Abstract
Objective:
Conduct a systematic review and use meta-analytic techniques to estimate the proportion of total treatment effect that can be attributable to contextual effects (PCE) in adults receiving nonpharmacological, nonsurgical (NPNS) treatments for knee osteoarthritis (OA).
Design:
We reviewed the published literature to identify five frequently studied NPNS treatments for knee OA: exercise, acupuncture, ultrasound, laser, and transcutaneous electrical nerve stimulation (TENS). We searched for randomized controlled trials (RCTs) of these treatments and abstracted pre- and post-intervention pain scores for groups receiving placebo and active treatments. For each study we calculated the PCE by dividing the change in pain in the placebo group by the change in pain in the active treatment group. We log transformed the PCE measure and pooled across studies using a random effects model.
Results:
We identified 25 studies for analysis and clustered the RCTs into two groups: acupuncture and topical energy modalities (TEM). 13 acupuncture studies included 1,653 subjects and 12 TEM studies included 572 subjects. The combined PCE was 0.61 (95% CI 0.46 – 0.80) for acupuncture and 0.69 (95% CI 0.54 – 0.88) for TEM.
Conclusion:
Our findings suggest that about 61% and 69% of the total treatment effect experienced by subjects receiving acupuncture and TEM treatments, respectively, for knee OA pain may be explained by contextual effects. Contextual effects may include the placebo effect, changes attributable to natural history, and effects of co-therapies. These data highlight the important role of contextual effects in the response to NPNS OA treatments.
Keywords: knee osteoarthritis, contextual effect, placebo effect, pain
Introduction
Symptomatic knee osteoarthritis (OA) is a disabling condition affecting about 14 million adults in the US [1]. Knee OA is characterized pathologically by damage to the articular cartilage, meniscus and subchondral bone with osteophyte formation and synovial proliferation. Clinically, this condition presents with pain and functional limitation, but no treatments have been shown to reverse or arrest structural damage. Therefore, current treatments for knee OA are largely directed at reducing pain and improving function.
Nonpharmacological, nonsurgical (NPNS) therapies are important first-line approaches in the management of knee OA as they present low-risk, low-cost options [2–6]. Acupuncture, for example, was conditionally recommended in the 2019 American College of Rheumatology Treatment Guidelines [7]. However, prior work has shown that NPNS approaches to OA treatment are underused; barriers to the provision of NPNS care include clinicians’ perceived lack of expertise, perceived lack of evidence-based treatment, and suboptimal organization of care [8]. As such, an understanding of the therapeutic effects of NPNS approaches would inform management. In addition to a direct physiologic effect (DE), the total therapeutic effect (TE) of treatment approaches may be attributable to contextual effects (CE; TE = DE + CE, Figure 1) [9–11]. CE may include the placebo effect, changes attributable to natural history, and effects of co-therapies. These factors can influence therapeutic outcomes substantially [12]; increasing contextual effect in treatment may lead to larger patient-perceived pain relief. Thus, even if NPNS treatments largely consist of contextual effects, they could nonetheless play an important role in optimizing the analgesic effect experienced by patients. We examine these effects in studies of knee OA, as knee OA is among the most common sites of OA and is a major threat to independent mobility [13].
Figure 1: Conceptual illustration of total effect, direct effect, and placebo effect.
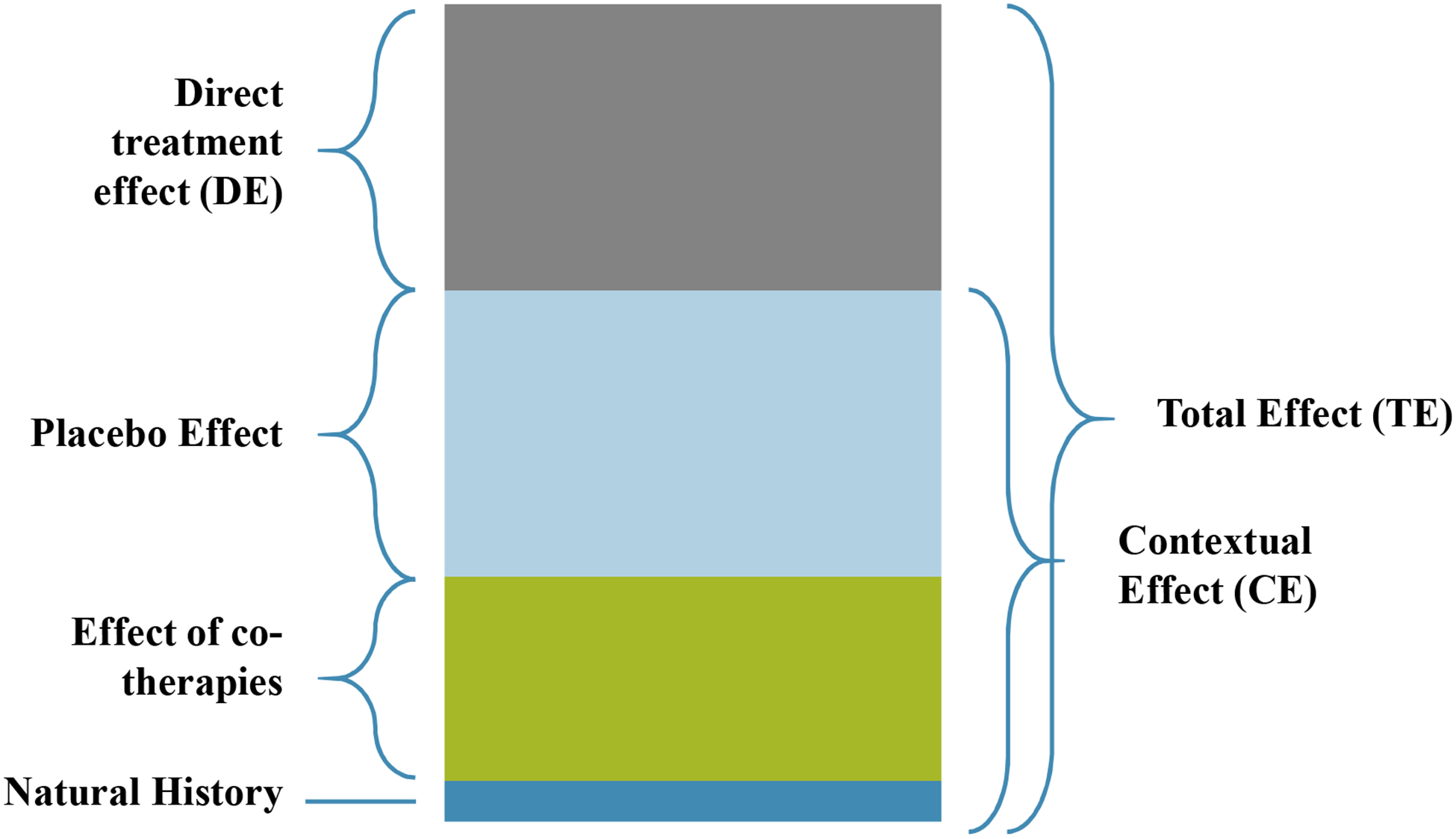
Total pain relief of an active treatment may be attributable to multiple factors. In this figure, the height of the bar represents the total treatment effect experienced by patients. “Natural History” refers to the natural pain relief that a patient may experience with the passage of time. “Effect of co-therapies” represents the relief provided by concurrent therapies for pain. “Placebo Effect” shows the improvement that patients receiving a placebo treatment in a randomized controlled trial may experience on top of the relief from natural history and co-therapies. Together, these three components constitute the contextual effect (CE) of a treatment. The remaining pain relief that patients in active treatment groups might experience – that is, the additional benefit after accounting for contextual effect – is the “Direct Effect (DE)”. The sum of all CE and DE equates to an active treatment’s “Total Effect (TE).” This figure is conceptual in nature, drawn to represent the factors contributing to therapeutic effect, and does not represent actual data.
To our knowledge, contextual effects in NPNS knee OA management are largely unexplored. In 2016, Zou et al. published a meta-analysis of OA trials that examined the proportion of total treatment effect attributable to contextual effect (PCE) in diverse treatments of osteoarthritis of various joints [14]. Of the eleven treatment modalities studied, only two (pulsed electromagnetic field therapy (PEMF) and acupuncture) were NPNS approaches; the PCE for these treatments were 0.80 (95% CI 0.64 – 0.99; PEMF) and 0.85 (95% CI 0.74 – 0.97; acupuncture). Another meta-analysis specifically examined the placebo effect in a range of nonpharmacological, pharmacological, and surgical treatments for knee OA and found that overall, placebo was effective at relieving pain and improving function [15]. Most recently, Huang et al. published a study [16] on placebo response to treatment of OA of the knee, hip, foot, or hand, and found a PCE of 0.44 (95% CI 0.23 – 0.65). Notably, Huang and colleagues only examined pharmacological approaches.
As safe, low-tech options for treating knee OA [4–6], non-pharmacological and non-surgical approaches to therapy deserve a more focused, comprehensive review. Published literature suggests that PCE varies by treatment delivery (e.g., surgical vs. oral) [14], so in contrast to previous work, this study focused exclusively on NPNS treatments, homing in on modalities that are frequently used in clinical settings. We aimed to clarify the role of contextual effect in widely used NPNS treatments for knee OA by conducting a systematic literature review of NPNS knee OA trials and comparing baseline and follow-up pain outcomes for active and placebo treatment groups. We expected the active treatments to confer distinguishable physiologic pain benefits, but also expected contextual effects to contribute substantially to the total pain relief reported by patients. Results of this study shed light on alternative, yet important, approaches to treating knee OA.
Methods
This study follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Data sources and selection process
We searched five databases – MEDLINE (Ovid Medline), Embase, Web of Science, CINAHL, and Cochrane Central. We identified knee OA RCTs using index terms related to ‘tibiofibular,’ ‘osteoarthrosis,’ and ‘randomized controlled trial.’ Our initial search included all treatments mentioned in the OARSI guidelines for nonpharmacological interventions [3], as well as associated terms identified using the Medical Subject Headings (MeSH) vocabulary thesaurus [17]. The Appendix details the exact search string used. We uploaded the results from the search into Covidence [18].
The first author (AC) searched PubMed to identify commonly used nonpharmacological, nonsurgical (NPNS) therapies for knee OA. Results of this step indicated that exercise therapy, laser therapy, ultrasound therapy, acupuncture therapy, and transcutaneous electrical nerve stimulation (TENS) therapy were five of the most frequently studied modalities. We limited study inclusion to these therapeutic approaches.
The first author (AC) then screened titles and abstracts based on a list of defined inclusion and exclusion criteria. In this paper, we considered “placebo treatment” to be approaches that physically resembled the active treatment without delivering the therapy. For example, sham ultrasound typically consisted of using the ultrasound machine exactly as in the active arm, but with the ultrasound output set to zero. Similarly, the placebo in acupuncture studies included the placement of needles into sham acupuncture points or the use of non-penetrating sham needles. Studies passing preliminary evaluation for inclusion were moved forward for a full-text review. Any equivocal references, including those with missing abstracts, were also moved forward to the full-text review.
During the full-text review, two authors (AC, JS) independently reviewed the full-length text of each published report to confirm eligibility. All discrepancies were discussed and resolved between the two authors, and the senior author (JNK) was consulted if necessary.
Inclusion and exclusion criteria
A study was included if it: 1) was a randomized controlled trial, 2) included a placebo arm, 3) only enrolled individuals with knee OA that had not received a total knee replacement (TKR), 4) involved an active treatment modality that was either exercise, laser, ultrasound, acupuncture, or transcutaneous electrical nerve stimulation (TENS) therapy, 5) reported a pain outcome, 6) included a follow-up assessment 1–3 months (28–91 days) after baseline measurements, 7) reported an improvement of pain from baseline, 8) had a published full-length manuscript that could be retrieved, 9) received a Physiotherapy Evidence Database (PEDro) scale [19] quality-assessment score greater than 5 points, and 10) was published in English.
Inter-rater reliability of the title and abstract screen
We randomly selected 100 citations excluded during the title and abstract screen and combined them with 102 citations that the first author moved forward to the full-text review. The second reviewing author (JS) evaluated these 202 studies’ titles and abstracts for inclusion eligibility. We assessed inter-rater reliability of the title and abstract screening using Cohen’s kappa statistic [20].
Data abstraction
We recorded title, author, year, country, demographic characteristics of the study sample, and treatment modalities. We also noted if co-therapies – such as exercise or nonsteroidal anti-inflammatory drugs (NSAIDs) – were reported to be administered in conjunction with the placebo and active treatments. We grouped ultrasound, TENS and laser modalities into an overarching “topical energy modality (TEM)” category to distinguish between acupuncture and energy-based therapeutic treatments. If a study had two outcome measures reported in the timeframe of interest (1–3 months), we used the later value in our analysis. We abstracted mean (SD) baseline and follow-up pain measurements for placebo and active treatment arms in each study. When reported, we also abstracted mean (SD) change in pain. For studies that reported outcomes in graphical form, we used ImageJ [21] to estimate outcome measures.
Two reviewers (AC, JS) independently abstracted all study details and resolved any discrepancies through discussion. Each reviewer also performed a quality assessment of all studies using the PEDro scale, which evaluates trials on criteria such as study eligibility, randomization, and blinding to rate methodological quality on a 10-point scale.
Data analysis
Heterogeneity tests
We assessed between-study heterogeneity in outcomes using the I2 statistic [22], which describes the percentage of variation across studies that is due to heterogeneity in the outcome measure rather than chance. Larger values of the I2 statistic indicate increasing heterogeneity. We assessed the contribution to overall heterogeneity of each study using an influence term, which was calculated by comparing the overall heterogeneity with and without each study.
Calculating effect size and PCE
For each study, we calculated an effect size to capture the total treatment effect (EStotal) and the contextual effect (EScontext) by dividing the mean difference – the change in average pain between baseline and follow-up measurements – by the pooled standard deviation of change between active and placebo arms (SDchange,pooled):
We imputed standard deviations (SD) of change in pain for studies in which the value was not explicitly reported [23]. To do this, we used
where SDA,baseline and SDA,follow-up represent the standard deviations of baseline and follow-up measures of pain in the active treatment group, respectively. The correlation coefficient, Corr, was derived from studies that did report SD of change in pain using
and used to calculate missing SDs of change under the assumption that similar correlations applied across all studies. SDs of change for placebo groups (SDP,change) were imputed in the same manner. We pooled SDs of change across active and placebo groups using
where NA and NP were the number of participants analyzed in the active and placebo treatment arms, respectively.
Estimating the PCE
Our methods to estimate the PCE reflect those used by Whiteside et al., 2018 [24]. We calculated the proportion attributable to contextual effect (PCE) for each study by dividing the mean improvement in pain – the improvement in average pain between baseline and follow-up measurements – of the placebo group by the mean improvement in pain of the active group . We log-transformed PCE to normalize the distribution, and then used Hedges method to calculate standard errors of log(PCE) [25] for each study. We used the random effects model to pool the log(PCE) across studies, and then transformed the results back to PCE by exponentiating. Taking the approach of Zou et al. 2016 [14], we capped the PCE at 1 (in other words, we set the PCE to 1 for studies in which the placebo group had a greater mean improvement in pain than the active group). We also excluded studies in which no improvement or worsening of pain from baseline was observed [24]; a worsening of pain from baseline may indicate nocebo effect (which is logically incompatible with PCE), and the PCE measure does not allow negative values due to the log transformation [14]. We used SAS PROC MIXED to pool PCE across studies using a maximum likelihood random effects model. Statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
Sensitivity analyses
We conducted five sensitivity analyses. First, because co-therapies may influence pain reporting, we excluded studies with treatments that reported the use of a co-therapy. Second, we excluded studies with large contributions to heterogeneity, as indicated by influence term values greater than 1. In a third sensitivity analysis, we excluded outliers, which we defined as studies with standardized mean changes in pain in either the placebo or active treatment arm greater than 3 standard errors away from the pooled change in pain. In the fourth sensitivity analysis, we excluded studies that had PEDro scores of 7 or below to examine how study quality might affect our results. Finally, we tested how results would change if studies excluded for worsening pain after baseline were included in our analysis.
Results
Study selection
Figure 2 illustrates the selection process. 5,321 citations met our initial search criteria. 6 of these were identified as duplicates by Covidence and automatically excluded, so the primary author screened 5,315 titles and abstracts for potential study inclusion according to the established inclusion criteria. 272 references moved forward for full-text review. After two authors independently reviewed the full-length text of each study to confirm eligibility, 27 studies remained; most studies excluded at this stage were not full-length papers (n=116). One study was excluded after scoring below 5 on the PEDro quality assessment scale [26]. 13 [27–39] of the 27 studies were acupuncture studies, 13 [40–52] were TEM studies, and 1 was an exercise study. With only one exercise study [53], we did not have enough data to warrant meta-analytic techniques for an exercise treatment group. Thus, we did not include this study in our final analysis. Additionally, one study [52] in which the mean pain of the placebo group worsened over time was also excluded from data analysis. Our final group consisted of 25 total acupuncture and TEM studies.
Figure 2: Study selection.

Study inclusion criteria were as follows: 1) RCT, 2) placebo treatment, 3) participants with knee OA without prior TKR, 4) exercise, laser, ultrasound, acupuncture, or transcutaneous electrical nerve stimulation (TENS) therapy, 5) pain outcome, 6) follow-up in 1–3 months (28–91 days), 7) observed pain improvement, 8) full-length manuscript, 9) over 5 points on the PEDro quality-assessment score, and 10) published in English.
Reliability of title and abstract screen
Of the random sample of 202 titles and abstracts (100 excluded citations and 102 full-text review citations), the primary and secondary reviewers (AC, JS) disagreed on 9 determinations, giving a Cohen’s kappa statistic of 0.91 (the range for ‘almost perfect agreement’ is 0.81 – 1.00 [54]) and an overall agreement of 96%. Discrepancies were discussed and resolved. None of the disagreements altered the final set of included studies.
Trial characteristics
Table 1 presents study characteristics. Dates of study publication within the acupuncture and TEM groups ranged from 1994–2013 and 2002–2017, respectively. Mean subject age ranged from 57 to 71 for acupuncture studies and from 53 to 69 for TEM studies, and the range of mean BMI for the two groups was virtually the same (26 to 33 for acupuncture, 27 to 33 for TEM).
Table 1:
Study characteristics
| Acupuncture studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| First author, year | Country | # of subjects analyzed | Age (SD) | % Female | BMI Mean (SD) | Co-therapy | Total # of treatment sessions | Treatment duration (weeks) |
| Berman, 2004 [23] | USA | 330 | 66(12) | 63 | NR | NA | 23 | 26 |
| Chen, 2013 [24] | USA | 181 | 60(16) | 52 | 33 | Exercise | 12 | 12 |
| Itoh, 2008 [15] | Japan | 16 | 72(10) | NR | NR | NA | 5 | 21 |
| Jubb, 2008 [25] | UK | 62 | 65(2) | 81 | 32(10) | NA | 10 | 5 |
| Mavrommatis, 2012 [26] | Greece | 78 | 61(15) | 73 | 31(7) | NSAIDs | 16 | 8 |
| Miller, 2011 [27] | Israel | 41 | 71(12) | 69 | NR | NA | 16 | 8 |
| Min, 2009 [28] | Korea | 65 | 59(8) | 82 | 26(4) | NA | 8 | 4 |
| Sangdee, 2002 [29] | Thailand | 91 | 64(8) | 77 | NR | Placebo tablet | 12 | 4 |
| Spaeth, 2013 [16] | USA | 20 | 57(12) | 30 | NR | NA | 6 | 4 |
| Suarez-Almazor, 2010 [30] | USA | 422 | NR | NR | NR | NA | 12 | 6 |
| Takeda, 1994 [31] | Canada | 40 | 62(13) | 50 | 33(9) | NA | 9 | 3 |
| Vas, 2004 [32] | Spain | 88 | 67(14) | 40 | 33(8) | NSAIDs | 12 | 12 |
| Witt, 2005 [33] | Germany | 219 | 64(9) | 68 | 29(7) | NA | 12 | 8 |
| Topical Energy Modality (TEM) Studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| First author, year | Country | Modality | # of subjects analyzed | Age (SD) | % Female | BMI Mean (SD) | Co-therapy | Total # of treatment sessions | Treatment duration (weeks) |
| Alfredo, 2017 [34] | Brazil | Laser | 40 | 62(10) | 77 | 30(6) | Exercise | 9 | 3 |
| Atamaz, 2012 [35] | Turkey | TENS | 67 | 61(9) | 78 | 29(5) | Exercise | 15 | 3 |
| Cheing, 2002 [36] | Hong Kong | TENS | 32 | 65(10) | 91 | 28(5) | NA | 20 | 4 |
| Gur, 2003 [37] | Turkey | Laser | 60 | 60(9) | 82 | 31(5) | Exercise | 10 | 2 |
| Helianthi, 2016 [38] | Indonesia | Laser | 59 | 69(8) | 71 | 27(6) | NA | 10 | 5 |
| Inal, 2016 [39] | Turkey | TENS | 60 | 64(2) | 100 | 33(1) | Exercise, hot pack, ultrasound | 10 | 2 |
| Kheshie, 2014 [41] | Saudi Arabia | Laser | 35 | 54(13) | 0 | 29(5) | Exercise | 12 | 6 |
| Loyola-Sanchez, 2012 [42] | Canada | Ultrasound | 25 | 62(15) | 78 | 32(10) | NA | 24 | 8 |
| Pietrosimone, 2011 [43] | USA | TENS | 20 | NR | 58 | 29(11) | NA | 12 | 4 |
| Yegin, 2017 [44] | Turkey | Ultrasound | 62 | NR | NR | NR | NA | 10 | 2 |
| Yildiz, 2015 [45] | Turkey | Ultrasound | 60 | 57(10) | 85 | 32(7) | NA | 10 | 2 |
| Yurtkuran, 2007 [46] | Turkey | Laser | 52 | 53(10) | 96 | 32(10) | Exercise | 10 | 2 |
SD: Standard Deviation; BMI: Body Mass Index; NSAIDs: Nonsteroidal anti-inflammatory drugs; NR: Not Reported; NA: Not Applicable
Sample size varied: the smallest acupuncture trial analyzed data from 16 participants (across both active and placebo groups) while the largest had a sample size of 422. The range in sample size for TEM studies was 20 to 67. Acupuncture studies included a total of 1,653 subjects, while TEM included 572. Ten studies (4 acupuncture, 6 TEM) reported a co-therapy: 7 included exercise [28, 40, 41, 43, 45, 46, 51], 2 included administration of an NSAID [31, 38], and 1 involved administration of a placebo tablet [34]. All studies included a follow-up assessment of knee pain 28–91 days after the baseline measurement (acupuncture studies: mean 61.8 days, median 63 days; TEM studies: mean 59.3 days, median 56 days).
Heterogeneity and quality assessment
Acupuncture and TEM study groups both exhibited high overall heterogeneity (acupuncture I2 = 0.85, 95% CI [0.76–0.91]; TEM I2 = 0.89, 95% CI [0.83–0.93]). In the acupuncture cohort, four [27, 31, 36, 38] of thirteen studies carried notably larger influence than the rest; two TEM studies [44, 46] contributed substantially more than others to heterogeneity (see Appendix: heterogeneity assessment plots). We used a random effects model to account for the high between-study variability. PEDro scores of the final set of studies for analysis ranged from 7 to 10, with a mean of 8 (moderate to high-quality studies score ≥6 [55]).
Primary analysis
Acupuncture results
Table 2 details mean changes in pain, effect sizes, and PCEs for the acupuncture studies. Effect sizes for the contextual effect (EScontext) ranged from 0.02 [35] to 2.00 [31], and effect sizes for the total treatment effect (EStotal) ranged from 0.54 [33] to 3.94 [31]. The pooled PCE was 0.61 (95% CI 0.46 – 0.80); that is, across the acupuncture studies, contextual effects accounted for about 61% of the pain relief experienced by patients receiving active treatment. Figure 3 illustrates the proportions of contextual effect constituting total treatment effect for individual acupuncture studies.
Table 2:
Acupuncture Results
| Author | Year | PEDro Quality Score | NP | Δ pain, placebo | Δ pain SD, placebo | NA | Δ pain, active | Δ pain SD, active | SDpooled | EScontext | EStotal | PCE | 95% CI lower bound | 95% CI upper bound |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Berman [23] | 2004 | 9 | 161 | 2.7 | 3.3 | 169 | 3.2 | 3.8 | 3.6 | 0.75 | 0.89 | 0.84 | 0.83 | 0.86 |
| Chen [24] | 2013 | 9 | 94 | 2.4 | 3.0 | 87 | 2.8 | 4.2 | 3.6 | 0.65 | 0.78 | 0.83 | 0.81 | 0.86 |
| Itoh (2008) [25] | 2008 | 7 | 7 | 12.6 | 14.9 | 9 | 24.7 | 13.0 | 13.9 | 0.91 | 1.79 | 0.51 | 0.40 | 0.65 |
| Jubb [26] | 2008 | 9 | 32 | 2.0 | 23.1 | 30 | 14.0 | 26.6 | 24.8 | 0.08 | 0.56 | 0.14 | 0.09 | 0.24 |
| Mavrommatis [27] | 2012 | 9 | 39 | 20.9 | 9.3 | 39 | 41.2 | 11.5 | 10.5 | 2.00 | 3.94 | 0.51 | 0.50 | 0.52 |
| Miller [28] | 2008 | 7 | 20 | 3.8 | 10.0 | 21 | 7.7 | 14.2 | 12.3 | 0.31 | 0.63 | 0.49 | 0.40 | 0.61 |
| Min [29] | 2009 | 7 | 31 | 0.9 | 3.0 | 34 | 2.0 | 4.3 | 3.7 | 0.24 | 0.54 | 0.45 | 0.38 | 0.53 |
| Sangdee [30] | 2002 | 9 | 45 | 22.9 | 27.0 | 46 | 48.2 | 24.4 | 25.7 | 0.89 | 1.88 | 0.47 | 0.46 | 0.49 |
| Spaeth [31] | 2013 | 8 | 10 | 0.2 | 12.8 | 10 | 11.9 | 14.6 | 13.7 | 0.02 | 0.87 | 0.02 | 0.00 | 1.00 |
| Suarez-Almazor [32] | 2010 | 9 | 283 | 20.7 | 22.9 | 139 | 22.1 | 28.8 | 25.0 | 0.83 | 0.88 | 0.94 | 0.93 | 0.95 |
| Takeda [33] | 1994 | 7 | 20 | 4.7 | 13.6 | 20 | 7.4 | 12.4 | 13.0 | 0.36 | 0.56 | 0.63 | 0.50 | 0.80 |
| Vas [34] | 2004 | 9 | 41 | 23.1 | 20.5 | 47 | 48.3 | 12.3 | 16.6 | 1.39 | 2.91 | 0.48 | 0.46 | 0.49 |
| Witt [35] | 2005 | 8 | 73 | 5.6 | 10.3 | 146 | 11.5 | 14.3 | 13.1 | 0.43 | 0.88 | 0.49 | 0.47 | 0.50 |
PEDro: Physiotherapy Evidence Database, scored from 0–10 (0 = lowest quality, 10 = highest quality); NP:Number of subjects analyzed in placebo arm; NA:Number of subjects analyzed in active arm; SDpooled: Pooled SD of change in pain levels between active and placebo arms; EScontext: Effect size, contextual effect; EStotal: Effect size, total treatment effect; PCE: Proportion attributable to Contextual Effect; 95% CI: 95% Confidence Interval
Figure 3: Proportion of total treatment attributable to contextual effect (PCE) in acupuncture studies.

Bars represent the proportion of total treatment effect that may be attributable to contextual effects (PCE). These were calculated by dividing the effect size of the contextual effects by the effect size of the total effect . Table 2 lists the effect size values for each group in the acupuncture studies. Error bars show the associated 95% confidence intervals.
TEM results
Results for TEM studies are detailed in Table 3. Across studies, effect sizes for the contextual effect ranged from 0.08 [47] to 6.05 [46], and the range of effect sizes for total treatment effect ranged from 0.19 [47] to 8.68 [46]. PCEs for each study are shown in Figure 4. The pooled PCE across TEM studies was 0.69 (95% CI 0.54 – 0.88); that is, contextual effects in TEM studies that used laser, ultrasound, and TENS modalities may account for about 69% of the total treatment effect.
Table 3:
Topical Energy Modality (TEM) Results
| Author | Year | PEDro Quality Score | NP | Δ pain, placebo | Δ pain SD, placebo | NA | Δ pain, active | Δ pain SD, active | SDpooled | EScontext | ESactive | PCE | 95% CI lower bound | 95% CI upper bound |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alfredo [36] | 2017 | 9 | 20 | 1.7 | 2.7 | 20 | 2.9 | 3.1 | 2.9 | 0.58 | 1.01 | 0.57 | 0.50 | 0.65 |
| Atamaz [37] | 2012 | 10 | 35 | 27.1 | 20.1 | 32 | 24.6 | 21.4 | 20.7 | 1.31 | 1.19 | 1.00 | 1.00 | 1.00 |
| Cheing [38] | 2002 | 7 | 16 | 51.4 | 42.2 | 16 | 56.3 | 30.3 | 36.7 | 1.40 | 1.53 | 0.91 | 0.84 | 0.99 |
| Gur [39] | 2003 | 9 | 30 | 2.4 | 1.6 | 30 | 3.7 | 2.0 | 1.8 | 1.35 | 2.07 | 0.65 | 0.63 | 0.68 |
| Helianthi [40] | 2016 | 7 | 29 | 1.3 | 6.0 | 30 | 40.5 | 14.8 | 11.4 | 0.11 | 3.56 | 0.03 | 0.03 | 0.04 |
| Inal [41] | 2016 | 9 | 30 | 3.7 | 3.0 | 30 | 4.1 | 3.2 | 3.1 | 1.19 | 1.32 | 0.90 | 0.86 | 0.95 |
| Kheshie [42] | 2014 | 8 | 15 | 3.9 | 0.6 | 20 | 5.7 | 0.7 | 0.7 | 6.05 | 8.68 | 0.70 | 0.69 | 0.71 |
| Loyola-Sanchez [43] | 2012 | 9 | 13 | 0.3 | 4.3 | 12 | 0.8 | 3.4 | 3.9 | 0.08 | 0.19 | 0.40 | 0.08 | 1.00 |
| Pietrosimone [44] | 2011 | 8 | 10 | 2.7 | 4.9 | 10 | 4.7 | 4.6 | 4.7 | 0.57 | 0.99 | 0.57 | 0.43 | 0.76 |
| Yegin [45] | 2017 | 9 | 32 | 2.7 | 4.4 | 30 | 2.9 | 3.5 | 4.0 | 0.68 | 0.73 | 0.93 | 0.85 | 1.00 |
| Yildiz [46] | 2015 | 7 | 30 | 1.7 | 2.3 | 30 | 5.1 | 2.2 | 2.2 | 0.78 | 2.27 | 0.34 | 0.32 | 0.36 |
| Yurtkuran [47] | 2007 | 9 | 25 | 1.3 | 3.0 | 27 | 0.9 | 2.0 | 2.6 | 0.49 | 0.35 | 1.00 | 1.00 | 1.00 |
PEDro: Physiotherapy Evidence Database, scored from 0–10 (0 = lowest quality, 10 = highest quality); NP:Number of subjects analyzed in placebo arm; NA:Number of subjects analyzed in active arm; SDpooled: Pooled SD of change in pain levels between active and placebo arms; EScontext: Effect size, contextual effect; EStotal: Effect size, total treatment effect; PCE: Proportion attributable to Contextual Effect; 95% CI: 95% Confidence Interval
Figure 4: Proportion of total treatment attributable to contextual effect (PCE) in topical energy modality (TEM) studies.

Bars represent the proportion of total treatment effect that may be attributable to contextual effects (PCE). These were calculated by dividing the effect size of the contextual effects by the effect size of the total effect Table 3 lists the effect size values for each group. Error bars show the associated 95% confidence intervals.
Sensitivity analyses results
Excluding studies with reported co-therapies:
After excluding four acupuncture studies with co-therapies [28, 31, 34, 38], the pooled PCE for acupuncture increased to 0.73 (95% CI 0.55 – 0.98). In contrast, the exclusion of six TEM studies with co-therapies [40, 41, 43, 45, 46, 51] dropped the pooled PCE to 0.44 (95% CI 0.15 – 1.00).
Excluding studies with large contributions to heterogeneity:
Three acupuncture studies [31, 36, 38] had influence scores greater than 1 (Appendix Figure 1a), indicating high contribution to overall heterogeneity. After excluding these studies, the pooled PCE for acupuncture remained nearly unchanged at 0.63 (95% CI 0.47 – 0.83). We excluded two TEM studies [44, 46], and the pooled PCE for TEM studies was 0.73 (95% CI 0.55 – 0.97).
Excluding outliers:
Primary analysis results yielded four acupuncture study outliers [29, 31, 34, 38] and one TEM outlier [46]. After exclusion of outliers, pooled PCE estimates were 0.80 (95% CI 0.67 – 0.97) and 0.67 (95% CI 0.48 – 0.94) for acupuncture and TEM studies, respectively.
Excluding low-quality studies:
We excluded studies with a PEDro quality score of 7 – the lowest quality score in the final set of studies – which included four acupuncture studies [29, 32, 33, 37] and three TEM studies [42, 44, 50]. The pooled PCEs for acupuncture (0.63 (95% CI 0.48 – 0.81)) and TEM (0.72 (95% CI 0.65 – 0.79)) remained similar to those documented in the primary analysis.
Excluding studies with pain that worsened after baseline:
Only one study [52] had a placebo arm with worsening pain after baseline. After including that study in the TEM group and recalculating, the pooled PCE was 0.61.
Discussion
We used meta-analytic techniques to evaluate the contribution of contextual effects to the total analgesic effect experienced by knee OA patients on acupuncture or topical energy modality (TEM) treatment plans. Our results indicate that about 61% of pain relief experienced by knee OA acupuncture patients may be attributable to contextual effects – including placebo, changes in natural history, and co-therapies – and contextual effects may account for about 69% of pain relief from treatments involving a topical energy modality. The results remained largely unchanged in a series of sensitivity analyses that excluded studies with large contributions to the overall heterogeneity, reported exceptionally high changes in pain, or were of low trial quality (PEDro score 7 or below).
Our findings were robust across several sensitivity analyses because we examined relative pain differences between the placebo and active treatment arms in RCTs, rather than absolute change; factors that affected both arms largely did not affect the PCE estimate. Inclusion of a study with a placebo arm that had worsening pain after baseline yielded a slight decrease in the pooled PCE of the TEM study group, but the overall PCE of 0.61 was consistent with the base case results showing a substantial contextual effect. However, when we excluded six TEM studies with co-therapies, the pooled PCE decreased to 0.44 (95% CI 0.15 – 1.00). This is likely due to the small number of studies that remained after excluding studies with co-therapies (six TEM studies remained) and the outsized influence of one with a low PCE and a small standard deviation (PCE 0.34, SDpooled 2.23) [50]. The imprecision in this estimate is reflected by its wide confidence interval. Future studies to examine the sources of variability in the pooled PCE estimate could use meta-regression, which was beyond the scope of this study.
Our analyses suggest that a substantial portion of total analgesic effect experienced by patients receiving acupuncture or TEM treatments may be attributed to contextual effects. This is the first study that attempts to quantify the role of these factors in nonpharmacological, nonsurgical (NPNS) treatments as they apply specifically to knee OA. Our methodological approach aligned with the study by Zou et al. [14], which found an average PCE of 0.85 (95% CI 0.74–0.97) across 17 acupuncture studies – higher than our PCE of 0.61 (95% CI 0.46 – 0.80). However, our study includes several key distinctions. We focused exclusively on trials of knee OA and limited our study inclusion to trials with follow-up results reported 1–3 months after baseline evaluation to reduce heterogeneity in our sample. In contrast, Zou and colleagues included OA studies of any joint – including the spine, hip, knee, hand, foot, and TMJ – and did not specify a window for follow-up reporting. Finally, we included two commonly used NPNS methods for treating knee OA, ultrasound and TENS, that were not previously studied by Zou et al [14]. Our findings, in conjunction with those of Zou and colleagues, provide a clear summary of the role of CE in commonly prescribed NPNS treatments for knee OA.
Studies of the contextual effect in knee OA are limited, but previous work has examined the placebo effect – an important component of contextual effect. In 2008, Zhang et al. studied hand, hip and knee osteoarthritis patients and determined the effect size of placebo treatments (ES 0.51; 95% CI [0.46–0.55]) to be considerably greater than the ES in untreated controls (ES 0.03; 95% CI [−0.13–0.18]) [15]. Comparing placebo treatments to untreated controls in this manner allowed Zhang and colleagues to separate the placebo effect from natural history (Figure 1); the large difference in their findings suggests that the placebo effect may comprise most of the contextual effect.
We acknowledge several important limitations of our study. First, this study was not preregistered. Additionally, despite our selection criteria, there was still wide variation in study design. For example, trial eligibility criteria often differed (some studies included participants with Kellgren-Lawrence Grade 4, or bone-on-bone, arthritis; others stated minimal pain requirements for participant enrollment), and treatment details varied (e.g., different active treatment frequencies or intensities, or different approaches to administering placebo acupuncture). This heterogeneity may have reduced the comparability of studies, as prior studies suggest that symptom severity [56] and dosing frequency [57] may affect outcome.
Additionally, the inclusion of studies with reported co-therapies in the primary analysis might falsely bolster the reported changes in pain; we accounted for this with a sensitivity analysis that excludes all such studies and found that results for acupuncture remained similar (the pooled PCE increased to 0.73 (95% CI 0.55 – 0.98)), while the PCE for TEM studies dropped to 0.44 (95% CI 0.15 – 1.00)). As noted, this drop may be due in part to the small sample size of studies without co-therapies. Although knee OA pain has been shown to be associated with knee OA disability [58], our findings of PCE in pain outcomes should not be generalized to functional measures (such as walking distance or knee range of motion) since effects on performance-based and psychometric measures can vary after treatment [16, 59].
Finally, despite the abundance of literature on exercise-based approaches to treating knee OA, this meta-analysis did not include any such studies. Only one exercise or physical therapy trial included a placebo control (the others had active controls). Because placebo treatment is necessary for the evaluation of contextual effect, studies lacking a placebo were ineligible for this analysis. In the single exercise-based study that included a placebo arm [53], the PCE was 0.91 at 12 weeks after baseline. This finding should be viewed cautiously, as the placebo approach (sham ultrasound, light application of non-therapeutic gel) was conspicuously different from the active exercise treatment. The dearth of placebo-controlled exercise trials highlights the challenge of designing an apt placebo approach to exercise.
This systematic review shows that factors other than the direct effect of an active treatment may play an important role in the analgesic effects experienced by knee OA patients receiving acupuncture or TEM. If such a large proportion of pain treatment effect in nonpharmacological, nonsurgical treatment of knee OA is indeed contextual, then finding ways to ethically bolster contextual effects may present an opportunity to enhance clinical care. Aspects of care such as a patient’s expectations towards therapy, the clinician’s behavior, and the clinician’s touch have been previously shown to influence a patient’s pain perception [12]. Additionally, numerous studies have identified clinical benefits of the nondeceptive use of placebo [60–64]. Further research is needed to determine how contextual effects vary across treatment approaches – including exercise – and outcome measures, and how to harness the benefits of contextual effects in ways that align with patient goals.
Supplementary Material
Figure 5: Pooled proportion of total treatment attributable to contextual effect (PCE) estimates for acupuncture and topical energy modality (TEM) studies.

The y-axis measures the proportion of the total treatment effect that may be attributable to contextual effects in acupuncture and TEM treatments. Pooled estimates were calculated using a random effects model. Approximately 61% and 69% of acupuncture and TEM treatment effects may be attributable to contextual effects, respectively.
Funding Support
This research was funded by National Institutes of Health (NIH-NIAMS) grants K24-AR057827 to Dr. Losina, P30-AR072577 and U01-AR071658, and by the Rheumatology Research Foundation Investigator Award to Dr. Collins. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the NIH or the Federal government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interest statement: Dr. Collins reports grants from NIH NIAMS and Roche/Genentech, and consulting fees from Boston Imaging Core Labs. Dr. Losina reports grant support from Pfizer, Samumed, and the NIH, consulting fees from Velocity, and is deputy editor of the Journal of Bone and Joint Surgery. Dr. Katz reports grant funding from the NIH, Samumed and Flexion Therapeutics.
References
- 1.Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ, Messier SP, et al. Number of persons with symptomatic knee osteoarthritis in the us: Impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res (Hoboken) 2016; 68(12): 1743–1750. DOI: 10.1002/acr.22897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, Mcgowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012; 64(4): 465–474. [DOI] [PubMed] [Google Scholar]
- 3.Mcalindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014; 22(3): 363–388. DOI: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Bruyere O, Honvo G, Veronese N, Arden NK, Branco J, Curtis EM, et al. An updated algorithm recommendation for the management of knee osteoarthritis from the european society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (esceo). Semin Arthritis Rheum 2019; 49(3): 337–350. DOI: 10.1016/j.semarthrit.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Dziedzic KS, Hill JC, Porcheret M and Croft PR. New models for primary care are needed for osteoarthritis. Phys Ther 2009; 89(12): 1371–1378. DOI: 10.2522/ptj.20090003. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira RM, Duarte JA and Goncalves RS. Non-pharmacological and non-surgical interventions to manage patients with knee osteoarthritis: An umbrella review. Acta Reumatol Port 2018; 43(3): 182–200. [PubMed] [Google Scholar]
- 7.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2020; 72(2): 149–162. DOI: 10.1002/acr.24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selten EMH, Vriezekolk JE, Nijhof MW, Schers HJ, Van Der Meulen-Dilling RG, Van Der Laan WH, et al. Barriers impeding the use of non-pharmacological, non-surgical care in hip and knee osteoarthritis: The views of general practitioners, physical therapists, and medical specialists. J Clin Rheumatol 2017; 23(8): 405–410. DOI: 10.1097/RHU.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 9.Bennell KL, Egerton T, Martin J, Abbott JH, Metcalf B, Mcmanus F, et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: A randomized clinical trial. Jama 2014; 311(19): 1987–1997. DOI: 10.1001/jama.2014.4591. [DOI] [PubMed] [Google Scholar]
- 10.De Groef A, Van Kampen M, Vervloesem N, De Geyter S, Christiaens MR, Neven P, et al. Myofascial techniques have no additional beneficial effects to a standard physical therapy programme for upper limb pain after breast cancer surgery: A randomized controlled trial. Clin Rehabil 2017; 31(12): 1625–1635. DOI: 10.1177/0269215517708605. [DOI] [PubMed] [Google Scholar]
- 11.Garcia AN, Costa L, Hancock MJ, Souza FS, Gomes G, Almeida MO, et al. Mckenzie method of mechanical diagnosis and therapy was slightly more effective than placebo for pain, but not for disability, in patients with chronic non-specific low back pain: A randomised placebo controlled trial with short and longer term follow-up. Br J Sports Med 2018; 52(9): 594–600. DOI: 10.1136/bjsports-2016-097327. [DOI] [PubMed] [Google Scholar]
- 12.Rossettini G, Carlino E and Testa M. Clinical relevance of contextual factors as triggers of placebo and nocebo effects in musculoskeletal pain. BMC Musculoskeletal Disorders 2018; 19(1): 27 DOI: 10.1186/s12891-018-1943-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lespasio MJ, Piuzzi NS, Husni ME, Muschler GF, Guarino A and Mont MA. Knee osteoarthritis: A primer. Perm J 2017; 21: 16–183. DOI: 10.7812/TPP/16-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou K, Wong J, Abdullah N, Chen X, Smith T, Doherty M, et al. Examination of overall treatment effect and the proportion attributable to contextual effect in osteoarthritis: Meta-analysis of randomised controlled trials. Annals of the Rheumatic Diseases 2016; 75(11): 1964 DOI: 10.1136/annrheumdis-2015-208387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Robertson J, Jones AC, Dieppe PA and Doherty M. The placebo effect and its determinants in osteoarthritis: Meta-analysis of randomised controlled trials. Ann Rheum Dis 2008; 67(12): 1716–1723. DOI: 10.1136/ard.2008.092015. [DOI] [PubMed] [Google Scholar]
- 16.Huang Z, Chen J, Hu QS, Huang Q, Ma J, Pei FX, et al. Meta-analysis of pain and function placebo responses in pharmacological osteoarthritis trials. Arthritis Research & Therapy 2019; 21(1): 173 DOI: 10.1186/s13075-019-1951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Center for Biotechnology Information. MeSH: Medical subject headings. Available from https://www.ncbi.nlm.nih.gov/mesh.
- 18.Covidence Systematic Review Software. Melbourne, Australia, Veritas Health Innovation. Available from www.covidence.org. [Google Scholar]
- 19.De Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust J Physiother 2009; 55(2): 129–133. [DOI] [PubMed] [Google Scholar]
- 20.Viera AJ and Garrett JM. Understanding interobserver agreement: The kappa statistic. Fam Med 2005; 37(5): 360–363. [PubMed] [Google Scholar]
- 21.Schneider Ca RW, Eliceiri Kw. NIH image to ImageJ: 25 years of image analysis. Nature methods 2012; 9(7): 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ and Altman DG. Measuring inconsistency in meta-analyses. Bmj 2003; 327(7414): 557–560. DOI: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP and Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Available from www.handbook.cochrane.org. [Google Scholar]
- 24.Whiteside N, Sarmanova A, Chen X, Zou K, Abdullah N, Doherty M, et al. Proportion of contextual effects in the treatment of fibromyalgia-a meta-analysis of randomised controlled trials. Clin Rheumatol 2018; 37(5): 1375–1382. DOI: 10.1007/s10067-017-3948-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedges LV and Olkin I. Statistical Methods for Meta-Analysis. San Diego, Academic Press; 1985. [Google Scholar]
- 26.Shen X, Zhao L, Ding G, Tan M, Gao J, Wang L, et al. Effect of combined laser acupuncture on knee osteoarthritis: A pilot study. Lasers Med Sci 2009; 24(2): 129–136. DOI: 10.1007/s10103-007-0536-9. [DOI] [PubMed] [Google Scholar]
- 27.Berman BM, Lao L, Langenberg P, Lee WL, Gilpin AM and Hochberg MC. Effectiveness of acupuncture as adjunctive therapy in osteoarthritis of the knee: A randomized, controlled trial. Ann Intern Med 2004; 141(12): 901–910. [DOI] [PubMed] [Google Scholar]
- 28.Chen LX, Mao JJ, Fernandes S, Galantino ML, Guo W, Lariccia P, et al. Integrating acupuncture with exercise-based physical therapy for knee osteoarthritis: A randomized controlled trial. J Clin Rheumatol 2013; 19(6): 308–316. DOI: 10.1097/RHU.0b013e3182a21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itoh K, Hirota S, Katsumi Y, Ochi H and Kitakoji H. Trigger point acupuncture for treatment of knee osteoarthritis--a preliminary RCT for a pragmatic trial. Acupunct Med 2008; 26(1): 17–26. DOI: 10.1136/aim.26.1.17. [DOI] [PubMed] [Google Scholar]
- 30.Jubb RW, Tukmachi ES, Jones PW, Dempsey E, Waterhouse L and Brailsford S. A blinded randomised trial of acupuncture (manual and electroacupuncture) compared with a non-penetrating sham for the symptoms of osteoarthritis of the knee. Acupunct Med 2008; 26(2): 69–78. DOI: 10.1136/aim.26.2.69. [DOI] [PubMed] [Google Scholar]
- 31.Mavrommatis CI, Argyra E, Vadalouka A and Vasilakos DG. Acupuncture as an adjunctive therapy to pharmacological treatment in patients with chronic pain due to osteoarthritis of the knee: A 3-armed, randomized, placebo-controlled trial. Pain 2012; 153(8): 1720–1726. DOI: 10.1016/j.pain.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Miller E, Maimon Y, Rosenblatt Y, Mendler A, Hasner A, Barad A, et al. Delayed effect of acupuncture treatment in osteoarthritis of the knee: A blinded, randomized, controlled trial. Evid Based Complement Alternat Med 2011; 2011: 792975 DOI: 10.1093/ecam/nen080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Min M-H, Choi Y-G, Kim Y-J, Park H-J, Lee S-C, Joo H-N, et al. The effect of sa-am acupuncture on knee osteoarthritis 2009.
- 34.Sangdee C, Teekachunhatean S, Sananpanich K, Sugandhavesa N, Chiewchantanakit S, Pojchamarnwiputh S, et al. Electroacupuncture versus diclofenac in symptomatic treatment of osteoarthritis of the knee: A randomized controlled trial. BMC Complementary and Alternative Medicine 2002; 2(1): 3 DOI: 10.1186/1472-6882-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spaeth RB, Camhi S, Hashmi JA, Vangel M, Wasan AD, Edwards RR, et al. A longitudinal study of the reliability of acupuncture deqi sensations in knee osteoarthritis. Evid Based Complement Alternat Med 2013; 2013: 204259 DOI: 10.1155/2013/204259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suarez-Almazor ME, Looney C, Liu Y, Cox V, Pietz K, Marcus DM, et al. A randomized controlled trial of acupuncture for osteoarthritis of the knee: Effects of patient-provider communication. Arthritis Care Res (Hoboken) 2010; 62(9): 1229–1236. DOI: 10.1002/acr.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeda W and Wessel J. Acupuncture for the treatment of pain of osteoarthritic knees. Arthritis Care Res 1994; 7(3): 118–122. [DOI] [PubMed] [Google Scholar]
- 38.Vas J, Mendez C, Perea-Milla E, Vega E, Panadero MD, Leon JM, et al. Acupuncture as a complementary therapy to the pharmacological treatment of osteoarthritis of the knee: Randomised controlled trial. Bmj 2004; 329(7476): 1216 DOI: 10.1136/bmj.38238.601447.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witt C, Brinkhaus B, Jena S, Linde K, Streng A, Wagenpfeil S, et al. Acupuncture in patients with osteoarthritis of the knee: A randomised trial. Lancet 2005; 366(9480): 136–143. DOI: 10.1016/s0140-6736(05)66871-7. [DOI] [PubMed] [Google Scholar]
- 40.Alfredo PP, Bjordal JM, Junior WS, Lopes-Martins RÁB, Stausholm MB, Casarotto RA, et al. Long-term results of a randomized, controlled, double-blind study of low-level laser therapy before exercises in knee osteoarthritis: Laser and exercises in knee osteoarthritis. Clin Rehabil 2017; 32(2): 173–178. DOI: 10.1177/0269215517723162. [DOI] [PubMed] [Google Scholar]
- 41.Atamaz FC, Durmaz B, Baydar M, Demircioglu OY, Iyiyapici A, Kuran B, et al. Comparison of the efficacy of transcutaneous electrical nerve stimulation, interferential currents, and shortwave diathermy in knee osteoarthritis: A double-blind, randomized, controlled, multicenter study. Arch Phys Med Rehabil 2012; 93(5): 748–756. DOI: 10.1016/j.apmr.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 42.Cheing GL, Hui-Chan CW and Chan KM. Does four weeks of TENS and/or isometric exercise produce cumulative reduction of osteoarthritic knee pain? Clin Rehabil 2002; 16(7): 749–760. DOI: 10.1191/0269215502cr549oa. [DOI] [PubMed] [Google Scholar]
- 43.Gur A, Cosut A, Sarac AJ, Cevik R, Nas K and Uyar A. Efficacy of different therapy regimes of low-power laser in painful osteoarthritis of the knee: A double-blind and randomized-controlled trial. Lasers Surg Med 2003; 33(5): 330–338. DOI: 10.1002/lsm.10236. [DOI] [PubMed] [Google Scholar]
- 44.Helianthi DR, Simadibrata C, Srilestari A, Wahyudi ER and Hidayat R. Pain reduction after laser acupuncture treatment in geriatric patients with knee osteoarthritis: A randomized controlled trial. Acta Med Indones 2016; 48(2): 114–121. [PubMed] [Google Scholar]
- 45.Inal E, Eroğlu P, Yücel SH and Orhan H. Which is the appropriate frequency of TENS in managing knee osteoarthritis: High or low frequency? 2016.
- 46.Kheshie AR, Alayat MS and Ali MM. High-intensity versus low-level laser therapy in the treatment of patients with knee osteoarthritis: A randomized controlled trial. Lasers Med Sci 2014; 29(4): 1371–1376. DOI: 10.1007/s10103-014-1529-0. [DOI] [PubMed] [Google Scholar]
- 47.Loyola-Sanchez A, Richardson J, Beattie KA, Otero-Fuentes C, Adachi JD and Macintyre NJ. Effect of low-intensity pulsed ultrasound on the cartilage repair in people with mild to moderate knee osteoarthritis: A double-blinded, randomized, placebo-controlled pilot study. Arch Phys Med Rehabil 2012; 93(1): 35–42. DOI: 10.1016/j.apmr.2011.07.196. [DOI] [PubMed] [Google Scholar]
- 48.Pietrosimone BG, Saliba SA, Hart JM, Hertel J, Kerrigan DC and Ingersoll CD. Effects of transcutaneous electrical nerve stimulation and therapeutic exercise on quadriceps activation in people with tibiofemoral osteoarthritis. J Orthop Sports Phys Ther 2011; 41(1): 4–12. DOI: 10.2519/jospt.2011.3447. [DOI] [PubMed] [Google Scholar]
- 49.Yegin T, Altan L and Kasapoglu Aksoy M. The effect of therapeutic ultrasound on pain and physical function in patients with knee osteoarthritis. Ultrasound Med Biol 2017; 43(1): 187–194. DOI: 10.1016/j.ultrasmedbio.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 50.Yildiz SK, Ozkan FU, Aktas I, Silte AD, Kaysin MY and Badur NB. The effectiveness of ultrasound treatment for the management of knee osteoarthritis: A randomized, placebo-controlled, double-blind study. Turk J Med Sci 2015; 45(6): 1187–1191. [DOI] [PubMed] [Google Scholar]
- 51.Yurtkuran M, Alp A, Konur S, Ozcakir S and Bingol U. Laser acupuncture in knee osteoarthritis: A double-blind, randomized controlled study. Photomed Laser Surg 2007; 25(1): 14–20. DOI: 10.1089/pho.2006.1093. [DOI] [PubMed] [Google Scholar]
- 52.Jia L, Wang Y, Chen J and Chen W. Efficacy of focused low-intensity pulsed ultrasound therapy for the management of knee osteoarthritis: A randomized, double blind, placebo-controlled trial. Sci Rep 2016; 6: 35453 DOI: 10.1038/srep35453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bennell KL, Hinman RS, Metcalf BR, Buchbinder R, Mcconnell J, Mccoll G, et al. Efficacy of physiotherapy management of knee joint osteoarthritis: A randomised, double blind, placebo controlled trial. Ann Rheum Dis 2005; 64(6): 906–912. DOI: 10.1136/ard.2004.026526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mchugh ML. Interrater reliability: The kappa statistic. Biochem Med (Zagreb) 2012; 22(3): 276–282. [PMC free article] [PubMed] [Google Scholar]
- 55.PEDro statistics. 2019, Available from https://www.pedro.org.au/english/downloads/pedro-statistics/.
- 56.Evans KR, Sills T, Wunderlich GR and Mcdonald HP. Worsening of depressive symptoms prior to randomization in clinical trials: A possible screen for placebo responders? J Psychiatr Res 2004; 38(4): 437–444. DOI: 10.1016/j.jpsychires.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Hoekman DR, Zeevenhooven J, Van Etten-Jamaludin FS, Douwes Dekker I, Benninga MA, Tabbers MM, et al. The placebo response in pediatric abdominal pain-related functional gastrointestinal disorders: A systematic review and meta-analysis. J Pediatr 2017; 182: 155–163.e157. DOI: 10.1016/j.jpeds.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 58.Cubukcu D, Sarsan A and Alkan H. Relationships between pain, function and radiographic findings in osteoarthritis of the knee: A cross-sectional study. Arthritis 2012; 2012: 984060 DOI: 10.1155/2012/984060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harden RN, Saracoglu M, Connolly S, Kirsling A, Comstock K, Khazey K, et al. “Managing” the placebo effect: The single-blind placebo lead-in response in two pain models. Pain Med 2016; 17(12): 2305–2310. DOI: 10.1093/pm/pnv109. [DOI] [PubMed] [Google Scholar]
- 60.Carvalho C, Caetano JM, Cunha L, Rebouta P, Kaptchuk TJ and Kirsch I. Open-label placebo treatment in chronic low back pain: A randomized controlled trial. Pain 2016; 157(12): 2766–2772. DOI: 10.1097/j.pain.0000000000000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Charlesworth JEG, Petkovic G, Kelley JM, Hunter M, Onakpoya I, Roberts N, et al. Effects of placebos without deception compared with no treatment: A systematic review and meta-analysis. J Evid Based Med 2017; 10(2): 97–107. DOI: 10.1111/jebm.12251. [DOI] [PubMed] [Google Scholar]
- 62.Kaas BM, Humbyrd CJ and Pantelyat A. Functional movement disorders and placebo: A brief review of the placebo effect in movement disorders and ethical considerations for placebo therapy. Mov Disord Clin Pract 2018; 5(5): 471–478. DOI: 10.1002/mdc3.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, et al. Placebos without deception: A randomized controlled trial in irritable bowel syndrome. PLoS One 2010; 5(12): e15591 DOI: 10.1371/journal.pone.0015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mundt JM, Roditi D and Robinson ME. A comparison of deceptive and non-deceptive placebo analgesia: Efficacy and ethical consequences. Ann Behav Med 2017; 51(2): 307–315. DOI: 10.1007/s12160-016-9854-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


