INTRODUCTION
Comparison data regarding anti-tumor necrosis factor (anti-TNF) drug concentrations in inflammatory bowel disease (IBD) between the enzyme-linked immunosorbent assay (ELISA) and the homogenous mobility shift assay (HMSA) are scarce.1–3 As decisions in clinical practice depend on the thresholds that define a therapeutic drug concentration, it is important to determine if this varies based on the type of assay used for therapeutic drug monitoring (TDM).4 We recently showed a discrepancy between a commercially available ELISA and the HMSA for both infliximab and adalimumab concentrations in patients with IBD.5 Based on the results of this study, Prometheus Laboratories initiated a comprehensive review of their HMSA assays and found that there was an upward drift for both infliximab (from December 2017 to May 2019) and adalimumab (from August 2017 to May 2019), including when our study was performed. Prometheus corrected the errant values and reported the revised drug concentrations to physicians (supplementary material). We aimed to compare the corrected infliximab and adalimumab concentrations to the original ELISA values.
METHODS
These are described in the supplementary material.
RESULTS
For infliximab, 45 samples were analyzed (supplementary Table 1). Following the implementation of corrective measures for the HMSA infliximab concentrations (median [interquartile range, IQR]) were still significantly higher when measured by the HMSA compared to ELISA (9 [7.1–12.4] vs. 5.7 [4.8–9] μg/ml; p<0.001, respectively). The correlation between assays remained very good (r=0.873, p<0.001, Figure 1A). However, agreement between assays, although improved, was only moderate (ICC=0.658, 95%CI: −0.080 to 0.892, p<0.001). A Bland–Altman plot of infliximab concentrations to compare the two assays is shown in Figure 1C. A Passing and Bablok plot is shown in Figure 1E. Qualitative agreement in drug concentration status (therapeutic vs. sub-therapeutic), although improved, was only minimal between assays using >5 μg/ml (K = 0.299, p=0.005), >7 μg/ml (K = 0.303, p=0.005) or >10 μg/ml (K = 0.323, p=0.003) as therapeutic drug concentrations. Supplementary Table 2 describes negative, positive and total agreement between assays based on different cut-offs for therapeutic infliximab concentrations.
Figure 1.
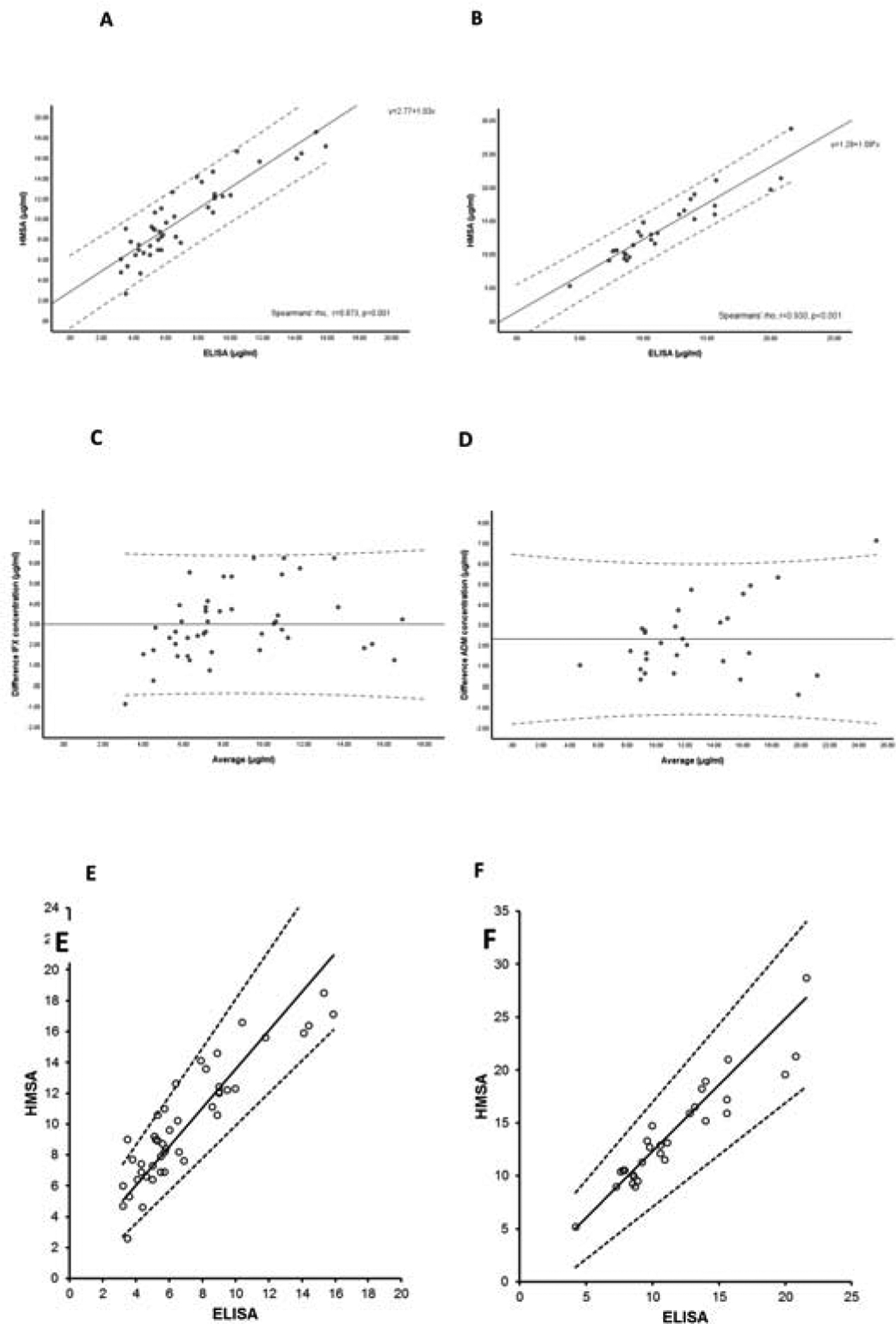
Correlation of infliximab (A) and adalimumab (B) concentrations between assays. Scatter plot with solid line representing fitted regression line and dashed lines the 95% confidence interval for the regression line. Bland–Altman plot of infliximab (C) and adalimumab (D) concentrations to compare the two assays: the difference between two measurements (mg/L) is plotted on the Y-axis and the average of the two measurements (mg/L) on the X-axis. Dotted lines represent the 5–95% limits of the mean difference (solid line). Passing and Bablok plots for infliximab (E) and adalimumab (F) according to assay. Scatter plot with solid line representing fitted regression line and dashed lines the 95% confidence (CI) interval for the regression line. For infliximab (slope: 1.25; 95%CI: 1.05–1.57 and intercept: 1.04; 95%CI: −0.63 to 2.37) and for adalimumab (slope: 1.25; 95%CI: 0.98–1.47 and intercept: −0.24; 95%CI: −2.76 to 2.21).
IFX: infliximab; ADM: adalimumab.
For adalimumab, 29 samples were analysed (supplementary Table 1). Following the implementation of corrective measures for the HMSA adalimumab concentrations (median [IQR]) were still significantly higher when measured by the HMSA compared to ELISA (12.9 [10.3–16.9] vs. 10.6 [8.6–14] μg/ml; p=0.036, respectively). The correlation between assays remained very good (r=0.930, p<0.001 Figure 1B). Agreement between assays was now strong (ICC = 0.826, 95%CI: −0.066 to 0.949, p<0.001). A Bland–Altman plot of adalimumab concentrations comparing the two assays is shown in Figure 1D. A Passing and Bablok plot is shown in Figure 1F. Qualitative agreement in adalimumab concentration status (therapeutic or sub-therapeutic) markedly improved reaching a total agreement of 97% for both >5 μg/ml and >7 μg/ml adalimumab concentrations; however was still weak between assays using >10 μg/ml as therapeutic drug concentrations (K = 0.437, p=0.004). Supplementary Table 2 describes negative, positive and total agreement between assays based on different cut-offs for therapeutic adalimumab concentrations.
DISCUSSION
Although the implementation of corrective measures for the HMSA made the two assays more comparable for both infliximab and adalimumab, there are still quantitative and qualitative discrepancies in drug concentrations. While the correlation remained very good for both infliximab and adalimumab the qualitative agreement between assays, although improved, was still only minimal for infliximab concentrations >5 μg/ml (K=0.299), >7 μg/ml (K=0.303), or >10 μg/ml (K=0.323), respectively, and weak for adalimumab concentrations >10 μg/ml (K=0.437).
These differences between assays may also apply to other biologics as well. A recent cross-sectional study comparing ustekinumab serum concentrations between two commercial ELISAs manufactured by Progenika (Dynacare Labs) and Theradiag and the HMSA in patients with CD showed that there was poor agreement between the HMSA and both ELISA tests. There was an almost 2-fold increased difference in the absolute ustekinumab serum concentrations between the HMSA and both ELISA tests.6 Moreover, differences in the quantitative results may apply also to different ELISAs as demonstrated by two recent studies, one for infliximab7 and one for golimumab.8
Limitations of the study include the relatively small sample size and the fact that most of the samples were drawn applying proactive TDM limiting the evaluation of clinically meaningful differences at lower drug concentrations.
In conclusion, although the correlation between the ELISA and the HMSA was very good for both infliximab and adalimumab, our data suggest that it may be difficult to compare absolute concentrations between assays. Until commercial assays are accurately cross-validated and standardized, clinicians should consider assay-dependent drug concentration thresholds and follow patients over time utilizing the same assay, if possible.
METHODS
Implementation of corrective measures
The mathematical corrections were determined utilizing wet-lab experiments by comparing the assay standards (calibrators) in place when the drift occurred to new gold standards freshly prepared from original pure infliximab and adalimumab vials. For Infliximab, both the assay infliximab standard in place at the time of the drift and the new infliximab gold standard were also compared to an International standard for Infliximab that had recently become available from the World Health Organization (WHO). The new infliximab gold standard and the WHO International infliximab standard were observed to match, confirming both the accuracy of the new infliximab gold standard, as well as the method used to prepare the new standards from the pure drug source vials. Subsequently, additional lots of standards were prepared for both infliximab and adalimumab using this method (measured drug from the pure drug source vial) by different personnel to further compare the accuracy of both the gold and the current drug standards. Lastly, spike and recovery experiments were also performed using measured levels of pure drug spiked into buffer at various concentrations spanning across the standards range for each of the drug assays. These experiments further confirmed the accuracy of the gold standards as well as the values obtained for unknown patient samples using these standards in an assay.
Statistical analysis
This was a follow up of a previous single-center prospective cross-sectional study comparing drug concentrations of infliximab and adalimumab utilizing two different commercially available assays, an ELISA (InformTx™, Inform Diagnostics, Irving, TX, USA) and the HMSA (ANSER®, Prometheus Laboratories Inc. San Diego, CA, USA).5 Peripheral venous blood samples from consecutive patients with IBD either on infliximab or adalimumab therapy were collected and sent the same day in separate tubes to be evaluated both with ELISA (for research purposes) and the HMSA (as part of routine clinical care) following the implementation of corrective measures for the latter by Prometheus. Per the reported limit of quantification of the assays, infliximab concentrations of < 0.3 μg/mL were considered undetectable with the ELISA and concentrations < 1 μg/mL were considered as undetectable with the HMSA assay. Adalimumab concentrations of < 0.5 μg/mL were deemed undetectable with the ELISA, and concentrations < 1.6 μg/mL were deemed undetectable with the HMSA assay. Samples with no absolute values were excluded from the final analyses. All patients consented to participate in the study. Approval was obtained from the Institutional Review Board at Beth Israel Deaconess Medical Center.
Comparisons of drug concentrations between assays were performed using the Wilcoxon-signed rank test. To quantify the correlation between drug concentrations from the ELISA and the HMSA, the Spearman’s’ rho was calculated, whereby a value of 1 represents an ideal correlation between the two methods. Correlation coefficients were compared using a linear regression analysis. Comparison of the two assays was also performed by Passing Bablok regression analysis. The intraclass correlation coefficient (ICC) was calculated using the two-way mixed single measures test (absolute agreement) to quantify the agreement between drug concentrations for the two assays. ICC were interpreted as follows: lack of agreement (0–0.3), weak agreement (0.31–0.5), moderate agreement (0.51–0.7), strong agreement (0.71–0.9) and very strong agreement (>0.91). A Bland–Altman plot was used to present the data in which the difference between two measurements is plotted on the Y-axis, and the average of two measurements on the X-axis. Qualitative agreement in drug concentration status between assays was performed using the method described by Fleiss31 and expressed as the positive and negative percent agreement (correlating with therapeutic and sub-therapeutic classification, respectively) using different cut-offs for therapeutic drug concentrations. Coefficient of agreement was reported using Cohen’s kappa (K) and classified as almost perfect (>0.9), strong (0.8–0.9), moderate (0.6–0.79), weak (0.4–0.59), minimal (0.21–0.39), and none (0–0.2). The statistical software package SPSS version 25.0 (IBM, New York, NY, USA) and GraphPad Prism version 5.03 for Windows (GraphPad Software, San Diego, CA, USA) were used for all analyses.
Supplementary Material
DISCLOSURES:
NVC reports personal fees from Janssen, Pfizer, Progenity and Prometheus, grants and personal fees from Takeda and UCB Pharma and grants from R-Biopharm; A.S.C: received consultancy fees from AbbVie, Janssen, Takeda, Ferring, Miraca, AMAG, Arena, Samsung, Prometheus and Pfizer, and research support from Miraca; G.Y.M has received research funding from Pfizer and is a consultant for Abbvie, Boehringer-Ingelheim, Celgene, Jannsen, Medtronic, Pfizer, Samsung Bioepis, Takeda; P.M.I is on the Advisory Board and Speaker’s Bureau for Abbvie, MSD, and Takeda; C.A.S. Consultant/Advisory Board: Abbvie, Amgen, BMS, Celgene, Lilly, Janssen, Sandoz, Pfizer, Prometheus, Sebela, Takeda, Speaker for CME activities: Abbvie, Celgene, Janssen, Pfizer, Takeda, Grant support: Crohn’s and Colitis Foundation, AHRQ (1R01HS021747-01) Broad Medical Research Program, Abbvie, Janssen, Pfizer, Takeda, Intellectual property: MiTest Health, LLC has a patent pending for a “System and Method of Communicating Predicted Medical Outcomes”, filed 3/34/10. Dr. Corey Siegel and Dr. Lori Siegel are inventors. Colonary Concepts, LLC has Unites States Patents on “Dietary Purgatives” and “Foods, Systems, Methods, and Kits for Providing Electrolyte Replacement.” Dr. Corey Siegel is an inventor Equity Interest Dr. Corey Siegel and Dr. Lori Siegel are cofounders of MiTest Health, LLC Dr. Corey Siegel is a co-founder of Colonary Concepts, LLC; K.P. received a lecture fee from Mitsubishi Tanabe Pharma; the remaining authors disclose no conflicts of interest.
GRANT SUPPORT:
This study was funded by Inform Diagnostics. N.V.C. holds a Research Scholar Award from the American Gastroenterological Association. K.P. is supported by the Ruth L. Kirschstein NRSA Institutional Research Training Grant (5T32DK007760-18).
ABBREVIATIONS:
- ELISA
enzyme-linked immunosorbent assay
- HMSA
homogeneous mobility shift assay
- IBD
inflammatory bowel disease
- ICC
intraclass correlation coefficient
- TDM
therapeutic drug monitoring
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
WRITING ASSISTANCE: None.
Guarantor of the article: Adam S. Cheifetz, MD.
REFERENCES
- 1.Vande Casteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013;108:962–71. [DOI] [PubMed] [Google Scholar]
- 2.Steenholdt C, Bendtzen K, Brynskov J, Thomsen O, Ainsworth MA. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in crohn’s disease: Post hoc analysis of a randomized controlled trial. Am J Gastroenterol 2014;109:1055–64. [DOI] [PubMed] [Google Scholar]
- 3.Bodini G, Giannini EG, Furnari M, et al. Comparison of two different techniques to assess adalimumab trough levels in patients with Crohn’s disease. J Gastrointes Liver Dis 2015;24:451– [DOI] [PubMed] [Google Scholar]
- 4.Vermeire S, Dreesen E, Papamichael K, et al. How, when, and for whom should we perform therapeutic drug monitoring? Clin Gastroenterol Hepatol. 2019. October 4. pii: S1542–3565(19)31092–4. doi: 10.1016/j.cgh.2019.09.041. [DOI] [PubMed] [Google Scholar]
- 5.Clarke WT, Papamichael K, Vande Casteele N, et al. Infliximab and adalimumab concentrations may vary between the enzyme-linked immunosorbent assay and the homogeneous mobility shift assay in patients with inflammatory bowel disease: a prospective cross-sectional observational study. Inflamm Bowel Dis 2019;25: e143–e145. [DOI] [PubMed] [Google Scholar]
- 6.Verdon C, Vande Casteele N, Heron V, et al. Comparison of serum concentrations of ustekinumab obtained by three commercial assays in patients with Crohn’s disease. Gastroenterology 2019;156 (6_suppl 1): S–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertin D, Serrero M, Grimaud JC, et al. Monitoring of infliximab trough levels and anti-infliximab antibodies in inflammatory bowel diseases: A comparison of three commercially available ELISA kits. Cytokine. 2020. February;126:154859. [DOI] [PubMed] [Google Scholar]
- 8.Berger AE, Duru G, de Vries A, et al. Comparison of immunoassays for measuring serum levels of golimumab and antibodies against golimumab in ulcerative colitis: a retrospective observational Study. Ther Drug Monit 2019;41:459–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


