Abstract
Purpose
Identify genes associated with ocular sarcoidosis (OS).
Methods
We genotyped 1.1 million genetic variants to identify significant OS associations, defined as those that achieved p<5×10−8 in a genome-wide comparison of OS cases to healthy controls in our European- or African-American cohorts (EA, AA). Potential functional roles of all associated variants were assessed.
Results
Eight significant non-HLA variants were found in AA OS cases compared to healthy controls and confirmed as at least suggestive when comparing OS to non-OS cases. Seven of these were within MAGI1 and include transcription factor binding sites and expression quantitative trait loci. Our EA cohort, while showing similar effect sizes at variants within MAGI1, had no significant variants. Association analysis of HLA-DRB1 alleles confirmed association to OS in EA to *04:01.
Conclusion
Our results support organ-specific genetic risk in OS in a compelling candidate, MAGI1, known to be associated with barrier function and autoimmunity.
Keywords: sarcoidosis, GWAS, ocular. MAGI1, HLA-DRB1*04:01
Introduction
Sarcoidosis is a complex inflammatory disease characterized by the formation of granulomas in any number of affected organs. Ocular sarcoidosis (OS) may involve any part of the eye.1 Uveitis is reported in 30%–70% and conjunctivitis in up to 40% of sarcoidosis patients.2 Sarcoidosis impacts individuals of all races, but African Americans (AA) have a three-fold higher lifetime risk (2.4% versus 0.85%) and age-adjusted annual incidence (35.5 per 100,000 versus 10.9 per 100,000) than do European Americans (EA).3, 4 AA are also more likely to have increased disease severity5 and potentially more OS compared to EA.6, 7
While the etiology of sarcoidosis is unclear, significant genetic associations to susceptibility,8 organ-specific manifestations,9–11 and gene by environment interactions12 are well documented and can differ by ancestry.13 A subset of sarcoidosis-associated variants have been found to be enriched in OS by candidate gene and HLA typing studies, including variants in RAB23, ANXA11, multiple HLA alleles, and HSP70-hom.14–16 Other OS-associated variants in CTLA-417 and CFH18 appear to associate only with the OS phenotype of sarcoidosis; although OS-specific CFH variants also associate with ocular inflammatory conditions age-related macular degeneration and multifocal choroiditis. Notably, HLA-DRB1*04, particularly HLA-DRB1*04:01, has been shown to be protective against sarcoidosis susceptibility but is a significant risk factor for OS and for Heerfordt’s syndrome, a subtype of sarcoidosis characterized by the presence of uveitis, parotid or salivary gland enlargement and cranial nerve palsies.19–21
We hypothesized, based on previous findings of organ-specific effects,9–11, 21 that genes conveying specific risk for ocular manifestations of sarcoidosis exist but may have been missed in candidate gene and HLA typing studies of OS. To identify these OS-specific genetic variants, we performed genome-wide association screens (GWAS) in our AA and EA cohorts independently, comparing OS cases to healthy controls and then confirming in a comparison of OS cases to those with no ocular involvement (non-OS). In addition to identification of novel, organ-specific effects via GWAS, we sought to assess previously reported associations to HLA-DRB1 alleles and to investigate probable functional significance of all associated variants.
Materials and Methods
Ethics Statement
Institutional Review Board (IRB) approval was obtained prior to sample collection or data acquisition for each of the participating studies, including A Case Control Etiologic Study of Sarcoidosis (ACCESS) Group, Sarcoidosis Genetic Analysis (SAGA) study, Henry Ford Family Study (HFFS), and Oklahoma Medical Research Foundation (OMRF) Sarcoidosis Genetic Studies (SGS). Complete genetic data is available via dbGaP (pht005793.v1.p1). Informed consent was obtained and enrollment was HIPAA-compliant and adhered to the tenets of the Declaration of Helsinki.
Participants
Participants for this study were taken from existing cohorts of both AA and EA sarcoidosis patients, family members and controls, specifically ACCESS,6, 22 SAGA,10 and HFFS. After genotyping quality control measures below, our cohort included 1271 AA sarcoidosis cases, 1551 AA controls, 332 EA cases and 2046 EA controls. While the majority of patients had biopsy-proven sarcoidosis, 17.2% (218/1271) of AA cases and 3.6% (12/332) of EA cases were designated as highly probable, defined as having chest radiographs showing bilateral hilar adenopathy and either a history of erythema nodosum or at least 2 years observation during which time no other disease was found to explain radiographic abnormalities.
As in previous ACCESS and SAGA studies,6, 10, 22 we defined definite ocular involvement in sarcoidosis (OS) as presence of one or more of the following: positive conjunctiva biopsy, positive sclera biopsy, lacrimal gland swelling, uveitis, or optic neuritis. Probable OS was defined as blindness (visual acuity less than 20/200 with corrective lenses). Criteria for possible OS were glaucoma, cataract, and sicca syndrome. For GWAS analyses, both probable OS (1/260 or 0.4% of AA OS cases, 0/35 or 0% of EA OS cases) and possible OS (67/260, 26%; 12/35, 34%) were included in the OS group. Of the cases, 61% (773/1271) AA cases and 77% (256/332) of EA cases were confidently categorized as OS or non-OS. Demographic information is summarized in Table 1.
Table 1.
Demographics of subjects used for AA and EA GWAS.
| AA Cases | AA Controls | EA Cases | EA Controls | |
|---|---|---|---|---|
| Total | 1271 | 1551 | 331 | 2046 |
| Ocular | 260 (20.5%) | n/a | 35 (10.6%) | n/a |
| Non-Ocular | 513 (40.4%) | n/a | 221 (66.7%) | n/a |
| Females | 949 (74.7%) | 1107 (71.4%) | 195 (58.7%) | 1172 (57.3%) |
| Males | 322 (25.3%) | 444 (28.6%) | 137 (41.3%) | 874 (42.7%) |
| Age (Average, SD) | 48.31, 8.05 | 59.10, 14.46 | 43.33, 10.66 | 43.85, 10.41 |
Genotyping
Genotype data used in our association analyses were obtained through the Clinical Genomics Core (CGC) at the Oklahoma Medical Research Foundation (Oklahoma City, OK) using the Illumina Human Omni1-Quad platform, which measures approximately 1.1 million single-nucleotide polymorphisms (SNPs) across the genome. The details of sample collection and genotyping have been described previously.8 All SNPs analyzed met the following quality control criteria for inclusion for each population: minor allele frequency >0.01, Hardy-Weinberg proportion test p>0.001. Only autosomal chromosomes were included in the analysis. Subjects with more than 1% of SNPs missing were removed from the analyses. Due to the possibility of self-reported ethnicity deviating from actual ancestry, principal components calculated from autosomal ancestry informative SNPs were used to assess population outliers from within each of our AA and EA cohorts.23
HLA-DRB1 allele counts were imputed and confirmed with observed HLA sequencing (SSOP method) as previously described in our paper24 using HLA Genotype Imputation with Attribute Bagging (HIBAG)25: an accurate package for imputing HLA types using SNP data without requiring a training dataset. The imputation was performed using post-QC SNPs in the HLA region. Imputed DRB1 alleles were verified a subset of our samples (n=325) that were HLA-typed via the SSOP method24.
Whole genome sequencing (WGS) data collected on a subset of the AA cohort including 285 cases (73 OS and 166 non-OS) and 345 controls were used as a reference for imputation. These data were obtained on the Illumina HiSeq XTen platform at the Human Genome Sequencing Center, Baylor College of Medicine (Houston, TX) as a part of the NHLBI Trans-Omics for Precision Medicine (TOPMed) program following the manufacturer’s protocols. Genotype imputation for AA subjects was imputed from WGS as a reference using SHAPEIT 2.7 and Minimac 3.2; genotype imputation for EA individuals was conducted using the 1000 Genomes Project haplotypes as the reference panel and the IMPUTE2 program. Imputed SNPs with R2<0.5 between expected and observed (Minimac 3.2) were removed. In total, the AA and EA studies had 13,976,150 SNPs and 4,755,887 SNPs, respectively, after quality control.
Association Analyses
Single-marker association tests were performed using logistic regression in PLINK226 assuming an additive genetic model. Due to the known association of sex and ancestry with ocular sarcoidosis phenotype and the admixed nature of AA genotypes, sex and the first five genomic principal components were included as covariates in the analysis. The first five genomic principal components serve to reduce spurious associations driven by population stratification within the AA subjects while still capturing the majority of genomic variance without unnecessarily complicating or reducing the power of the association test. The same covariates were included in both the AA and EA subjects to avoid model bias. OS-specific SNPs were defined within ancestry groups as those reaching genome-wide significance (p<5×10−8) in the comparison of OS to healthy controls. For a small number of OS to non-OS comparisons, the association had to be calculated by PLINK to achieve convergence.
HIBAG-imputed HLA-DRB1 allele counts were tested independently by Fisher’s Exact test and corrected for multiple comparisons by false discovery rate with Q<0.05 considered significant.
Bioinformatics Analyses
All OS-specific variants were examined separately for genomic location (i.e., exonic, intronic, within a promoter) via exon annotation (ENCODE, GTEx Portal exon reference file). SNPs were also assessed for potential function, including mapping to known transcription factor binding sites (TFBS; SNP2TFBS27 SNPSelect tool), expression quantitative trait loci (eQTLs; Haploreg28 and SNP2TFBS), and enhancer regions (specifically assessed with reference to retina tissue; ENCODE, EnhancerAtlas29). To identify molecular pathways in which OS-specific variants demonstrated effects, pathway analyses (Ingenuity Pathway Analysis, IPA) were performed on all genes containing or in close proximity to an OS-associated variant as well as transcription factors associated with OS-specific SNPs.
Results
Cohort Characteristics
Our AA cohort comprises 260 OS cases of 773 total phenotyped cases (33.6%), while our EA cohort comprises 35 OS cases of 346 total (10.12%). All EA OS and non-OS cases were collected as a part of ACCESS,6 within 6 months of diagnosis, and therefore lower prevalence of OS in our EA compared to AA cohort is likely due to the ascertainment of incident rather than prevalent cases in this cohort. Eye involvement in EA was not associated with sex, but EA OS cases were significantly older (mean and SD of age: 47.83, 10.80) than non-OS cases (42.62, 10.45; p=0.012). Insufficient clinical data were present to assess associations of disease state or organ involvement with OS in EA cases.
Within AA sarcoidosis cases with complete sex and ocular information, females had more OS (212 of 589 sarcoidosis patients, 36.0%) than males (48 of 184 sarcoidosis patients, 26.1%) [p=0.01; OR 1.59 (95% C.I.: 1.10–2.32)]. Cases with OS had similar organ (excluding eye) involvement as non-OS cases and there were no differences in age (Table 1) or persistent versus resolved disease between AA OS and AA non-OS cases. Similarly, time since diagnosis did not differ between AA OS (mean and SD: 11.86, 9.4) and AA non-OS patients (12.37, 8.6), suggesting our findings are not an artifact of differences in disease course or severity.
GWAS
We performed independent GWAS analyses in AA and EA using observed and imputed genotype data. A total of 13,976,150 SNPs in our AA cohort (n=2822) and 4,755,887 SNPs in our EA GWAS cohort (n=2377) passed quality control checks.
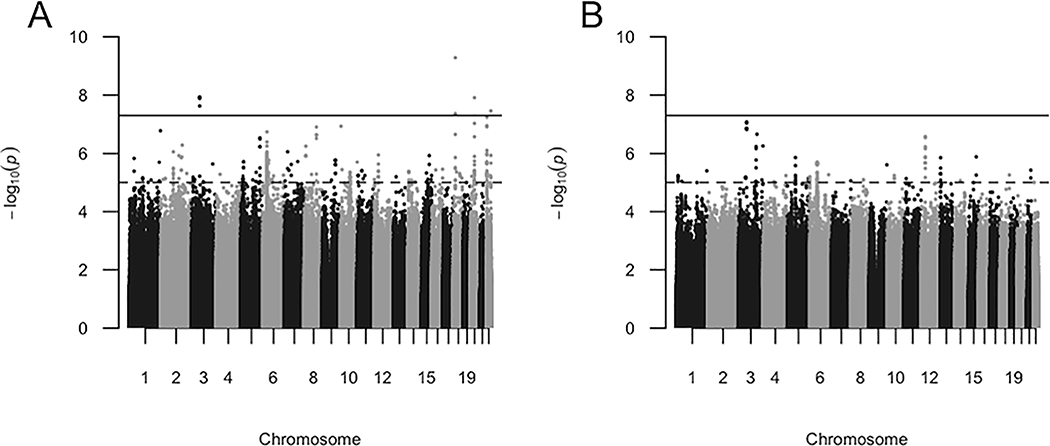
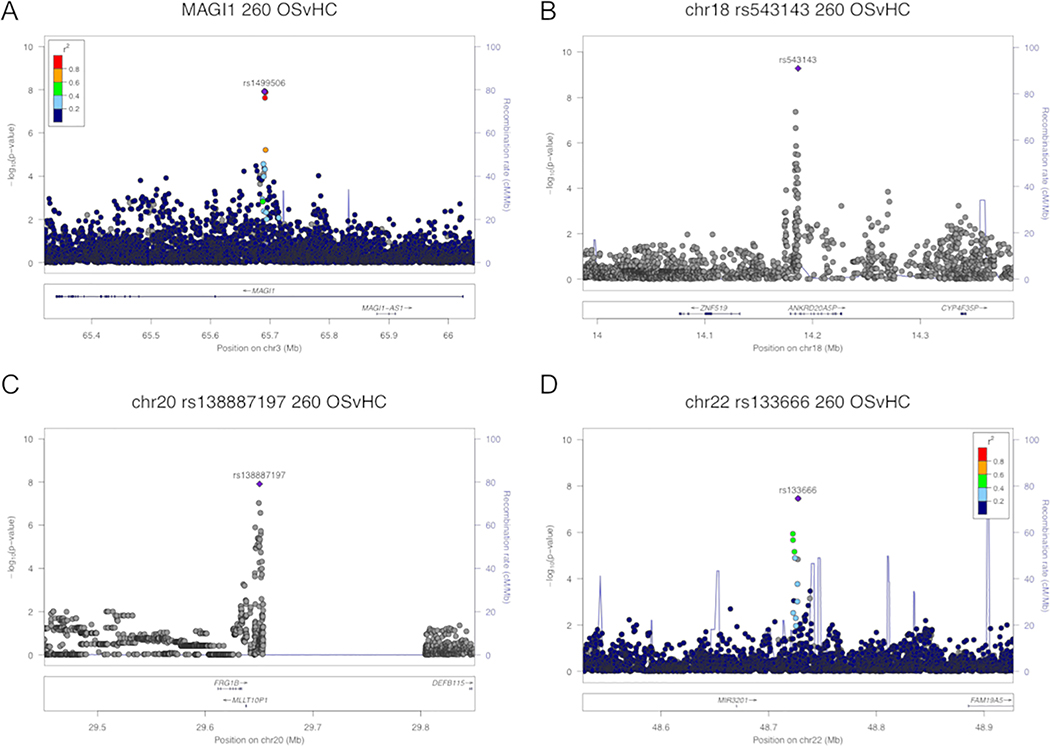
Comparing AA OS cases (n=260) to healthy controls (n=1551) generated a total of 11 SNPs in four independent regions that reached genome-wide significance (p<5×10−8, Figure 1A). None of these 11 OS-associated SNPs were associated with sarcoidosis when all patients were compared to controls (Table 2, p>10−5). While 4 AA OS-associated SNPs occurred alone or in pseudogenes, MAGI1 (Figure 2A) contained 7 AA OS-specific SNPs (peak signal at rs1499506, odds ratio [OR], 0.51; 95% confidence interval [CI], 0.40–0.64), p=1.20×10−8). Only these 7 SNPs in MAGI1 were suggestively associated with OS when compared to non-OS cases. When AA cases with possible or probable OS were removed from the OS group, p values of the 11 OS-associated SNPs increased to suggestive, but not significant values.
Figure 1.
AA OS GWAS analyses suggest OS-specific risk alleles. Manhattan plots of AA OS cases compared to AA healthy controls (a) and AA OS cases to non-OS cases (b). Negative logarithms of the p values of each SNP (dot) are shown. Grayscale blocks indicate distinct chromosomes. Red and blue lines indicate genome-wide significant and suggestive levels, respectively.
Table 2.
AA GWAS analysis suggests OS-specific risk alleles.
| Characteristics | AA OS v HC | AA CC | AA OS v NOS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHR | BP | SNP | Gene | Minor Allele | OR | 95% CIs | P | OR | 95% CIs | P | OR | 95% CIs | P |
| 3 | 65690367 | rs1499506 | MAGI1 | C | 0.51 | 0.40–0.64 | 1.2×10^−8 | 0.89 | 0.79–1.00 | 4.7×10^−2 | 0.49 | 0.38–0.64 | 9.1×10^−8 |
| 3 | 65690647 | rs4437178 | MAGI1 | T | 0.51 | 0.40–0.64 | 1.2×10^−8 | 0.89 | 0.79–1.00 | 4.1×10^−2 | 0.49 | 0.38–0.64 | 1.4×10^−7 |
| 3 | 65690975 | rs59337986 | MAGI1 | CA | 0.51 | 0.40–0.64 | 1.2×10^−8 | 0.89 | 0.79–1.00 | 4.1×10^−2 | 0.49 | 0.38–0.64 | 1.4×10^−7 |
| 3 | 65691043 | rs17073522 | MAGI1 | G | 0.51 | 0.40–0.64 | 1.2×10^−8 | 0.89 | 0.79–1.00 | 4.1×10^−2 | 0.49 | 0.38–0.64 | 1.4×10^−7 |
| 3 | 65691280 | rs7618480 | MAGI1 | T | 0.51 | 0.40–0.64 | 1.2×10^−8 | 0.89 | 0.79–1.00 | 4.1×10^−2 | 0.49 | 0.38–0.64 | 1.4×10^−7 |
| 3 | 65691371 | rs7640824 | MAGI1 | A | 0.51 | 0.41–0.65 | 2.3×10^−8 | 0.90 | 0.80–1.01 | 6.1×10^−2 | 0.49 | 0.38–0.64 | 1.5×10^−7 |
| 3 | 65692374 | rs17073533 | MAGI1 | G | 0.51 | 0.41–0.65 | 1.3×10^−8 | 0.90 | 0.80–1.01 | 6.1×10^−2 | 0.50 | 0.39–0.64 | 8.3×10^−8 |
| 18 | 14184043 | rs4101176 | ANKRD20A5P | A | 2.55 | 1.83–3.57 | 4.3×10^−8 | 1.35 | 1.07–1.70 | 1.1×10^−2 | 2.05 | 1.36–3.09 | 6.5×10^−4 |
| 18 | 14186391 | rs543143 | ANKRD20A5P | C | 2.53 | 1.89–3.38 | 5.2×10^−10 | 1.41 | 1.17–1.71 | 4.3×10^−4 | 1.93 | 1.36–2.74 | 2.33×10^−4 |
| 20 | 29650348 | rs138887197 | FRG1BP | T | 1.86 | 1.50–2.30 | 1.2×10^−8 | 1.25 | 1.10–1.41 | 6.5×10^−4 | 1.72 | 1.32–2.24 | 6.25×10^−5 |
| 22 | 48727259 | rs133666 | NA | G | 1.79 | 1.45–2.20 | 3.4×10^−8 | 1.17 | 1.03–1.33 | 1.8×10^−2 | 1.90 | 1.47–2.45 | 1.08×10^−6 |
To be defined as an OS-specific SNP, the variant had to be significant (p<5×10−8) in OS compared to controls. Known characteristics of each variant are included: chromosome (CHR), base pair (BP), reference SNP (SNP), and closest gene. Bolded p values indicate genome-wide significance. Italicized p values indicate that to achieve convergence, the association was calculated by PLINK. OR: Odds Ratio. CIs: confidence intervals. HC: healthy control. CC: case-control. NOS: non-OS.
Figure 2.
Zoom plots of AA OS-specific regions. Variants in four regions were found to be associated with OS within our AA cohort: MAGI1 (a) and regions on chromosomes 18 (b), 20 (c), and 22 (d). Locations of all peak SNPs within each region are shown, with respective GWAS p values (y axis) and linkage disequilibrium (r2) values relative to peak signal within region when available (color-coded legend). Conditioning on most significant SNP (c and d) indicates variants are nonindependent.
GWAS of EA OS (n=35) versus controls (n=2046) yielded no significant SNPs, likely due to small sample size and thus lack of power. Notably, there was no overlap between SNPs even suggestively associated with OS in AA and EA (p<10−5); the closest suggestive SNPs were over 1430 kb apart (eTables 1 and 2). However, ORs of the 7 MAGI1 OS-specific SNPs in EA subjects (0.525–0.601) were similar to those in AA subjects, suggesting the lack of significance was due to lack of power and thus a larger study of EA may find similar effects.
As mentioned previously, HLA-DRB1*04:01 has been found to be associated with OS in multiple candidate gene or HLA typing studies.19–21 In the ACCESS study, a subset of the samples used here, HLA-DRB1*04:01 was associated with ocular manifestations of sarcoidosis in EA samples but not AA samples.21 We thus assessed by Fisher’s exact test the association of all represented four-digit HLA-DRB1 alleles with OS in this expanded collection of both EA and AA subjects. We confirmed the OS association with DRB1*04:01 in EA OS compared to non-OS (OR, 4.97; 95% CI, 2.043, 11.701; p=0.018), suggesting the effect is isolated to ocular manifestations and not associated with sarcoidosis as a whole. In contrast, DRB1*04:01 was not associated with AA OS compared to controls (OR, 0.521; 95% CI, 0.102, 1.667; p=0.351) or non-OS (OR, 0.844; 95% CI, 0.140, 3.735; p=1). However, DRB1*03:01 (OR, 0.368; 95% CI, 0.194, 0.644; p=0.00175), *11:01 (OR, 1.594; 95% CI, 1.182, 2.136; p=0.01983), and *12:01 (OR, 2.201; 95% CI, 1.474, 3.238; p=0.00175) were associated with OS when AA OS cases were compared to controls, but not when OS was compared to non-OS. Thus, the effects of *03:01, *11:01, and *12:01 appear to be on sarcoidosis in general, consistent with a previous sarcoidosis study.24
As expected with loci on separate chromosomes, subsequent analyses of MAGI1 for conditional or interaction effects of HLA-DRB1 alleles found there was no significant interaction between the peak signal of MAGI1 (rs1499506) and HLA-DRB1*03:01, *04:01, *11:01, and *12:01 on rs1499506 in AA OS compared to controls (p=0.557, 0.976, 0.604, and 0.177, respectively) (Table 3).
Table 3.
HLA-DRB1 associations with OS.
| AA OSvHC | AA OSvNOS | EA OSvHC | EA OSvNOS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DRB1 Allele | OR (95% CI) | P | Pint | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| 03:01 | 0.368 (0.194–0.644) | 0.002 | 0.557 | 0.565 (0.281–1.068) | 0.608 | 0.657 (0.222–1.623) | 1.000 | 0.583 (0.188–1.526) | 1.000 |
| 04:01 | 0.521 (0.102–1.667) | 0.768 | 0.972 | 0.844 (0.14–3.735) | 1.000 | 1.844 (0.83–3.898) | 0.717 | 4.678 (1.859–11.484) | 0.018 |
| 11:01 | 1.594 (1.182–2.136) | 0.020 | 0.604 | 0.938 (0.672–1.305) | 1.000 | 2.814 (1.145–6.293) | 0.248 | 1.431 (0.549–3.441) | 1.000 |
| 12:01 | 2.201 (1.474–3.238) | 0.002 | 0.177 | 1.091 (0.705–1.67) | 1.000 | 0.830 (0.02–5.104) | 1.000 | 0.622 (0.014–4.626) | 1.000 |
Verified, imputed four-digit HLA-DRB1 alleles were tested independently by Fisher’s Exact test and corrected for multiple comparisons by false discovery rate (FDR) with Q<0.05 considered significant. P: p value of association of indicated HLA-DRB1 allele with indicated subject group. Pint: p value of the interaction between the peak signal of MAGI1 (rs1499506) and indicated DRB1 allele in AA OS compared to controls. OR: Odds Ratio. CI: confidence interval. HC: healthy control. NOS: non-OS.
Bioinformatics analyses
OS-specific variants were analyzed for genomic location and potential function, including known TFBSs, enhancer regions or eQTLs. Of the 11 OS-specific SNPs, rs4437178 was located within a known TFBS for MZF-1 and eQTLs known to affect serum concentration of metabolites.30 None of the OS-specific SNPs were in exons or were known enhancers in retinal tissue. MAGI1 is known to function in PTEN signaling and Epithelial Adherens Junction Signaling (IPA).
Discussion
Previous genetic studies of ocular sarcoidosis have been limited to candidate gene studies of up to 38 SNPs and HLA typing studies.14–21 Here, we perform the first GWAS and largest genetic association of OS. Demographic characteristics in our cohorts were similar to those found previously published associations and were used to inform the inclusion of sex, ethnicity, and HLA,3–5 as covariates in our GWAS analyses.
We confirmed association of OS to HLA-DRB1*04:01 in EA, and *03:01, *11:01, and *12:01 to sarcoidosis in AA. All identified HLA-DRB1 effects were independent of other genetic associations. Thus, in the first large, ethnically diverse genetic association study of HLA we found that the HLA-DRB1*04:01 association with OS is ancestry-specific.
We further identified a compelling candidate gene that encodes for the scaffolding protein MAGI1 (membrane-associated guanylate kinase WW and PDZ domain-containing protein 1). MAGI1 is integral to the multiprotein complexes of tight junctions and expressed in many tissues, including the lens and retina.31, 32 Together with other proteins, MAGI1 ensures that the epithelial barrier in the eyes and other organs is highly selective in its permeability.33, 34 SNPs in MAGI1 have been associated with inflammatory disorders in which barrier integrity is compromised, including spontaneous glomerulosclerosis,34 celiac disease,35 medically refractory ulcerative colitis,36 and the stricturing Crohn’s disease phenotype.37 In a rodent model of experimental autoimmune uveitis mediated by peptide-specific CD4+ T cells, MAGI1 was upregulated in T cells capable of inducing remitting disease as compared to those that induce monophasic disease.38 MAGI1 at OS-associated SNP rs4437178 is bound by the transcription factor MZF-1, which has known immune-associated targets such as NOS1 (IPA). MAGI1 is also thought to regulate type I interferon, and contains TFBSs for immune regulating transcription factors BLIMP-1, STAT1, and NFkB.39
In summary, this is the first report of a genome-wide association study of OS and the largest genetic association study of HLA-DRB1 in OS. We report a novel association of MAGI1 with sarcoidosis that is organ-specific. The location of our signal implies that the loss of barrier integrity is a risk factor for the development of ocular sarcoidosis, and that risk pathways are shared between multiple inflammatory diseases. We also confirmed previous associations of HLA-DRB1*04:01 with OS in EA. Further studies of how OS-specific variants, particularly within MAGI1, influence ocular sarcoidosis are warranted, as are further investigations into the growing body of evidence that supports etiologic differences between sarcoidosis cases of African and European ancestries.
Supplementary Material
Acknowledgements
This work was supported by the Foundation for Sarcoidosis research (Chicago, IL), and the National Institutes of Health under Grant R01HL113326-05, P30 GM110766-01, and U54GM104938-06.
Footnotes
The authors have no disclosures to report.
References
- 1.Acharya NR, Browne EN, Rao N, Mochizuki M, International Ocular Sarcoidosis Working Group. Distinguishing Features of Ocular Sarcoidosis in an International Cohort of Uveitis Patients. Ophthalmology. August 16 2017. [DOI] [PubMed] [Google Scholar]
- 2.Rothova A Ocular involvement in sarcoidosis. Br J Ophthalmol. January 2000;84(1):110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cozier YC, Berman JS, Palmer JR, Boggs DA, Serlin DM, Rosenberg L. Sarcoidosis in black women in the United States: data from the Black Women’s Health Study. Chest. January 2011;139(1):144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rybicki BA, Major M, Popovich J Jr., Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. February 1 1997;145(3):234–241. [DOI] [PubMed] [Google Scholar]
- 5.Dubrey S, Shah S, Hardman T, Sharma R. Sarcoidosis: the links between epidemiology and aetiology. Postgrad Med J. October 2014;90(1068):582–589. [DOI] [PubMed] [Google Scholar]
- 6.Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. November 15 2001;164(10 Pt 1):1885–1889. [DOI] [PubMed] [Google Scholar]
- 7.Evans M, Sharma O, LaBree L, Smith RE, Rao NA. Differences in clinical findings between Caucasians and African Americans with biopsy-proven sarcoidosis. Ophthalmology. February 2007;114(2):325–333. [DOI] [PubMed] [Google Scholar]
- 8.Adrianto I, Lin CP, Hale JJ, et al. Genome-wide association study of African and European Americans implicates multiple shared and ethnic specific loci in sarcoidosis susceptibility. PLoS One. 2012;7(8):e43907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bello GA, Adrianto I, Dumancas GG, et al. Role of NOD2 Pathway Genes in Sarcoidosis Cases with Clinical Characteristics of Blau Syndrome. American Journal of Respiratory and Critical Care Medicine. November/01 2015;192(9):1133–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rybicki BA, Sinha R, Iyengar S, et al. Genetic linkage analysis of sarcoidosis phenotypes: the sarcoidosis genetic analysis (SAGA) study. Genes and immunity. July 2007;8(5):379–386. [DOI] [PubMed] [Google Scholar]
- 11.Lareau CA, Adrianto I, Levin AM, Iannuzzi MC, Rybicki BA, Montgomery CG. Fine mapping of chromosome 15q25 implicates ZNF592 in neurosarcoidosis patients. Ann Clin Transl Neurol. October 2015;2(10):972–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Yang J, Levin AM, et al. Efficient generalized least squares method for mixed population and family-based samples in genome-wide association studies. Genet Epidemiol. July 2014;38(5):430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lareau CA, DeWeese CF, Adrianto I, et al. Polygenic risk assessment reveals pleiotropy between sarcoidosis and inflammatory disorders in the context of genetic ancestry. Genes and immunity. 03//print 2017;18(2):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davoudi S, Chang VS, Navarro-Gomez D, et al. Association of genetic variants in RAB23 and ANXA11 with uveitis in sarcoidosis. Mol Vis. 2018;24:59–74. [PMC free article] [PubMed] [Google Scholar]
- 15.Bogunia-Kubik K, Koscinska K, Suchnicki K, Lange A. HSP70-hom gene single nucleotide (+2763 G/A and +2437 C/T) polymorphisms in sarcoidosis. Int J Immunogenet. April 2006;33(2):135–140. [DOI] [PubMed] [Google Scholar]
- 16.Spagnolo P, Sato H, Marshall SE, et al. Association between heat shock protein 70/Hom genetic polymorphisms and uveitis in patients with sarcoidosis. Invest Ophthalmol Vis Sci. July 2007;48(7):3019–3025. [DOI] [PubMed] [Google Scholar]
- 17.Hattori N, Niimi T, Sato S, et al. Cytotoxic T-lymphocyte antigen 4 gene polymorphisms in sarcoidosis patients. Sarcoidosis Vasc Diffuse Lung Dis. March 2005;22(1):27–32. [PubMed] [Google Scholar]
- 18.Thompson IA, Liu B, Sen HN, et al. Association of complement factor H tyrosine 402 histidine genotype with posterior involvement in sarcoid-related uveitis. Am J Ophthalmol. June 2013;155(6):1068–1074 e1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darlington P, Tallstedt L, Padyukov L, et al. HLA-DRB1* alleles and symptoms associated with Heerfordt’s syndrome in sarcoidosis. Eur Respir J. November 2011;38(5):1151–1157. [DOI] [PubMed] [Google Scholar]
- 20.Sato H, Woodhead FA, Ahmad T, et al. Sarcoidosis HLA class II genotyping distinguishes differences of clinical phenotype across ethnic groups. Hum Mol Genet. October 15 2010;19(20):4100–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossman MD, Thompson B, Frederick M, et al. HLA-DRB1*1101: a significant risk factor for sarcoidosis in blacks and whites. Am J Hum Genet. October 2003;73(4):720–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager H Jr., Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. March 1999;16(1):75–86. [PubMed] [Google Scholar]
- 23.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. August 2006;38(8):904–909. [DOI] [PubMed] [Google Scholar]
- 24.Levin AM, Adrianto I, Datta I, et al. Association of HLA-DRB1 with Sarcoidosis Susceptibility and Progression in African Americans. Am J Respir Cell Mol Biol. August 2015;53(2):206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng X, Shen J, Cox C, et al. HIBAG--HLA genotype imputation with attribute bagging. Pharmacogenomics J. April 2014;14(2):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. September 2007;81(3):559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar S, Ambrosini G, Bucher P. SNP2TFBS - a database of regulatory SNPs affecting predicted transcription factor binding site affinity. Nucleic Acids Res. January 4 2017;45(D1):D139–D144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward LD, Kellis M. HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. January 4 2016;44(D1):D877–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao T, He B, Liu S, Zhu H, Tan K, Qian J. EnhancerAtlas: a resource for enhancer annotation and analysis in 105 human cell/tissue types. Bioinformatics. December 1 2016;32(23):3543–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suhre K, Shin SY, Petersen AK, et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature. August 31 2011;477(7362):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen MM, Nguyen ML, Caruana G, Bernstein A, Lambert PF, Griep AE. Requirement of PDZ-containing proteins for cell cycle regulation and differentiation in the mouse lens epithelium. Mol Cell Biol. December 2003;23(24):8970–8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamagata M, Sanes JR. Synaptic localization and function of Sidekick recognition molecules require MAGI scaffolding proteins. J Neurosci. March 10 2010;30(10):3579–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaessinger S, Zhou Y, Bray SJ, Tapon N, Djiane A. Drosophila MAGI interacts with RASSF8 to regulate E-Cadherin-based adherens junctions in the developing eye. Development. March 15 2015;142(6):1102–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni J, Bao S, Johnson RI, et al. MAGI-1 Interacts with Nephrin to Maintain Slit Diaphragm Structure through Enhanced Rap1 Activation in Podocytes. J Biol Chem. November 18 2016;291(47):24406–24417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jauregi-Miguel A, Fernandez-Jimenez N, Irastorza I, Plaza-Izurieta L, Vitoria JC, Bilbao JR. Alteration of tight junction gene expression in celiac disease. J Pediatr Gastroenterol Nutr. June 2014;58(6):762–767. [DOI] [PubMed] [Google Scholar]
- 36.Haritunians T, Taylor KD, Targan SR, et al. Genetic predictors of medically refractory ulcerative colitis. Inflamm Bowel Dis. November 2010;16(11):1830–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonso A, Domenech E, Julia A, et al. Identification of risk loci for Crohn’s disease phenotypes using a genome-wide association study. Gastroenterology. April 2015;148(4):794–805. [DOI] [PubMed] [Google Scholar]
- 38.Wildner G, Kaufmann U. What causes relapses of autoimmune diseases? The etiological role of autoreactive T cells. Autoimmun Rev. September 2013;12(11):1070–1075. [DOI] [PubMed] [Google Scholar]
- 39.Kumar M, Liu H, Rice AP. Regulation of interferon-beta by MAGI-1 and its interaction with influenza A virus NS1 protein with ESEV PBM. PLoS One. 2012;7(7):e41251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.