SUMMARY
Attention to breath reduces pain independent of endogenous opioids, and may be an effective chronic-pain treatment due to a lack of cross-tolerance with traditional pain-therapies.
INTRODUCTION
The endogenous opioidergic system is characterized as one of the central physiological networks supporting the cognitive modulation of pain [8; 75; 80]. Analgesia produced by a spectrum of non-pharmacological approaches including placebo [4; 29; 41; 49; 90], conditioned pain modulation [46], distraction [69], transcranial magnetic stimulation [28] and hypnosis [34; 70] are driven by opioidergically mediated descending inhibition of pain [10; 66]. Mindfulness meditation is a promising, cost-effective pain therapy, although the specific mechanisms supporting mindfulness have not comprehensively identified [22; 37; 39; 56; 89]. As adapted in our laboratory, mindfulness-based pain-relief is associated with multiple supraspinal mechanisms supporting the cognitive-affective regulation (greater orbitofrontal/ ventrolateral prefrontal cortex activation) of ascending nociceptive information (greater thalamic deactivation) [84; 87]. We have proposed [85; 89] that this unique corticothalamo-cortical gating mechanism bypasses opioidergically mediated descending inhibition to reduce pain [52; 83]. This model is supported further by our recent findings [84] demonstrating that mindfulness meditation reliably deactivates the periaqueductal gray matter (PAG), a central node of opioidergically mediated descending pain inhibition. It has been postulated that meditation-based pain-relief engages mechanisms supporting placebo (expectations, conditioning; beliefs)[68], reductions in respiration rate [39], non-reactive attention to somatic sensations (i.e., interoception)[30], and reappraisal[84]. One way to bridge this explanatory gap is to determine if the potential non-specific components such as slower breathing rate, expectations for pain-relief, and attention to breath that underlie mindfulness-based analgesia differentially engage the endogenous opioid system.
The present randomized, crossover double-blinded study built upon our prior work [83] and employed a graded analytical approach to determine if antagonizing endogenous opioids reverses reductions in pain produced during mindfulness meditation, slow-paced breathing, and sham-mindfulness meditation. We aimed to employ robust control conditions to tease apart the role of endogenous opioids corresponding to the potential non-specific effects including expectations, breathing, beliefs that may be evoked by mindfulness. Based on our previous work [52; 83], we postulated that mindfulness-based pain reductions do not engage endogenous opioids due to the role of supraspinally mediated reappraisal processes [83; 89]. Sham-mindfulness meditation engages brain and autonomic mechanisms supporting placebo analgesia [3; 84; 86], thus, we predicted that sham-mindfulness meditation-induced analgesia would be facilitated by endogenous opioids. A variety of slow, paced breathing techniques attenuate both experimentally-induced and clinical pain [5; 19; 71; 79; 82; 91]. Brainstem respiratory control mechanisms are modulated by direct input from prefrontal mechanisms [54; 59], and slow-breathing based analgesia is not associated with spinally mediated mechanisms [5; 51]. Yet, it remains unknown if slow-breathing engages endogenous opioid systems. Give that endogenous opioids play a significant role in the control of breathing [18; 55; 72], we predicted that pain-relief produced by slow-breathing also would be mediated by endogenous opioids.
METHODS AND MATERIALS
Participants
Eighty-seven healthy, pain-free and meditation-naive participants were recruited from the local community via flyers, social media advertisements, and the Wake Forest clinical trial registry. Wake Forest School of Medicine’s Institutional Review Board approved all study procedures. Inclusion criteria included individuals 18 – 65 years of age, no prior meditative experience, and pain-free. Exclusion criteria included those currently taking opioids, pregnant, and those with a history of syncope, fear of needles and blood. The study was registered on clinicaltrials.gov (NCT03419858) prior to study initiation (data collection = March 6th -July, 13th 2017). All subjects provided written, informed consent at the initial study visit with all methods clearly explained, acknowledging that (1) they would experience painful heat stimuli, and (2) they were free to withdraw from the study at any time without prejudice.
Twenty-six of the 87 participants that were screened over the phone were excluded at or after the initial in-person visit (Figure 1). Subjects were dismissed for low pain sensitivity (n=2), concerns with time commitments (n=7), ongoing chronic pain (n=1), ongoing opioid use (n=1), history of syncope (n=1), “no shows” (n=7), procedural errors (n=4), lymphedema (n=1), issues involving a prosthetic leg (n=1) and one participant was excluded because our targeted sample size had been reached during said session 1’s data collection (Figure 1). After completing the slow-breathing intervention, a subject (female) experienced an adverse effect (light-headed; heavy perspiration) during naloxone administration, the infusion was immediately discontinued, she received clearance from a medical evaluation by the study physician, and was discharged.
Figure 1.
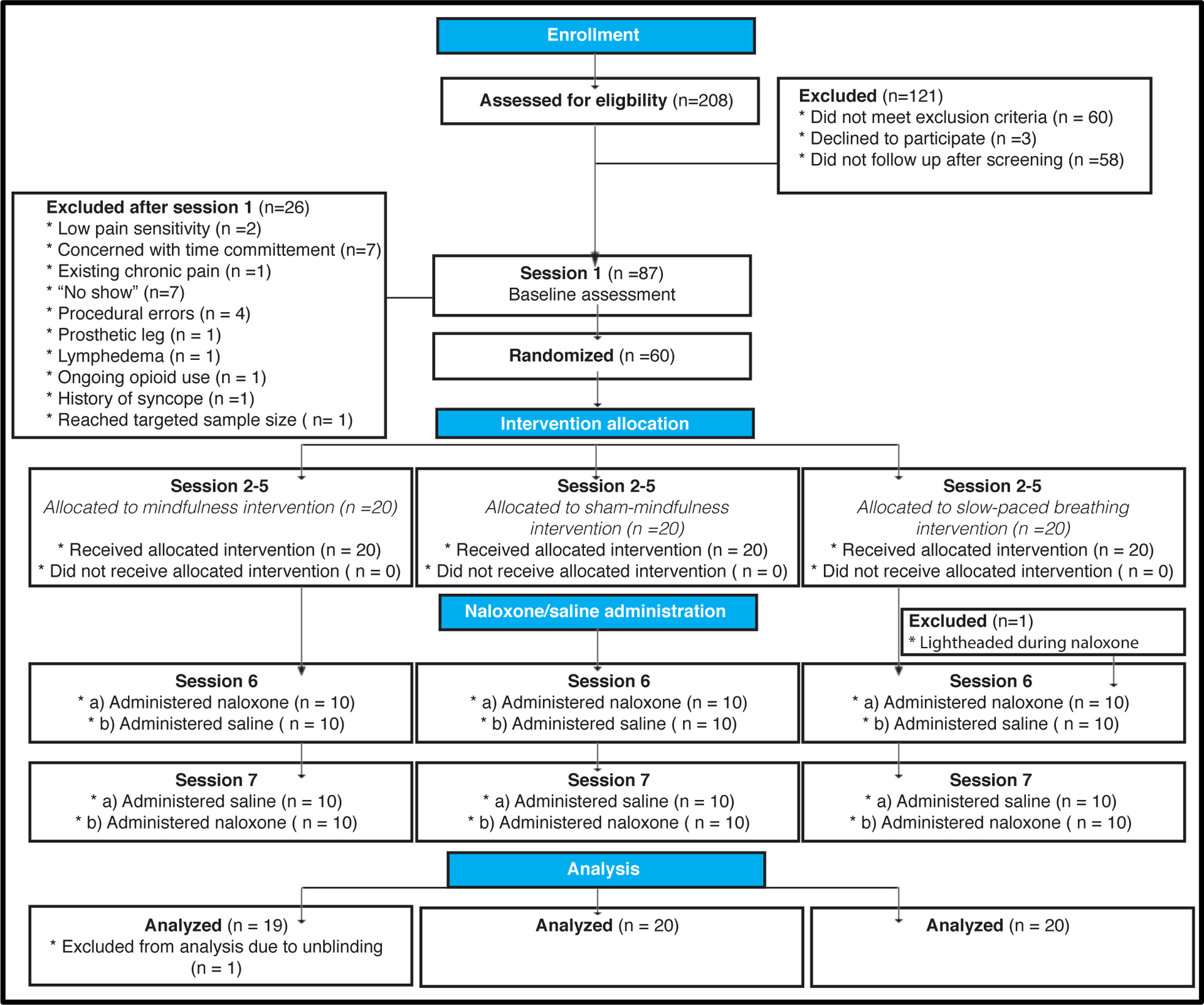
Experimental Design. The proposed mechanistically focused, crossover and double-blinded clinical trial included 7 separate sessions. After potential participants met study inclusion/exclusion criteria, participants completed study session 1, which served as a baseline control. After study session 1, 60 participants were randomized into one of three groups (mindfulness; sham-mindfulness; book-listening control). After their respective four-day interventions (Session 2–5), subjects participated in the post-intervention experimental session (session 6). Here, we assessed the effects of each respective manipulation during naloxone/saline infusion and noxious stimulation. One participant, in the slow-paced breathing group, was dismissed during naloxone infusion during session 6. Session 7’s experimental procedures were matched to session 6 except for the respective drug assignment. One participant from the mindfulness meditation group was dismissed from the final analysis because said subject’s drug assignment was unintentionally revealed and subsequently unblinded. Thus, a total of 59 subject’s data were included in the present study.
Randomization Procedure
Randomization was stratified by sex. Groups were matched on gender (10/group); each sex had their respective list of randomization codes. Males and females were randomized without replacement across a block of 60 codes using a random number generator by a research technician not involved in any part of the study. Drug order was counterbalanced across group and gender. Participants were informed of their respective group assignment after session 1.
Sixty participants (53 right-handed; mean age = 27 years ± 7 years; 30 males; 30 females) successfully completed all study procedures (42 = White, 13 = Black, 3 = Hispanic, 1 = Asian, and 1 = Native American). One participant (female; mindfulness group) was excluded from the final analysis due to the subject’s misunderstanding of the study nurse’s directives and was consequently un-blinded to her drug assignment during her first post-intervention testing session. Thus, 59 participants are included in the final study analyses (Table 1).
Table 1.
Group demographics, weight [kilograms (kg)], baseline pain ratings, and naloxone symptom assessments.
| Characteristic | Slow breathing* | Mindfulness* | Sham-mindfulness* | F | P value |
|---|---|---|---|---|---|
| Sociodemographics | |||||
| Age, Years (SD) | 26.70 (1.40) | 26.68 (2.09) | 28.30 (1.76) | .28 | .76 |
| Weight & Resulting drug/saline Dosage | |||||
| Weight (kg | 82.83 (5.15) | 76.32 (3.88) | 78.10 (3.62) | .02 | .98 |
| Drug/saline dosage (mg) | 14.49 (.90) | 13.36 (.66) | 13.76 (.63) | .25 | .78 |
| Baseline Pain Ratings | |||||
| Baseline VAS pain intensity pre | 5.30 (.48) | 4.82 (.49) | 4.18 (.40) | 1.54 | .22 |
| Baseline VAS pain unpleasantness pre | 5.78 (.48) | 5.06 (.58) | 4.46 (.40) | 1.87 | .16 |
| Baseline VAS pain intensity post | 5.62 (.52) | 4.88 (.45) | 4.25 (.43) | 2.20 | .12 |
| Baseline VAS pain unpleasantness post | 5.75 (.45) | 5.09 (.51) | 4.59 (.45) | 1.56 | .22 |
| Naloxone Symptom Assessment | |||||
| Saline + dry mouth | .45 (.18) | .84 (.28) | 1.40 (.30) | 3.46 | .04* |
| Naloxone + dry mouth | .60 (.24) | .95 (.31) | 1.35 (.36) | 1.48 | .24 |
| Saline + dry skin | .25 (.14) | .32 (.17) | .55 (.28) | .57 | .57 |
| Naloxone + dry skin | .45 (.22) | .37 (.16) | .35 (.24) | .06 | .94 |
| Saline + blurred vision | .10 (.06) | .11 (.11) | .05 (.05) | .16 | .86 |
| Naloxone + blurred vision | .05 (.05) | .16 (.12) | .10 (.06) | .44 | .65 |
| Saline + sedation | .25 (.14) | .11 (.07) | .35 (.15) | .91 | .41 |
| Naloxone + sedation | .25 (.17) | .37 (.19) | .65 (.25) | .50 | .61 |
| Saline + nausea | 0 | 0 | .05 (.05) | .97 | .38 |
| Naloxone + nausea | .05 (.05) | .11 (.07) | 0 | 1.10 | .34 |
| Saline + dizziness | .15 (.11) | .16 (.12) | .20 (.12) | .06 | .95 |
| Naloxone + dizziness | .20 (.12) | .21 (.16) | .30 (.18) | .13 | .88 |
| Saline + headache | 0 | .05 (.05) | .10 (.07) | 1.02 | .37 |
| Naloxone + headache | 0 | .21 (.12) | .10 (.10) | 1.35 | .27 |
| Saline + drowsy | 1.84 (2.12) | 1.47 (1.87) | 1.85 (2.13) | .21 | .81 |
| Naloxone + drowsy | 2.11 (2.31) | 1.68 (1.86) | 2.75 (2.67) | 1.11 | .34 |
| Saline + excited | 1.53 (1.90) | 2.32 (2.50) | 1.60 (1.64) | .88 | .42 |
| Naloxone + excited | 1.42 (1.95) | 1.84 (2.32) | 1.85 (1.81) | .40 | .67 |
| Saline + feeble | 2.21 (2.30) | 1.37 (1.57) | 1.30 (1.84) | 1.33 | .27 |
| Naloxone + feeble | 2.00 (2.08) | 1.79 (2.39) | 2.65 (2.83) | .72 | .49 |
| Saline + clear-headed | 8.39 (1.85) | 8.58 (2.06) | 8.95 (1.82) | .43 | .66 |
| Naloxone + clear-headed | 8.06 (2.21) | 7.95 (2.20) | 7.70 (3.16) | .72 | .49 |
| Saline + clumsy | 2.16 (2.89) | 1.68 (2.52) | 1.45 (1.79) | .43 | .66 |
| Naloxone + clumsy | 1.95 (2.09) | 1.63 (2.61) | 1.55 (2.11) | .09 | .91 |
| Saline + energetic | 7.00 (2.54) | 6.42 (2.84) | 7.15 (2.16) | .45 | .64 |
| Naloxone + energetic | 7.32 (2.08) | 6.26 (2.90) | 6.15 (2.70) | 1.43 | .25 |
| Saline + discontented | 1.32 (1.83) | 1.32 (1.70) | 1.10 (1.07) | .13 | .88 |
| Naloxone + discontented | 1.37 (1.71) | 1.95 (2.92) | .75 (1.12) | 1.70 | .19 |
| Saline + tranquil | 8.42 (1.87) | 9.00 (1.67) | 7.90 (2.27) | 1.54 | .22 |
| Naloxone + tranquil | 8.53 (1.84) | 8.47 (1.87) | 8.85 (1.39) | .25 | .78 |
| Saline + quick-witted | 7.58 (2.32) | 8.26 (1.66) | 8.10 (2.36) | .53 | .59 |
| Naloxone + quick-witted | 7.21 (2.20) | 7.63 (2.52) | 7.30 (3.23) | .09 | .92 |
| Saline + relaxed | 8.42 (2.12) | 7.53 (2.88) | 8.35 (1.60) | .94 | .40 |
| Naloxone + relaxed | 8.32 (2.03) | 8.05 (2.44) | 7.05 (2.70) | 1.71 | .19 |
| Saline + dreamy | 3.16 (2.91) | 1.79 (2.20) | 1.55 (1.88) | 2.60 | .08 |
| Naloxone + dreamy | 2.58 (2.67) | 2.21 (2.15) | 2.65 (3.07) | .13 | .88 |
| Saline + proficient | 8.37 (1.71) | 8.74 (1.49) | 9.00 (.92) | 1.00 | .38 |
| Naloxone + proficient | 8.37 (1.64) | 8.58 (1.71) | 8.80 (1.61) | .23 | .80 |
| Saline + sad | 1.79 (2.23) | 1.11 (1.73) | 1.40 (2.14) | .54 | .59 |
| Naloxone + sad | 2.11 (2.13) | 2.00 (2.69) | 1.05 (1.32) | 1.34 | .27 |
| Saline + amicable | 8.42 (1.92) | 9.16 (1.26) | 9.30 (1.08) | 2.01 | .14 |
| Naloxone + amicable | 8.63 (1.74) | 8.47 (2.17) | 8.85 (1.42) | .22 | .81 |
| Saline + bored | 2.11 (2.45) | 2.53 (2.34) | 1.80 (2.44) | .45 | .64 |
| Naloxone + bored | 1.63 (1.57) | 2.32 (2.26) | 2.50 (2.31) | 1.18 | .32 |
| Saline + gregarious | 7.53 (1.95) | 7.89 (2.13) | 7.60 (2.04) | .17 | .84 |
| Naloxone + gregarious | 7.58 (2.27) | 6.95 (2.51) | 7.60 (1.82) | .66 | .52 |
| Saline + insecure | 1.32 (1.92) | .63 (.90) | .45 (.76) | 2.42 | .10 |
| Naloxone + insecure | 1.26 (2.26) | 1.26 (1.66) | .75 (1.16) | .51 | .60 |
All responses are mean (SD) unless otherwise specified
Sample Size Determination
Based on our recent study [83], a sample size of 18 participants per group provided 85% power with an alpha level set at 0.05 to detect a partial reversal of pain reductions corresponding to an large effect size of ηp2 =.18 (g-power software 3.0.1). However, to better account for potential variability in naloxone effectiveness, we recruited a total of 60 participants (20/group). This sample size was calculated to provide > 90% power to detect a significant group × session × pain type interaction.
Stimuli
As previously conducted [83; 87], a thermal sensory analyzer (TSA-II, Medoc, Inc., Raleigh, NC) fitted with a 16 mm2 surface area thermal probe delivered all thermal stimuli. To minimize habituation, the thermal probe was moved to a new stimulation site after each experimental series. Subjects were free to escape stimuli at any time by lifting their limb away from the probe-holder.
Psychophysical Assessment of Pain
As previously employed [83; 84], pain intensity and unpleasantness ratings were assessed with a 15 cm sliding visual analog scale (VAS) [67]. The minimum rating (“0”) was designated as “no pain sensation” and “not at all unpleasant” whereas the maximum (“10”) was labeled as “most intense pain sensation imaginable” or “most unpleasant sensation imaginable”, respectively.
Drug Administration
The weight of each participant (measured at session 1) was used to calculate their appropriate saline/naloxone dosage. A 0.15 mg/kg bolus dose of naloxone dissolved in 25 ml normal saline (Naloxone HCI, Amphastar Pharmaceuticals, Inc., Rancho Cucamonga, California) or 25 ml normal saline alone was administered over 10 minutes via an intravenous (IV) line inserted into the antecubital vein of the non-dominant arm. Onset of naloxone-induced opioidergic antagonism occurs within two minutes and exhibits an average half-life of 64 minutes (see Summary of Product, Amphastar Pharmaceuticals, Inc.). Importantly, the duration of the experiment from the onset of naloxone infusion to completion was 22 minutes. To ensure that naloxone antagonized opioid receptors throughout the entire experimental session, we administered a supplementary continuous IV infusion of 0.1 mg/kg/hour naloxone or saline immediately after bolus infusion ceased till the end of the experimental session (~12 minutes). This large dose comprehensively antagonizes endogenous opioids [48] and is larger than dosages used to reverse analgesia produced by placebo [4; 11; 41; 49], electrical stimulation of periventricular gray matter [1; 45], transcranial magnetic stimulation [28; 73], acupuncture [53], or hypnosis [70]. Only the study physician, pharmacist, and study-coordinator were aware of participant-drug assignment. Subjects, nurses, and experimenters were blinded to drug assignment.
Experimental Design
Experimental Session 1 (Psychophysical Training + Baseline Pain Testing):
Participants first reported to Wake Forest’s Clinical Research Unit (CRU) and were positioned in a custom-made chair with their right calf placed on a custom-made thermal probe holder. Study volunteers were initially familiarized with 32, 5s duration thermal stimuli (35 – 49°C) and use of the VAS [87]. Stimuli were delivered to the ventral aspect of the left forearm. We then assessed baseline psychophysical responses to noxious heat by administering a total of four heat series. Each of these heat series (4 min and 24 s duration) consisted of ten alternating 12 s plateaus of 49°C and 35°C stimulation delivered to the back of the right calf. The thermal probe was moved to a different region on the back of the right calf after completion of each heat series. VAS pain intensity and unpleasantness ratings were collected after each series.
Throughout session 1, participants were instructed to remain still and sit quietly. After the first two heat series, participants were instructed to continue to sit quietly for 10 minutes to control for the time elapsed in the subsequent heat testing-pharmacologic experimental sessions. After 10 minutes passed, two heat series were administered and pain ratings were collected. After successful completion of sensory testing, participants were informed of their respective group assignment.
Experimental Session 2–5: Group Training Sessions
Certified meditation teachers facilitated all the interventions.
Mindfulness Meditation Training Regimen:
As in our previous studies [84; 87], subjects in the mindfulness meditation group participated in four separate sessions (20 minutes each) of mindfulness-based mental training. Across all of the meditation training sessions, subjects were instructed to focus on the changing sensations of the breath while employing a non-evaluative cognitive state. Mindfulness-based instructions emphasized acknowledging arising thoughts, feelings and/or emotions without judgment or emotional reaction and to “simply return their attention back to the breath sensations” whenever such discursive events occurred. Subjects were instructed not to explicitly change their breathing rate and to practice outside of training to reduce inter-individual variability in practice time.
Sham-mindfulness Meditation Training Regimen:
As previously conducted [84; 86], the main purpose of this intervention was to lead subjects to believe they were practicing mindfulness meditation without instructions related to mindfully attending to the breath in a non-evaluative manner. Participants were first told they were randomly assigned to the mindfulness meditation group. In each of the four training sessions (20 minutes each), subjects were instructed to sit with a straight posture, close their eyes, and every 2–3 minutes were instructed to take a deep, slow breath “as we sit here in meditation” [84; 86]. Importantly, there were no instructions related to attending to the breath sensations and/or to reduce judgments/reactions to arising sensory events. All aspects of the mindfulness meditation training were matched to the sham-mindfulness meditation intervention including training room, posture, and training facilitators.
Slow-Breathing Exercise Training Regimen:
The purpose of the slow breathing exercise regimen was to train individuals to independently lower their breathing rate in a rhythmic fashion. In training sessions 1–3 (20 minutes each), a validated [19] slow-breathing paced breathing program entitled EZ-air Light™ (Thought Technology, Montreal, QC) was used which utilized a fluctuating light to guide participants to lower their respective respiration at a rate of 5.5 breaths/minute and slow breathing (inspiration total = 1.5 seconds). In session 4, subjects were instructed to practice slow, paced breathing without the EZ-air Light™ device and eyes closed.
Experimental Session 6 and 7:
A double-blind crossover design was used to evaluate the effect of the opioid antagonist, naloxone and placebo-saline across two separate sessions. Ten subjects in each group were administered naloxone in Session 6 and saline in Session 7, and vice versa.
After successful completion of each group’s respective intervention, subjects reported to the CRU to complete Session 6 and 7 on separate days (separated by 3 −10 days). In both sessions, CRU nurses first administered a urine drug screen to confirm that subjects were not using opioids and to minimize withdrawal symptoms. No subjects tested positive for opioids. Weight was subsequently measured to confirm the prescribed drug dosage. A CRU nurse then inserted an intravenous (IV) catheter into the non-dominant arm of each subject. Blood pressure, respiration rate, oxygen saturation, and heart rate data were systematically monitored and recorded throughout the experimental session (only respiration rate will be presented here). The same testing procedures occurred on Session 6 and 7. The only difference was the administration of either the assigned naloxone or saline
Testing Phase
Rest:
Subjects were instructed to relax without explicitly altering their respiration rate. Two “heat” series were then administered and VAS pain ratings were collected after each series.
Naloxone/saline administration:
After the first two heat series, a research nurse administered the naloxone (0.15 mg/kg) or placebo-saline bolus and all subjects in the mindfulness and sham-mindfulness meditation groups were instructed to “begin meditation and to continue until the end of the experiment”. Subjects in the slow-breathing exercise group were instructed to “begin the slow-breathing practice matched to that in your training sessions until the end of the experiment.” The EZ-Light stimulus was not used during the experimental sessions to minimize distraction effects [15]. Participants were provided 10 minutes to practice their respective manipulation during bolus administration prior to heat stimulation [83].
Manipulation:
After bolus delivery, the maintenance infusion (0.1mg/kg/hour) was administered until the end of the study. Two more heat series were delivered as participants continued to practice their respective manipulation. VAS pain ratings were collected after each heat series. Subjects were then queried for potential naloxone-related symptoms.
Analysis of Behavioral Data
Behavioral data were analyzed with SPSS 26.0 software (IBM, Armonk, New York, USA).
Pain Ratings
The primary outcomes of the study were VAS pain intensity and unpleasantness ratings. A 3 (group) × 2 (pain type; pain intensity vs. unpleasantness) × 3 (no-infusion vs. saline vs. naloxone session) × 2 (within session pre vs. post pain ratings) repeated measures ANOVA (RM ANOVA) was conducted to determine if change in pain varied by group during saline and naloxone administration. Planned analyses examined the percent change in pain ratings between rest (pre) and manipulation (post) in the naloxone and saline infusion sessions, respectively. Significant (p < .05) main effects and interactions were investigated with simple effects tests [24; 74].
Respiration Rate
A three-factor ANOVA examined if the percent change in respiration rate (manipulation-induced respiration – rest-induced respiration rate /rest-induced respiration rate), controlling for drug order, varied by group and drug administration. Significant main effects and interactions were investigated with simple effects tests.
Secondary Outcomes
Demographics and Naloxone Symptoms
Univariate ANOVA analyses examining potential group differences on demographics, drug dosage, and naloxone symptoms checklist [12; 29; 83] were conducted.
Qualitative assessment of group manipulations
After session 7, subjects were asked, “what did you do during your practice (slow-breathing, meditation)?” We examined the frequency of subject responses, per group, that stated whether attention or focus on the breath was employed during each respective manipulation. A one-way ANOVA tested for significant between group differences. Significant main effects were investigated with planned post hoc tests (least significant difference) [24; 74]. An exploratory bivariate correlational analysis was also conducted to determine if self-reported focus on the breath, across all individuals, predicted percent changes in pain intensity ratings during saline and naloxone infusion, respectively.
Naloxone-related side effects
After completion of session 7 and 8, subjects rated 17-items [12; 29; 64; 83] assessing adverse effects of naloxone. Each symptom was rated as “inexistent” (0) - “extremely strong” (6). One-way ANOVAs tested for significant between group differences. After each infusion session, participants provided a “yes” or “no” response to the following assessment, “are you aware if you received naloxone or saline?”
RESULTS
Pain Ratings
Primary Analyses
Slow-breathing and mindfulness-induced pain reductions were not reversed by opioidergic antagonism
Post-intervention session:
There was a significant group × pain type × session × within session pre vs. post pain ratings interaction, F (4, 112) = 3.20, p = .02, η2p= .10. To interpret the 4-way interaction, a 3 (group) × 2 (percent Δ in VAS pain intensity vs. unpleasantness) × 2 (saline vs. naloxone) RM ANOVA was conducted and exhibited a significant session × pain rating type × group interaction effect, F (2, 56) = 4.08, p = .02, η2p= .13.
To translate this interaction and to test our primary aims, simple effects tests were performed and revealed no significant differences in the percent change in pain intensity (p = .79; 95% CI −12%; 16%) and pain unpleasantness (p = .76; CI −16%; 21%) ratings during mindfulness meditation between saline (intensity = −7%; unpleasantness = −18%) and naloxone (intensity = −5%; unpleasantness = −15%) sessions (Figure 2; Table 2). There were no significant differences in pain intensity (p = .79; CI −16%; 12%) and pain unpleasantness (p = .41; CI −25%; 10%) ratings during slow-breathing between saline (intensity = −10%; unpleasantness = −11%) and naloxone (intensity = −11%; unpleasantness = −18%) sessions. Sham-mindfulness meditation produced an increase in pain intensity ratings during saline (+9%) and naloxone (+9%) infusion sessions, and there were no significant differences between sessions (p = .93). Importantly, sham-mindfulness based pain unpleasantness reductions were reversed (p = .02; CI −3%; 38%) by naloxone (+12%) when compared to saline (−8%) infusion (Figure 2).
Figure 2.
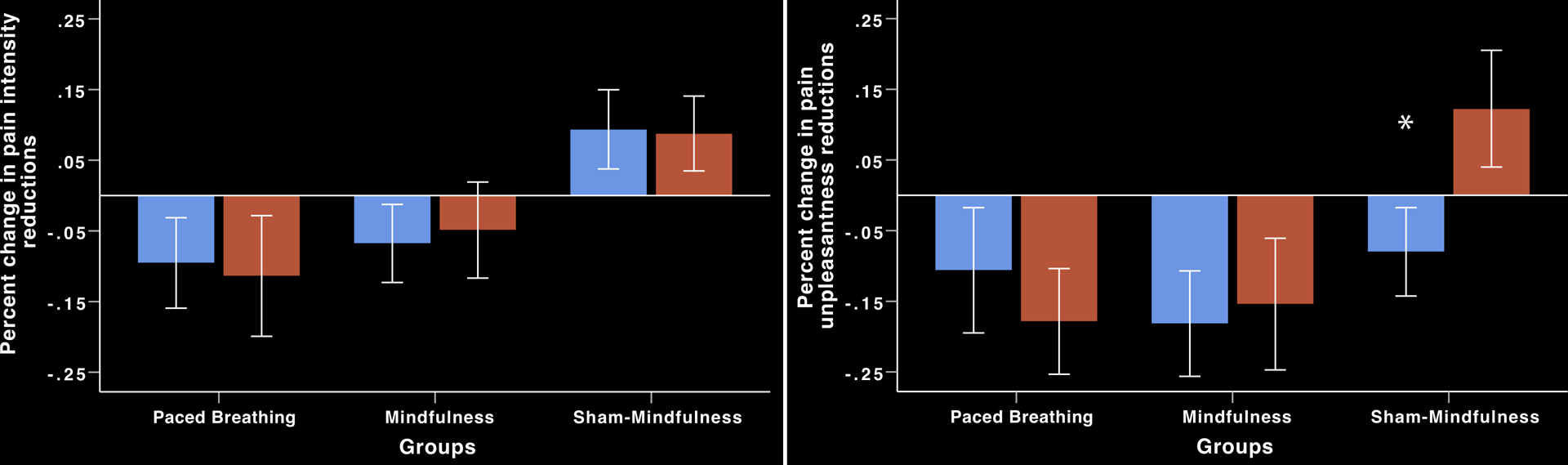
Percent change in pain ratings from rest to manipulation during noxious heat stimulation and saline (blue) and naloxone (red) infusion (± 95% confidence intervals). Left graph: There were no significant differences in pain intensity reductions between saline and naloxone infusion during mindfulness meditation or slow-paced, breathing. Sham-mindfulness did not reduce pain intensity ratings (p > .4). Right graph: There were no significant differences in pain unpleasantness reductions between saline and naloxone infusion during mindfulness meditation or slow-paced, breathing. *Sham-mindfulness reduced pain unpleasantness ratings during saline infusion but naloxone infusion reversed sham-mindfulness induced pain unpleasantness reductions (p =.02).
Table 2.
VAS pain intensity and pain unpleasantness ratings during saline and naloxone infusion sessions during Session 6 & 7
| Slow breathing* | Mindfulness* | Sham-mindfulness* | F | P value | |
|---|---|---|---|---|---|
| Saline pain intensity- rest | 4.60 (.35) | 3.95 (.35) | 3.56 (.40) | 2.08 | .14 |
| Saline pain intensity -manipulation | 4.16 (.44) | 3.55 (.33) | 3.73 (.41) | .63 | .53 |
| Naloxone pain intensity- rest | 4.95 (.49) | 4.34 (.47) | 3.67 (.39) | 2.04 | .14 |
| Naloxone pain intensity- manipulation | 4.19 (.51) | 3.87(.40) | 3.97 (.43) | .13 | .88 |
| Saline pain unpleasantness - rest | 4.59 (.41) | 3.97 (.45) | 3.87 (.45) | .82 | .45 |
| Saline pain unpleasantness - manipulation | 4.03 (.49) | 3.00 (.37) | 3.29 (.42) | 1.51 | .23 |
| Naloxone pain unpleasantness - rest | 4.94 (.52) | 4.22 (.57) | 3.73 (.45) | 1.41 | .25 |
| Naloxone pain unpleasantness -manipulation | 4.04 (.57) | 3.05 (.42) | 3.85 (.44) | 1.15 | .32 |
Responses are: average pain rating on 0–10 scale (standard deviation)
Secondary Analyses
Pre-intervention session:
There were no significant between group differences in pain ratings during the pre-intervention, session 1, F(2, 56) = 1.53, p = .23, η2p= .05 (Table 1).
Although not registered in clinicaltrials.gov, the following supplementary simple effects tests were conducted to test the efficacy of each respective manipulation and to better interpret the primary aims of the study.
Mindfulness meditation group:
There was no significant change in pain intensity (p = .79; CI −.36; .47) and unpleasantness (p = .93; CI −.49; .54) ratings from pre to post during session 1.
In experimental session 7, mindfulness produced a significant reduction in pain unpleasantness ratings during saline (p = .01; CI −1.71; −.23) and naloxone (p = .006; CI −2.00; −.35) infusion sessions. However, mindfulness meditation did not significantly reduce pain intensity ratings during saline (p = .15; CI −.96; .15) and naloxone (p = .14; CI −1.09; .15) infusion sessions when compared to rest.
Slow-paced breathing group:
There was no significant change in pain intensity (p = .12; CI −.08; .72) and unpleasantness (p = .89; CI −.54; .47) ratings during session 1.
When compared to rest, slow-controlled breathing significantly reduced pain intensity ratings during naloxone administration (p = .01; CI −1.37; −.16) but not saline (p = .11; CI −.99; .10). Similarly, slow-paced breathing elicited a significant reduction in pain unpleasantness ratings during naloxone (p = .03; CI −1.70; −.09) but not saline (p = .13; CI −1.29; .16) infusion sessions.
Sham-mindfulness meditation group:
In session 1, there was no significant change in pain intensity (p = .75; CI −.34; .47) and unpleasantness (p = .61; CI −.38; .63) ratings.
Sham-mindfulness meditation increased pain intensity ratings across the saline (p = .53; CI −.37; .71) and naloxone (p = .33; CI .90; −.31) infusion sessions. Sham-mindfulness did not significantly reduce pain unpleasantness ratings during saline (p=.11; CI −1.30; .14) or naloxone (p=.76; CI −.68; .93) administration.
Respiration Rate
All group manipulations significantly reduced respiration rate by, on average, 19% from rest and these reductions did not vary by session, F(1, 55) = .42, p =.52, η2p= .008 or group, F(2, 55) = .16, p =.86, η2p= .006 (Table 3).
Table 3.
Respiration rate across during noxious heat and saline and naloxone infusion sessions
| Slow breathing | Mindfulness | Sham-mindfulness | |
|---|---|---|---|
| Saline - rest | 14.19 (.37) | 14.33 (.36) | 14.65 (.25) |
| Saline -manipulation | 11.54 (.53) | 12.23 (.42) | 12.13 (56) |
| Naloxone - rest | 13. 98 (.37) | 14.15 (.33) | 14.67 (.22) |
| Naloxone - manipulation | 10.56 (.58) | 11.31 (.51) | 11.76 (.35) |
Naloxone-related side effects
Naloxone did not produce potentially un-blinding subjective effects (ps > .24; Table 1). To further confirm this, all participants were also asked whether they “were able to identify if they received naloxone or saline?” All participants responded with a “no” in both infusion sessions.
Qualitative assessment of group manipulations
Nineteen out of the 20 slow-breathing, 19 out of the 19 mindfulness, and 6 out of the 20 sham-mindfulness group members reported “focusing on the breath” during their respective practices (Figure 3). There was significant between group difference, F(2,58)= 32.82, p <.001 on frequency of individuals reporting “focusing on the breath” driven by the sham-mindfulness group reporting lower focus on breath frequency when compared to the other groups (p < .001; Figure 3). Individuals, across groups, that self-reported focusing on the breath exhibited a greater percent decrease in pain intensity during saline (r = −.34, p = .009) and naloxone infusion (r = −.31, p = .02).
Figure 3.
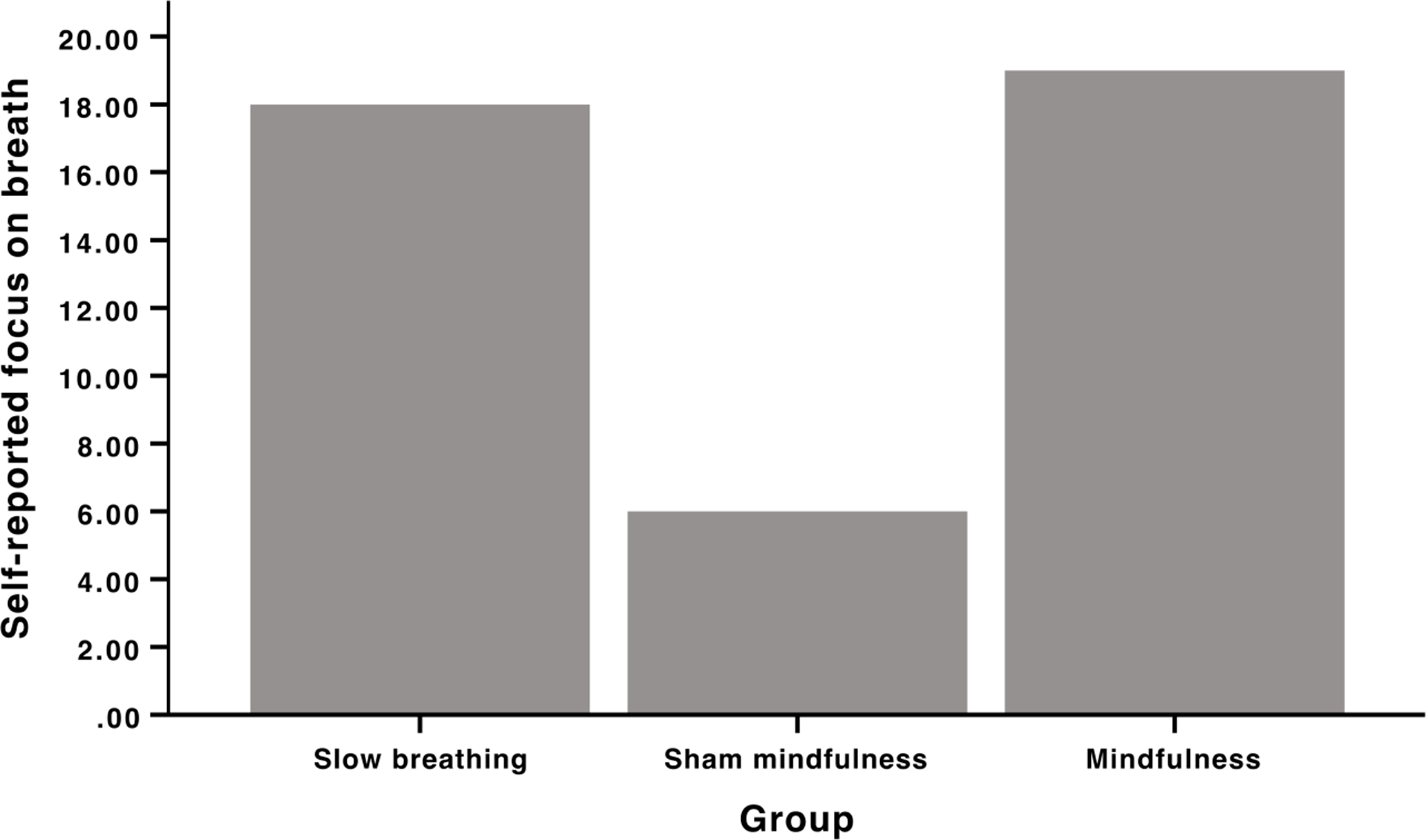
Qualitative assessment of “self-reported focus on the breath”. Nineteen out of the 20 slow-breathing, 19 out of the 19 mindfulness and 6 out of the 20 sham mindfulness group members reported “focusing on the breath”.
DISCUSSION
The ancient practice of slow, paced breathing [44] (i.e., qigong; pranayama; Buteyko; meditation) is one of the most widely used techniques to promote well-being [23]. Yet, the specific mechanisms supporting pain reductions by paced breathing are not known. To bridge this explanatory gap, the present crossover designed study employed double-blinded high-dose, IV administration of naloxone versus placebo saline to identify the potential role of endogenous opioids in pain reductions produced by mindful-based breath focus, paced breathing, and deep breathing without self-directed attention to the breath (i.e., sham-mindfulness), respectively. In the present study, pain reductions produced by mindfulness-based meditation and slow-paced breathing were insensitive to naloxone infusion when compared to saline administration (Figure 2). Importantly, pain unpleasantness reductions produced by sham-mindfulness meditation observed during saline infusion that were abolished by naloxone indicate a significant role for endogenous opioids in the analgesia produced by this behavioral paradigm. In contrast to our previous work [3; 84], sham-mindfulness did not lower pain intensity ratings. This surprising effect may be associated with the utilization of different a) experimental procedures (IV infusion) and b) meditation facilitators when compared to prior work [3; 84].
All three behavioral techniques significantly lowered respiration rate. Thus, we cannot explicitly conclude that slow-breathing practices, per se, lowered pain independently from endogenous opioids. Of critical importance, however, volitional attending to the breath/body sensations were a distinct operational feature particular to mindfulness and the slow-breathing group. All mindfulness subjects (100%) and the large majority of the slow-breathing (95%) subjects reported focusing on the breath during their respective practices and delivery of noxious heat stimuli. In contrast, only 30% of the sham-mindfulness practitioners reported attending specifically to sensations related to breathing (Figure 3). Sham-mindfulness is characterized as a meditative practice, but in contrast to mindfulness and paced breathing, is passively operationalized by slow-breathing in a non-focused cognitive stance likely engaging a combination of placebo and relaxation to alleviate pain. Taken together, we provide novel evidence that pain reductions achieved by volitional directed attention to, and regulation of, the breath sensations is not mediated by opioid-related mechanisms.
Self-directed attention to breath sensations bridges the interoceptive processes of bodily awareness (i.e., rise/fall of chest and abdomen; somatic sensations within the nostrils) and executive control of motoric processes regulating respiration with exteroceptive awareness of the individual’s internal and external sensory environment [33]. Attention to the breath is thought to increase meta-cognitive processes that promote reappraisal of sensations, feelings and emotions in a present-centered and non-reactive, non-judgmental fashion [25; 31; 88]. Therefore, the normally high salience of noxious thermal stimuli may be reappraised by voluntarily redirecting attention non-reactively to the breath thereby diminishing the sensory/affective intensity of said sensory events. This suggests a divided attention mechanism, however that is likely not the case, since distraction-induced pain relief has been repeatedly implicated to be associated with endogenous opioid systems mediated via descending inhibition at the level of the spinal cord [69; 77]. Yet, other effortful reappraisal-based practices reduce pain independent of opioidergically-driven systems [13; 52; 83]. Volitional attention to breath could also very likely engage a combination of interoceptively driven (i.e., breath; pain; mind wandering) mechanisms integrating 1) divided attention, 2) motor control (paced breathing), and 3) unique reappraisal processes [40].
Volitional attention to breath sensations is associated with higher-order, regulation of somatosensory processes when compared to sub-conscious breath regulation [20; 21]. This distinction is important to note because allocation of attention to continuously monitor the breath sensations promotes interoceptive and reappraisal-based processes [2; 9; 14; 26; 27; 32; 36; 43; 60; 61; 63]. Slow-breathing practices reliably reduce pain [5; 17; 47; 51; 82; 91], and endogenous opioids are engaged during volitional breath control [6; 7; 55; 59; 65; 72; 76]. This unique form of interoceptive control may engage a sensory filtering mechanism mediated by prefrontal control of serial thalamic projections to somatosensory targets [42; 57; 58]. We have postulated [83–85; 87; 89] that mindfulness meditations employs this PFC-thalamo-cortical facilitated pain modulatory pathway. This pathway is mediated by glutamatergic projects from the PFC to the GABA-ergic thalamic reticular nuclei to reduce modulate activity of ascending thalamocortical projections [42; 57; 58]. Attention to breath-related pain relief is insensitive to opioid antagonism, an effect potentiated by GABA-ergic inhibition of endogenous opioidergic transmission (and vice versa) at the level of the PAG [16; 78]. Supplementary evidence is provided from neuroimaging studies that reveal that mindful attention to breath reliably deactivates the PAG when compared to rest and sham-mindfulness mediation [84; 87]. Mindfulness-based analgesia is also repeatedly associated with greater PFC activation and thalamic deactivation [84; 87]. Thus, a key distinction between attending to breath, mindfully or otherwise, is mechanistically multi-modal integrating an interoceptive filtering mechanism between higher (cognitive control, reappraisal) and lower (slower respiration) analgesic properties. We postulate that the proposed PFC-thalamo-cortical gating mechanism is driven by executive shifts in attention between breath sensations, appraising distractions (noxious heat; ruminations) and reorienting attention back to breath to modulate pain prior to the elaboration of nociceptive information into a subjective sensory experience.
The present results provide novel evidence that controlling/attention to the breath reliably reduces pain independent of endogenous opioids. However, they are limited in scope in that tidal volume, minute ventilation, and arterial pO2 was not collected. These parameters could provide important insight into the physiological parameters supporting the interaction between respiration rate, pain and endogenous opioids. In our previous work [1; 10–12], mindfulness significantly reduced pain intensity and unpleasantness ratings. However, in the present study, mindfulness did not significantly reduce pain intensity but rather only pain unpleasantness ratings. Interestingly, the majority of mindfulness and pain studies demonstrate greater reductions in the affective dimension of pain when compared to pain intensity [1; 3–5; 7]. These studies generally examined a Vipassana (choice-less awareness) meditative practice. This technique is postulated to reflect the ability to attend to sensory aspects of pain and modulate affective appraisals of the corresponding experience. In the present study, we employed different mindfulness facilitators from on our previous work. Said teachers utilized a slightly different training format, in that they taught a more Vipassana (choice-less awareness) aligned mindfulness-based didactic in addition to our traditional Shamatha (focused attention) mindfulness training. As previous work [1; 3–5; 7] has demonstrated, this slight training deviation may explain why only pain unpleasantness reductions, in the present study, were significantly reduced from rest.
The effectiveness of slow breathing in reducing pain is critical for pain patients seeking a “user-friendly”, self-regulated, and non-opioidergic therapy. It is postulated that slow-paced breathing is easier to perform than mindful attention to the breath because it is not as cognitive demanding and less operationally nebulous [40]. This is an important caveat for pain conditions that exhibit cognitive deficiencies such as fibromyalgia, chronic fatigue syndrome and multiple sclerosis. However, mindfulness-based practices that engage cognitive and affective processing may provide more of an impact in clinical pain conditions that so often involve significant cognitive and affective dysregulation, producing more effective and longer-lasting pain analgesia over paced-breathing. Further, since meditative practitioners focus on regularly scheduled practices, it may allow individuals to build the skillset needed for pain analgesia as a function of greater meditative frequency. Nevertheless, we found that the increased capacity to focus on the breath with either paced breathing or mindfulness meditation following brief mental training can effectively reduce the subjective experience of pain and may engage a novel, pain modulatory pathway that bypasses opioidergically mediated descending inhibition of ascending nociceptive processes.
FUNDING AND DISCLOSURES
This work was supported by the NIH’s National Center for Complementary and Integrative Health (NCCIH) (K99/R00-AT008238; R21-AT007247; F32-AT006949; R01-AT009693; R21-AT010352, Dr. Zeidan) (F30-AT009165, Dr. Adler-Neal), (K23AT008406, Dr. Wells), the Mind and Life Institute, and the Wake Forest Translational Science Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no competing financial interests.
REFERENCES
- [1].Adams JE. Naloxone reversal of analgesia produced by brain stimulation in the human. Pain 1976;2(2):161–166. [PubMed] [Google Scholar]
- [2].Adler D, Herbelin B, Similowski T, Blanke O. Breathing and sense of self: visuo-respiratory conflicts alter body self-consciousness. Respiratory physiology & neurobiology 2014;203:68–74. [DOI] [PubMed] [Google Scholar]
- [3].Adler-Neal AL, Waugh CE, Garland EL, Shaltout HA, Diz DI, Zeidan F. The role of heart rate variability in mindfulness-based pain relief. J Pain 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 1999;19(1):484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Arsenault M, Ladouceur A, Lehmann A, Rainville P, Piche M. Pain modulation induced by respiration: phase and frequency effects. Neuroscience 2013;252:501–511. [DOI] [PubMed] [Google Scholar]
- [6].Atweh SF, Kuhar MJ. Autoradiographic localization of opiate receptors in rat brain. II. The brain stem. Brain Res 1977;129(1):1–12. [DOI] [PubMed] [Google Scholar]
- [7].Atweh SF, Kuhar MJ. Distribution and physiological significance of opioid receptors in the brain. Br Med Bull 1983;39(1):47–52. [DOI] [PubMed] [Google Scholar]
- [8].Bandura A, O’Leary A, Taylor CB, Gauthier J, Gossard D. Perceived self-efficacy and pain control: opioid and nonopioid mechanisms. J Pers Soc Psychol 1987;53(3):563–571. [DOI] [PubMed] [Google Scholar]
- [9].Barttfeld P, Wicker B, McAleer P, Belin P, Cojan Y, Graziano M, Leiguarda R, Sigman M. Distinct patterns of functional brain connectivity correlate with objective performance and subjective beliefs. Proceedings of the National Academy of Sciences of the United States of America 2013;110(28):11577–11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 1984;7:309–338. [DOI] [PubMed] [Google Scholar]
- [11].Benedetti F, Arduino C, Amanzio M. Somatotopic activation of opioid systems by target-directed expectations of analgesia. J Neurosci 1999;19(9):3639–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bentley P, Husain M, Dolan RJ. Effects of cholinergic enhancement on visual stimulation, spatial attention, and spatial working memory. Neuron 2004;41(6):969–982. [DOI] [PubMed] [Google Scholar]
- [13].Berna C, Leknes S, Ahmad AH, Mhuircheartaigh RN, Goodwin GM, Tracey I. Opioid-Independent and Opioid-Mediated Modes of Pain Modulation. The Journal of neuroscience : the official journal of the Society for Neuroscience 2018;38(42):9047–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Berner LA, Simmons AN, Wierenga CE, Bischoff-Grethe A, Paulus MP, Bailer UF, Ely AV, Kaye WH. Altered interoceptive activation before, during, and after aversive breathing load in women remitted from anorexia nervosa. Psychological medicine 2018;48(1):142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Birnie KA, Chambers CT, Spellman CM. Mechanisms of distraction in acute pain perception and modulation. Pain 2017;158(6):1012–1013. [DOI] [PubMed] [Google Scholar]
- [16].Bramham CR, Sarvey JM. Endogenous activation of mu and delta-1 opioid receptors is required for long-term potentiation induction in the lateral perforant path: dependence on GABAergic inhibition. J Neurosci 1996;16(24):8123–8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Busch V, Magerl W, Kern U, Haas J, Hajak G, Eichhammer P. The effect of deep and slow breathing on pain perception, autonomic activity, and mood processing--an experimental study. Pain Med 2012;13(2):215–228. [DOI] [PubMed] [Google Scholar]
- [18].Butland RJ, Woodcock AA, Gross ER, Geddes DM. Endogenous opioids (endorphins) and the control of breathing. N Engl J Med 1981;305(18):1096. [DOI] [PubMed] [Google Scholar]
- [19].Chalaye P, Goffaux P, Lafrenaye S, Marchand S. Respiratory effects on experimental heat pain and cardiac activity. Pain Med 2009;10(8):1334–1340. [DOI] [PubMed] [Google Scholar]
- [20].Chan PY, Cheng CH, Jhu YJ, Chen CL, von Leupoldt A. Being Anxious, Thinking Positively: The Effect of Emotional Context on Respiratory Sensory Gating. Frontiers in physiology 2016;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chan PY, Davenport PW. Respiratory-related-evoked potential measures of respiratory sensory gating in attend and ignore conditions. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society 2009;26(6):438–445. [DOI] [PubMed] [Google Scholar]
- [22].Cherkin DC, Sherman KJ, Balderson BH, Cook AJ, Anderson ML, Hawkes RJ, Hansen KE, Turner JA. Effect of Mindfulness-Based Stress Reduction vs Cognitive Behavioral Therapy or Usual Care on Back Pain and Functional Limitations in Adults With Chronic Low Back Pain: A Randomized Clinical Trial. JAMA 2016;315(12):1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Clarke TC, National Center for Health Statistics (U.S.). Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS data brief,. pp. 1 online resource (7 pages, 1 unnumbered page). [PubMed] [Google Scholar]
- [24].Cohen BH, Lea RB. Essentials of statistics for the social and behavioral sciences. Hoboken, N.J.: Wiley, 2004. [Google Scholar]
- [25].Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosom Med 2007;69(6):560–565. [DOI] [PubMed] [Google Scholar]
- [26].Critchley HD, Nicotra A, Chiesa PA, Nagai Y, Gray MA, Minati L, Bernardi L. Slow breathing and hypoxic challenge: cardiorespiratory consequences and their central neural substrates. PLoS One 2015;10(5):e0127082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Daubenmier J, Sze J, Kerr CE, Kemeny ME, Mehling W. Follow your breath: respiratory interoceptive accuracy in experienced meditators. Psychophysiology 2013;50(8):777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].de Andrade DC, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Neuropharmacological basis of rTMS-induced analgesia: the role of endogenous opioids. Pain 2011;152(2):320–326. [DOI] [PubMed] [Google Scholar]
- [29].Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 2009;63(4):533–543. [DOI] [PubMed] [Google Scholar]
- [30].Farb NA, Segal ZV, Anderson AK. Mindfulness meditation training alters cortical representations of interoceptive attention. Soc Cogn Affect Neurosci 2013;8(1):15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Farb NA, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, Anderson AK. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience 2007;2(4):313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Faull OK, Cox PJ, Pattinson KTS. Cortical processing of breathing perceptions in the athletic brain. NeuroImage 2018;179:92–101. [DOI] [PubMed] [Google Scholar]
- [33].Faull OK, Subramanian HH, Ezra M, Pattinson KTS. The midbrain periaqueductal gray as an integrative and interoceptive neural structure for breathing. Neuroscience and biobehavioral reviews 2019;98:135–144. [DOI] [PubMed] [Google Scholar]
- [34].Frid M, Singer G. Hypnotic analgesia in conditions of stress is partially reversed by naloxone. Psychopharmacology (Berl) 1979;63(3):211–215. [DOI] [PubMed] [Google Scholar]
- [35].Gard T, Holzel BK, Sack AT, Hempel H, Lazar SW, Vaitl D, Ott U. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb Cortex 2012;22(11):2692–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Garfinkel SN, Manassei MF, Hamilton-Fletcher G, In den Bosch Y, Critchley HD, Engels M. Interoceptive dimensions across cardiac and respiratory axes. Philosophical transactions of the Royal Society of London Series B, Biological sciences 2016;371(1708). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Garland EL, Brintz CE, Hanley AW, Roseen EJ, Atchley RM, Gaylord SA, Faurot KR, Yaffe J, Fiander M, Keefe FJ. Mind-Body Therapies for Opioid-Treated Pain: A Systematic Review and Meta-analysis. JAMA Intern Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Garland EL, Gaylord SA, Palsson O, Faurot K, Douglas Mann J, Whitehead WE. Therapeutic mechanisms of a mindfulness-based treatment for IBS: effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J Behav Med 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Grant JA, Rainville P. Pain sensitivity and analgesic effects of mindful states in Zen meditators: a cross-sectional study. Psychosomatic Medicine 2009;71(1):106–114. [DOI] [PubMed] [Google Scholar]
- [40].Grant JA, Zeidan F. Employing pain and mindfulness to understand consciousness: a symbiotic relationship. Curr Opin Psychol 2019;28:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Grevert P, Albert LH, Goldstein A. Partial antagonism of placebo analgesia by naloxone. Pain 1983;16(2):129–143. [DOI] [PubMed] [Google Scholar]
- [42].Guglietti CL, Daskalakis ZJ, Radhu N, Fitzgerald PB, Ritvo P. Meditation-related increases in GABAB modulated cortical inhibition. Brain Stimul 2013;6(3):397–402. [DOI] [PubMed] [Google Scholar]
- [43].Hassanpour MS, Simmons WK, Feinstein JS, Luo Q, Lapidus RC, Bodurka J, Paulus MP, Khalsa SS. The Insular Cortex Dynamically Maps Changes in Cardiorespiratory Interoception. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2018;43(2):426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ho PY, Lisowski FP. A brief history of Chinese medicine. Singapore; River Edge, N.J.: World Scientific, 1997. [Google Scholar]
- [45].Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science 1977;197(4299):183–186. [DOI] [PubMed] [Google Scholar]
- [46].King CD, Goodin B, Kindler LL, Caudle RM, Edwards RR, Gravenstein N, Riley JL 3rd, Fillingim RB. Reduction of conditioned pain modulation in humans by naltrexone: an exploratory study of the effects of pain catastrophizing. J Behav Med 2013;36(3):315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Larsen KL, Brilla LR, McLaughlin WL, Li Y. Effect of Deep Slow Breathing on Pain-Related Variables in Osteoarthritis. Pain Res Manag 2019;2019:5487050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Levine JD, Gordon NC. Method of administration determines the effect of naloxone on pain. Brain Res 1986;365(2):377–378. [DOI] [PubMed] [Google Scholar]
- [49].Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet 1978;2(8091):654–657. [DOI] [PubMed] [Google Scholar]
- [50].Lutz A, McFarlin DR, Perlman DM, Salomons TV, Davidson RJ. Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage 2013;64:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Martin SL, Kerr KL, Bartley EJ, Kuhn BL, Palit S, Terry EL, DelVentura JL, Rhudy JL. Respiration-induced hypoalgesia: exploration of potential mechanisms. J Pain 2012;13(8):755–763. [DOI] [PubMed] [Google Scholar]
- [52].May LM, Kosek P, Zeidan F, Berkman ET. Enhancement of Meditation Analgesia by Opioid Antagonist in Experienced Meditators. Psychosom Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mayer DJ, Price DD, Rafii A. Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res 1977;121(2):368–372. [DOI] [PubMed] [Google Scholar]
- [54].McKay LC, Evans KC, Frackowiak RS, Corfield DR. Neural correlates of voluntary breathing in humans. Journal of applied physiology 2003;95(3):1170–1178. [DOI] [PubMed] [Google Scholar]
- [55].McQueen DS. Opioid peptide interactions with respiratory and circulatory systems. Br Med Bull 1983;39(1):77–82. [DOI] [PubMed] [Google Scholar]
- [56].Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: a randomized controlled pilot study. Pain 2008;134(3):310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nakajima M, Schmitt LI, Halassa MM. Prefrontal Cortex Regulates Sensory Filtering through a Basal Ganglia-to-Thalamus Pathway. Neuron 2019;103(3):445–458 e410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci 2002;5(11):1203–1209. [DOI] [PubMed] [Google Scholar]
- [59].Pattinson KT, Governo RJ, MacIntosh BJ, Russell EC, Corfield DR, Tracey I, Wise RG. Opioids depress cortical centers responsible for the volitional control of respiration. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009;29(25):8177–8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Paulus MP. The breathing conundrum-interoceptive sensitivity and anxiety. Depression and anxiety 2013;30(4):315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Paulus MP, Flagan T, Simmons AN, Gillis K, Kotturi S, Thom N, Johnson DC, Van Orden KF, Davenport PW, Swain JL. Subjecting elite athletes to inspiratory breathing load reveals behavioral and neural signatures of optimal performers in extreme environments. PloS one 2012;7(1):e29394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Perlman DM, Salomons TV, Davidson RJ, Lutz A. Differential effects on pain intensity and unpleasantness of two meditation practices. Emotion 2010;10(1):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Petersen S, Schroijen M, Molders C, Zenker S, Van den Bergh O. Categorical interoception: perceptual organization of sensations from inside. Psychological science 2014;25(5):1059–1066. [DOI] [PubMed] [Google Scholar]
- [64].Petrovic P, Pleger B, Seymour B, Kloppel S, De Martino B, Critchley H, Dolan RJ. Blocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and losses. J Neurosci 2008;28(42):10509–10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Pokorski M, Grieb P, Wideman J. Opiate system influences central respiratory chemosensors. Brain Res 1981;211(1):221–226. [DOI] [PubMed] [Google Scholar]
- [66].Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science 2000;288(5472):1769–1772. [DOI] [PubMed] [Google Scholar]
- [67].Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain 1994;56(2):217–226. [DOI] [PubMed] [Google Scholar]
- [68].Salomons TV, Kucyi A. Does Meditation Reduce Pain through a Unique Neural Mechanism? J Neurosci 2011;31(36):12705–12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Buchel C. Attention modulates spinal cord responses to pain. Curr Biol 2012;22(11):1019–1022. [DOI] [PubMed] [Google Scholar]
- [70].Stephenson JB. Reversal of hypnosis-induced analgesia by naloxone. Lancet 1978;2(8097):991–992. [DOI] [PubMed] [Google Scholar]
- [71].Stone CI, Demchik-Stome DA, Horan JJ. Coping with pain: a component analysis of Lamaze and cognitive-behavioral procedures. J Psychosom Res 1977;21(6):451–456. [DOI] [PubMed] [Google Scholar]
- [72].Takita K, Herlenius EA, Lindahl SG, Yamamoto Y. Actions of opioids on respiratory activity via activation of brainstem mu-, delta- and kappa-receptors; an in vitro study. Brain Res 1997;778(1):233–241. [DOI] [PubMed] [Google Scholar]
- [73].Taylor JJ, Borckardt JJ, Canterberry M, Li X, Hanlon CA, Brown TR, George MS. Naloxone-Reversible Modulation of Pain Circuitry by Left Prefrontal rTMS. Neuropsychopharmacology 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Toothaker LE. Multiple Comparison Procedures, Vol. 07–089 Newbury Park, CA: Sage; 1993. [Google Scholar]
- [75].Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci 2002;22(7):2748–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Uhl GR, Goodman RR, Kuhar MJ, Childers SR, Snyder SH. Immunohistochemical mapping of enkephalin containing cell bodies, fibers and nerve terminals in the brain stem of the rat. Brain Res 1979;166(1):75–94. [DOI] [PubMed] [Google Scholar]
- [77].Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, Erhard P, Tolle TR. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain 2004;109(3):399–408. [DOI] [PubMed] [Google Scholar]
- [78].Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature 1997;390(6660):611–614. [DOI] [PubMed] [Google Scholar]
- [79].Villemure C, Ceko M, Cotton VA, Bushnell MC. Insular cortex mediates increased pain tolerance in yoga practitioners. Cereb Cortex 2014;24(10):2732–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A 2007;104(26):11056–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wallace BA. The Attention Revolution: Unlocking the Power of the Focused Mind. Somerville: Wisdom Publications, 2006. [Google Scholar]
- [82].Zautra AJ, Fasman R, Davis MC, Craig AD. The effects of slow breathing on affective responses to pain stimuli: an experimental study. Pain 2010;149(1):12–18. [DOI] [PubMed] [Google Scholar]
- [83].Zeidan F, Adler-Neal AL, Wells RE, Stagnaro E, May LM, Eisenach JC, McHaffie JG, Coghill RC. Mindfulness-Meditation-Based Pain Relief Is Not Mediated by Endogenous Opioids. J Neurosci 2016;36(11):3391–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, Coghill RC. Mindfulness Meditation-Based Pain Relief Employs Different Neural Mechanisms Than Placebo and Sham Mindfulness Meditation-Induced Analgesia. J Neurosci 2015;35(46):15307–15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zeidan F, Grant JA, Brown CA, McHaffie JG, Coghill RC. Mindfulness meditation-related pain relief: evidence for unique brain mechanisms in the regulation of pain. Neurosci Lett 2012;520(2):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zeidan F, Johnson SK, Gordon NS, Goolkasian P. Effects of brief and sham mindfulness meditation on mood and cardiovascular variables. J Altern Complement Med 2010;16(8):867–873. [DOI] [PubMed] [Google Scholar]
- [87].Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci 2011;31(14):5540–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zeidan F, Salomons T, Farris SR, Emerson NM, Adler-Neal A, Jung Y, Coghill RC. Neural Mechanisms Supporting the Relationship between Dispositional Mindfulness and Pain. Pain 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zeidan F, Vago DR. Mindfulness meditation-based pain relief: a mechanistic account. Ann N Y Acad Sci 2016;1373(1):114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci 2005;25(34):7754–7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zunhammer M, Eichhammer P, Busch V. Do cardiorespiratory variables predict the antinociceptive effects of deep and slow breathing? Pain Med 2013;14(6):843–854. [DOI] [PubMed] [Google Scholar]


