Abstract
Background
Effects of clinical practice changes on ICU delirium are not well understood.
Objectives
Determine ICU delirium rates over time.
Methods
Data from a previously described screening cohort of the Pharmacological Management of Delirium trial was analyzed. Richmond Agitation-Sedation Scale (RASS) and Confusion Assessment Method for the ICU (CAM-ICU) were assessed twice daily. We defined: Any delirium (positive CAM-ICU at any time during ICU stay) and ICU-acquired delirium (1st CAM-ICU negative with a subsequent positive CAM-ICU). Mixed-effects logistic regression models were used to test for differences.
Results
2742 patient admissions were included. Delirium occurred in 16.5%, any delirium decreased [22.7% to 10.2% (p < 0.01)], and ICU-acquired delirium decreased [8.4% to 4.4% (p = 0.01)]. Coma decreased from 24% to 17.4% (p = 0.04). Later ICU years and higher mean RASS scores were associated with lower odds of delirium.
Conclusions
Delirium rates were not explained by the measured variables and further prospective research is needed.
Keywords: Delirium, Time trend, Intensive care unit, Outcomes
Introduction
Delirium is a form of acute brain failure associated with poor health outcomes, including increased mortality, longer duration of mechanical ventilation and hospital stay, increased risk of dementia, and post-discharge institutionalization.1–11 As the population continues to age and utilization of intensive care unit (ICU) services increase, delirium is likely to have a significant health impact in the United States. In prior studies, up to 80% of intensive care unit (ICU) patients experienced delirium.2 Given its association with poor outcomes in the critically ill, efforts to proactively screen for and mitigate precipitating delirium risk factors, such as use of benzodiazepines for sedation, have received increased attention.8,12–16 Whether these efforts have led to changes in clinical practice, however, and what effect these changes have had on rates of delirium over time has not been well described. A better understanding of recent trends in delirium may permit further refinement of delirium management strategies. Therefore, we conducted this analysis to describe changes in delirium rates at our intensive care unit (ICU) and identify factors associated with the change. We hypothesized that our delirium rates have decreased over time mediated by reduced use of benzodiazepines, and increased level of consciousness due to reduced sedation.
Materials and methods
Selection and sample populations
Data from a previously described screening cohort of the Pharmacological Management of Delirium (PMD) clinical trial was used for this analysis.11,15 Consecutive patients admitted to the medical-surgical ICU at Eskenazi Health, a busy urban academic hospital in Indianapolis, Indiana, between July 1, 2010 and December 30, 2014 were included in this cohort if they met the following criteria.
Inclusion criteria
English-speaking adult patients aged 18 years or older, admitted to the ICU.
Exclusion criteria were
(1) non-English speaking, (2) hearing impaired, (3) legally blind, (4) admitted with alcohol intoxication, (5) currently incarcerated (as unable to give consent), (6) having an axis 1 psychiatric disorder, (7) persistently comatose as defined by Richmond Agitation Sedation Scale of −4 or −5 throughout ICU stay, or (8) pregnant or nursing (due to parent trial eligibility criteria). For the present analysis, we excluded admissions which occurred: a) before July 1, 2010 or after December 31, 2014 as delirium assessments occurred once daily during this time; b) when patients were admitted to a step-down/progressive-care unit rather than the ICU; or, c) when records from a particular admission event could not be matched with patient or hospital data through the Regenstrief Medical Record System. Beginning at ICU admission, patients were assessed for level of consciousness and delirium using the Richmond Agitation-Sedation Scale (RASS)17 and the Confusion Assessment Method for the ICU (CAM-ICU), respectively.18 Trained research assistants performed twice daily RASS and CAM-ICU assessments after ICU admission until the patients became delirious, died, or were discharged from the ICU. As the screening phase for the study ended once the patient developed delirium, subtypes of delirium were identified at the time of first positive CAM-ICU assessment, and mixed typed phenotypes could not be reported. Hyperactive delirium was defined as a positive CAM-ICU score along with a positive RASS score (+1 to +4), while hypoactive delirium was defined as a positive CAM-ICU score along with a negative RASS score (−3 to 0). The study received approval from the Institutional Review Board.
We defined any delirium as a positive CAM-ICU result at any time during the ICU stay. We defined ICU-acquired delirium as the subset of patients who had a negative 1st delirium screening in ICU (CAM-ICU negative), with subsequent positive CAM-ICU.
Other data collection
Data not collected from in-person assessments was retrieved using the Regenstrief Medical Record System (RMRS), a local electronic medical record system.19 RMRS was used to identify patients’ age, gender, race, insurance status, smoking and alcohol use, length of hospital stay and mortality. International Classification of Diseases, 9th Edition (ICD-9) codes in the RMRS were used to determine chronic comorbidities and admission diagnoses. Drug exposure was assessed by active orders identified in RMRS. Diagnoses, including acute respiratory failure, acute renal failure, trauma, and hypoalbuminemia (albumin <3) were identified through RMRS.
Statistical analysis
ICU admissions were grouped into five calendar years by date of ICU admission (2010–2014). Rates of any delirium, hyperactive or hypoactive delirium, and other binary clinical variables were compared among the five years using mixed effect logistic models in the complete study sample and in the sub-set of patients who were initially delirium free. The mixed effects logistic regression models included a random effect to account for within-patient variation for those patients who had multiple ICU stays and fixed effects for patient characteristics including admission diagnoses. Similarly, mixed effects models were used to determine if continuous patient characteristics changed over time. To determine whether delirium rates change over the five study years, mixed effects logistic models for any delirium and ICU-acquired delirium were used with delirium as the binary outcome variable and ICU admission time as a categorical variable (with five levels representing the calendar years of ICU admission) and as a continuous variable as the number of years from the start of the study (i.e. number of years from 2010) while adjusting for other covariates. A significant ICU admission time effect in the mixed effects logistic models indicates changes in delirium rates over time. As the results were similar, we chose to present our analysis with time as a continuous variable. All analyses were conducted using SAS v9.4 (SAS Institute, Cary, NC).
Results
After exclusion criteria were applied, 2742 unique ICU patient admissions were included in the screening cohort, as detailed in Fig. 1. All 2742 patient admissions had at least one CAM-ICU evaluation and were included in the screening for any delirium. Only those with an initial negative CAM-ICU result and one or more subsequent CAM-ICU assessments were included in the subset screened for ICU-acquired delirium (n = 2117). Delirium occurred in 452/2742 (16.5%) patients, 308/2742 (11.2%) had a positive CAM-ICU on the first assessment, and 144/2117 (7%) had ICU-acquired delirium (Fig. 1). The mean age of participants in the analysis was 57.7 years (SD: 16 years), 46% of patients were African American, and 37% received mechanical ventilation. Delirium occurred in 17.2% of patients age 50 years and older, and 17.1% of female patients experienced delirium. Supplementary Tables 1 and 2 provide characteristics of the study sample. As shown in Supplementary Table 1, 37% (n = 1011) of all patients experienced mechanical ventilation, 37% were diagnosed with respiratory failure (n = 1026), 31% (n = 845) experienced acute renal failure, and 19% were admitted due to trauma (n = 511). Patients in the study also had various comorbidities; hypertension (77%, n = 2100), dementia (12%, n = 322), smoking/tobacco use (44%, n = 1215), and sleep disorders (24%, n = 670).
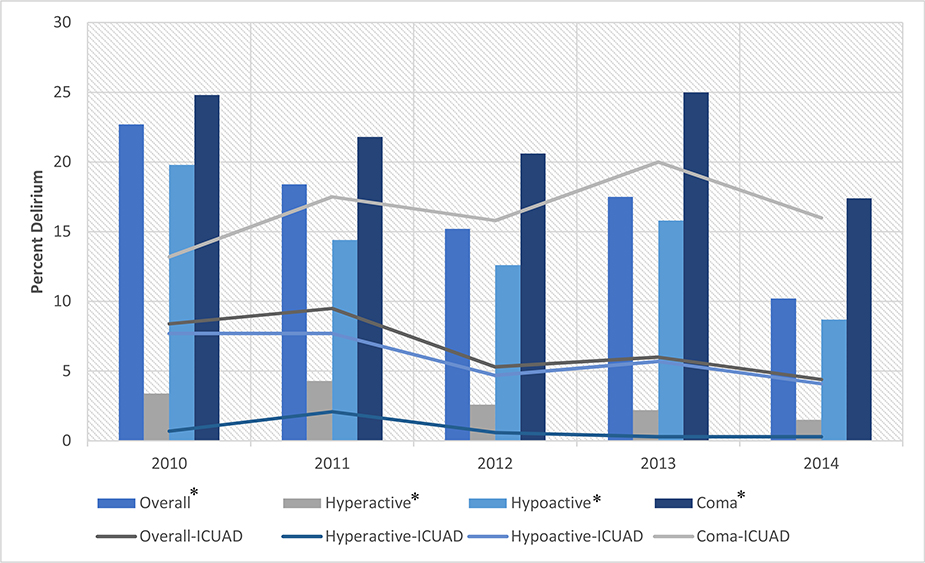
Fig. 1.
Patients assessed for eligibility for the screening cohort. n: Unique patient admissions. Any delirium: 1st delirium screening in ICU positive, or positive on subsequent CAM-ICU. ICU-acquired delirium: 1st delirium screening in ICU negative, with subsequent positive CAM-ICU.
Table 1 presents rates of any delirium, ICU-acquired delirium, and clinical outcomes by year of ICU admission. Rates of any delirium (Fig. 2) significantly decreased over time in the study sample: 23% (year 2010), 18% (2011), 15% (2012), 18 (2013) and 10% (2014), p < 0.001. There was also a decrease in coma; 24% (2010), 22% (2011), 21% (2012), 25% (2013) and 17% (2014), p = 0.035. Consistent with this decrease in coma, mean RASS scores at ICU admission increased over time, from −0.3 IQR: −2,0 (2010) to 0 IQR: −0.4, 0 (2014), p < 0.01. Table 1 provides additional outcomes including length of stay, respiratory failure, trauma, hypoalbuminemia, and opioid orders. There were no significant differences in benzodiazepine orders over time, while opioid orders increased: 66% (2010), 72% (2011), 74% (2014), p = 0.046.
Table 1.
Delirium rates and clinical outcomes for the screening cohort and the subset of patients screened for ICU-acquired delirium.
| All Patients Screened for Delirium in Study (n = 2742) | ||||||
| Outcomes | 2010**** (n = 383) | 2011**** (n = 706) | 2012**** (n = 650) | 2013**** (n = 463) | 2014**** (n = 540) | P-value |
| Any Delirium* (%) | 22.7 | 18.4 | 15.2 | 17.5 | 10.2 | <0.001 |
| Hyperactive delirium** (%) | 3.4 | 4.1 | 2.6 | 1.9 | 1.5 | 0.057 |
| Hypoactive delirium** (%) | 19.3 | 14.3 | 12.6 | 15.6 | 8.7 | <0.001 |
| Coma (%) | 24.0 | 21.8 | 20.6 | 25.0 | 17.4 | 0.035 |
| Admission mean RASS1 – median IQR2 | −0.3 (−2, 0) | −0.5 (−1.5, 0) | −0.5 (−1.3,−0.1) | 0 (−1, 0) | 0 (−0.4, 0) | <0.001 |
| LOS days median (IQR) | 7 (5–13) | 8 (5–15) | 8 (5–13) | 9 (6–16) | 8 (5–12) | 0.026 |
| Hospital mortality (%) | 3.4 | 4.2 | 4.3 | 3.5 | 3.5 | 0.873 |
| Mechanical Ventilation (%) | 36.0 | 36.5 | 33.8 | 41.2 | 37.8 | 0.166 |
| Respiratory failure (%) | 26.6 | 32.4 | 40.0 | 42.3 | 44.3 | <0.001 |
| Acute renal failure (%) | 29.5 | 32.7 | 29.8 | 32.8 | 28.7 | 0.490 |
| Trauma (%) | 15.9 | 17.0 | 16.5 | 21.0 | 23.3 | 0.011 |
| Hypoalbuminemia | 26.6 | 25.8 | 23.8 | 25.9 | 42.2 | <0.001 |
| Unit | <0.001 | |||||
| MICU, n (%) | 66.8 | 67.0 | 69.5 | 62.0 | 55.2 | |
| SICU, n (%) | 33.2 | 33.0 | 30.5 | 38.0 | 44.8 | |
| Medication Orders | ||||||
| Benzodiazepines | 20.4 | 22.1 | 24.8 | 21.6 | 21.1 | 0.486 |
| Opioids | 66.3 | 72.1 | 70.6 | 75.4 | 74.4 | 0.046 |
| Subset of Patients Screened for ICU-Acquired Delirium (n = 2117) | ||||||
| Outcomes | 2010**** (n = 287) | 2011**** (n = 570) | 2012**** (n = 531) | 2013**** (n = 366) | 2014**** (n = 363) | P-value |
| ICU-acquired Delirium*** (%) | 8.4 | 9.5 | 5.3 | 6.0 | 4.4 | 0.014 |
| Hyperactive delirium** (%) | 0.7 | 1.9 | 0.6 | 0.3 | 0.3 | 0.061 |
| Hypoactive delirium** (%) | 7.7 | 7.5 | 4.7 | 5.7 | 4.1 | 0.113 |
| Coma (%) | 13.2 | 17.5 | 15.8 | 20.0 | 16.0 | 0.211 |
| Admission mean RASS1 – median IQR2 | 0 (−0.7, 0) | −0.3 (−1.0, 0) | −0.4 (−0.9, −0.1) | 0 (−0.5, 0) | 0 (−0.3, 0) | <0.001 |
| LOS days median (IQR) | 7 (4–11) | 8 (5–14) | 8 (5–12) | 8 (5–15) | 9 (6–13) | 0.001 |
| Hospital mortality (%) | 3.1 | 3.3 | 2.8 | 3.0 | 1.9 | 0.798 |
| Mechanical Ventilation (%) | 24.0 | 30.7 | 26.0 | 33.9 | 35.5 | 0.003 |
| Respiratory failure (%) | 20.2 | 29.3 | 34.6 | 37.2 | 41.3 | <0.001 |
| Acute renal failure (%) | 27.8 | 33.0 | 27.1 | 32.0 | 27.8 | 0.202 |
| Trauma, n (%) | 14.3 | 15.8 | 14.9 | 19.7 | 25.3 | 0.001 |
| Hypoalbuminemia | 23.7 | 23.5 | 21.7 | 23.5 | 41.9 | <0.001 |
| Unit | <0.001 | |||||
| MICU, n (%) | 65.8 | 66.8 | 69.7 | 61.2 | 50.4 | |
| SICU, n (%) | 34.2 | 33.2 | 30.3 | 38.8 | 49.6 | |
| Medication Orders | ||||||
| Benzodiazepines | 20.2 | 21.0 | 24.1 | 21.0 | 20.7 | 0.680 |
| Opioids | 67.9 | 72.6 | 71.0 | 77.0 | 79.3 | 0.009 |
n: Unique patient admissions.
Any delirium: Patients with delirium at any time during the screening phase (at first assessment or during subsequent screenings in the intensive care unit);.
Delirium subtypes were determined based on first positive delirium screening;.
ICU-acquired delirium: 1st delirium screening in ICU negative, with subsequent positive CAM-ICU.
Time was regarded as a categorical variable with 5 levels (year 2010 to 2014).
Fig. 2.
Rates of delirium in all patients screened and the subset with ICU-acquired delirium. Graph showing the trends of any delirium and ICU-acquired delirium between 2010 and 2014. The bar graph also shows the percentage of each subtype of delirium (hypo vs. hyperactive). *Rates for any delirium. ICUAD: rates for ICU-acquired delirium. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In the subset of patients who were initially delirium free, rates of ICU-acquired delirium decreased over time (as shown in Table 1), from 8% (2010), 9.5% (2011), 5.3% (2012), 6% (2013) to 4% (2014), p = 0.014. In contrast to those with any delirium, rates of coma in the patients with ICU-acquired delirium did not change; 13% (2010) to 16% (2014), p = 0.211. Level of consciousness by RASS and other outcomes are shown in Table 1; benzodiazepine orders were unchanged over time, while opioid orders increased [68% (2010), 79% (2014), p = 0.009). Multivariate Analysis
Any delirium
In mixed effects logistic regression models (Table 2), younger age defined as age 49 or less was associated with decreased odds of delirium (OR 0.27 95% CI: 0.17–0.44). Increased level of consciousness measured by mean RASS was associated with lower odds of delirium (OR 0.43 95% CI: 0.38–0.49). Exposures associated with higher odds of delirium were: mechanical ventilation (OR 6.74 95% CI: 4.21–10.77); acute renal failure (OR 1.64 95% CI: 1.21–2.22); hypoalbuminemia (OR 1.89 95% CI: 1.4–2.54); alcohol abuse (OR 1.55 95% CI: 1.05–2.28) and benzodiazepine exposure (OR 1.60 95%CI: 1.15–2.22). Later years of ICU stay, defined as number of years from 2010, were associated with decreased odds of delirium (OR 0.82 95%CI: 0.73–0.92).
Table 2.
Results of mixed effects logistic regression models for delirium in the screening cohort and for subset of patients screened for ICU-acquired delirium.
| All Patients Screened for Delirium (n = 2742) |
Subset Screened for ICU-acquired Delirium (n = 2117) |
|||
|---|---|---|---|---|
| Characteristics | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Age | ||||
| 18–49 | 0.27 (0.17, 0.44) | <0.001 | 0.25 (0.12, 0.54) | 0.001 |
| 50–64 | 0.6 (0.41, 0.88) | 0.009 | 0.55 (0.31, 0.98) | 0.041 |
| 65+ (Reference) | 1.0 | 1.0 | ||
| Race | ||||
| African-American | 1.24 (0.92, 1.67) | 0.161 | 1.01 (0.64, 1.60) | 0.962 |
| Caucasian (Reference) | 1.0 | 1.0 | ||
| Insurance | ||||
| Medicaid | 1.34 (0.84, 2.13) | 0.215 | 1.84 (0.87, 3.90) | 0.111 |
| Self-pay (Reference) | 1.0 | 1.0 | ||
| Clinical Characteristics | ||||
| Mechanical Ventilation | 6.74 (4.21, 10.77) | <0.001 | 4.46 (2.19, 9.07) | <0.001 |
| Acute Respiratory Failure | 1.34 (0.92, 1.95) | 0.121 | 0.95 (0.53, 1.71) | 0.873 |
| Acute Renal Failure | 1.64 (1.21, 2.22) | 0.002 | 1.84 (1.15, 2.96) | 0.012 |
| Trauma | 1.38 (0.94, 2.03) | 0.104 | 0.87 (0.47, 1.60) | 0.645 |
| Hypoalbuminemia | 1.89 (1.40, 2.54) | <0.001 | 2.42 (1.53, 3.84) | <0.001 |
| Dementia | 1.62 (1.05, 2.49) | 0.029 | 1.64 (0.87, 3.11) | 0.128 |
| Smoking | 0.84 (0.61, 1.14) | 0.253 | 0.90 (0.56, 1.45) | 0.663 |
| Alcohol Abuse History | 1.55 (1.05, 2.28) | 0.028 | 1.35 (0.71, 2.57) | 0.363 |
| MICU vs. SICU | 1.11 (0.78, 1.59) | 0.553 | 0.69 (0.41, 1.16) | 0.155 |
| Benzodiazepines | 1.60 (1.15, 2.22) | 0.005 | 1.66 (1.03, 2.69) | 0.038 |
| Opioids | 1.03 (0.74, 1.44) | 0.856 | 1.45 (0.83, 2.56) | 0.194 |
| Mean RASS Score | 0.43 (0.38, 0.49) | <0.001 | 0.38 (0.30, 0.47) | <0.001 |
| ICU Admission Time* | ||||
| Number of years from 2010* | 0.82 (0.73, 0.92) | 0.001 | 0.80 (0.66, 0.97) | 0.021 |
Time in years as a continuous variable for the number of years that ICU admission from the first study year of 2010.
n: Unique patient admissions. MICU: Medical ICU; RASS: Richmond Agitation Sedation Scale; SICU: Surgical ICU.
ICU-acquired delirium
In the subset of patients who were initially delirium-free, younger age (OR 0.25 95% CI: 0.12–0.54) and increased level of consciousness (OR 0.38 95% CI: 0.30–0.47) were associated with decreased odds of delirium (Table 2). Mechanical ventilation (OR 4.46 95% CI: 2.19–9.07), acute renal failure (OR 1.84 95% CI: 1.15–2.96), hypoalbuminemia (OR 2.42 95% CI 1.53–3.84) and benzodiazepine exposure (OR 1.66 95% CI: 1.03–2.69) were associated with higher odds of ICU-acquired delirium. Later years of ICU stay (number of years from 2010) were associated with decreased odds of ICU-acquired delirium (OR 0.82 95%CI: 0.66–0.97).
Discussion
In our study, we found a decrease in yearly rates of any delirium and coma in critically ill patients. While there was a decrease in delirium over time in the subset of patients with ICU-acquired delirium, rates of coma in that subset remained unchanged. Surprisingly, despite efforts in recent years to limit benzodiazepine use, orders for benzodiazepines did not change, while there was an increase in opioid orders. We also found that younger age, increased level of consciousness (higher mean RASS score), and later years of ICU stay were associated with decreased odds of delirium. Consistent with prior studies, mechanical ventilation, acute renal failure, low serum albumin, and benzodiazepines were associated with increased odds of developing ICU-acquired delirium. We believe this scientific work provides a valuable update to previously quoted literature (where delirium rates can be as high as 40–80%), improves our understanding of which factors have changed over time, and points to additional areas needing study in order to appropriately address the challenging syndrome of delirium from a clinical practice perspective.
In contrast to early studies in ICU delirium which found delirium rates as high as 80%, we found lower delirium rates in our study.3,8,20 Recent multi-center studies of ICU delirium have also found lower rates.21 In the REDUCE trial which selectively enrolled patients at high risk for delirium, incidence of delirium was approximately 33%.13 Delirium rates in our study may be lower than some high-risk ICU populations for the following reasons: a) our analysis is limited to patients in a screening cohort rather than all patients admitted to the ICU; b) delirium rates presented in our analysis did not include patients with persistent coma throughout the ICU stay who could not be assessed for delirium; and c) implementation of a spontaneous awakening and breathing protocol at our center occurred during our study.22 The protocol was implemented in 2009 and mandated a daily safety screen with subsequent discontinuation of sedatives, a spontaneous breathing trial on the ventilator, and resumption of sedatives at half their previous dose if ventilator liberation was not performed. Implementation of this protocol also incorporated judicious use of sedatives and screening for delirium. As previously published, implementation of this program was associated with increased level of consciousness for patients admitted to the ICU.22 The effects of this protocol, which implemented ABCD components of the ABCDEF bundle, may at least partially explain the trend of decreasing yearly delirium and coma rates, as well as the increasing level of consciousness.
Despite the above-mentioned factors, we believe our study findings reiterate the important clinical connection between respiratory failure, receipt of mechanical ventilation, and risk of developing delirium. While rates of delirium in our study cohort differed from recent studies, the odds of delirium in mechanically ventilated patients remained consistent with other literature.23 This association of respiratory failure and delirium occurred despite the study ICU’s academic environment where clinical trials focused on delirium are conducted, and where protocolized spontaneous awakening and breathing protocols were in place. Therefore, we believe the study’s statistical description of risk factors and clinical management trends (including orders for medications) remain generalizable to critically ill patient populations in a variety of healthcare settings and general clinical practice. Consistent with previous studies and despite recent advances in ventilator and sedation practices, our study found mechanical ventilation, benzodiazepines, older age, renal failure and low albumin levels remain associated with higher odds of delirium.24–25 Time in years from 2010 was associated with decreased odds of delirium, likely representative of recent changes in practice including implementation of ABCDEF at our center. Another contributing factor may be the increased use of non-invasive ventilation for acute respiratory failure; endo-tracheal intubation procedures often require deep sedation.26 In contrast to findings in patients with any delirium, the subset of patients with ICU-acquired delirium had increasing trends of mechanical ventilation and unchanged rates of coma. This re-emphasizes the important connection between respiratory failure, coma and delirium in this population. Our study has important clinical and research implications. Despite advances in delirium management over time including increased delirium awareness, improved respiratory technology including non-invasive ventilation, and revised society guidelines emphasizing assessment and management of pain and titration of sedation (which may explain increased orders for opioids), educational efforts to improve sedation practices, mechanical ventilation remains a powerful risk factor for delirium in the intensive care setting.27 This emphasizes the need to further study respiratory failure and delirium pathophysiology in order to develop effective pharmacological and non-pharmacological interventions. The identification of mechanically ventilated patients as high-risk for delirium despite recent efforts to reduce the delirium burden highlights a priority subgroup to receive bundled delirium management strategies in the critical care setting.
Our study has important limitations. First, as previously mentioned, our study sample was limited to patients eligible for a larger delirium study rather than all critically ill patients, which may have resulted in underestimation of the true delirium rate. Second, while results of spontaneous awakening and breathing protocols at our center have been previously published, we did not collect patient-level data on ABCDEF adherence for this study thus we were not able to account for the influence from the ABCDEF program. Third, as CAM-ICU assessments were stopped once a patient screened positive for delirium, we were unable to report mixed subtype delirium. Fourth, while comorbidities and rates of organ failure are provided in this analysis and have been shown to predict mortality in respiratory failure, our analysis did not adjust for severity of illness or sepsis. Fifth, our analysis included medication orders as doses of medications administered (including benzodiazepines and opioids) were not available; therefore, our analysis does not answer whether doses of medications changed over time. Finally, the study was performed at an academic hospital with ongoing clinical research in delirium; clinical practices may have been affected limiting the generalizability of our findings.
Strengths of our study include use of a large prospective screening cohort representative of medical and surgical ICU populations with high rates of mechanical ventilation and advanced comorbidities, robust sedation and delirium assessments, and collection of a comprehensive set of clinical outcomes.
Conclusion
Our study found a decrease in delirium rates in ICU stays from 2010 to 2014. This finding was not entirely explained by the measuredvariables, including time in years. Further investigation isneeded to understand reasons for this decrease.
Supplementary Material
Acknowledgements
We thank members of the Team Vitality Research Core and the patients and care providers who made this work possible.
Funding
MB is supported by NIA R01AG052493. BK is supported by NIA R01AG055391, R01HL131730. S.W. is funded by K23AG062555-01 and NIA 2P30AG010133.
Footnotes
Declaration of Competing Interest
To the best of our knowledge, no conflicts of interest, financial orother, exists.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.hrtlng.2020.03.006.
References
- 1.Khan BA, Zawahiri M, Campbell NL, et al. Delirium in hospitalized patients: implications of current evidence on clinical practice and future avenues for research–a systematic evidence review. J Hosp Med. 2012;7(7):580–589. 10.1002/jhm.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salluh Jorge I, Han Wang, Eric Schneider, Neeraja Nagaraja, Gayane Yenokyan,Abdulla Damluji et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis BMJ 2015; 350 :h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14): 1753–1762. [DOI] [PubMed] [Google Scholar]
- 4.Girard TD, Thompson JL, Pandharipande PP, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6(3):213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes CG, Patel MB, Brummel NE, et al. Relationships between markers of neurologic and endothelial injury during critical illness and long-term cognitive impairment and disability. Intensive Care Med. 2018;44(3):345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasilevskis EE, Chandrasekhar R, Holtze CH, et al. The cost of ICU delirium and coma in the intensive care unit patient. Med Care. 2018;56(10):890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin S-M, Liu C-Y, Wang C-H, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32(11):2254–2259. [DOI] [PubMed] [Google Scholar]
- 8.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34–41. 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pisani MA, Kong SYJ, Kasl SV, Murphy TE, Araujo KLB, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Amer J Resp Crit Care Med. 2009;180:1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandharipande PP, Girard TD, Jackson JC, et al. Long–term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan BA, Perkins A, Hui SL, et al. Relationship between african-american race and delirium in the ICU. Crit Care Med. 2016;44(9):1727–1734. 10.1097/CCM.0000000000001813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pun TB, Balas MC, MA Barnes-Daly, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. 2018. 10.1097/CCM.0000000000003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Boogaard M, Slooter AJC, Bruüggemann RJM, et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium. REDUCE Random Clin Trial JAMA. 2018;319(7):680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girard TD, Exline MC, Carson SS, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. New Engl J Med. 2018;379(26):2506–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan BA, Perkins AJ, Campbell NL, Gao S, Farber MO, Wang S, et al. Pharmacological management of delirium in the intensive care unit: a randomized pragmatic clinical trial. J Am Geriatr Soc. 2019. May;67(5):1057–1065. 10.1111/jgs.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell NL, Perkins AJ, Khan BA, Gao S, Farber MO, Khan S, et al. Deprescribing in the pharmacologic management of delirium: a randomized trial in the intensive care unit. J Am Geriatr Soc. 2019. April;67(4):695–702. 10.1111/jgs.15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sessler CN, Gosnell MS, Grap MJ, et al. The richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 18.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703–2710. [DOI] [PubMed] [Google Scholar]
- 19.McDonald CJ, Overhage JM, Tierney WM, et al. The Regenstrief medical record system: a quarter century experience. Int J Med Inform. 1999;54(3):225–253. [DOI] [PubMed] [Google Scholar]
- 20.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salluh JI, Soares M, Teles JM, et al. Delirium epidemiology in critical care (DECCA): an international study. Crit Care. 2010;14(6):R210 10.1186/cc9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan BA, Fadel WF, Tricker JL, et al. Effectiveness of implementing a wake up and breathe program on sedation and delirium in the ICU. Crit Care Med. 2014;42(12): e791–e795. 10.1097/CCM.0000000000000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh SJ, Soto GJ, Hope AA, Ponea A, Gong MN. The association between acute respiratory distress syndrome, delirium, and in-hospital mortality in intensive care unit patients. Am J Respir Crit Care Med. 2015;191(1):71–78. 10.1164/rccm.201409-1690OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Y, Jia P, Zhang J, et al. Prevalence and risk factors of postoperative delirium in elderly hip fracture patients. J Int Med Res. 2016;44(2):317–327. 10.1177/0300060515624936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walkey AJ, Wiener RS. Use of noninvasive ventilation in patients with acute respiratory failure, 2000–2009: a population-based study. Ann Am Thorac Soc. 2013;10 (1):10–17. 10.1513/AnnalsATS.201206-034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30 (1):119–141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.