who were blinded to exposure and outcome. A diagnosis of CD or UC was made based on accepted clinical criteria incorporating symptoms, endoscopic, histologic, radiographic, or operative findings22,23. Disagreements on case definition were infrequent and resolved through consensus.
Assessment of lifestyle factors
Current smoking status and pack-years of smoking were inquired in each questionnaire cycle. BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2). For physical activity, weekly energy expenditure was estimated by summing the metabolic equivalent task (MET) value per week from all individual activities24,25. Diet quality was assessed through FFQs26,27 and was quantified using the alternate Mediterranean diet score (aMED)28 which includes the following nine components: vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated-to-saturated fatty acid ratio, red and processed meats, and moderate alcohol intake. Participants received 1 point if: (a) red and processed meat intake was below the median intake; (b) alcohol intake between 5–15 g/d; (c) other categories above the median intake; otherwise they received 0 points. The possible aMED score ranges from 0–9, with higher score representing a closer resemblance to a Mediterranean diet. Alcohol consumption was calculated by multiplying the frequency of consumption by the alcohol content in each alcoholic beverage and summing up alcohol intake from all alcoholic beverages29.
Our primary exposure was number of healthy lifestyle factors following IBD diagnosis. Based upon previous publications demonstrating an association with mortality, the following were considered “healthy” lifestyle factors: (a) never smoking13; (b) BMI 18.5–24.9 kg/m2 12; (c) vigorous physical activity in the highest 50% of the cohort with non-zero value (equivalent to two hours per week of jogging, running, biking, swimming or playing tennis)9; (d) aMED score greater than or equal to 410; and (e) light alcohol consumption (0.1–5.0 g/d)11. For the primary analysis, we examined all-cause mortality among individuals with 0, 1, 2, and 3–5 healthy lifestyle factors.
Ascertainment of death
Deaths were identified through reports from state statistics records, next of kin and the National Death Index30. All-cause mortality was ascertained between date of IBD diagnosis after study baseline (June 1, 1986 for the NHS/HPFS; June 1, 1991 for the NHS II) and the end of follow-up (June 1, 2014 for the NHS/HPFS; June 1, 2015 for the NHS II).
Statistical analysis
Person-years were calculated from the date of return of the first post-diagnosis questionnaire to death from any cause or the end of follow-up, whichever occurred first. Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) adjusting for relevant confounders. For our primary analysis, we used exposure information obtained from the first post-diagnosis questionnaire. If the first post-diagnosis questionnaire was missing, we used the next available questionnaire. To compare distribution of lifestyle factors between participants with and without IBD, we used the data from questionnaires immediately after the median date of disease diagnosis in the three cohorts (2000 for the NHS, 1999 for the NHS II, 2000 for the HPFS) for non-IBD participants to ensure comparable calendar time periods of assessment given secular trends in the lifestyle factors. All multivariable-adjusted models stratified by age at diagnosis, year of diagnosis, and cohort, adjusted for age at diagnosis group, and race. Multivariable-adjusted models of each individual risk factor were mutually adjusted for the other lifestyle factors. The two-tailed P value for the linear trend test was calculated by treating the number of healthy factors as a continuous variable for the analysis of healthy lifestyle; for analysis of individual lifestyle factors, pack-years of smoking, BMI, and alcohol consumption were treated as continuous variables whereas physical activity was analyzed using median of each quintile as a continuous variable.
We performed two a priori defined sensitivity analyses. First, we allowed for an additional lag period between diagnosis of IBD and assessment of exposure modeled exposures with a lag of one questionnaire cycle, namely 2–4 years after diagnosis of IBD. We also repeated our analysis adjusting for family history of IBD and disease severity defined as need for immunosuppressive therapy or bowel-resection surgery assessed in the supplemental questionnaire. Additionally, we examined whether the association of healthy lifestyle with mortality differed between adult-onset (18–59 years) and elderly-onset (≥ 60 years) IBD. Tests for interaction were conducted by introducing a cross-product interaction term in the regression model. We conducted all analyses using the SAS software (SAS Institute, Inc., Version 9.4, Cary, NC). All statistical analyses were two-sided with a p-value less than 0.05 indicating statistical significance.
RESULTS
Our study included 363 and 465 patients with CD and UC contributing to 4,741 and 6,061 person-years of follow-up, respectively. Among these, there were 83 and 80 deaths among those with CD and UC. Cancer (CD: 17 [20%], UC: 13 [16%]) and cardiovascular disease (CD: 9 [11%], UC: 14 [18%]) were the leading causes of death. The age- and cohort-standardized baseline characteristics according to number of healthy lifestyle factors are summarized in Table 1 and Supplementary Table 1. The median age of IBD diagnosis was 55 years (range 28–85 years). While patients with IBD were broadly similar to non-IBD participants in distribution of lifestyle behaviors, those with IBD were more likely to be smokers and less likely to drink alcohol; CD patients had lower physical activity compared with non-IBD participants or those with UC (Supplementary Table 2).
Table 1.
Basic characteristics of participants with inflammatory bowel disease, stratified by number of healthy lifestyle factorsa
| Variables | Number of Healthy Lifestyle Factors | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3–5 | |
| No. of participants | 59 | 192 | 287 | 290 |
| Age, years | 45 | 45 | 45 | 44 |
| Crohn’s disease, % | 54 | 48 | 44 | 42 |
| Female, % | 88 | 88 | 88 | 92 |
| White % | 94 | 93 | 94 | 94 |
| Smoking, pack-year | 26.0 (18.3) | 16.2 (18.2) | 10.5 (16.1) | 7.6 (14.4) |
| Never, % | 0 | 30 | 52 | 70 |
| Former, % | 88 | 58 | 42 | 26 |
| Current, % | 12 | 12 | 6 | 4 |
| Body mass index, kg/m2 | 32.0 (6.5) | 30.2 (7.9) | 25.9 (4.9) | 24.0 (4.0) |
| <18.5 kg/m2, % | 1 | 2 | 2 | 1 |
| 18.5–24.9 kg/m2, % | 0 | 16 | 47 | 77 |
| 25–29.9 kg/m2, % | 47 | 44 | 32 | 14 |
| ≥30 kg/m2, % | 52 | 38 | 17 | 8 |
| Physical activity, MET hr/wk, mean (SD) | 6.1 (8.8) | 12.2 (15.5) | 15.2 (16.9) | 27.8 (25.4) |
| Alternate Mediterranean diet score, median (IQR) | 2 (1–3) | 3 (2–4) | 4 (2–5) | 5 (4–6) |
| Alcohol consumption, g/d, mean (SD) | 7.2 (13.8) | 5.2 (12.2) | 4.6 (9.4) | 4.7 (6.9) |
| Use of immunomodulator, % | 18 | 16 | 13 | 14 |
| History of surgery for IBD complication, % | 30 | 33 | 28 | 24 |
Abbreviations: SD, standard deviation; MET, metabolic equivalent of task; IQR, interquartile range; IBD, inflammatory bowel disease.
The baseline characteristics are presented by number of healthy lifestyle factors. All variables are standardized to the age and cohort distribution of the study population, except for age which is standardized to cohort. Mean (SD) is presented for continuous variables, median (IQR) for ordinal variables, and percentage of participants for categorical variables. All variables are derived from the first questionnaire reported after IBD diagnosis.
Number of healthy lifestyle factors and mortality
We found an inverse association between number of healthy lifestyle factors and mortality (Figure 1). Compared to individuals with no healthy lifestyle factors, those with 1, 2, and 3–5 healthy lifestyle factors had a HR for death of 0.57 (95% CI 0.33–1.00), 0.29 (95% CI 0.17–0.51), and 0.29 (95% CI 0.16–0.52), respectively (Ptrend < 0.0001) (Table 2). Separate analysis in those with CD (HR 0.34 to 0.16; Ptrend 0.003) and UC (HR 0.70 to 0.24; Ptrend 0.0003) revealed similar statistically significant reductions in mortality with an increasing number of healthy lifestyle factors. Adjusting additionally for family history of IBD, use of immunosuppressive therapy, and surgery did not alter this association (Supplementary Table 3).
Figure 1. Number of healthy lifestyle factors and all-cause mortality in patients with inflammatory bowel disease.
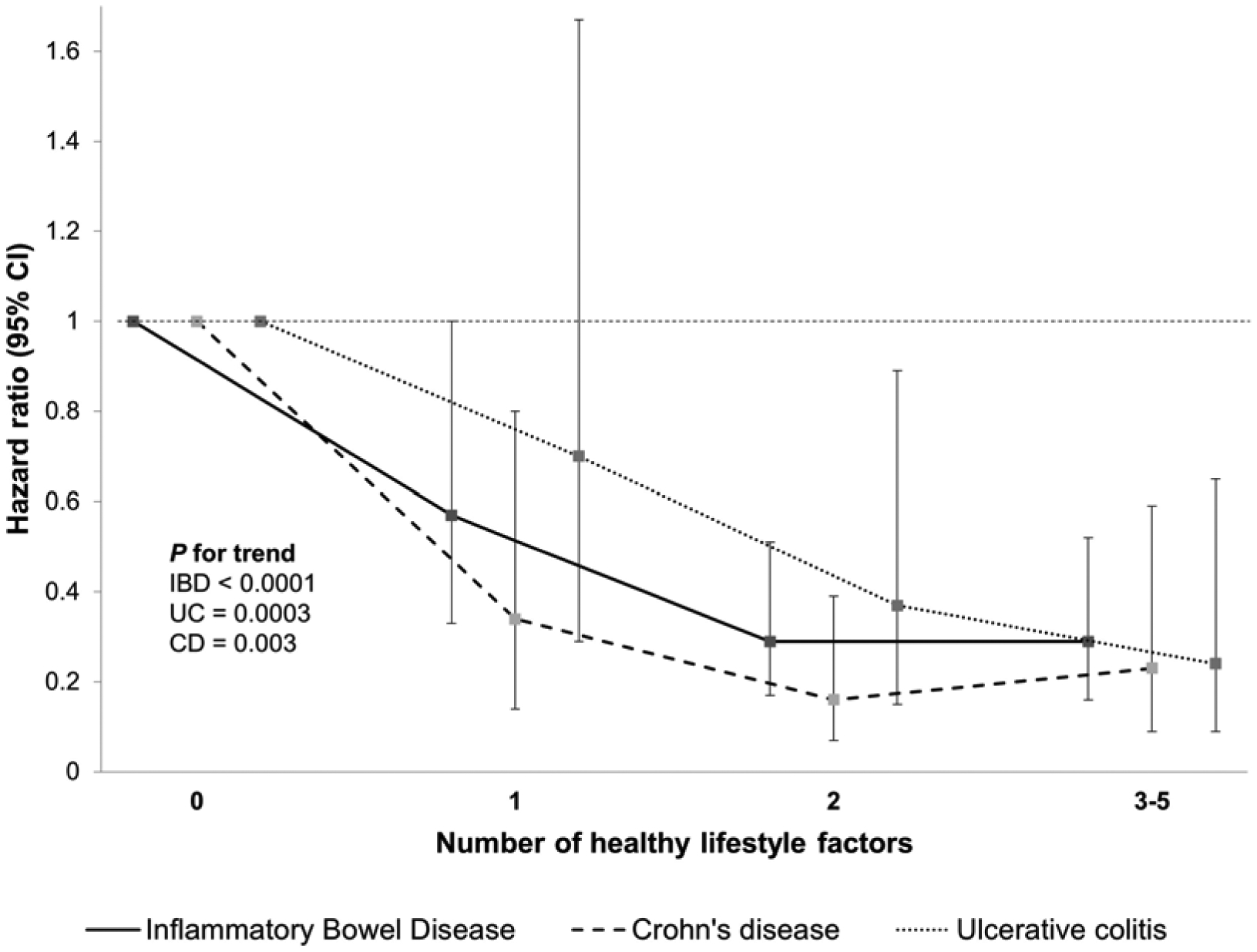
Healthy lifestyle factors were defined as never smoker, normal body weight (BMI 18.5–24.9 kg/m2), vigorous physical activity in the highest 50% with non-zero value, alternate Mediterranean diet score ≥ 4, light drinking (0.1–5 g/d).
Table 2.
All-cause mortality among participants with inflammatory bowel disease according to number of healthy lifestyle factors
| Disease type | Number of Healthy Lifestyle Factors | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3–5 | Ptrendd | |
| Inflammatory bowel disease | |||||
| No. of deaths | 21 | 55 | 59 | 28 | |
| Person-years | 684 | 2339 | 3900 | 3880 | |
| Age-adjusted HR (95% CI)b | 1 (Reference) | 0.56 (0.33–0.97) | 0.28 (0.17–0.49) | 0.29 (0.16–0.52) | <0.0001 |
| Multivariable-adjusted HR (95% CI)c | 1 (Reference) | 0.57 (0.33–1.00) | 0.29 (0.17–0.51) | 0.29 (0.16–0.52) | <0.0001 |
| Crohn’s disease | |||||
| No. of deaths | 12 | 29 | 26 | 16 | |
| Person-years | 296 | 1155 | 1764 | 1527 | |
| Age-adjusted HR (95% CI)b | 1 (Reference) | 0.32 (0.15–0.68) | 0.15 (0.07–0.34) | 0.21 (0.09–0.50) | 0.001 |
| Multivariable-adjusted HR (95% CI)c | 1 (Reference) | 0.34 (0.14–0.80) | 0.16 (0.07–0.39) | 0.23 (0.09–0.59) | 0.003 |
| Ulcerative colitis | |||||
| No. of deaths | 9 | 26 | 33 | 12 | |
| Person-years | 388 | 1183 | 2137 | 2354 | |
| Age-adjusted HR (95% CI)b | 1 (Reference) | 0.70 (0.29–1.67) | 0.37 (0.15–0.90) | 0.25 (0.09–0.66) | 0.0003 |
| Multivariable-adjusted HR (95% CI)c | 1 (Reference) | 0.70 (0.29–1.67) | 0.37 (0.15–0.89) | 0.24 (0.09–0.65) | 0.0003 |
Abbreviations: NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study; HR, hazard ratio; CI, confidence interval; BMI, body mass index; aMED, alternate Mediterranean diet score.
Healthy lifestyle factors included never smoker, normal body weight (BMI 18.5–24.9 kg/m2), vigorous physical activity in the top 2 quartiles after excluding participants with no vigorous physical activity, aMED ≥4, light drinking (0.1–5 g/d).
Cox proportional hazards regression model stratified by age at diagnosis (in 5-year intervals), year of diagnosis (<1990, 1990–1999, 2000–2009, ≥2010), cohort (NHS, NHS II, HPFS), with additional adjustment for age at diagnosis (continuous, year).
Further adjusted for race (Southern European/Mediterranean, Scandinavian, other Caucasian, other races).
P for trend was calculated by treating the number of healthy lifestyle factors (0, 1, 2, 3, 4, 5) as a continuous variable.
Individual lifestyle risk factors and mortality
Current smoking was associated with a four-fold increase in risk of death (HR 4.08; 95% CI 2.28–7.30) (Table 3). The association of pack-years of smoking was strongest for those with a > 25 pack-year history of smoking (HR 1.92; 95% CI 1.24–2.97) and a statistically significant trend was noted across strata of smoking burden (Ptrend < 0.0001). For physical activity, compared with the first quintile, IBD patients in the third, fourth, and fifth quintiles had lower mortality risk, with the HRs ranging from 0.31 to 0.55 (Ptrend 0.001). An aMED score ≥ 4 was associated with lower mortality compared to a score < 4 (HR 0.69; 95% CI 0.49–0.98). For alcohol intake, compared with never drinkers, decreased risk of death was observed in light drinkers (HR 0.61; 95% CI 0.39–0.95; Ptrend ≤ 5 g/d 0.03) whereas increased mortality was observed in drinkers who consumed > 15 g/d (HR 1.84; 95% CI 1.02–3.32; Ptrend > 0 g/d 0.0003).
Table 3.
All-cause mortality among participants with inflammatory bowel disease according to lifestyle factors
| Variables | Inflammatory bowel disease | |||
|---|---|---|---|---|
| Person-years | No. of deaths | Age-adjusted HR (95% CI)a | Multivariable-adjusted HR (95% CI)b | |
| Smoking status | ||||
| Never smoker | 5321 | 54 | 1 (reference) | 1 (reference) |
| Past smoker | 4710 | 87 | 1.25 (0.86–1.83) | 1.25 (0.83–1.87) |
| Current smoker | 772 | 22 | 3.89 (2.27–6.68) | 4.08 (2.28–7.30) |
| Pack-years of smoking | ||||
| Never smoker | 5077 | 47 | 1 (reference) | 1 (reference) |
| ≤10 | 1698 | 14 | 1.00 (0.54–1.86) | 1.06 (0.55–2.03) |
| 10.1–25 | 2006 | 34 | 1.48 (0.91–2.42) | 1.45 (0.86–2.45) |
| >25 | 2022 | 68 | 1.92 (1.26–2.91) | 1.92 (1.24–2.97) |
| P for trendc | <0.0001 | <0.0001 | ||
| Body mass index | ||||
| <18.5 kg/m2 | 171 | 5 | 1.38 (0.54–3.52) | 1.66 (0.62–4.47) |
| 18.5–24.9 kg/m2 | 5314 | 71 | 1 (reference) | 1 (reference) |
| 25–29.9 kg/m2 | 3303 | 55 | 1.03 (0.70–1.53) | 1.10 (0.73–1.67) |
| ≥30 kg/m2 | 2015 | 32 | 1.52 (0.97–2.37) | 1.64 (1.03–2.63) |
| P for trend <25 kg/m2c | 0.12 | 0.08 | ||
| P for trend ≥18.5 kg/m2c | 0.04 | 0.08 | ||
| Physical activity | ||||
| Quintile 1 | 2120 | 49 | 1 (reference) | 1 (reference) |
| Quintile 2 | 2034 | 34 | 0.56 (0.35–0.90) | 0.55 (0.33–0.92) |
| Quintile 3 | 2326 | 38 | 0.45 (0.28–0.73) | 0.43 (0.26–0.71) |
| Quintile 4 | 2182 | 22 | 0.33 (0.19–0.57) | 0.31 (0.17–0.57) |
| Quintile 5 | 2041 | 20 | 0.41 (0.23–0.74) | 0.35 (0.19–0.65) |
| P for trendd | 0.003 | 0.001 | ||
| Alternate Mediterranean diet score | ||||
| <4 | 4776 | 87 | 1 (reference) | 1 (reference) |
| ≥4 | 6027 | 76 | 0.66 (0.47–0.93) | 0.69 (0.49–0.98) |
| P | 0.02 | 0.04 | ||
| Alcohol consumption | ||||
| Nondrinker | 4751 | 78 | 1 (reference) | 1 (reference) |
| 0.1–5 g/d | 3363 | 38 | 0.63 (0.41–0.97) | 0.61 (0.39–0.95) |
| 5.1–15 g/d | 1727 | 26 | 0.85 (0.53–1.37) | 1.08 (0.65–1.81) |
| >15 g/d | 962 | 21 | 1.34 (0.78–2.30) | 1.84 (1.02–3.32) |
| P for trend ≤5 g/dc | 0.07 | 0.03 | ||
| P for trend >0 g/dc | 0.0004 | 0.0003 | ||
Abbreviations: NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study; HR, hazard ratio; CI, confidence interval; BMI, body mass index; MET, metabolic equivalent of task; aMED, alternate Mediterranean diet score.
Cox proportional hazards regression model stratified by age at diagnosis (in 5-year intervals), year of diagnosis (<1990, 1990–1999, 2000–2009, ≥2010), cohort (NHS, NHS II HPFS), with additional adjustment for age at diagnosis (continuous, year).
Further adjusted for race (Southern European/Mediterranean, Scandinavian, other Caucasian, other races), post-diagnostic smoking status (never smoker, 0.1–10, 10.1–25, >25 pack-years), BMI (<18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2), physical activity (in quintiles, MET-hr/wk), aMED (in quartiles), and alcohol consumption (nondrinker, 0.1–5, 5.1–15, >15 g/d).
P for trend was calculated by treating the exposure as a continuous variable.
P for trend was calculated using the median of each quintile as a continuous variable.
We observed associations between lifestyle factors and mortality for which the association was observed primarily for either CD or UC (Table 4). Current smoking and > 25 pack-years of smoking were associated with increased risk of death in CD patients (current smoking: HR 6.32; 95% CI 2.66–14.99; > 25 pack-years: HR 2.77; 95% CI 1.38–5.55; Ptrend 0.0005) but not UC patients. BMI < 18.5 kg/m2 was associated with nearly eight-fold greater mortality (HR 7.87; 95% CI 1.32–46.85; ) in UC patients compared with the reference group (BMI 18.5–24.9 kg/m2). For physical activity, there was significant reduction in mortality with increasing physical activity in UC patients, with HR across quintiles ranging from 0.41 to 0.14 (Ptrend 0.002). CD patients with an aMED score ≥ 4 had lower mortality risk compared to those with score < 4 (HR 0.50; 95% CI, 0.29–0.87). Compared with never drinkers, CD patients who consumed > 15 g/d had significantly higher mortality (HR 2.41; 95% CI 1.04–5.62; Ptrend > 0 g/d 0.0005).
Table 4.
All-cause mortality among participants with Crohn’s disease or ulcerative colitis according to lifestyle factors
| Variables | Crohn’s disease | Ulcerative colitis | ||||
|---|---|---|---|---|---|---|
| Death/Person-year | Model 1 HR (95% CI)a | Model 2 HR (95% CI)b | Death/Person-year | Model 1 HR (95% CI)a | Model 2 HR (95% CI)b | |
| Smoking status | ||||||
| Never smoker | 26/2299 | (reference) | 1 (reference) | 28/3022 | 1 (reference) | 1 (reference) |
| Past smoker | 41/1935 | 1.54 (0.86–2.78) | 1.66 (0.87–3.16) | 46/2776 | 1.09 (0.63–1.88) | 1.06 (0.57–1.97) |
| Current smoker | 16/508 | 6.85 (3.07–15.30) | 6.32 (2.66–14.99) | 6/264 | 2.32 (0.86–6.24) | 2.75 (0.88–8.63) |
| Pack-years of smoking | ||||||
| Never smoker | 23/2119 | 1 (reference) | 1 (reference) | 24/2958 | 1 (reference) | 1 (reference) |
| ≤10 | 6/568 | 1.64 (0.60–4.49) | 1.72 (0.58–5.13) | 8/1130 | 0.82 (0.35–1.94) | 1.10 (0.44–2.75) |
| 10.1–25 | 20/1008 | 2.22 (1.08–4.59) | 2.07 (0.89–4.82) | 14/999 | 1.01 (0.45–2.26) | 0.75 (0.30–1.89) |
| >25 | 34/1047 | 2.26 (1.20–4.28) | 2.77 (1.38–5.56) | 34/975 | 1.89 (1.01–3.57) | 1.34 (0.65–2.76) |
| P for trendc | 0.0005 | 0.0005 | 0.004 | 0.13 | ||
| Body mass index | ||||||
| <18.5 kg/m2 | 2/102 | 0.65 (0.15–2.90) | 0.63 (0.12–3.23) | 3/69 | 6.75 (1.66–27.41) | 7.87 (1.32–46.85) |
| 18.5–24.9 kg/m2 | 38/2215 | 1 (reference) | 1 (reference) | 33/3099 | 1 (reference) | 1 (reference) |
| 25–29.9 kg/m2 | 25/1488 | 0.93 (0.51–1.69) | 1.04 (0.51–2.14) | 30/1815 | 1.48 (0.82–2.68) | 1.49 (0.75–2.97) |
| ≥30 kg/m2 | 18/936 | 1.31 (0.68–2.52) | 1.56 (0.76–3.21) | 14/1079 | 1.71 (0.77–3.79) | 1.56 (0.63–3.85) |
| P for trend <25 kg/m2c | 0.59 | 0.48 | 0.19 | 0.007 | ||
| P for trend ≥18.5 kg/m2c | 0.25 | 0.13 | 0.05 | 0.26 | ||
| Physical activity | ||||||
| Quintile 1 | 21/965 | 1 (reference) | 1 (reference) | 27/1179 | 1 (reference) | 1 (reference) |
| Quintile 2 | 16/961 | 0.85 (0.40–1.83) | 0.73 (0.30–1.76) | 18/1151 | 0.42 (0.21–0.83) | 0.41 (0.19–0.92) |
| Quintile 3 | 16/905 | 0.66 (0.32–1.38) | 0.65 (0.29–1.44) | 18/1381 | 0.28 (0.14–0.60) | 0.27 (0.12–0.64) |
| Quintile 4 | 14/1026 | 0.60 (0.27–1.29) | 0.74 (0.30–1.79) | 9/1154 | 0.19 (0.08–0.46) | 0.21 (0.08–0.56) |
| Quintile 5 | 16/884 | 1.13 (0.53–2.41) | 0.81 (0.34–1.89) | 8/1196 | 0.13 (0.04–0.39) | 0.14 (0.04–0.46) |
| P for trendd | 0.74 | 0.82 | 0.0001 | 0.002 | ||
| Alternate Mediterranean diet score | ||||||
| <4 | 48/2256 | 1 (reference) | 1 (reference) | 39/2520 | 1 (reference) | 1 (reference) |
| ≥4 | 35/2486 | 0.54 (0.33–0.89) | 0.50 (0.29–0.87) | 41/3528 | 0.69 (0.42–1.15) | 1.25 (0.68–2.28) |
| P | 0.01 | 0.01 | 0.16 | 0.47 | ||
| Alcohol consumption | ||||||
| Nondrinker | 42/2202 | 1 (reference) | 1 (reference) | 36/2549 | 1 (reference) | 1 (reference) |
| 0.1–5 g/d | 19/1429 | 0.85 (0.46–1.58) | 0.82 (0.43–1.56) | 19/1934 | 0.42 (0.22–0.83) | 0.53 (0.25–1.10) |
| 5.1–15 g/d | 10/716 | 0.92 (0.43–1.96) | 1.01 (0.44–2.30) | 16/1011 | 0.82 (0.42–1.58) | 1.17 (0.53–2.61) |
| >15 g/d | 12/395 | 2.26 (1.06–4.83) | 2.41 (1.04–5.62) | 9/567 | 0.62 (0.25–1.55) | 0.86 (0.31–2.42) |
| P for trend ≤5 g/dc | 0.33 | 0.26 | 0.13 | 0.49 | ||
| P for trend >0 g/dc | 0.01 | 0.005 | 0.19 | 0.08 | ||
Abbreviations: NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study; HR, hazard ratio; CI, confidence interval; BMI, body mass index; MET, metabolic equivalent of task; aMED, Alternate Mediterranean Diet Score.
Cox proportional hazards regression model stratified by age at diagnosis (in 5-year intervals), year of diagnosis (<1990, 1990–1999, 2000–2009, ≥2010), cohort (NHS, NHS II, HPFS), with additional adjustment for age at diagnosis (continuous, year).
Further adjusted for race (Southern European/Mediterranean, Scandinavian, other Caucasian, other races), post-diagnostic smoking status (never smoker, 0.1–10, 10.1–25, >25 pack-years), BMI (<18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2), physical activity (in quintiles, MET-hr/wk), aMED (in quartiles), and alcohol consumption (nondrinker, 0.1–5, 5.1–15, >15 g/d).
P for trend was calculated by treating the exposure as a continuous variable.
P for trend was calculated using the median of each quintile as a continuous variable.
Sensitivity & stratified analysis
We tested our results by replacing the first post-diagnostic questionnaire with the second questionnaire (Supplementary Table 4). The results were largely similar and unchanged after adjusting for family history of IBD, immunomodulator use, and surgery. A healthy lifestyle was associated with lower mortality in both adult-onset and elderly-onset IBD (Supplementary Table 5). Although no statistical difference was observed (Pinteraction 0.44), the magnitude of association was numerically stronger among elderly-onset IBD.
DISCUSSION
In this study, we demonstrate that adherence to five healthy lifestyle factors was associated with lower all-cause mortality in older patients with CD and UC. These include moderate-to-high physical activity, maintenance of normal BMI, light alcohol consumption, non-smoking, and adherence to a Mediterranean diet. Our results support the importance of educating both patients and providers on adherence to these important lifestyle measures to improve life expectancy in patients with IBD.
Patients with IBD generally have a higher age-specific mortality compared to the general population5–8. While mortality directly from intestinal inflammation is rare, common causes of death as in the general population are cardiovascular diseases and cancer5,6. In the general population, a significant fraction of these deaths may be preventable by adhering to a healthy lifestyle31. However, many healthy lifestyle behaviors may be challenging to maintain in IBD patients due to dietary (self-imposed or physician-guided) or physical restrictions15 and the risk of cardiovascular disease and cancer is often increased by persistent overt and subclinical systemic inflammation32,33. Additionally, there has been a significant increase in the burden of prevalent disease in older population18. Reducing the risk of death is important in these patients as they may be particularly vulnerable to not only the impact of disease and its treatment17, but also to competing influences from co-morbid diseases. Consequently, the extent to which modifiable lifestyle behaviors impact mortality in this population is an important question that has significant implications for clinical practice.
IBD has considerable influence on patients’ dietary and activity pattern. Whether or not adherence to a healthy lifestyle offers the same mortality benefit to these patients compared with the general population is unknown. A meta-analysis of 15 studies suggested that roughly 60% of premature deaths could be attributed to five lifestyle behaviors, namely, smoking, diet, excess weight, alcohol, and physical inactivity31. Using data from the NHS and HPFS, Li et al.34 reported lower all-cause, cancer, and cardiovascular mortality and longer life expectancy among those with higher number of healthy lifestyle factors. Specifically, the HR for all-cause mortality in adults with 5 compared with zero low-risk factors were 0.26 (95% CI, 0.22–0.31), which is similar to what we observed for IBD patients with 3–5 healthy lifestyle factors. Separately, abstinence from smoking13, maintaining a normal weight12, regular physical activity9, healthy dietary pattern10, and light alcohol consumption11 have each been associated with lower mortality in previous publications. This is generally in agreement with our analysis. The similar associations of individual lifestyle factors with risk of death between IBD patients and the general population suggest that lifestyle modification is an equally effective strategy to prevent premature death in the IBD population. Furthermore, the effects of lifestyle factors do not appear to be mediated by disease severity as we found no consistent association between these factors and use of immunosuppressive therapy or surgery in our population and the magnitude of benefit remained unchanged upon adjusting for those parameters.
When comparing associations with individual lifestyle factors between CD and UC, we observed some differences. For example, smoking appeared to influence mortality in CD but not UC. This is similar to the associations observed for incident disease where current smoking is associated with greater risk of CD but decreased risk of UC. This difference in effect has been postulated to be due to the differential immunologic effects of smoking on CD and UC35. The theoretical cardiovascular benefit conferred by smoking cessation in UC may also be countered by more active disease and inflammatory burden as a consequence14. Low BMI following diagnosis was a risk factor for death in UC and possibly an indicator of disease severity, which is most apparent in the first two years after diagnosis. For physical activity, the mortality benefit seemed to be more apparent in UC patients. This may be due to lower physical activity in CD patients, including those in our cohorts, compared with UC patients and the general population36.
To our knowledge, this is the first study examining the impact of lifestyle on longevity after a diagnosis of IBD. There are several strengths to our study. First, we utilized three large cohorts that prospectively ascertained diet and lifestyle information. This minimizes bias related to differential recall in lifestyle factors. Second, our IBD cases were confirmed through detailed medical record review by board-certified gastroenterologists and deaths were systematically confirmed through validated measures.
We acknowledge several limitations. First, we were unable to specifically examine the association of lifestyle with cause-specific mortality due to limited number of deaths that could be attributable to any given cause. Second, while we obtained information on immunosuppressive therapy and surgery in patients with confirmed IBD, this was not systematically performed longitudinally. Growing data indicate distinct effects of immunosuppressive therapies on the risk of infections and cancers17, thus any association with medication use or disease severity in our study has to be interpreted with caution. Third, as with all other observational studies, we cannot exclude the possibility of unmeasured and residual confounding. Fourth, the present study focused on older IBD patients and thus our findings may not be applicable to younger patients. Fifth, we recognize the important contribution of each lifestyle factors to good health and longevity in IBD, therefore we did not assign different weights to different lifestyle behaviors. Last, our participants were mostly healthcare professionals and Caucasians, therefore generalizing findings to population of different race or socioeconomic status should be performed with caution.
In conclusion, providers are often faced with the challenge of advising patients who seek to actively modify their diet and lifestyle to improve their health. We demonstrate that adherence to key lifestyle factors was associated with lower mortality in patients with IBD. Assessment of healthy lifestyle behaviors should be routinely performed in IBD patients and adherence to such behaviors should be encouraged to improve longevity and promote healthy aging. Future work should examine the impact of changes in lifestyle behavior on mortality with accrual of more cases and longer follow-up period.
Supplementary Material
What you need to know.
Background:
A healthy lifestyle might reduce mortality of patients with inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC).
Findings
In an analysis of data from 3 large cohort studies, we associated adherence to a healthy lifestyle (never smoking, not being overweight, physical activity, Mediterranean diet score, and light consumption of alcohol) with reduced mortality in patients with CD or UC.
Implications for patient care:
Patients with IBD should be educated about the importance of a healthy lifestyle on survival.
ACKNOWLEDGEMENTS
We would like to thank the participants and staff of the Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Grant Support: This work was supported by the U.S. National Institutes of Health (U01 CA167552, U01 CA186107, U01 CA176726; K24 DK098311 to A.T.C.), the Crohn’s and Colitis Foundation (to H.K., A.T.C., A.N.A.), the Chleck Family Foundation (to A.N.A.), and Beker Foundation (to H.K.). The funders had no role in the design and conduct of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Abbreviations:
- NHS
Nurses’ Health Study
- HPFS
Health Professionals Follow-up Study
- IBD
inflammatory bowel disease
- CD
Crohn’s disease
- UC
ulcerative colitis
- BMI
body mass index
- FFQ
food frequency questionnaire
- MET
metabolic equivalent task
- aMED
alternate Mediterranean diet score
- HR
hazard ratio
- CI
confidence interval
- IQR
interquartile range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
J.M.R is a consultant to Policy Analysis Inc and Takeda Pharmaceuticals. A.T.C. serves as a consultant for Janssen, Pfizer, and Bayer Pharma AG for work unrelated to the topic of this manuscript. A.N.A. has served as a Scientific Advisory Board member for Abbvie, Gilead, Kyn therapeutics and received research grants from Pfizer and Merck. No other conflict of interest exists.
REFERENCES
- 1.Loftus EV. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 2004;126:1504–1517. [DOI] [PubMed] [Google Scholar]
- 2.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 2015;12:205–217. [DOI] [PubMed] [Google Scholar]
- 3.Ananthakrishnan AN, Bernstein CN, Iliopoulos D, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol 2018;15:39–49. [DOI] [PubMed] [Google Scholar]
- 4.Piovani D, Danese S, Peyrin-Biroulet L, et al. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology 2019;157:647–659.e4. [DOI] [PubMed] [Google Scholar]
- 5.Survival Jess T. and cause specific mortality in patients with inflammatory bowel disease: a long term outcome study in Olmsted County, Minnesota, 1940–2004. Gut 2006;55:1248–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Persson P, Bernell O, Leijonmarck C, et al. Survival and cause-specific mortality in inflammatory bowel disease: a population-based cohort study. Gastroenterology 1996;110:1339–1345. [DOI] [PubMed] [Google Scholar]
- 7.Jess T, Frisch M, Simonsen J. Trends in overall and cause-specific mortality among patients with inflammatory bowel disease from 1982 to 2010. Clinical Gastroenterology and Hepatology 2013;11:43–48. [DOI] [PubMed] [Google Scholar]
- 8.Olén O, Askling J, Sachs MC, et al. Increased mortality of patients with childhood-onset inflammatory bowel diseases, compared with the general population. Gastroenterology 2019;156:614–622. [DOI] [PubMed] [Google Scholar]
- 9.Andersen LB, Schnohr P, Schroll M, et al. All-cause mortality associated with physical activity during leisure time, work, sports, and cycling to work. Arch Intern Med 2000;160:1621–1628. [DOI] [PubMed] [Google Scholar]
- 10.Mitrou PN. Mediterranean dietary pattern and prediction of all-Cause mortality in a US population: results From the NIH-AARP Diet and Health Study. Arch Intern Med 2007;167:2461. [DOI] [PubMed] [Google Scholar]
- 11.Di Castelnuovo A Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 2006;166:2437. [DOI] [PubMed] [Google Scholar]
- 12.Bhaskaran K, dos-Santos- Silva I, Leon DA, et al. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. The Lancet Diabetes & Endocrinology 2018;6:944–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter BD, Abnet CC, Feskanich D, et al. Smoking and Mortality — Beyond Established Causes. N Engl J Med 2015;372:631–640. [DOI] [PubMed] [Google Scholar]
- 14.Beaugerie L, Massot N, Carbonnel F, et al. Impact of cessation of smoking on the course of ulcerative colitis. Am J Gastroenterol 2001;96:2113–2116. [DOI] [PubMed] [Google Scholar]
- 15.Vagianos K, Clara I, Carr R, et al. What are adults with inflammatory bowel disease (IBD) eating? A closer look at the dietary habits of a population-based canadian ibd cohort. JPEN J Parenter Enteral Nutr 2016;40:405–411. [DOI] [PubMed] [Google Scholar]
- 16.Bielawska B, Day AG, Lieberman DA, et al. Risk factors for early colonoscopic perforation include non-gastroenterologist endoscopists: a multivariable analysis. Clinical Gastroenterology and Hepatology 2014;12:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaugerie L, Kirchgesner J. Balancing benefit vs risk of immunosuppressive therapy for individual patients with inflammatory bowel diseases. Clinical Gastroenterology and Hepatology 2019;17:370–379. [DOI] [PubMed] [Google Scholar]
- 18.Taleban S, Colombel J-F, Mohler MJ, et al. Inflammatory bowel disease and the elderly: a review. Journal of Crohn’s and Colitis 2015;9:507–515. [DOI] [PubMed] [Google Scholar]
- 19.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6:49–62. [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. The Lancet 1991;338:464–468. [DOI] [PubMed] [Google Scholar]
- 21.Khalili H, Huang ES, Ananthakrishnan AN, et al. Geographical variation and incidence of inflammatory bowel disease among US women. Gut 2012;61:1686–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ordás I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012;380:1606–1619. [DOI] [PubMed] [Google Scholar]
- 23.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012;380:1590–1605. [DOI] [PubMed] [Google Scholar]
- 24.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–999. [DOI] [PubMed] [Google Scholar]
- 25.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology 1996;7:81–86. [DOI] [PubMed] [Google Scholar]
- 26.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. American Journal of Epidemiology 1988;127:188–199. [DOI] [PubMed] [Google Scholar]
- 27.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. Journal of the American Dietetic Association 1993;93:790–796. [DOI] [PubMed] [Google Scholar]
- 28.Fung TT, Rexrode KM, Mantzoros CS, et al. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009;119:1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Q, Townsend MK, Okereke OI, et al. Alcohol consumption at midlife and successful ageing in women: a prospective cohort analysis in the Nurses’ Health Study Hay PJ, ed. PLoS Med 2011;8:e1001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol 1984;119:837–839. [DOI] [PubMed] [Google Scholar]
- 31.Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Preventive Medicine 2012;55:163–170. [DOI] [PubMed] [Google Scholar]
- 32.Schicho R, Marsche G, Storr M. Cardiovascular complications in inflammatory bowel disease. Curr Drug Targets 2015;16:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. WJG 2016;22:4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Pan A, Wang DD, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation 2018;138:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parkes GC, Whelan K, Lindsay JO. Smoking in inflammatory bowel disease: Impact on disease course and insights into the aetiology of its effect. Journal of Crohn’s and Colitis 2014;8:717–725. [DOI] [PubMed] [Google Scholar]
- 36.Langenberg DR van, Papandony MC, Gibson PR. Sleep and physical activity measured by accelerometry in Crohn’s disease. Aliment Pharmacol Ther 2015;41:991–1004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


