Abstract
Mammalian sirtuins (SIRTs 1-7), are a family of NAD+-dependent deacetylases with distinct subcellular localization and biological functions that regulate various important cellular processes. Among these, SIRTs −3, −4, and −5 are located in the mitochondria and have been implicated in caloric restriction, oxidative stress, aging, and various human diseases. Emerging evidence has found dysregulation of mitochondrial sirtuins in multiple dermatological conditions, including responses to ultraviolet radiation (UVR), suggesting their importance in maintaining skin health. In this review, we discuss the roles and implications of mitochondrial sirtuins in cutaneous cellular processes, and their emerging potential as a target for the management of skin diseases, including skin cancer. Among mitochondrial sirtuins, SIRT3 is the most studied and linked to multiple skin conditions and diseases (keratinocyte differentiation, wound healing, chronological aging, UVR and ozone response, systemic sclerosis, melanoma, basal cell carcinoma (BCC), and squamous cell carcinoma (SCC)). SIRT4 has been connected to keratinocyte differentiation, chronological aging, UVR response, alopecia, BCC and SCC. Further, SIRT5 has been associated with keratinocyte differentiation, melanoma, BCC and SCC. Overall, while there is compelling evidence for the involvement of mitochondrial sirtuins in skin, additional detailed studies are needed to understand their exact roles in skin and skin cancers.
Keywords: mitochondrial sirtuins, ultraviolet radiation, skin, skin cancers
Graphical Abstract
Mitochondrial sirtuins (SIRTs −3, −4, and −5) have been implicated in caloric restriction, oxidative stress, aging, and various human diseases. Emerging evidence has found dysregulation of mitochondrial sirtuins in multiple dermatological conditions, including responses to ultraviolet radiation (UVR), suggesting their importance in maintaining skin health. In this review, we discuss the roles and implications of mitochondrial sirtuins in cutaneous cellular processes and their emerging potential as a target for the management of skin diseases. In this graphical abstract, the involvement of SIRTs −3, −4, and −5 are indicated in multiple dermatological conditions including skin cancer.
INTRODUCTION
The founding member of the nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase (HDAC) sirtuin family, Sir2, was described initially in S. cerevisiae and was associated with stress response and longevity (1). Since then, Sir2 homologs have been identified in numerous species, and mammalian sirtuins have been found to play key roles in cellular processes such as metabolism, cell cycle, transcriptional regulation, and cell division, as well as the pathogenesis of a variety of diseases, including cancer (2-4). There are seven known mammalian sirtuins (SIRTs 1 to 7), and all of them have a conserved Sir2 catalytic core domain, which is key in their NAD+-dependent deacetylase activity (5). Each sirtuin has been found to have distinct subcellular localizations, as well as discrete protein deacetylation or other catalytic activities (6, 7). Although certain sirtuins may be found throughout the cell, their localization and site of action allows each sirtuin to have distinct functions within cells. SIRT1 and SIRT2 are primarily found in the nucleus and cytoplasm, respectively, although certain cellular conditions may cause their localization to the other cellular compartments (8, 9). SIRT3, SIRT4 and SIRT5, which we will focus on in this review, are referred to as the mitochondrial sirtuins, as they are located primarily in the mitochondria. Their localizations suggest that they are principally involved in metabolism, although they may have other effects within the cell (10). SIRT6 and SIRT7 are found in the nucleus, where they facilitate transcription and epigenetic regulation (11).
While SIRT3, SIRT4 and SIRT5 are predominantly localized in the mitochondria, each has a unique catalytic activity and contributes to mitochondrial homeostasis via distinct mechanisms. SIRT3, the most studied mitochondrial sirtuin, is one of the key mitochondrial deacetylases in regards to regulation of energy production (12). SIRT4, on the other hand, shows weak deacetylation activity (13), and behaves as a deacylase (14), lipoamidase (15) and ADP-ribosyltransferase (16). SIRT5 also has weak deacetylation activity (17), and is active as a lysine demalonylase, desuccinylase (18) and deglutarylase (19). Due to their distinct catalytic activities, mitochondrial sirtuins have been found to be involved in a number of diverse pathways (20), and their dysregulation has been observed in multiple metabolic and aging-related diseases, including skin conditions and skin cancers (21).
ROLE OF MITOCHONDRIAL SIRTUINS IN SKIN AGING AND IN RESPONSE TO UVR AND OZONE EXPOSURE
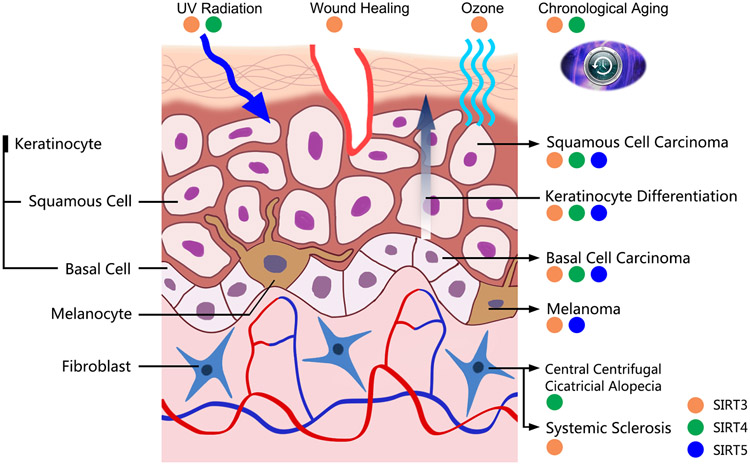
As the primary barrier between the human body and the environment, the skin is constantly exposed to damages from environmental factors as well as biological mishaps. Mitochondrial sirtuins have been implicated in the skin’s response to many of these hazards, including the external factors such as ultraviolet radiation (UVR), as well as internal factors such as aging. Below, we discuss the roles that have been uncovered for mitochondrial sirtuins in the skin aging and in response to UVR and ozone exposure. (Figure 1, Table 1).
Figure 1. The involvement of mitochondrial sirtuins in skin and skin cancers.
Mitochondrial sirtuins have been studied in several skin conditions and diseases. SIRT3 is involved in keratinocyte differentiation, wound healing, chronological aging, UV radiation/ozone response, Systemic Sclerosis, melanoma, basal cell carcinoma, and squamous cell carcinoma. SIRT4 has been associated with keratinocyte differentiation, chronological aging, UVR response, central centrifugal cicatricial alopecia, basal cell carcinoma, and squamous cell carcinoma. SIRT5 has been implicated in keratinocyte differentiation, melanoma, basal cell carcinoma, and squamous cell carcinoma.
Table 1.
The expression level and molecular associations of mitochondrial sirtuins in skin and skin cancers.
| Condition/Disease | SIRT3 | SIRT4 | SIRT5 | Molecular Association |
|---|---|---|---|---|
| Keratinocyte Differentiation | ↓ | ↑ | ↑ | Keratin-1 and −10, loricrin, filaggrin, mtROS |
| Wound Healing | ↓ | Unk | Unk | IL1β, FAB4 |
| Chronological Aging | ↓ | ↑ | Unk | miR-15b |
| UV Radiation | ↓ | ↑ | Unk | mtROS |
| Ozone | ↓ | Unk | Unk | IL-1α, mtROS |
| Systemic Sclerosis | ↓ | Unk | Unk | TGF-ß, mtROS |
| Central Centrifugal Cicatricial Alopecia | Unk | ↓ | Unk | Unk |
| Melanoma | ↑ | Unk | ↑↓ | P16, P21, MnSOD, mtROS |
| Basal Cell Carcinoma | ↓ | - | - | Unk |
| Squamous Cell Carcinoma | ↑ | ↑ | ↑ | Unk |
Unk, Unknown; -, No change; ↑, Upregulated; ↓, Downregulated
Role in chronological skin aging
Skin aging is a complicated process involving both intrinsic and extrinsic factors and is accompanied by a loss of morphologic and physiologic characteristics. There are two main, distinct types of aging scenarios that affect the skin: chronological skin aging and photoaging. Chronological aging is a spontaneous process that proceeds with elapsed time, and throughout the body, mitochondrial sirtuins have been found to play an important role in chronological aging and aging-related diseases (22). For example, in one mouse model, the loss of SIRT3 was found to accelerate the aging process (23). Another study found reduced levels of SIRT3 in various organs and tissues in aged mice, including adipose, kidney, and lung tissues, although no change was seen in the skin (24). Interestingly, an in vitro study revealed an upregulation of SIRT4 in human foreskin fibroblasts with increased passage numbers (25), associated with the downregulation of miR-15b, a repressor of SIRT4 mRNA. This study suggested that SIRT4 may be linked to aging in human fibroblasts. Although lack of SIRT5 has been shown to exacerbate aging-related diseases like Parkinson’s disease (26), there is so far no data available addressing the role of SIRT5 in skin aging. The scarcity of available research on mitochondrial sirtuins in chronological skin aging makes it difficult to reach a conclusion at this time, though their roles in other aging-related conditions/diseases strongly suggest that SIRT3, SIRT4 and SIRT5 need to be further explored.
Role in ultraviolet radiation (UVR) response and photoaging
Photoaging is premature aging of the skin primarily instigated by frequent UVR exposure (27, 28). Solar UVR that reaches the atmosphere is comprised of 90–99% UVA (320–400 nm) and 1–10% UVB (280–320 nm). Most of the high energy UVR (UVC, 200-290 nm) is absorbed by stratospheric ozone. Both UVA as well as UVB have been implicated in various cutaneous conditions. Indeed, UVR has numerous effects on the skin, both beneficial and harmful. While UVR catalyzes the synthesis of vitamin D in the skin and may be used to treat certain skin disorders like psoriasis, it also is a primary contributor to detrimental processes such as photoaging, DNA damage, and ROS generation (29, 30). A study by Iwahara and colleagues suggested a UVR-responsive role for SIRT3 by showing that in HeLa and U2OS cells, the full-length form of SIRT3 was directly degraded in the nucleus after exposure to UVR (70 J/m2), which resulted in increased stress-responsive genes (31). Another study found that UV exposure in normal human epidermal keratinocytes (NHEKs) increased mitochondrial reactive oxygen species (mtROS), as well as dysregulated SIRT3 and SIRT4 (32). In this study, NHEKs were exposed to UVB (10 mJ / cm2) and monitored for up to 10 hours. They observed a sustained reduction of SIRT3 mRNA, although SIRT4 mRNA levels were only transiently reduced at 3 hours and ultimately upregulated by 10 hours (32). Fluctuating SIRT4 levels were reported in another study that employed the broader light spectrum of solar simulated light (SSL, 4.152 J/cm2 UVA and 193.8 mJ/cm2 UVB) on NHEKs, where SIRT4 mRNA levels were shown to be increased at 5 hours post-treatment, and maintained it until 8 hours (33). The discrepancy between increased SIRT4 mRNA (by 5 hours) and protein (8 hours and later) is likely due to the time lag between transcription and translation (33). A similar trend was observed in another recent study that found an increase in SIRT4 at 72 hours after UVR (30 mJ/cm2 of 75% UVB and 25% UVA) (34). Although there is currently no direct evidence linking SIRT5 to photoaging or UVR, it appears that SIRT5 may contribute to the retrieval of cell viability after UVR in keratinocytes due to its role in glycolysis regulation (35), as the augmentation of glycolysis can recover UV-stressed keratinocytes (60 mJ/cm2 of 68% UVB and 32% of UVA ) (36). Overall, the available data suggest that mitochondrial sirtuins, especially SIRT4 may play a key role in the response of human keratinocytes to UV damage, and may, therefore, be potential targets to combat photoaging. However, more research needs to be done on this subject to fully understand the roles and significance of mitochondrial sirtuins in cutaneous aging, especially in in vivo models.
Role in ozone exposure response
Another environmental hazard that the skin is exposed to is ozone. Ozone is a gas that consists of three oxygen atoms (O3) that naturally occur in the Earth’s atmosphere. Interestingly, ozone in the upper atmosphere is beneficial to the skin by blocking harmful UVR from passing through the atmosphere (37). In the last century, depletion of ozone in the atmosphere has increased the amount of UVR that the skin is exposed to and likely has contributed to the heightened incidence of skin cancer (38). Although ozone can be used to treat certain skin diseases (39), atmospheric ozone is categorized as an air pollutant (40). In terms of its interactions with the skin, ozone reacts with lipids on the surface of the skin, generating several inflammable products such as 4-OPA and 6-MHO (41). Together with ozone, these products are able to penetrate skin barrier (42, 41), which has been found to induce inflammation, reduction of antioxidants, and affect mitochondrial ROS in a mouse model (43). Evidence for the mitochondrial sirtuin-related response to ozone was found in a study where ozone (0.8 ppm) exposure to NHEKs was shown to cause a 48.2% reduction in SIRT3 levels (44). This study also found increased H2O2 levels, decreased ATP levels, increased DNA damage, and an increased inflammatory response as measured by IL-1α ELISA, suggesting an association between SIRT3 and cellular ozone damage response.
Although no direct study has been done to determine the role of SIRT4 in the skin’s response to ozone exposure, it appears that SIRT4 possesses tissue-specific effects on ROS perturbation. For example, SIRT4 induces ROS to accelerate cardiac hypertrophy by inhibiting the activity of MnSOD (45), but suppresses ROS and podocyte apoptosis to protect against diabetic nephropathy (46). A similar lack of studies exploring links between ozone and SIRT5 exists, although this protein may still serve as a guardian of the skin by eliminating ROS. Since it has been reported that SIRT5 was able to desuccinylate SOD1 to eliminate ROS (47), SIRT5 may play a similar role to SIRT3 in ROS scavenging in response to ozone exposure.
ROLE OF MITOCHONDRIAL SIRTUINS IN SKIN AND SKIN DISEASES
Mitochondrial sirtuins are integral to key protective processes employed by the skin against insults generated from environmental factors as well as biological mishaps. Keratinocyte differentiation is one such process. Below, we discuss the roles that have been discovered for mitochondrial sirtuins in keratinocyte differentiation, wound healing as well as in response to autoimmune diseases (Figure 1, Table 1).
Role in keratinocyte differentiation
Keratinocytes make up the largest portion of the epidermal biomass, and are involved in many cutaneous activities, such as skin inflammation (48), protection against UV damage (49), and wound healing (50). Keratinocyte proliferation and differentiation are essential in maintaining a functional and intact epidermis, and successful differentiation requires the involvement of specific molecules and proteins, including mtROS and SIRT3 protein. Increased mtROS has been shown to be involved in the activation of differentiation promoting proteins, including Notch and β-catenin (51). As they differentiate, keratinocytes detach from the basal layer of the epidermis and express early differentiation markers Keratins-1 and −10 (52). Bause et al. demonstrated that SIRT3 expression was decreased during keratinocyte differentiation, and the knockdown of SIRT3 in keratinocytes leads to elevated mtROS as well as increased differentiation markers Keratins −1 and −10 (53). Accordingly, overexpression of SIRT3 reversed the process, resulting in decreased mtROS and differentiation markers including loricrin and filaggrin (53). This suggests that SIRT3 is an important regulator of keratinocyte differentiation.
The involvement of mitochondrial sirtuins in keratinocyte differentiation is expected, as keratinocyte metabolism is continuously adjusted during this process. Although there are limited studies regarding the roles of SIRT4 and SIRT5 in keratinocyte differentiation, their roles in energy metabolism suggest they may also have roles in this important process. In one study, SIRT4 was found to be present in NHEK, and its expression was shown to be in opposition to SIRT3, likely due to their opposite roles in metabolism, especially in that SIRT3 activates glutamate dehydrogenase (GDH) while SIRT4 does the opposite (32, 54). However, the role of SIRT4 in differentiation has not been explored. In a different in vitro study, Benavente et al. simulated the differentiation process by growing undifferentiated keratinocytes with a media-air interface (33). They observed a transient increase of SIRT4 and SIRT5 expression, with their levels returned to the normal range upon terminal differentiation, suggesting that a transient increase in SIRT4 and/or SIRT5 may be involved in the differentiation process. Interestingly, although no concrete work has been done in keratinocytes, SIRT4 and SIRT5 have been found to regulate the differentiation of adipocytes. In one study, SIRT4 was shown to be upregulated in subcutaneous bovine adipocyte differentiation (55); while another group found that SIRT5 was necessary in the browning of subcutaneous white adipose tissue (56). These findings suggest that mitochondrial sirtuins, especially SIRT3, may be key modulators in the harmony and regulation of cellular differentiation.
Role in wound healing
Cutaneous wound healing is a complex but integrated process involving four distinct phases: homeostasis, inflammation, proliferation, and remodeling (57). A successful wound closure requires a subtle collaboration between the skin cells and non-skin cells, such as blood cells, immune cells, and stem cells. A recent mouse study found that SIRT3 plays an important role in wound repair, especially in wound macrophages (58). This study demonstrated significantly increased SIRT3 in wound macrophages during the transition from an inflammatory to a reparative stage. It was further demonstrated that FAB4, a fatty acid-binding protein, was inversely correlated with SIRT3 in wound macrophages (58). Further, the SIRT3 deficient mice displayed impaired wound repair. In addition, the wound macrophages isolated from SIRT3-deficient mice showed an increase in pro-inflammatory factors IL1β, Tnfα, and Nos2, which were reversed by the adoptive transfer of macrophages from SIRT3-competent mice, resulting in improved healing (58). In addition, this study also found that under pre-diabetic conditions, SIRT3 was decreased in wound macrophages, likely due to the upregulation of FAB4, which resulted in sustained secretion of pro-inflammatory factors (58). Another group found that the administration of MC2562, a SIRT3 activator, accelerated the process of wound healing in mice (59). However, this acceleration may not primarily result from the activation of SIRT3, as MC2562 also activates SIRT1 and SIRT2, albeit at higher concentrations (6). There has been no work done on wound healing in the other mitochondrial sirtuins, although their roles in cellular metabolism suggest that they may be involved and should be explored further.
Role in fibrotic and scarring diseases
Systemic sclerosis (SS), also known as scleroderma, is a rare autoimmune disease characterized by vasculopathy and fibrosis of the skin and internal organs, with a 74.9% five-year survival rate from diagnosis (60). SS is marked by the replacement of normal skin tissues with extracellular matrix and collagen, as well as decreased expression of collagenase in skin fibroblasts (61). Although the exact etiology of SS is unknown, abnormalities in several cell signaling events have been implicated in the fibroblasts of SS patients, including cytokines and growth factors (62), as well as SIRT3 signaling (63). In a mouse study, SIRT3 knockout was found to promote tissue fibrosis in multiple organs through induction of fibroblastic TGF-β (64), a critical signaling molecule in SS and other fibrotic diseases involved in the production of the extracellular matrix (65). In a study by Akamata et al., SIRT3 was found to be reduced in SS skin biopsies and was associated with increased ROS and TGF-β-induced fibrotic gene expression (66). Further, it was also found that induction of SIRT3 by a novel fluorinated synthetic honokiol analogue hexafluoro or genetic overexpression attenuated mtROS and TGF-β expression, while overexpression of TGF-β in normal fibroblasts lead to reduced SIRT3 and increased ROS (66). Therefore, SIRT3 appears to have potential anti-fibrotic roles in SS fibroblasts, and the activation of SIRT3 may be a therapeutic strategy in the treatment of SS through inhibition of the TGF-β pathway. This appears to be a different regulatory mechanism from the macrophage-based SIRT3 modulation that occurs during wound healing, suggesting that SIRT3 is linked to multiple skin healing pathways.
Although there have been no reports of SIRT4 in SS, this sirtuin has been suggested to play a role in other fibrosis-involved diseases, such as central centrifugal cicatricial alopecia (CCCA). CCCA is common in African American females and is marked by hair loss (primarily in the central part of the scalp) and scarring alopecia marked by activation of fibroblast (66). In a transcriptome analysis of scalps from five CCCA patients, SIRT4 was found to be downregulated, indicating a potentially protective role of SIRT4 in CCCA (67). However, this data is far from conclusive, and further research is needed to investigate the roles of SIRT4 and the rest sirtuins in CCCA and other fibrosis-related diseases that can affect the skin.
ROLE OF MITOCHONDRIAL SIRTUINS IN SKIN CANCERS
The known functions of mitochondrial sirtuins in metabolism and energy production point towards their possible roles in cancer progression. However, the role of mitochondrial sirtuins in cancer appears to be complex, with evidence suggesting both tumor promoter and suppressor functions. The conflicting evidence based on a number of studies suggests that all three mitochondrial sirtuins can act either as a tumor suppressor or as a tumor promoter, likely depending on the cellular and/or tissue context. This has been discussed further in several recent reviews (68-70), and persists in the context of skin cancer as well.
Skin cancers are the most common human cancer, and its main types are divided into melanoma and non-melanoma skin cancer (NMSC), which includes basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC). UV exposure has been identified as one of the major epidemiologic risk factors of all three types of skin cancer (29), as UV-induced DNA photoproducts can be highly mutagenetic (71). In addition to DNA mutations, epigenetic abnormities and altered cellular metabolism exist widely in skin cancer, providing a rationale for the role of the mitochondrial sirtuins in cutaneous neoplasms. Below, we discuss the roles for mitochondrial sirtuins that have been observed in the three main types of skin cancers (Figure 1, Table 1).
Role in melanoma
Arising from the melanocytes in the epidermis, melanoma is one of the deadliest types of skin cancer, and its incidence has been increasing over the past 30 years (72). Environmental and behavioral changes are likely responsible for this, with increased UVR exposure leading signature UV-mediated C→T base pair conversions. As the most frequently mutated gene in melanoma, BRAF mutations exist in over 50% of melanoma patients (73, 74). The BRAF mutation has two major roles in the progression from normal skin to melanoma: 1) it initiates the genesis of nevi from melanocytes (75) and 2) it then cooperates with subsequent driver mutations, such as TERT (76) and/or p53 (77), to promote the transition from nevi to melanoma.
The involvement of mitochondrial sirtuins in melanoma development and progression is not very well understood. Recently, we have demonstrated that SIRT3 may act as a tumor promoter in melanoma (78). In our study, we found that SIRT3 is significantly overexpressed in human melanoma cell lines as well as clinical melanoma tissue samples. Additionally, knockdown of SIRT3 by small hairpin RNA resulted in decreased cell growth, G1-phase cell cycle arrest and senescence induction, as evidenced by increased senescence-associated beta-galactosidase activity and p16 and p21 protein expression in melanoma cell lines. Moreover, forced overexpression of SIRT3 promoted the proliferation of melanoma cells as well as normal immortalized Mel-ST melanocytes. In a xenograft mouse model, knockdown of SIRT3 inhibited tumor growth and improved overall survival rate, emphasizing the potential tumor promotor role of SIRT3 in melanoma (78). The tumor promotor role of SIRT3 is also supported by a very recent study by Torrens-Mas et al., where the authors found that mutant p53 induces SIRT3/MnSOD axis to moderate ROS production in melanoma (79). In this study, the mutant p53 in MeWo melanoma cells was shown to promote SIRT3, which enhanced the activity of manganese superoxide dismutase (MnSOD), leading to a tempered ROS level that helps the melanoma cells survive the cytotoxic ROS environment. In a separate study from our laboratory, we demonstrated the effect of 4’-bromo-resveratrol, a dual SIRT1 and 3 inhibitor (80), against human melanoma cells (81). In this study, we found that 4’-bromo-resveratrol treatment imparted antiproliferative effects against human melanoma cells through metabolic reprogramming and effects on the cell cycle and apoptosis signaling. This further confirms the tumor promoter role of SIRT3 in melanoma (81).
The role of SIRT4 in melanoma, on the other hand, has not been explored other than a study that found an upregulation of SIRT4 in melanoma after administration of melphalan, a chemotherapy drug (82). Consistent with the tumor suppressor vs. promoter debate in other cancers, there are differing studies regarding the role of SIRT5 in melanoma. In one study, knockdown of SIRT5 decreased the proliferation of melanoma cells both in vivo and in vitro (83). However, another study using a BRAFV600E mouse model showed that SIRT5 knockout did not affect melanoma development or progression (84). Therefore, additional research is needed to understand the exact roles of SIRT4 and SIRT5 in melanoma.
Role in basal cell carcinoma (BCC)
BCC of the skin is derived from keratinocytes located in the basal layer of the epidermis, and is the most common human cancer, with millions of people affected by this neoplasm every year in the United States (85). Although low in metastatic and mortality rates, BCC can be associated with significant morbidity, and patients diagnosed with this neoplasm are at a greater risk for developing melanoma (86). Similar to melanoma, UV is a major environmental carcinogen of BCC. UV-mediated C→T conversions are observed in several tumor suppressor genes in BCC patients, including patched 1 (PTCH1) and p53 (88). Although direct research on mitochondrial sirtuins and their response to UV in this neoplasm are severely lacking, the links between SIRT3 and p53, especially those discussed above in the melanoma section, suggest that further research will uncover a clear relationship in BCC. In a recent epidemiology study, it was found that people receiving a high level of occupational UV exposure were associated with an increased risk of BCC (87). In this paper, Temel et al. sampled both BCC and normal skin biopsies from patients, and analyzed the mRNA expressions of all the sirtuins (89). For the mitochondrial sirtuins, they found that SIRT3 mRNA was decreased in BCC samples as compared to normal tissues, suggesting a tumor-suppressive role for SIRT3 in BCC. SIRT4 and SIRT5 were found to have no change between normal and BCC tissues, which suggests they may not be involved in BCC progression, although significantly more research needs to be done on all three mitochondrial sirtuins to better elucidate their mechanisms in BCC.
Role in squamous cell carcinoma (SCC)
Depending on its anatomic site, SCC can be separated into four sub-types: cutaneous SCC (cSCC), head and neck SCC (HNSCC), esophageal SCC (ESCC), and lung SCC (LUSC) (90). Although they can all be classified as SCC, the only cSCC is classified as skin cancer and will be discussed here. cSCC is a neoplasm of keratinocytes arising from the squamous layer of the epidermis and is the second most common skin cancer in the United States with a trend of increasing incidence (91, 85). Similar to BCC, UV exposure is the major cause of cSCC (92). A UV signature characterized by C→T conversions exists in most cSCC patients (93), but whether this is related to mitochondrial sirtuins are unknown. Interestingly, to date, the only evidence that exists for all three mitochondrial sirtuins suggests that they play a tumor promotor role in cSCC (33). The research compared sirtuin expression of actinic keratosis, a pre-cSCC lesion, and cSCC biopsies to their normal adjacent skin, and found that SIRT3, SIRT4 and SIRT5 were upregulated in both actinic keratosis and cSCC. Although the evidence points towards a potential tumor promotor role for the mitochondrial sirtuins in cSCC, more extensive in vitro and in vivo research is needed to draw any definitive conclusions.
CONCLUSIONS
Mitochondrial sirtuins have been found to play important roles in maintaining skin homeostasis against environmental stress, and the alteration of mitochondrial sirtuins is closely related to skin dysfunctions and cancers. The finding that SIRT3, SIRT4 and SIRT5 are dysregulated in several dermatological conditions may provide us avenues to maintaining skin health. Additionally, the dysregulation of these sirtuins in skin diseases and cancers may provide an opening to develop new therapeutic strategies. However, the mechanism of mitochondrial sirtuins in dermatological conditions has not been fully discovered, especially that of SIRT4 and SIRT5. Therefore, future research is needed to uncover the functional relevance of mitochondrial sirtuins in skin diseases, including skin cancer.
ACKNOWLEDGEMENTS
This work was partially supported by funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant number R01AR059130 to NA), and the Department of Veterans Affairs (VA Merit Review Awards I01CX001441 and I01BX004221, and a Research Career Scientist Award IK6BX003780 to NA). We also acknowledge the core facilities supported by the Skin Diseases Research Center Core Grant P30AR066524 from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- 1.Imai S, Armstrong CM, Kaeberlein M and Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800. [DOI] [PubMed] [Google Scholar]
- 2.Blander G and Guarente L (2004) The Sir2 family of protein deacetylases. Annu. Rev. Biochem 73, 417–435. [DOI] [PubMed] [Google Scholar]
- 3.Saunders LR and Verdin E (2007) Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene 26, 5489–5504. [DOI] [PubMed] [Google Scholar]
- 4.Haigis MC and Sinclair DA (2010) Mammalian sirtuins: biological insights and disease relevance. Annu. Rev. Pathol 5, 253–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang JH, Kim HC, Hwang KY, Lee JW, Jackson SP, Bell SD and Cho Y (2002) Structural basis for the NAD-dependent deacetylase mechanism of Sir2. J. Biol. Chem 277, 34489–34498. [DOI] [PubMed] [Google Scholar]
- 6.Carafa V, Rotili D, Forgione M, Cuomo F, Serretiello E, Hailu GS, Jarho E, Lahtela-Kakkonen M, Mai A and Altucci L (2016) Sirtuin functions and modulation: from chemistry to the clinic. Clin. Epigenetics 8, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kupis W, Palyga J, Tomal E and Niewiadomska E (2016) The role of sirtuins in cellular homeostasis. J. Physiol. Biochem 72, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michan S and Sinclair D (2007) Sirtuins in mammals: insights into their biological function. Biochem. J 404, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michishita E, Park JY, Burneskis JM, Barrett JC and Horikawa I (2005) Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell 16, 4623–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He W, Newman JC, Wang MZ, Ho L and Verdin E (2012) Mitochondrial sirtuins: regulators of protein acylation and metabolism. Trends Endocrinol. Metab 23, 467–476. [DOI] [PubMed] [Google Scholar]
- 11.Lee N, Kim D-K, Kim E-S, Park SJ, Kwon J-H, Shin J, Park S-M, Moon YH, Wang HJ, Gho YS and Choi KY (2014) Comparative interactomes of SIRT6 and SIRT7: Implication of functional links to aging. Proteomics 14, 1610–1622. [DOI] [PubMed] [Google Scholar]
- 12.Bause AS and Haigis MC (2013) SIRT3 regulation of mitochondrial oxidative stress. Exp. Gerontol 48, 634–639. [DOI] [PubMed] [Google Scholar]
- 13.Laurent G, German Natalie J., Saha Asish K., de Boer Vincent C. J., Davies M, Koves Timothy R., Dephoure N, Fischer F, Boanca G, Vaitheesvaran B, Lovitch Scott B., Sharpe Arlene H., Kurland Irwin J., Steegborn C, Gygi Steven P., Muoio Deborah M., Ruderman Neil B. and Haigis Marcia C. (2013) SIRT4 Coordinates the Balance between Lipid Synthesis and Catabolism by Repressing Malonyl CoA Decarboxylase. Mol. Cell 50, 686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pannek M, Simic Z, Fuszard M, Meleshin M, Rotili D, Mai A, Schutkowski M and Steegborn C (2017) Crystal structures of the mitochondrial deacylase Sirtuin 4 reveal isoform-specific acyl recognition and regulation features. Nat. Commun 8, 1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathias RA, Greco TM, Oberstein A, Budayeva HG, Chakrabarti R, Rowland EA, Kang Y, Shenk T and Cristea IM (2014) Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell 159, 1615–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P and Verdin E (2007) Regulation of Insulin Secretion by SIRT4, a Mitochondrial ADP-ribosyltransferase. J. Biol. Chem 282, 33583–33592. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa T, Lomb DJ, Haigis MC and Guarente L (2009) SIRT5 Deacetylates Carbamoyl Phosphate Synthetase 1 and Regulates the Urea Cycle. Cell 137, 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q and Lin H (2011) Sirt5 Is a NAD-Dependent Protein Lysine Demalonylase and Desuccinylase. Science 334, 806–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan M, Peng C, Anderson Kristin A., Chhoy P, Xie Z, Dai L, Park J, Chen Y, Huang H, Zhang Y, Ro J, Wagner Gregory R., Green Michelle F., Madsen Andreas S., Schmiesing J, Peterson Brett S., Xu G, Ilkayeva Olga R., Muehlbauer Michael J., Braulke T, Mühlhausen C, Backos Donald S., Olsen Christian A., McGuire Peter J., Pletcher Scott D., Lombard David B., Hirschey Matthew D. and Zhao Y (2014) Lysine Glutarylation Is a Protein Posttranslational Modification Regulated by SIRT5. Cell Metab. 19, 605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh CK, Chhabra G, Ndiaye MA, Garcia-Peterson LM, Mack NJ and Ahmad N (2018) The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal 28, 643–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osborne B, Bentley NL, Montgomery MK and Turner N (2016) The role of mitochondrial sirtuins in health and disease. Free Radic. Biol. Med 100, 164–174. [DOI] [PubMed] [Google Scholar]
- 22.van de Ven RAH, Santos D and Haigis MC (2017) Mitochondrial Sirtuins and Molecular Mechanisms of Aging. Trends Mol. Med 23, 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonnell E, Peterson BS, Bomze HM and Hirschey MD (2015) SIRT3 regulates progression and development of diseases of aging. Trends Endocrinol. Metab 26, 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon Y, Kim J, Lee C-Y and Kim H (2015) Expression of SIRT1 and SIRT3 varies according to age in mice. Anat. Cell Biol 48, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang A, Grether-Beck S, Singh M, Kuck F, Jakob S, Kefalas A, Altinoluk-Hambüchen S, Graffmann N, Schneider M, Lindecke A, Brenden H, Felsner I, Ezzahoini H, Marini A, Weinhold S, Vierkötter A, Tigges J, Schmidt S, Stühler K, Köhrer K, Uhrberg M, Haendeler J, Krutmann J and Piekorz RP (2016) MicroRNA-15b regulates mitochondrial ROS production and the senescence-associated secretory phenotype through sirtuin 4/SIRT4. Aging (Albany NY) 8, 484–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Peritore C, Ginsberg J, Shih J, Arun S and Donmez G (2015) Protective role of SIRT5 against motor deficit and dopaminergic degeneration in MPTP-induced mice model of Parkinson’s disease. Behav. Brain Res 281, 215–221. [DOI] [PubMed] [Google Scholar]
- 27.Fisher GJ, Kang S, Varani J and et al. (2002) Mechanisms of photoaging and chronological skin aging. Arch. Dermatol 138, 1462–1470. [DOI] [PubMed] [Google Scholar]
- 28.Helfrich YR, Sachs DL and Voorhees JJ (2008) Overview of skin aging and photoaging. Dermatol. Nurs 20, 177. [PubMed] [Google Scholar]
- 29.D’Orazio J, Jarrett S, Amaro-Ortiz A and Scott T (2013) UV radiation and the skin. Int. J. Mol. Sci 14, 12222–12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Archier E, Devaux S, Castela E, Gallini A, Aubin F, Le Maitre M, Aractingi S, Bachelez H, Cribier B, Joly P, Jullien D, Misery L, Paul C, Ortonne JP and Richard MA (2012) Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J. Eur. Acad. Dermatol. Venereol 26 Suppl 3, 22–31. [DOI] [PubMed] [Google Scholar]
- 31.Iwahara T, Bonasio R, Narendra V and Reinberg D (2012) SIRT3 Functions in the Nucleus in the Control of Stress-Related Gene Expression. Mol. Cell. Biol 32, 5022–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong K, Pelle E, Yarosh DB and Pernodet N (2012) Sirtuin 4 identification in normal human epidermal keratinocytes and its relation to sirtuin 3 and energy metabolism under normal conditions and UVB-induced stress. Exp. Dermatol 21, 231–233. [DOI] [PubMed] [Google Scholar]
- 33.Benavente CA, Schnell SA and Jacobson EL (2012) Effects of niacin restriction on sirtuin and PARP responses to photodamage in human skin. PLoS One 7, e42276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen Y, Stanislauskas M, Li G, Zheng D and Liu L (2017) Epigenetic and genetic dissections of UV-induced global gene dysregulation in skin cells through multi-omics analyses. Sci. Rep 7, 42646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishida Y, Rardin Matthew J., Carrico C, He W, Sahu Alexandria K., Gut P, Najjar R, Fitch M, Hellerstein M, Gibson Bradford W.and Verdin E (2015) SIRT5 Regulates both Cytosolic and Mitochondrial Protein Malonylation with Glycolysis as a Major Target. Mol. Cell 59, 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schütz R, Kuratli K, Richard N, Stoll C and Schwager J (2016) Mitochondrial and glycolytic activity of UV-irradiated human keratinocytes and its stimulation by a Saccharomyces cerevisiae autolysate. J. Photochem. Photobiol. B 159, 142–148. [DOI] [PubMed] [Google Scholar]
- 37.Brian D (2004) Climate change, ozone depletion and the impact on ultraviolet exposure of human skin. Phys. Med. Biol 49, R1–11. [DOI] [PubMed] [Google Scholar]
- 38.Diepgen T and Mahler V (2002) The epidemiology of skin cancer. Br. J. Dermatol 146, 1–6. [DOI] [PubMed] [Google Scholar]
- 39.Zeng J and Lu J (2018) Mechanisms of action involved in ozone-therapy in skin diseases. Int. Immunopharmacol 56, 235–241. [DOI] [PubMed] [Google Scholar]
- 40.Nuvolone D, Petri D and Voller F (2018) The effects of ozone on human health. Environ. Sci. Pollut. Res. Int 25, 8074–8088. [DOI] [PubMed] [Google Scholar]
- 41.Lakey PSJ, Wisthaler A, Berkemeier T, Mikoviny T, Pöschl U and Shiraiwa M (2017) Chemical kinetics of multiphase reactions between ozone and human skin lipids: Implications for indoor air quality and health effects. Indoor Air 27, 816–828. [DOI] [PubMed] [Google Scholar]
- 42.Travagli V, Zanardi I, Valacchi G and Bocci V (2010) Ozone and ozonated oils in skin diseases: a review. Mediators Inflamm. 610418, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valacchi G, Pagnin E, Corbacho AM, Olano E, Davis PA, Packer L and Cross CE (2004) In vivo ozone exposure induces antioxidant/stress-related responses in murine lung and skin. Free Radic. Biol. Med 36, 673–681. [DOI] [PubMed] [Google Scholar]
- 44.McCarthy JT, Pelle E, Dong K, Brahmbhatt K, Yarosh D and Pernodet N (2013) Effects of ozone in normal human epidermal keratinocytes. Exp. Dermatol 22, 360–361. [DOI] [PubMed] [Google Scholar]
- 45.Luo Y-X, Tang X, An X-Z, Xie X-M, Chen X-F, Zhao X, Hao D-L, Chen H-Z and Liu D-P (2017) SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur. Heart J 38, 1389–1398. [DOI] [PubMed] [Google Scholar]
- 46.Shi J-X, Wang Q-J, Li H and Huang Q (2017) SIRT4 overexpression protects against diabetic nephropathy by inhibiting podocyte apoptosis. Exp. Ther. Med 13, 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Lin Z-F, Xu H-B, Wang J-Y, Lin Q, Ruan Z, Liu F-B, Jin W, Huang H-H and Chen X (2013) SIRT5 desuccinylates and activates SOD1 to eliminate ROS. Biochem. Biophys. Res. Commun 441, 191–195. [DOI] [PubMed] [Google Scholar]
- 48.Johnston A, Fritz Y, Dawes SM, Diaconu D, Al-Attar PM, Guzman AM, Chen CS, Fu W, Gudjonsson JE and McCormick TS (2013) Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J. Immunol 190, 2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao C and Wan Y (2009) Parameters of protection against ultraviolet radiation-induced skin cell damage. J. Cell Physiol 220, 277–284. [DOI] [PubMed] [Google Scholar]
- 50.Li D, Li XI, Wang A, Meisgen F, Pivarcsi A, Sonkoly E, Stahle M and Landen NX (2015) MicroRNA-31 Promotes Skin Wound Healing by Enhancing Keratinocyte Proliferation and Migration. J. Invest. Dermatol 135, 1676–1685. [DOI] [PubMed] [Google Scholar]
- 51.Hamanaka RB, Glasauer A, Hoover P, Yang S, Blatt H, Mullen AR, Getsios S, Gottardi CJ, DeBerardinis RJ, Lavker RM and Chandel NS (2013) Mitochondrial Reactive Oxygen Species Promote Epidermal Differentiation and Hair Follicle Development. Sci. Signal 6, ra8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu S, Oh H-S, Shim M, Sterneck E, Johnson PF and Smart RC (1999) C/EBPβ modulates the early events of keratinocyte differentiation involving growth arrest and keratin 1 and keratin 10 expression. Mol. Cell. Biol 19, 7181–7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bause AS, Matsui MS and Haigis MC (2013) The Protein Deacetylase SIRT3 Prevents Oxidative Stress-induced Keratinocyte Differentiation. J. Biol. Chem 288, 36484–36491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verdin E, Hirschey MD, Finley LW and Haigis MC (2010) Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem. Sci 35, 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong J, Li S, Wang X, Mei C and Zan L (2018) Study of expression analysis of SIRT4 and the coordinate regulation of bovine adipocyte differentiation by SIRT4 and its transcription factors. Biosci. Rep 38, BSR20181705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shuai L, Zhang L-N, Li B-H, Tang C-L, Wu L-Y, Li J and Li J-Y (2019) SIRT5 Regulates Brown Adipocyte Differentiation and Browning of Subcutaneous White Adipose Tissue. Diabetes 68, 1449–1461. [DOI] [PubMed] [Google Scholar]
- 57.Guo S and DiPietro LA (2010) Factors Affecting Wound Healing. J. Dent. Res 89, 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boniakowski AM, denDekker AD, Davis FM, Joshi A, Kimball AS, Schaller M, Allen R, Bermick J, Nycz D, Skinner ME, Robinson S, Obi AT, Moore BB, Gudjonsson JE, Lombard D, Kunkel SL and Gallagher KA (2019) SIRT3 Regulates Macrophage-Mediated Inflammation in Diabetic Wound Repair. J. Invest. Dermatol 139, 2528–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spallotta F, Cencioni C, Straino S, Nanni S, Rosati J, Artuso S, Manni I, Colussi C, Piaggio G and Martelli F (2013) A nitric oxide-dependent cross-talk between class I and III histone deacetylases accelerates skin repair. J. Biol. Chem 288, 11004–11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Denton CP and Khanna D (2017) Systemic sclerosis. Lancet 390, 1685–1699. [DOI] [PubMed] [Google Scholar]
- 61.Takeda K, Hatamochi A, Ueki H, Nakata M and Oishi Y (1994) Decreased collagenase expression in cultured systemic sclerosis fibroblasts. J. Invest. Dermatol 103, 359–363. [DOI] [PubMed] [Google Scholar]
- 62.Kawaguchi Y, Hara M and Wright TM (1999) Endogenous IL-1α from systemic sclerosis fibroblasts induces IL-6 and PDGF-A. J. Clin. Invest 103, 1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akamata K, Wei J, Bhattacharyya M, Cheresh P, Bonner MY, Arbiser JL, Raparia K, Gupta MP, Kamp DW and Varga J (2016) SIRT3 is attenuated in systemic sclerosis skin and lungs, and its pharmacologic activation mitigates organ fibrosis. Oncotarget 7, 69321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sundaresan NR, Bindu S, Pillai VB, Samant S, Pan Y, Huang JY, Gupta M, Nagalingam RS, Wolfgeher D, Verdin E and Gupta MP (2015) SIRT3 Blocks Aging-Associated Tissue Fibrosis in Mice by Deacetylating and Activating Glycogen Synthase Kinase 3beta. Mol. Cell. Biol 36, 678–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ayers NB, Sun C-M and Chen S-Y (2018) Transforming growth factor-β signaling in systemic sclerosis. J. Biomed. Res 32, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Subash J, Alexander T, Beamer V and McMichael A (2018) A proposed mechanism for central centrifugal cicatricial alopecia. Exp. Dermatol, 29, 190–195. [DOI] [PubMed] [Google Scholar]
- 67.Aguh C, Dina Y, Talbot CC Jr. and Garza L (2018) Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J. Am. Acad. Dermatol 79, 904–912 e901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen Y, Fu LL, Wen X, Wang XY, Liu J, Cheng Y and Huang J (2014) Sirtuin-3 (SIRT3), a therapeutic target with oncogenic and tumor-suppressive function in cancer. Cell Death Dis. 5, e1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang G and Zhu G (2018) Sirtuin-4 (SIRT4), a therapeutic target with oncogenic and tumor-suppressive activity in cancer. Onco. Targets Ther 11, 3395–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bringman-Rodenbarger LR, Guo AH, Lyssiotis CA and Lombard DB (2018) Emerging Roles for SIRT5 in Metabolism and Cancer. Antioxid. Redox Signal 28, 677–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pfeifer GP and Besaratinia A (2012) UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem. Photobiol. Sci 11, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siegel RL, Miller KD and Jemal A (2019) Cancer statistics, 2019. CA Cancer J. Clin 69, 7–34. [DOI] [PubMed] [Google Scholar]
- 73.Ascierto PA, Kirkwood JM, Grob J-J, Simeone E, Grimaldi AM, Maio M, Palmieri G, Testori A, Marincola FM and Mozzillo N (2012) The role of BRAF V600 mutation in melanoma. J. Transl. Med 10, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhatia P, Friedlander P, Zakaria EA and Kandil E (2015) Impact of BRAF mutation status in the prognosis of cutaneous melanoma: an area of ongoing research. Ann. Transl. Med 3, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yeh I, von Deimling A and Bastian BC (2013) Clonal BRAF Mutations in Melanocytic Nevi and Initiating Role of BRAF in Melanocytic Neoplasia. J. Natl. Cancer Inst 105, 917–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y, Cheng HS, Chng WJ and Tergaonkar V (2016) Activation of mutant TERT promoter by RAS-ERK signaling is a key step in malignant progression of BRAF-mutant human melanomas. Proc. Natl. Acad. Sci 113, 14402–14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shain AH, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A, Dummer R, North J, Pincus L, Ruben B, Rickaby W, D’Arrigo C, Robson A and Bastian BC (2015) The Genetic Evolution of Melanoma from Precursor Lesions. N. Engl. J. Med 373, 1926–1936. [DOI] [PubMed] [Google Scholar]
- 78.George J, Nihal M, Singh CK, Zhong W, Liu X and Ahmad N (2016) Pro-proliferative function of mitochondrial sirtuin deacetylase SIRT3 in human melanoma. J. Invest. Dermatol 136, 809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Torrens-Mas M, Cordani M, Mullappilly N, Pacchiana R, Riganti C, Palmieri M, Pons DG, Roca P, Oliver J and Donadelli M (2020) Mutant p53 induces SIRT3/MnSOD axis to moderate ROS production in melanoma cells. Arch. Biochem. Biophys 679, 108219. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen GT, Gertz M and Steegborn C (2013) Crystal structures of Sirt3 complexes with 4’-bromo-resveratrol reveal binding sites and inhibition mechanism. Chem Biol 20, 1375–1385. [DOI] [PubMed] [Google Scholar]
- 81.George J, Nihal M, Singh CK and Ahmad N (2019) 4’-Bromo-resveratrol, a dual Sirtuin-1 and Sirtuin-3 inhibitor, inhibits melanoma cell growth through mitochondrial metabolic reprogramming. Mol. Carcinog 58, 1876–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wouters J, Stas M, Govaere O, Van den Eynde K, Vankelecom H and van den Oord JJ (2012) Gene expression changes in melanoma metastases in response to high-dose chemotherapy during isolated limb perfusion. Pigment Cell Melanoma Res. 25, 454–465. [DOI] [PubMed] [Google Scholar]
- 83.Park J, Chen K, Park J, Pak M, Verhaegen M, Fullen D, Scott D, Osterman A, Wang M, Andea A, Prichard A, Scolyer R, Wilmott J and Lombard DB (2016) 293 - Human Melanoma Cell Need SIRT5 to Survive. Free Radic. Biol. Med 100, S128. [Google Scholar]
- 84.Moon H, Zhu J and White AC (2019) Sirt5 is dispensable for Braf(V600E) -mediated cutaneous melanoma development and growth in vivo. Exp. Dermatol 28, 83–85. [DOI] [PubMed] [Google Scholar]
- 85.Rogers HW, Weinstock MA, Feldman SR and Coldiron BM (2015) Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol 151, 1081–1086. [DOI] [PubMed] [Google Scholar]
- 86.Marzuka AG and Book SE (2015) Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J. Biol. Med 88, 167–179. [PMC free article] [PubMed] [Google Scholar]
- 87.Schmitt J, Haufe E, Trautmann F, Schulze HJ, Elsner P, Drexler H, Bauer A, Letzel S, John SM, Fartasch M, Bruning T, Seidler A, Dugas-Breit S, Gina M, Weistenhofer W, Bachmann K, Bruhn I, Lang BM, Bonness S, Allam JP, Grobe W, Stange T, Westerhausen S, Knuschke P, Wittlich M, Diepgen TL and Group FBS (2018) Occupational UV-Exposure is a Major Risk Factor for Basal Cell Carcinoma: Results of the Population-Based Case-Control Study FB-181. J. Occup. Environ. Med 60, 36–43. [DOI] [PubMed] [Google Scholar]
- 88.Dessinioti C, Antoniou C, Katsambas A and Stratigos AJ (2010) Basal cell carcinoma: what’s new under the sun. Photochem. Photobiol 86, 481–491. [DOI] [PubMed] [Google Scholar]
- 89.Temel M, Koç MN, Ulutaş S and Göğebakan B (2016) The expression levels of the sirtuins in patients with BCC. Tumor Biol. 37, 6429–6435. [DOI] [PubMed] [Google Scholar]
- 90.Yan W, Wistuba II, Emmert-Buck MR and Erickson HS (2011) Squamous cell carcinoma – similarities and differences among anatomical sites. Am. J. Cancer Res 1, 275–300. [PMC free article] [PubMed] [Google Scholar]
- 91.Karia PS, Han J and Schmults CD (2013) Cutaneous squamous cell carcinoma: Estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J. Am. Acad. Dermatol 68, 957–966. [DOI] [PubMed] [Google Scholar]
- 92.Schmitt J, Seidler A, Diepgen TL and Bauer A (2011) Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: a systematic review and meta-analysis. Br. J. Dermatol 164, 291–307. [DOI] [PubMed] [Google Scholar]
- 93.Pickering CR, Zhou JH, Lee JJ, Drummond JA, Peng SA, Saade RE, Tsai KY, Curry JL, Tetzlaff MT, Lai SY, Yu J, Muzny DM, Doddapaneni H, Shinbrot E, Covington KR, Zhang J, Seth S, Caulin C, Clayman GL, El-Naggar AK, Gibbs RA, Weber RS, Myers JN, Wheeler DA and Frederick MJ (2014) Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin. Cancer Res 20, 6582–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]