Abstract
This case study evaluated the effect of implanted multi-joint neuromuscular electrical stimulation (NMES) gait assistance on oxygen consumption relative to walking without NMES after stroke. The participant walked slowly with an asymmetric gait pattern after stroke. He completed repeated six-minute walk tests at a self- selected walking speed with and without hip, knee and ankle stimulation assistance. His walking speed with NMES more than doubled from 0.28±0.01 m/s to 0.58±0.04 m/s while average step length and cadence increased by 0.12 m and 24 steps/min, respectively. As a result, energy cost of walking with NMES decreased by 0.19 ml O2/kg/m as compare to walking without stimulation while oxygen consumption increased by 1.1 METs (3.9 ml O2/kg/min). These metabolic demands are similar to those reported for stroke survivors capable of walking at equivalent speeds without stimulation, suggesting the increase in oxygen consumption and decreased energy cost result from improved efficiency of faster walking facilitated by NMES. While the effect of NMES on gait economy has implications for community walking within the user’s metabolic reserves, this case study’s results should be interpreted with caution and the hypothesis that multi-joint NMES improves metabolic efficiency should be tested in a wide population of stroke survivors with varied deficits.
Keywords: stroke, oxygen consumption, neuromuscular electrical stimulation, walking
I. Introduction
Many stroke survivors walk slowly and are at an increased risk of falls1. Clinically prescribed assistive devices primarily focus on assisting ankle dorsiflexion by means of ankle foot orthoses (AFO) or peroneal nerve stimulation (PNS) to ensure swing foot clearance to prevent tripping2,3. Alternatively, or in addition, stroke survivors often adopt compensatory strategies such as hip hiking and circumduction to compensate for increased extensor tone, drop foot, and weak knee and hip flexion and joint incoordination. Although assistive devices prevent drop foot2, they do not provide substantial assistance at the hip and knee joints2,3 or generate clinically significant improvements in walking speed (>0.2 m/s)4,5. In a recent case report, neuromuscular electrical stimulation (NMES) to activate muscles controlling multiple joints during gait significantly increased walking speed6. While increased walking speed is related to functional independence and community ambulation, patients are unlikely to adopt assistive devices that significantly increase metabolic effort7. Considering stroke survivors already expend more energy to maintain the same walking speeds as their able-bodied counterparts8–12 it is worthwhile to determine whether neuromuscular stimulation facilitating faster walking creates an undue metabolic demand. A study showed walking with PNS resulted in similar metabolic energy expenditure and energy cost as walking with an ankle foot orthosis, therefore stimulation alone did not significantly affect oxygen consumption13. While PNS assists primarily with dorsiflexion, additional stimulation for hip and knee flexion that significantly increased walking speed6 may also significantly affect energy consumption. Although faster gait speeds are expected to increase energy consumption and decrease energy cost, it is not clear how these effects change in response to stimulation increasing walking speed. Stimulation could reduce relative oxygen consumption by reducing gait inefficiencies stroke survivors often exhibit. Alternatively, poorly timed over stimulation could increase relative oxygen consumption. If assistive devices, such as a neural stimulation system, substantially increase energy expenditure beyond levels associated with increased gait speed, they may be more appropriate for exercise and may not be realistic for daily community ambulation. This study evaluated changes in oxygen consumption when walking with implanted multi-joint NMES assistance in a single stroke survivor. Implanted electrodes were used in this study because they more selectively recruit target muscles that are difficult to activate with surface electrodes while bypassing skin sensory fibers, reducing discomfort. Implanted electrodes also simplify donning and doffing to facilitate daily use. We hypothesized that applying NMES to assist hip, knee, and ankle movement to increase gait speed would improve metabolic efficiency of gait within the user’s metabolic reserves for community ambulation.
II. Methods
Study participant
A 69-year old male, 1.9 m tall, weighing 108 kg (Body Mass Index=29.0 kg/m2), 6 years post stroke participated in this study. He suffered a hemorrhagic stroke resulting in left sided hemiparesis including weakness throughout the lower limb with hypertonia presenting as an extensor synergy pattern and spasticity in response to passive stretching. He did not report any pain. He walked slowly with a step-to gait pattern. Steps were asymmetric with shorter steps with his less involved leg. He used a cane for balance and wore an ankle foot orthosis (AFO) to prevent foot drop. He was implanted with an 8-channel implanted pulse generator (IPG) which improved his walking speed and endurance6. Intramuscular electrodes were inserted near the nerves innervating muscles to ameliorate his deficits of weak hip and knee flexion and extension, and ankle dorsi and plantar flexion: 1) tensor fasciae latae, 2) sartorius, 3) gluteus maximus, 4) quadriceps, 5) tibialis anterior, 6) peroneus longus, 7) short head of biceps femoris (SHBF), and 8) gastrocnemius. Electrode leads were routed to the IPG subcutaneously.
NMES device
An external control unit provided the IPG power and stimulus timing by means of Radio-Frequency (RF) transmission coupling. Gastrocnemius and SHBF were not incorporated into the stimulation pattern due to 1) difficulties establishing proper timing of gastrocnemius stimulation for push-off and 2) stimulus spill over to triceps surae when stimulating SHBF during initial swing. Swing and stance phase stimulation patterns were triggered via a heel switch placed inside the AFO. Heel off and heel strike initiated stimulation for swing and stance phase, respectively. Once the gait event was detected, the next stimulation phase was initiated as shown in Figure 1.
Figure 1:
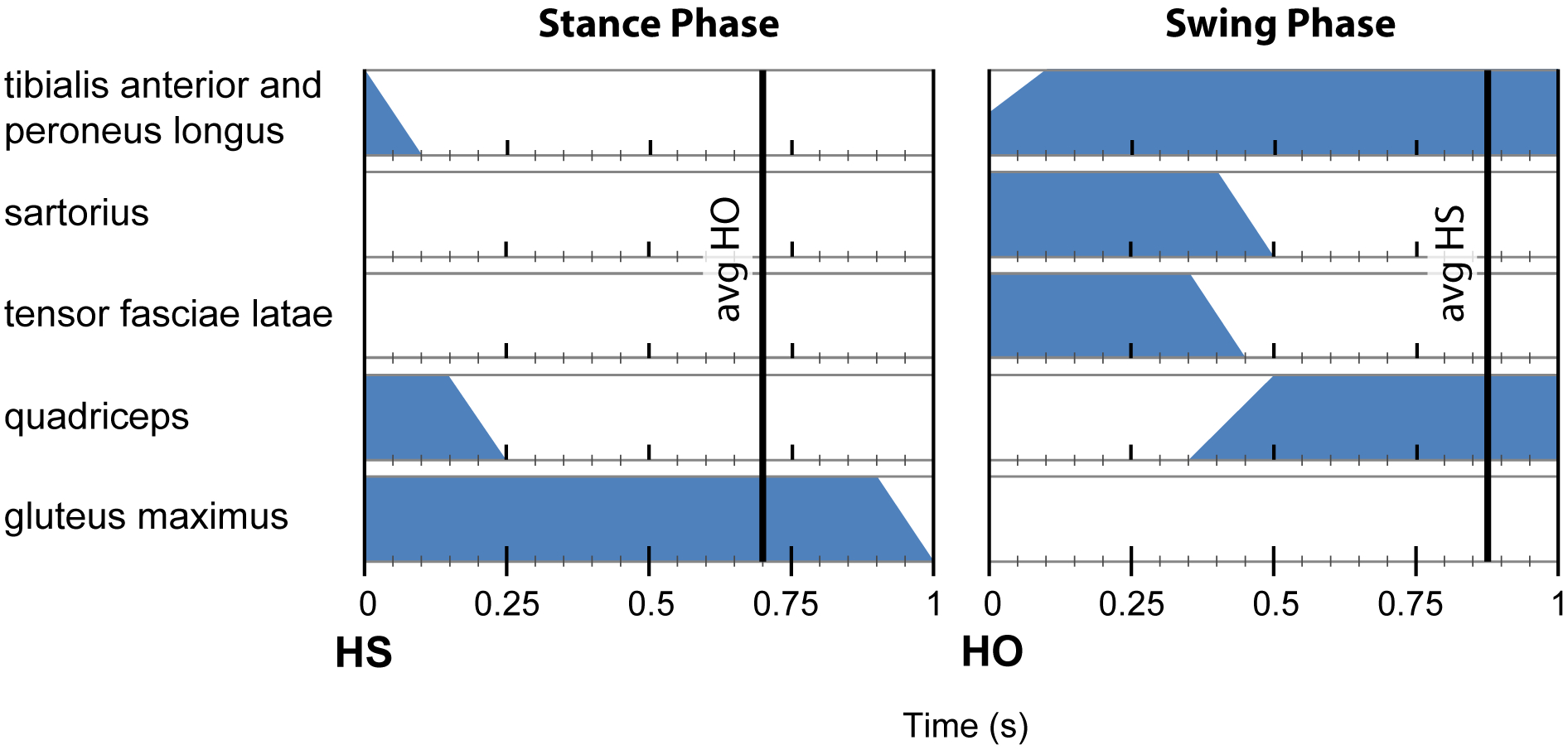
Stimulation pattern with left leg muscle activation timing relative to gait events (HS – Heel Strike, HO – Heel Off). Vertical lines indicate average timing of subsequent gait event detection. Stimulation phase transitions occur immediately following heel switch detection (HS and HO).
Written informed consent approved by the Louis Stokes Cleveland Veterans Affairs Medical Center Institutional Review Board was obtained prior to study initiation. The implanted NMES device has an Investigational Device Exemption approval by the United States Food and Drug Administration for research use only. This study conforms to all CARE guidelines and reports the required information accordingly (see Supplementary Checklist).
Data collection
Oxygen consumption was measured with a K4b2 Metabolic Analyzer (Cosmed, Italy) on 3 different days during 6-minute walks14. Each walk was completed in hallways free from obstacles. The shortest walkway was over 90 m long, requiring one or two wide turns depending on walking speed. The subject walked in random order with and without stimulation each day with five total trials under each condition. Walking speed was not controlled; he walked at a self-selected speed in each condition. At least six minutes of rest were provided between walks. Resting heart rate was measured prior to each walk to confirm it reached the resting level before initiating each trial. Data for analysis was averaged over the last two minutes of each walk during steady state metabolic energy consumption. In addition, distance was measured and the number of steps were counted during each walk to calculate walking speed, cadence and average step length. Heart rate (HR) in beats per minute (BPM) was measured manually before and immediately after each walk. All activities were approved by the local Institutional Review Board. The registry number is NCT01570816.
Data analysis
Metabolic energy and gait outcome measures were calculated for the last two-minute period of each 6-minute walk. Metabolic energy was analyzed as oxygen consumption in Metabolic Equivalent of Task (MET) (1MET = 3.5 ml of O2/kg/min) which is oxygen consumed over time and energy cost of transport (ml of O2/kg/meter(m)) which is oxygen consumed as a function of distance walked. Data were checked for normality. Differences in outcome measures between walking with and without stimulation were tested for significant changes using a Student’s t-test (p<0.05).
III. Results
The study participant walked significantly faster (0.3 m/s) with stimulation (0.58±0.04 m/s) than without stimulation assistance (0.28±0.01 m/s) as shown in Table 1. Stimulation assistance enabled him to transition from a step-to to a reciprocal gait pattern. Average step length increased by 0.12 m and cadence increased by 24 steps/min while walking with stimulation assistance as compared to no stimulation. Oxygen consumption increased by 1.1 METs (3.9 ml O2/kg/min) while energy cost decreased by 0.19 ml O2/kg/m when walking with stimulation. All changes were statistically significant (p<0.05). There were no adverse events during the course of this study.
Table 1:
Outcome Measures of Metabolic Analysis and Gait
| Outcome | At rest mean (SD) | Volitional mean (SD) | Stimulation mean (SD) | P value |
|---|---|---|---|---|
| Energy expenditure (METs) | 0.84 (0.12) | 2.9 (0.2) | 4.0 (0.4) | <0.01 |
| (ml of O2/kg/min) | 3.1 (0.4) | 10.1 (0.8) | 14.0 (1.3) | <0.01 |
| Energy cost (ml of O2/kg/m) | N/A | 0.59 (0.05) | 0.40 (0.04) | <0.01 |
| Walking speed (m/s) | N/A | 0.28 (0.01) | 0.58 (0.04) | <0.01 |
| Cadence (steps/min) | N/A | 45.5 (2.6) | 69.4 (3.8) | <0.01 |
| Average Step Length (m) | N/A | 0.38 (0.05) | 0.50 (0.05) | <0.01 |
| Heart rate (BPM) | 64 | 96 | 103 | 0.04 |
IV. Discussion
The individual walked twice as fast with stimulation assisting hip, knee, and ankle movement than with an AFO without stimulation. Cadence and step length increased commensurate with increases in walking speed. Energy cost decreased by 32% with increased walking speed while energy expenditure increased by 28% compared to walking without stimulation.
Oxygen consumption in stroke survivors
Since maximal oxygen uptake (VO2 max) was not measured, user reserve effort was based on available values in the literature. User reserve effort is the oxygen capacity between resting consumption and maximum consumption. Deconditioned stroke survivors reported VO2 max of 19.0 ml of O2/kg/min (5.4 METs) at an average speed of 0.76 m/s while walking at an average incline of 11%15. Assuming similar conditioning, our participant was using ~68% of his user reserve effort with stimulation.
Energy expenditure and energy cost of walking without stimulation assistance were similar to those reported in stroke survivors walking at comparable speeds (0.28m/s)11,16–20. Two studies both with average walking speeds of 0.27 m/s on a treadmill reported energy expenditures of 10.3 and 10.8 ml of O2/kg/min (~3METs)16,18 which is comparable to the 10.1 ml of O2/kg/min reported here without stimulation.
While walking with stimulation assistance (0.58m/s), energy expenditure and energy cost were similar to those reported in studies of stroke survivors walking at comparable speeds without stimulation11,20–25. Two studies reporting similar walking speeds had oxygen consumption above resting values of 10.2 and 8.5 ml of O2/kg/min respectively, which are slightly lower than 10.9 ml of O2/kg/min shown here walking with stimulation24,25. A subset of individuals in another study walking at a comparable average speed consumed an average of 12.9 (±2.6) ml of O2/kg/min21, with some individuals wearing a partial body weight support, comparable to 14.0 ml of O2/kg/min in our participant walking with stimulation. Studies reporting energy cost of walking at similar speeds without stimulation also showed similar results as ours walking with NMES20,27. These results suggest that the addition of stimulation assistance to improve walking speed by increased cadence and step length does not significantly increase the metabolic burden of walking beyond the increase normally observed in higher functioning stroke survivors.
Relevance to exercise
While increased oxygen consumption means the participant worked harder to maintain a faster speed, it also provides an opportunity for exercise he may not otherwise have. The participant reported enjoying walking with stimulation assistance, in part because walking with stimulation was a form of exercise that incorporated his paretic limb and enabled him to walk significantly faster as compared to without stimulation. In addition to increased function and mobility, multi-joint NMES provides another opportunity to walk for exercise, which is important for maintaining cardiovascular health, especially in a patient population that is at a greater risk of a sedentary lifestyle and deconditioning28. Based on a VO2 max of 19.0 ml O2/kg/min for population matched stroke survivors, he would be at 68% of his reserve while walking with stimulation assistance, which is within the limits recommended for aerobic exercise29,30. However, his effort walking without stimulation would be at 44% of his metabolic reserve, which is below the level recommended for aerobic exercise. While other exercise modalities such as recumbent bicycling can elicit necessary metabolic load31 and incorporate neural stimulation32, stimulation assisted walking provides another opportunity to walk and exercise and could reduce the negative effects of a sedentary lifestyle by increasing muscle mass and improving cardiovascular health and quality of life.
Limitations
The primary limitation of this study was that VO2 max was not measured in this study, requiring estimates of oxygen reserve based on available literature. Since the participant in this study walked at different speeds with and without stimulation assistance, we are similarly limited to comparisons within the literature of stroke survivors walking at matched speeds. Although this study provides insight into the impact of implanted multi-joint NMES on energy consumption and cost, it is limited to a single individual with stimulation applied to a specific set of muscles and does not generalize to stroke survivors or to multi-joint implanted or surface NMES in general. Incorporating additional muscles into the stimulation pattern with a more sophisticated control algorithm could potentially improve walking speed and efficiency. While the individual increased his walking distance with stimulation assistance over time6, the impact of implanted multi-joint NMES training on VO2 max was not measured either.
Conclusions
An implanted multi-joint neuromuscular stimulation system in a stoke survivor decreased energy cost with increased oxygen consumption commensurate with significantly increased walking speed. Comparison to the literature suggests changes in energy consumption and energy cost primarily result from faster walking rather than a change in metabolic inefficiency of NMES. Additional study with comparisons at matched speeds and VO2 max measurements on a larger population of stroke survivors with various gait deficits are necessary to validate these conclusions and generalize the results. Faster walking speeds represent potential increased community access4 and metabolic demands may facilitate secondary health benefits through walking exercise.
Supplementary Material
Funding –
This work was supported by Merit Review Award No. B7692R from the United States Department of Veterans Affairs Rehabilitation Research and Development Service. RJ Triolo was supported by the Senior Research Career Scientist Award No. A9259-L: from the U.S. Department of Veterans Affairs Rehabilitation Research and Development Service. NS Makowski was supported by the National Institutes of Health National Center for Advancing Translational Sciences Award No. KL2TR002547.
Footnotes
Competing interests – none
Details of prior presentation – a subset of the data were presented in a poster at the Translational Science 2017 meeting
References
- 1.McKevitt C, Fudge N, Redfern J, et al. Self-reported long-term needs after stroke. Stroke. 2011;42(5):1398–1403. [DOI] [PubMed] [Google Scholar]
- 2.Tyson SF, Sadeghi-Demneh E, Nester CJ. A systematic review and meta-analysis of the effect of an ankle-foot orthosis on gait biomechanics after stroke. Clinical rehabilitation. 2013;27(10):879–891. [DOI] [PubMed] [Google Scholar]
- 3.Prenton S, Hollands KL, Kenney LP. Functional electrical stimulation versus ankle foot orthoses for foot-drop: A meta-analysis of orthotic effects. Journal of rehabilitation medicine. 2016;48(8):646–656. [DOI] [PubMed] [Google Scholar]
- 4.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26(6):982–989. [DOI] [PubMed] [Google Scholar]
- 5.Tilson JK, Sullivan KJ, Cen SY, et al. Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Physical therapy. 2010;90(2):196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makowski NS, Kobetic R, Lombardo LM, et al. Improving Walking with an Implanted Neuroprosthesis for Hip, Knee, and Ankle Control After Stroke. American journal of physical medicine & rehabilitation. 2016;95(12):880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waters RL, Hislop HJ, Perry J, Antonelli D. Energetics: application to the study and management of locomotor disabilities. Energy cost of normal and pathologic gait. Orthop Clin North Am. 1978;9(2):351–356. [PubMed] [Google Scholar]
- 8.Bard G. Energy expenditure of hemiplegic subjects during walking. Archives of Physical Medicine and Rehabilitation. 1963;44:368–370. [PubMed] [Google Scholar]
- 9.Platts MM, Rafferty D, Paul L. Metabolic cost of over ground gait in younger stroke patients and healthy controls. Medicine and science in sports and exercise. 2006;38(6):1041–1046. [DOI] [PubMed] [Google Scholar]
- 10.Serra MC, Treuth MS, Hafer-Macko CE, Ryan AS. Increased Energy Cost of Mobility in Chronic Stroke. J Gerontol Geriatr Res. 2016;5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer S, Johnson L, Bernhardt J, Cumming T. Energy Expenditure and Cost During Walking After Stroke: A Systematic Review. Archives of Physical Medicine and Rehabilitation. 2016;97(4):619–632 e611. [DOI] [PubMed] [Google Scholar]
- 12.Compagnat M, Mandigout S, David R, Lacroix J, Daviet JC, Salle JY. Compendium of physical activities strongly underestimates the oxygen cost during activities of daily living in stroke patients. American journal of physical medicine & rehabilitation. 2018. [DOI] [PubMed] [Google Scholar]
- 13.Schiemanck S, Berenpas F, van Swigchem R, et al. Effects of implantable peroneal nerve stimulation on gait quality, energy expenditure, participation and user satisfaction in patients with post-stroke drop foot using an ankle-foot orthosis. Restor Neurol Neurosci. 2015;33(6):795–807. [DOI] [PubMed] [Google Scholar]
- 14.Stookey AD, McCusker MG, Sorkin JD, et al. Test-retest reliability of portable metabolic monitoring after disabling stroke. Neurorehabilitation and neural repair. 2013;27(9):872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serra MC, Balraj E, DiSanzo BL, et al. Validating accelerometry as a measure of physical activity and energy expenditure in chronic stroke. Topics in stroke rehabilitation. 2017;24(1):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung T, Ozaki Y, Lai B, Vrongistinos K. Comparison of energy expenditure between aquatic and overground treadmill walking in people post-stroke. Physiotherapy research international : the journal for researchers and clinicians in physical therapy. 2014;19(1):55–64. [DOI] [PubMed] [Google Scholar]
- 17.Delussu AS, Morone G, Iosa M, Bragoni M, Traballesi M, Paolucci S. Physiological responses and energy cost of walking on the Gait Trainer with and without body weight support in subacute stroke patients. Journal of neuroengineering and rehabilitation. 2014;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danielsson A, Sunnerhagen KS. Oxygen consumption during treadmill walking with and without body weight support in patients with hemiparesis after stroke and in healthy subjects. Archives of Physical Medicine and Rehabilitation. 2000;81(7):953–957. [DOI] [PubMed] [Google Scholar]
- 19.van Nunen MP, Gerrits KH, de Haan A, Janssen TW. Exercise intensity of robot-assisted walking versus overground walking in nonambulatory stroke patients. Journal of rehabilitation research and development. 2012;49(10):1537–1546. [DOI] [PubMed] [Google Scholar]
- 20.Compagnat M, Mandigout S, Chaparro D, Salle JY, Daviet JC. Predicting the oxygen cost of walking in hemiparetic stroke patients. Ann Phys Rehabil Med. 2018;61(5):309–314. [DOI] [PubMed] [Google Scholar]
- 21.Fredrickson E, Ruff RL, Daly JJ. Physiological Cost Index as a proxy measure for the oxygen cost of gait in stroke patients. Neurorehabilitation and neural repair. 2007;21(5):429–434. [DOI] [PubMed] [Google Scholar]
- 22.Manns PJ, Haennel RG. SenseWear Armband and Stroke: Validity of Energy Expenditure and Step Count Measurement during Walking. Stroke Res Treat. 2012;2012:247165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ijmker T, Houdijk H, Lamoth CJ, et al. Effect of balance support on the energy cost of walking after stroke. Archives of Physical Medicine and Rehabilitation. 2013;94(11):2255–2261. [DOI] [PubMed] [Google Scholar]
- 24.Bleyenheuft C, Caty G, Lejeune T, Detrembleur C. Assessment of the Chignon dynamic ankle-foot orthosis using instrumented gait analysis in hemiparetic adults. Ann Readapt Med Phys. 2008;51(3):154–160. [DOI] [PubMed] [Google Scholar]
- 25.Stoquart GG, Detrembleur C, Palumbo S, Deltombe T, Lejeune TM. Effect of botulinum toxin injection in the rectus femoris on stiff-knee gait in people with stroke: a prospective observational study. Arch Phys Med Rehabil. 2008;89(1):56–61. [DOI] [PubMed] [Google Scholar]
- 26.Miller PC, Kobetic R, Lew RD. Energy costs of walking and standing using functional electrical stimulation Paper presented at: RESNA 13th Annual Conference1990; Washington D.C. [Google Scholar]
- 27.Boyne P, Dunning K, Carl D, et al. High-Intensity Interval Training and Moderate-Intensity Continuous Training in Ambulatory Chronic Stroke: Feasibility Study. Phys Ther. 2016;96(10):1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fanchamps MHJ, de Kam D, Sneekes EM, Stam HJ, Weerdesteyn V, Bussmann JBJ. Effect of different operationalizations of sedentary behavior in people with chronic stroke. Disability and rehabilitation. 2018:1–7. [DOI] [PubMed] [Google Scholar]
- 29.Nathoo C, Buren S, El-Haddad R, et al. Aerobic Training in Canadian Stroke Rehabilitation Programs. J Neurol Phys Ther. 2018;42(4):248–255. [DOI] [PubMed] [Google Scholar]
- 30.Physical activity trends--United States, 1990–1998. MMWR Morb Mortal Wkly Rep. 2001;50(9):166–169. [PubMed] [Google Scholar]
- 31.Linder SM, Rosenfeldt AB, Bazyk AS, Koop MM, Ozinga S, Alberts JL. Improved lower extremity pedaling mechanics in individuals with stroke under maximal workloads. Top Stroke Rehabil. 2018;25(4):248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szecsi J, Krewer C, Muller F, Straube A. Functional electrical stimulation assisted cycling of patients with subacute stroke: kinetic and kinematic analysis. Clin Biomech (Bristol, Avon). 2008;23(8):1086–1094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


