Metal ions in signaling: looking beyond Calcium
Cell signaling —the processes by which cells relay information from their environment to the intracellular machinery—is essential for cells to adjust to changes at the organismal and tissue level and produce a coordinated physiological response. Metal ions play a prominent role in these processes. Calcium(II), in particular, is widely recognized as a ubiquitous second messenger that impacts almost every aspect of cell physiology, ranging from cell motility to cell death.[1] Fully validated examples of other divalent main group cations and d-block metals in a signaling role, however, are much more difficult to pinpoint. This gap stems from a combination of pervasive conceptual and methodological limitations.
Conceptually, d-block metals have been traditionally considered to play structural and catalytic roles as static cofactors in proteins, thus controlling basal metabolic activity in the cell. The fraction of the total metal content that is “free” or, more fittingly, kinetically available is maintained at very low levels to prevent cytotoxicity, especially for redox-active metals that may otherwise generate harmful reactive oxygen species. This fact seems paradoxical with the occurrence of large transients in free ions first thought to be required for signaling. A recent account by Maret [2*] discusses how such transients can be generated for a tightly bound metal such as Zn2+. For example, either release from subcellular compartments or mobilization of the bound pool upon chemical modification of thiol-based metal binding sites by small redox-reactive species (e.g. nitric oxide action on metallothioneins) can change the levels of labile ion. Analogous models could be invoked for the mobilization of other tightly bound d-block metals, consistent with their unique bioinorganic chemistry. For these, it is clear that the term “free” metal does not apply in the same way as with Ca2+, and that changes in metal availability must be considered instead. With these considerations in mind, the possibility of d-block metal signaling has started to gain traction [3*], although whether the cations are involved as effectors of canonical signaling pathways (e.g. Ras/MAPK), regulators of second messengers (Ca2+), or acting as signal carriers of their own, is still matter of debate in many systems.
The notion of magnesium playing a role as a signaling ion has been considered for decades [4], but is still plagued by similar conceptual challenges to other metals at the other end of the concentration spectrum. The basal levels of intracellular free Mg2+ are relatively high—Mg2+ is the most abundant divalent cation in the cell—leaving little room for large transients [5,6*]. Furthermore, the extra-and intracellular concentrations of this metal are similar, raising questions about the feasibility of large fluxes in the absence of marked concentration gradients across membranes (though an electrochemical gradient is present). Yet an important, but often ignored, aspect of Mg2+ biology is that the main species comprising the “bound” pool are polyphosphates (e.g. MgATP) whose concentrations are highly dynamic. As such, localized changes in metal availability triggered by processes that alter the rate of synthesis or conversion of these species, altering the free to bound ratio of the metal, are possible. Moreover, based on the well-tuned binding affinity of some Mg2+-regulated proteins such as kinases and phosphatases, it is possible that relatively small transients—much smaller than previously recognized—are sufficient to generate a downstream effect. A bona fide sensor protein capable of carrying forward the effect of a Mg2+ signal, however, has remained elusive [7].
Full validation of a signaling event requires the demonstration of transient changes in the availability of the signaling species, completed with the identification of a source and a target. Changes in the free or available metal pools can be detected with small molecule fluorescent indicators that bind to kinetically accessible metals without disrupting the total cellular buffer. Armed with the right indicators, fluorescence microscopy can be a powerful tool for the study of metal signaling, providing the spatial resolution required to visualize the transient mobilization of intracellular metal cations in response to stimuli, combined with the temporal resolution to distinguish acute transients with reversible downstream effects from the chronic changes leading to long-term regulation. We present herein the most recent advances in the development of fluorescent probes and imaging techniques for understudied ions in cellular signaling, discussing remaining challenges and opportunities in the field. The reader is directed to excellent literature that discusses Zn2+ [2*,8] and Cu+ signaling [3*,9], and presents recent advances in the detection of labile Fe2+/3+ [10,11], which are not covered here in detail. Instead, we focus the discussion on Mg2+, an ion that is most often overlooked but that exemplifies the kind of paradigm shift posed by metal signaling beyond calcium.
Magnesium, a controversial second messenger:
In marked contrast with its heavier Group II metal congener, magnesium has received little attention in the context of signaling. A possible role for this metal in signal transduction was first investigated with regards to the cellular response to insulin, and the proposal that Mg2+ ions act as second messengers in this context has been out in the field since the 1970s [12]. But this notion has been met with skepticism. Evidence for a connection between hypomagnesemia and glucose-stimulated insulin response has been contradictory [13–15] and the relevant mechanistic details have remained elusive.
Within the last decade, the idea of Mg2+ participating directly in signal transduction was re-examined by Lenardo and coworkers, who demonstrated that this cation meets the criteria—originally delineated based on cAMP [16]—to be designated as a second messenger in the context of T-cell activation. Their mechanistic proposal invokes an influx of Mg2+ through MagT1 in response to antigen receptor stimulation, leading to activation of phospholipase C-γ (PLCγ1) and changes in Ca2+ influx [17]. This mechanism has since been revised and refined [18**,19] to account for the fact that MagT1 functions as an accessory subunit of the oligosaccharyl-transferase, OST—and perhaps not as a magnesium transporter, after all— playing a role in Mg2+-dependent glycosylation of NKG2D (also known as killer cell lectin-like receptor K1) and thus affecting the function of cytotoxic immune cells [19]. The basic features of the model, however, are thought to remain, though the source of the observed cytosolic Mg2+ transient upon T-cell activation is unclear.
More recently, Oka and coworkers demonstrated an increase in cytosolic Mg2+ upon γ-aminobutyric acid (GABA) receptor stimulation in young neurons. The transient was proposed to originate from mobilization of Mg2+ from mitochondria, and it was shown to activate the mammalian Target of Rapamycin (mTOR) and transcription factor CREB, independent of Ca2+, thus suggesting a role of Mg2+ as a second messenger in early stages of neuronal development (Figure 1) [20**].
Figure 1.
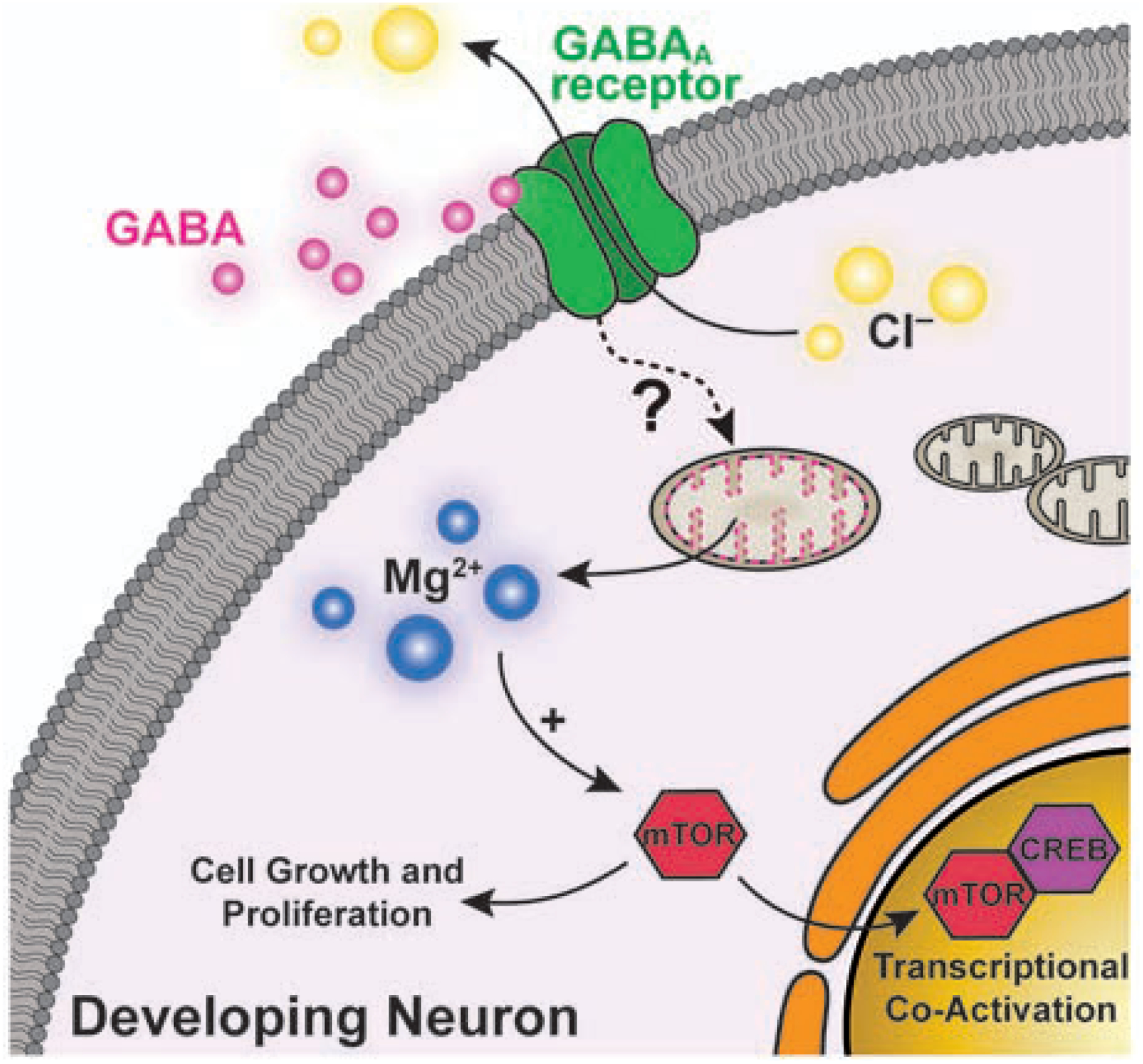
Schematic representation of the proposed mechanism for the GABA-induced, Mg2+-mediated activation of mTOR in developing neurons. Stimulation of GABA receptors is shown to elicit the release of mitochondrial Mg2+ into the cytosol by an unidentified mechanism. An increase in [Mg2+]cyto leads to the activation of mTOR and CREB, which activates transcriptional programs toward dendritic growth.
Imaging cellular free Mg2+:
The selectivity problem:
One of the most important challenges in the study of Mg2+ in a signaling context is disentangling its role from that of Ca2+. The complex interplay between the two ions has been difficult to examine given the poor selectivity of currently available molecular tools for detection of the former. Specifically, most small molecule fluorescent sensors for the hard Mg2+ ion bear metal recognition moieties rich in carboxylate groups, which also bind other hard biologically-relevant divalent cations leading to interferences in cellular imaging [21]. The aminophenol triacetic acid (APTRA) moiety, most commonly used in commercial Mg2+ indicators, is notorious for this limitation [22].
Recent efforts toward the design of better, more selective chelators [23,24**] have ushered important advances in fluorescent sensor development. The groups of Mizukami and Kikuchi developed the MGQ series of sensors (Figure 2) based on a novel 2,8-dicarboxyquinoline metal binding moiety that exhibits good selectivity for Mg2+ over Ca2+ and is well tuned to the low millimolar physiological concentrations of Mg2+ (e.g. KD Mg2+= 0.24 mM for MGQ-2) [24,25]. Both green and red-emitting derivatives exhibit a turn-off fluorescent response, less desirable for imaging applications. Nevertheless, combination with a turn-on sensor of a different color enabled ratiometric imaging of Mg2+ extrusion with enhanced sensitivity in cells overexpressing CNNM4, a Mg2+ transporter [25]. Highly selective, ratiometric imaging based on individual small molecule probes remains an important unmet need in the field, particularly relevant to proper quantification of Mg2+ transients.
Figure 2.
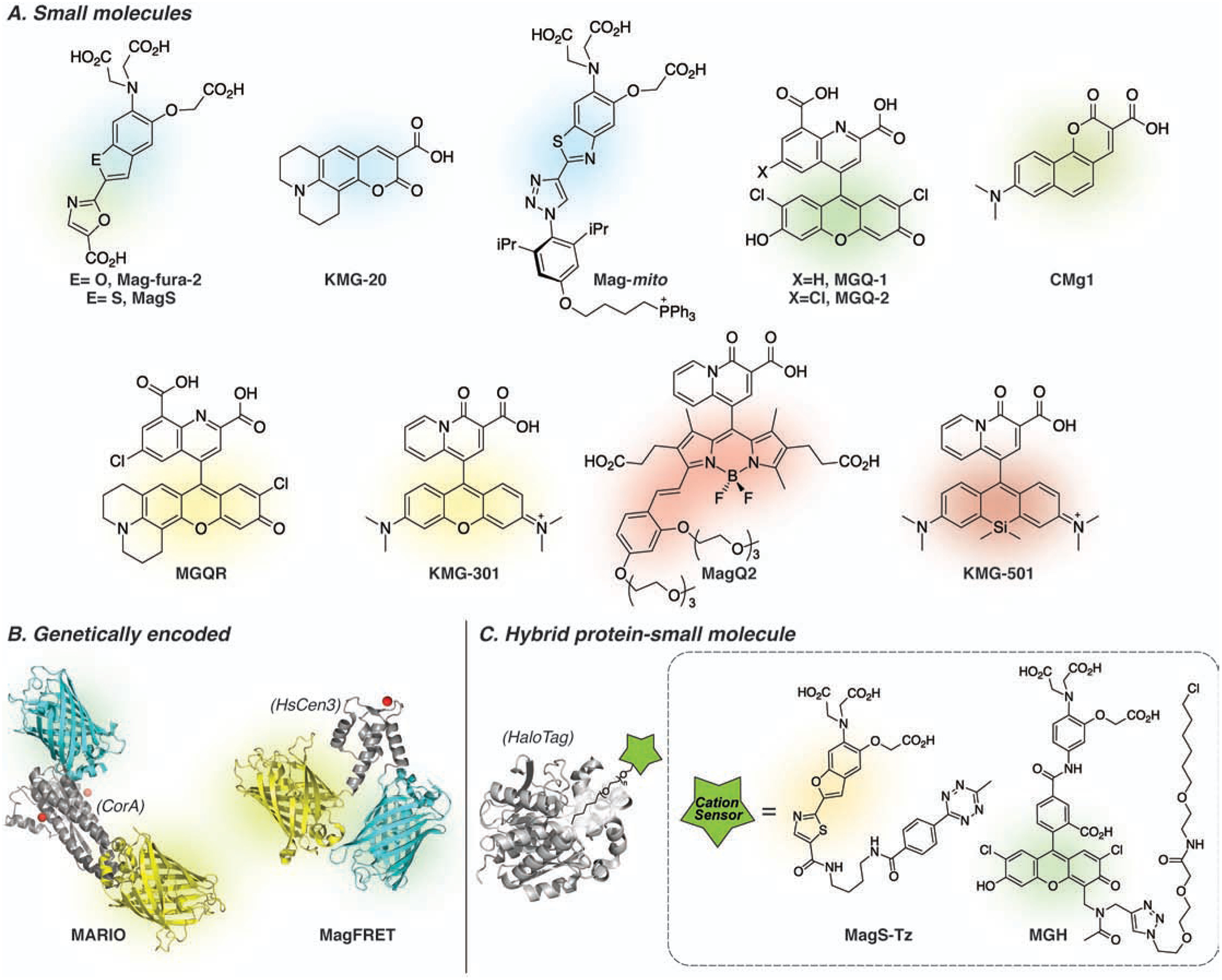
Selected fluorescent indicators used for imaging cellular free Mg2+. (A) Small molecule indicators. (B) Genetically encoded, all-protein-based indicators. MARIO is comprised of the metal binding cytoplasmic domain of CorA (PDBID: 2HN2), flanked by ECFP (PDBID: 2YDZ) and Venus (PDBID: 1MYW) fluorescent proteins; MagFRET contains the N terminus of sCen3 as metal binding domain (drawn based on HsCen2, PDBID: 2GGM) flanked by Cerulean (PDBID: 2Q57) and Citrine (PDBID: 1HUY). (C) Hybrid indicators comprised of a small molecule sensing component and a protein carrier. The basal concentration of free Mg2+ in most cells is 0.5–1.0 mM, requiring indicators with low affinity for maximum sensitivity under physiological conditions.
Sensors based on β-dicarbonyl chelators, including the KMG series [30–32,40,41] and related compounds [33,35] (Figure 2), also show an excellent metal selectivity profile. Due to their low denticity, however, these compounds easily form ternary complexes with Mg2+-bound biomolecules [42*], encumbering the distinction of free—available for signaling—versus bound metal. In recent work, Murata et al [41] used a near infrared-emitting member of the KMG family in parallel to a genetically encoded MgATP indicator to monitor rapid changes in magnesium upon mitochondria uncoupling. This combined imaging approach ruled out a major direct effect of MgATP on the fluorescence output of the small molecule. Similar approaches are likely necessary for the unequivocal study of free Mg2+ with these indicators in systems in which the highly dynamic pools of biomolecule-bound Mg2+ may change. Oka and coworkers capitalized on a combination of green and mitochondria-targeted, red-emitting members of the KMG family, namely KMG-104 and KMG-301, respectively, to show GABA-induced release of Mg2+ from mitochondria leading to activation of the mTOR and CREB signaling pathways in the maturation of neural networks. This work constitutes the most recent demonstration that Mg2+ participates in signal transduction, with a clearly identified source and molecular target [20**].
Looking for the source of ion mobilization
Despite mounting evidence toward Mg2+ acting as a second messenger, questions remain regarding the identity of the cellular stores that act as sources of the free metal, especially relevant to mobilization in a signaling context. Mitochondria have been found to perform this function [20,31,43], but studies have also shown that the pool of biomolecule-bound Mg2+, including the highly abundant pool of MgATP available in many compartments, could be a major contributor to intracellular changes in free Mg2+ under certain circumstances [39*]. The development of targeted fluorescent sensors is crucial for probing organelle-specific levels of metals and investigating patterns of ion accumulation and mobilization. Through favorable combinations of lipophilicity and positive charge, mitochondria targeting of Mg2+ indicators can be readily achieved [28*,32]. But more general design strategies, suitable for targeted imaging of other compartments of choice, remain scarce.
Buccella and coworkers have tackled the challenge of organelle-targeted Mg2+ detection by developing a hybrid system that combines a small molecule fluorescent sensor, Mag-S-Tz, and a HaloTag fusion protein that acts as intracellular directing group [36*]. The small molecule fluorophore is installed on the protein via in situ fluorogenic reaction with a dual reactive chloroalkane ligand. The fluorogenic conjugation step is key to enabling selective activation of fluorescence in the chosen subcellular locale, minimizing spurious signal from fluorophores that remain trapped in undesired compartments and compromise spatial resolution. In experiments done with HEK 239T cells, the hybrid system enabled comparison of the relative levels of free Mg2+ in various organelles, showing differences in basal concentrations of the compartments as well as the strengths and generality of this approach for potentially probing sources and destinations of intracellular metal trafficking. Another important advantage of hybrid protein-small molecule sensing designs is their increased intracellular retention compared to the freely diffusing small molecule counterparts. This feature, demonstrated by the groups of Kikuchi and Mizukami with a HaloTag-conjugated Magnesium Green derivative, MGH [37], enables imaging of ions over long periods without loss of signal, thus facilitating the distinction of short term signals versus long term changes involved in chronic regulation.
Quantifying the amplitude of a signal: a major challenge across metals
Quantification of the magnitude of a transient—the amplitude of a signal—remains a major challenge for most metals. Ratiometric detection, typically based on two wavelengths of fluorescence emission or excitation, normalizes the signal and minimizes the effect of most analyte-independent factors that affect the fluorescence output in microscopy experiments. Reversible, ratiometric metal-responsive indicators are thus preferred when quantitative rather than qualitative information is sought about the target metal.
Genetically encoded, entirely protein-based FRET sensors are ideal candidates for ratiometric detection of ions with subcellular resolution. This class of sensors has been used extensively to study the roles of Ca2+ and Zn2+ in cell signaling [44,45]. For Mg2+, however, the scarcity of protein domains that (i) bind the metal with the right affinity and selectivity, (ii) exhibit a large conformational change upon metal binding, and (iii) retain in cellulo the binding properties optimized in vitro, have been major roadblocks. Merkx and coworkers reported MagFRET, the first genetically-encoded Mg2+ sensor, based on modified human centrin-3 (HsCen3) for magnesium recognition [38]. Though MagFRET-1 showed favorable properties in vitro, it was unresponsive to procedures known to alter cellular Mg2+ levels, possibly due to unanticipated interactions of the probe with other proteins in the cellular milieu. Most recently, Maeshima and coworkers reported MARIO, a new genetically-encoded Mg2+ indicator that incorporates the cytosolic domain of the E.coli Mg2+ transporter CorA for metal binding, flanked by an ECFP/Venus FRET pair for ratiometric detection [39*]. A nuclear-targeted variant was employed to monitor relative Mg2+ levels during mitosis in the first—and only—successful example of fluorescence detection of changes in cellular free Mg2+ by a genetically encoded magnesium indicator in live cells. Metal quantification based on calibration of fluorescence ratio from dual wavelength Mg2+ indicators, however, is still primarily achieved with less selective small molecule indicators and remains to be demonstrated with the newer genetically encoded counterparts. Advances toward this goal are important for assessing the amplitude of the Mg2+ transients, which are notoriously difficult to quantify over the background of a high basal concentration.
Conclusions and Perspective:
Advances in imaging technologies combined with the development of increasingly selective, sensitive fluorescent indicators have revolutionized the study of the cell biology of metals. Fluorescent indicators developed by Roger Tsien, such as fura-2 [46], were instrumental in attaining our current understanding of calcium signaling, and they established much of the foundation for the design of tools that are now ushering the re-evaluation of the roles of other metals in biology. Furnished with the right indicators, fluorescence microscopy is particularly well suited to reveal in real time the transient changes in metal availability required for signaling to take place. Furthermore, the high resolution offered by fluorescence imaging with targeted indicators can deliver information on the spatial localization of such signals and help uncover intraor extracellular sources of ion mobilization, thus providing a more complete picture of the information flow.
As exposed herein through the lens of magnesium, the study of metals in signaling roles is pushing the design of a better toolbox to track metals at the sub-cellular level. Each metal cation brings unique parameters and technical challenges to the development of fluorescent indicators. Yet the final requirements are similar for all, and include well-tuned affinity, high selectivity for the target cation over others, ability to quantify transient changes in ion concentration, and controllable localization to enable visualization of patterns of metal accumulation and translocation to and from subcellular compartments. Undoubtedly, much progress has been made in various fronts. Careful molecular design, based on consideration of the unique coordination chemistry of each metal, has enabled the development of indicators with sufficient selectivity to probe, at last, transients of Mg2+ without the interference from Ca2+ fluxes. New genetically encoded indicators have provided access to metals on specific cellular compartments of interest. Finally, hybrid indicators are filling some of the gaps left by small molecules and protein-based ones, combining some of the best features of both classes. But a number of challenges still remain, and reliable quantification is perhaps at the top of the list.
The notion of d-block metals and magnesium playing a role in signaling represents a clear departure from the current paradigm in which these metals are viewed exclusively as controlling basal metabolic activity. This shift forces us to rethink the basic mechanistic aspects of information transfer and to question the magnitude of a transient required to carry a signal. In principle, the vast differences in the concentrations of the different metals—and of the affinities and concentrations of their binding partners, which ultimately determine both metal availability and the concentration range at which a downstream effect can be triggered—open the possibility of multiple ions operating in parallel, carrying different signals with virtually no crosstalk. Probing the molecular aspects of such model poses an exciting challenge for chemists and biologists alike, presenting a fertile ground for innovation in tool design.
Table 1.
Spectroscopic properties of selected fluorescent indicators for imaging free Mg2+.
| Probe | Absorption λmax (nm) | Fluorescence Emission λmax (nm) | Φ(free), Φ(bound) | KD, Mg2+ 25 °C (mM) | KD, Mg2+ 37 °C (mM) | Ref. |
|---|---|---|---|---|---|---|
| Small molecules | ||||||
| Mag-Fura-2 | 369, 330a | 511, 491a | 0.24, 0.30 | 1.9b | 1.5 | [26,27] |
| Mag-mito | 356, 330a | 495, 482a | 0.42, 0.25c | 6.7 | N.R. | [28*] |
| Mag-S | 396, 350a | 572, 547a | 0.17, 0.30 | 3.2 | 1.97 | [29] |
| KMG-20 | 425, 445a | 485 | 0.63 | N.R. | 10 | [30] |
| KMG-104 | 504 | 523 | N.R., 0.02 | 2.1 | N.R. | [31] |
| MGQ-1 | 515 | 536 | 0.36, <0.01 | N.R. | 0.14 | [24**] |
| MGQ-2 | 516 | 536 | 0.33, <0.01 | N.R. | 0.27 | [24**] |
| MGQR | 561 | 588 | 0.29, <0.01 | N.R. | 0.29 | [25] |
| KMG-301 | 563 | 590 | N.R., 0.15 | 4.5 | N.R. | [32] |
| MagQ2 | 600 | 634 | 0.0099, 0.34 | 1.51 | N.R. | [33] |
| KMG-501 | 663 | 684 | 0.004, 0.05 | 3.2 | N.R. | [34] |
| CMg1 | 820, 880d | 559 | 0.29, 0.28 | 1.7 | N.R. | [35] |
| Hybrid Sensors | ||||||
| Mag-S-Tz | 404, 358a | 595 | 0.1, 0.24 | 3.1 | N.R. | [36*] |
| MGH | 515 | 538 | 0.19, 0.56 | N.R. | 1.3 | [37] |
| Genetically Encoded | ||||||
| MagFRET-1 | Cerulean, Citrine | 0.15e | - | [38] | ||
| MARIO | ECFP, Venus | 7.2 | N.R. | [39*] | ||
Ratiometric sensor; two values represent absorption or emission maxima in the metal-free and -bound form, respectively.
Dissociation constant reported at 22 °C.
Quantum yields determined on reference, non-targeted analogue.
Two-photon absorption maxima.
Temperature not specified N.R. = not reported.
Acknowledgements
The authors are grateful to the National Institutes of Health (CA 217817) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Clapham DE: Calcium Signaling. Cell 2007, 131:1047–1058. [DOI] [PubMed] [Google Scholar]
- 2.*.Maret W: Zinc in cellular regulation: the nature and significance of “zinc signals”. Int JMol Sci 2017, 18:2285. [DOI] [PMC free article] [PubMed] [Google Scholar]; Current understanding of the nature of zinc signals is reviewed, discussing mechanisms by which transients of this tightly bound metal cation can be generated.
- 3.*.Chang CJ: Searching for harmony in transition-metal signaling. Nat Chem Biol 2015, 11:744–747. [DOI] [PubMed] [Google Scholar]; Discusses the emergence of signaling roles for d-block metals, featuring examples in which Zn2+, Cu+ or Fe2+ seem to play a major role.
- 4.Takaya J, Higashino H, Kobayashi Y: Can magnesium act as a second messenger? Current data on translocation induced by various biologically active substances. Magnes Res 2000, 13:139–146. [PubMed] [Google Scholar]
- 5.Romani AM: Cellular magnesium homeostasis. Arch Biochem Biophys 2011, 512:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.**.de Baaij JH, Hoenderop JG, Bindels RJ: Magnesium in man: implications for health and disease. Physiol Rev 2015, 95:1–46. [DOI] [PubMed] [Google Scholar]; Expansive review on the roles of magnesium in human health, including an overview of the potential role in signaling and some of the questions that the surround the controversy about the designation of Mg2+ as second messenger.
- 7.Stangherlin A, O’Neill JS: Signal Transduction: Magnesium Manifests as a Second Messenger. Curr Biol 2018, 28:R1403–R1405. [DOI] [PubMed] [Google Scholar]
- 8.Fukada T, Kambe T: Zinc Signaling edn 2nd: Springer; Singapore; 2019. [Google Scholar]
- 9.Ackerman CM, Chang CJ: Copper signaling in the brain and beyond. J Biol Chem 2018, 293:4628–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter KP, Young AM, Palmer AE: Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem Rev 2014, 114:4564–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aron AT, Reeves AG, Chang CJ: Activity-based sensing fluorescent probes for iron in biological systems. Curr Opin Chem Biol 2018, 43:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lostroh AJ, Krahl ME: Magnesium, a second messenger for insulin: Ion translocation coupled to transport activity. Adv Enzyme Regul 1974, 12:73–81. [DOI] [PubMed] [Google Scholar]
- 13.Gommers LMM, Hill TG, Ashcroft FM, de Baaij JHF: Low extracellular magnesium does not impair glucose-stimulated insulin secretion. PLoS One 2019, 14:e0217925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Legrand C, Okitolonda W, Pottier AM, Lederer J, Henquin JC: Glucose homeostasis in magnesium-deficient rats. Metabolism 1987, 36:160–164. [DOI] [PubMed] [Google Scholar]
- 15.Reis MA, Latorraca MQ, Carneiro EM, Boschero AC, Saad MJ, Velloso LA, Reyes FG: Magnesium deficiency improves glucose homeostasis in the rat: studies in vivo and in isolated islets in vitro. Br J Nutr 2001, 85:549–552. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland EW: Studies on the mechanism of hormone action. Science 1972, 177:401–408. [DOI] [PubMed] [Google Scholar]
- 17.Chaigne-Delalande B, Li FY, O’Connor GM, Lukacs MJ, Jiang P, Zheng L, Shatzer A, Biancalana M, Pittaluga S, Matthews HF, et al. : Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2 D. Science 2013, 341:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.**.Li FY, Chaigne-Delalande B, Kanellopoulou C, Davis JC, Matthews HF, Douek DC, Cohen JI, Uzel G, Su HC, Lenardo MJ: Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011, 475:471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rapid influx of Mg2+ is observed upon antigen receptor stimulation in T cells. Studies in lymphocytes from patients with inherited MagT1 deficiency revealed impaired Mg2+ flux compared to normal controls. Decreased Mg2+ influx leads to defective activation of phopholipase Cγ1 and impaired Ca2+ influx. Mg2+ is proposed to act as second messenger, coupling cell surface receptor activation to intracellular effectors.
- 19.Matsuda-Lennikov M, Biancalana M, Zou J, Ravell JC, Zheng L, Kanellopoulou C, Jiang P, Notarangelo G, Jing H, Masutani E, et al. : Magnesium transporter 1 (MAGT1) deficiency causes selective defects in N-linked glycosylation and expression of immune-response genes. J Biol Chem 2019, 294:13638–13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.**.Yamanaka R, Shindo Y, Hotta K, Suzuki K, Oka K: GABA-Induced Intracellular Mg2+ Mobilization Integrates and Coordinates Cellular Information Processing for the Maturation of Neural Networks. Curr Biol 2018, 28:3984–3991.e3985. [DOI] [PubMed] [Google Scholar]; A combination of a small molecule Mg2+ indicator, KMG-104, and a reference fluorescent protein reveal an increase in cytosolic Mg2+ as a result of GABA stimulation in young neurons. A mitochondria-targeted analogue indicator, KMG-301, reveals a decrease in mitochondrial Mg2+ suggesting these organelles act as ion source. Released Mg2+ is proposed to act as second messenger, activating mTOR and CREB signaling pathways.
- 21.Liu M, Yu X, Li M, Liao N, Bi A, Jiang Y, Liu S, Gong Z, Zeng W: Fluorescent probes for the detection of magnesium ions Mg2+: from design to application. RSC Advances 2018, 8:12573–12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brady M, Piombo SD, Hu C, Buccella D: Structural and spectroscopic insight into the metal binding properties of the o-aminophenol-N,N,O-triacetic acid (APTRA) chelator: implications for design of metal indicators. Dalton Trans 2016, 45:12458–12464. [DOI] [PubMed] [Google Scholar]
- 23.Walter ERH, Fox MA, Parker D, Williams JAG: Enhanced selectivity for Mg2+ with a phosphinate-based chelate: APDAP versus APTRA. Dalton Trans 2018, 47:1879–1887. [DOI] [PubMed] [Google Scholar]
- 24.**.Matsui Y, Sadhu KK, Mizukami S, Kikuchi K: Highly selective tridentate fluorescent probes for visualizing intracellular Mg2+ dynamics without interference from Ca2+ fluctuation. Chem Commun (Camb) 2017, 53:10644–10647. [DOI] [PubMed] [Google Scholar]; A fluorescent indicator with a new quinoline-based tridentate metal binding motif exhibits 10-fold selectivity for Mg2+ over Ca2+, enabling the detection of free Mg2+ under conditions of high Ca2+ influx in live cells.
- 25.Matsui Y, Mizukami S, Kikuchi K: Ratiometric Imaging of Intracellular Mg2+ Dynamics Using a Red Fluorescent Turn-off Probe and a Green Fluorescent Turn-on Probe. Chem Lett 2018, 47:23–26. [Google Scholar]
- 26.The Molecular Probes Handbook: A Guide Fluorescent Probes and Labeling Technologies: edn 11th United States of America: Life Technologies Corporation; 2010. [Google Scholar]
- 27.Raju B, Murphy E, Levy LA, Hall RD, London RE: A fluorescent indicator for measuring cytosolic free magnesium. Am J Physiol-Cell Physiol 1989, 256:C540–C548. [DOI] [PubMed] [Google Scholar]
- 28.*.Zhang G, Gruskos JJ, Afzal MS, Buccella D: Visualizing changes in mitochondrial Mg2+ during apoptosis with organelle-targeted triazole-based ratiometric fluorescent sensors. Chem Sci 2015, 6:6841–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]; A targeted ratiometric indicator, Mag-mito, enables quantification of free Mg2+ in mitochondria in whole live cells, and reveals a transient rise in [Mg2+]mito in early stages of apoptosis, preceding Ca2+ mobilization from the endoplasmic reticulum. Concentration of free Mg2+ in these organelles is shown to roughly triple upon induction of apoptosis.
- 29.Afzal MS, Pitteloud J-P, Buccella D: Enhanced ratiometric fluorescent indicators for magnesium based on azoles of the heavier chalcogens. Chem Commun 2014, 50:11358–11361. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, Komatsu H, Ikeda T, Saito N, Araki S, Citterio D, Hisamoto H, Kitamura Y, Kubota T, Nakagawa J: Design and synthesis of Mg2+-selective fluoroionophores based on a coumarin derivative and application for Mg2+ measurement in a living cell. Anal Chem 2002, 74:1423–1428. [DOI] [PubMed] [Google Scholar]
- 31.Kubota T, Shindo Y, Tokuno K, Komatsu H, Ogawa H, Kudo S, Kitamura Y, Suzuki K, Oka K: Mitochondria are intracellular magnesium stores: investigation by simultaneous fluorescent imagings in PC12 cells. Biochim Biophys Acta 2005, 1744:19–28. [DOI] [PubMed] [Google Scholar]
- 32.Shindo Y, Fujii T, Komatsu H, Citterio D, Hotta K, Suzuki K, Oka K: Newly developed Mg2+-selective fluorescent probe enables visualization of Mg2+ dynamics in mitochondria. PLoS One 2011, 6:e23684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Q, Buccella D: Highly selective, red emitting BODIPY-based fluorescent indicators for intracellular Mg2+ imaging. Journal of Materials Chemistry B 2018, 6:7247–7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murata O, Shindo Y, Ikeda Y, Iwasawa N, Citterio D, Oka K, Hiruta Y: Near-Infrared Fluorescent Probes for Imaging of Intracellular Mg2+ and Application to Multi-Color Imaging of Mg2+, ATP and Mitochondrial Membrane Potential. Anal Chem 2020, 92:966–974. [DOI] [PubMed] [Google Scholar]
- 35.Kim HM, Yang PR, Seo MS, Yi J-S, Hong JH, Jeon S-J, Ko Y-G, Lee KJ, Cho BR: Magnesium Ion Selective Two-Photon Fluorescent Probe Based on a Benzo[h]chromene Derivative for in Vivo Imaging. The Journal of Organic Chemistry 2007, 72:2088–2096. [DOI] [PubMed] [Google Scholar]
- 36.*.Gruskos JJ, Zhang G, Buccella D: Visualizing Compartmentalized Cellular Mg2+ on Demand with Small-Molecule Fluorescent Sensors. J Am Chem Soc 2016, 138:14639–14649. [DOI] [PubMed] [Google Scholar]; Through the use of a hybrid sensor design that combines a ratiometric small molecule indicator and a HaloTag fusion protein anchor, targeted detection of free Mg2+ in subcellular compartments is achieved. Ratiometric enables comparison of relative levels of free Mg2+ in various organelles.
- 37.Matsui Y, Funato Y, Imamura H, Miki H, Mizukami S, Kikuchi K: Visualization of long-term Mg2+ dynamics in apoptotic cells using a novel targetable fluorescent probe. Chemical Science 2017, 8:8255–8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindenburg LH, Vinkenborg JL, Oortwijn J, Aper SJ, Merkx M: MagFRET: the first genetically encoded fluorescent Mg2+ sensor. PLoS One 2013, 8:e82009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.*.Maeshima K, Matsuda T, Shindo, Imamura, Tamura S, Imai, Kawakami S, Nagashima R, Soga T, Noji H, et al. : A Transient Rise in Free Mg2+ Ions Released from ATP-Mg Hydrolysis Contributes to Mitotic Chromosome Condensation. Curr Biol 2018, 28:444–451 e446. [DOI] [PubMed] [Google Scholar]; A new genetically encoded Mg2+ indicator, MARIO, is introduced. A nuclear-targeted variant is employed to track relative concentrations of free Mg2+ through the cell cycle, revealing an increase in ion concentration during mitosis in HeLa cells. The study represents the first successful demonstration of the application of a genetically encoded indicator for detecting changes in Mg2+ levels in live cells. Quantification of the metal, however, is conducted with a small molecule ratiometric indicator.
- 40.Komatsu H, Iwasawa N, Citterio D, Suzuki Y, Kubota T, Tokuno K, Kitamura Y, Oka K, Suzuki K: Design and synthesis of highly sensitive and selective fluorescein-derived magnesium fluorescent probes and application to intracellular 3D Mg2+ imaging. Journal of the American Chemical Society 2004, 126:16353–16360. [DOI] [PubMed] [Google Scholar]
- 41.Murata O, Shindo Y, Ikeda Y, Iwasawa N, Citterio D, Oka K, Hiruta Y: Near-Infrared Fluorescent Probes for Imaging of Intracellular Mg2+ and Application to Multi-Color Imaging of Mg2+, ATP, and Mitochondrial Membrane Potential. Analytical Chemistry 2019. [DOI] [PubMed] [Google Scholar]
- 42.*.Schwartz SC, Pinto-Pacheco B, Pitteloud JP, Buccella D: Formation of ternary complexes with MgATP: effects on the detection of Mg2+ in biological samples by bidentate fluorescent sensors. Inorg Chem 2014, 53:3204–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]; A word of caution: a study on the metal binding properties of low denticity indicators reveal their tendency to form indicator-Mg2+-biomolecule ternary complexes, which exhibit a fluorescence output comparable to that elicited by the formation of binary indicator-Mg2+ complexes. The formation of ternary complexes precludes clear distinction of “free” vs. tightly bound Mg2+ with β-dicarbonyl-based indicators.
- 43.Yamanaka R, Tabata S, Shindo Y, Hotta K, Suzuki K, Soga T, Oka K: Mitochondrial Mg2+ homeostasis decides cellular energy metabolism and vulnerability to stress. Sci Rep 2016, 6:30027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carpenter MC, Palmer AE: Native and engineered sensors for Ca2+ and Zn2+: lessons from calmodulin and MTF1. Essays Biochem 2017, 61:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park JG, Palmer AE: Quantitative measurement of Ca2+ and Zn2+ in mammalian cells using genetically encoded fluorescent biosensors. Methods Mol Biol 2014, 1071:29–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grynkiewicz G, Poenie M, Tsien RY: A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry 1985, 260:3440–3450. [PubMed] [Google Scholar]


