Abstract
Objective
Acute kidney injury (AKI) is common in critically ill children; however, the incidence of septic shock-associated AKI and impact on functional status are unknown. We evaluated functional outcomes of children with septic shock-associated AKI.
Design
Secondary analysis of patients with septic shock from the prospective Life after Pediatric Sepsis Evaluation (LAPSE) study. We defined AKI using Kidney Disease Improving Global Outcomes criteria, comparing patients with absent/Stage 1 AKI to those with Stage 2/3 AKI (Severe AKI). Our primary outcome was a composite of mortality or new functional morbidity at day 28 of hospitalization or discharge. We also assessed poor long-term outcome, defined as mortality or a persistent, serious deterioration in health-related quality of life at 3 months.
Setting
Twelve academic pediatric intensive care units in the United States.
Patients
Critically ill children, 1 month-18 years, with community-acquired septic shock requiring vasoactive-inotropic support
Interventions
None
Measurements and Main Results
More than 50% (176/348) of patients developed severe AKI; of those, 21.6% (38/176) required renal replacement therapy. Twice as many patients with severe AKI died or developed new substantive functional morbidity (38.6 vs 16.3%; p<0.001). After adjustment for age, malignancy, and initial illness severity, severe AKI was independently associated with mortality or new substantive morbidity (adjusted odds ratio 2.78; 95% CI 1.63–4.81, p<0.001). Children with severe AKI had poorer health-related quality of life at 3 months (adjusted effect size 2.46; 95% CI 1.44–4.20; p=0.002). Children with severe AKI required longer duration of mechanical ventilation (11.0 vs 7.0 days; p<0.001) and PICU stay (11.7 vs 7.1 days; p<0.001).
Conclusions
Among children with septic shock, severe AKI was independently associated with increased risk of death or new substantive functional morbidity. Survivors of sepsis with severe AKI were more likely to have persistent, serious health-related quality of life deterioration at 3 months.
Keywords: Acute kidney injury, critical care outcomes, health-related quality of life, recovery of function, functional status, renal replacement therapy, sepsis, septic shock
Introduction
In the United States, sepsis accounts for approximately 8% of pediatric critical care admissions.(1, 2) Acute kidney injury (AKI) is diagnosed in up to one-quarter of children admitted to PICUs, and is associated with increased mortality and length of stay.(3, 4) One previous study found that severe AKI occurred in 21% of PICU patients with sepsis.(5) The prevalence of sepsis in children is increasing, and with the decrease in sepsis-related mortality, functional outcomes and long-term health related quality of life (HRQL) among survivors have become a focus of pediatric critical care outcomes investigators.(6) Most prior research has focused on reducing AKI-associated mortality and/or improving hospital outcomes. With improvements in clinical care, the majority of children with AKI now survive their hospitalization. However, approximately half demonstrate residual impairment in kidney function, which may include an increased risk of hypertension, proteinuria and chronic kidney disease (CKD).(7, 8)
With an increased recognition of the long-term kidney-related sequalae of AKI, the impact of AKI on HRQL is increasingly important. HRQL is recognized as a patient-centered and clinically meaningful outcome. Recognition of impairments on HRQL is the first step toward improving quality of life. Research investigating functional outcomes and HRQL among children with AKI have solely focused on short-term HRQL and found that AKI is associated with poor short term outcomes.(5, 9)
The Life After Pediatric Sepsis Evaluation (LAPSE, R01HD073362) investigation was a prospective descriptive cohort-outcome study that enrolled children with community-acquired septic shock in 12 academic PICUs (10, 11). LAPSE described the trajectory of HRQL among critically ill children surviving septic shock by comparing baseline and serial follow-up assessments over the year following the sepsis event. We used data from the LAPSE study to assess the association between severe acute kidney injury and poor short-term functional outcomes, defined as a composite of mortality or new functional morbidity 28 days following admission for septic shock or hospital discharge, whichever occurred first. We also assessed poor long-term outcome, defined as mortality or a persistent, serious deterioration in health-related quality of life (HRQL) at 3 months. We hypothesized a priori that among children with septic shock, severe acute kidney injury would be associated with poor functional status and HRQL outcomes.
Materials and Methods
LAPSE was a prospective, descriptive cohort study which assessed the long-term mortality and morbidity of children ages 1 month to 18 years following an encounter of septic shock. The details of enrollment and data collection have been previously published.(10, 11) Patients at each site were screened upon admission for septic shock and initial and daily clinical data were collected for the duration of PICU admission. Institutional review boards (central or local) approved the LAPSE Protocol for each site. Study procedures were conducted only after informed, documented permission from the parent or guardian. In addition, developmentally appropriate subjects provided assent for their own study participation around the time of PICU discharge. All children with preexisting kidney-related comorbidities were excluded.
Baseline clinical data included patient demographics, illness severity assessment,(12) baseline organ dysfunction,(13) infection-related data, and baseline measurements of functional status and HRQL.(14–16) Chronic comorbid conditions were classified according to the Pediatric Medical Complexity Algorithm.(17) Information related to vasoactive-inotropic infusions and ventilator settings were recorded twice daily while patients remained in the PICU.(18, 19) Laboratory monitoring and clinical care included hemodynamic resuscitation, renal replacement therapy, extracorporeal life support, and nutritional management, and occurred at the discretion of the responsible attending physician and were not mandated by study protocol.
Acute Kidney Injury Definition
Many of the hospitalized children enrolled in the study were previously healthy and therefore did not have a creatinine measurement to use as baseline. In these children, baseline kidney function was assumed to be normal. As has been utilized in previous studies of AKI in critically ill children, we estimated a baseline serum creatinine value for each patient by assuming a normal glomerular filtration rate of 120 mL/min/1.73 m2 and back-calculating a creatinine using the bedside Schwartz equation [creatinine (mg/dL) = 0.413 x height (cm)/120].(5, 9, 20, 21) Preexisting kidney disease was determined based on chronic comorbidity reporting at the time of study entry. Children without a recorded height as well as without serum creatinine or urine output measurements were also excluded from this secondary analysis.
AKI was defined using Kidney Disease Improving Global Outcomes (KDIGO) criteria, and classified using both creatinine and urine output criteria.(Supplemental Table 1) (22, 23) The outcome of AKI could have occurred at any point during the first 28 days of hospital admission, based on KDIGO criteria. We defined severe AKI as stage 2 or 3 AKI (serum creatinine level ≥2 times the calculated baseline or urine output <0.5mL/kg/hr for ≥12 hours) as these stages are associated with increased morality in studies of children. (3, 8, 21) When serum creatinine and urine output criteria resulted in different stages, we chose the higher stage. Participants were then divided into two groups. Children with no AKI and Stage 1 AKI were categorized as having absent or mild AKI. Children with Stage 2 and Stage 3 AKI were categorized as having severe AKI. These two groups were used for all analyses.
Outcomes
Functional status was longitudinally assessed utilizing Pediatric Overall Performance Category (POPC) and Pediatric Cerebral Performance Category (PCPC) scores and the Functional Status Scale obtained at study entry (reflecting baseline pre-sepsis status during the month prior to PICU admission), study day 7, and study day 28 or hospital discharge (whichever occurred first).(24, 25) Similarly, participating families completed serial parent-proxy assessments of their child’s HRQL utilizing the Pediatric Quality of Life Inventory 4.0 Generic Core Scales (PedsQL™) or PedsQL™ Infant Scales or the Stein-Jessop Functional Status Scale (FSII-R) at study entry (reflecting baseline pre-sepsis status),(15, 26) study day 7 and 1, 3, 6, and 12 months following PICU admission.(16, 27) Both scales employ a 0–100 point range.(15, 26)
Our primary outcome was short-term functional status, utilizing a composite of new substantive morbidity or death. We defined new substantive functional morbidity as a Functional Status Scale increase of 3 or more points from baseline to day 28/hospital discharge.(14) Functional Status Scale is a standardized pediatric scale created to assess adaptive status, that includes mental status, sensory, communication, motor functioning, feeding and respiratory domains.(15) A score change of 3 or more is considered clinically significant.(14) As a secondary outcome, we assessed poor long-term HRQL outcome, defined as death or persistent, serious HRQL deterioration ≥25% below baseline at 3 months following PICU admission.
Statistical Analysis
To characterize the LAPSE cohort by AKI status, patient factors were summarized using counts and percentages for categorical variables, and the median and interquartile range for continuous variables in both the absent/mild AKI group and the severe AKI group. The association between categorical variables compared across AKI status was evaluated using the likelihood-ratio test, while ordinal variables were evaluated using the Wilcoxon rank-sum test. (Tables 1, 2, and 4)
Table 1.
Demographics and baseline characteristics of subjects with and without severe acute kidney injury
| Acute Kidney Injury Group |
|||
|---|---|---|---|
| Demographic and baseline characteritics | Absent/Stage 1 AKI (N = 172) | Stage 2/3 AKI (N = 176) | P-value |
| Male | 94 (54.7%) | 92 (52.3%) | 0.6571 |
| Age (years) | 5.7 [1.7, 11.8] | 7.4 [1.5, 13.8] | 0.2432 |
| Race | 0.3841 | ||
| White | 110 (64.0%) | 98 (55.7%) | |
| Black or African American | 30 (17.4%) | 39 (22.2%) | |
| Multiracial | 6 (3.5%) | 6 (3.4%) | |
| Other | 12 (7.0%) | 18 (10.2%) | |
| Unknown or not reported | 14 (8.1%) | 15 (8.5%) | |
| Ethnicity | 0.9611 | ||
| Hispanic or Latino | 42 (24.4%) | 42 (23.9%) | |
| Not Hispanic or Latino | 129 (75.0%) | 131 (74.4%) | |
| Unknown or Not Reported | 1 (0.6%) | 3 (1.7%) | |
| Weight at PICU admission (kg) | 19.1 [11.2, 37.0] | 23.8 [9.8, 49.9] | 0.1932 |
| Height at PICU admission (cm) | 111.0 [80.3, 137.0] | 117.0 [75.5, 150.5] | 0.3902 |
| PRISM (excluding creatinine) | 8.0 [4.0, 14.0] | 12.0 [8.0, 18.5] | <.0012 |
| Medical complexity algorithm category | 0.3221 | ||
| No chronic comorbid conditions | 84 (48.8%) | 88 (50.0%) | |
| Chronic comorbid conditions (non-complex) | 12 (7.0%) | 6 (3.4%) | |
| Chronic comorbid conditions (complex) | 78 (44.2%) | 81 (46.0%) | |
| Immune-related comorbid conditions | |||
| Malignancy | 13 (7.6%) | 8 (4.5%) | 0.2361 |
| Subject immunocompromised | 33 (19.2%) | 32 (18.2%) | 0.8101 |
| Solid organ transplant | 0 (0.0%) | 2 (1.1%) | 0.0981 |
| Bone marrow or stem cell transplantation | 2 (1.2%) | 1 (0.6%) | 0.5451 |
| Sickle cell disease | 2 (1.2%) | 1 (0.6%) | 0.5451 |
| FSS at baseline | 0.3372 | ||
| Good (6 – 7) | 103 (59.9%) | 94 (53.4%) | |
| Mildly abnormal (8 – 9) | 11 (6.4%) | 23 (13.1%) | |
| Moderately abnormal (10 – 15) | 36 (20.9%) | 40 (22.7%) | |
| Severely abnormal (16 – 21) | 19 (11.0%) | 15 (8.5%) | |
| Very severely abnormal (≥ 22) | 3 (1.7%) | 4 (2.3%) | |
| Criteria for AKI diagnosis | |||
| No AKI diagnosis | - | 0 (0.0%) | |
| Serum creatinine | - | 93 (52.8%) | |
| Urine output | - | 28 (15.9%) | |
| Serum creatinine and urine output | - | 55 (31.3%) | |
Likelihood ratio test.
Wilcoxon rank-sum test.
Abbreviations: AKI, acute kidney injury; FSS, Functional Status Scale; PRISM, Pediatric Risk of Mortality Score; PICU, Pediatric Intensive Care Unit
Table 2.
Comparison of outcomes in subjects with and without severe acute kidney injury
| Acute Kidney Injury Group |
|||
|---|---|---|---|
| Outcomes | Absent/Stage 1 AKI (N = 172) | Stage 2/3 AKI (N = 176) | P-value |
| In-hospital mortality | 7 (4.1%) | 27 (15.3%) | <.0011 |
| New substantive morbidity | 21 (12.2%) | 50 (28.4%) | <.0011 |
| In-hospital mortality or new substantive morbidity | 28 (16.3%) | 68 (38.1%) | <.0011 |
| Cardiopulmonary arrest or chest compressions | 7 (4.1%) | 26 (14.8%) | <.0011 |
| Return to renal baseline3 | 117 (68.0%) | 83 (47.2%) | <.0011 |
| Ventilator-free days | 21.0 [17.0, 24.0] | 17.0 [2.5, 22.0] | <.0012 |
| Vasoactive-inotropic-free days | 26.0 [24.5, 27.0] | 24.0 [19.0, 26.0] | <.0012 |
| Hospital length of stay (days) | 14.1 [7.8, 20.7] | 20.6 [11.8, 35.2] | <.0012 |
| PICU length of stay (days) | 7.1 [4.3, 12.0] | 11.7 [7.2, 21.7] | <.0012 |
| Survived to hospital discharge | |||
| Total | 165 | 149 | |
| New substantive FSS morbidity | 21 (12.7%) | 41 (27.5%) | 0.0011 |
| Ventilator-free days | 22.0 [18.0, 24.0] | 19.0 [11.0, 23.0] | <.0012 |
| Vasoactive-inotropic-free days | 26.0 [25.0, 27.0] | 24.0 [21.0, 26.0] | <.0012 |
| FSS (Day 28/hospital discharge) | 0.0042 | ||
| Good (6 – 7) | 76 (46.1%) | 45 (30.2%) | |
| Mildly abnormal (8 – 9) | 21 (12.7%) | 25 (16.8%) | |
| Moderately abnormal (10 – 15) | 38 (23.0%) | 43 (28.9%) | |
| Severely abnormal (16 – 21) | 21 (12.7%) | 26 (17.4%) | |
| Very severely abnormal (≥ 22) | 6 (3.6%) | 8 (5.4%) | |
| Unknown | 3 (1.8%) | 2 (1.3%) | |
| Δ FSS (baseline to Day 28/hospital discharge) | 0.0 [0.0, 1.0] | 0.0 [0.0, 3.0] | <0.0012 |
| HRQL Outcomes | |||
| Total | 161 | 165 | |
| Substantively reduced HRQL or mortality at Day 28 | 52 (32.1%) | 76 (46.1%) | 0.0151 |
| Substantively reduced HRQL or mortality at 3 months | 36 (22.4%) | 71 (43.0%) | <0.0011 |
| HRQL Outcomes excluding Day 28 deaths | |||
| Total | 153 | 136 | |
| Substantively reduced HRQL or mortality at Day 28 | 45 (29.6%) | 52 (38.2%) | 0.1501 |
| Substantively reduced HRQL or mortality at 3 months | 28 (18.3%) | 42 (30.9%) | 0.0151 |
Likelihood ratio test.
Wilcoxon rank-sum test.
Return to renal baseline considered a last serum creatinine that was within 0.3mg/dL of the initial value.
Abbreviations: AKI, acute kidney injury; FSS, Functional Status Scale; FSII-R, Stein-Jessop Functional Status Scale; HRQL, Health Related Quality of Life; PedsQL™, Pediatric Quality of Life Inventory 4.0 Generic Core Scales; PICU, Pediatric Intensive Care Unit
Table 4.
Comparison of therapies used for patients with and without severe acute kidney injury
| Acute Kidney Injury Group |
|||
|---|---|---|---|
| Absent/Stage 1 AKI (N = 172) | Stage 2/3 AKI (N = 176) | P-value | |
| Vasoactive-inotropic use | 157 (91.3%) | 172 (97.7%) | 0.0061 |
| Vasoactive-inotropic use (days) | 2.0 [1.0, 3.0] | 4.0 [2.0, 8.0] | <.0012 |
| Mechanical ventilation (days) | 6.0 [4.0, 10.0] | 10.0 [6.0, 18.0] | <.0012 |
| Sum of vasoactive-inotropic scores | 19.5 [7.0, 53.5] | 54.8 [20.9, 127.4] | <.0012 |
| Blood product use | 77 (44.8%) | 115 (65.3%) | <.0011 |
| Immunomodulating medication given | 28 (16.3%) | 40 (22.7%) | 0.1281 |
| ECMO or VAD | 3 (1.7%) | 20 (11.4%) | <.0011 |
| Renal replacement therapy | 0 (0.0%) | 38 (21.6%) | <.0011 |
| Treatment for increased intracranial pressure | 3 (1.7%) | 9 (5.1%) | 0.0781 |
| Plasma exchange | 3 (1.7%) | 19 (10.8%) | <.0011 |
| Corticosteroid use | 105 (61.0%) | 131 (74.4%) | 0.0071 |
| Neuromuscular blockade | 120 (69.8%) | 135 (76.7%) | 0.1431 |
| Parental nutrition | 58 (33.7%) | 96 (54.5%) | <.0011 |
| Indewelling catheter use | |||
| Central venous | 161 (93.6%) | 171 (97.2%) | 0.1101 |
| Urinary | 143 (83.1%) | 159 (90.3%) | 0.0461 |
| Arterial | 130 (75.6%) | 155 (88.1%) | 0.0021 |
Likelihood ratio test.
Wilcoxon rank-sum test.
Abbreviations: AKI, acute kidney injury; ECMO, extracorporeal membrane oxygenation; VAD, ventricular assist device
Associations between AKI status and binary outcomes such as in-hospital mortality and reduced HRQL or mortality were investigated using logistic regression. Factors considered confounding were specified in each model as covariates were determined a priori and included: age (< 1 year, 1 – 11 years, ≥ 12 years), malignancy, and PRISM III (excluding the creatinine component).(12) Reported statistics for these models include the adjusted odds ratio and corresponding 95% confidence interval (CI). Associations with AKI status and ordinal outcomes such as ΔPedsQL™ and ΔFSII-R were modeled separately using linear regression with the same covariates as specified for the logistic models. The adjusted estimated effect size and 95% CI are reported for these models. Change from baseline for continuous measures at different time points are denoted with the symbol delta (Δ). (Table 3) Summaries and analyses were performed using SAS 9.4 (SAS Institute; Cary, NC)
Table 3.
Association of severe acute kidney injury with outcomes
| Outcomes | Adjusted odds ratio (95% CI) | Adjusted effect (95% CI) | P-value |
|---|---|---|---|
| Outcomes (clinical cohort, N=348) | |||
| In-hospital mortality | 3.78 (1.61, 10.02) | 0.002 | |
| In-hospital mortality or new substantive morbidity1 | 2.78 (1.63, 4.81) | <.001 | |
| New substantive morbidity1 (among hospital survivors) | 2.31 (1.25, 4.35) | 0.007 | |
| Δ FSS at Day 28 or hospital discharge1 (among hospital survivors) | 1.01 (0.17, 1.86) | 0.019 | |
| Outcomes (HRQL cohort, N=326) | |||
| Reduced HRQL or mortality at Month 1 | 1.77 (1.06, 2.94) | 0.029 | |
| Reduced HRQL at Month 1 (among survivors2) | 1.56 (0.90, 2.72) | 0.117 | |
| Reduced HRQL or mortality at Month 3 | 2.46 (1.44, 4.20) | 0.001 | |
| Reduced HRQL at Month 3 (among survivors2) | 1.96 (1.05, 3.63) | 0.035 | |
| Outcomes (PedsQLTM Month 3 survival cohort2, N=179) | |||
| Psychosocial summary score | −3.35 (−8.86, 2.16) | 0.234 | |
| Emotional function domain score | −4.12 (−10.84, 2.59) | 0.229 | |
| Social function domain score | −2.73 (−10.51, 5.04) | 0.490 | |
| Physical summary score | −9.00 (−17.12, -0.87) | 0.030 | |
| Δ PedsQL™ | −4.64 (−11.31, 2.04) | 0.174 | |
| Outcomes (FSII-R Month 3 survival cohort2, N=110) | |||
| Δ FSII-R | −9.79 (−19.96, 0.39) | 0.061 |
All models control for age (< 1 year, 1 year - < 12 years, ≥ 12 years), malignancy, and PRISM III (without creatinine component).
There were 5 patients surviving hospitalization without FSS at Day 28 or hospital discharge.
Patient survival was verified for all LAPSE subjects regardless of survey completion.
Abbreviations: AKI, acute kidney injury; FSS, Functional Status Scale; FSII-R, Stein-Jessop Functional Status Scale; HRQL, Health Related Quality of Life; PedsQL™, Pediatric Quality of Life Inventory 4.0 Generic Core Scales
To account for subjects lost to follow-up and to reduce the potential bias ignoring such loss may have had on analyses, clinical data for all subjects surviving to hospital discharge and completed HRQL data were used to estimate missing longitudinal HRQL data (33% at Month 3).(28) The imputation methods used in this secondary LAPSE investigation and the detailed methodology for imputing and analyzing data sets with missing data have been published.(10, 11) In summary, ten multiples of imputed data sets were created independently using observed data values and a sequence of regression models to replace missing HRQL values. By independently creating ten imputed data sets, random perturbation of the imputed values was intentionally introduced. Each imputed data set was analyzed separately, and the results were combined using the MIANALYSE procedure. A complete-case sensitivity analysis was performed disregarding data from subjects without complete 3-Month follow-up.
Results
AKI Incidence
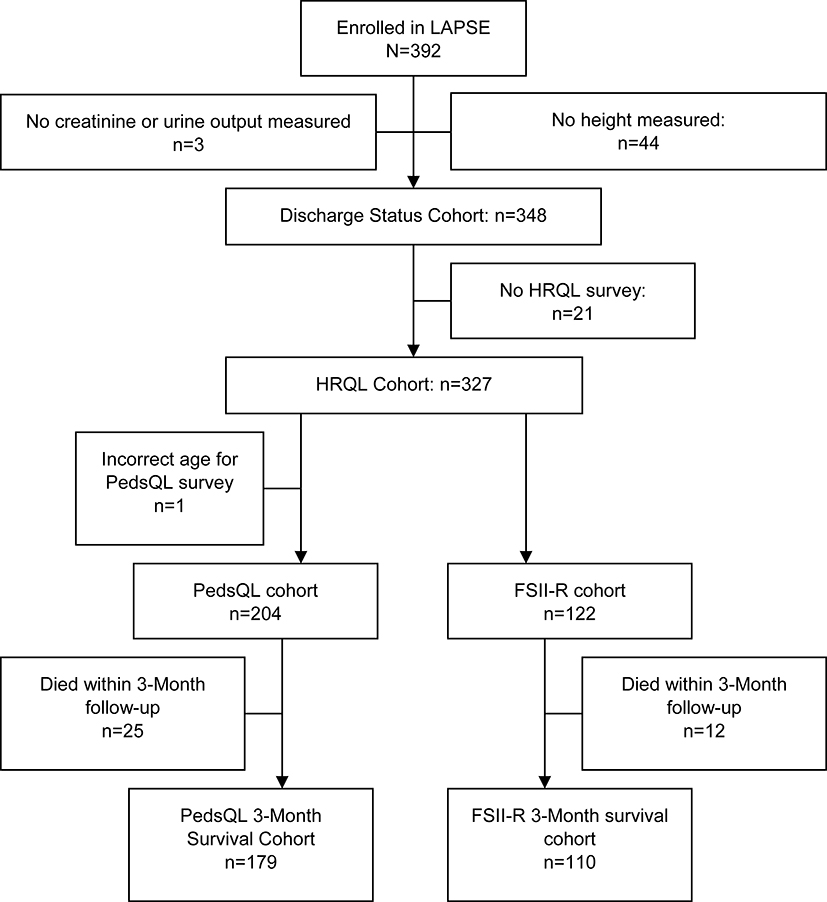
From January 1, 2014 through June 30, 2017, 838 patients were screened; 632 were eligible, 570 (90%) were approached and 392 (69% of those approached) were enrolled into the LAPSE study. Of the 392 patients enrolled in LAPSE, 348 patients met inclusion criteria for this AKI secondary study, and were included in this analysis (Figure 1). No patients had chronic kidney-related morbidity at study entry. Patients were excluded for not having an available height necessary to estimate baseline kidney function (n=44) as well as insufficient serum creatinine and urine output measurement to determine acute kidney injury (n=3). Of those, 172 had absent/mild AKI, and 176 (50.6%) had severe AKI. Renal replacement therapy (RRT) was provided to 38 (21.6%) of those with severe AKI. Of those with severe AKI, 93 (52.8%) were diagnosed with AKI based on changes in serum creatinine, and 28 (15.9%) on urine output decline alone, while the remainder 55 (31.3%) met both serum creatinine and urine output criteria. There were no significant differences with respect to sex, age, race or ethnicity between patients with and without severe AKI (Table 1). Additionally, there were no significant difference between patients with and without severe AKI in the occurrence of medical complexity or immune-related comorbid conditions (Table 1).
Figure 1.
LAPSE Flow Diagram
Failure to return to baseline creatinine by hospital discharge or 28 days was common in all subjects; however, it was shown to occur even more frequently in patients with severe AKI (68.0% vs 47.2%, p <0.001). (Table 2)
Severe AKI and Higher Illness Severity and Complexity
Patients with severe AKI had higher median PRISM III scores (excluding creatinine) (12.0 vs 8.0; p < 0.001) (Table 1). Children with severe AKI had longer PICU stays (11.7 vs 7.1 days; p < 0.001), and longer duration of mechanical ventilation (10.0 vs 6.0 days; p <0.001) (Table 2). Those with severe AKI were treated more often with blood products (65.3 vs 44.8%; p <0.001), and corticosteroids (74.4 vs 61.0%; p = 0.007) (Table 4).
AKI and In-Hospital Death/Substantive Functional Morbidity
AKI was associated with the death or new substantive functional morbidity, which occurred among 39% of those with severe AKI compared to 16% of those with absent/mild AKI (p <0.001) (Table 2). In-hospital death occurred in 15.3% of the severe AKI group and 4.1% of the no/mild AKI group (p <0.001). New substantive functional morbidity occurred in 27.5% of survivors with severe AKI versus 12.7% of those with no/mild AKI (p = 0.001). Among the 38 patients requiring renal replacement therapy, 36.8% died (14/38), and new substantive functional morbidity occurred in 29.2% (7/24)
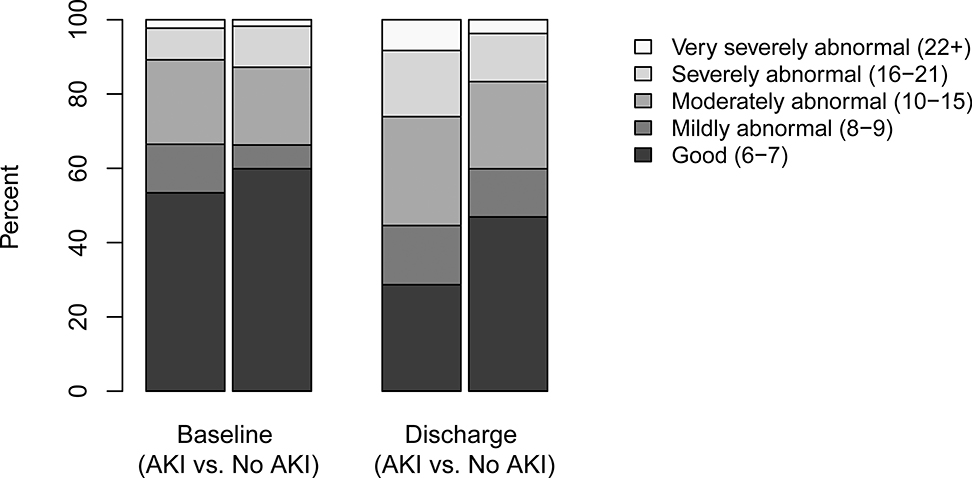
In multivariable regression modeling, severe AKI remained independently associated with death or new substantive functional morbidity after adjustment for age, history of malignancy, and severity of illness (adjusted odds ratio 2.78, 95% CI 1.63–4.81, p <0.001 (Table 3). Those with severe AKI had an almost four-fold increased odds of death (adjusted odds ratio 3.78, 95% CI 1.61–10.02, p = 0.002). Despite a similar FSS at baseline, children with severe AKI demonstrated a larger increase in their score comparing baseline and day 28/hospital discharge (indicating a deterioration of functional status) with an adjusted effect of 1.01 in linear regression modeling (adjusted effect 1.01, 95% CI 0.17–1.86, p = 0.019).
AKI and Long-Term HRQL
Children with septic shock complicated with severe AKI exhibited an increased likelihood of poor long-term HRQL outcomes. Those with severe AKI were 2.5 times more likely to have persistent, serious deterioration in their HRQL or have died by three months following ICU admission (adjusted odds ratio 2.46, 95% CI 1.44–4.20, p = 0.001) (Table 3). This difference was also evident at the 1-month post-discharge (adjusted odds ratio 1.77, 95% CI 1.06–2.94, p = 0.029).
In the subgroup of patients surviving 3 months and assessed with the PedsQL™ (n=179), physical summary scores were lower in the severe AKI group (adjusted effect −8.83, 95% CI −17.04- −0.62, p=0.035). There were no statistically significant differences in emotional, social or psychosocial summary scores in this subgroup (Table 3). In the subgroup of surviving patients assessed using FSII-R (n=110), there were no statistically significant differences seen at 3 months (p=0.061).
Discussion
Severe AKI occurred in over half of children with community-acquired septic shock in this prospective cohort, and was independently associated with decreases in short -term functional status and long-term HRQL outcomes. Children with severe AKI in the setting of septic shock had more than twice the odds of death or new substantive functional morbidity at 28 days/hospital discharge than children with absent/mild AKI. Those with severe AKI who survived also had persistent, serious deterioration of HRQL at 3 months and an increased prevalence of persistent abnormal kidney function. This is the first study to assess the association of AKI with long-term HRQL outcomes in critically ill children with severe AKI. This study indicates that the increased risk of both short and long-term morbidity is additive among children with both septic shock and severe AKI.
There is growing evidence suggesting that children with AKI have poor HRQL outcomes, however this is the first study to assess these outcomes beyond hospital discharge.(5, 9) While patients with severe AKI in this study received more intensive treatment and presented with higher illness severity, the association of severe AKI and poor HRQL outcomes persisted after adjustment for these factors in our primary analysis. Similar to findings of previous studies of HRQL, this difference was driven by changes in physical functional status.(9) Evidence from other studies of AKI demonstrate that AKI is a systemic disease with wide-ranging targets, including impacts to the neurologic system.(29, 30) Animal models of acute kidney injury following ischemia and reperfusion have shown marked neurologic changes evidenced by inflammatory changes and microvascular dysfunction.(29) These changes were particularly notable in the hippocampus, an area essential for behavioral regulation and learning.(30) The findings of this study add credence to the ongoing work evaluating functional patient outcomes in critically ill patients with concomitant AKI.(31)
We also note that a large percentage of patients in both groups (both those with and without severe AKI) were discharged from the PICU with an abnormal creatinine. This suggests that a number of patients may have residual impairment in their kidney function. Previous studies of pediatric AKI survivors have shown that those with AKI may develop chronic kidney disease (CKD) that is apparent as early as 6–12 months after AKI.(7, 8) The early findings of CKD can be subtle, and include hypertension, proteinuria, and mild changes in glomerular filtration rate. Early detection of these abnormalities allows earlier intervention and can slow progression of CKD.(32, 33) Previous studies suggest that while up to 25% of patients leave the PICU with an abnormal creatinine, only a minority return for follow-up with nephrology.(34) Frequently AKI is not listed as a discharge diagnosis, and families and the care team often are not aware of the kidney injury that has occurred.(21, 35) This underscores the importance of long-term kidney-related follow-up for these patients.
There are several limitations of this study. First, this was a secondary analysis of an existing cohort and therefore depended on existing data. Accordingly, determination of AKI relied on urine output and serum creatinine data collected as part of usual clinical care. Secondly, as baseline creatinine data were not available, we calculated a baseline based on an assumption of normal renal function. While we recognize this as a limitation, this is a strategy that has been utilized in many studies of pediatric AKI.(5, 21, 24, 36) To decrease the likelihood of misclassification due to unknown baseline kidney status, we categorized patients into dichotomous AKI outcomes. Additionally, it is possible that despite our best attempts to control for potential confounders, children with severe sepsis may be at greater risk for AKI and demonstrate poor functional outcomes due to the underlying severity of their critical illness. Finally, LAPSE had ~30% loss to follow-up by 3 months following discharge. Multiple imputation techniques were utilized for missing data to account for subjects lost to follow-up and to reduce the potential bias ignoring such loss may have had on analyses.
Despite these limitations, this cohort-outcome study has several strengths that provide additional knowledge to the field. These include a moderate-sized prospectively enrolled cohort, including patients from twelve tertiary PICUs across the United States. Therefore, our findings are likely to be generalizable among pediatric academic centers where most patients with severe AKI in the setting of sepsis receive care. Other studies examining long term HRQL among children surviving sepsis have been hampered by lack of baseline HRQL and functional status measures, small cohort numbers, and variable time to follow-up. Children in LAPSE were assessed for chronic comorbid conditions, and underwent baseline functional status and HRQL evaluations.
Conclusions
In conclusion, over half of the children in this community-acquired septic shock cohort developed severe AKI. This is much higher than previously reported in this clinical population. Severe AKI is an independent risk factor for death or clinically substantive decrease in functional status at PICU discharge, as well as late mortality or persistent serious HRQL deterioration 3 months after PICU admission for the sepsis encounter.
Previous studies of patients with severe AKI have focused on kidney outcomes, such as the risk of developing chronic kidney disease or hypertension. This observation remains important, as we report a large percentage of patients with septic shock failed to return to their baseline creatinine by PICU discharge. However, we also note a strong association between AKI and new clinically substantive morbidity among children surviving septic shock, further underscoring the importance of long-term follow-up not only for functional morbidity and HRQL outcomes among survivors, but also for kidney function, as well as other organ systems potentially impacted by sepsis associated-AKI.
Supplementary Material
Figure 2.
Distribution of Function Status Scale in subjects with and without severe acute kidney injury
Acknowledgements
The LAPSE Investigators thank all subjects and families for participating in the LAPSE investigation.
Following is a summary of LAPSE Performance Sites, Principal Investigators (PI), Co-investigators (CI), Research Coordinators (RC), and Allied Research Personnel (AP).
Children’s Hospital of Michigan, Detroit, MI: Kathleen L. Meert, PI; Sabrina Heidemann, CI; Ann Pawluszka, RC; Melanie Lulic, RC.
Children’s Hospital of Philadelphia, Philadelphia, PA: Robert A Berg, PI; Athena Zuppa, CI; Carolann Twelves, RC; Mary Ann DiLiberto, RC.
Children’s National Medical Center, Washington, DC: Murray Pollack, PI; David Wessel, PI; John Berger, CI; Elyse Tomanio, RC; Diane Hession, RC; Ashley Wolfe, RC.
Children’s Hospital of Colorado, Denver, CO: Peter Mourani, PI; Todd Carpenter, CI; Diane Ladell, RC; Yamila Sierra, RC; Alle Rutebemberwa, RC.
Nationwide Children’s Hospital, Columbus, OH: Mark Hall, PI; Andy Yates, CI; Lisa Steele, RC; Maggie Flowers, RC; Josey Hensley, RC.
Mattel Children’s Hospital, University of California Los Angeles, Los Angeles, CA: Anil Sapru, PI; Rick Harrison, CI, Neda Ashtari, RC; Anna Ratiu, RC.
Children’s Hospital of Pittsburgh, University of Pittsburgh Medical Center, Pittsburgh, PA: Joe Carcillo, PI; Michael Bell, CI; Leighann Koch, RC; Alan Abraham, RC.
Benioff Children’s Hospital, University of California, San Francisco, San Francisco, CA: Patrick McQuillen, PI; Anne McKenzie, RC; Yensy Zetino, RC.
Children’s Hospital of Los Angeles, Los Angeles, CA: Christopher Newth, PI; Jeni Kwok, RC; Amy Yamakawa, RC.
CS Mott Children’s Hospital, University of Michigan, Ann Arbor, MI: Michael Quasney, PI; Thomas Shanley, CI; CJ Jayachandran, RC.
Cincinnati Children’s Hospital, Cincinnati, OH: Ranjit Chima PI; Hector Wong, CI; Kelli Krallman, RC; Erin Stoneman, RC; Laura Benken, RC; Toni Yunger, RC.
Seattle Children’s Hospital, Seattle Children’s Research Institute (LAPSE Follow-up Center), University of Washington, Seattle, WA: Jerry J Zimmerman, PI; Catherine Chen, RC; Erin Sullivan, RC; Courtney Merritt, RC; Deana Rich, RC; Julie McGalliard, AP; Wren Haaland, AP; Kathryn Whitlock, AP; Derek Salud, AP.
University of Utah (LAPSE Data Coordinating Center), Salt Lake City, UT: J Michael Dean, PI; Richard Holubkov, CI; Whit Coleman, RC; Samuel Sorenson, RC; Ron Reeder, AP; Russell Banks, AP; Angie Webster, AP; Jeri Burr, AP; Stephanie Bisping, AP; Teresa Liu, AP; Emily Stock, AP; Kristi Flick, AP.
Texas A&M University, College Station, TX: James Varni, AP
Financial Support: This investigation was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, R01HD073362, and was supported, in part, by the following cooperative agreements: UG1HD050096, UG1HD049981, UG1HD049983, UG1HD063108, UG1HD083171, UG1HD083166, UG1HD083170, U10HD050012, U10HD063106, and U01HD049934.
Copyright form disclosure: Dr. Starr disclosed that this investigation was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH), Department of Health and Human Services, T32DK007662, R01HD073362, and was supported, in part, by the following cooperative agreements: UG1HD050096, UG1HD049981, UG1HD049983, UG1HD063108, UG1HD083171, UG1HD083166, UG1HD083170, U10HD050012, U10HD063106, and U01HD049934. Drs. Starr, Banks, Reeder, Pollack, Meert, McQuillen, Mourani, Chima, Sorenson, Varni, Hingorani, and Zimmerman received support for article research from the NIH.Drs. Banks, McQuillen, Sorenson, and Zimmerman’s institutions received funding from the NICHD. Drs. Banks and Sorenson disclosed government work. Drs. Reeder, Pollack, Meert, and Mourani’s institutions received funding from the NIH. Dr. Varni holds the copyright and the trademark for the PedsQL™ and receives financial compensation from the Mapi Research Trust, which is a nonprofit research institute that charges distribution fees to for-profit companies that use the Pediatric Quality of Life Inventory™. Dr Varni provided consultation on original study design and final manuscript edits but played no role in data acquisition or analysis. Dr. Zimmerman’s institution received funding from Immunexpress, and he received funding from Elsevier and the Society of Critical Care Medicine. Dr. Fitzgerald disclosed that she does not have any potential conflicts of interest.
Footnotes
Conflicts of Interest: No performance site investigators disclose financial interests, activities, relationships, or affiliations that could be construed as real or potential conflicts of interest related to the manuscript or the related investigation. Dr. Varni holds the copyright and the trademark for the PedsQL™ and receives financial compensation from the Mapi Research Trust, which is a nonprofit research institute that charges distribution fees to for-profit companies that use the Pediatric Quality of Life Inventory™. Dr Varni provided consultation on original study design and final manuscript edits, but played no role in data acquisition or analysis.
No reprints will be ordered.
References
- 1.Ruth A, McCracken CE, Fortenberry JD, et al. : Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med 2014; 15:828–838 [DOI] [PubMed] [Google Scholar]
- 2.Balamuth F, Weiss SL, Neuman MI, et al. : Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med 2014; 15:798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaddourah A, Basu RK, Bagshaw SM, et al. : Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med 2017; 376:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider J, Khemani R, Grushkin C, et al. : Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med 2010; 38:933–939 [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald JC, Basu RK, Akcan-Arikan A, et al. : Acute Kidney Injury in Pediatric Severe Sepsis: An Independent Risk Factor for Death and New Disability. Crit Care Med 2016; 44:2241–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farris RWD, Weiss NS, Zimmerman JJ: Functional outcomes in pediatric severe sepsis: further analysis of the researching severe sepsis and organ dysfunction in children: a global perspective trial. Pediatr Crit Care Med 2013; 14:835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mammen C, Al Abbas A, Skippen P, et al. : Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis 2012; 59:523–530 [DOI] [PubMed] [Google Scholar]
- 8.Askenazi DJ, Feig DI, Graham NM, et al. : 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 2006; 69:184–189 [DOI] [PubMed] [Google Scholar]
- 9.Richardson KL, Watson RS, Hingorani S: Quality of life following hospitalization-associated acute kidney injury in children. J Nephrol 2018; 31:249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmerman J, Banks R, Berg R: Trajectory of mortality and health related quality of life morbidity following community-acquired pediatric septic shock. Crit Care Med 2020; 48:329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann J, Banks R, Berg R: Critical illness variables associated with long-term mortality and/or persistent, serious health-related quality of life morbidity following community acquired septic shock. Crit Care Med 2020; 48:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollack MM, Patel KM, Ruttimann UE: PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 1996; 24:743–752 [DOI] [PubMed] [Google Scholar]
- 13.Leteurtre S, Duhamel A, Salleron J, et al. : PELOD-2: an update of the Pediatric logistic organ dysfunction score. Crit Care Med 2013; 41:1761–1773 [DOI] [PubMed] [Google Scholar]
- 14.Pollack MM, Hulubkov R, Funai T, et al. : Pediatric Intensive Care Outcomes: Development of New Morbidities During Pediatric Critical Care. Pediatr Crit Care Med 2014; 15:821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein RE, Jessop DJ: Functional status II(R). A measure of child health status. Med Care 1990; 28:1041–1055 [DOI] [PubMed] [Google Scholar]
- 16.Varni JW, Limbers CA, Burwinkle TM: Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes 2007; 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon TD, Cawthon ML, Stanford S, et al. : Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics 2014; 133:e1647–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McIntosh AM, Tong S, Deakyne SJ, et al. : Validation of the Vasoactive-Inotropic Score in Pediatric Sepsis. Pediatr Crit Care Med 2017; 18:750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khemani RG, Thomas NJ, Venkatachalam V, et al. : Comparison of SpO2 to PaO2 based markers of lung disease severity for children with acute lung injury. Crit Care Med 2012; 40:1309–1316 [DOI] [PubMed] [Google Scholar]
- 20.Schwartz GJ, Brion LP, Spitzer A: The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 1987; 34:571–590 [DOI] [PubMed] [Google Scholar]
- 21.Zappitelli M, Parikh CR, Akcan-Arikan A, et al. : Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol 2008; 3:948–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutherland SM, Byrnes JJ, Kothari M, et al. : AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol 2015; 10:554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellum JA, Lameire N, KDIGO AKI Guideline Work Group: Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013; 17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollack MM, Holubkov R, Glass P, et al. : Functional Status Scale: new pediatric outcome measure. Pediatrics 2009; 124:e18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiser DH, Long N, Roberson PK, et al. : Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med 2000; 28:2616–2620 [DOI] [PubMed] [Google Scholar]
- 26.Aspesberro F, Fesinmeyer MD, Zhou C, et al. : Construct Validity and Responsiveness of the Pediatric Quality of Life Inventory 4.0 Generic Core Scales and Infant Scales in the PICU. Pediatr Crit Care Med 2016; 17:e272–279 [DOI] [PubMed] [Google Scholar]
- 27.Varni JW, Limbers CA, Newman DA, et al. : Longitudinal factorial invariance of the PedsQL 4.0 Generic Core Scales child self-report Version: one year prospective evidence from the California State Children’s Health Insurance Program (SCHIP). Qual Life Res 2008; 17:1153–1162 [DOI] [PubMed] [Google Scholar]
- 28.Liu M, Taylor JM, Belin TR: Multiple imputation and posterior simulation for multivariate missing data in longitudinal studies. Biometrics 2000; 56:1157–1163 [DOI] [PubMed] [Google Scholar]
- 29.Yap SC, Lee HT: Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology 2012; 116:1139–1148 [DOI] [PubMed] [Google Scholar]
- 30.Liu M, Liang Y, Chigurupati S, et al. : Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol 2008; 19:1360–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curley MAQ, Zimmerman JJ: Alternative outcome measures for pediatric clinical sepsis trials. Pediatr Crit Care Med 2005; 6:S150–156 [DOI] [PubMed] [Google Scholar]
- 32.Wühl E, Trivelli A, et al. for the ESCAPE Trial Group: Strict blood-pressure control and progression of renal failure in children. N Engl J Med 2009; 361:1639–1650 [DOI] [PubMed] [Google Scholar]
- 33.Fathallah-Shaykh SA, Flynn JT, Pierce CB, et al. : Progression of pediatric CKD of nonglomerular origin in the CKiD cohort. Clin J Am Soc Nephrol 2015; 10:571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alkandari O, Eddington KA, Hyder A, et al. : Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care 2011; 15:R146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutherland SM, Ji J, Sheikhi FH, et al. : AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol 2013; 8:1661–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basu RK, Wong HR, Krawczeski CD, et al. : Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol 2014; 64:2753–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.