Abstract
Design and fabrication of scaffolds with three-dimensional (3D) topological cues inducing regeneration of the neo-tissue comparable to native one remains a major challenge in both scientific and clinical fields. Here, we developed a well-designed vascular graft with 3D highly interconnected and circumferentially oriented microchannels by using the sacrificial sugar microfiber leaching method. The microchannels structure was capable of promoting the migration, oriented arrangement, elongation, and the contractile phenotype expression of vascular smooth muscle cells (VSMCs) in vitro. After implantation into the rat aorta defect model, the microchannels in vascular grafts simultaneously improved the infiltration and aligned arrangement of VSMCs and the oriented deposition of extracellular matrix (ECM), as well as the recruitment and polarization of macrophages. These positive results also provided protection and support for ECs growth, and ultimately accelerated the endothelialization. Our research provides a new strategy for the fabrication of grafts with the capability of inducing arterial regeneration, which could be further extended to apply in preparing other kinds of oriented scaffolds aiming to guide oriented tissue in situ regeneration.
Keywords: vascular smooth muscle cells regeneration, melt-spinning, circumferentially oriented microchannels, vascular grafts, sugar template leaching method
1. Introduction
Cardiovascular diseases (CVDs) remain the leading cause of mortality and morbidity in the world and continue to rise at an alarming rate. According to the report of the world health organization (WHO), 17.9 million people died from CVDs in 2016. Bypass surgery was one of the main treatments for CVDs [1–3]. Autologous grafts, including the great saphenous vein, the internal mammary artery, and the radial artery remain the first choice for bypass procedure. However, there are no available autologous grafts for a large percentage of patients, due to previous employment of autotransplantation or disease conditions. Accordingly, there is an urgent need to develop a small diameter vascular graft (SDVG) capable of guiding the regeneration of new blood vessels comparable to a native artery in structure and function to replace injured arteries [4]. Generally, the native arteries are mainly composed of three layers: intima, media, and adventitia. Different types of cells in the wall of the native artery have their unique arrangement, which contributes to their particular function [5]. The endothelial cells (ECs) are oriented longitudinally in the intima, while the vascular smooth muscle cells (VSMCs) with a spiral orientation are distributed in the media and perpendicular to the direction of blood flow. Fibroblasts are randomly distributed in the adventitia. Hereinto, the VSMCs, as well as secreted extracellular matrix (ECM), play a central role in vascular physiology function and mechanical properties [6].
Based on the principles of bionics, the design and construction of SDVG by simulating the inherent structure of a native artery is a promising strategy [7–10]. Recently, the development of degradable polymer vascular grafts with capability of transforming in situ from a polymeric graft to a living, artery-like structure has received increasing attention [9]. Many technologies like electrospinning [11–13], phase separation [14–16], particle leaching (such as salt-leaching, paraffin, gelatin leaching) [7, 9, 10, 13, 15, 16] have been developed for preparing porous polymeric vascular scaffolds. However, the scaffolds generated from these methods have no aligned topological cues to induce VSMCs circumferential oriented regeneration. Meanwhile, researchers are committed to developing other controllable topological structures using different technologies to guide the VSMCs aligned regeneration [8, 19–23]. JY Shen et al. generated a degradable polymer scaffold with oriented microchannel structure, which could guide VSMCs oriented growth and enhance the expression of smooth muscle α-actin of VSMCs [23]. Human fetal aortic smooth muscle cells (HASMCs) were cultured on polydimethylsiloxane (PDMS) with mono-layer circumferentially aligned microchannels fabricated by soft lithography and exhibited an in vivo-like cell phenotype with high alignment and viability [22]. These and other similar studies showed that the oriented microchannel (OM) structure could induce VSMCs oriented arrangement and phenotypic transformation to contractility [8, 19–23]. However, the structure of microchannels prepared in these studies were single-layer in contrast to the multi-layers of native arteries. Also, these studies were limited to in vitro proof-of-concept, and lack in vivo evidence of their effectiveness. Therefore, the design and fabrication of vascular grafts with circumferentially oriented microchannels and in vivo verification of their guiding effects on VSMCs remain to be solved.
Previous studies demonstrated that melting extrusion printing technology, which has been widely used in 3D printing, could accurately control the size and arrangement of extruded fibers [24–30]. JS Miller et al. printed a sacrificed 3D scaffolds templates with a mixture of glucose, sucrose, and dextran to generate vascular network in tissue engineering scaffolds [25]. Inspired by this, we selected the sugar fibers as sacrificial porogen leaching materials to prepare SDVGs with an oriented microchannels structure to guide VSMCs circumferentially aligned regeneration. A sacrificial oriented sugar fiber template was prepared by melt spinning technology, and the poly (L-lactic-co-ε-caprolactone) (PLCL) solution was filled into the interspace among the sugar fibers. After removing the sugar fibers, an SDVG with multi-layer aligned microchannels was obtained. In order to prevent bleeding after implantation, random electrospinning (ES) thin fibers were added as outer layer sheath. The structure and mechanical properties of the grafts were characterized. Regulation of VSMCs behavior in vitro and artery regeneration in the abdominal aorta model in rats demonstrated the performance of the vascular grafts with circumferentially oriented microchannels.
2. Materials and Methods
2.1. Materials
PLCL pellets (viscosity 2.1) were purchased from Jinan Daigang Biomaterials Inc. (Jinan, China). Hexafluoroisopropanol (HFIP) was purchased from Aladdin (Shanghai China). Ethanol, chloroform, and alcohol were purchased from Tianjin Chemical Reagent Company (Tianjin, China). Maltitol was purchased from solarbio life science technology (Tianjin China). Sprague Dawley rats (male, weight 280–320 g) were purchased from SPF (Beijing) Biotechnology Co., Ltd. (Beijing, China). Animal experiments were approved by the Animal Experiments Ethical Committee of Nankai University and complied with the Guideline for Care and Use of Laboratory Animals.
2.2. Grafts fabrication
The fabrication process of bi-layered grafts was schematically presented in Fig. 1A. Firstly, a sacrificial aligned maltitol-fiber template was produced by melt spinning. The maltitol powder was loaded into a metal syringe and then heated to 180°C until it melted. The melting maltitol was kept at 165°C and extruded from a 20G injection needle. A rotating mandrel (2.0 mm in diameter) was used as a collector. The fibers’ diameter was controlled by changing the mandrel rotation and reciprocating speed. Secondly, the sugar fiber template was immersed in a 10% PLCL/CHCl3 (w/v) solution. After the interspace among the sugar fibers was filled with a polymer solution, the composite composed of sugar filaments and polymer was taken out to evaporate the solvent. Subsequently, the composite was immersed in water to remove the sugar fibers, generating circumferentially aligned micro-channels in the vascular graft. Finally, the scaffold was covered by random PLCL electrospinning thin fibers sheath prepared according to the following conditions: 16% PLCL solution (w/v in HFIP), 21G needle, a voltage of 15 kV, collection distance of 10 cm, electrospinning time of 12 min. The prepared grafts were further dried under a vacuum for 2 days to remove residual solvent.
Fig. 1.

Fabrication and characterization of bi-layered vascular grafts. A: Schematic illustration of the fabrication process of bi-layered vascular grafts with circumferentially oriented microchannel in the inner layer and thin fibers in the outer layer; The sugar microfibers template was fabricated by the melting spinning method (a). Then, the sugar fiber template was immersed into the 10% (w/v) PLCL/chloroform solution (b), and taken out to volatilize the chloroform solvent (c). After removal of the sugar template, the tubular scaffold with circumferentially aligned microchannels was obtained (d). Finally, a layer of randomly distributed thin fibers fabricated by electrospinning was covered on the outside of the internal layer with a microchannels scaffold (e, f). B: Macroscopic image of the sugar fibers template, scale bar: 1 mm; C: Local amplification of B, scale bar: 200 μm; D: Macroscopic picture of bi-layered vascular grafts, scale bar: 5 mm; E: No kinking formed when vascular graft was folded to 180°, scale bar: 1 cm; F: SEM images showing the cross-section structure of the bi-layered graft, scale bar: 1 mm; G: Local amplification of F, scale bar: 200 μm; H: Magnified SEM image of the longitudinal structure of the bi-layered grafts, scale bar: 500 μm, Inset: Low multiple SEM image; I: SEM image showing the randomly distributed thin fibers on the external layer of the grafts, scale bar: 100 μm; J-M: X-ray micro-CT images showing the 3D structure of inner layer (J, K) and two layers of vascular grafts (L, M); N: porosity of the vascular grafts. O: the stress-strain curve of the vascular grafts. Data are represented as the mean ± SEM for each group. *:˖p < 0.05; **: p< 0.01; ***: p < 0.001; (n > 6, Single comparisons were carried out using an unpaired student’s t-test).
The electrospinning PLCL vascular scaffolds with an inner diameter of 2 mm were used as controls and prepared according to the following conditions: PLCL solution (16%, w/v in HFIP), 21G needle, a voltage of 15 kV, a collection distance of 10 cm, electrospinning time of 30 min.
Before implantation, the grafts were cut into 1.1 cm lengths and sterilized by immersing in 75% ethanol for one h, and then washed with sterile phosphate buffer saline (PBS, pH 7.0) for five times.
2.3. Mechanical properties
Mechanical properties in circumferential direction were tested by using tensile-testing machine (Instron-3345, Norwood, MA) at room temperature. Grafts (total length, 2.0 mm) were pulled at a strain rate of 5 mm/min until rupture. The stress-strain curve was determined by accessing the tensile force on the sample and the corresponding strain. The elastic modulus, ultimate tensile strength and failure strain were measured. The elastic modulus was calculated from the initial linear region of the stress-strain curve.
In order to measure the burst pressure, a graft of 2 cm in length was filled with Vaseline, and then clamped on one end, and the other end was hermetically sealed to a vascular catheter. After incubation at 37°C for 30 min, the sample was filled with nitrogen at a slow rate. The burst pressure was recorded until the graft wall burst.
2.4. Characterization of vascular grafts
The cross-sections and luminal surfaces of PLCL grafts were attached to the metal sample table with conductive adhesive cloth and sputter-coated with gold. The grafts were observed under a scanning electron microscope (SEM, HITACHI, X-650, Japan) at an accelerating a voltage of 15 kV. For explanted grafts, the samples were firstly fixed in 2.5% (v/v) glutaraldehyde for 12 h at 4°C and dehydrated in gradient ethanol before observation.
3D X-ray micro-CT (nanoVoxel 3502, Sanying Precision Instruments Co., Ltd, Tianjin) was used to characterize the spatial structure of the grafts at voltage of 15 kV, current of 40 μA, and resolution of 1.6 μm. The tomography stacks were reconstructed by using nano Voxel CT-Pro software. In this study, the porosity of the samples was obtained based on CT data.
2.5. Analysis of VSMCs morphology and orientation in vitro
Membranous scaffolds were cut into circular discs and sterilized in 75% ethanol for 1 h. The VSMCs cell line bought from ScienCell (USA) were seeded onto the scaffolds with a density of 5×103/well and cultured in DMEM high glucose medium (Hyclone, USA) with 10% fetal bovine serum (FBS) for 1, 4 and 7 days. At the determined time points, CCK8 dilution in DMEM (1:10) was added in each well after the media was removed. Upon incubation for 3h at 37°C, 100 μL supernatant was taken out from each well and added to a 96-well plate. The optical density (OD) value was measured at 450nm using a microplate reader (Bio-Rad). For cytoskeleton staining, the VSMCs were fixed with 4% paraformaldehyde solution for 30 min and then incubated in a Triton X-100 (0.1%) for 10 min before staining with FITC-phalloidin (green) for 1 h. The morphology of VSMCs was quantitatively evaluated by nuclear shape index (NSI) and angel orientation (θ) [22]. The cell assumes a linear, elongated morphology when NSI approaches 0, while it becomes more circular when NSI is 1. The θ was defined as the angle between the microchannel direction and the cell’s major axis, ranging from 0 (parallel to the microchannel direction) to 90° (perpendicular to the microchannel direction). More than 50 individual cells cultured on each scaffold were used to evaluate θ statistically. In order to investigate the contractibility of the VSMCs cultured on the different scaffolds, we performed immunohistochemical staining, including alpha-smooth muscle actin (α-SMA, 1:100, Abcam, ab7817, USA) and smooth muscle myosin heavy chain I (SM-MHC, 1:500, Abcam, ab212657, USA).
2.6. Analysis of VSMCs proliferation and migration in vitro
VSMCs labeled with a DiI Vybrant Solution (Invitrogen, USA) were seeded on OM and ES scaffolds to evaluate cell migration [31]. After the cells adhered to the scaffolds, live-cell imaging was performed by using an Infinity 3 2D array confocal scanner (Visitech Intl. Ltd. Norway). Images were taken every 5 min for 6 h. “Manual tracking class” plug-in of Image J software was used to track the movement of each cell. The initial position of all cells was normalized to the original point (0, 0). Chemotaxis and Migration Tool 2.0 (IBIDI, Germany) was used to calculate the velocity and Euclidean distance (S) of cell migration and counts of VSMCs migration at a different direction. More than 50 individual cells were tracked in each group.
2.7. In vivo implantation
Sixty-one Sprague Dawley rats were randomly divided into two groups for transplantation of OM and ES vascular grafts. The transplantation procedure was the same as our previous study [11]. At the predetermined time point (3 days, 2 weeks, 4 weeks, and 12 weeks), the rats were anesthetized to analyze the patency of the grafts by high-resolution ultrasound (Vevo 2100 System, Visualsonics, Canada). After that, the rats were sacrificed by injection of overdose chloral hydrate, and the implanted grafts were collected. Each explanted graft was cut in half in the middle. One part was put into optimal cutting temperature (OCT, Tissue Tek) compound and snap-frozen by liquid nitrogen for frozen cross-sections. The other part was longitudinally cut into two pieces. After observed under a stereomicroscope (LEICA S8AP0, Germany), one piece was embedded in OCT snap-frozen by liquid nitrogen for longitudinal sections for CD31 staining. The other piece was fixed by 2.5% glutaraldehyde for the SEM examination, as noted in 2.4.
2.8. Histological evaluation
For histological analysis, cross-sections of explanted grafts (6 μm in thickness) were stained with hematoxylin and eosin (H&E), Masson’s trichrome, Verhoeff Van-Gieson (VVG), Safranin O, and Sirius Red. Slides were observed under an upright microscope (Leica DM3000, Germany), and images were acquired with a digital camera (Leica DFC450, Germany).
Collagen and elastin autofluorescence within the explanted grafts wall were analyzed by using an Olympus Fluoview FV1200-MPE Multiphoton laser scanning microscope. The vascular graft or native aorta was cut into 2 mm-thick cross-section rings, and the cross-section was observed under the following condition: excitation wavelength was set to 870 nm, and the emission wavelength range was set to 420–460 nm (collagen) and 495–540 nm (elastin) [32].
2.9. Immunofluorescence staining
Frozen sections were fixed in acetone at −20°C for 10 min, air-dried, and rinsed with 0.01 mM PBS for three times. For intracellular antigen staining, Triton X-PBS (0.1%) was used to permeate the cell membrane. Slides were blocked with 5% normal goat serum for 45 min at 4°C, and then sections were incubated with the following antibodies for 12 h at 4°C. The mouse anti-CD31 (1:100, Abcam, ab24590, USA) antibody was used to mark the endothelial cells. The mouse anti-CD68 (1:200, Abcam, ab31630, USA), rabbit anti-CD206 (ab64693), and rabbit anti-iNOS (ab15323) antibodies were used to mark the macrophages. The mouse anti-α-SMA (1:100, Abcam, ab7817, USA) and mouse anti-SM-MHC (1:500, Abcam, ab212657, USA) antibodies were used to mark the VSMCs. The mouse anti-collagen I (1:200, Abcam, ab6308, USA) antibody was used to mark the collagen I deposition. After completing the incubation with primary antibodies, the slides were washed 6 times with PBS and then incubated with appropriate secondary antibodies for 2 h at room temperature. The cell nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich) mounting solution. The slides were then observed under a fluorescence microscope (Zeiss Axio Imager Z1, Germany).
The cross-sections stained with anti-CD68, anti-iNOS, anti-CD206 antibodies were used to count the number of macrophages. The sections stained with anti-collagen I antibody were used to quantitatively analyze the positive area ratio of collagen I, using Image J 1.46 software (National Institutes of Health).
All CD31 positive capillaries within graft walls were counted to assess vascularization. The ECs coverage rate was quantified by adding the length of the CD31-positive monolayer and dividing this sum by the length of the longitudinal section of the graft.
2.10. Evaluation of polymer degradation
The polymer degradation was analyzed by two methods. The structural morphology of the explants was detected by SEM. The molecular weight of the explants was analyzed by gel permeation chromatography (GPC) (Waters, USA) after dissolved in tetrahydrofuran.
2.11. Statistical analysis
GraphPad Prism Software Version 8.0 (San Diego, CA, USA) was used for statistical analysis. Single comparisons were carried out using an unpaired student’s t-test. Multiple comparisons were performed using a two-way ANOVA and Tukey’s post hoc analysis. The significant difference was accepted at a p-value below 0.05.
3. Results
3.1. Preparation and characterization of vascular grafts
According to our design, the vascular graft was fabricated by the process presented in the schematic illustration (Fig. 1A). The sugar microfibers templates with size distribution in the range from 30μm to 140μm can be fabricated by the melting spinning method (Fig. S1A and movie 1). The microfibers with the cross angle of 36° and uniform diameter of 54.6 ± 8.4 μm were selected as sacrificial templates to produce microchannels (Fig. S1A and Fig. 1B, C). Then, the sugar fiber template was immersed into the 10% (w/v) PLCL/chloroform solution, and taken out to volatilize the chloroform solvent. After removal of the sugar template, the tubular scaffold with circumferentially aligned microchannels was obtained. Finally, a layer of randomly distributed thin fibers fabricated by electrospinning (ES) was covered on the outside of the internal layer with microchannels scaffold (Fig. 1A). No kinking occurred when the graft was folded to 180° (Fig. 1D and E). SEM images of the cross-section showed that the two layers stuck together, and the interconnected microchannels were aligned circumferentially in the internal layer (Fig. 1F, G). Moreover, the SEM images of the longitudinal section demonstrated the oriented microchannels distributed not only in the graft wall but also in the luminal surface (Fig. 1H). The diameter of the aligned microchannels was 56.4 ± 7.9 μm according to the statistical analysis of SEM images (Table S1). The outer layer was composed of dense thin fibers with diameter of 2.3 ± 0.6 μm, as observed by SEM images (Fig. 1I and Table S1). Furthermore, the spatial structure of the vascular grafts reconstructed by 3D micro-CT showed that highly interconnected and circumferentially aligned microchannels distributed in the inner layer and the thin dense ES fibers distributed in the outer layer, which was consistent with SEM observation (Fig. 1J–M, movie 2). Based on these Micro-CT analyses, the porosity of the OM graft was significantly higher than that of the ES graft (72.6% ± 1.7% vs. 60.8% ± 3.8%) (Fig. 1N). The mechanical properties of the OM and ES grafts, including elastic modulus at the early phase, ultimate tensile strength, and failure strain, were significantly higher than that of native arteries (Table S2). The stress-strain curves of the OM grafts were closer to those of the native arteries compared to ES grafts (Fig. 1O). The burst pressure of OM grafts and ES grafts were 1787 ± 118 mmHg and 2181 ± 122 mmHg, respectively, which approached or were higher than that of the saphenous vein of 1700 mmHg [33].
3.2. Aligned microchannels induce VSMCs oriented growth in vitro
The effects of aligned microchannels on VSMCs proliferative rate, morphology and contractile protein expression were evaluated in vitro. The result showed that VSMCs proliferation rate cultured on the OM groups were significantly higher than that on ES groups during the culture times in vitro (Fig. S2). Confocal microscopy images showed that the cytoskeleton F-actin of VSMCs stained by FITC labelled phalloidin was gradually elongated and grew aligned along the microchannels over time on OM scaffolds (Fig. 2A). While, the VSMCs cultured on ES scaffolds were randomly arranged within 7 days (Fig. 2A). The ratio of VSMCs with a small orientated angle on OM scaffold increased over time. After being cultured for 7 days, over 80% of the cells had an angle of orientation below 15°, while the angle of VSMCs on the ES scaffold was randomly distributed from 0° to 90° during the 7 days (Fig. 2B). Furthermore, the value of NSI in OM scaffold was gradually decreased and remained constant after 4 days, indicating that the cells were elongated. NSI of VSMCs on ES scaffolds was significantly higher than that on OM scaffolds and almost unchanged throughout 7 days (Fig. 2C). The expression of protein α-SMA+ and SM-MHC of VSMCs cultured in OM scaffold displayed no significant differences with that cultured in ES scaffold (Fig. 2D–G). These results revealed that oriented microchannel enabled cell alignment and enhanced the expression of contractile proteins of VSMCs.
Fig. 2.
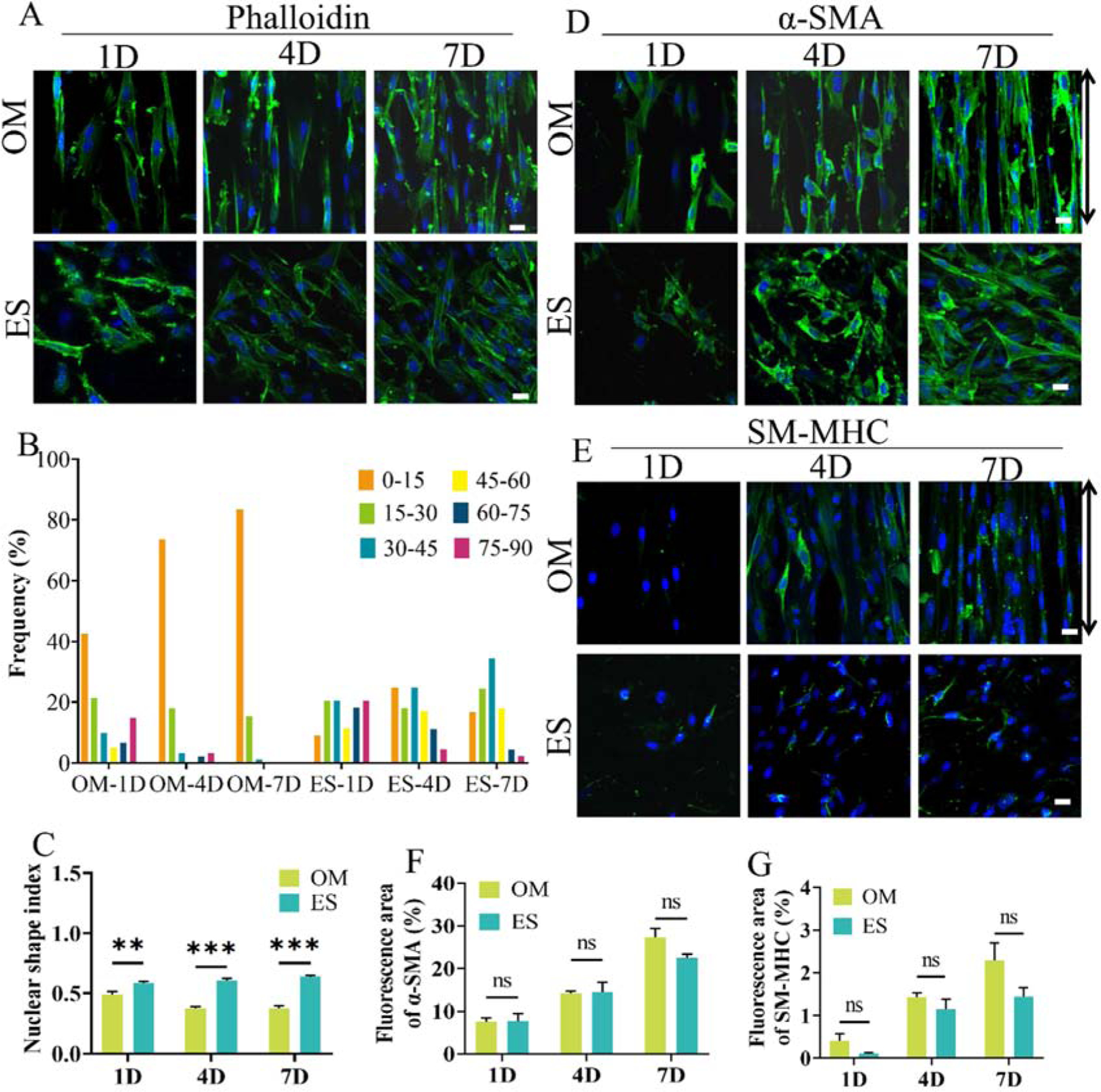
Morphology, expression of contractile protein markers of VSMCs cultured on the OM and ES scaffolds. A: Cytoskeleton staining with FITC labeled phalloidin (green) showing the cell morphology. B: Distribution frequency of VSMCs at different angles of orientation (θ); C: Nuclear shape index (NSI); D, E: immunofluorescence staining showing expression of α-SMA and SM-MHC; F, G: Quantitative analysis of α-SMA and SM-MHC expression (immunofluorescence area). The black arrow indicates the orientation of microchannels. 1D, 4D, and 7D mean 1 days, 4 days and 7 days, respectively. Data are represented as the mean ± SEM for each group. *:˖p < 0.05; **: p< 0.01; ***: p < 0.001; (n > 50, two-way-ANOVA followed by Tukey’s post hoc analysis). Scale bar: 50 μm.
3.3. Aligned microchannels promoted VSMCs migration
The migration of VSMCs on two different scaffolds was also detected and quantitatively analyzed. The VSMCs on the OM scaffold migrated parallel to the microchannels over a certain distance (Fig. 3A, movie 3). However, VSMCs on the ES scaffold vibrated around the initial position (Fig. 3A, movie 4). The trajectory of VSMCs on OM scaffolds exhibited a slender radial pattern; in contrast, the cells migrated randomly around the initial position on ES scaffolds (Fig. 3B, movies 5 and 6). Most of VSMCs on OM scaffolds moved along with the direction of the microchannel with a low angle of migration, while cells on ES scaffolds migrated irregularly in all directions (Fig. 3C). The velocity of VSMCs on the OM scaffold was significantly faster than that on the ES scaffold, and the distance of VSMCs on OM scaffold was also significantly further than that on ES scaffold (Fig. 3D and E). These findings demonstrated that the microchannel structure accelerated the migration of VSMCs.
Fig. 3.

Migration behavior of VSMCs cultured on the OM and ES scaffolds. A: Distribution of DiI-labeled VSMCs at 0 h, 3 h and 6 h, Arabic numbers indicates typical cells; B: Migration traces of DiI labeled VSMCs; C: Counts of VSMCs migration at a different direction. D, E: Velocity and Euclidian distance of VSMCs migration. The migration of VSMCs was continuously tracked every 5 min for 6 h. The black arrow indicates the orientation of microchannels. 1D, 4D, and 7D mean 1 days, 4 days and 7 days, respectively. Data are represented as the mean ± SEM for each group. *:˖p < 0.05; **: p< 0.01; ***: p < 0.001; (n=50, single comparisons were carried out using unpaired student’s t-test). Scale bar: 50 μm.
3.4. Aligned microchannels facilitate cell migration and orientated arrangement in vivo
The vascular grafts were implanted into rat abdominal aorta and harvested at 3 days, 2 weeks, 4 weeks, and 12 weeks post-implantation. Cellularization within the wall of the vascular grafts was firstly detected based on DAPI staining. Cells rapidly migrated into the wall of OM grafts at 3 days, and distributed uniformly throughout the graft wall until 12 weeks. The number of cells gradually increased over time (Fig. 4A, B). In contrast, cells mainly distributed on the outside of the ES graft wall at 3 days and 2 weeks, and occupied the whole graft wall at 4 weeks (Fig. 4A, B). The number of cells gradually increased over 4 weeks and then decreased at 12 weeks (Fig. 4A, B). These cells infiltrated into the OM grafts aligned along the direction of microchannels and gradually elongated over time, while the cell morphology in the wall of ES grafts was almost unchanged (Fig. 4A, B). Further quantitative analysis revealed that the NSI of cells in the OM graft gradually decreased over time and was close to that of the native artery (0.53 ± 0.12 vs. 0.55 ± 0.16). Nevertheless, the NSI of cells in ES grafts was significantly higher than that of cells in OM grafts at 4 and 12 weeks (Fig. 4C). These results indicated that the OM structure facilitated cell migration and aligned arrangement in vivo.
Fig. 4.

Cell migration and arrangement in vivo. A: DAPI staining showing the distribution of the cells within the wall of OM and ES grafts (white dashed line indicates the lumen surface); B: Quantitative analysis of cell number. C: The nuclear shape index of cells within OM and ES grafts. 3D, 2W, 4W and 12W mean 3 days, 2 weeks, 4 weeks, and 12 weeks, respectively. The red dashed line in the images are showing the interface between scaffold and the neointima. Data are represented as the mean ± SEM for each group. *:˖p < 0.05; **: p< 0.01; ***: p < 0.001; n = 6, two-way-ANOVA followed by Tukey’s post hoc analysis. Scale bar: 50 μm.
3.5. Oriented microchannel structures enhance the regeneration of VSMCs in vivo
VSMCs regeneration was also detected by immunofluorescent staining and corresponding statistical analysis. Immunofluorescent staining with α-SMA anti-body showed that α-SMA+ cells appeared in the wall of OM grafts as early as 2 weeks and gradually increased over time (Fig. 5A). While only a few α-SMA+ cells appeared at 4 weeks, they were distributed on the luminal surface of ES grafts until 12 weeks (Fig. 5A). Moreover, the contractile VSMCs were evaluated by SM-MHC antibody staining (Fig. 5B). The results demonstrated that SM-MHC+ cells in the wall of OM grafts appeared at 4 weeks, and the expression increased until 12 weeks (Fig. 5B). Only a few SM-MHC+ cells distributed in the luminal surface of ES grafts (Fig. 5B). Also, quantitative analysis showed that the number of α-SMA+ cells in OM grafts was significantly more than that of ES grafts from 2 to 12 weeks (Fig. 5C). Correspondingly, the number of SM-MHC+ cells in OM grafts was significantly higher than that of ES grafts after implantation for 4 weeks and 12 weeks (Fig. 5D). These results suggested that aligned microchannels structure enhanced the expression of the contractile protein of VSMCs in vivo.
Fig. 5.
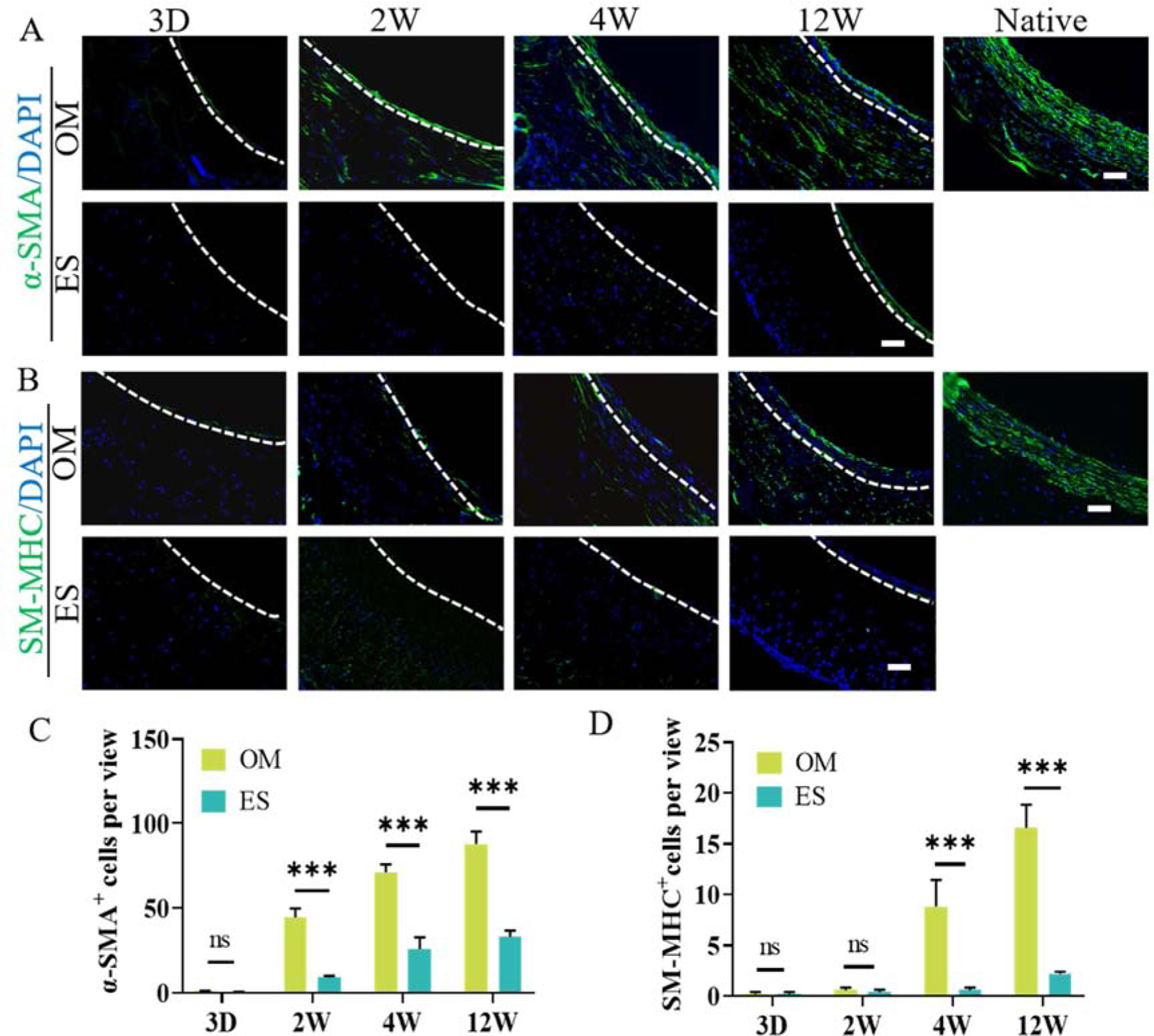
VSMCs regeneration in vivo. A, B: VSMCs were detected by immunofluorescent staining with α-SMA and SM-MHC anti-body; C, D: Quantitative analysis showing the number of α-SMA+ and SM-MHC+ cells per view. The immunofluorescence area of vascular grafts was normalized to that of the native aorta. White dashed lines indicate the interface between the scaffolds and the neointima. All the immunofluorescent micrographs were counterstained for nuclei by DAPI (blue). 3D, 2W, 4W and 12W mean 3 days, 2 weeks, 4 weeks, and 12 weeks, respectively. Data are represented as the mean ± SEM for each group. *:˖p<0.05; **: p < 0.01. ***: p < 0.001 (n = 6, two-way-ANOVA followed by Tukey’s post hoc analysis). Scale bar: 50 μm.
3.6. ECM deposition and organization
ECM, including collagen, elastin, and glycosaminoglycans (GAGs), was evaluated by histological and immunofluorescent staining (Fig. 6 and Fig. S3). H&E staining showed that a large number of cells infiltrated into the OM graft wall and secreted substantial ECM with high density at 12 weeks, despite being lower than that of native arteries (Fig. 6A). Collagen fibrils in native arteries are circumferentially oriented and provide mechanical support [6]. Masson’s and Sirius Red staining confirmed that substantial collagen was deposited in a circumferentially aligned fashion and became denser and denser in the wall of OM grafts over time (Fig. 6B, C). In addition, immunofluorescence staining for collagen I further confirmed its deposition and arrangement (Fig. 6D). Statistical analysis showed that the fluorescent area of collagen I in OM grafts was significantly larger than that in ES grafts from 2 to 12 weeks (Fig. 6H). Elastin in native arteries provides elasticity and compliance [6, 34]. Verhoeff staining showed that the expression of elastin in the wall of OM grafts was gradually increased and approached that of native arteries at 12 weeks (Fig. 6E). GAGs contribute to the biological and mechanical function of the vascular tissues [30]. Second-harmonic generation (SHG) also showed that, the collagen and elastin was circumferentially aligned in the OM wall of vascular grafts, and presented a stronger signal compared with that in ES group at 12 weeks (Fig.6 G). Safranin O staining disclosed that the GAGs in the wall of OM grafts were gradually increased and closed to that in native arteries at 12 weeks (Fig. 6F). However, only a small amount of ECM, including collagen, elastin, and GAGs, were scattered in the wall of ES grafts, even at 12 weeks (Fig. 6 and Fig. S3). These results exhibited that the grafts with aligned microchannels were able to improve the oriented deposition of ECM.
Fig. 6.

ECM deposition and organization within the OM and ES grafts over time. A: H&E staining; B, C: Masson’s trichrome (blue) staining and Sirius red (red) showing the distribution of collagen. D: Immunofluorescence staining (green) showing the distribution of collagen I; E: Verhoeff’s staining (black) showing the presence of elastin. F: Safranin O (pink) staining showing the distribution of GAGs. G: SHG showing elastin and collagen architecture in native aorta and vascular grafts at 12 weeks. H: Statistical analysis of the immunofluorescence area rate of collagen I. The immunofluorescence area of vascular grafts was normalized by that of the native aorta. The dashed line indicates the interface between scaffold and the neointima. Nuclei were counterstained by DAPI (blue). 3D, 2W, 4W and 12W mean 3 days, 2 weeks, 4 weeks and 12 weeks, respectively. Data are represented as the mean ± SEM for each group. *:˖p < 0.05; **: p < 0.01; ***: p < 0.001 (n = 6, two-way-ANOVA followed by Tukey’s post hoc analysis). Scale bar: 100 μm.
3.7. High patency and rapid endothelialization
Patency is a key factor for evaluating the performance of vascular grafts, and a prerequisite for vascular regeneration. Color Doppler ultrasound showed a high patency rate for both the OM grafts (30/31) and the ES grafts (30/30) (Fig. 7A and Table S3). When observed under the stereomicroscope, the lumen of the OM graft was covered by a layer of neo-tissue at 4 weeks post-implantation, and the luminal surfaces of the grafts were clean and smooth without intimal hyperplasia or thrombosis (Fig. 7B and inset). SEM images further confirmed that the luminal surface was covered by a layer of cobblestone-like cells parallel to the direction of blood flow (Fig. 7B, C and Fig. S4). Moreover, most of the microchannels distributed in the OM graft wall were filled with neo-tissue. Immunofluorescence staining demonstrated that about 74% of the length of the lumen of the OM grafts was covered with monolayer CD31 positive cells at 4 weeks (Fig. 7D, J). In contrast, only about 44% of length of lumen surface of the ES grafts was covered with the monolayer CD31 positive cells (Fig. 7E, J). The statistical analysis based on CD31 immunofluorescence staining displayed that the endothelialization rate in OM grafts was significantly higher than that in ES grafts at 2 and 4 weeks, although both of them achieved complete endothelialization at 12 weeks (Fig. 7J). In addition, a large number of capillaries were distributed within the wall of OM grafts, while only a few capillaries scattered within the wall of ES grafts (Fig. 7H, I). Further quantitative analysis results confirmed that the number of capillaries within OM grafts was significantly higher than that within ES grafts from two to 12 weeks, although the capillaries number gradually decreased with the prolongation of implantation time (Fig. 7K).
Fig. 7.
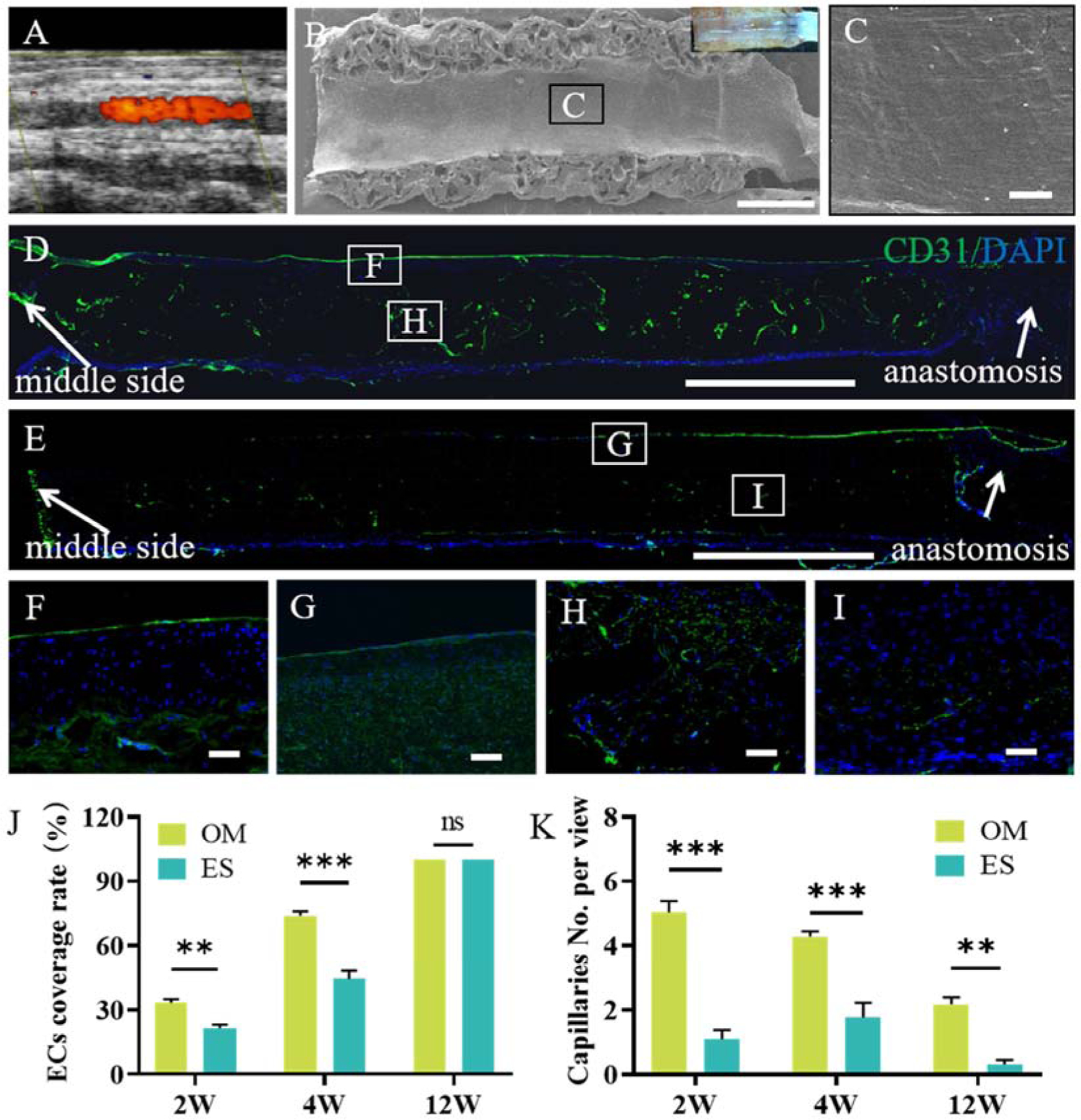
High patency and endothelialization rate of the OM and ES grafts during the implantation period. A: Typical color Doppler ultrasound showing patency of the OM graft; B: SEM image of lumen surface in OM vascular grafts at 4 weeks, inset: stereomicroscope observation; C: Local amplification of B; D, E: CD31 antibody (green) staining marked ECs in OM and ES grafts at 4 weeks post-implantation; F, G, H, I: Local amplification of D and E; J: Endothelial coverage rate of the implanted grafts over time. K: Capillaries number within grafts over time. Nuclei were counterstained by DAPI (blue). 2W, 4W, and 12W mean 2 weeks, 4 weeks, and 12 weeks, respectively. Data are represented as the mean ± SEM for each group. *: p < 0.05; **: p < 0.01.***: p < 0.001 (n = 6, two-way-ANOVA followed by Tukey’s post hoc analysis). Scale bar for B, D, E: 1 mm; for C, F, G, H, I: 50 μm.
3.8. Macrophage infiltration and polarization
Macrophages with different phenotypes (M1 and M2) have diverse functions in tissue repair. CD68 positive macrophages were uniformly distributed within the OM vascular grafts. Their number gradually increased from 3 days to 12 weeks (Fig. 8A–C), while the number of CD68 positive cells within the wall of ES grafts increased until 4 weeks and then decreased at 12 weeks (Fig. 8A, C). The number of CD68 positive cells in the OM grafts was significantly more than that in the ES grafts at 3 days, 2- and 12-weeks post-implantation (Fig. 8C). The number of both iNOS and CD68 positive cells within OM and ES grafts increased until 4 weeks and then decreased at 12 weeks (Fig. 8A and E). The number of CD206 positive cells in OM vascular grafts was higher than that in ES grafts throughout the implantation period and showed significant differences at 2 and 4 weeks (Fig. 8B and D). Also, the number of iNOS positive cells within OM grafts was lower than that of ES grafts at 4 and 12 weeks, although its number within OM grafts was higher than that within ES grafts at 3 days and 2 weeks (Fig. 8E). The value of CD206+/iNOS+, indicating the ratio of M2/M1 macrophages, was used to evaluate the phenotype switch of macrophages (Fig. 8F). The ratio of M2/M1 macrophages of OM grafts was lower than that of ES grafts at 3 days and 2 weeks, but significantly higher at 4 and 12 weeks (Fig. 8F). These results showed that the structure of the aligned microchannels enhanced the recruitment and phenotype switch of macrophages.
Fig. 8.
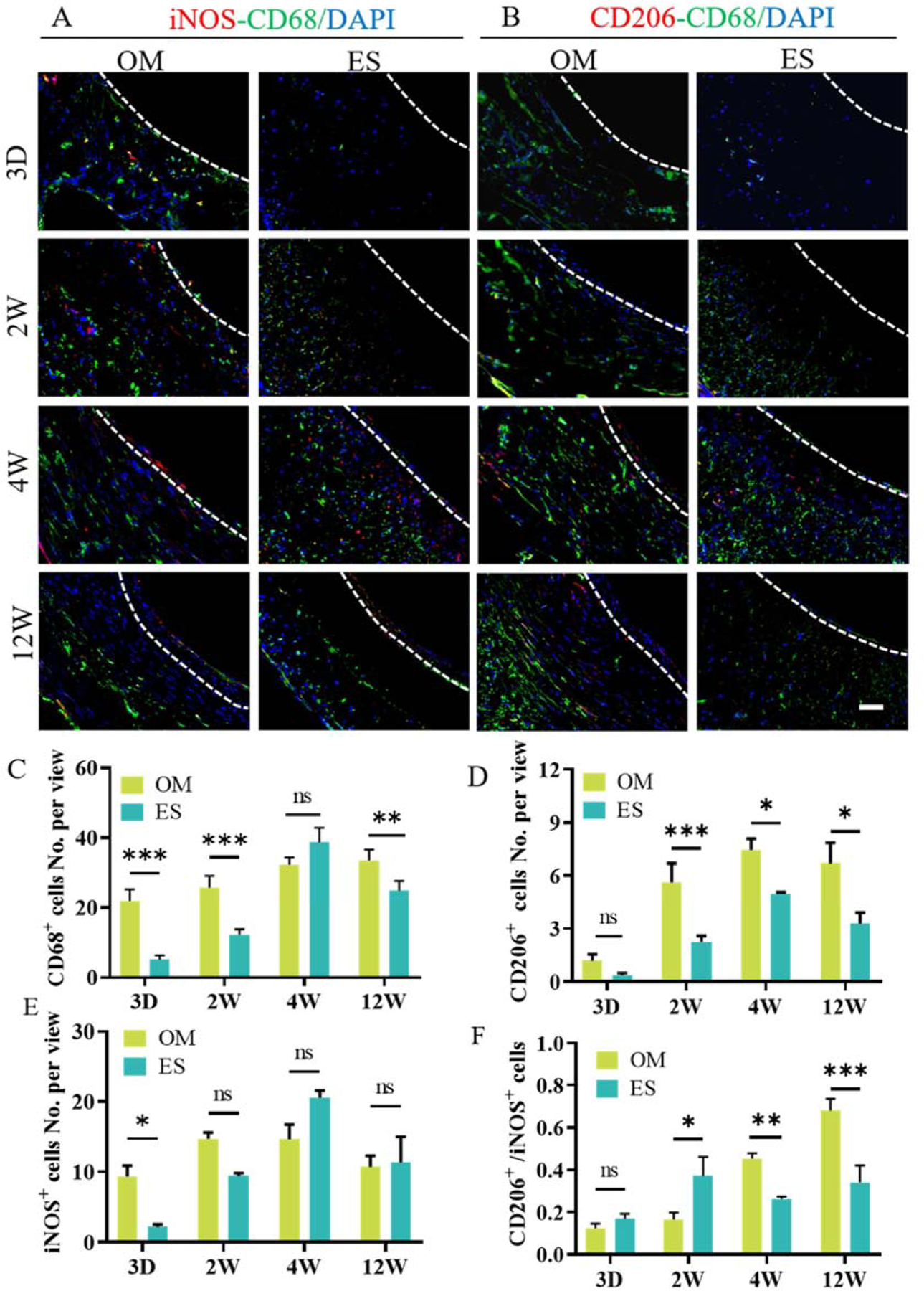
Macrophages infiltration and polarization. A, B: Representative images of immunofluorescence co-staining with the CD68 (green)-iNOS (red) and CD68 (green)-CD206 (red) antibody. C, D, and E: Quantification of the number of CD68+ cells, CD206+ cells, and iNOS+ cells. F: The ratio of CD206+ to iNOS+ cells. Nuclei were counterstained by DAPI (blue). 3D, 2W, 4W and 12W mean 3 days, 2 weeks, 4 weeks and 12 weeks, respectively. The white dashed line indicates the interface between scaffold and the neointima. Data are represented as the mean ± SEM for each group. *:˖p < 0.05; **: p < 0.01; ***: p < 0.001, n = 6, two-way-ANOVA followed by Tukey’s post hoc analysis. Scale bar: 50 μm.
3.9. Polymer degradation
The morphology of the explanted grafts was observed by SEM after implantation for 3 days, 2, 4 and 12 weeks, respectively (Fig. S5A–K). No obvious changes of structure morphology in the vascular grafts could be observed before 4 weeks. Local rupture occurred in the pore wall of the OM grafts, and the fibers were broken into fragment in ES graft after implantation for 12 weeks. Moreover, GPC analysis showed that Mn and Mw of OM graft was all smaller than that of ES graft at 12 weeks (Fig. S5L and Table S4), which indicated that the OM grafts showed a faster degradation rate compared with ES grafts.
4. Discussion
Tissue engineering scaffolds should been fabricated with suitable physical and biochemical properties to provide an appropriate environment for cells adhesion, proliferation, migration, and differentiation [5]. However, inadequate structural design and manufacturing techniques limited improvement in the performance of scaffolds. The basic principle for the design and fabrication of a scaffold was the simulation of the inherent structure of native tissues [35]. In this study, we developed a new type of SDVG with a highly interconnected and circumferentially oriented microchannels structure, which could effectively induce VSMCs alignment and elongation, enhance expression of the contractile protein, and promote the cell migration in vitro. After implantation into rat abdominal aorta defects, the oriented microchannels promoted cell infiltration, circumferentially oriented VSMCs regeneration, SM-MHC expression, aligned ECM fibrils deposition, rapid endothelialization and an increased M2/M1 ratio of macrophages. In contrast, the traditional ES grafts resulted in limited vascular regeneration, including restricted cell infiltration, slight SM-MHC expression, low M2/M1 ratio of macrophages, little and irregular ECM deposition, and a slow rate of endothelialization, as summarized in Fig. 9.
Fig. 9.
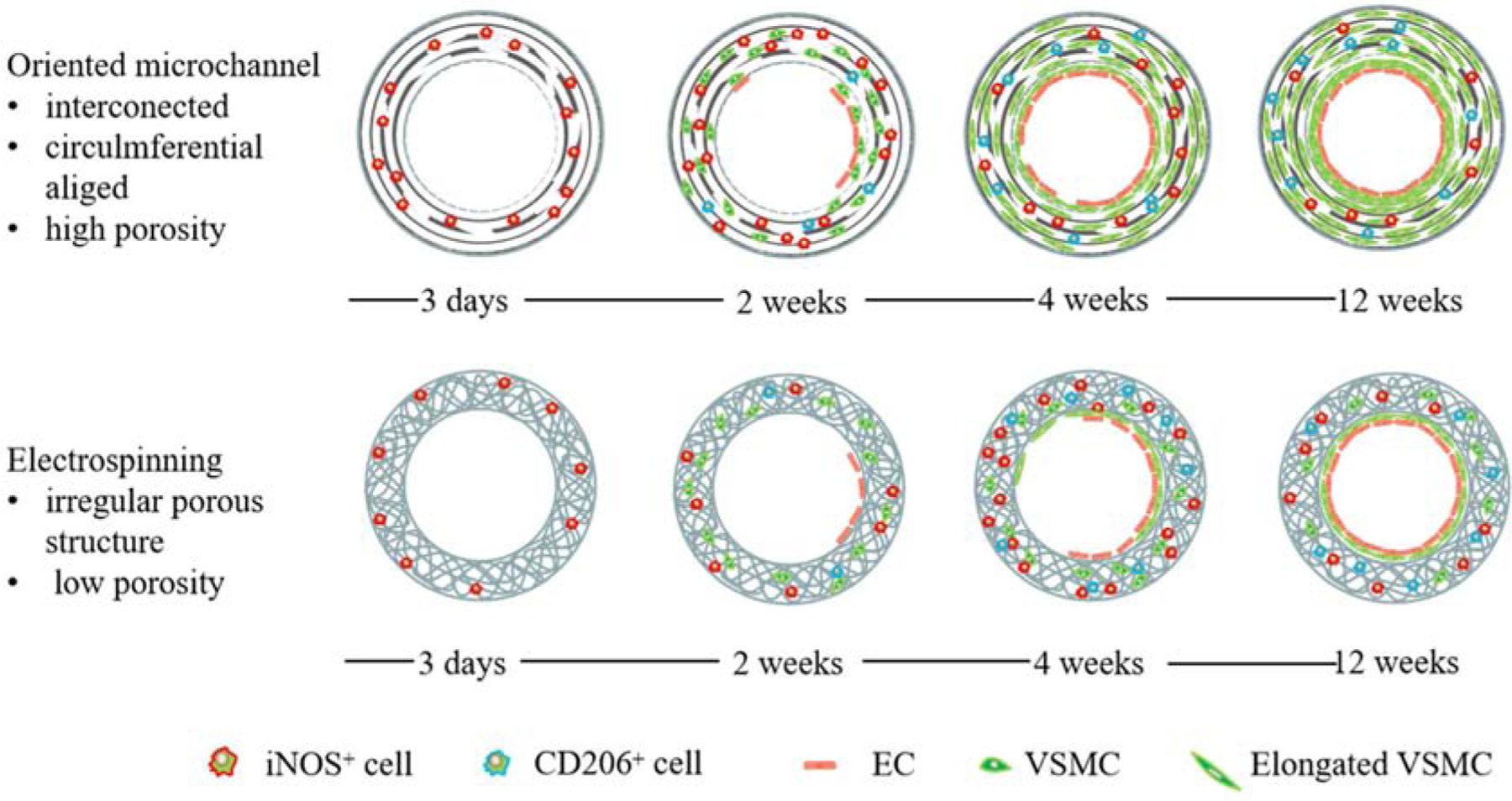
Schematic illustration of the vascular regeneration induced by the different topological structures.
Scaffolds with topological cues, including aligned fibers and microchannels, could regulate cell behavior and guide oriented tissue regeneration [8, 19–23, 36–38]. Oriented microchannel structures prepared in previous studies were monolayer structures, which couldn’t simulate the multilayer 3D structure of native arteries [8, 19–23]. To solve this problem, we developed a sugar microfiber template leaching method to prepare a vascular graft with a circumferentially aligned multi-layer microchannels structure. Maltitol was selected as a sacrificial fiber template due to its good printability, thermal stability and non-toxicity. In addition, it is soluble in water rather than chloroform, which enables the morphology and structure of the sugar fiber template to remain stable in the solution of PLCL in chloroform. After evaporation of the chloroform, the microchannels were generated when the sugar template was eluted with water. According to the crossing angle of collagen fiber in the native artery [35], we printed sugar fibers with an intercrossing angle of 36 degree by adjusting the collector rotating speed and reciprocating moving rate. The diameter of microchannels (about 50 μm) in this study, was consistent with the size of channels in the previous study, which also reported improved VSMCs elongation and growth [20, 23].
Rapid cell infiltration is the first and crucial step for tissue regeneration. The cells were uniformly distributed in the wall of OM grafts, even as early as 3 days post-implantation, while the cells were mainly distributed in the outer layer of ES grafts even at 2 weeks post-implantation (Fig. 4). The reasons are as follows: firstly, aligned microchannels accelerated the cell migration rate compared with the irregular porous structure in ES scaffolds, which was consistent with the in vitro results (Fig. 3). Secondly, interconnected pores among the microchannels and the high porosity promoted cell infiltration all over the channels [7, 9, 10, 14, 15, 17, 18]. In contrast, the dense structure of ES grafts limited the infiltration of cells in the early stage, and ultimately led to cell and vascular degeneration at 12 weeks post-implantation; a similar finding has been confirmed by other researchers [39].
Many kinds of grafts with various pore structures have been developed to promote vascular regeneration in vivo. Recent studies have shown that vascular grafts with a highly interconnected pore structure prepared by phase separation or salt leaching method can achieve rapid cell migration and promote vascular remodeling [7, 14, 15, 17, 18]. However, an irregular pore structure cannot guide the circumferential regeneration of VSMCs in the wall of these vascular grafts, especially in the early stage after implantation. In our study, the highly interconnected microchannels initially promoted cell migration and then began to exert a guiding effect on the oriented growth of the VSMCs. A large number of α-SMA positive cells distributed in the wall of OM grafts, and VSMCs within the wall of OM grafts were elongated and circumferentially aligned over time (Fig. 4 and Fig. 5). In contrast, the morphology of VSMCs within the wall of the ES grafts almost had minimal elongation during the implantation period (Fig. 4 and Fig. 5). The elongation of VSMCs induced the cells to switch to a contractile phenotype, a finding which has been verified by other studies and our in vitro results [40, 41]. Indeed, the expression of SM-MHC positive protein was synchronous with the noticeable elongation of the nucleus. The regeneration of VSMCs was also associated with the participation of macrophages. Compared with ES grafts, a larger number of CD68 positive macrophages appeared in the wall of the OM grafts as early as 3 days post-implantation and were transformed into an elongated M2 phenotype after 2 weeks [42]. Our results provide compelling evidence that the oriented microchannels structure enhance phenotypic switch of macrophages and VSMCs.
Collagen, elastin and GAGs are the main ECM components in the native artery, and play a critical role in maintaining the mechanical properties and structure required for an artery’s biological function [6]. Collagen fibrils in the media layer are oriented circumferentially to provide mechanical support, while the elastin fibrils provide elasticity and compliance. GAGs can serve hydrate to ECM, form bridges between fibrils, and bind with many cell-surface receptors with high specificity [34, 43–45]. In our study, the ECM, including elastin, collagen, and GAGs in the wall of OM grafts, were densely arranged in an orderly manner post-implantation, showing a comparable structure to that of the native artery (Fig. 6). However, only a small amount of disordered ECM was scattered in the walls of ES grafts, even 12 weeks after implantation. The topological structure of OM grafts promoted the VSMCs proliferation and regeneration, and thus, more ECM was secreted and deposited in the walls of the grafts. Also, the interconnected oriented microchannels with high porosity provided sufficient space for the ECM secretion. Except for the density, the arrangement of the ECM contributed to vascular physiological function [6, 46–49]. The VSMCs grew along the direction of microchannels in the wall of OM grafts, and naturally directed the aligned deposition of ECM [49].
Rapid endothelialization of vascular grafts is vital for long-term patency, because it can effectively inhibit acute thrombosis and intimal hyperplasia [4–5]. In this study, without any bioactive modification for accelerating the regeneration of the ECs, the endothelization rate of OM graft was significantly faster than that of ES grafts (Fig. 7). The rapid regeneration of VSMCs and ECM deposition in OM grafts provided a favorable microenvironment, which protected and supported ECs growth and ultimately accelerated the endothelialization [50, 51]. In addition, the recruitment and M2 polarization of macrophages in OM grafts also contributed to rapid endothelialization, and this effect has been verified by several studies [52–54].
Although our newly designed vascular grafts exhibited a positive effect on guiding artery regeneration in a rodent model, several issues need further exploration. Whether the degradation of the material could promote the recovery of the physiological function of the explanted vascular grafts after long term implantation or not, is worth of studying. The origin of various immune cells besides macrophage and vascular stem cells, and mechanism of their dynamic interactions in vascular remodeling remains to be studied. Such regenerative efficacy in the animal model of vascular disease and aging as well as large animal models, also need further investigation, and bioactive modification may be necessary.
5. Conclusion
We successfully developed a bi-layered vascular graft with a circumferentially aligned microchannels structure and demonstrated its guiding effect in vivo and in vitro. Compared with the traditional ES structure, the OM structure promotes VSMCs migration and aligned arrangement, the expression of contractile protein, ECM oriented deposition and rapid endothelialization. Overall, our study proved the effectiveness of structural design of SDVGs and provided a new strategy for the development of novel biomimetic biodegradable polymeric scaffolds.
Supplementary Material
Acknowledgements:
We thank Professor Phillip Bryant for his assistance with proofreading. This work was supported by the National Natural Science Foundation of China (NSFC) projects (31700845, 81972063, 81530059, 31670990), National Key Research and Development Program of China (2017YFC1103500), Innovative Research Group Project (81921004), the China Postdoctoral Science Foundation (2017M620090), National Science Foundation (NSF-DMR award number 1508511 and NIAMS award number 1R01AR067859). This work was also supported by the CSC Scholarships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Reference
- [1].Caliskan E, de Souza DR, Boning A, Liakopoulos OJ, Choi YH, Pepper J, Gibson CM, Perrault LP, Wolf RK, Kim KB, Emmert MY. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat. Rev. Cardiol 2019. 10.1038/s41569-019-0249-3. [DOI] [PubMed] [Google Scholar]
- [2].Arsalan M, Mack MJ. Coronary artery bypass grafting is currently underutilized. Circulation. 133 (2016) 1036–1045. [DOI] [PubMed] [Google Scholar]
- [3].Ciuspin EB Weinberg B. A blood vessel model constructed. Sci. Transl. Med (1986) 397–231. [DOI] [PubMed] [Google Scholar]
- [4].Seifu DG, Purnama A, Mequanint K, Mantovani D. Small-diameter vascular tissue engineering. Nat. Rev. Cardiol 10 (2013) 410–421. [DOI] [PubMed] [Google Scholar]
- [5].Thomas AC, Campbell GR, Campbell JH. Advances in vascular tissue engineering. Cardiovasc. Pathol 12 (2003) 271–276. [DOI] [PubMed] [Google Scholar]
- [6].Bourget JM, F. A, Germain L, Guillemette M, Veres T, Alignment of cells and extracellular matrix within tissue- engineered substitutes, in: Pignatello R (Eds.), Advances in biomaterials science and biomedical applications, InTech pulishing, Croatia, (2013) 365–390. [Google Scholar]
- [7].Wu W, Allen RA, Wang Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat. Med 18 (2012) 1148–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tijore A, Behr JM, Irvine SA, Baisane V, Venkatraman S. Bioprinted gelatin hydrogel platform promotes smooth muscle cell contractile phenotype maintenance. Biomed Microdevices. 20 (2018) 32. [DOI] [PubMed] [Google Scholar]
- [9].Stowell CET, Wang Y. Quickening: Translational design of resorbable synthetic vascular grafts. Biomaterials.173 (2018) 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Osorio M, Fernandez-Morales P, Ganan P, Zuluaga R, Kerguelen H, Ortiz I, Castro C. Development of novel three-dimensional scaffolds based on bacterial nanocellulose for tissue engineering and regenerative medicine: Effect of processing methods, pore size, and surface area. J Biomed Mater Res A. 107 (2019) 348–359. [DOI] [PubMed] [Google Scholar]
- [11].Wang Z, Cui Y, Wang J, Yang X, Wu Y, Wang K, Gao X, Li D, Li Y, Zheng XL, Zhu Y, Kong D, Zhao Q. The effect of thick fibers and large pores of electrospun poly(epsilon-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials. 35 (2014) 5700–5710. [DOI] [PubMed] [Google Scholar]
- [12].Du F, Wang H, Zhao W, Li D, Kong D, Yang J, Zhang Y. Gradient nanofibrous chitosan/poly ε -caprolactone scaffolds as extracellular microenvironments for vascular tissue engineering. Biomaterials. 33 (2012) 762–770. [DOI] [PubMed] [Google Scholar]
- [13].Zheng W, Wang Z, Song L, Zhao Q, Zhang J, Li D, Wang S, Han J, Zheng X, Yang Z, Kong Deling. Endothelialization and patency of RGD-functionalized vascular grafts in a rabbit carotid artery model. Biomaterials 33 (2012) 2880–2891. [DOI] [PubMed] [Google Scholar]
- [14].Hu J, Sun X, Ma H, Xie C, Chen YE, Ma PX. Porous nanofibrous PLLA scaffolds for vascular tissue engineering. Biomaterials. 31 (2010) 7971–7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].He W, Nieponice A, Soletti L, Hong Y, Gharaibeh B, Crisan M, Usas A, Peault B, Huard J, Wagner WR, Vorp DA. Pericyte-based human tissue engineered vascular grafts. Biomaterials. 31 (2010) 8235–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guan J, Fujimoto KL, Sacks MS, Wagner WR. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials.26 (2005) 3961–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhou Q, Gong Y, Gao C. Microstructure and mechanical properties of poly(L-lactide) scaffolds fabricated by gelatin particle leaching method. J. Appl. Polym. Sci 98 (2005) 1373–1379. [Google Scholar]
- [18].Wright LD, Andric T, Freeman JW. Utilizing NaCl to increase the porosity of electrospun materials. Mater. Sci. Eng. C 31 (2011) 30–36. [Google Scholar]
- [19].Zhao X, Irvine SA, Agrawal A, Cao Y, Lim PQ, Tan SY, Venkatraman SS. 3D patterned substrates for bioartificial blood vessels - The effect of hydrogels on aligned cells on a biomaterial surface. Acta biomaterialia. 26 (2015) 159–168. [DOI] [PubMed] [Google Scholar]
- [20].Sarkar S, Dadhania M, Rourke P, Desai TA, Wong JY. Vascular tissue engineering: microtextured scaffold templates to control organization of vascular smooth muscle cells and extracellular matrix. Acta biomaterialia. 1 (2005) 93–100. [DOI] [PubMed] [Google Scholar]
- [21].Heath DE, Kang GC, Cao Y, Poon YF, Chan V, Chan-Park MB. Biomaterials patterned with discontinuous microwalls for vascular smooth muscle cell culture: biodegradable small diameter vascular grafts and stable cell culture substrates. J Biomater. Sci. Polym. Ed 27 (2016) 1477–1494. [DOI] [PubMed] [Google Scholar]
- [22].Choi JS, Piao Y, Seo TS. Circumferential alignment of vascular smooth muscle cells in a circular microfluidic channel. Biomaterials. 35 (2014) 63–70. [DOI] [PubMed] [Google Scholar]
- [23].Shen JY, Chan-park MB, He B, Zhu AP, Zhu X, Beuerman RW, Yang EB, Chen W, Chan V. Three-dimensional microchannels in biodegradable polymeric films for control orientation and phenotype of vascular smooth muscle cells. Tissue Eng. 12 (2006) 2229–2240. [DOI] [PubMed] [Google Scholar]
- [24].Tarafder S, Koch A, Jun Y, Chou C, Awadallah MR, Lee CH. Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication. 8 (2016) 025003. [DOI] [PubMed] [Google Scholar]
- [25].Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN, Chen CS. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 11 (2012) 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Melchiorri AJ, Hibino N, Best CA, Yi T, Lee YU, Kraynak CA, Kimerer LK, Krieger A, Kim P, Breuer CK, Fisher JP. 3D-printed biodegradable polymeric vascular grafts. Adv. Healthc. Mater 5 (2016) 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, Park SJ, Kotikian A, Nesmith AP, Campbell PH, Vlassak JJ, Lewis JA, Parker KK. Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater 16 (2017) 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li T, Zhai D, Ma B, Xue J, Zhao P, Chang J, Gelinsky M, Wu C. 3D printing of hot dog-like biomaterials with hierarchical architecture and distinct bioactivity. Adv. Sci (2019) 1901146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. U S A 113 (2016) 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Blaeser A, Duarte Campos DF, Puster U, Richtering W, Stevens MM, Fischer H. Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater 5 (2016) 326–333. [DOI] [PubMed] [Google Scholar]
- [31].Dong X, Yuan X, Wang L, Liu J, Midgley AC, Wang Z, Wang K, Liu J, Zhu M, Kong D. Construction of a bilayered vascular graft with smooth internal surface for improved hemocompatibility and endothelial cell monolayer formation. Biomaterials 181 (2018) 1–14. [DOI] [PubMed] [Google Scholar]
- [32].Zoumi A, Lu X, Kassab GS, Tromberg BJ. Imaging coronary artery microstructure using second-harmonic and two-photon fluorescence microscopy. Biophys. J 87(2004) 2778–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jungst T, Pennings I, Schmitz M, Rosenberg AJWP, Groll J, Gawlitta D. Heterotypic Scaffold Design Orchestrates Primary Cell Organization and Phenotypes in Cocultured Small Diameter Vascular Grafts. Adv. Funct. Mater (2019) 1905987. [Google Scholar]
- [34].Wade RJ, Burdick JA. Engineering ECM signals into biomaterials. Mater. Today 15 (2012) 454–459. [Google Scholar]
- [35].Akentjew TL, Terraza C, Suazo C, Maksimcuka J, Wilkens CA, Vargas F, Zavala G, Ocana M, Enrione J, Garcia-Herrera CM, Valenzuela LM, Blaker JJ, Khoury M, Acevedo JP. Rapid fabrication of reinforced and cell-laden vascular grafts structurally inspired by human coronary arteries. Nat. Commun 10 (2019) 3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhu M, Wu Y, Li W, Dong X, Chang H, Wang K, Wu P, Zhang J, Fan G, Wang L, Liu J, Wang H, Kong D. Biodegradable and elastomeric vascular grafts enable vascular remodeling. Biomaterials. 183 (2018) 306–318. [DOI] [PubMed] [Google Scholar]
- [37].Zhu M, Wang Z, Zhang J, Wang L, Yang X, Chen J, Fan G, Ji S, Xing C, Wang K, Zhao Q, Zhu Y, Kong D, Wang L. Circumferentially aligned fibers guided functional neoartery regeneration in vivo. Biomaterials. 61 (2015) 85–94. [DOI] [PubMed] [Google Scholar]
- [38].Li W, Wu P, Zhang Y, Midgley AC, Yuan X, Wu Y, Wang L, Wang Z, Zhu M, Kong D. Bilayered polymeric micro- and nanofiber vascular grafts as abdominal aorta replacements: long-term in vivo studies in a rat model. ACS Applied Bio Materials. 2 (2019) 4493–4502. [DOI] [PubMed] [Google Scholar]
- [39].de Valence S, Tille JC, Mugnai D, Mrowczynski W, Gurny R, Moller M, Walpoth BH. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials. 33 (2012) 38–47. [DOI] [PubMed] [Google Scholar]
- [40].Cao Y, Poon YF, Feng J, Rayatpisheh S, Chan V, Chan-Park MB. Regulating orientation and phenotype of primary vascular smooth muscle cells by biodegradable films patterned with arrays of microchannels and discontinuous microwalls. Biomaterials. 31 (2010) 6228–6238. [DOI] [PubMed] [Google Scholar]
- [41].Williams C, Brown XQ. Bartolak-suki E, Ma H, Chikkoti A, Wong JY. The use of micropatterning to control smooth muscle myosin heavy chain expression and limit the response to transforming growth factor β1 in vascular smooth muscle cells. Biomaterials.32 (2011) 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Smith TD, Nagalla RR, Chen EY, Liu WF. Harnessing macrophage plasticity for tissue regeneration. Adv. Drug Deliver. Rev 114 (2017) 193–205. [DOI] [PubMed] [Google Scholar]
- [43].Rouet V, Hamma-Kourbali Y, Petit E, Panagopoulou P, Katsoris P, Barritault D, Caruelle JP, Courty J. A synthetic glycosaminoglycan mimetic binds vascular endothelial growth factor and modulates angiogenesis. J Biol Chem. 280 (2005) 32792–32800. [DOI] [PubMed] [Google Scholar]
- [44].Mattson JM, Wang Y, Zhang Y. Contributions of glycosaminoglycans to collagen fiber recruitment in constitutive modeling of arterial mechanics. J Biomech. 82 (2019) 211–219. [DOI] [PubMed] [Google Scholar]
- [45].Bingley JA, Hayward IP, Campbell GR, Campbell JH. Relationship of glycosaminoglycan and matrix changes to vascular smooth muscle cell phenotype modulation in rabbit arteries after acute injury. J. Vasc. Surg 33 (2001) 155–164. [DOI] [PubMed] [Google Scholar]
- [46].Xu C. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 25 (2004) 877–886. [DOI] [PubMed] [Google Scholar]
- [47].Mukhatyar VJ, Salmeron-Sanchez M, Rudra S, Mukhopadaya S, Barker TH, Garcia AJ, Bellamkonda RV. Role of fibronectin in topographical guidance of neurite extension on electrospun fibers. Biomaterials.32 (2011) 3958–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Huang AH, Balestrini JL, Udelsman BV, Zhou KC, Zhao L, Ferruzzi J, Starcher BC, Levene MJ, Humphrey JD, Niklason LE. Biaxial stretch improves elastic fiber maturation, collagen arrangement, and mechanical properties in engineered arteries. Tissue Eng. Part C-Me 22 (2016) 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Guillemette MD, Cui B, Roy E, Gauvin R, Giasson CJ, Esch MB, Carrier P, Deschambeault A, Dumoulin M, Toner M, Germain L, Veres T, Auger FA. Surface topography induces 3D self-orientation of cells and extracellular matrix resulting in improved tissue function. Integr. Biol 1 (2009) 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wu HC, Wang TW, Kang PL, Tsuang YH, Sun JS, Lin FH. Coculture of endothelial and smooth muscle cells on a collagen membrane in the development of a small-diameter vascular graft. Biomaterials. 28 (2007) 1385–1392. [DOI] [PubMed] [Google Scholar]
- [51].Wallace CS, Champion JC, Truskey GA. Adhesion and function of human endothelial cells co-cultured on smooth muscle cells. Ann. Biomed. Eng 35 (2007) 375–386. [DOI] [PubMed] [Google Scholar]
- [52].Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 37 (2015) 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 35 (2014) 4477–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Li X, Cho B, Martin R, Seu M, Zhang C, Zhou Z, Choi JS, Jiang X, Chen L, Walia G, Yan J, Callanan M, Liu H, Colbert K, Morrissette-McAlmon J, Grayson W, Reddy S, Sacks JM, Mao H-Q. Nanofiber-hydrogel composite–mediated angiogenesis for soft tissue reconstruction. Sci. Transl. Med eaau6210 (2019) 1–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


