Abstract
Allogeneic hematopoietic cell transplantation (alloHCT) is a life-saving technology that can cure otherwise incurable diseases, but imposes significant physiologic stress upon recipients. This stress leads to short term toxicity and mid to long term physical function impairment in some recipients. Exercise interventions have demonstrated preliminary efficacy in preserving physical function in HCT recipients, but the role of these intervention prior to HCT (“prehabilitative”) is less known. We tested a 5-12 week, prehabilitative higher intensity home-based aerobic exercise intervention in a randomized study of alloHCT candidates. Of 113 patients screened, 34 were randomized to control or intervention groups, 16 underwent pre- and post-intervention peak oxygen consumption (VO2peak)testing, and 12 underwent pre- and post-intervention six minute walk distance (6MWD) testing. No significant differences in VO2peak or 6MWD were seen pre- to post-intervention between intervention and control groups, but final numbers of evaluable participants in each group were too small to draw inferences regarding the efficacy of the intervention. We conclude that the design of our prehabilitative intervention was not feasible in this pilot randomized study, and make recommendations regarding the design of future exercise intervention studies in alloHCT.
Keywords: Allogeneic transplants, Hematopoietic stem cell transplantation, Exercise, Exercise therapy, Exercise test, High-intensity interval training
Introduction
Allogeneic hematopoietic-cell transplantation (alloHCT) cures otherwise incurable diseases.1 However, physiologic stress from alloHCT results in early toxicities that can lead to transplant-related complications and long term physical impairments in some survivors.2–5 Stressors related to alloHCT include chemotherapy or radiation-related organ dysfunction, infection, and graft versus host disease. These stressors cause symptoms, which are prevalent before transplant from prior disease and therapy, and increase significantly in the early post-transplant period. Symptoms are associated with physical function impairment.6–8 Transplant patients, caregivers and health care providers consistently identify preservation of physical function as an important long-term goal following transplant.9, 10 Patients with better physical function before transplant and in the early post-transplant period are more likely to survive and to have better long-term health.11, 12
Physical function is closely related to another underlying health concept, cardiorespiratory fitness (CRF). Though the concepts are not exactly the same, individuals with impaired function are more likely to have lower cardiorespiratory fitness, and individuals with higher function are likely to have higher cardiorespiratory fitness. Cardiorespiratory fitness is an objective measure of maximal oxygen extraction during intense exertion, obtained by cardiorespiratory exercise testing.13, 14 CRF is so strongly associated with survival in large patient populations that the American Heart Association has recommended that it should be measured routinely as a new “vital sign.”15 At least two recent studies have demonstrated that pre-HCT CRF predicts post-HCT survival. CRF is also linked to survival in other cancer patient populations..16–19
Because pre-HCT CRF is a prognostic marker that can be measured with gold standard testing, it is an attractive target for interventions designed to determine whether improving pre-HCT CRF might improve post-HCT survival and long term physical function. In other settings, such as surgery, pre-treatment interventions (“prchabilitation”) have been studied. Though the evidence is mixed, in part because of small studies with heterogeneous designs, it appears that prehabilitative interventions prior to surgery may be associated with improved pre-surgical functional capacity, reduced post-surgical toxicities, and preserved post-surgical function, in some settings.20–25
However, prehabilitative interventions have not been well studied prior to HCT. Because exercising at moderate to high intensity has been demonstrated to be an efficient way to improve CRF in a short period of time26–29 our group has been interested in applying interval-based higher intensity aerobic training (hereafter called “interval exercise training” or IET) to the pre-HCT period as a prehabilitative intervention. In a previous nonrandomized study, we demonstrated that home-based prehabilitative IET prior to HCT appeared to be associated with improvements in CRF, with more pronounced effects prior to alloHCT.30 Therefore, we designed a follow-up study for patients preparing to undergo alloHCT in which we planned to randomize participants to home-based IET (intervention) or usual care (control) for a 5-12 week intervention period. We hypothesized that pre-alloHCT IET would be associated with greater improvements in CRF prior to alloHCT compared with usual care.
Methods
This was a two-arm, single center randomized trial intended to assess the pre-transplant effectiveness of an interval exercise intervention among patients preparing to undergo allogeneic HCT. Participants between the ages of 18 and 75 years were recruited at the University of North Carolina from December 2015 to March 2017. To participate, patients had to be considered candidates for allogeneic HCT, with a schedule that would accommodate at least a 5 week pre-HCT exercise intervention, but not anticipated to have more than 12 weeks before HCT. Participants could not have comorbid illness that would preclude either maximal effort during exercise testing or participation in regular exercise programming as determined by the treating physician or study exercise physiologist.
All participants underwent cardiopulmonary exercise testing at baseline and at the time of HCT with cycle ergometry for the determination of peak oxygen uptake (VO2peak) and maximum heart rate (MHR) for exercise prescription, and additionally underwent 6-minute walk distance testing, as previously described according to published guidelines. 13, 16, 30, 31 The graded exercise test was completed on a cycle ergometer (Monark 828E, Goteborg, Sweden). Participants were fitted with a facemask (NRB1, Hans Rudolph Inc., Kansas City, MO, USA) in order to ensure a secure seal around the nose and mouth. Respiratory gases were monitored continuously and analyzed with open-circuit spirometry using a calibrated metabolic cart (K4b2 Cosmed, Rome, Italy). Heart rate was monitored continuously throughout the duration of the protocol using a polar heart-rate strap (Model FT1, Polar Inc., Lake Success, NY, USA).
All participants were provided with a study accelerometer (Fitbit Surge, Fitbit Inc., San Francisco, CA, USA) at baseline and were instructed to wear the accelerometer throughout the duration of the study. All participants completed baseline and periodic symptom, quality of life, and physical function surveys using 16 questions from the PRO-CTCAE, the PROMIS 10-item Global Health instrument, and the PROMIS 20-item Physical Function instrument.
Participants were randomly assigned using numbered, sealed, envelopes to intervention or control groups, which determined subsequent activities between baseline evaluation and HCT. Exercise physiologists administering VO2peak and 6MWD tests were blinded to group assignment.
Intervention group participants were given verbal and paper instructions on how to perform IET. In brief, participants were individually counseled to discuss available local training resources and were encouraged to choose one or more modes of exercise (from a list that included walking, jogging, running, cycling, elliptical, or stair climbing) for IET sessions. Participants were directed to observe their heart rates on the FitBit Surge during IET sessions in order to achieve pre-defined and programmed target heart rates, calculated for each participant as 80% of the maximum heart rate (80% MHR) as determined by the CPET. During the first week of the intervention period, participants were asked to engage in 30 minutes of walking, jogging and/or running at any intensity for 3-4 days. During weeks two and beyond, participants were asked to perform exercise sessions consisting of a five minute warm-up followed by five 2-minute intervals targeting 80% MHR or beyond, interspersed with 3-minute bouts of lower-intensity recovery intervals. Each exercise session was designed to be 30 minutes in length. Participants were asked to engage in 3-4 exercise sessions per week during the IET intervention period. Participants were also provided with weekly motivational phone calls, and were given personalized step targets on the Fitbit Surge, with a goal of increasing average steps per day by approximately 10% each week.
Control group participants were provided with Fitbit Surges but did not receive instructions or information about IET, and did not receive instructions on how to use the Fitbit Surges for IET or step goal-setting beyond the basic functions of the Fitbit Surge. Control group participants did receive weekly scripted calls to match the number of contacts between groups, but were not provided with motivational messaging to increase total physical activity or physical activity intensity each week.
Statistical analysis
For the primary endpoint, change in VO2peak, a total sample size of 60 was chosen in order to have 85% power using a two-sided test at the 0.05 level to detect a 3.8ml/kg*min increase in VO2peak in the intervention relative to the control arm30. This calculation assumed a common standard deviation of VO2peak of 7ml/kg*min and a correlation between repeated measurements of 0.8, together implying a change score standard deviation of 4.5ml/kg*min.
For the primary endpoint, intention to treat and per protocol analyses were planned. These analyses used an analysis of covariance (ANCOVA) linear model, controlling for the baseline VO2peak, sex, age, and receipt of myeloablative vs reduced intensity conditioning, to compare change in VO2peak across arms. This test was conducted at the two-sided 5% significance level. The same model was used to obtain an estimated treatment effect along with 95% confidence intervals. All analyses were conduct in SAS, version 9.4 (SAS Institute, Cary, NC).
Results
A total of 113 patients who were potential candidates for alloHCT were screened for participation in the study (Figure 1). Of these, 18 were felt to be uncertain candidates for HCT, and 20 were felt not to be candidates for alloHCT on further review. 17 patients had insufficient time remaining before HCT to participate in the planned pre-HCT exercise protocol before the study could be discussed with them. 2 patients were too sick to participate in an exercise program. 4 had no working email address, which was a requirement for participation. 2 were unable to provide informed consent. 16 patients were approached for the study but declined participation. Thus, a total of 34 patients were enrolled and randomized. Enrollment stopped short of the planned 60 patient sample size because of challenges encountered with recruitment and feasibility.
Figure 1: CONSORT Diagram.
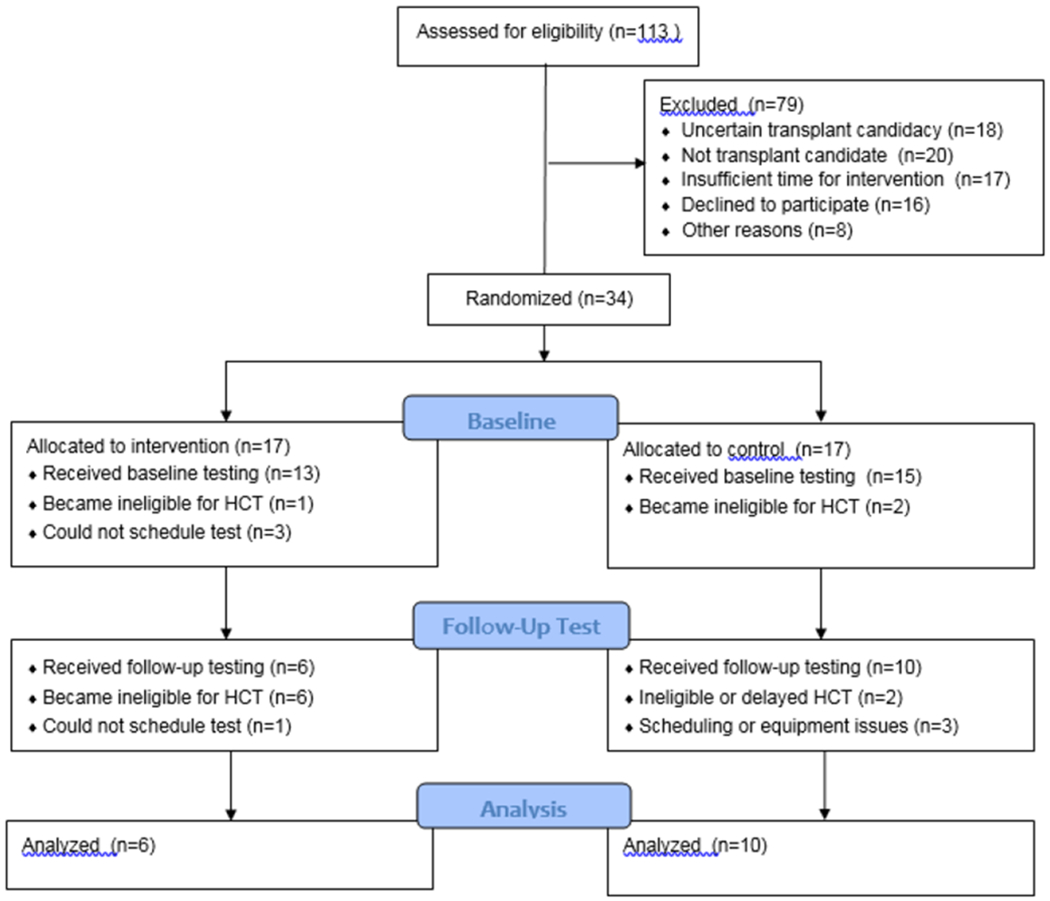
113 patients were assessed for eligibility prior to allogeneic hematopoietic cell transplantation, of which 34 were randomized to intervention or control groups, 28 underwent baseline testing, and 16 completed follow-up testing and were available for endpoint analysis.
Among the 34 patients enrolled, 17 were randomized to the intervention group and 17 were randomized to the control group. Of these, 11 patients did not proceed to HCT and came off study; these reasons included inadequate disease control (2), social concerns precluding transplantation (3), inability to identify a donor (3), comorbidity (1), and patient choice (2). Three additional patients did proceed to HCT but could not complete baseline physiological testing because of transportation and scheduling issues. Four patients underwent baseline testing and ultimately proceeded to HCT, but could not undergo follow-up physiological testing for response evaluation; 3 of these patients encountered scheduling issues with the follow-up test, and in 1 patient, equipment technical difficulties precluded the follow-up test.
Thus, a total of 16 patients (6 intervention, 10 control) underwent baseline and follow-up testing and were evaluable for response assessment. Four of these patients completed the follow-up VO2peak test but not the 6MWD.
Characteristics of the 28 patients who completed baseline testing are presented in Table 1. The median age of this population was 52 years (range 28-73), and the most common underlying diagnosis was acute myeloid leukemia. The population was almost evenly split between planned myeloablative and reduced intensity conditioning. Most were white (68%), and non-Hispanic (86%). 50% of patients had at least a college degree, and over half of the participants were married or living with someone (61%). Approximately 30% of participants had a body mass index (BMI) greater than 30. Selected baseline patient reported outcomes are shown in Table 2. At baseline, participants reported significant symptom burden and impaired physical function. Approximately half reported at least mild levels of pain, fatigue, anxiety, and depressive symptoms, and 25% reported at least mild baseline shortness of breath. Baseline PROMIS mental health scores were comparable to the general population (median score 51, population norm 50) while physical health and physical function scores were somewhat lower (median scores of 45 and 46 with population norms of 50 each).
Table 1: Baseline Patient Characteristics.
Data provided in this table correspond to the 28 patients who underwent baseline testing in the intervention (N=13) or control (N=15) groups. Groups are combined for purposes of overall cohort description.
| Characteristic | N (%) |
|---|---|
| Age | Median (range) in years: 52 (28-73) |
| Gender | |
| Male | 16 (57%) |
| Female | 12 (43%) |
| Race | |
| White | 19 (68%) |
| Black | 6 (21%) |
| Other | 3 (11%) |
| Ethnicity | |
| Non-Hispanic | 24 (86%) |
| Hispanic | 1 (4%) |
| Other | 3 (11%) |
| Education | |
| At least a college degree | 13 (47%) |
| No college degree reported | 15 (53%) |
| Social support | |
| Living with anyone | 17 (61%) |
| Did not report living with anyone | 11 (39%) |
| Body Mass Index | |
| BMI ≥ 30 | 8 (29%) |
| BMI < 30 | 20 (71%) |
| Diagnosis | |
| AML | 15 (54%) |
| MDS | 3 |
| ALL | 3 |
| CML | 1 |
| Hodgkin lymphoma | 1 |
| Multiple myeloma | 1 |
| Myelofibrosis | 1 |
| Aplastic anemia | 1 |
| Mantle cell lymphoma | 1 |
| HLH | 1 |
| Conditioning regimen intensity | |
| Myeloblative | 13 (46%) |
| Reduced | 15 (54%) |
Table 2: Baseline Patient Reported Outcomes.
Data provided in this table correspond to the 25 patients who provided baseline patient reported outcomes. Groups are combined for purposes of overall cohort description. Selected patient reported outcomes are shown to reflect selected symptom prevalence and patient function at baseline.
| Characteristic | N (%) |
|---|---|
| Pain frequency (PRO-CTCAE) | |
| Never | 10 (36%) |
| Rarely | 7 (25%) |
| Occasionally | 5 (18%) |
| Frequently | 1 (4%) |
| Almost constantly | 2 (7%) |
| Fatigue severity (PRO-CTCAE) | |
| None | 8 (29%) |
| Mild | 9 (32%) |
| Moderate | 5 (18%) |
| Severe or very severe | 3 (11%) |
| Shortness of breath severity (PRO-CTCAE) | |
| None | 18 (64%) |
| Mild | 5 (18%) |
| Moderate | 2 (7%) |
| Depressive symptoms frequency (PRO-CTCAE) | |
| Never | 12 (43%) |
| Rarely | 7 (25%) |
| Occasionally | 4 (14%) |
| Frequently | 2 (7%) |
| Anxiety symptoms frequency (PRO-CTCAE) | |
| Never | 11 (39%) |
| Rarely | 7 (25%) |
| Occasionally | 6 (21%) |
| Frequently | 1 (4%) |
| Physical health (PROMIS Global) | Median T score (range): 45 (30-62) |
| Mental health (PROMIS Global) | Median T score (range): 51 (25-68) |
| Physical function (PROMIS Physical Function) | Median T score (range): 46 (36-70) |
For the 16 patients who had baseline and follow-up physiological testing data available, and were thus evaluable for the primary endpoint, the median age was 52 (range 34-72), and over half (9) had acute myeloid leukemia as an underlying diagnosis. For these 16 patients, the median time between baseline testing and follow-up testing was 8.5 weeks (range 3-20 weeks), which represented the intervention period. The median intervention period in the intervention group was 11 weeks (range 6-20 weeks) and the corresponding median period in the control group was 7 weeks (range 3-14 weeks). For the evaluable patients, the median time between follow-up testing and HCT was 5 days, which was similar in both intervention and control patients.
Results for the evaluable intervention and control patients are shown in Table 3. There were no significant differences in change scores for VO2peak or 6MWD between intervention and control groups, though the final evaluable population was too small to draw any meaningful conclusions about the effect of the intervention.
Table 3: VO2peak and 6 minute walk distance results.
Data provided in this table correspond to the 16 patients who underwent baseline and follow-up VO2peak testing (results in ml/kg*min), and the 12 patients who underwent baseline and follow-up 6 minute walk distance testing (results in meters). There were no significant differences from baseline to follow-up in each group, nor were there significant differences between groups.
| Group | Variable | Median |
|---|---|---|
| Control (N=10) | Baseline VO2peak Follow-up VO2peak Change in VO2peak |
15.8 ml/kg/min 17.2 ml/kg/min +1.2 ml/kg/min |
| Intervention (N=6) | Baseline VO2peak Follow-up VO2peak Change in VO2peak |
20.3 ml/kg/min 20.7 ml/kg/min +0.4 ml/kg/min |
| Control (N=8) | Baseline 6MWD Follow-up 6MWD Change in 6MWD |
444.1 meters 331.4 meters −34.2 meters |
| Intervention (N=4) | Baseline 6MWD Follow-up 6MWD Change in 6MWD |
405.3 meters 412.2 meters +5.3 meters |
Discussion
We designed a randomized controlled trial to study the effect of a 5-12 week pre-alloHCT home-based prehabilitative interval exercise training program on cardiorespiratory fitness as measured by CPET and 6MWD. The most important message from our experience was that the intervention, as designed and tested in this study, was not feasible in the setting of a randomized controlled trial. Thus, we were unable to effectively test the hypothesis that higher intensity aerobic interval exercise improves fitness during the pre-transplant period relative to usual care. This was a surprising conclusion in light of our previous nonrandomized prehabilitative exercise study that suggested that the intervention was feasible and efficacious.30 It is likely that chance variation resulting in a favorable cohort in our earlier study, combined with the enrollment time pressures in the randomized study, exposed vulnerabilities in our prehabilitative intervention design. However, we believe that there are several important lessons learned from our experience which we hope will inform future exercise intervention efforts in the field.
First, the timing for transplant exercise interventions may be critical. We initially avoided a peri-transplant design in part because of the negative results of the BMT CTN 0902 study. BMT CTN 0902 was a phase III trial that randomized participants to exercise training, stress management, combined exercise training and stress management, or neither intervention, with a primary endpoint of change in self-reported physical and mental function at Day 100.32 This peri-transplant intervention was feasible, but the trial results did not demonstrate that either or both interventions resulted in an improvement in the primary endpoint. There were several potential explanations offered, including that the intervention was of insufficient intensity to result in quality of life improvement. Indeed, a partially supervised exercise program of higher intensity, also during the peri-transplant period, was associated with functional preservation and possibly improved survival.33, 34 These data are consistent with other studies suggesting benefit to exercise around the time of transplant.35–40 Nonetheless, rather than working to optimize a peri-transplant intervention, we focused on the pre-transplant period as a potential widow of opportunity.
There are a few studies looking at entirely prehabilitative transplant exercise interventions, as was the case in our protocol. While feasible in the peri-HCT setting, it may be that exercise interventions that are entirely prehabilitative are less feasible. A primary reason for this is that, unlike surgical settings in which most candidates eventually proceed to surgery, the application of HCT is limited by an intention to treat issue. As our CONSORT diagram illustrated, many potential trial candidates were excluded on the basis of transplant ineligibility or uncertain transplant candidacy, or by an inability to appropriately time the intervention for those patients who were in fact proceeding to HCT. Even of the 34 patients randomized, including a selection for those likely to proceed to SCT, only 68% ultimately proceeded to transplant. In addition to the feasibility concerns for this particular study, this observation also raises broader issues about the generalizability of findings in any interventional HCT trial to the broader population of patients who might be considered eligible for HCT.
For future HCT exercise studies, we recommend that the timing and objectives of the intervention are carefully considered. If the intent is truly prehabilitative, then other populations with narrower and more achievable outcomes could be considered – such as exercise as an adjuvant to hematopoietic cell mobilization in patients preparing for autologous transplant, for which there are emerging data.41–43 Alternatively, exercise intervention could be studied as a means for improving transplant eligibility, by helping otherwise marginal candidates to become better candidates for the procedure. On the other hand, if the intent is to determine the impact of exercise upon post-transplant outcomes, then it may be prudent to return to the peri-transplant period and focus on patients who are certain to proceed, by starting more intensive interventions closer to initiation of the transplant process and continuing through the peri-transplant period, as has been done in previous transplant exercise studies.
This brings us back to the issue of optimizing peri-transplant exercise intervention intensity. To that end, another important lesson learned from our study was the need for testing procedures to be as patient-friendly as possible in order to enable broad participation. In our study, we chose to use cardiopulmonary exercise testing to assess our primary outcome, as the gold standard assessment for aerobic fitness. We found out however, that despite having testing available on our transplant unit and administered by exercise physiologists who were part of the research team, many patients were not willing to come back to the transplant center for additional testing visits, and others were not able to complete testing because of other issues. Pragmatically, clinic-administered or home-based data collection may be preferable for future exercise intervention studies in order to avoid these issues. The emerging field of digital biomarkers may help in the future to provide solutions like this.44 In the past, home-based VO2max estimates have been technically challenging to develop45, 46 though technology continues to improve47 with projects ongoing (http://sagebionetworks.org/research-projects/cardiorespiratory-fitness-module/).
We also found that though we implemented a standardized home-based interval exercise testing program in order to try to control dose intensity of the intervention, the rigidity of the protocol was challenging from an adherence standpoint and may not have been optimally suited for participants of varying levels of baseline motivation and fitness. We recommend that future transplant exercise studies consider a more pragmatic and individually tailored approach to maximize adherence and acceptability. At the same time, these interventions must have sufficient intensity to overcome the presumed limitations of BMT CTN 0902. One approach would be to maintain a high frequency of remote patient contact through a longitudinal coaching program, while using wearable sensors to optimize home-based intensity in a way that is more individually tailored and achievable for participants of all levels of baseline fitness. This is the approach we are currently using as a response to the results of this study, and our experience to date reflects improvements in feasibility and adherence with high patient satisfaction.
In conclusion, we conducted a randomized study of a home-based, prehabilitative, interval exercise testing intervention for patients preparing for allogeneic hematopoietic cell transplantation. Unlike our earlier nonrandomized study, we encountered barriers to successfully completing our randomized study and did not find that the intervention was feasible. However, we hope that the lessons learned from our study can inform future exercise interventions in transplant so that patients can maximally benefit from exercise and hopefully improve transplant outcomes by increasing physical function and fitness.
ACKNOWLEDGMENTS
Financial disclosure: This work was supported in part by National Cancer Institute Grant 1R21CA192127-01A1.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
CONFLICT OF INTEREST There are no conflicts of interest to report. I have full control of all primary data and I agree to allow the journal to review data if requested.
REFERENCES
- 1.D’Souza A, Lee S, Zhu X, Pasquini M. Current Use and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant 2017; 23(9): 1417–1421. e-pub ahead of print 2017/06/14; doi: 10.1016/j.bbmt.2017.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armenian SH, Horak D, Scott JM, Mills G, Siyahian A, Berano Teh J et al. Cardiovascular Function in Long-Term Hematopoietic Cell Transplantation Survivors. Biol Blood Marrow Transplant 2017; 23(4): 700–705. e-pub ahead of print 2017/01/10; doi: 10.1016/j.bbmt.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arora M, Sun CL, Ness KK, Teh JB, Wu J, Francisco L et al. Physiologic Frailty in Nonelderly Hematopoietic Cell Transplantation Patients: Results From the Bone Marrow Transplant Survivor Study. JAMA Oncol 2016; 2(10): 1277–1286. e-pub ahead of print 2016/06/03; doi: 10.1001/jamaoncol.2016.0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia S, Armenian SH, Landier W. How I monitor long-term and late effects after blood or marrow transplantation. Blood 2017; 130(11): 1302–1314. e-pub ahead of print 2017/07/27; doi: 10.1182/blood-2017-03-725671 [DOI] [PubMed] [Google Scholar]
- 5.Inamoto Y, Lee SJ. Late effects of blood and marrow transplantation. Haematologica 2017; 102(4): 614–625. e-pub ahead of print 2017/02/25; doi: 10.3324/haematol.2016.150250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood 2009; 114(1): 7–19. e-pub ahead of print 2009/04/02; doi: 10.1182/blood-2008-10-182592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosher CE, Redd WH, Rini CM, Burkhalter JE, DuHamel KN. Physical, psychological, and social sequelae following hematopoietic stem cell transplantation: a review of the literature. Psychooncology 2009; 18(2): 113–127. e-pub ahead of print 2008/08/05; doi: 10.1002/pon.1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiuza-Luces C, Simpson RJ, Ramirez M, Lucia A, Berger NA. Physical function and quality of life in patients with chronic GvHD: a summary of preclinical and clinical studies and a call for exercise intervention trials in patients. Bone Marrow Transplant 2016; 51(1): 13–26. e-pub ahead of print 2015/09/15; doi: 10.1038/bmt.2015.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bevans M, El-Jawahri A, Tierney DK, Wiener L, Wood WA, Hoodin F et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: The Patient-Centered Outcomes Working Group Report. Biol Blood Marrow Transplant 2017; 23(4): 538–551. e-pub ahead of print 2016/09/19; doi: 10.1016/j.bbmt.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman AT, Stover AM, Grover NS, Shea TC, Reeve BB, Wood WA. Patient perspectives on physical function after allogeneic hematopoietic stem cell transplantation: a qualitative study. Bone Marrow Transplant 2017; 52(10): 1483–1484. e-pub ahead of print 2017/08/08; doi: 10.1038/bmt.2017.176 [DOI] [PubMed] [Google Scholar]
- 11.Wood WA, Le-Rademacher J, Syrjala KL, Jim H, Jacobsen PB, Knight JM et al. Patient-reported physical functioning predicts the success of hematopoietic cell transplantation (BMT CTN 0902). Cancer 2016; 122(1): 91–98. doi: 10.1002/cncr.29717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw BE, Brazauskas R, Millard HR, Fonstad R, Flynn KE, Abernethy A et al. Centralized patient-reported outcome data collection in transplantation is feasible and clinically meaningful. Cancer 2017; 123(23): 4687–4700. e-pub ahead of print 2017/08/18; doi: 10.1002/cncr.30936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003; 167(2): 211–277. e-pub ahead of print 2003/01/14; doi: 10.1164/rccm.167.2.211 [DOI] [PubMed] [Google Scholar]
- 14.Jones LW, Eves ND, Haykowsky M, Joy AA, Douglas PS. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol 2008; 9(8): 757–765. doi: 10.1016/S1470-2045(08)70195-5 [DOI] [PubMed] [Google Scholar]
- 15.Ross R, Blair SN, Arena R, Church TS, Despres JP, Franklin BA et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation 2016; 134(24): e653–e699. e-pub ahead of print 2016/11/25; doi: 10.1161/cir.0000000000000461 [DOI] [PubMed] [Google Scholar]
- 16.Wood WA, Deal AM, Reeve BB, Abernethy AP, Basch E, Mitchell SA et al. Cardiopulmonary fitness in patients undergoing hematopoietic SCT: a pilot study. Bone Marrow Transplant 2013; 48(10): 1342–1349. doi: 10.1038/bmt.2013.58 [DOI] [PubMed] [Google Scholar]
- 17.Jones LW, Watson D, Herndon JE 2nd, Eves ND, Haithcock BE, Loewen G et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer 2010; 116(20): 4825–4832. doi: 10.1002/cncr.25396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol 2012; 30(20): 2530–2537. doi: 10.1200/JCO.2011.39.9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelsey CR, Scott JM, Lane A, Schwitzer E, West MJ, Thomas S et al. Cardiopulmonary exercise testing prior to myeloablative allo-SCT: a feasibility study. Bone Marrow Transplant 2014; 49(10): 1330–1336. doi: 10.1038/bmt.2014.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boujibar F, Bonnevie T, Debeaumont D, Bubenheim M, Cuvellier A, Peillon C et al. Impact of prehabilitation on morbidity and mortality after pulmonary lobectomy by minimally invasive surgery: a cohort study. J Thorac Dis 2018; 10(4): 2240–2248. e-pub ahead of print 2018/06/01; doi: 10.21037/jtd.2018.03.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillis C, Fenton TR, Sajobi TT, Minnella EM, Awasthi R, Loiselle SE et al. Trimodal prehabilitation for colorectal surgery attenuates post-surgical losses in lean body mass: A pooled analysis of randomized controlled trials. Clin Nutr 2018. e-pub ahead of print 2018/07/22; doi: 10.1016/j.clnu.2018.06.982 [DOI] [PubMed] [Google Scholar]
- 22.Minnella EM, Awasthi R, Loiselle SE, Agnihotram RV, Ferri LE, Carli F. Effect of Exercise and Nutrition Prehabilitation on Functional Capacity in Esophagogastric Cancer Surgery: A Randomized Clinical Trial. JAMA Surg 2018. e-pub ahead of print 2018/09/08; doi: 10.1001/jamasurg.2018.1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moran J, Guinan E, McCormick P, Larkin J, Mockler D, Hussey J et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: A systematic review and meta-analysis. Surgery 2016; 160(5): 1189–1201. e-pub ahead of print 2016/10/30; doi: 10.1016/j.surg.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 24.Sebio Garcia R, Yanez-Brage MI, Gimenez Moolhuyzen E, Salorio Riobo M, Lista Paz A, Borro Mate JM. Preoperative exercise training prevents functional decline after lung resection surgery: a randomized, single-blind controlled trial. Clin Rehabil 2017; 31(8): 1057–1067. e-pub ahead of print 2017/07/22; doi: 10.1177/0269215516684179 [DOI] [PubMed] [Google Scholar]
- 25.Treanor C, Kyaw T, Donnelly M. An international review and meta-analysis of prehabilitation compared to usual care for cancer patients. J Cancer Surviv 2018; 12(1): 64–73. e-pub ahead of print 2017/09/14; doi: 10.1007/s11764-017-0645-9 [DOI] [PubMed] [Google Scholar]
- 26.Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol 2007; 25(28): 4396–4404. doi: 10.1200/JCO.2006.08.2024 [DOI] [PubMed] [Google Scholar]
- 27.Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM et al. Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol 2009; 27(27): 4605–4612. doi: 10.1200/JCO.2008.20.0634 [DOI] [PubMed] [Google Scholar]
- 28.Dolan LB, Gelmon K, Courneya KS, Mackey JR, Segal RJ, Lane K et al. Hemoglobin and aerobic fitness changes with supervised exercise training in breast cancer patients receiving chemotherapy. Cancer Epidemiol Biomarkers Prev 2010; 19(11): 2826–2832. doi: 10.1158/1055-9965.EPI-10-0521 [DOI] [PubMed] [Google Scholar]
- 29.van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM et al. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J Clin Oncol 2015. doi: 10.1200/JCO.2014.59.1081 [DOI] [PubMed] [Google Scholar]
- 30.Wood WA, Phillips B, Smith-Ryan AE, Wilson D, Deal AM, Bailey C et al. Personalized home-based interval exercise training may improve cardiorespiratory fitness in cancer patients preparing to undergo hematopoietic cell transplantation. Bone Marrow Transplant 2016; 51(7): 967–972. e-pub ahead of print 2016/03/21; doi: 10.1038/bmt.2016.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166(1): 111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 32.Jacobsen PB, Le-Rademacher J, Jim H, Syrjala K, Wingard JR, Logan B et al. Exercise and stress management training prior to hematopoietic cell transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biol Blood Marrow Transplant 2014; 20(10): 1530–1536. doi: 10.1016/j.bbmt.2014.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiskemann J, Dreger P, Schwerdtfeger R, Bondong A, Huber G, Kleindienst N et al. Effects of a partly self-administered exercise program before, during, and after allogeneic stem cell transplantation. Blood 2011; 117(9): 2604–2613. doi: 10.1182/blood-2010-09-306308 [DOI] [PubMed] [Google Scholar]
- 34.Wiskemann J, Kleindienst N, Kuehl R, Dreger P, Schwerdtfeger R, Bohus M. Effects of physical exercise on survival after allogeneic stem cell transplantation. Int J Cancer 2015; 137(11): 2749–2756. e-pub ahead of print 2015/06/11; doi: 10.1002/ijc.29633 [DOI] [PubMed] [Google Scholar]
- 35.Wiskemann J, Huber G. Physical exercise as adjuvant therapy for patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 2008; 41(4): 321–329. doi: 10.1038/sj.bmt.1705917 [DOI] [PubMed] [Google Scholar]
- 36.Persoon S, Kersten MJ, van der Weiden K, Buffart LM, Nollet F, Brug J et al. Effects of exercise in patients treated with stem cell transplantation for a hematologic malignancy: a systematic review and meta-analysis. Cancer Treat Rev 2013; 39(6): 682–690. e-pub ahead of print 2013/03/15; doi: 10.1016/j.ctrv.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 37.van Haren IE, Timmerman H, Potting CM, Blijlevens NM, Staal JB, Nijhuis-van der Sanden MW. Physical exercise for patients undergoing hematopoietic stem cell transplantation: systematic review and meta-analyses of randomized controlled trials. Phys Ther 2013; 93(4): 514–528. e-pub ahead of print 2012/12/12; doi: 10.2522/ptj.20120181 [DOI] [PubMed] [Google Scholar]
- 38.Jarden M, Baadsgaard MT, Hovgaard DJ, Boesen E, Adamsen L. A randomized trial on the effect of a multimodal intervention on physical capacity, functional performance and quality of life in adult patients undergoing allogeneic SCT. Bone Marrow Transplant 2009; 43(9): 725–737. e-pub ahead of print 2009/02/24; doi: 10.1038/bmt.2009.27 [DOI] [PubMed] [Google Scholar]
- 39.Hacker ED, Collins E, Park C, Peters T, Patel P, Rondelli D. Strength Training to Enhance Early Recovery after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 2017; 23(4): 659–669. e-pub ahead of print 2017/01/04; doi: 10.1016/j.bbmt.2016.12.637 [DOI] [PubMed] [Google Scholar]
- 40.Dimeo F, Fetscher S, Lange W, Mertelsmann R, Keul J. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood 1997; 90(9): 3390–3394. [PubMed] [Google Scholar]
- 41.Agha NH, Baker FL, Kunz HE, Graff R, Azadan R, Dolan C et al. Vigorous exercise mobilizes CD34+ hematopoietic stem cells to peripheral blood via the beta2-adrenergic receptor. Brain Behav Immun 2018; 68: 66–75. e-pub ahead of print 2017/10/12; doi: 10.1016/j.bbi.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker JM, Nederveen JP, Parise G. Aerobic exercise in humans mobilizes HSCs in an intensity-dependent manner. J Appl Physiol (1985) 2017; 122(1): 182–190. e-pub ahead of print 2016/11/25; doi: 10.1152/japplphysiol.00696.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emmons R, Niemiro GM, De Lisio M. Exercise as an Adjuvant Therapy for Hematopoietic Stem Cell Mobilization. Stem Cells Int 2016; 2016: 7131359 e-pub ahead of print 2016/03/31; doi: 10.1155/2016/7131359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coravos A, Khozin S, Mandl KD. Developing and adopting safe and effective digital biomarkers to improve patient outcomes. NPJ Digit Med 2019; 2(1). e-pub ahead of print 2019/03/15; doi: 10.1038/s41746-019-0090-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altini M, Casale P, Penders J, Ten Velde G, Plasqui G, Amft O. Cardiorespiratory fitness estimation using wearable sensors: Laboratory and free-living analysis of context-specific submaximal heart rates. J Appl Physiol (1985) 2016; 120(9): 1082–1096. e-pub ahead of print 2016/03/05; doi: 10.1152/japplphysiol.00519.2015 [DOI] [PubMed] [Google Scholar]
- 46.Altini M, Penders J, Amft O. Estimating Oxygen Uptake During Nonsteady-State Activities and Transitions Using Wearable Sensors. IEEE J Biomed Health Inform 2016; 20(2): 469–475. e-pub ahead of print 2015/01/17; doi: 10.1109/jbhi.2015.2390493 [DOI] [PubMed] [Google Scholar]
- 47.Cook AJ, Ng B, Gargiulo GD, Hindmarsh D, Pitney M, Lehmann T et al. Instantaneous VO2 from a wearable device. Med Eng Phys 2018; 52: 41–48. e-pub ahead of print 2018/01/17; doi: 10.1016/j.medengphy.2017.12.008 [DOI] [PubMed] [Google Scholar]


