Summary:
Until recently, endocytic trafficking and its regulators were thought to function almost exclusively on membrane-bound organelles and/or vesicles containing a lipid bilayer. Recent studies have demonstrated that endocytic regulatory proteins play much wider roles in trafficking regulation, and influence a variety of non-endocytic pathways, including trafficking to/from mitochondria and peroxisomes. Moreover, new studies also suggest that endocytic regulators also control trafficking to and from cellular organelles that lack membranes, such as the centrosome. While endocytic membrane trafficking clearly impacts pathways downstream of the centrosome, such as ciliogenesis (including transport to and from cilia), mitotic spindle formation, and cytokinesis, relatively few studies have focused on the growing role for endocytic membrane trafficking more directly on centrosome biogenesis, maintenance and control throughout cell cycle, and centrosome duplication. Indeed, a growing number of endocytic regulatory proteins have been implicated in centrosome regulation, including various Rab proteins (among them Rab11) and the leucine rich repeat kinase 2 (LRRK2). In this review, we will examine the relationship between centrosomes and endocytic membrane trafficking, focusing primarily on how endocytic membrane trafficking directly influences the centrosome.
Introduction
A traditional depiction of endocytic membrane trafficking (EMT) includes its primary roles in the internalization of receptors and lipids from the plasma membrane and their sorting and delivery to internal endocytic compartments and subsequent recycling to the plasma membrane and/or ultimate degradation in lysosomes. However, a growing number of recent studies have expanded the definition of endocytic trafficking to include previously unforeseen roles that include transport to and from mitochondria and peroxisomes as well as control of their fission and fusion [1–4], trafficking of Bcl-2 family apoptotic regulatory proteins [5], involvement in ciliogenesis [6], and regulation of centriole disengagement and centrosome duplication through the removal of key inhibitory proteins by their transport to the mitotic spindle midbody [7] (Fig. 1).
Fig. 1. Membrane trafficking pathways controlled by endocytic regulatory proteins.
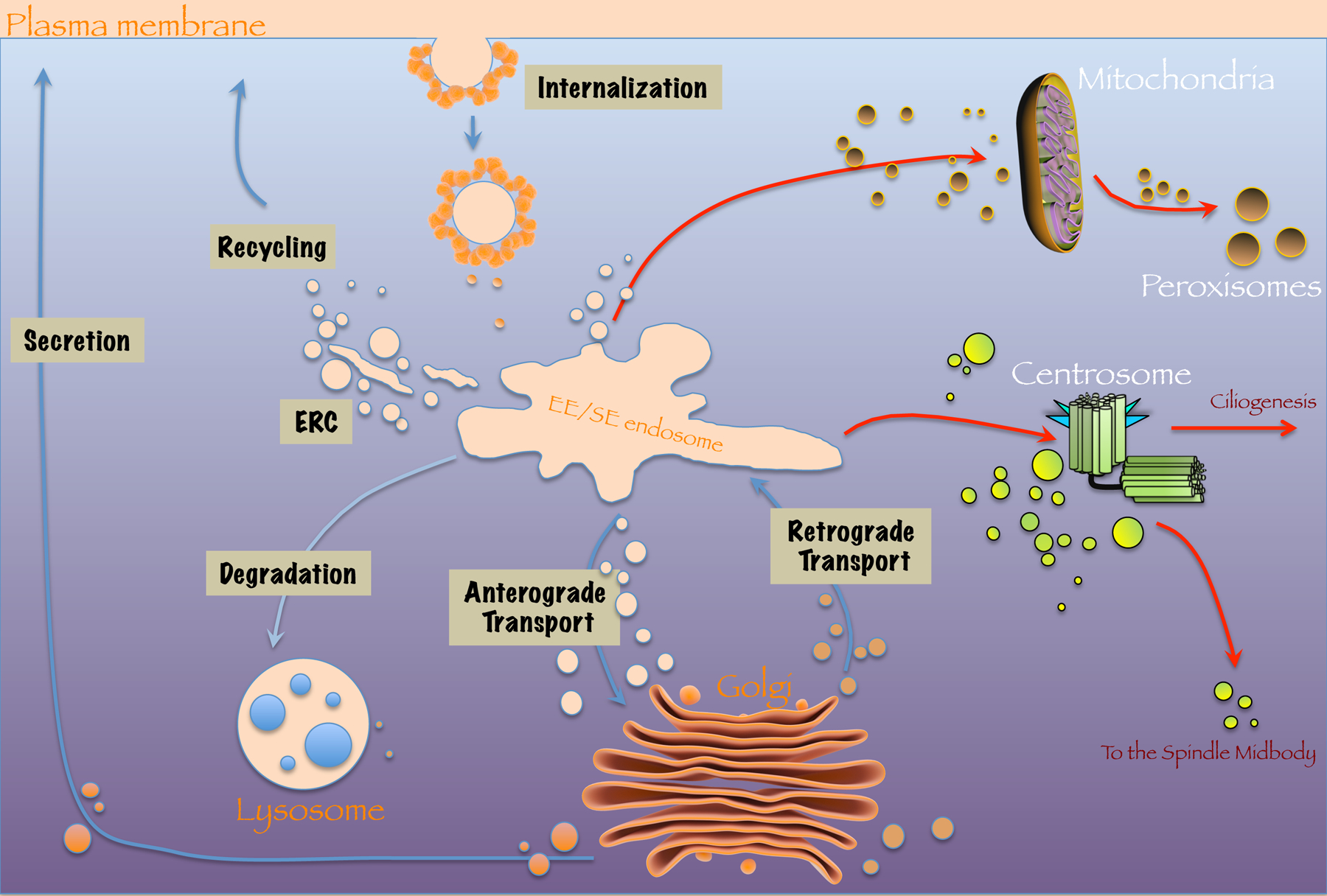
Endocytic trafficking traditionally includes the internalization of receptors and lipids from the plasma membrane, the fusion of internalized vesicles with the sorting endosome, recycling to the plasma membrane (often through a transitory compartment known as the Endocytic Recycling Compartment or ERC), transport to the lysosome, and trafficking to and from the Golgi apparatus in anterograde and retrograde transport (blue arrows). Somewhat surprisingly, recent studies have also demonstrated roles for vesicular transport and endocytic trafficking at mitochondria, peroxisomes and the centrosome (red arrows).
The Centrosome
The centrosome or “center-body” is the major microtubule-organizing center in the cell, and among its primary functions are the generation of mitotic spindles and cilia [8]. In comparison with endosomes, endocytic organelles and even mitochondria, at first glance the centrosome is an unlikely target organelle for regulation by EMT because it is devoid of a limiting membrane (Fig. 2). Indeed, the centrosome is a tiny organelle composed of a pair of barrel-shaped proteinaceous centrioles and surrounded by a shell of pericentriolar material (PCM) that nucleates the growth of microtubules [9]. Centrioles are structures of about 0.5 μm in length that are composed of nine-triplet microtubule bundles and comprise the duplicating elements of centrosomes [10]. To ensure accurate chromosome segregation during mitosis, centriole duplication must be regulated closely to allow a single occurrence each cell division [11,12]. In G1-phase, cells typically contain a single centrosome comprised of a mother-daughter centriole pair docked at the plasma membrane, forming a basal body that nucleates a ciliary axoneme when ciliogenesis occurs. During S-phase, cilia are reabsorbed and each ‘mother’ centriole assembles a new single ‘daughter’ that initially forms as a short procentriole, positioned orthogonally to the mother. In G2-phase, the newer daughter centrioles elongate, and centrosomes recruit a highly ordered PCM shell, comprised of tens of different proteins. The PCM itself is involved in regulation of the centriole duplication process and facilitates mitotic spindle assembly [13]. During mitotic exit, each daughter cell receives a single, tightly-knit mother-daughter centriole pair that must ‘disengage’ from one another prior to duplication [14,15]. The main functions of centrioles are to: 1) form basal bodies that assemble cilia and, 2) during mitosis, recruit a PCM to nucleate spindle microtubules and form mitotic spindle poles.
Fig. 2. Schematic representation of the centrosome, a membrane-free organelle.
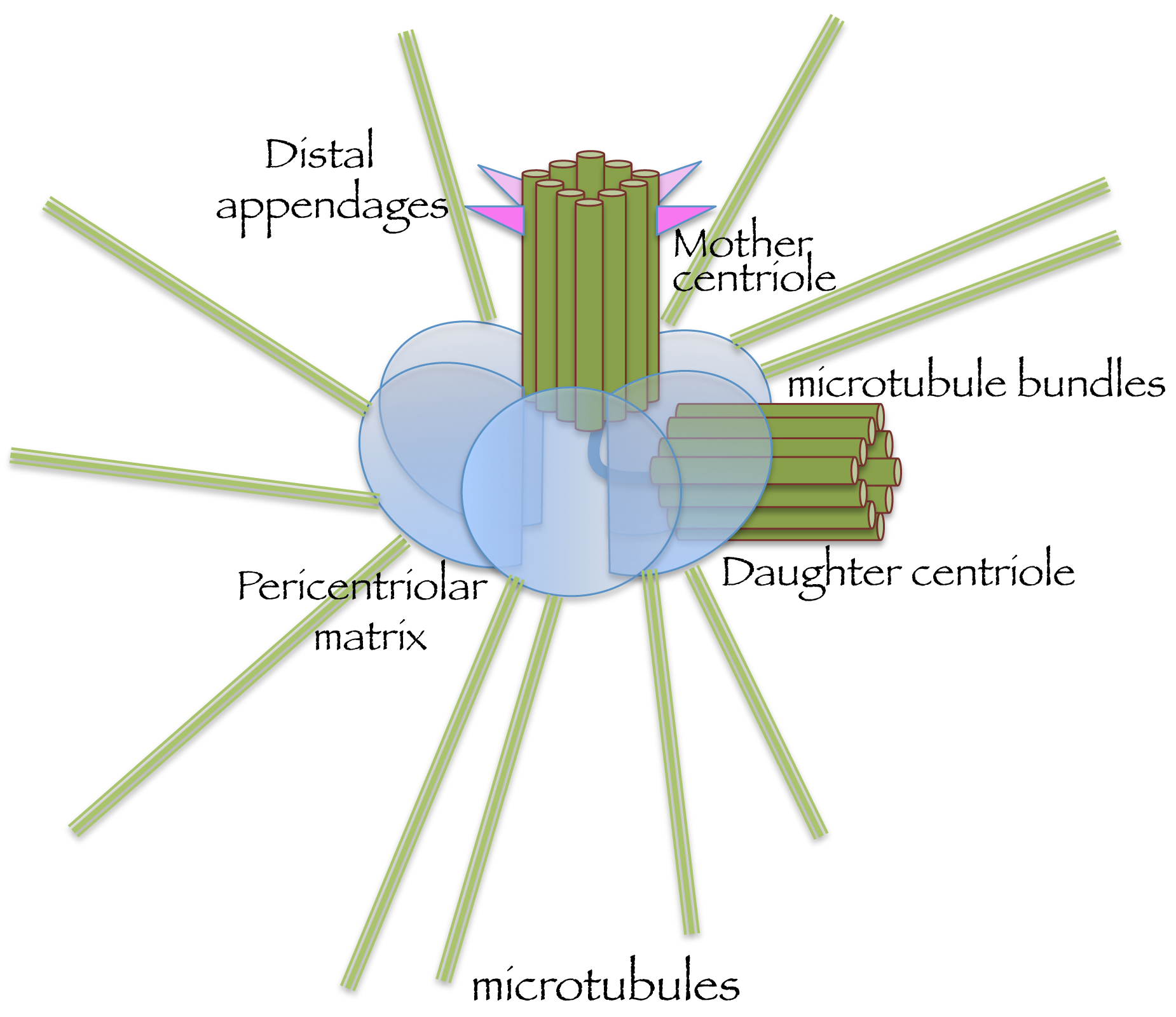
The centrosome is comprised of a mother centriole (marked by distal appendages), an orthogonally positioned daughter centriole, and a surrounding pericentriolar matrix from which microtubules emanate.
Close Encounters of the Golgi-Centrosome Kind
The spatial proximity of the centrosome to the endocytic recycling compartment (ERC) [16], the Golgi apparatus, and the intermediate compartment [17] has long led to speculation of a functional relationship between the centrosome and endosomes, but until recently, little evidence has been found to directly link these organelles. However, despite the fact that centrosomes do not have a limiting membrane, recent studies now suggest a number of intriguing connections between centrosomes and EMT. Although there is ample evidence of significant EMT roles in downstream centrosome-mediated events, such as ciliogenesis (reviewed in [18,19]) and spindle pole generation (reviewed in [20]), fewer studies have addressed the more direct potential impact of EMT on the centrosome itself and regulation of its biogenesis, maintenance and duplication. Herein, we will explore this connection by addressing newer studies that implicate EMT events in regulation of the centrosome, and examine recent research demonstrating that EMT influences key centrosomal events, such as centriole disengagement, centrosome cohesion, and removal of key centrosome inhibitory proteins to the spindle midbody to facilitate centrosome duplication.
Endocytic Membrane Trafficking Directly Affects the Centrosome
There is a considerable body of literature that addresses the role of EMT in the regulation of ciliogenesis (reviewed in [18, 19]), with a role documented for EMT both in the delivery of preciliary vesicles to the distal appendages of the mother centriole in a myosin-Va-dependent manner [21], and the subsequent Rab11-Rab8 cascade [6,22] that also involves the Eps15 Homology Domain protein, EHD1 [23] and its interaction partner, MICAL-L1 [24]. However, as noted above, less focus has been placed on the impact of EMT directly on the centrosome itself, including its biogenesis, homeostasis, and regulation throughout cell cycle in non-ciliated as well as ciliated cells. One of the initial key findings directly linking centrosomes and EMT came from the study of Gromley and co-workers, who demonstrated that centriolin, a key mother centriole-specific protein, interacts directly with the exocyst complex subunit Sec15 [25]. In turn, Sec15 serves as an effector for the recycling endosome regulator, Rab11 [26], thus establishing a physical connection between centrosomes and EMT. Soon afterward, Rab11 itself and the Rab11 GTPase activating protein (GAP) Evi5 [27], were also identified localizing to the distal appendages of mother centrioles and interacting with two mother centriole resident proteins, centriolin and cenexin [28]. Intriguingly, depletion of centriolin and/or loss of Evi5 led to enhanced Rab11 association with centrosomes, indicating that Rab11-centrosome binding is guanine nucleotide dependent [28].
The localization of Rab11 and its regulatory proteins to the distal appendage of the mother centriole likely has important bearing on the key functions of the centrosome. Indeed, evidence supports a role for vesicles containing Rab11, along with the PCM protein, γ-tubulin, and the motor protein, dynein, as potential vesicular carriers of proteins to the mitotic spindle poles [29]. Moreover, recruitment of Rab11 to the mother centriole appears to be crucial for the initiation of the Rab11-Rab8 cascade required for downstream functions, such as generation of the ciliary vesicle and ciliogenesis [6,22,23].
Intriguingly, a series of very recent studies has linked the EMT protein, Rab8a, directly to centrosomal regulation, independent of downstream ciliogenesis. As noted above, centrosomes normally duplicate in S-phase, by initially spawning procentrioles orthogonally to both mother and daughter centrioles; ultimately the linker connecting the original centriole pair is severed, facilitating separation of the two newly formed centriolar pairs [13]. However, it was observed that centrosomes in cells expressing a pathogenic form of the leucine rich repeat kinase 2 (LRRK2) gene that is causative for familial Parkinson’s disease [30,31] induced a centrosomal defect with impaired centrosomal cohesion [32]. Whereas a recent study showed downstream effects of LRRK2 on centrosomes through its phosphorylation of Rab10 and impairment of ciliogenesis [33], LRRK2 also impacts the centrosome more directly via Rab8a, which was also unambiguously identified as a key substrate for phosphorylation by the LRRK2 kinase [34]. Indeed, LRRK2-mediated phosphorylation of Rab8a induces its enhanced centrosomal localization that leads to premature disruption of the centriolar linker, ultimately causing a centrosome cohesion defect [32]. Moreover, the Rab7L1/Rab29 protein, also implicated in Parkinson’s disease [35,36], functions in the same pathway, recruiting LRRK2 to the Golgi region and facilitating Rab8a phosphorylation, which in turn impacts centrosome cohesion [37]. In addition, Rab-family related proteins, such as RabL6a, regulate centrosome duplication and have been observed localizing to the centrosome [38], whereas proteins such RabL2, which binds to Cep164 and Cep83 on the mother centriole, as well as Rab34, appear to function primarily downstream in ciliogenesis [39,40].
The notion that centrosomes are regulated by EMT and endocytic regulatory proteins is not a new one, but it has received new impetus recently. Initially, PCM proteins such as ninein, were also found localized to the mitotic spindle midbody during cell division [41]. Later, studies demonstrated that ninein was also released from centrosomes and transported toward the spindle midbody in a microtubule-dependent manner, suggesting potential involvement of vesicles from EMT pathway [42]. However, it remains possible that ninein could be trafficked directly by dynein motor protein movement along microtubules in a vesicle-independent manner, similar to the mechanism of transport proposed for ninein and other proteins that localize to centriolar satellites [43].
Additional evidence supporting a role for EMT in control of centriolar events comes from a recent study demonstrating that knock-down of EHD1, an endocytic regulatory protein involved in the recycling of receptors [44], impaired centriole disengagement and centrosome duplication [7]**. In this study, it was demonstrated that endocytic vesicles containing EHD1 interacted with centrosome regulatory proteins such as Cep215/Cdkrap2 and pericentrin, as a mechanism for centrosomal removal and transport from the centrosome to the mitotic spindle midbody [7] (see Fig. 3). Overall, this highlights a new role for EMT in the control of centrosome duplication through its selective removal of proteins that inhibit centrosome duplication and their trafficking to the spindle midbody.
Fig. 3. Removal of the centriolar inhibitory protein Cep215/Cdkrap2 to the mitotic spindle midbody occurs by endocytic membrane trafficking.
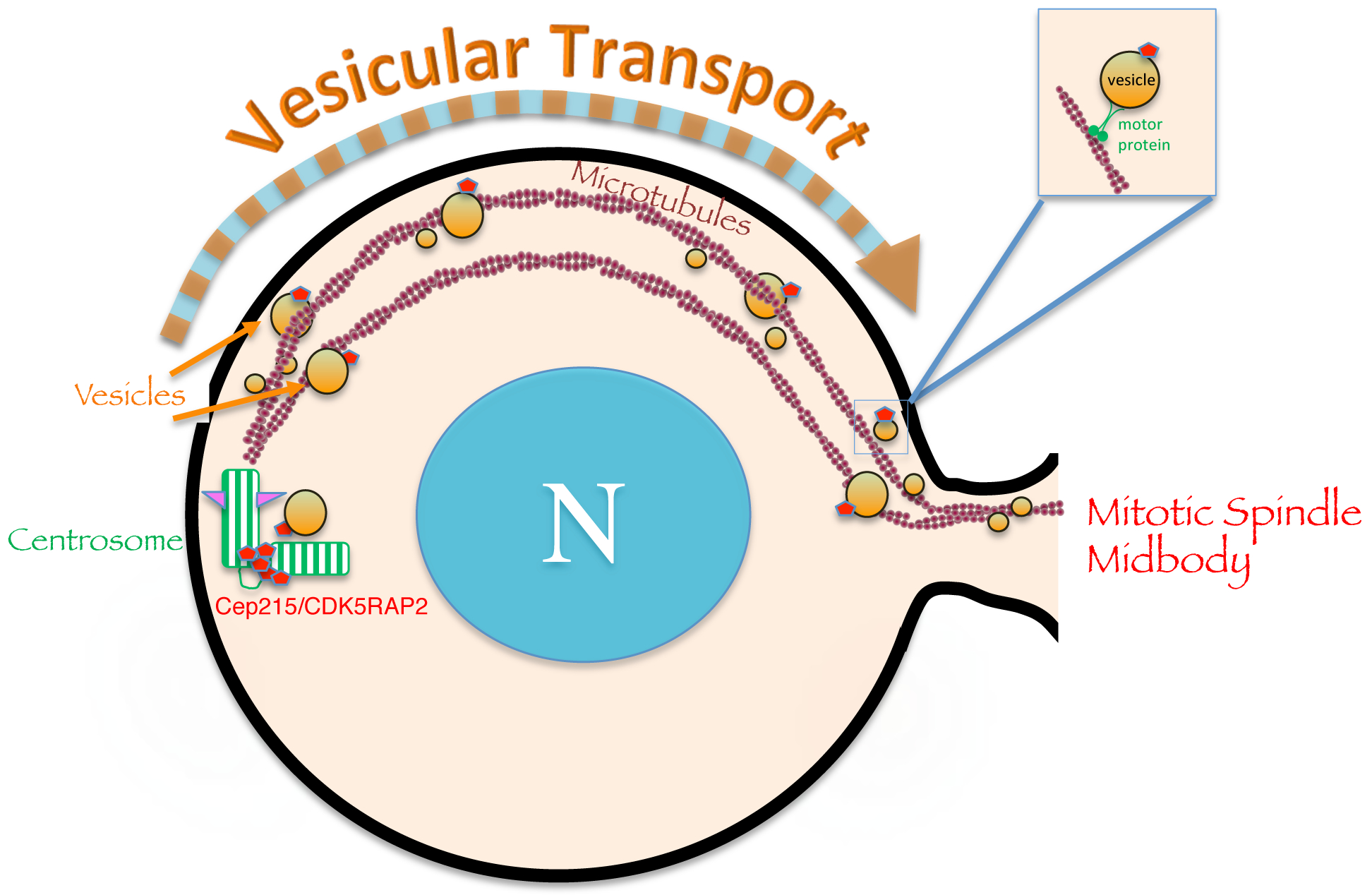
Centriolar proteins such as Cep215/Cdkrap2 link the procentriole to the mother centriole and prevent premature disengagement and duplication. Vesicular transport provides a mechanism by which endocytic regulatory proteins such as EHD1 can interact with Cep215/Cdkrap2 to facilitate its removal from the centriole to the spindle midbody, thus facilitating centriole disengagement and duplication.
Concluding Remarks and Future Directions
In summary, while the vast majority of regulation by EMT on the centrosome is directed downstream at functions such as ciliogenesis and mitotic spindle formation, a growing number of recent studies indicate that centrosomal processes such as centriole disengagement, centrosome duplication and separation of centrosomes throughout cell cycle may be regulated by EMT. The increasing number of centrosomal proteins that are trafficked to the spindle midbody suggests that in addition to merely removing inhibition at the centrosome, the relocation of centrosomal proteins to the spindle midbody may have additional functional consequences that have yet to be determined, and this may become a priority for future studies. In addition, as the role for EMT continues to gain traction as a regulator of centrosomal function, the spotlight will undoubtedly move to the centrosomal satellites, and the potential for EMT to control the movement of satellite proteins from this important ‘reservoir’ to the centrosome. Remarkably, EMT impacts the centrosome cycle and its function despite the centrosome’s lack of a surrounding lipid membrane, thus highlighting the possibility that EMT may play a broader role in the cell. Recent studies have suggested a different type of “membraneless organelle” that results from either liquid/liquid or liquid/solid phase separations in the cell. Indeed the recent focus on these non-stoichiometric “membraneless organelles” derived from quinary assemblies hints at the possibility of a new role for EMT in the trafficking to and from these organelles, a topic that will likely receive significant attention in the coming years.
Acknowledgments
The authors acknowledge support from the National Institutes of General Medical Sciences (1R01GM123557 and P30GM106397).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Soubannier V, McLelland GL, Zunino R, Braschi E, Rippstein P, Fon EA, McBride HM: A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol 2012, 22:135–141. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Wang X, Fujioka H, Hoppel C, Whone AL, Caldwell MA, Cullen PJ, Liu J, Zhu X: Parkinson’s disease-associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat Med 2016, 22:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W, Ma X, Zhou L, Liu J, Zhu X: A conserved retromer sorting motif is essential for mitochondrial DLP1 recycling by VPS35 in Parkinson’s disease model. Hum Mol Genet 2017, 26:781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang FL, Liu W, Hu JX, Erion JR, Ye J, Mei L, Xiong WC: VPS35 Deficiency or Mutation Causes Dopaminergic Neuronal Loss by Impairing Mitochondrial Fusion and Function. Cell Rep 2015, 12:1631–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farmer T, O’Neill KL, Naslavsky N, Luo X, Caplan S: Retromer facilitates the localization of Bcl-xL to the mitochondrial outer membrane. Mol Biol Cell 2019, 30:1138–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W: Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A 2010, 107:6346–6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.**.Xie S, Reinecke JB, Farmer T, Bahl K, Yeow I, Nichols BJ, McLamarrah TA, Naslavsky N, Rogers GC, Caplan S: Vesicular trafficking plays a role in centriole disengagement and duplication. Mol Biol Cell 2018:mbcE18040241. [DOI] [PMC free article] [PubMed] [Google Scholar]; Xie et al., 2019, (#7) identify a direct link between endocytic membrane trafficking and centrosome duplication by demonstrating that the endocytic regulatory protein EHD1 is required for the removal of Cep215/CDK5RAP2 from centrosome. By trafficking Cep215/CDK5RAP2 away from centrosomes to the mitotic spindle midbody by vesicular transport, EHD1 paves the way for centriole disengage and ensuing centrosome duplication.
- 8.Vertii A, Hehnly H, Doxsey S: The Centrosome, a Multitalented Renaissance Organelle. Cold Spring Harb Perspect Biol 2016, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conduit PT, Wainman A, Raff JW: Centrosome function and assembly in animal cells. Nat Rev Mol Cell Biol 2015, 16:611–624. [DOI] [PubMed] [Google Scholar]
- 10.Bettencourt-Dias M, Glover DM: Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol 2007, 8:451–463. [DOI] [PubMed] [Google Scholar]
- 11.Nigg EA, Cajanek L, Arquint C: The centrosome duplication cycle in health and disease. FEBS Lett 2014, 588:2366–2372. [DOI] [PubMed] [Google Scholar]
- 12.Nigg EA, Stearns T: The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol 2011, 13:1154–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu J, Hagan IM, Glover DM: The centrosome and its duplication cycle. Cold Spring Harb Perspect Biol 2015, 7:a015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee K, Rhee K: Separase-dependent cleavage of pericentrin B is necessary and sufficient for centriole disengagement during mitosis. Cell Cycle 2012, 11:2476–2485. [DOI] [PubMed] [Google Scholar]
- 15.Pagan JK, Marzio A, Jones MJ, Saraf A, Jallepalli PV, Florens L, Washburn MP, Pagano M: Degradation of Cep68 and PCNT cleavage mediate Cep215 removal from the PCM to allow centriole separation, disengagement and licensing. Nat Cell Biol 2015, 17:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takatsu H, Katoh Y, Ueda T, Waguri S, Murayama T, Takahashi S, Shin HW, Nakayama K: Mitosis-coupled, microtubule-dependent clustering of endosomal vesicles around centrosomes. Cell Struct Funct 2013, 38:31–41. [DOI] [PubMed] [Google Scholar]
- 17.Marie M, Dale HA, Sannerud R, Saraste J: The function of the intermediate compartment in pre-Golgi trafficking involves its stable connection with the centrosome. Mol Biol Cell 2009, 20:4458–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Dynlacht BD: The regulation of cilium assembly and disassembly in development and disease. Development 2018, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nachury MV, Mick DU: Establishing and regulating the composition of cilia for signal transduction. Nat Rev Mol Cell Biol 2019, 20:389–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miserey-Lenkei S, Colombo MI: Small RAB GTPases Regulate Multiple Steps of Mitosis. Front Cell Dev Biol 2016, 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu CT, Chen HY, Tang TK: Myosin-Va is required for preciliary vesicle transportation to the mother centriole during ciliogenesis. Nat Cell Biol 2018, 20:175–185. [DOI] [PubMed] [Google Scholar]
- 22.Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, et al. : Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A 2011, 108:2759–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Q, Insinna C, Ott C, Stauffer J, Pintado PA, Rahajeng J, Baxa U, Walia V, Cuenca A, Hwang YS, et al. : Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat Cell Biol 2015, 17:531. [DOI] [PubMed] [Google Scholar]
- 24.Xie S, Farmer T, Naslavsky N, Caplan S: MICAL-L1 coordinates ciliogenesis by recruitment of EHD1 to the primary cilium. J Cell Sci 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ: Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell 2005, 123:75–87. [DOI] [PubMed] [Google Scholar]
- 26.Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T: Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem 2004, 279:43027–43034. [DOI] [PubMed] [Google Scholar]
- 27.Dabbeekeh JT, Faitar SL, Dufresne CP, Cowell JK: The EVI5 TBC domain provides the GTPase-activating protein motif for RAB11. Oncogene 2007, 26:2804–2808. [DOI] [PubMed] [Google Scholar]
- 28.Hehnly H, Chen CT, Powers CM, Liu HL, Doxsey S: The centrosome regulates the Rab11- dependent recycling endosome pathway at appendages of the mother centriole. Curr Biol 2012, 22:1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hehnly H, Doxsey S: Rab11 endosomes contribute to mitotic spindle organization and orientation. Dev Cell 2014, 28:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, et al. : Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 2004, 44:595–600. [DOI] [PubMed] [Google Scholar]
- 31.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, et al. : Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004, 44:601–607. [DOI] [PubMed] [Google Scholar]
- 32.**.Madero-Perez J, Fdez E, Fernandez B, Lara Ordonez AJ, Blanca Ramirez M, Gomez-Suaga P, Waschbusch D, Lobbestael E, Baekelandt V, Nairn AC, et al. : Parkinson disease-associated mutations in LRRK2 cause centrosomal defects via Rab8a phosphorylation. Mol Neurodegener 2018, 13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Madero-Perez et al., 2018, (#32) show that a pathogenic LRRK2 Parkinson’s disease mutant with upregulated kinase activity affects centrosome positioning/polarity and cohesion through its phosphorlation of Rab8a. These findings link a key Rab protein implicated in intracellular trafficking and recycling directly with centrosome structure and function, independently of its effects on ciliogenesis.
- 33.Dhekne HS, Yanatori I, Gomez RC, Tonelli F, Diez F, Schule B, Steger M, Alessi DR, Pfeffer SR: A pathway for Parkinson’s Disease LRRK2 kinase to block primary cilia and Sonic hedgehog signaling in the brain. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, Wachter S, Lorentzen E, Duddy G, Wilson S, et al. : Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A, et al. : Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet 2009, 41:1303–1307. [DOI] [PubMed] [Google Scholar]
- 36.Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, et al. : Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet 2009, 41:1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.*.Madero-Perez J, Fernandez B, Lara Ordonez AJ, Fdez E, Lobbestael E, Baekelandt V, Hilfiker S: RAB7L1-Mediated Relocalization of LRRK2 to the Golgi Complex Causes Centrosomal Deficits via RAB8A. Front Mol Neurosci 2018, 11:417. [DOI] [PMC free article] [PubMed] [Google Scholar]; Madero-Perez et al., 2018, (#37) demonstrate here that LRRK2 is recruited to the Golgi complex by Rab7L1, which causes enhanced Rab8a phosphorylation and centrosome cohesion deficits similar to those seen with the LRRK1 G2019S hyperactive kinase mutant. These findings further support a mechanism by which Rab8a and its phosphorylation directly impact centrosomes.
- 38.Zhang X, Hagen J, Muniz VP, Smith T, Coombs GS, Eischen CM, Mackie DI, Roman DL, Van Rheeden R, Darbro B, et al. : RABL6A, a novel RAB-like protein, controls centrosome amplification and chromosome instability in primary fibroblasts. PLoS One 2013, 8:e80228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.*.Dateyama I, Sugihara Y, Chiba S, Ota R, Nakagawa R, Kobayashi T, Itoh H: RABL2 positively controls localization of GPCRs in mammalian primary cilia. J Cell Sci 2019, 132. [DOI] [PubMed] [Google Scholar]; Dateyama et al., 2019, (#39) find that the RabL2 protein is recruited to the mother centriole by an interaction with Cep164 and Cep83 on distal appendages, where it plays a downstream role on centrosome function by trafficking G-protein coupled receptors to the ciliary base of mammalian cells.
- 40.*.Xu S, Liu Y, Meng Q, Wang B: Rab34 small GTPase is required for Hedgehog signaling and an early step of ciliary vesicle formation in mouse. J Cell Sci 2018, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]; Xu et al., 2018, (#40) show that Rab34 is required for ciliogenesis because it is needed for the successive fusion of preciliary vesicles to generate the ciliary vesicle and for migration of the mother centriole to the plasma membrane.
- 41.Bouckson-Castaing V, Moudjou M, Ferguson DJ, Mucklow S, Belkaid Y, Milon G, Crocker PR: Molecular characterisation of ninein, a new coiled-coil protein of the centrosome. J Cell Sci 1996, 109 (Pt 1):179–190. [DOI] [PubMed] [Google Scholar]
- 42.Moss DK, Bellett G, Carter JM, Liovic M, Keynton J, Prescott AR, Lane EB, Mogensen MM: Ninein is released from the centrosome and moves bi-directionally along microtubules. J Cell Sci 2007, 120:3064–3074. [DOI] [PubMed] [Google Scholar]
- 43.Betleja E, Nanjundappa R, Cheng T, Mahjoub MR: A novel Cep120-dependent mechanism inhibits centriole maturation in quiescent cells. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caplan S, Naslavsky N, Hartnell LM, Lodge R, Polishchuk RS, Donaldson JG, Bonifacino JS: A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J 2002, 21:2557–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]


