Abstract
Objective
The anterior insular cortex (AI), which is a part of the “salience network,” is critically involved during visual awareness, multisensory perception, and social and emotional processing, among other functions. In children and adolescents with autism spectrum disorders (ASDs), evidence has suggested aberrant functional connectivity (fc) of AI compared to typically developing (TD) peers. While recent studies have primarily focused on the functional connections between salience and social networks, much less is known about connectivity between AI and primary sensory regions, including visual areas, and how these patterns may be linked to autism symptomatology.
Method
The current investigation implemented functional magnetic resonance imaging (MRI) to examine resting state fc patterns of salience and visual networks in children and adolescents with ASDs compared to TD controls, and to relate them to behavioral measures.
Results
Functional underconnectivity was found in the ASD group between left AI and bilateral visual cortices. Moreover, in an ASD subgroup with more atypical visual sensory profiles, functional connectivity was positively correlated with abnormal social motivational responsivity.
Conclusion
Findings of reduced fc between salience and visual networks in ASDs potentially suggest deficient selection of salient information. Moreover, in children with ASDs who show strongly atypical visual sensory profiles, connectivity at seemingly more neurotypical levels may be paradoxically associated with greater impairment of social motivation.
Keywords: autism, vision, salience network, anterior insula, functional connectivity MRI
Introduction
The anterior insular cortex (AI) is critically involved in a multitude of sensory processes including visual perception (e.g., alertness and awareness; for a review, see1), multisensory integration,2–4 and oddball detection.2,5 In addition, this putative “hub” mediates interactions between brain networks involved in internally- and externally-oriented processing,6 and is implicated in social and emotional awareness,7 interoceptive processing,8,9 and general mediation of awareness.10
Likely related to these multifunctional roles, the AI, along with the anterior cingulate cortex, is considered a key node in the “salience network” (SN), which serves to identify salient stimuli and integrate sensory inputs with relevant homeostatic, visceral, and emotional information in order to guide behavior.8,11–13 Given the diverse functioning of AI and the central role it plays in salience processing,14 aberrations in functional connectivity (fc) or response patterns of this region are believed to contribute to the atypical salience processing and behavioral impairments observed in clinical populations such as autism.15–17 Autism spectrum disorders (ASDs) are pervasive neurodevelopmental disorders that are characterized by impairments in social communication, repetitive and restricted interests and behaviors, and abnormal sensory reactivity.18,19
In children and adolescents with ASDs, evidence has suggested aberrant connectivity of the SN and AI compared to typically developing (TD) peers. Magnetic resonance imaging (MRI) studies examining resting state fc have predominantly indicated underconnectivity within SN20–24 (but see25), as well as decreased connectivity between SN nodes and “social” brain regions such as the amygdala.20,26 In task-based neuroimaging studies of individuals with ASDs, findings have consistently revealed AI to be a locus of hypoactivity in a wide range of social cognitive paradigms27,28 (for meta-analysis, see29).
Extensive evidence suggests that visual processing may be relatively spared in ASDs, possibly including islands of superior function.30–33 While recent studies have primarily focused on the connectivity between salience, default mode, central-executive, and “social” networks,8,11,22,26,34 much less is known about fc patterns between salience and visual networks and their relation to autism symptomatology. This presents a critical gap in the literature, as atypical access to visual information may conceivably contribute to atypical SN function in ASDs. Therefore, resting state fc and behavioral measures in children and adolescents with ASDs were used to: a) investigate the links between salience and visual areas, and b) examine the relation between fc patterns of these regions and core symptomatology in autism. We predicted that cross-network fc between AI and visual nodes in ASDs would be atypically reduced, based on the predominance of findings indicating reduced between-network connectivity with SN regions (cf.25), and furthermore, that this atypicality would be related to autism symptom severity (i.e., greater fc abnormality would be correlated with increased severity).
Method
Participants
Fifty children and adolescents with ASDs and 43 TD participants between 7 and 18 years of age were included in the study. Diagnoses of ASDs were established using the Autism Diagnostic Interview-Revised (ADI-R35), the Autism Diagnostic Observation Schedule (ADOS36), and expert clinical decision according to DSM-5.18 Participants with known history of autism-related medical conditions (e.g., Fragile-X syndrome, tuberous sclerosis) or other neurological conditions (e.g., epilepsy, Tourette’s Syndrome) were excluded from the ASD group. Any TD participants with reported history of ASDs or any other neurological or psychiatric conditions were also excluded. There were no significant differences between the ASD and TD groups on gender, handedness, age, nonverbal IQ, or in-scanner head motion (Table 1). All TD participants scored above the cutoff for intellectual disability (Full Scale IQ > 70) on the Wechsler Abbreviated Scale of Intelligence–2nd ed. (WASI-II37). Hand preference was assessed with the Edinburgh Handedness Inventory.38 Parent-report measures including the Sensory Profile Caregiver Questionnaire (SP39), Social Responsiveness Scale (SRS40), and Behavior Rating Inventory of Executive Function–2nd ed. (BRIEF-241), were obtained for all participants (BRIEF-2 reports from 2 participants in the ASD1 subgroup, as well as 6 TD participants, were unavailable). The experimental protocol was approved by the Institutional Review Boards of San Diego State University and University of California San Diego. Each participant and caregiver provided written informed assent and consent, respectively, and was compensated for their time.
Table 1.
Participant Characterization per Group
| ASD (N = 50) | TD (N = 43) | Statistical Comparison | |||||
|---|---|---|---|---|---|---|---|
| Mean | (SD) | Range | Mean | (SD) | Range | ||
| Gender | 8 female participants | 5 female participants | χ2(1) = 0.37, p = 0.544 | ||||
| Handedness | 9 left | 5 left | χ2(1) = 0.73, p = 0.392 | ||||
| Age (years) | 13.5 | (2.7) | 7.4–18.0 | 13.6 | (2.6) | 8.1–17.7 | t(91) = −0.17, p = 0.865 |
| WASI-II | |||||||
| Verbal IQ | 100.5 | (17.6) | 59–147 | 106.8 | (9.2) | 87–127 | t(91) = −2.19, p = 0.032 |
| Non-verbal IQ | 105.3 | (18.2) | 53–140 | 104.5 | (13.4) | 62–129 | t(91) = 0.24, p = 0.810 |
| Full-Scale IQ | 103.3 | (16.9) | 61–141 | 106.1 | (10.6) | 79–126 | t(91) = −0.97, p = 0.333 |
| RMSD | 0.06 | (0.03) | 0.02–0.13 | 0.06 | (0.03) | 0.02–0.13 | t(91) = 0.57, p = 0.569 |
| ADOSa | |||||||
| Social interaction | 7.8 | (2.6) | 4–14 | – | – | – | – |
| Communication | 4.0 | (1.7) | 0–8 | – | – | – | – |
| Rep./Restricted | 2.2 | (1.5) | 0–5 | – | – | – | – |
| ADI-Rb | – | – | – | ||||
| Social interaction | 18.2 | (4.6) | 6–28 | – | – | – | – |
| Communication | 13.2 | (5.0) | 2–24 | – | – | – | – |
| Rep. behaviors | 5.8 | (2.2) | 1–12 | – | – | – | – |
| Psychotropic medication use | 26 reported | – | – | – | – | ||
| Comorbiditiesc | 20 reported | – | – | – | – | ||
| SRS | |||||||
| Awareness | 71.7 | (14.1) | 39–97 | 44.2 | (9.2) | 30–73 | t(90) = 10.81, p < 0.001 |
| Cognition | 75.9 | (11.8) | 45–99 | 43.6 | (6.2) | 36–56 | t(90) = 16.28, p < 0.001 |
| Communication | 80.8 | (11.3) | 62–110 | 43.6 | (5.5) | 36–60 | t(90) = 19.43, p < 0.001 |
| Motivation | 75.6 | (11.9) | 56–104 | 45.9 | (5.6) | 37–56 | t(90) = 15.05, p < 0.001 |
| ASD mannerisms | 86.4 | (15.8) | 58–126 | 44.6 | (5.9) | 40–65 | t(90) = 16.31, p < 0.001 |
Note: ADI-R = Autism Diagnostic Interview-Revised; ADOS = Autism Diagnostic Observation Schedule; ASD = autism spectrum disorder; IQ = intelligence quotient; Rep. = repetitive; RMSD = root mean squared displacement; SD = standard deviation; SRS = Social Responsiveness Scale; TD = typically developing; WASI-II = Wechsler Abbreviated Scale of Intelligence, 2nd edition.
Subdomain data not available for 1 participant with ASD.
Subdomain data not available for 1 participant with ASD.
Comorbid psychiatric conditions reported in the ASD group include attention-deficit/hyperactivity disorder (n = 11), depression (n = 5), and anxiety (n = 12); 6 out of 20 participants reported more than one diagnosed comorbid condition.
Data Acquisition and Image Preprocessing
Functional and structural imaging data were acquired on a GE 3T MR750 scanner (GE Healthcare, Chicago, Illinois) with an 8-channel head coil at the University of California San Diego Center for Functional MRI. High-resolution structural images were collected using a standard fast spoiled gradient echo T1-weighted sequence (TR: 8.136 ms; TE: 3.172 ms; flip angle: 8°; field of view [FOV]: 25.6 cm; matrix: 256 × 192; 172 slices; resolution: 1 mm3). Resting-state functional T2*-weighted images were obtained using a single-shot gradient-recalled, echo-planar pulse sequence. A single 6 min 10 s scan was acquired consisting of 185 whole-brain volumes (TR: 2000 ms; TE: 30 ms; slice thickness: 3.4 mm; flip angle: 90°; FOV: 22.0 cm; matrix: 64 × 64; in-plane resolution: 3.4 mm2). Field maps were acquired using a 2D interleaved single shot gradient echo pulse sequence (TR: 614 ms; TE: 6.5 ms; slice thickness: 3.4 mm; flip angle: 45°; receiver bandwidth: 31.25 kHz, FOV: 22.0 cm; matrix: 64 × 64; in-plane resolution: 3.4 mm2). To allow for equilibration effects, the first five time points were discarded, leaving 180 remaining time points for analysis. Participants were instructed: “Keep your eyes on the cross-hair, relax, let your mind wander, and try not to fall asleep.” Eye status was monitored throughout the duration of the scan using an in-bore video camera to ensure participant compliance.
Functional MRI data were processed with Analysis of Functional NeuroImages (AFNI42) and FMRI software library (FSL43). Functional images underwent slice-time correction in order to compensate for temporal offset between slice acquisitions, motion correction to align acquired volumes, and field-map correction to minimize any effects of magnetic field inhomogeneity. Registration between functional and structural images was conducted using FLIRT in FSL.44 Normalization of structural images to the MNI152 template was performed using FNIRT, a nonlinear registration tool in FSL, and the resultant transformation matrix was then applied to the functional images for similar spatial normalization; functional images were resampled to 3 mm isotropic voxels. Functional MRI time series were bandpass filtered (.008 < f < .08 Hz) using a second-order Butterworth filter to isolate spontaneous low-frequency blood oxygen level-dependent (BOLD) fluctuations.45 Datasets were effectively smoothed to a Gaussian full-width half-maximum (FWHM) of 6 mm. Masks of cerebral white matter and lateral ventricles for individual participants were created with FAST automated segmentation in FSL,46 and an averaged time-series for each was extracted. Time courses for white matter, ventricles, and six rigid-body motion parameters, each with their first derivatives, were bandpass filtered and input as nuisance regressors.
Several quality assurance measures were adopted during data preprocessing and analysis to minimize the impact of head motion on BOLD correlations.47,48 Specifically, six rigid-body motion parameters and their first derivatives were used as nuisance regressors. Time points with head motion > 0.5 mm, including two subsequent volumes, were censored and excluded from subsequent analyses. All participants selected for the current investigation retained a minimum of 80% of total time points (or 144 out of 180 volumes). Furthermore, while no significant group differences were discovered in the root mean squared displacement (RMSD; Table 1) – suggesting that any detected connectivity differences were unlikely related to differences in motion – statistical analyses included motion as a covariate. No age-related effects in terms of significant correlations with fc were detected at the group level, but given extensive evidence of age-related changes in fc in children and adolescents,16 age was nevertheless included as a covariate.
Regions-of-Interest Selection and Functional Connectivity Analyses
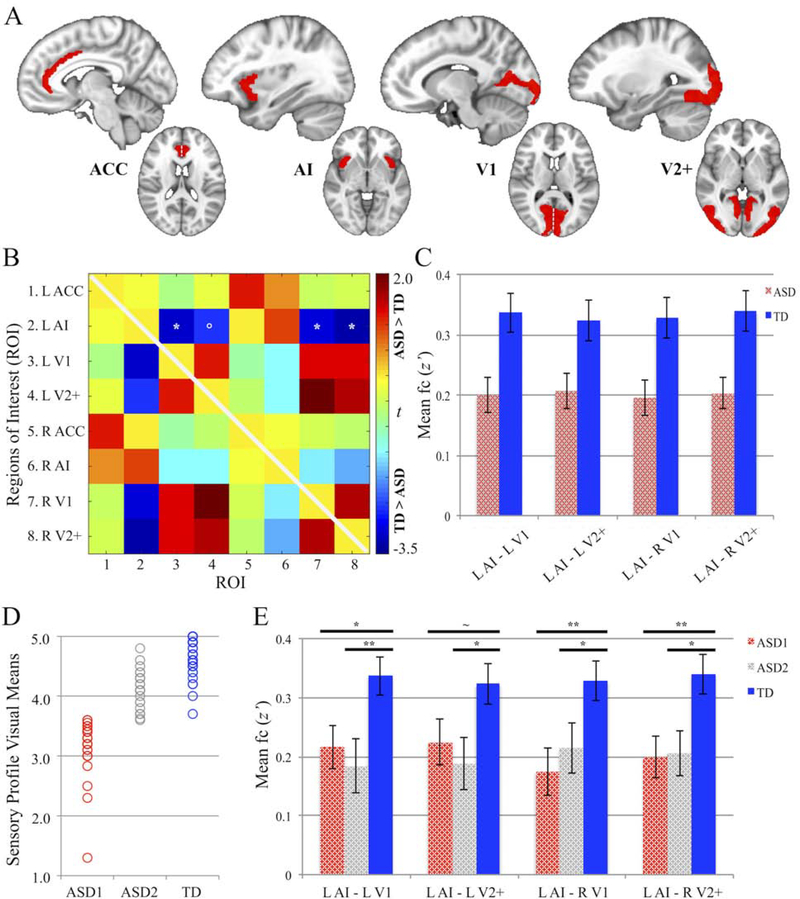
Regions-of-Interest (ROIs) were derived from the Harvard-Oxford (HO) and Jülich Histological (JH) atlases.49–53 Four areas were identified, separately in the left and right hemispheres: two in the SN – anterior insula (AI) and anterior cingulate cortex (ACC); and two in the visual network – primary visual cortex (V1) and secondary visual plus extrastriate cortices (V2+; Figure 1A). The insular region of the HO atlas was parcellated along its midpoint on the MNI y-axis (i.e., y ≥ 5) to obtain the anterior aspect of the insula. The ACC ROI was selected from the HO atlas. The ROIs derived from the HO atlas were grey-matter masked. The JH regional probability maps, which provide greater spatial specificity in sensory areas, were used to extract visual ROIs. After a winner-take-all step to determine the most likely identity of each voxel (e.g., V1 vs. V2), each JH probability map was further binarized and grey-matter masked. The V1 region, corresponding to Brodmann Area (BA) 17, was extracted as a single ROI, while our V2+ ROI combined the V2 mask (corresponding to BA 18) with extrastriate masks from V3v, V4, and V5. The resulting masks were then applied to the individual participants.
Figure 1.
Functional Connectivity Patterns Between Visual and Salience Network Regions
Note: (A) Regions-of-Interest (ROIs) derived from Harvard-Oxford and Jülich Histological atlases; dashed white line in axial slice (ACC, V1) delineates left (L) and right (R) hemisphere ROIs. (B) Between-group differences (ASD vs. TD) in ROI-to-ROI functional connectivity (t; both triangles identical); significance levels are FDR-corrected. (C) Group mean functional connectivity (z’) for four underconnected ROI-to-ROI pairings. (D) Two ASD subgroups (ASD1, ASD2) determined using median split of mean scores from the Sensory Profile Visual Processing section; visual means shown in comparison to TD group. (E) Between-group statistical comparisons of mean functional connectivity (z’) for the four underconnected ROI-to-ROI pairings in 2 ASD subgroups and TD group. Error bars represent SE. ACC = anterior cingulate cortex; AI = anterior insula; ASD = autism spectrum disorder; FDR = false discovery rate; TD = typically developing; V1 = primary visual cortex; V2+ = secondary visual and extrastriate cortices.
** p≤ .01, * p≤ .05, ~p ≤ .075, ° p≤ 0.10
Averaged time-series were extracted from each of the eight ROIs and Pearson-correlated with the time-series of all other ROIs. The resultant correlations were normalized using Fisher’s r-to-z’ transformations. Analyses of variance (ANOVA) models controlling for age and head motion RMSD were used to determine between-group effects for all unique ROI-to-ROI pairings, and were adjusted for multiple comparisons via FDR correction.54 Effect sizes (Cohen’s d) were also calculated for each pair.
Additionally, functional connectivity (z’) between all unique pairs of regions within each network (i.e., SN, visual) was averaged for each participant in the ASD and TD groups. Two-tailed t-tests were conducted to examine group differences in within-network connectivity strength.
Whole-brain analyses using the ROIs as seed regions were also performed. In group-level comparisons (ASD vs. TD), however, there were no seed-to-whole brain effects that survived cluster correction for any of the ROIs.
ASD Subgrouping
Given the known heterogeneity in autism, particularly regarding sensory processing abilities,55–57 two ASD subsamples (ASD1, ASD2) were determined using a median split of mean scores of the nine visual items from the Visual Processing section of the SP measure (Figure S1A, available online). This method of subgrouping was performed to maximize sample size within each subgroup, as there was no clear bimodal distribution of mean visual SP scores (Figure S1B, available online). The parent-report questionnaire rates individual items on a Likert scale from 1 to 5 (1 = Always; 5 = Never), such that lower scores indicate more atypical sensory processing responses. For instance, one visual item reads: “Is bothered by bright lights after others have adapted to the light.”
Based on the median split, one ASD subgroup encompassed the range of mean scores presented in the TD group, thus resulting in a “more atypical” ASD subgroup (ASD1) and a “less atypical” ASD subgroup (ASD2) with respect to visual sensory profiles. ANOVA models controlling for age and head motion RMSD were used to determine between-group effects for the ROI pairings with medium to large effect sizes (i.e., Cohen’s |d| > 0.5). Post-hoc two-tailed t-tests were performed to isolate the pairwise group differences.
Correlational Analyses
Functional connectivity (z’) data from the ROI-to-ROI pairs for which medium to large between-group (ASD vs. TD) effect sizes (Cohen’s d) were detected were entered into correlational analyses with subscores of the ADOS (Social interaction, Communication, Repetitive and restricted interests), ADI-R (Social interaction, Communication, Repetitive behaviors), and SRS (Motivation, Awareness, Cognition, Communication, Autistic mannerisms) within each ASD subgroup to explore the links between neural measures and autism symptomatology. Covariates including age and head motion (RMSD) were included in these analyses. Due to a significant difference (and large variance) in verbal IQ between ASD and TD groups, verbal IQ was also included as a covariate. Separate partial correlations controlling for nonverbal IQ and full-scale IQ, in addition to age and RMSD, followed very similar patterns.
Results
Patterns of Functional Connectivity
ANOVA models of between-group comparisons (ASD vs. TD) for the entire pairwise ROI-to-ROI connectivity matrix revealed significant and trending main effects of group between left anterior insula (L AI) and bilateral primary visual (V1) and secondary visual and extrastriate (V2+) cortices (Table 2; Figure 1B; see Figure S2A, available online, for within-group patterns). Across all ROI-to-ROI pairings, medium to large between-group effect sizes (i.e., Cohen’s |d| > 0.5) were detected in four ROI pairs (L AI – L V1: |d| = 0.65; L AI – L V2+: |d| = 0.54; L AI – R V1: |d| = 0.62; L AI – R V2+: |d| = 0.68). Mean fc (z’) of the four ROI pairings in the ASD and TD groups are presented in Figure 1C.
Table 2.
Between-group Statistical Comparisons
| ROI Pairing | Statistical Testa | p-value | MSe | Corrected p-valueb | |
|---|---|---|---|---|---|
| ASD vs. TD | L AI – L V1 | F(1,89) = 9.68 | 0.003 | 0.42 | p = 0.037 |
| L AI – L V2+ | F(1,89) = 6.44 | 0.013 | 0.30 | p = 0.091 | |
| L AI – R V1 | F(1,89) = 8.68 | 0.004 | 0.39 | p = 0.037 | |
| L AI – R V2+ | F(1,89) = 10.14 | 0.002 | 0.41 | p = 0.037 | |
| ASD1 vs. ASD2 vs. TD | L AI – L V1 | F(2,88) = 4.97 | 0.009 | 0.22 | -- |
| L AI – L V2+ | F(2,88) = 3.34 | 0.040 | 0.16 | -- | |
| L AI – R V1 | F(2,88) = 4.51 | 0.014 | 0.21 | -- | |
| L AI – R V2+ | F(2,88) = 5.03 | 0.009 | 0.21 | -- | |
| ASD1 vs. ASD2 | L AI – L V1 | t(48) = 0.56 | 0.581 | -- | -- |
| L AI – L V2+ | t(48) = 0.62 | 0.540 | -- | -- | |
| L AI – R V1 | t(48) = −0.68 | 0.503 | -- | -- | |
| L AI – R V2+ | t(48) = −0.11 | 0.914 | -- | -- | |
| ASD1 vs. TD | L AI – L V1 | t(66) = −2.37 | 0.021 | -- | -- |
| L AI – L V2+ | t(66) = −1.84 | 0.071 | -- | -- | |
| L AI – R V1 | t(66) = −2.83 | 0.006 | -- | -- | |
| L AI – R V2+ | t(66) = −2.71 | 0.009 | -- | -- | |
| ASD2 vs. TD | L AI – L V1 | t(66) = −2.79 | 0.007 | -- | -- |
| L AI – L V2+ | t(66) = −2.41 | 0.019 | -- | -- | |
| L AI – R V1 | t(66) = −2.10 | 0.044 | -- | -- | |
| L AI – R V2+ | t(66) = −2.53 | 0.014 | -- | -- | |
Note: AI = anterior insula; ASD = autism spectrum disorder; L = left; R = right; ROI = Region-of-Interest; TD = typically developing; V1 = primary visual cortex; V2+ = secondary visual and extrastriate cortices; MSe = mean squared error.
Only significant and trending F-tests (controlling for age and RMSD) of between-group effects are reported; post-hoc two-tailed t-tests reported for ROI pairings with medium to large effect sizes (i.e., Cohen’s |d| > 0.5).
False Discovery Rate (FDR) correction for multiple comparisons.
Within-network group comparisons (ASD vs. TD) showed marginally greater visual network fc in the ASD cohort (t(91) = 1.80, p = 0.076). There was no significant group difference for fc within the SN (t(91) = 0.54, p = 0.592).
Subgrouping of ASDs Based on SP Visual Processing
Sensory processing abnormalities in the visual domain (based on parent-report from the SP) were not found to be correlated with fc patterns in the overall ASD group. In subsequent analyses, the ASD cohort was divided into two ASD subgroups using SP visual mean scores. A median split (median = 3.6) into greater (ASD1: mean = 3.13, SD = 0.50) or fewer (ASD2: mean = 4.11, SD = 0.32) atypical responses was used (Figure 1D). According to the SP, a score of 3 indicates that responses made in the manner described in the items occur occasionally; in the ASD1 subgroup, these behaviors occurred approximately 50 to 100% of the time. A score of 4 on the SP indicates that those responses seldom occur; in the ASD2 subgroup (and TD group), the behaviors occurred less than 25% of the time. The median split resulted in two subsamples that were significantly different from the TD group (TD: mean = 4.67, SD = 0.29; ASD1 vs. TD: t(66) = −16.21, p< 0.001; ASD2 vs. TD: t(66) = −7.47, p< 0.001; ASD1 vs. ASD2: t(48) = −8.33, p< 0.001). The two ASD subsamples, however, did not significantly differ on demographic or diagnostic measures, or on overall intellectual functioning (Table 3). Moreover, the two subgroups were largely similar regarding fc patterns (Figure S2B, available online), and did not show any significant between-group differences after FDR correction for multiple comparisons (Figure S2C, available online). Although there was a main effect of group for all four of the ROI pairings described in the preceding subsection, these effects were driven solely by significantly greater mean fc (z’) in the TD group compared to the ASD1 and ASD2 subgroups (Table 2; Figure 1E; see Figure S2D, available online, for group comparisons of the entire fc matrix).
Table 3.
Autism Spectrum Disorder Subgroup Characterization
| ASD1 (n = 25) | ASD2 (n = 25) | Statistical Comparison | |||||
|---|---|---|---|---|---|---|---|
| Mean | (SD) | Range | Mean | (SD) | Range | ||
| Gender | 3 female participants | 5 female participants | χ2(1) = 0.60, p = 0.440 | ||||
| Handedness | 4 left | 5 left | χ2(1) = 0.14, p = 0.713 | ||||
| Age (years) | 13.7 | (2.5) | 9.2–18.0 | 13.3 | (2.9) | 7.4–17.8 | t(48) = 0.56, p = 0.580 |
| WASI-II | |||||||
| Verbal IQ | 101.5 | (21.2) | 59–147 | 99.6 | (13.5) | 70–128 | t(48) = 0.39, p = 0.698 |
| Non-verbal IQ | 100.9 | (18.4) | 53–134 | 109.7 | (17.2) | 70–140 | t(48) = −1.75, p = 0.086 |
| Full-Scale IQ | 101.6 | (19.0) | 61–141 | 105.0 | (14.8) | 79–139 | t(48) = −0.71, p = 0.483 |
| RMSD | 0.06 | (0.03) | 0.02–0.13 | 0.07 | (0.03) | 0.02–0.12 | t(48) = −0.44, p = 0.661 |
| ADOSa | |||||||
| Social interaction | 7.3 | (2.2) | 4–12 | 8.2 | (2.9) | 4–14 | t(47) = −1.21, p = 0.233 |
| Communication | 3.6 | (1.7) | 0–6 | 4.4 | (1.6) | 2–8 | t(47) = −1.55, p = 0.128 |
| Rep./Restricted | 2.2 | (1.5) | 0–5 | 2.3 | (1.6) | 0–5 | t(47) = −0.40, p = 0.692 |
| ADI-Rb | |||||||
| Social interaction | 18.4 | (5.0) | 6–28 | 18.0 | (4.3) | 12–26 | t(47) = 0.33, p = 0.743 |
| Communication | 13.0 | (5.5) | 2–24 | 13.3 | (4.5) | 6–22 | t(47) = −0.17, p = 0.862 |
| Rep. behaviors | 6.0 | (2.2) | 3–12 | 5.5 | (2.2) | 1–9 | t(47) = 0.86, p = 0.392 |
| Psychotropic med. use | 13 reported | 13 reported | χ2(1) = 0.00, p = 1.000 | ||||
| Comorbidities | 11 reported | 9 reported | χ2(1) = 0.33, p = 0.564 | ||||
| SRS | |||||||
| Awareness | 74.0 | (13.2) | 46–95 | 69.4 | (14.9) | 39–97 | t(48) = 1.16, p = 0.254 |
| Cognition | 77.7 | (11.1) | 50–96 | 74.0 | (12.4) | 45–99 | t(48) = 1.12, p = 0.268 |
| Communication | 83.2 | (10.0) | 66–105 | 78.4 | (12.2) | 62–110 | t(48) = 1.52, p = 0.135 |
| Motivation | 75.2 | (12.0) | 56–97 | 76.1 | (11.9) | 58–104 | t(48) = −0.26, p = 0.796 |
| ASD mannerisms | 91.4 | (16.1) | 60–126 | 81.4 | (14.0) | 58–112 | t(48) = 2.34, p = 0.023 |
Note: ADI-R = Autism Diagnostic Interview-Revised; ADOS = Autism Diagnostic Observation Schedule; IQ = intelligence quotient; Rep. = repetitive; RMSD = root mean squared displacement; SD = standard deviation; SRS = Social Responsiveness Scale; WASI-II = Wechsler Abbreviated Scale of Intelligence, 2nd edition.
Subdomain data not available for 1 ASD2 participant.
Subdomain data not available for 1 ASD2 participant.
Post-hoc Examination of ASD Subgroups
To further characterize the two ASD subgroups, and given evidence suggesting that ACC and AI are involved in executive functioning,58,59 a post-hoc examination of executive function in ASD1 and ASD2 was conducted. BRIEF-2 indices of behavioral, emotional, and cognitive regulation were compared between the two subgroups using two-tailed t-tests, which revealed greater impairments in ASD1 compared to ASD2 in emotional regulation (t(46) = 2.19, p = 0.033; ASD1: mean = 73.52, SD = 9.12; ASD2: mean = 67.40, SD = 10.16), as well as cognitive regulation (t(46) = 2.43, p = 0.019; ASD1: mean = 68.43, SD = 8.28; ASD2: mean = 62.36, SD = 8.98). Behavioral regulation, however, was not significantly different between the two ASD subgroups (t(46) = 1.25, p = 0.217; ASD1: mean = 65.30, SD = 9.03; ASD2: mean = 61.68, SD = 10.85). As expected, both ASD subgroups combined showed increased dysregulation compared to the TD group across all three domains of executive functioning: behavior (t(83) = 9.58, p < 0.001; ASD: mean = 63.42, SD = 10.08; TD: mean = 45.35, SD = 6.21), emotion (t(83) = 13.40, p < 0.001; ASD: mean = 70.33, SD = 10.06; TD: mean = 45.59, SD = 5.65), and cognition (t(83) = 9.03, p < 0.001; ASD: mean = 65.27, SD = 9.09; TD: mean = 48.41, SD = 7.76).
Correlations with Autism Symptomatology
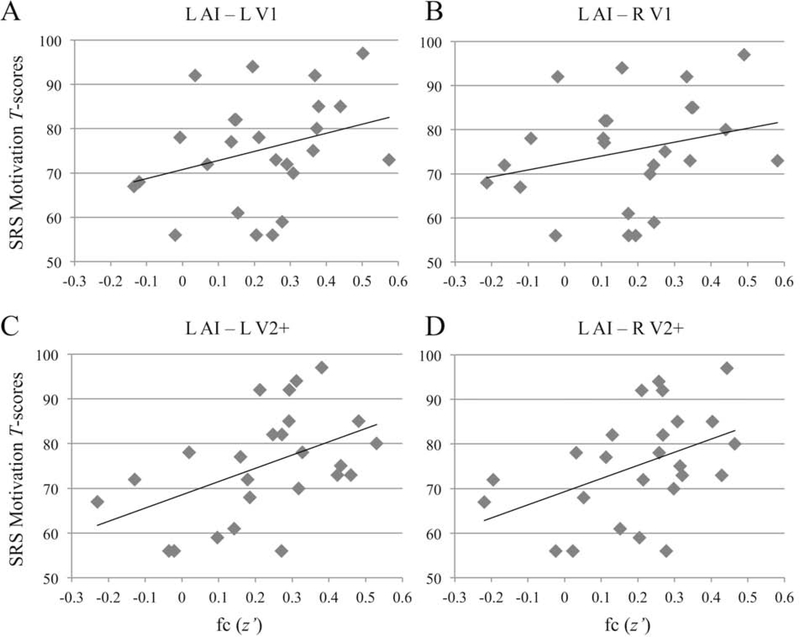
In order to explore the functional relevance of fc findings, correlations with ADOS, ADI-R, and SRS scores were performed with specific focus on ASD1 (i.e., the subgroup with relatively more atypical visual SP scores). While there were few neural or diagnostic differences between the two ASD subgroups, partial correlations (controlling for age, RMSD, and verbal IQ) in the ASD1 subgroup revealed positive relationships of medium to large effect size between fc (z’) and SRS Motivation T-scores for each of the four ROI pairings, two of which survived FDR correction (Figure 2; L AI – L V1: r(20) = 0.50, p = 0.019, corrected p = 0.279; L AI – L V2+: r(20) = 0.65, p = 0.001, corrected p = 0.044; L AI – R V1: r(20) = 0.46, p = 0.033, corrected p = 0.363; L AI – R V2+: r(20) = 0.62, p = 0.002, corrected p = 0.044). This particular SRS subscale represents the motivation to engage in social-interpersonal behaviors (e.g., “Seems much more fidgety in social situations than when alone”). Given the T-scores distribution in which elevated scores (above the mean of 50) indicate greater social impairment, with scores > 65 indicating clinically significant impairment, lower AI to visual connectivity—that is, greater underconnectivity—was associated with decreased impairments in this particular social domain in the ASD1 subgroup (which had relatively severe visual processing abnormalities on the SP).
Figure 2.
Correlational Findings Between Social Motivation and Functional Connectivity
Note: Plots depict zero-order correlations in the ASD1 subgroup between SRS Motivation subscale T-scores and functional connectivity (z’) in the ROI-to-ROI pairings from left AI to: (A) left V1; (B) right V1; (C) left V2+; (D) right V2+. ACC = anterior cingulate cortex; AI = anterior insula; ROI = Region-of-Interest; SRS = Social Responsiveness Scale; V1 = primary visual cortex; V2+ = secondary visual and extrastriate cortices.
No significant correlations were observed between fc (z’) and SRS Motivation T-scores in the ASD2 subgroup or TD group (Figure S3, available online), or in the ASD group overall (Table S1, available online). No correlations were found between fc (z’) of the four ROI pairings and other SRS subdomains, or ADOS or ADI-R scores.
Discussion
Our findings in children and adolescents with ASDs indicate robust functional underconnectivity between bilateral visual cortices and left anterior insula – a region that mediates sensory perception and social and emotional awareness in neurotypical individuals1 and is considered a hub of the SN. Despite the known heterogeneity of ASDs, such underconnectivity was found both in ASD participants with high and with low levels of atypical sensory responses to visual stimuli. However, a link with social impairment was only detected in a small subgroup with more pronounced sensory abnormalities in the visual domain (ASD1). In this subgroup, increased functional underconnectivity was unexpectedly associated with lower impairments in social motivation, suggesting a potential neural mechanism that supports certain social behaviors.
Functional Underconnectivity Between Salience and Visual Networks
Previous studies have reported predominant underconnectivity between salience and other networks in ASDs,20,21,26 but have not focused on connectivity with visual cortex. Our study shows a distinct pattern of functional underconnectivity between left AI and bilateral visual cortices in children and adolescents with ASDs, compared to TD peers; this pattern was also reproduced in a larger in-house sample (Figure S4 and Table S2, available online). The AI evaluates the intensity and saliency of external stimuli, and integrates this information to coordinate interactions between the SN and large-scale brain networks.8,17 Underconnectivity of this region may therefore reflect uncoupling between AI and sensory regions, and could indicate decreased salience detection and deficient allocation of resources for guiding social behaviors.11,17,25 According to the salience network dysfunction hypothesis,11,60 abnormally reduced SN activity (and connectivity) may stem from an inappropriate neural response during cognitively challenging tasks (e.g., social processing), which results in the ineffective selection of salient sensory information and reduced attention to relevant social stimuli. Alternatively, deficits in the ability to recognize social stimuli may result in decreased engagement of SN during salience detection of relevant information, which would manifest as aberrant downstream AI connectivity. While these theories have yet to be tested thoroughly, they nevertheless suggest that dysfunctional connectivity of AI with sensory networks may play a crucial role in social cognition in autism.11,20,61
Regarding within-network connectivity, studies examining local fc have found evidence of atypically increased functional connectivity between visual regions, namely primary visual and extrastriate cortices, in ASD compared to TD groups.25,62,63 Although within-network fc was only marginally increased for the visual ROIs used in the current investigation, our findings may be in line with overconnectivity observed in these earlier studies and consistent with evidence for a special status of vision in autism.64 In any case, they suggest integrity of visual network fc, implying that underconnectivity with SN was not caused by, or associated with, disruption within the visual system itself. We did not find significant group differences within SN. While other investigations have shown decreased within-network connectivity for salience nodes, this may be attributed to age differences in the samples (i.e., mostly older participants in20,65).
Left and right AI are frequently coactivated, which likely reflects generally strong interhemispheric coupling of homotopic regions.66 Right AI, however, predominates in task-based activation studies targeting SN functions (for a review, see11), and is often selected as a hub within SN in the resting state literature of children with ASDs.20,67 In the current study, while primary findings occurred in left AI, similar patterns (though sub-threshold) were observed in the right hemisphere. The nuances of hemispheric asymmetries for AI were beyond the scope of the current study.
Brain and Behavior Links in Children with More Severe Visual Abnormalities
ASDs are highly heterogeneous, particularly with regard to sensory functioning (for review, see57). Here, the ASD cohort was median-split to maximize two subsamples based on parent-reported sensory abnormalities in the visual domain: ASD1 showed greater levels of atypical visual responses; ASD2 had fewer abnormal responses to visual stimuli (with mean SP visual scores falling into the range observed in the TD group). Given the limited sample size and the resultant concerns regarding statistical power, in addition to the lack of a clear, clinical cutoff on the SP, the following findings should be interpreted with caution. While similar fc patterns between salience and visual networks were observed in both subgroups, only children and adolescents with relatively higher levels of reported atypical visual processing behaviors (ASD1) showed distinct brain-behavior links, with greater underconnectivity associated with less severe impairments in social motivation. (Please note that while these effects emerge from greater abnormalities in visual processing behaviors, they do not demonstrate the presence of a distinct subtype.) These findings are contrary to our hypothesis – and most intuitive expectation – of greater fc abnormalities relating to increased core ASD symptomatology. They instead suggest that atypical visual processing behaviors (and correspondingly atypical connectivity) may be protective of certain social abilities (i.e., social motivation) in children with more severe sensory abnormalities in the visual domain. According to the ‘sensory hypersensitivity’ theory64 and the enhanced perceptual functioning model of autism,68 individuals with ASDs are extremely sensitive to sensory information and even show enhanced sensory perception, particularly in the visual domain.33,69 In these individuals, the salience network may perhaps “gate” or limit the input from visual cortex (reflected by underconnectivity), such that greater underconnectivity aids in reducing sensory overload (particularly in higher-order extrastriate cortices given the prominent correlations with V2+ found in the current study) and allowing for more efficient processing of social information (i.e., lower symptom severity). More research, however, is needed to substantiate this theory.
In a previous study of a relatively small sample of children and adolescents with ASDs,67 sensory over-responsivity (SOR) was correlated with increased resting state fc between salience and sensory network nodes, including somatosensory and auditory cortices; that is, lower fc between salience and primary sensory regions was associated with reduced SOR. This may appear consistent with the current findings of a positive relation between social symptomatology and fc between SN and visual regions in ASD. However, Green and colleagues also reported different patterns for visual regions, for which greater underconnectivity was associated with increased SOR.67 Importantly, in the current study, the positive correlations linking fc to core symptoms, specifically social motivation, were observed only in a subsample of ASD participants with more atypical sensory responses to visual stimuli. This extends beyond the scope of the study by Green and colleagues,67 and further highlights the intimate links between sensory processing, particularly in the visual domain, and certain aspects of sociocommunicative symptomatology.
More recently, Xu and colleagues reported functional underconnectivity between left AI (namely, ventral agranular and dorsal dysgranular insula) and bilateral precuneus, as well as right cuneus, in children with ASDs compared to TD peers,22 which is consistent with the current findings. The underconnectivity observed by Xu et al.22 between left ventral agranular insula and right precuneus, however, were negatively correlated with ADOS Total and Social scores. Based on these results, the authors argued that underconnectivity between AI and precuneus, both of which participate in social-cognitive processing, contributed to the social interaction deficits observed in children with ASDs. Notably, Xu and colleagues did not investigate sensory abnormalities within their cohort.22 In an earlier study examining the auditory domain in children with ASDs, we showed that atypically increased thalamocortical fc was correlated with improved social functioning.70 Compared to the current results, this association between increasingly atypical fc and less severe social impairment suggests a similar, counterintuitive mechanism at play. Combined, the findings from these studies suggest that heterogeneity in sensory processing abilities must be taken into consideration prior to making inferences regarding links between functional connectivity of sensory cortices and social symptomatology. Although findings from previous studies by Green et al.67 and Xu et al.22 do not fully converge with those reported here, they broadly support the importance of neurobehavioral links between social and sensory processing in ASDs.
It is important to note that the two ASD subgroups were further differentiated (beyond visual processing abilities) with respect to executive functioning, with greater impairments reported in the ASD1 than ASD2 subgroup. Moreover, the ASD participants overall demonstrated increased emotional and cognitive dysregulation compared to TD participants, which is consistent with previous reports.71 Although there is evidence of anterior insular contributions to emotional and social impairments in ASDs,29,58,61 direct links between neural connectivity patterns and emotional and cognitive regulatory processes were not found in the current study. Nevertheless, this additional characterization suggests that visual processing deficiencies may be part of a broader cognitive impairment,72 including general executive functioning, which may impact social symptomatology in autism.
Several limitations are worth mentioning. First, we selected the anterior half of the insula, as derived from the Harvard Oxford atlas, as seed approximating the insular SN node. Given the functional diversity within the insular cortex22 and the limited spatial resolution of fMRI, some partial volume effects (i.e., inclusion of some BOLD variance from neighboring insular parcels) cannot be ruled out. Second, the individuals with ASDs in this study were able to follow explicit instructions and remain supine for extended periods of time so that useable, low-motion MRI data could be acquired. Therefore, this cohort may not be fully representative of individuals at the lower end of the spectrum. Third, the patterns of functional underconnectivity in the ASD vs. TD group were reproduced within a larger in-house, rather than external, dataset. We deliberately opted not to use publically available data collections such as the ABIDE initiative,73,74 as recent studies have shown poor replication across sites.75,76Moreover, no single site contributes datasets that are fully comparable to our in-house dataset in size, age and IQ ranges and distributions, gender ratio, and symptom severity in the ASD sample. Other potentially crucial information, such as medication status and history, is largely unavailable as well. This choice, however, impacted the available sample size of the study. Relatedly, there was a limited sample size in analyses of ASD subgroups, and findings therefore need to be interpreted with caution. Lastly, parent-report measures such as the SRS and SP are inherently limited in their capacity to capture specific idiosyncrasies of social abilities and sensory processing fully (particularly the latter). As such, more objective behavioral measures of sensory processing abilities will be desirable in future studies.
In summary, using resting state fMRI, patterns of functional connectivity between salience and sensory network nodes were examined in ROI analyses and correlated with social impairments in children and adolescents with ASDs. Findings showed underconnectivity between AI and visual cortex in ASD compared to TD participants. These disruptions may implicate deficient selection of salient sensory information as part of autism symptomatology. Furthermore, in an ASD subgroup with relatively higher levels of reported visual processing abnormalities, we found a seemingly paradoxical link between more atypical connectivity and reduced deficits in a particular domain of social processing (i.e., social motivation), which may indicate a potentially protective neural mechanism.
Supplementary Material
Acknowledgements
The authors thank members of the Brain Development Imaging Laboratories, and the participating children and families for their time and patience.
Funding: This research was supported by the National Institutes of Health (K01-MH113819 to RJJK; R01-MH081023 and R01-MH101173 to RAM; K01-MH097972 to IF). The funding sources had no role in study design, writing of the report, or the decision to submit the article for publication.
Footnotes
Conflicts of Interest: Dr. Jao Keehn, Ms. Pueschel, Ms. Gao, Ms. Jahedi, Mr. Alemu, Dr. Carper, Dr. Fishman, and Dr. Müller report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
R. Joanne Jao Keehn, Brain Development Imaging Laboratories, San Diego State University, CA.
Ellyn B. Pueschel, Brain Development Imaging Laboratories, San Diego State University, CA.
Yangfeifei Gao, Brain Development Imaging Laboratories, San Diego State University, CA; San Diego State University/University of California, San Diego Joint Doctoral Program in Clinical Psychology, CA.
Afrooz Jahedi, Brain Development Imaging Laboratories, San Diego State University, CA; San Diego State University/Claremont Graduate University Joint Doctoral Program in Computational Statistics, CA.
Kalekirstos Alemu, Brain Development Imaging Laboratories, San Diego State University, CA.
Ruth Carper, Brain Development Imaging Laboratories, San Diego State University, CA; San Diego State University/University of California, San Diego Joint Doctoral Program in Clinical Psychology, CA.
Inna Fishman, Brain Development Imaging Laboratories, San Diego State University, CA; San Diego State University/University of California, San Diego Joint Doctoral Program in Clinical Psychology, CA.
Ralph-Axel Müller, Brain Development Imaging Laboratories, San Diego State University, CA; San Diego State University/University of California, San Diego Joint Doctoral Program in Clinical Psychology, CA.
References
- 1.Sterzer P, Kleinschmidt A. Anterior insula activations in perceptual paradigms: often observed but barely understood. Brain Struct Funct. 2010;214(5–6):611–622. [DOI] [PubMed] [Google Scholar]
- 2.Kim H Involvement of the dorsal and ventral attention networks in oddball stimulus processing: A meta-analysis. Hum Brain Mapp. 2014;35(5):2265–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calvert GA. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cereb Cortex. 2001;11(12):1110–1123. [DOI] [PubMed] [Google Scholar]
- 4.Amedi A, von Kriegstein K, van Atteveldt NM, Beauchamp MS, Naumer MJ. Functional imaging of human crossmodal identification and object recognition. Exp Brain Res. 2005;166(3–4):559–571. [DOI] [PubMed] [Google Scholar]
- 5.Odriozola P, Uddin LQ, Lynch CJ, Kochalka J, Chen T, Menon V. Insula response and connectivity during social and non-social attention in children with autism. Soc Cogn Affect Neurosci. 2016; 11:433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31(50):18578–18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N. Volitional Control of Anterior Insula Activity Modulates the Response to Aversive Stimuli. A Real-Time Functional Magnetic Resonance Imaging Study. Biol Psychiatry. 2010;68(5):425–432. [DOI] [PubMed] [Google Scholar]
- 8.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002;3(8):655–666. [DOI] [PubMed] [Google Scholar]
- 10.Craig AD. How do you feel - now? The anterior insula and human awareness. Nat Rev Neurosci 2009;10(1):59–70. [DOI] [PubMed] [Google Scholar]
- 11.Uddin LQ, Menon V. The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev. 2009;33(8):1198–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103(26):10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uddin LQ, Kinnison J, Pessoa L, Anderson ML. Beyond the tripartite cognition-emotion-interoception model of the human insular cortex. J Cogn Neurosci. 2014;26(1):16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greicius M Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21(4):424–430. [DOI] [PubMed] [Google Scholar]
- 16.Uddin LQ, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front Hum Neurosci. 2013;7:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16(1):55–61. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association; 2013. [Google Scholar]
- 19.Zablotsky B, Black LI, Maenner MJ, Schieve LA, and Blumberg SJ CDC. Estimated Prevalence of Autism and Other Developmental Disabilities Following Questionnaire Changes in the 2014 National Health Interview Survey. Natl Health Stat Report. 2015;87:1–20. [PubMed] [Google Scholar]
- 20.Ebisch SJ, Gallese V, Willems RM, et al. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum Brain Mapp. 2011;32(7):1013–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbott AE, Nair A, Keown CL, et al. Patterns of Atypical Functional Connectivity and Behavioral Links in Autism Differ Between Default, Salience, and Executive Networks. Cereb Cortex (New York, NY). 2016;26(10):4034–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J, Wang H, Zhang L, et al. Both hypo-connectivity and hyper-connectivity of the insular subregions associated with severity in children with autism spectrum disorders. Front Neurosci 2018;12(APR):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang M-X, Liu X-H, Zhang Z-J, et al. Functional connection between the stereotyped behavior and the motor front area in children with autism. Br J Neurosurg. December 2018:1–4. [DOI] [PubMed] [Google Scholar]
- 24.Francis SM, Camchong J, Brickman L, et al. Hypoconnectivity of Insular Resting-State Networks in Adolescents with Autism Spectrum Disorders. Psychiatry Res Neuroimaging. December 2019; 283:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uddin LQ, Supekar K, Lynch CJ, et al. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013;70(8):869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von dem Hagen EA, Stoyanova RS, Baron-Cohen S, Calder AJ. Reduced functional connectivity within and between “social” resting state networks in autism spectrum conditions. Soc Cogn Affect Neurosci. 2013;8(6):694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hubl D, Bolte S, Feineis-Matthews S, et al. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61(9):1232–1237. [DOI] [PubMed] [Google Scholar]
- 28.Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: an fMRI study.Soc Neurosci. 2008;3(2):97–112. [DOI] [PubMed] [Google Scholar]
- 29.Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eussen ML, Gool AR, Louwerse A, Verhulst FC, Greaves-Lord K. Superior Disembedding Performance in Childhood Predicts Adolescent Severity of Repetitive Behaviors: A Seven Years Follow-Up of Individuals With Autism Spectrum Disorder. Autism Res 2016;9(2):282–291. [DOI] [PubMed] [Google Scholar]
- 31.Horlin C, Black M, Falkmer M, Falkmer T. Proficiency of individuals with autism spectrum disorder at disembedding figures: A systematic review. Dev Neurorehabil. 2016;19(1):54–63. [DOI] [PubMed] [Google Scholar]
- 32.Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36(1):27–43. [DOI] [PubMed] [Google Scholar]
- 33.O’Riordan MA, Plaisted KC, Driver J, Baron-Cohen S. Superior visual search in autism. J Exp Psychol Hum Percept Perform. 2001;27(3):719–730. [DOI] [PubMed] [Google Scholar]
- 34.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. [DOI] [PubMed] [Google Scholar]
- 36.Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule—Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. J Autism Dev Disord 2000;30(3):205–223. [PubMed] [Google Scholar]
- 37.Wechsler D Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, Texas: Psychological Corporation; 1999. [Google Scholar]
- 38.Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 39.Dunn W The Sensory Profile: User’s Manual. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 40.Constantino & Gruber, C.P. JN. Social Responsiveness Scale. Los Angeles, CA: Western Psychological Services; 2005. [Google Scholar]
- 41.Gioia GA, Isquith PK, Guy SC, Kenworthy L. (BRIEF-2) Behavior Rating Inventory of Executive Function. 2nd ed. Lutz, FL: PAR Inc.; 2015. [Google Scholar]
- 42.Cox R AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(29):162–173. [DOI] [PubMed] [Google Scholar]
- 43.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(SUPPL. 1):208–219. [DOI] [PubMed] [Google Scholar]
- 44.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. [DOI] [PubMed] [Google Scholar]
- 45.Cordes D, Haughton VM, Arfanakis K, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. [DOI] [PubMed] [Google Scholar]
- 47.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage. 2013;76:439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 50.Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann’s Areas 17 and 18 Brought into Stereotaxic Space—Where and How Variable? Neuroimage. 2000;11(1):66–84. [DOI] [PubMed] [Google Scholar]
- 51.Rottschy C, Eickhoff SB, Schleicher A, et al. Ventral visual cortex in humans: Cytoarchitectonic mapping of two extrastriate areas. Hum Brain Mapp. 2007;28(10):1045–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilms M, Eickhoff SB, Specht K, et al. Human V5/MT+: Comparison of functional and cytoarchitectonic data. In: Anatomy and Embryology. Vol 210 2005:485–495. [DOI] [PubMed] [Google Scholar]
- 53.Malikovic A, Amunts K, Schleicher A, et al. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: A probabilistic, stereotaxic map of area hOc5. Cereb Cortex. 2007;17(3):562–574. [DOI] [PubMed] [Google Scholar]
- 54.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57(1):289–300. [Google Scholar]
- 55.Marco EJ, Hinkley LBN, Hill SS, Nagarajan SS. Sensory processing in autism: A review of neurophysiologic findings. Pediatr Res. 2011;69(5 PART 2):48R–54R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ausderau K, Sideris J, Furlong M, Little LM, Bulluck J, Baranek GT. National survey of sensory features in children with ASD: Factor structure of the sensory experience questionnaire (3.0). J Autism Dev Disord. 2014;44(4):915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeBoth KK, Reynolds S. A systematic review of sensory-based autism subtypes. Res Autism Spectr Disord. 2017;36:44–56. [Google Scholar]
- 58.Yamada T, Itahashi T, Nakamura M, et al. Altered functional organization within the insular cortex in adult males with high-functioning autism spectrum disorder: evidence from connectivity-based parcellation. Mol Autism. 2016;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. [DOI] [PubMed] [Google Scholar]
- 60.Toyomaki A, Murohashi H. “Salience network” dysfunction hypothesis in autism spectrum disorders. Jpn Psychol Res. 2013;55(2):175–185. [Google Scholar]
- 61.Caria A, de Falco S. Anterior insular cortex regulation in autism spectrum disorders. Front Behav Neurosci. 2015;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maximo JO, Keown CL, Nair A, Müller R-A. Approaches to local connectivity in autism using resting state functional connectivity MRI. Front Hum Neurosci. 2013;7(October):605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keown C, Shih P, Nair A, Peterson N, Mulvey M, Müller RA. Local functional overconnectivity in posterior brain regions is associated with symptom severity in autism spectrum disorders. Cell Rep. 2013;5(3):567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baron-Cohen S, Ashwin E, Ashwin C, Tavassoli T, Chakrabarti B. Talent in autism: Hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philos Trans R Soc B Biol Sci. 2009;364(1522):1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory Control in High-Functioning Autism: Decreased Activation and Underconnectivity in Inhibition Networks. Biol Psychiatry. 2007;62(3):198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stark DE, Margulies DS, Shehzad ZE, et al. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci. 2008;28(51):13754–13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Green SA, Hernandez L, Bookheimer SY, Dapretto M. Salience Network Connectivity in Autism Is Related to Brain and Behavioral Markers of Sensory Overresponsivity. J Am Acad Child Adolesc Psychiatry. 2016;55(7):618–626.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mottron L, Burack JA. Enhanced perceptual functioning in the development of autism. In: The Development of Autism: Perspectives from Theory and Research. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 2001:131–148. [Google Scholar]
- 69.Ashwin E, Ashwin C, Rhydderch D, Howells J, Baron-Cohen S. Eagle-eyed visual acuity: an experimental investigation of enhanced perception in autism. Biol Psychiatry. 2009;65(1):17–21. [DOI] [PubMed] [Google Scholar]
- 70.Linke AC, Jao Keehn RJ, Pueschel EB, Fishman I, Müller R-A. Children with ASD show links between aberrant sound processing, social symptoms, and atypical auditory interhemispheric and thalamocortical functional connectivity. Dev Cogn Neurosci. 2018;29:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Res. 2009;166(2–3):210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reiter MA, Mash LE, Linke AC, Fong CH, Fishman I, Müller RA. Distinct Patterns of Atypical Functional Connectivity in Lower-Functioning Autism. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(3):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Martino A, Yan CG, Li Q, et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry. 2014;19(6):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di Martino A, O’Connor D, Chen B, et al. Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci Data. 2017;4:170010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.King JB, Prigge MBD, King CK, et al. Generalizability and reproducibility of functional connectivity in autism. Mol Autism. 2019;10(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He Y, Byrge L, Kennedy DP. Non-replication of functional connectivity differences in autism spectrum disorder across multiple sites and denoising strategies. bioRxiv. October 2019:640797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.