Abstract
Background
Adherence to antiretroviral therapy (ART) is imperative for viral suppression and reducing HIV transmission, but many people living with HIV report difficultly sustaining long-term adherence. Long-acting injectable (LAI) ART has the potential to transform HIV treatment and prevention. However, little LAI ART-related behavioral research has occurred among women, particularly outside of clinical trials.
Setting
Six Women’s Interagency HIV Study (WIHS) sites: New York, Chicago, Washington DC, Atlanta, Chapel Hill, and San Francisco.
Methods
We conducted 59 in-depth interviews with women living with HIV across six WIHS sites (10 per site; 9 at Washington DC). We interviewed women were who were not included in LAI ART clinical trials, but who receive care at university settings that will administer LAI ART once it is approved. Interviews were recorded, transcribed, and analyzed using thematic content analysis.
Results
Most women enthusiastically endorsed monthly LAI ART and would prefer it over pills. Three reasons emerged for this preference: 1) convenience and confidentiality; 2) avoiding daily reminders about living with HIV; and 3) believing that shots are more effective than pills. Challenges remain, however, specifically around: 1) medical mistrust; 2) concerns about safety and effectiveness; 3) pill burden for HIV and other conditions; and 4) barriers to additional medical visits.
Conclusion
Most women preferred LAI ART over daily pills given its benefits including convenience, privacy, and perceived effectiveness. Future research should incorporate more women into LAI ART trials in order to better understand and align development with user concerns and preferences in order to enhance uptake.
Keywords: long-acting injectable, biomedical prevention, HIV anti-retroviral therapy, women, adherence
INTRODUCTION
Nearly one-quarter of people living with HIV (PLWH) in the United States (U.S.) are women. Of these, 89% know their diagnosis, 65% receive care, and 51% are virally suppressed.1 Women living with HIV (WLWH) have historically been underrepresented in HIV treatment research and face myriad barriers, including gender-specific barriers, to HIV care continuum progression.2,3 Despite oral antiretroviral therapy (ART), in 2016 only 58% of diagnosed WLWH were virally suppressed, in part due to suboptimal adherence. WLWH have poorer adherence,5,6 viral suppression,7,8 and long-term clinical outcomes9,10 than men, and are more likely to receive a simultaneous HIV and AIDS diagnosis (i.e., a late diagnosis).7,8 Although women face unique adherence barriers, they are underrepresented in HIV clinical trials and the trial data from primarily men does represent their needs. As such, women do not equally benefit from technological advances that aim to improve HIV prevention and treatment.11
In addition to individual health benefits, the treatment of persons living with HIV decreases transmission (i.e. Treatment as Prevention) and, while studies remain limited, research suggests that increased treatment coverage is associated with declines in community viral load in South Africa,12 San Francisco,13 and British Columbia.14 Mathematical modeling suggests it would also be successful in Washington DC.15 Obtaining and maintaining this reduction in community viral load, however, requires medication adherence over the lifespan. Demonstrated barriers to adherence include side effects,16 substance use17 or mental health18, provider- and clinic-level challenges (e.g. medical mistrust or poor communication),19,20 and social and structural barriers (e.g. stigma,2,3 care access,21 and gender norms22). Many PLWH face structural barriers that constrain adherence (e.g. homelessness, food insecurity), yet the majority of behavioral interventions to promote adherence focus on the individual level. As such, the majority of behavioral adherence interventions have not been successful. Given the importance of ART in maintaining the health of PLWH, and limiting the risk of further HIV transmission, new strategies are needed to facilitate adherence.
With a goal to address these barriers, phase III clinical trials are currently testing the efficacy of long-acting injectable (LAI) ART (e.g., Antiretroviral Therapy as Long-Acting Suppression (ATLAS23) and First Long-Acting Injectable Regimen (FLAIR24)). Results to date indicate non-inferiority to oral ART in terms of viral suppression and low frequency of virologic failure.23 Participants report that the side effects (e.g. fatigue, fever, headache and nausea) have been generally well-tolerated and only rarely led to discontinuation.23–27 Trial participants reported a high preference (97% in FLAIR, 91% in ATLAS) for LAI ART over oral ART. ATLAS data has already been submitted to the U.S. Food and Drug Administration for approval; ATLAS 2M, which is exploring the efficacy of bimonthly shots, is ongoing.23–27 In their current form, these injections require visits with HIV providers though over time they may be administered in pharmacies or by other types of providers.
LAI ART may help alleviative some barriers to oral medication but significant challenges remain as its current form requires one or more injections per visit and frequent injections (i.e. monthly visits were tested in ATLAS, and bimonthly visits in ATLAS 2M).31 The majority of LAI ART trials include males, particularly men who have sex with men (MSM) and White participants: in ATLAS only one-third were women, in FLAIR about one-fifth were women, and in LATTE-2 only 8% of participants were women.23–27 While oral ART has improved, WLWH still face significant barriers to daily pill-taking, some of which are gender-specific. These include at the individual level (e.g., forgetfulness,2,3 pill fatigue,2,3 drug use;28,29 interpersonal level (e.g., care-taking responsibilities that complicate clinic visits, stigmatization,2,30 unintended disclosure);31,32 clinic level (e.g., medical mistrust and patient-provider communication;) and structural level (e.g., unstable employment, transportation, food insecurity,33 and care access.)31,32 Some of these multi-level barriers might be addressed, or alleviated, by LAI ART.
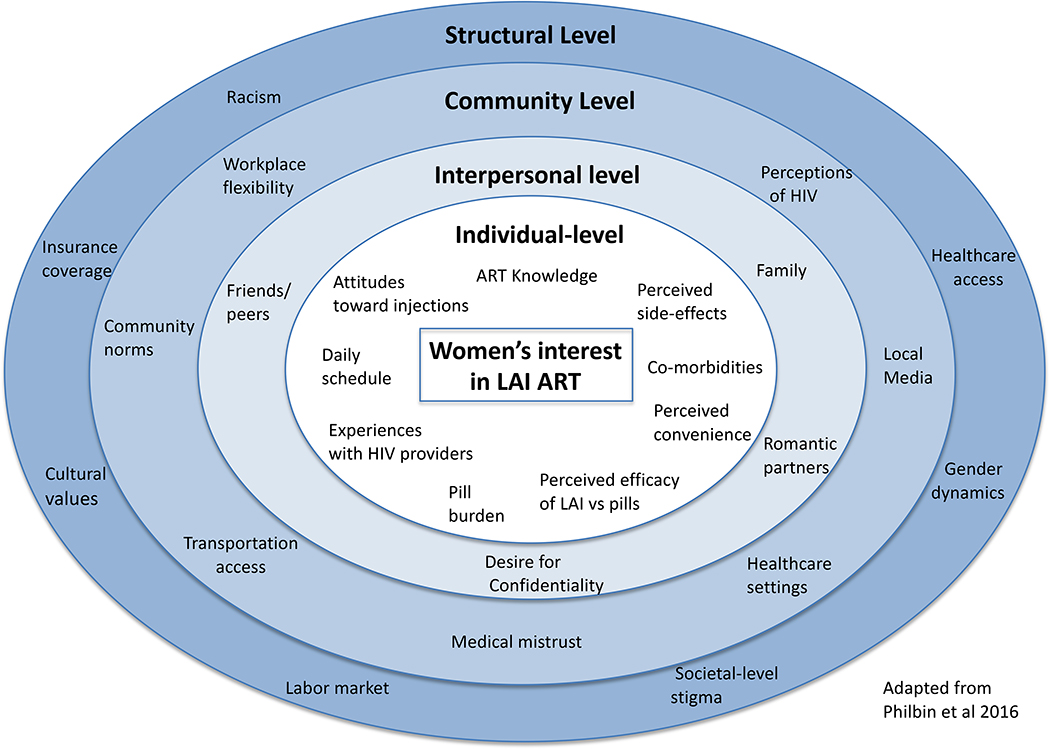
Given the myriad level factors that impact WLWH’s interest in, and access to, LAI ART, we applied Bronfenbrenner’s34 (1979) ecological model, adapted from a model tailored to PrEP uptake35, to frame our analytic approach (Figure 1). To better understand women’s unique experiences with HIV medication adherence and care access, and to explore their interest in LAI ART, with a focus on perceived barriers and facilitators to uptake, we conducted interviews with WLWH in six sites across the U.S.
Figure 1:
Ecological model of factors that impact Women’s interest in LAI ART
METHODS
Data were collected from the Women’s Interagency HIV Study (WIHS), the largest national prospective cohort study of women living with and at risk for HIV infection.36 WIHS participants are followed biannually and study visits include a physical examination, interviewer-administered questionnaire, and ascertainment of medical history and psychosocial factors.
Six WIHS sites were included in this qualitative sub-study (Atlanta, Georgia; San Francisco, California; Washington, D.C.; Chapel Hill, North Carolina; Bronx, New York; Chicago, Illinois). Details of the WIHS have previously described.37 The 59 in-depth interviews (10 at each site and 9 in Washington D.C.) were conducted from November 2017 – October 2018. The sites recruited participants to ensure they varied by characteristics that might influence their interest in, and willing to use LAI ART (e.g., age, job status, caregiving responsibilities and time since diagnosis). The interviews lasted approximately 60 minutes, were digitally recorded and transcribed verbatim. Interview questions were open-ended and explored women’s experience with injectable medication, related knowledge and attitudes, and the perceived barriers and facilitators towards using injectable HIV medications for ART. Participants provided informed consent and were compensated $50. The IRBs at all participating sites provided approval.
Data were analyzed using the constant comparative method38,39 to explore women’s responses to LAI ART, with a focus on barriers and facilitators. Three individuals conducted line-by-line open coding on the first five interviews to develop a provisional coding scheme focused primarily on identifying women’s perceived barriers and facilitators towards LAI ART uptake. Subsequently, thematic codes based on existing literature were added to ensure that theory-based and emergent concepts were included. The team members then cross-coded a random sample of 10 additional transcripts to refine the code dictionary and develop a codebook. This codebook was reviewed and amended by other team members.40 Two coders independently applied this final coding scheme to all interview transcripts, and any discrepancies were resolved during team meetings.
RESULTS
Participants’ median age was 53 years (range 32–72) and the majority were women of color (96%). One-third had less than a high school education, while just under one-third had a high school diploma or some college, and 15% had a graduate degree. Most women were unemployed (66%) and earned <$12,000/year (59%) (Table 1).
Table 1.
Participant Demographic Characteristics
| Characteristic | Total (N = 59) | Median | Percentage |
|---|---|---|---|
| Age in years (range: 34–72 years) | 53 | ||
| 34–39 | 7 | 12% | |
| 40–49 | 14 | 24% | |
| 50–59 | 28 | 47% | |
| 60+ | 10 | 17% | |
| Race | |||
| Black/African American | 45 | 76% | |
| Caucasian | 2 | 3% | |
| Hispanic | 4 | 7% | |
| Mixed | 6 | 10% | |
| Other | 2 | 3% | |
| Education | |||
| Less than high school | 12 | 20% | |
| Completed high school/GED | 19 | 32% | |
| Some college | 18 | 31% | |
| College or graduate school | 10 | 17% | |
| Household income | $15,876 | ||
| $0 – $11,999 | 24 | 41% | |
| $12,000+ | 34 | 58% | |
| Relationship status | |||
| Single | 27 | 46% | |
| Dating <6 months | 1 | 2% | |
| Dating >6 months | 12 | 20% | |
| Married/long-term partnership | 19 | 32% | |
| Children | |||
| Has children | 43 | 73% | |
| Does not have children | 16 | 27% | |
| Insurance | |||
| Uninsured | 4 | 7% | |
| Public insurance | 51 | 86% | |
| Private insurance | 3 | 5% | |
| Other insurance | 1 | 2% | |
| Year diagnosed with HIV | |||
| 1980–1989 | 14 | 24% | |
| 1990–1999 | 16 | 27% | |
| 2000–2009 | 22 | 37% | |
| 2010–2019 | 7 | 12% | |
| No. of years on ART | 12 | ||
| 0–9 | 25 | 42% | |
| 10–19 | 20 | 34% | |
| 20+ | 12 | 20% |
Over half (33/59), responded that they would choose LAI over daily pills, expressing sentiments such as, “Once a month? And I don’t have to take no damn pill no more? Man, shoot me fast” (45, Black, Atlanta). WLWH identified three primary factors that would facilitate LAI ART uptake: 1) convenience and confidentiality over daily pills; 2) pill fatigue and avoiding daily reminders of their sero-status; and 3) believing shots were more effective than pills. Four primary factors were found to limit enthusiasm for LAI ART: 1) medical mistrust; 2) potential side effects; 3) incomplete pill burden reduction; and 4) additional medical visits.
Facilitators of LAI ART uptake
Convenience and confidentiality
Women nearly uniformly described how LAI ART would make HIV medication more convenient. Specifically, LAI ART could be adopted in ways that would not interfere with their daily lives: “If I’m at my brother’s house and I forgot my meds, that nightmare. If [the shot] were there, you travel for a week, then you don’t have to worry” (50, Black, D.C.). LAI ART would eliminate the need to carry daily medication as it would only require a monthly doctor’s visit. This focus on convenience was particularly relevant for WLWH who had struggled with adherence to oral ART. Participants identified subgroups of WLWH who could most benefit from LAI ART, including youth, those with unstable housing, and incarcerated women.
WLWH also described LAI ART as a way to maintain confidentiality that is often challenging with pills. They frequently used words like “discreet” and “privacy” to describe LAI, which was important because of “nosy” friends: “Nobody knows you’re going to get a shot every month. [But if] you got any stuff jingling in your bag. A person there: “What you got in there?” Some pills. This is the stuff I’ve heard. That’s a plus for it [LAI], the discreet part” (34, Black, Chapel Hill). Another woman described how other medication-related items in her house could reveal her HIV status which limited her social life; “I don’t have people over because I don’t want them to see the massive fucking 36 binders of paperwork I have. I don’t care what anybody says, but there is a stigma, and it sucks” (51, Mixed race, San Francisco). These sentiments demonstrate how LAI might ameliorate the ways that women’s HIV status can govern how they interact with others, and help them lead ‘normal’ lives.
Pill fatigue and daily reminders of being HIV-positive
In addition to LAI being more “discreet,” women focused on how it might eliminate pill taking: “Once a month! I don’t have to take pills that associate me with HIV. It’s awesome! I’m stuck once a month and no pills! I’m so tired of taking pills” (50, Black, Bronx). Eliminating pills was particularly important because the pills provided a daily reminder of women’s HIV status and related stigma. This stigma challenged their ability to remain adherent and maintain viral suppression: “Some days I get depressed and I’m like, “Why did this have to happen to me? I’m a good person. That could break you to a point where you might say, ‘I’m tired of taking the medicine. I got to be on this stuff for the rest of my life’” (62, Black, Atlanta). Using shots would also eliminate a tangible symbol of women’s illness—namely the pills: “It would make you feel more normal, because you don’t have to think about it every day, when I take my medicine, I’m like, ‘I got to take this because I’m HIV-positive’. With a shot I just know it would be so much better. (43, Black, Chapel Hill).
Many WLWH had been HIV-positive for decades, and described their frustration and struggle with daily pill-taking, “I used to be so good about it, and I noticed that within the last year I started not giving a shit. It’s almost like being a brat, thinking, ‘Ha, ha. I didn’t take my pills.’ Like I get so tired of having to do all of these things that I never wanted to do and don’t want to deal with” (51, Mixed race, San Francisco). Women who struggled with viral suppression frequently noted the challenges of pill fatigue and how LAI ART could help alleviate that and increase their adherence. Other women shared this fatigue with daily pill-taking and juxtaposed it directly with the perceived convenience of monthly shots: “A shot you know the date, you know to go get it and you ain’t got nothing else to worry about” (61, Black, Bronx). This suggests that LAI could potentially lower HIV-related stigma because women would not have daily reminders of their HIV status, and that it could also facilitate adherence because it would help address pill fatigue.
Shots as more effective than pills
Women were also excited about LAI because they believed that shots were more effective than pills, and that they worked faster. “I think it’s more effective in a shot...That’s just from drug use, there’s a difference between injecting cocaine and snorting it or smoking it, because you get everything that’s in there. You know it got into your system” (65, Black, San Francisco). These beliefs around the shot’s perceived effectiveness and efficiency are important to note given the fact that women also described injection-related fears, described below.
Barriers to LAI ART
Medical mistrust
Many WLWH described a mistrust of the medical system, particularly the fear of new—and perceived untested—injectable products. Women frequently expressed a desire to wait before considering LAI ART in order to ensure its safety. This was particularly true because women already took medication they knew was effective: “I want to make sure it has the same efficiency as my drugs I’m taking now. I would hate if I stop taking my medicines for a month while I’m testing this out, and my viral load shoots up because it’s not doing the same thing my cocktail is doing” (56, Black, D.C.). Lastly, a few women described a fear that speaks to the history of how medicine engaged with marginalized communities, “I heard about injectable HIV medication, I immediately thought, ‘Here it comes. They’re going to kill us off. They’re going to try to force us to take this injection and it’s going to end us.’ And that way they won’t have to pay for these pills, it’s just an easy way to kill us off” (51, Mixed race, San Francisco). Thus, while women described injectable medication as more convenient, certain historical precedents might dampen their enthusiasm and uptake.
Potential side effects
When asked about potential side effects, women described fears of injection-site pain, bruising, and nausea. One-third of women (39%) were somewhat or very concerned about potential injection site pain and responded with: “Shit, I know it’s going to hurt. Ouch” (57, Black, Atlanta) and “Not even very. Extremely concerned” (42, Hispanic, Bronx). In contrast, some women were not concerned and referenced familiarity with other shots and tattoos. Some women also worried about whether their age might impact their body’s ability to heal: “The older you get the longer it takes you to heal and stuff. I’d hate to have all this bruising or marks from six months of shots” (58, Caucasian, San Francisco). Approximately one-third (27%) said that the injection site location (i.e., the buttocks) would influence their decision to use LAI ART, with 24% noting that they would be more likely to use LAI ART if the injection could occur in the arm or stomach; 51% said injection location made no difference.
Additionally, some women shared a fear that they would have little recourse with side effects because the shot lasted for such a long time and could not be removed: “With a pill-- if I have a reaction, I can immediately go and flush it through my urine. With a shot, that stuff is in your blood stream which goes to your heart. That’s mind-boggling. I’d stick with the pill” (60, Black, D.C.). This was in direct contrast to the pill, which somebody could simply stop taking the next day. Lastly, a few women with a history of injection drug use worried that taking an injectable medication may serve as a ‘trigger’ and inadvertently facilitate reoccurrence of drug use.
Incomplete pill burden reduction
Women frequently took pills for multiple conditions, and reported mixed feelings about whether they would use LAI ART if it could only eliminate some of their pills (e.g., medication for HIV but not blood pressure). Specifically, some WLWH expressed that taking fewer pills wasn’t helpful; they would need all pills eliminated to use LAI ART: “I have bunch of medication to take, just because that one medication is injected, that doesn’t save me any trauma. I still have to worry about all these pills.” (50, Black, D.C.). Similarly, another woman described how, “You take all or you don’t take none. So, if you can’t take all my medicines away, I don’t want it” (36, Black, Atlanta). This was particularly challenging for older individuals with co-occurring conditions that required daily pills, since LAI would not eliminate all pills and also add additional medical visits.
Additional medical visits
While the majority said that time spent attending additional medical visits would not be a burden, many expressed wariness about accessing the clinic. Women, particularly in more rural sites (e.g., North Carolina) or in some communities (e.g., Atlanta), that may face transportation barriers, voiced concerns about getting to their appointments: “People that don’t have the transportation to get back and forth. Are they going to be able to get where they need to get these shots? Do they have the support system to get back and forth?” (58, Black, Chapel Hill). It was also challenging for women with full time jobs who might be unable to miss work with the frequency required for LAI medication. A number of women shared that they might prefer LAI ART if the doctor’s visits were less frequent, but that every month would be challenging, “It sounds promising. Only issue for me is the once a month. If it was more like every three months, every four or five months, I could do that. But once a month, no. I’m not anti the shot, it’s just the frequency” (34, Black, Atlanta). This suggests potential geographic disparities in LAI ART uptake as a result of women’s clinic access.
DISCUSSION
This paper explored perceived multi-level barriers and facilitators to LAI ART uptake as reported by WLWH across six distinct geographic areas in the U.S. HIV medication has improved drastically resulting in fewer side effects and the “one pill once a day” option. However, ART adherence is still a significant challenge.41 Researchers have therefore worked to develop LAI HIV therapy to improve individual patient outcomes and curb population-level HIV transmission.
The majority of women in this study would prefer LAI ART, particularly because of its convenience, privacy, and perceived effectiveness. Similar to other studies, participants described “forgetting” as a common reason for pill non-adherence, particularly while traveling, suggesting that eliminating daily pills may improve adherence.42 Injections could also occur in a doctor’s office, eliminating participants’ fears about friends discovering their medications. The focus on privacy points to the continued salience of HIV stigma in the lives of PLWH. Previous work has established a link between stigma and adherence to ART, suggesting that improved privacy may facilitate adherence.3,18,43
Additionally, participants described “pill fatigue” and the challenge of constant reminders of their HIV status. Women described monthly injections as an innovation that would allow them to more freely live their lives without the constant burden of treating their HIV. Treatment fatigue and increased regimen complexity are associated with decreased adherence to oral ART, which LAI ART may reduce and thus improve adherence.41,44,45 However, individuals who take daily pills to manage other conditions (e.g., mental health, diabetes) may prefer to continue their current regimen instead of adding monthly clinic visits to receive injections.
Many women expressed concerns about potential side effects and safety and about one-third of women questioned whether the medication was safe. As HIV disproportionately affects communities of color who have a history of mistreatment by the medical system, medical mistrust should be a consideration when implementing LAI ART.46–48 These findings are consistent with previous qualitative work among men that found concerns over the safety of newer treatment modalities.49 In addition, anticipated stigma in healthcare settings can also negatively impact ART adherence, raising concerns that the increased frequency of clinic visits may pose a problem if patients feel stigmatized by healthcare providers.2 This highlights the continued work that is necessary to address HIV-related stigma, particularly related to medication adherence.
Transportation challenges were also salient, though this varied by geography. Some felt that monthly visits would be a challenge, particularly for individuals who work hourly wages, have children, and live in areas without public transportation (often in the south).50 Previous work has demonstrated low adherence among rural and low-income mothers, supporting the notion that myriad socioeconomic factors may particularly disadvantage mothers.50,51 This also suggests that LAI ART uptake might improve if it could also be offered in additional accessible locations such as a local pharmacy or urgent care. In sum, findings demonstrate that LAI ART uptake and use will be most successful if potential barriers at all levels of the ecological framework are addressed. Specifically, our findings show the importance of incorporating individual-level factors such as perceived side-effects and current pill burden, interpersonal factors such as the need for confidentiality and caregiving burden, community-level factors such as workplace flexibility and ability to access transportation, and structural-level factors such as gender dynamics.
Current LAI ART clinical trials predominantly include men, and qualitative research has primarily explored men’s perceptions of LAI ART.23,24,49 While most men in LAI ART clinical trials reported injection-site side effects52, they remained enthusiastic about LAI ART and preferred it over oral ART.53,54 However, clinical trials frequently include participants with high adherence whose experiences may not mirror the general population. For example, only 49% of WLWH in WIHS would be eligible for AIDS Clinical Trials Group trials.37 A focus group study in the general population also found that PLWH were less enthusiastic about LAI ART than clinical trial participants.55 In addition, qualitative research with LAI ART clinical trial participants included only two women; WIHS women are representative of WLWH in the US, and it is imperative to explore their opinions regarding LAI ART since women have identified gender-specific challenges to oral ART uptake that may be ameliorated by LAI ART. These included disclosure concerns, difficulty obtaining childcare for more frequent doctors’ visits, and fears regarding whether the medication would influence women’s ability to get pregnant or subsequent fetal development.29 Specific to women, prior work suggests that non-daily contraceptive methods (e.g., depo-provera) increase adherence among women of childbearing age; these findings are potentially applicable to LAI ART.56,57
Future research should explore how diverse populations (e.g., by age, race/ethnicity, sexual orientation) perceive LAI ART, considering their disproportionate rates of HIV incidence. Trial recruiting efforts should be expanded to include more women of color.7,8 Research should be expanded to include younger women, particularly those who are newly diagnosed and therefore lack the experience of pill taking and women of childbearing age who may have unique concerns. Lastly, survey research with larger populations should be conducted so that those results can be synthesized with qualitative findings.
Strengths and Limitations
Interviews were conducted among women in six geographically diverse sites and could capture the experiences of women in various contexts (e.g. urban vs. rural, North vs. South). We also included women whose experiences might more accurately mirror those of the U.S. population—one study found that approximately 50% of women in WIHS would not be eligible for inclusion in clinical trials.58 Despite this strength, this study is limited by the fact that the sample population was older, and most individuals had been HIV-positive for many years and subsequently developed multiple coping and adherence strategies. Participants may therefore have different relationships to pills compared to younger HIV-positive individuals. We recruited women who varied by characteristics that could influence interest in WIHS, including those who face challenges attending clinic visits. We were only able to speak with women who presented at the clinic for their interview. Consequently, we may not have reached women who are less adherent to treatment and face transportation and geographical barriers, though we provided transportation funding to attend the interview. While women described a range of facilitators and barriers to LAI ART use, it is possible that the WLWH across the US may be less enthusiastic about LAI ART than the women we interviewed.
Conclusions
This study demonstrates that women living with HIV are open to LAI ART, and many believe it will provide distinct benefits over daily pills. Women shared many of the same concerns as men, however, they described unique challenges such as medical mistrust, the role of children and childbearing, caregiving responsibilities and privacy that LAI might help alleviate. LAI might also facilitate confidentiality and minimizing time spent dealing with pills or pharmacy refills. Efforts should be made to incorporate more women into LAI ART trials in order to better understand and align development with user concerns and preferences with a goal to enhance uptake. This highlights the need to tailor the scale-up of LAI ART to specific subpopulations; additional research should further explore potential differences in WLWH’s LAI ART interest by subgroup to further tailor specific interventions. For example, patient-provider communication strategies should include details about LAI ART efficacy and side-effects, and explicitly address medical mistrust; community and structural-level factors could be addressed through expansion of locations and times where LAI ART is administered. In order to realize the potential of LAI ART, future research needs to examine LAI ART can be integrated into the lives of WLWH across all levels of the ecological framework.
Supplementary Material
ACKNOWLEDGEMENTS
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos and Anjali Sharma), U01-AI-035004; Brooklyn WIHS (Deborah Gustafson and Tracey Wilson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye and Daniel Merenstein), U01-AI-034994; Miami WIHS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Bradley Aouizerat and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). Philbin is supported by K01DA039804A
Source of support: The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR). Dr. Philbin is supported by K01DA039804A
Conflicts of Interest Adaora A. Adimora-has received funding from Viiv, Merck, and Gilead, including a grant from Gilead; Anandi N. Sheth declares that Gilead has given research grants to her institution, but none are related to her current work. For the remaining authors none were declared.
References
- 1.Centers for Disease Control and Prevention. Women | Gender | HIV by Group | HIV/AIDS | CDC. CDC.gov. https://www.cdc.gov/hiv/group/gender/women/index.html. Published May 10, 2018. Accessed May 21, 2018.
- 2.Rice WS, Turan B, Fletcher FE, et al. A Mixed Methods Study of Anticipated and Experienced Stigma in Health Care Settings Among Women Living with HIV in the United States. AIDS Patient Care STDs. 2019;33(4):184–195. doi: 10.1089/apc.2018.0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turan B, Rice WS, Crockett KB, et al. Longitudinal association between internalized HIV stigma and antiretroviral therapy adherence for women living with HIV: the mediating role of depression. AIDS. 2019;33(3):571. doi: 10.1097/QAD.0000000000002071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Understanding the HIV Care Continuum.; 2018:4. [Google Scholar]
- 5.Knobel H, Urbina O, González A, et al. Impact of different patterns of nonadherence on the outcome of highly active antiretroviral therapy in patients with long-term follow-up. HIV Med 2009;10(6):364–369. doi: 10.1111/j.1468-1293.2009.00696.x [DOI] [PubMed] [Google Scholar]
- 6.Murphy P, Cocohoba J, Tang A, Pietrandoni G, Hou J, Guglielmo BJ. Impact of HIV-Specialized Pharmacies on Adherence and Persistence with Antiretroviral Therapy. AIDS Patient Care STDs. 2012;26(9):526–531. doi: 10.1089/apc.2012.0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Selected National HIV Prevention and Care Outcomes (2015, 2016). CDC.gov. https://www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-prevention-and-care-outcomes.pdf. Published December 2017. Accessed May 8, 2019.
- 8.Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Selected National HIV Prevention and Care Outcomes in the United States. Washington, DC: Centers for Disease Control and Prevention; 2018:3 https://www.cdc.gov/hiv/pdf/library/factsheets/cdc-hiv-national-hiv-care-outcomes.pdf. [Google Scholar]
- 9.Meditz AL, MaWhinney S, Allshouse A, et al. Sex, Race, and Geographic Region Influence Clinical Outcomes Following Primary HIV-1 Infection. J Infect Dis. 2011;203(4):442–451. doi: 10.1093/infdis/jiq085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy K, Hoover DR, Shi Q, et al. Association of self-reported race with AIDS death in continuous HAART users in a cohort of HIV-infected women in the United States. AIDS Lond Engl. 2013;27(15):2413–2423. doi: 10.1097/01.aids.0000432537.92958.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiv TL. New PrEP formulation approved…but only for some. Lancet HIV. 2019;6(11):e723. doi: 10.1016/S2352-3018(19)30350-9 [DOI] [PubMed] [Google Scholar]
- 12.Tanser F, Barnighausen T, Grapsa E, Newell M. Effect of ART Coverage on Rate of New HIV Infections in a Hyper-endemic, Rural Population: South Africa: In: 2012. [Google Scholar]
- 13.Das-Douglas M, Chu P, Santos G, et al. Decreases in Community Viral Load are Associated with a Reduction in New HIV Diagnoses in San Francisco. In: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montaner JSG, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. The Lancet. 2010;376(9740):532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walensky RP, Paltiel AD, Losina E, et al. Test and Treat DC: Forecasting the Impact of a Comprehensive HIV Strategy in Washington DC. Clin Infect Dis. 2010;51(4):392–400. doi: 10.1086/655130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services; 2018:K-14 https://aidsinfo.nih.gov/guidelines. Accessed April 11, 2019. [Google Scholar]
- 17.Zhang Y, Wilson TE, Adedimeji A, et al. The Impact of Substance Use on Adherence to Antiretroviral Therapy Among HIV-Infected Women in the United States. AIDS Behav. 2018;22(3):896–908. doi: 10.1007/s10461-017-1808-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Relf MV, Pan W, Edmonds A, Ramirez C, Amarasekara S, Adimora AA. Discrimination, medical distrust, stigma, depressive symptoms, antiretroviral medication adherence, engagement in care and quality of life among women living with HIV in North Carolina: a mediated structural equation model. JAIDS J Acquir Immune Defic Syndr. 2019;Publish Ahead of Print. doi: 10.1097/QAI.0000000000002033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanner AE, Philbin MM, Duval A, Ellen J, Kapogiannis B, Fortenberry JD. “Youth friendly” clinics: Considerations for linking and engaging HIV-infected adolescents into care. AIDS Care. 2014;26(2):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philbin MM, Tanner AE, DuVal A, et al. Factors Affecting Linkage to Care and Engagement in Care for Newly Diagnosed HIV-Positive Adolescents Within Fifteen Adolescent Medicine Clinics in the United States. AIDS Behav. 2014;18(8):1501–1510. doi: 10.1007/s10461-013-0650-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philbin MM, Tanner AE, DuVal A, Ellen J, Kapogiannis B, Fortenberry JD. Linking HIV-positive adolescents to care in 15 different clinics across the United States: creating solutions to address structural barriers for linkage to care. AIDS Care. 2014;26(1):12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philbin MM, Parker CM, Parker RG, Wilson PA, Garcia J, Hirsch JS. Gendered Social Institutions and Preventive Healthcare Seeking for Black Men Who Have Sex with Men: The Promise of Biomedical HIV Prevention. Arch Sex Behav. June 2018. doi: 10.1007/s10508-018-1211-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GSK Clinical Trials, ViiV Healthcare. Study Evaluating the Efficacy, Safety, and Tolerability of Switching to Long-acting Cabotegravir Plus Long-acting Rilpivirine From Current Antiretroviral Regimen in Virologically Suppressed HIV-1-infected Adults. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02951052. Published October 5, 2018. Accessed April 15, 2019. [Google Scholar]
- 24.GSK Clinical Trials, ViiV Healthcare. Study to Evaluate the Efficacy, Safety, and Tolerability of Long-acting Intramuscular Cabotegravir and Rilpivirine for Maintenance of Virologic Suppression Following Switch From an Integrase Inhibitor in HIV-1 Infected Therapy Naive Participants. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02938520. Published November 28, 2018. Accessed April 15, 2019. [Google Scholar]
- 25.Collins S. Phase 3 results with dual therapy cabotegravir/rilpivirine long-acting injections: ATLAS and FLAIR studies. HIV i-Base. http://i-base.info/htb/35812. Published March 12, 2019 Accessed April 15, 2019. [Google Scholar]
- 26.Orkin C Long-acting Cabotegravir + Rilpivirine for HIV maintenance: FLAIR week 48 results. Presented at the: CROI; March 4, 2019; Seattle, Washington: http://www.croiconference.org/sessions/long-acting-cabotegravir-rilpivirine-hiv-maintenance-flair-week-48-results. Accessed April 14, 2019. [Google Scholar]
- 27.Swindells S Long-acting Cabotegravir + Rilpivirine as maintenance therapy: ATLAS week 48 results. Presented at the: CROI; March 4, 2019; Seattle, Washington: http://www.croiconference.org/sessions/long-acting-cabotegravir-rilpivirine-maintenance-therapy-atlas-week-48-results. Accessed April 14, 2019. [Google Scholar]
- 28.Philbin MM, Feaster DJ, Gooden L, et al. The North-South Divide: Substance Use Risk, Care Engagement, and Viral Suppression Among Hospitalized Human Immunodeficiency Virus–Infected Patients in 11 US Cities. Clin Infect Dis. 2019;68(1):146–149. doi: 10.1093/cid/ciy506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter A, Roth EA, Ding E, et al. Substance Use, Violence, and Antiretroviral Adherence: A Latent Class Analysis of Women Living with HIV in Canada. AIDS Behav. 2018;22(3):971–985. doi: 10.1007/s10461-017-1863-x [DOI] [PubMed] [Google Scholar]
- 30.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;13(3 Suppl 2):18640. doi: 10.7448/IAS.16.3.18640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mugavero MJ, Amico KR, Horn T, Thompson MA. The State of Engagement in HIV Care in the United States: From Cascade to Continuum to Control. Clin Infect Dis. 2013;57(8):1164–1171. doi: 10.1093/cid/cit420 [DOI] [PubMed] [Google Scholar]
- 32.Mugavero M, Norton W, Saag M. Health care system and policy factors influencing engagement in HIV medical care: piecing together the fragments of a fractured health care delivery system. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011;52:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spinelli MA, Frongillo EA, Sheira LA, et al. Food Insecurity is Associated with Poor HIV Outcomes Among Women in the United States. AIDS Behav. 2017;21(12):3473–3477. doi: 10.1007/s10461-017-1968-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bronfenbrenner U The Ecology of Human Development. Boston, MA: Harvard University Press; 1979. [Google Scholar]
- 35.Philbin MM, Parker CM, Parker RG, Wilson PA, Garcia J, Hirsch JS. The Promise of Pre-Exposure Prophylaxis for Black Men Who Have Sex with Men: An Ecological Approach to Attitudes, Beliefs, and Barriers. AIDS Patient Care STDs. 2016;30(6):282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Institute of Allergy and Infectious Diseases (NIAID). Women’s Interagency HIV Study (WIHS). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00000797. Published October 1994 Accessed March 6, 2019. [Google Scholar]
- 37.Adimora AA, Ramirez C, Benning L, et al. Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol 2018;47(2):393–394i. doi: 10.1093/ije/dyy021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buetow S Thematic Analysis and Its Reconceptualization as ‘Saliency Analysis.’ J Health Serv Res Policy. 2010;15(2):123–125. doi: 10.1258/jhsrp.2009.009081 [DOI] [PubMed] [Google Scholar]
- 39.Glaser BG, Strauss AL. The Discovery of Grounded Theory : Strategies for Qualitative Research; 1967. [Google Scholar]
- 40.MacQueen K, McLellan E, Kay K. Codebook development for team-based qualitative analysis. Cult Anthropol Methods. 1998;10:31–36. doi: 10.1177/1525822X980100020301 [DOI] [Google Scholar]
- 41.Claborn KR, Meier E, Miller MB, Leffingwell TR. A Systematic Review of Treatment Fatigue among HIV-infected Patients Prescribed Antiretroviral Therapy. Psychol Health Med. 2015;20(3):255–265. doi: 10.1080/13548506.2014.945601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zahedi-Spung L, Young M, Haddad LB, Badell ML. Perceived Barriers to Antepartum HIV Medication Adherence in HIV Infected Pregnant Women. Infectious Diseases in Obstetrics and Gynecology. doi: 10.1155/2018/4049212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lipira L, Williams EC, Huh D, et al. HIV-Related Stigma and Viral Suppression Among African-American Women: Exploring the Mediating Roles of Depression and ART Nonadherence. AIDS Behav. October 2018. doi: 10.1007/s10461-018-2301-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stone VE, Hogan JW, Schuman P, et al. Antiretroviral regimen complexity, self-reported adherence, and HIV patients’ understanding of their regimens: survey of women in the her study. J Acquir Immune Defic Syndr 1999. 2001;28(2):124–131. [DOI] [PubMed] [Google Scholar]
- 45.Hanna DB, Hessol NA, Golub ET, et al. Increase in single-tablet regimen use and associated improvements in adherence-related outcomes in HIV-infected women. J Acquir Immune Defic Syndr 1999. 2014;65(5):587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bogart LM, Wagner GJ, Green HD, et al. Medical mistrust among social network members may contribute to antiretroviral treatment nonadherence in African Americans living with HIV. Soc Sci Med 1982. 2016;164:133–140. doi: 10.1016/j.socscimed.2016.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalichman SC, Eaton L, Kalichman MO, Grebler T, Merely C, Welles B. Race-based medical mistrust, medication beliefs and HIV treatment adherence: test of a mediation model in people living with HIV/AIDS. J Behav Med. 2016;39(6):1056–1064. doi: 10.1007/s10865-016-9767-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalichman SC, Eaton L, Kalichman MO, Cherry C. Medication beliefs mediate the association between medical mistrust and antiretroviral adherence among African Americans living with HIV/AIDS. J Health Psychol. 2017;22(3):269–279. doi: 10.1177/1359105315600239 [DOI] [PubMed] [Google Scholar]
- 49.Kerrigan D, Mantsios A, Gorgolas M, et al. Experiences with long acting injectable ART: A qualitative study among PLHIV participating in a Phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PloS One. 2018;13(1):e0190487. doi: 10.1371/journal.pone.0190487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iacob SA, Iacob DG, Jugulete G. Improving the Adherence to Antiretroviral Therapy, a Difficult but Essential Task for a Successful HIV Treatment—Clinical Points of View and Practical Considerations. Front Pharmacol. 2017;8. doi: 10.3389/fphar.2017.00831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castro A Adherence to Antiretroviral Therapy: Merging the Clinical and Social Course of AIDS. PLOS Med. 2005;2(12):e338. doi: 10.1371/journal.pmed.0020338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray M, Pulido F, Mills A, et al. Patient-reported tolerability and acceptability of cabotegravir + rilpivirine long-acting injections for the treatment of HIV-1 infection: 96-week results from the randomized LATTE-2 study. HIV Res Clin Pract. 2019;0(0):1–12. doi: 10.1080/25787489.2019.1661696 [DOI] [PubMed] [Google Scholar]
- 53.Margolis DA, Gonzalez-Garcia J, Stellbrink H-J, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet Lond Engl. 2017;390(10101):1499–1510. doi: 10.1016/S0140-6736(17)31917-7 [DOI] [PubMed] [Google Scholar]
- 54.Fernandez C, van Halsema CL. Evaluating cabotegravir/rilpivirine long-acting, injectable in the treatment of HIV infection: emerging data and therapeutic potential. HIVAIDS Auckl NZ. 2019;11:179–192. doi: 10.2147/HIV.S184642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simoni JM, Beima-Sofie K, Mohamed ZH, et al. Long-Acting Injectable Antiretroviral Treatment Acceptability and Preferences: A Qualitative Study Among US Providers, Adults Living with HIV, and Parents of Youth Living with HIV. AIDS Patient Care STDs. 2019;33(3):104–111. doi: 10.1089/apc.2018.0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fisher WA, Black A. Contraception in Canada: a review of method choices, characteristics, adherence and approaches to counselling. CMAJ Can Med Assoc J. 2007;176(7):953–961. doi: 10.1503/cmaj.060851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freeman S Nondaily hormonal contraception: considerations in contraceptive choice and patient counseling. J Am Acad Nurse Pract. 2004;16(6):226–238. [DOI] [PubMed] [Google Scholar]
- 58.Gandhi M, Ameli N, Bacchetti P, et al. Eligibility criteria for HIV clinical trials and generalizability of results: the gap between published reports and study protocols: AIDS. 2005;19(16):1885–1896. doi: 10.1097/01.aids.0000189866.67182.f7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.