Abstract
Background/Objective:
Less muscle mass has been associated with greater insulin resistance, but whether the association is independent of deleterious adipose depots in young adults with overweight/obesity who are at high risk for type 2 diabetes (T2DM) but are otherwise metabolically healthy is not known. The objective of this study was to determine whether muscle mass is independently associated with insulin sensitivity (IS) in young adults with overweight/obesity.
Subjects/Methods:
Cross-sectional Clinical Research Center study of 132 adults, 21–45yo, BMI ≥25 kg/m2 and metabolically healthy without T2DM. Primary independent variable: percent ideal appendicular lean mass (ALM) calculated as measured ALM divided by predicted ALM for age, weight, and height, calculated using validated NHANES data-based equation. Primary dependent variable: IS by Matsuda index.
Results:
Mean age was 34.3±6.8 years, and mean BMI 35.8±5.8 kg/m2 (mean±SD). Individuals in the highest % ideal ALM tertile had mean IS 45% higher than the lowest tertile [6.94±0.85 vs 4.80±0.56 (mean±SEM), p=0.008](sex interaction p=0.003). Men in the highest % ideal ALM tertile had mean IS twice the lowest tertile (5.47±0.68 vs 2.68±0.34, p=0.001), which remained significant controlling for visceral/subcutaneous and intermuscular adipose tissue, and intramyocellular and intrahepatic lipids (p=0.03). The association was not significant in women.
Conclusions:
Muscle mass is associated with IS independent of detrimental adipose depots in young men with overweight/obesity, at risk for T2DM but currently metabolically healthy. Muscle mass relative to sex, age, weight, and height-specific norms may be used to ascertain individual T2DM risk associated with low muscle mass.
Keywords: muscle, insulin sensitivity, obesity
Introduction
The worldwide increase in the incidence of type 2 diabetes (T2DM) has largely been attributed to the obesity epidemic. However, T2DM prevalence varies considerably among individuals of similar BMI, which is partially attributable to differences in detrimental adipose depots. For example, visceral adipose tissue (VAT) is more strongly associated with cardiometabolic risk than subcutaneous adipose tissue (SAT) (1, 2), which actually may be relatively protective (3). Intrahepatic lipids (IHL) are another known T2DM risk factor (4). Differences in skeletal muscle may also contribute to T2DM risk independent of adiposity. Preclinical and clinical models suggest that impaired insulin-stimulated glucose transport and glycogen synthesis in skeletal muscle contribute to insulin resistance (IR) and T2DM (5, 6). Less skeletal muscle mass (7–11), higher intermuscular adipose tissue (IMAT) (12–14), and higher intramyocellular lipids (IMCL) (15–18) have been associated with insulin resistance in adults of various ages, weights, and co-morbidities.
However, the association between muscle mass itself and insulin sensitivity (IS), independent of VAT/SAT, IMAT, IMCL, and IHL, in young adults with overweight/obesity who are otherwise metabolically healthy (without T2DM, hypertension, or hyperlipidemia) has not been studied. This is an important population to study because its lifetime risk of T2DM is high (19), age-related changes in skeletal muscle are yet to occur, and traditional methods to reduce T2DM risk such as weight loss are difficult to implement and maintain. In addition, no standard criterion exists on how best to define relative muscle mass given its dependence on age, sex, race, weight, and height. If greater muscle mass is independently associated with greater IS in this population without T2DM, muscle may be a modifiable T2DM risk factor.
We hypothesized that relatively more skeletal muscle mass is associated with greater IS independent of VAT/SAT, IMAT, IMCL, and IHL in young otherwise healthy men and women with overweight/obesity. We also explored whether there were sex and race differences. An association between % ideal ALM and IS has not been previously reported.
Methods
This study was Partners Human Research Committee institutional review board-approved and Health Insurance Portability and Accountability Act-compliant. Data were acquired after written informed consent was obtained from all subjects prior to the study.
The cross-sectional study was performed on a Clinical Research Center. The study group was comprised of 132 consecutive subjects (60 men, 72 women) with overweight or obesity (BMI ≥25 kg/m2) who were recruited over 8 years through advertisements seeking participation in an NIH-funded study. In order to be included in this substudy, participants had to be age 18 to 45 years and metabolically healthy as defined in previous studies (19), including no self-reported hypertension or hypercholesterolemia, no use of anti-hypertensive or lipid-lowering medications, and no T2DM. T2DM was defined as a fasting plasma glucose ≥126 mg/dL, a 2-hour plasma glucose ≥200 mg/dL on 75g oral glucose tolerance test (OGTT), self-reported T2DM, or use of hypoglycemics, including insulin. Additional exclusion criteria included cardiac disease or other chronic illness; amenorrhea in premenopausal women; smoking; estrogen, growth hormone or glucocorticoid use; and routine MRI contraindications.
All studies were performed after an 8-hour overnight fast. Participants underwent the following tests for body composition assessment: dual-energy x-ray absorptiometry (DXA) for measurement of ALM, computed tomography (CT) scan for assessment of VAT and SAT, IMAT and thigh muscle cross sectional areas, and proton magnetic resonance spectroscopy (1H-MRS) for quantification of IMCL and IHL. All subjects underwent a 75 g, 2-hour OGTT. The Matsuda Index, a measure of IS, was calculated from the OGTT (20). Although a standard cut-off for IS using the Matsuda index has not been defined, the 25th percentile in a Caucasian population was reported as 5.0 (21), and was therefore used as a cut-off in this study. Impaired fasting glucose was defined as a fasting plasma glucose between 100 and 125 mg/dL, and impaired glucose tolerance was defined as a two-hour plasma glucose during a 75 g OGTT between 140 and 199 mg/dL. Exercise was assessed using the Paffenbarger Questionnaire, and hours of vigorous activity (≥6 METs) a week was recorded (22).
Clinical characteristics, VAT, SAT, IMCL, and IHL from subjects in this cohort have been published in other reports (11, 17, 18, 23–27). However, % ideal ALM has not been calculated or reported.
Appendicular lean mass (ALM)
Since appendicular lean mass (ALM) is known to vary by sex, age, height, and weight, we used a measure of ALM relative to ideal ALM based on each subject’s sex, age, height, and weight (ALM/ideal ALM), calculated using a validated National Health and Nutrition Examination Survey (NHANES) equation, as follows. All subjects underwent DXA (Discovery A; Hologic Inc., Bedford, MA, USA) for measurement of ALM. Coefficient of variation of DXA in our laboratory is 2.4% for lean mass. After obtaining each subject’s measured ALM, each subject’s ideal ALM for sex, age, height, and weight was calculated using an anthropometric prediction equation for ALM. This prediction equation was developed and validated in 1999–2004 NHANES (28), and was chosen because it is well-established that ALM is dependent on sex, age, height and weight (29). Each subject’s % ideal ALM was then calculated as the ratio of measured ALM divided by ideal ALM multiplied by 100.
Computed Tomography (CT)
Subjects underwent single slice CT (LightSpeed Pro, GE Healthcare, Waukesha, WI, USA) of the abdomen through the mid-portion of the L4 level and the left mid-thigh (Supplemental Figure). Scan parameters were standardized: 144 mm table height, 80 kV and 70 mA for the abdomen, 120 kV and 170 mA for the thigh, scanning time of 2 seconds, 1 cm section thickness, and 48 cm field of view. Abdominal and thigh adipose tissue was identified using a threshold set for −50 to −250 Hounsfield units (30). Manual delineation was used to separate SAT, VAT, IMAT and muscle and cross-sectional areas (cm2) were obtained. The relative distribution of body fat in the abdomen was assessed using VAT/SAT ratio. Analyses were performed using Osirix software version 3.2.1 (www.osirix-viewer.com/index.html). The coefficient of variation of CT in our laboratory is 2.5% for VAT cross sectional area.
Proton MR Spectroscopy (1H-MRS)
Subjects underwent 1H-MRS of the soleus muscle to determine IMCL and of the liver to determine IHL after an overnight fast using a 3.0-T MR imaging system (Siemens Trio, Siemens Medical Systems, Erlangen, Germany) as previously described (11). Subjects were asked to avoid moderate or vigorous exercise or a high-fat diet 72 h prior to scanning. CV for measurements at our institution are 6% for IMCL and 8% for IHL quantification. Fitting of all 1H-MRS data was performed using LCModel (version 6.3–0K; Stephen Provencher, Oakville, Ontario, Canada) (31). For soleus muscle, IMCL (1.3 ppm) and extramyocellular lipids (1.5 ppm) methylene estimates were automatically scaled to unsuppressed water peak (4.7 ppm) and expressed as lipid-to-water ratio. Fitting algorithms specific for liver lipid estimates (0.9, 1.3, and 2.0 ppm) were scaled to unsuppressed water peak (4.7 ppm) and expressed as lipid-to-water ratio. A total of 82/132 subjects had IMCL data, and 71/132 subjects had IHL data.
Biochemical Analysis
Serum samples were run in real time or stored at −80°C. Serum glucose levels were run in real time using commercially available standardized assays. Serum insulin levels were measured using a radioimmunoassay (Linco, Research, Inc., St. Charles, MO, USA).
Statistical Analysis
JMP Statistical Database Software (version 12; SAS Institute, Cary, NC) was used for statistical analyses. Variables were assessed for normality using the Shapiro-Wilk test, and if non-normal or a small N (analyses within adults), variables were log-transformed. Percent ideal ALM was divided into tertiles, and t-testing was used to compare IS between the highest and lowest tertiles. Linear regression analyses with Pearson’s correlation coefficients were used to investigate associations between % ideal ALM and IS. Multivariate standard least squares and stepwise regression analyses were constructed to control for VAT/SAT, IMAT, IMCL, and IHL. An interaction term (sex*muscle) was included in all models given known sex differences in muscle, e.g. healthy men have more muscle mass than healthy women (29, 32). If the interaction term was significant, sex-stratified analyses were reported. Statistical significance was defined as a 2-tailed p≤0.05. Data are reported as mean ± SD, or n (%), unless otherwise noted. With an N=60, the probability was 80% that the study would detect a relationship between the dependent and independent variable at a 2-sided 0.05 significance level of 0.05 if the true difference in the dependent variable was 0.368 SD per 1 SD change in the dependent variable.
Results
Clinical characteristics
Clinical characteristics are shown in Table 1. The cohort was 56% female and 44% male, 77% White (n=101), 18% Black (n=24), 2% Asian (n=3), and 3% other or unknown (n=4). Mean age was 34.3 ± 6.8 years and mean BMI was 35.8 ± 5.8 kg/m2 (range 25.1–53.7 kg/m2). Mean % ideal ALM was 102.2 ± 9.5%. Mean Matsuda index was 5.7 ± 4.4, with 60% of subjects having a Matsuda index ≤5.0. Three percent of subjects had impaired fasting glucose, and 17% of subjects had impaired glucose tolerance.
Table 1.
Clinical characteristics of 132 adults with overweight/obesity
| mean ± SD | |
|---|---|
| Clinical characteristics | |
| Female (%) | 56% |
| Caucasian (%) | 77% |
| Age (y) | 34.3 ± 6.8 |
| Body mass index (BMI) (kg/m2) | 35.8 ± 5.8 |
| Physical activity (hrs/week) | 4.6 ± 5.2 |
| Dual-energy x-ray absorptiometry | |
| Appendicular lean mass (ALM) (kg) | 29.1 ± 7.4 |
| % ideal appendicular lean mass (%) | 102.2 ± 9.5 |
| Computed tomography | |
| Visceral adipose tissue (cm2) (VAT) | 130.4 ± 62.7 |
| Abdominal subcutaneous tissue (cm2) (SAT) | 461.6 ± 138.7 |
| VAT/SAT | 0.29 ± 0.16 |
| Thigh muscle area (cm2) | 5.2 ± 0.2 |
| Thigh muscle density (HU) | 48.3 ± 4.9 |
| Thigh intermuscular adipose tissue (IMAT) (cm2) | 8.8 ± 3.8 |
| Magnetic resonance spectroscopy | |
| Soleus intramyocellular lipids (lipid/water) | 0.04 ± 0.02 |
| Intrahepatic lipids (lipid/water) | 0.15 ± 0.24 |
| Glucose homeostasis | |
| Matsuda Index | 5.7 ± 4.4 |
| Impaired fasting glucose (%)* | 3% |
| Impaired glucose tolerance (%)** | 17% |
Impaired fasting glucose defined as fasting plasma glucose between 100 and 125 mg/dL
Impaired glucose tolerance defined as two-hour plasma glucose during a 75g oral glucose tolerance test between 140 and 199 mg/dL
Association of skeletal muscle mass with insulin sensitivity
Individuals in the highest tertile of % ideal ALM had mean IS 45% higher than the lowest tertile [6.94±0.85 vs 4.80±0.56 (mean ± SEM), p=0.008] (Figure 1a), which was not significant after controlling for VAT/SAT, IMAT, IMCL, and IHL (p=0.36). There was a sex interaction (p interaction=0.003), which remained significant after controlling for VAT/SAT, IMAT, IMCL, and IHL (p interaction=0.008), indicating a greater effect in men than women. Men in the highest tertile of % ideal ALM had mean IS twice as high as the lowest tertile [5.47±0.68 vs 2.68±0.34 (mean ± SEM), p=0.001], which remained significant after controlling for VAT/SAT, IMAT, IMCL, and IHL (p=0.03) (Figure 1a). The association was not significant in women (p=0.83) (Figure 1a).
Figure 1.
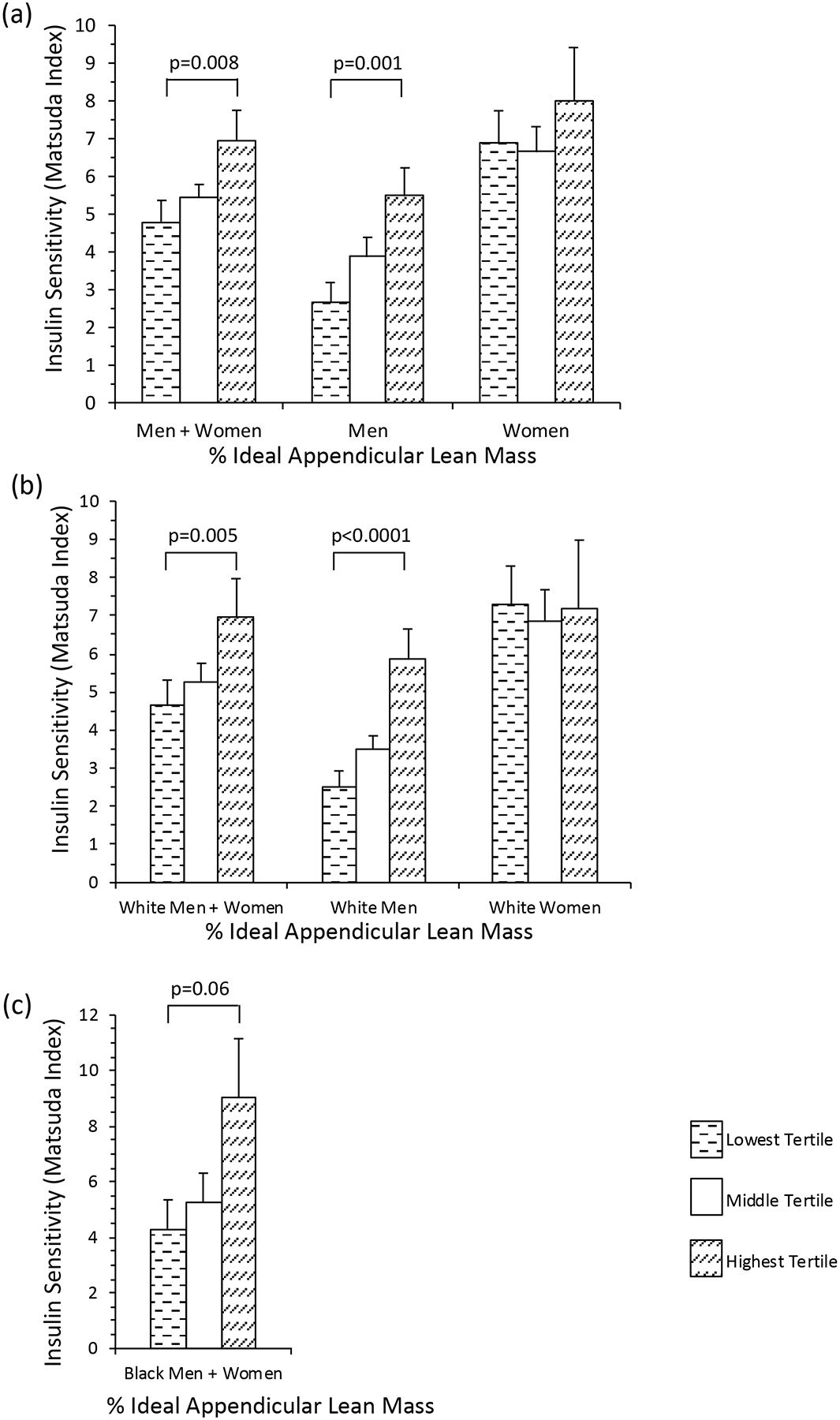
(a) Individuals with the highest tertile of % ideal appendicular lean mass (ALM) had mean insulin sensitivity (IS) 45% higher than the lowest tertile (p=0.008). There was a sex interaction (p interaction=0.003). Men in the highest tertile of % ideal ALM had mean IS twice as high as the lowest tertile (p=0.001). (b) White adults in the highest tertile of % ideal ALM had mean IS 50% higher than the lowest tertile (p=0.005). There was a sex interaction (p interaction=0.007). Men in the highest tertile of % ideal ALM had mean IS more than twice as high as the lowest tertile (p<0.0001). (c) Black adults in the highest tertile of % ideal ALM trended towards having higher mean IS than the lowest tertile (p=0.06). There was no sex interaction (p interaction=0.18). Data reported as mean ± SEM.
In the whole cohort, percent ideal ALM was positively associated with IS (R=0.29, p=0.0008) (Table 2), which was not significant after controlling for VAT/SAT, IMAT, IMCL, and IHL (p=0.26). There was a sex interaction (p interaction=0.01), which remained significant after controlling for VAT/SAT, IMAT, IMCL, and IHL (p interaction=0.006), indicating a greater effect in men than women. In men, % ideal ALM was positively associated with IS (R=0.41, p=0.001), which remained significant after controlling for VAT/SAT, IMAT, IMCL, and IHL (B1= 0.32, p=0.008) (Table 2). There was no significant association in women (p=0.99) (Table 2).
Table 2.
Association between % ideal appendicular lean mass and Matsuda index
| Unadjusted model | Adjusted model* | |||
|---|---|---|---|---|
| R | p | B1 | p | |
| Whole cohort | ||||
| Men + Women | 0.29 | 0.0008 | 0.26 | |
| Men | 0.41 | 0.001 | 0.32 | 0.008 |
| Women | 0.99 | 0.29 | ||
| White adults | ||||
| Men + Women | 0.31 | 0.002 | 0.5 | |
| Men | 0.46 | 0.001 | 0.42 | 0.008 |
| Women | 0.48 | −0.35 | 0.03 | |
| Black adults | ||||
| Men + Women | 0.34 | 0.099 | 0.36 | |
Adjusted for visceral/subcutaneous adipose tissue, intermuscular adipose tissue, intramyocellular lipids, and intrahepatic lipids
Skeletal muscle mass as an independent determinant of insulin sensitivity
In the whole cohort, IHL explained 49% of the variance in IS in a stepwise regression model including % ideal ALM, VAT/SAT, IMAT, IMCL, and IHL. In men, IHL explained 38%, and % ideal ALM explained 17%, of the variance in IS. In women, IHL explained 48% of the variance in IS; % ideal ALM was not a significant determinant of IS.
Association of skeletal muscle mass with insulin sensitivity in White adults
White adults in the highest tertile of % ideal ALM had mean IS 50% higher than the lowest tertile [6.96±1.01 vs 4.63±0.68 (mean ± SEM), p=0.005] (Figure 1b), which was not significant after controlling for VAT/SAT, IMAT, IMCL, and IHL (p=0.46). There was a sex interaction (p interaction=0.007), indicating a greater effect in men than women. Men in the highest tertile of % ideal ALM had mean IS more than twice as high as the lowest tertile [5.88±0.77 vs 2.52± 0.41 (mean ± SEM), p<0.0001], which remained significant after controlling for VAT/SAT, IMAT, IMCL, and IHL (p=0.002) (Figure 1b). The association was not significant in women (p=0.50) (Figure 1b).
In white adults, percent ideal ALM was positively associated with IS (R=0.31, p=0.002) (Table 2). There was a sex interaction (p interaction=0.007), indicating a greater effect in men than women. In men, % ideal ALM was positively associated with IS (R=0.46, p=0.001), which remained significant after controlling for VAT/SAT, IMAT, IMCL, and IHL (B1= 0.42, p=0.008) (Table 2). The association was not significant in women (p=0.48) (Table 2).
Association of skeletal muscle mass with insulin sensitivity in Black adults
Black adults in the highest tertile of % ideal ALM (N=8) trended towards having higher mean IS than the lowest tertile (N=8) [1.96±0.28 vs 1.23±0.27 (mean ± SEM), p=0.06] (Figure 1c), which was not significant after controlling for VAT/SAT, IMAT, IMCL, and IHL (p=0.17). There was no sex interaction (p interaction=0.18). Percent ideal ALM trended towards being positively associated with IS (R=0.34, p=0.099) (Table 2). There was no sex interaction (p interaction=0.43).
Discussion
Our data suggest that greater muscle mass is associated with greater insulin sensitivity in young adults, and the association is independent of detrimental adipose depots in young men, with overweight/obesity at risk for T2DM but who are currently in good metabolic health. Previous studies have demonstrated that lower muscle mass (7–11), higher IMAT (12–14), and higher IMCL content (15–18) are associated with greater IR in adults of various ages, weights, and co-morbidities. Although previous studies have reported that greater muscle mass may be relatively protective against T2DM, the association between muscle mass and IS independent of VAT/SAT, IMAT, IMCL, and IHL in young men and women with overweight/obesity who are otherwise metabolically healthy (without T2DM, hypertension, or hyperlipidemia) has not been reported. We demonstrate that in men, greater muscle mass is associated with greater IS independent of detrimental adipose depots that are thought to contribute to T2DM risk in obesity.
Muscle mass relative to sex, age, weight, and height-specific norms may be used to ascertain individual T2DM risk associated with low muscle mass and may be a modifiable T2DM risk factor. Muscle mass is greater in men than women, declines beginning at the end of the fifth decade of life, is linearly associated with height, and is nonlinearly associated with weight (i.e. muscle mass does not increase as much at higher weights) (29). Previous studies that investigated the association between relative muscle mass, defined as ALM/weight (8, 9) or ALM/BMI (7), and IS did not consistently control for sex, age, weight and height, which could have confounded the relationship between muscle mass and IS. Our findings are consistent with a previous study in older Native American adults that found that lower % ideal fat-free mass (defined as fat-free mass measured by bioelectrical impedance divided by predicted fat free mass) was associated with higher IR (33). Although large studies have demonstrated the effectiveness of lifestyle interventions and weight loss in the prevention of diabetes (34), the American Diabetes Association current clinical practice guidelines on the prevention of T2DM does not explicitly include lifestyle recommendations on maintaining or increasing muscle mass, although it does suggest that exercise regimens may include resistance training (35). Our data suggest that weight loss may be less effective in the prevention of T2DM if it results in the loss of both fat and muscle mass. Two studies in Korean adults reported that less muscle mass was associated with an increased risk for incident T2DM (36, 37), although other longitudinal studies investigating the association between muscle mass and incident T2DM have yielded inconsistent results (38, 39). This is an area that warrants further investigation.
The possible sex differences observed also warrant further study. In addition to having more muscle mass than women (29), men are diagnosed with T2DM at a lower mean age and BMI (40). Although some studies have reported relatively lower muscle mass is associated with IR or T2DM in men, but not women (9, 11, 41), no study had reported an interaction between sex and muscle in its association with IS, independent of adipose depots, in young adults with overweight/obesity but without T2DM, hypertension, or hyperlipidemia. Men in our cohort had a wider range of ALM than women (25.1 – 50.8 kg vs 16.7–31.6 kg) and a lower mean Matsuda index (4.0 ± 2.6 vs 7.2 ± 5.0), which may have enabled us to see significant associations in men, but not women. Therefore, we cannot rule out the possibility that we might have observed significant associations in women had we studied a larger cohort with a wider range of ALM and IS. It is also plausible that muscle may have evolved as a protective mechanism against T2DM in men, but not women, because women have more gluteofemoral fat, which may be relatively protective. If sex differences are replicated in future studies, they suggest that muscle mass may be a more modifiable T2DM risk in young men than women with overweight/obesity.
In a sub-analysis in which we stratified the cohort by race, we demonstrated that greater muscle mass independent of adipose depots is associated with greater insulin sensitivity in young White men. We cannot rule out the possibility that we might have observed significant associations in Black adults had we studied a larger cohort. Although racial differences in obesity and non-biological factors contribute to the higher incidence and prevalence of T2DM in Blacks vs Whites in the U.S. (42), differences in body composition may also play a causative role. Paradoxically, despite having greater muscle mass and lower VAT at a similar BMI, Blacks have higher insulin resistance than Whites (43). No study to date has investigated racial differences in the association between muscle mass and insulin sensitivity independent of adipose depots in young adults with overweight/obesity; our findings warrant further study.
The strengths of this study include its use of sophisticated imaging methods to characterize muscle and its strict criteria to select young adults with overweight/obesity who are otherwise metabolically healthy. Should sex, age, height and weight norms for appendicular skeletal muscle measurements by CT or MRI become available, studies investigating the relationship between measures of muscle mass and muscle characteristics with insulin sensitivity using these imaging modalities would be warranted. Exclusion of adults with diagnosed hypertension or taking lipid-lowering drugs also removed potential confounding as both hypertension (44) and statin use (45) have been associated with incident T2DM. A limitation of our study is its cross-sectional nature, such that the directionality of the relationship between muscle and IS cannot be determined. However, excluding adults with T2DM enabled us to minimize confounding by reverse causation, i.e. T2DM leading to loss of muscle mass. A second limitation is that we did not perform hyperinsulinemic euglycemic clamps, the gold standard for measuring skeletal muscle IS. However, IS as estimated by the Matsuda index correlates well with the euglycemic clamp (20). Finally, we cannot rule out a type 2 error as the explanation for the negative results in women. It is possible that an association between ALM and IS would have been detected in a larger cohort. Further studies are warranted to determine whether an association is present in women.
In conclusion, our data suggest that more muscle mass is associated with greater insulin sensitivity in young adults, and the association is independent of detrimental adipose depots in young men, with overweight/obesity at risk for T2DM, but currently in good metabolic health. Percent ideal ALM may be used to standardize muscle mass to sex, age, weight, and height-specific norms and to ascertain individual T2DM risk associated with low muscle mass. Given known sex differences in muscle mass and T2DM risk, the sex differences in our findings warrant further investigation in order to determine whether muscle may be a modifiable T2DM risk factor.
Supplementary Material
Acknowledgments
Sources of support: K23 DK113220; K23 DK115903; R01 HL-077674; K24 HL092902-03; K24 DK-109940; K23 RR-23090; P30 DK040561, NIH Nutrition and Obesity Research Center at Harvard; Women’s Wellness Foundation; 8 UL1 TR000170, Harvard Clinical and Translational Science Center, from the National Center for Advancing Translational Sciences; 1 UL1 RR025758, Harvard Clinical and Translational Science Center, from the National Center for Research Resources; and M01-RR-01066, from the National Center for Research Resources.
Footnotes
Competing Interests: The authors declare no competing financial interests.
References
- 1.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95(12):5419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48(2):301–8. [DOI] [PubMed] [Google Scholar]
- 4.Byrne CD, Targher G. Ectopic fat, insulin resistance, and nonalcoholic fatty liver disease: implications for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34(6):1155–61. [DOI] [PubMed] [Google Scholar]
- 5.Kim JK, Zisman A, Fillmore JJ, Peroni OD, Kotani K, Perret P, et al. Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4. J Clin Invest. 2001;108(1):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z, et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med. 1999;341(4):240–6. [DOI] [PubMed] [Google Scholar]
- 7.Batsis JA, Mackenzie TA, Jones JD, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and inflammation: Results from the 1999–2004 National Health and Nutrition Examination Survey. Clin Nutr. 2016;35(6):1472–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96(9):2898–903. [DOI] [PubMed] [Google Scholar]
- 9.Bower JK, Meadows RJ, Foster MC, Foraker RE, Shoben AB. The Association of Percent Body Fat and Lean Mass With HbA1c in US Adults. J Endocr Soc. 2017;1(6):600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalyani RR, Metter EJ, Ramachandran R, Chia CW, Saudek CD, Ferrucci L. Glucose and insulin measurements from the oral glucose tolerance test and relationship to muscle mass. J Gerontol A Biol Sci Med Sci. 2012;67(1):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schorr M, Dichtel LE, Gerweck AV, Valera RD, Torriani M, Miller KK, et al. Sex differences in body composition and association with cardiometabolic risk. Biol Sex Differ. 2018;9(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boettcher M, Machann J, Stefan N, Thamer C, Haring HU, Claussen CD, et al. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. J Magn Reson Imaging. 2009;29(6):1340–5. [DOI] [PubMed] [Google Scholar]
- 13.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71(4):885–92. [DOI] [PubMed] [Google Scholar]
- 14.Miljkovic-Gacic I, Gordon CL, Goodpaster BH, Bunker CH, Patrick AL, Kuller LH, et al. Adipose tissue infiltration in skeletal muscle: age patterns and association with diabetes among men of African ancestry. Am J Clin Nutr. 2008;87(6):1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42(1):113–6. [DOI] [PubMed] [Google Scholar]
- 16.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48(8):1600–6. [DOI] [PubMed] [Google Scholar]
- 17.Bredella MA, Torriani M, Thomas BJ, Ghomi RH, Brick DJ, Gerweck AV, et al. Peak growth hormone-releasing hormone-arginine-stimulated growth hormone is inversely associated with intramyocellular and intrahepatic lipid content in premenopausal women with obesity. J Clin Endocrinol Metab. 2009;94(10):3995–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bredella MA, Ghomi RH, Thomas BJ, Miller KK, Torriani M. Comparison of 3.0 T proton magnetic resonance spectroscopy short and long echo-time measures of intramyocellular lipids in obese and normal-weight women. J Magn Reson Imaging. 2010;32(2):388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckel N, Li Y, Kuxhaus O, Stefan N, Hu FB, Schulze MB. Transition from metabolic healthy to unhealthy phenotypes and association with cardiovascular disease risk across BMI categories in 90 257 women (the Nurses’ Health Study): 30 year follow-up from a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(9):714–24. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. [DOI] [PubMed] [Google Scholar]
- 21.Radikova Z, Koska J, Huckova M, Ksinantova L, Imrich R, Vigas M, et al. Insulin sensitivity indices: a proposal of cut-off points for simple identification of insulin-resistant subjects. Exp Clin Endocrinol Diabetes. 2006;114(5):249–56. [DOI] [PubMed] [Google Scholar]
- 22.Paffenbarger RS Jr., Blair SN, Lee IM, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25(1):60–70. [DOI] [PubMed] [Google Scholar]
- 23.Bredella MA, Ghomi RH, Thomas BJ, Ouellette HA, Sahani DV, Miller KK, et al. Breath-hold 1H-magnetic resonance spectroscopy for intrahepatic lipid quantification at 3 Tesla. J Comput Assist Tomogr. 2010;34(3):372–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bredella MA, Gill CM, Gerweck AV, Landa MG, Kumar V, Daley SM, et al. Ectopic and serum lipid levels are positively associated with bone marrow fat in obesity. Radiology. 2013;269(2):534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bredella MA, Lin E, Brick DJ, Gerweck AV, Harrington LM, Torriani M, et al. Effects of GH in women with abdominal adiposity: a 6-month randomized, double-blind, placebo-controlled trial. Eur J Endocrinol. 2012;166(4):601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, et al. Adiponectin is inversely associated with intramyocellular and intrahepatic lipids in obese premenopausal women. Obesity (Silver Spring). 2011;19(5):911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bredella MA, Gerweck AV, Lin E, Landa MG, Torriani M, Schoenfeld DA, et al. Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J Clin Endocrinol Metab. 2013;98(9):3864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian S, Mioche L, Denis JB, Morio B. A multivariate model for predicting segmental body composition. Br J Nutr. 2013;110(12):2260–70. [DOI] [PubMed] [Google Scholar]
- 29.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol (1985). 2000;89(1):81–8. [DOI] [PubMed] [Google Scholar]
- 30.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36(1):172–7. [DOI] [PubMed] [Google Scholar]
- 31.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–9. [DOI] [PubMed] [Google Scholar]
- 32.Machann J, Haring H, Schick F, Stumvoll M. Intramyocellular lipids and insulin resistance. Diabetes Obes Metab. 2004;6(4):239–48. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara LA, Capaldo B, Mancusi C, Lee ET, Howard BV, Devereux RB, et al. Cardiometabolic risk in overweight subjects with or without relative fat-free mass deficiency: the Strong Heart Study. Nutr Metab Cardiovasc Dis. 2014;24(3):271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Diabetes A. 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S29–S33. [DOI] [PubMed] [Google Scholar]
- 36.Son JW, Lee SS, Kim SR, Yoo SJ, Cha BY, Son HY, et al. Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: findings from the KoGES. Diabetologia. 2017;60(5):865–72. [DOI] [PubMed] [Google Scholar]
- 37.Kim HK, Lee MJ, Kim EH, Bae SJ, Choe J, Kim CH, et al. Longitudinal Changes of Body Composition Phenotypes and Their Association with Incident Type 2 Diabetes Mellitus during a 5-Year Follow-up in Koreans. Diabetes Metab J. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsen BA, Wassel CL, Kritchevsky SB, Strotmeyer ES, Criqui MH, Kanaya AM, et al. Association of Muscle Mass, Area, and Strength With Incident Diabetes in Older Adults: The Health ABC Study. J Clin Endocrinol Metab. 2016;101(4):1847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li JJ, Wittert GA, Vincent A, Atlantis E, Shi Z, Appleton SL, et al. Muscle grip strength predicts incident type 2 diabetes: Population-based cohort study. Metabolism. 2016;65(6):883–92. [DOI] [PubMed] [Google Scholar]
- 40.Logue J, Walker JJ, Colhoun HM, Leese GP, Lindsay RS, McKnight JA, et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. 2011;54(12):3003–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong VW, Bancks MP, Schreiner PJ, Lewis CE, Steffen LM, Meigs JB, et al. Insulin resistance since early adulthood and appendicular lean mass in middle-aged adults without diabetes: 20years of the CARDIA study. J Diabetes Complications. 2019;33(1):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golden SH, Brown A, Cauley JA, Chin MH, Gary-Webb TL, Kim C, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors--an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012;97(9):E1579–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knowles JW, Reaven G. Usual Blood Pressure and New-Onset Diabetes Risk: Evidence From 4.1 Million Adults and a Meta-Analysis. J Am Coll Cardiol. 2016;67(13):1656–7. [DOI] [PubMed] [Google Scholar]
- 45.Rochlani Y, Kattoor AJ, Pothineni NV, Palagiri RDR, Romeo F, Mehta JL. Balancing Primary Prevention and Statin-Induced Diabetes Mellitus Prevention. Am J Cardiol. 2017;120(7):1122–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


