Abstract
Objectives:
We aimed to identify longitudinal patterns and predictors of acute care use (emergency department [ED] visits and hospitalizations) among individuals with SLE enrolled in Medicaid, the largest U.S. public insurance.
Methods:
Using Medicaid data (29 states, 2000–2010) we identified 18–65-year-olds with SLE (≥3 SLE ICD-9 codes, 3rd code=index date), ≥12 months of enrollment prior to the index date and ≥24 months post. For each 90-day interval post index date, patients were assigned binary indicators (1=≥1 ED visit or hospitalization, 0=none). We used group-based trajectory models to graph patterns of acute care use overall and for SLE, and multinomial logistic regression models to examine predictors.
Results:
Among 40,381 SLE patients, the mean age was 40.8 (SD 11.9). Using a three-group trajectory model, 2,342 (6%) were recurrent all-cause high acute care utilizers, 12,932 (32%) moderate, 25,107 (62%) infrequent; 25% were moderate or high utilizers for SLE. There were higher odds of all-cause, recurrent high acute care use (vs. infrequent) among patients with severe vs. mild SLE (OR 3.37, 95% CI 3.0–3.78), chronic pain (OR 1.63, 95% CI 1.15–2.32), depression (OR 1.90 95% CI 1.74–2.09), and cardiovascular disease (OR 2.29, 9% CI 2.08–2.52). Older age, male sex and hydroxychloroquine use were associated with lower odds of recurrent acute care use, both all-cause and SLE.
Conclusion:
Nearly 40% of Medicaid beneficiaries with SLE are recurrent all-cause acute care utilizers; 25% have recurrent use for SLE. Modifiable factors including outpatient management of SLE and comorbidities may reduce avoidable acute care use.
Keywords: Systemic lupus erythematosus, health care utilization, health services research, health disparities
1.1. Introduction
Systemic lupus erythematosus (SLE) is a heterogenous autoimmune disease that disproportionately affects lower income individuals and racial/ethnic minorities. Prior studies demonstrate high rates of costly and frequent acute care use including hospitalizations, emergency department (ED) visits and readmissions among patients with SLE.(1, 2) Certain SLE patients appear to be particularly vulnerable including Medicaid beneficiaries, Hispanic and African American patients, patients with depressive symptoms and with more severe SLE, and those with high rates of nonadherence to their SLE-related medications.(2–5) Pain has also been shown to account for a significant percentage of ED visits particularly among SLE patients who repeatedly frequented the ED.(6)
Studies to date either have been limited to acute care use among a small cohort of patients, a single medical center, commercially insured patients, or to rates and predictors of hospital readmissions.(2, 3, 6) We derived our SLE cohort from nationwide beneficiaries of Medicaid, the largest U.S. public health insurance for low income individuals. Medicaid beneficiaries are known to suffer from a high burden of SLE as well as other chronic diseases and disproportionately experience adverse outcomes.(7, 8) We aimed to identify patterns of recurrent acute care use, defined as ED visits and/or hospitalizations, longitudinally over a 2-year period to delineate discrete groups at highest risk for repeated visits. We were interested both in overall acute care use for any cause, as well as for SLE. We also aimed to identify both demographic contributors and potentially modifiable predictors of the highest recurrent acute care use patterns in this especially vulnerable, nationwide SLE population.
1.2. Materials and Methods
1.2.1. Patient population
We conducted this study using the Medicaid Analytic eXtract (MAX), which includes demographic information, billing codes, claims and medication dispensing data for Medicaid beneficiaries from the 29 most populated states, 2000–2010. We restricted our population to age 18–65; individuals over 65 may be dually enrolled in Medicare and we did not have access to this information or to these claims. We defined prevalent SLE as ≥3 ICD-9 codes (710.0) separated by ≥30 days with the final code as the index date as described in prior studies.(7) For our secondary analyses, of incident SLE, we required ≥24 months of prior continuous enrollment in Medicaid without a SLE code prior to the first code.(7) The index date was similarly defined as the date of the last code. Our prevalent and incident cohorts were restricted to those with ≥24 months of continuous enrollment following the index date. We conducted sensitivity analyses among both incident and prevalent SLE patients requiring ≥90 days of follow-up rather than ≥24 months, and censored patients at death or disenrollment from Medicaid.
1.2.2. Outcome
Our primary outcome of interest was ED visits and hospitalizations occurring during the 24-month period following the day after the index date. For our primary analysis, we defined this broadly as any ED visit or hospitalization for any cause. We then restricted our outcome to ED visits or hospitalizations with a primary or secondary discharge diagnosis code for SLE to understand the degree of repeated acute care use directly attributable to SLE.
1.2.3. Predictors
We examined demographic factors including age at the index date, sex, race/ethnicity and geographic region. We also used U.S. Census data (9, 10) to examine zip code median household income. For all predictors unless otherwise specified, we used claims and medication data from the 12 months prior to and including the index date (the date of 3rd SLE code). We used a published claims-based algorithm applied previously to MAX data to classify patients as having mild, moderate or severe SLE.(5, 11) The algorithm includes a set of conditions, as well as immunosuppressive medications and corticosteroids that help categorize patients accordingly to SLE severity. We determined ever/never use of corticosteroids, hydroxychloroquine and immunosuppressive medications (rituximab, leflunomide, methotrexate, tacrolimus, sulfasalazine, mycophenolate mofetil/myfortic, azathioprine cyclosporine and cyclophosphamide) in the 12 months prior to the index date, as well as the total number of distinct medications at the index date. We examined comorbidities including diabetes mellitus, depression, diabetes, chronic pain, and cardiovascular disease using ICD-9 code and related medication data (see Appendix). We defined lupus nephritis using a previously validated algorithm.(12) Claims data do not include laboratory values, but we assessed number of SLE-related lab tests (anti-dsDNA, BUN, creatinine, urinalysis, C3, C4, ESR, CRP and CBC). We also included the number of outpatient visits during the baseline 12-month period.
1.2.4. Statistical Analysis
For each patient with SLE, we divided his/her 24-month follow-up into eight 90-day periods. Each 90-day period was assigned a “1” if the patient had one or more ED visit or hospitalization during that time and a “0” if there were none. We used these binary indicators to develop group-based trajectory models (GBTM) to classify patients by their acute care use, first overall (any discharge diagnosis) and then for SLE specifically. This method is applied to longitudinal data and allows for the identification of latent patterns over time.(13) Previously, GBTM was applied to understand patterns of medication adherence, including studies among Medicaid beneficiaries with SLE where the details of the methods are described.(14, 15)
We first fit multinomial logistic regression models without predictors to model the probability for each patient of belonging to a specific acute care use trajectory based on his/her 24-month acute care use pattern. We modeled time in quadratic and cubic forms. We examined GBTM ranging from three to six trajectory groups and compared model fit based on average posterior probabilities (aiming for ≥80%), the Bayesian information criterion (BIC, aiming for comparably small values), and reasonable distribution of patients across trajectory groups.(13) We also considered which models were both parsimonious and appropriately captured and explained our data.(13) When BICs were similar, we weighted the value least heavily as prior studies suggest that when conducting GBTM with large sample sizes, this may not be the most reliable indicator of model fit.(14) Once we chose the most appropriate group-based trajectory model, we used multivariable multinomial logistic regression models to examine the odds of belonging to the higher acute care use trajectories vs. the lowest based on different permutations of predictors of interest. We did not include SLE disease severity in the same models with medication use or comorbidities due to collinearity as certain comorbidities that are SLE-related (e.g. lupus nephritis) as well as medication use are incorporated into the severity algorithm. We additionally adjusted for the calendar year of the index date in all our models. We examined healthcare utilization and mortality, confirmed using the National Death Index, by trajectory group. We used multivariable Cox proportional hazards models to examine the risk of death in by trajectory group adjusting for demographic factors, calendar year of index date and SLE disease severity.
1.3. Results
We identified 40,381 individuals with prevalent SLE with ≥24 months of follow-up after the index date (Table 1). The mean age was 40.8 (SD 11.9), 93.7% were female, 40.6% black, 36.9% white, 2.6% Asian, 15.6% Hispanic, and 1% American Indian/Alaska Native. In this cohort, 45.9% were classified as having mild SLE, 36% moderate and 18.1% severe. Nearly half used corticosteroids during the baseline 12-month period, 38.5% hydroxychloroquine and 18.2% immunosuppressives. Cardiovascular disease and depression were prevalent in this cohort.
Table 1:
Baseline characteristics overall and by acute care use trajectory among individuals with prevalent SLE enrolled in Medicaid, 2000–2010
| Overall Prevalent SLE Cohort | Group 1 (Infrequent acute care use) |
Group 2 (Moderate acute care use) |
Group 3 (Persistently high acute care use) |
|
|---|---|---|---|---|
| N (% of total) | 40,381 | 25,107 (62.2) | 12,932 (32) | 2,342 (5.8) |
| Mean (SD) age | 40.8 (11.9) | 41.6 (11.9) | 39.5 (11.9) | 38.7 (11.2) |
| Age group -N (%) | ||||
| 18–34 years | 13635 (33.8) | 7854 (31.3) | 4898 (37.9) | 883 (37.7) |
| 35–50 years | 17611 (43.6) | 10982 (43.7) | 5502 (42.6) | 1127 (48.1) |
| 51–65 years | 9135 (22.6) | 6271 (25) | 2532 (19.6) | 332 (14.2) |
| Male - N (%) | 2549 (6.3) | 1707 (6.8) | 747 (5.8) | 95 (4.1) |
| Female - N (%) | 37832 (93.7) | 23400 (93.2) | 12185 (94.2) | 2247 (95.9) |
| Race/ethnicity - N (%) | ||||
| Black | 16374 (40.6) | 9764 (38.9) | 5593 (43.3) | 1017 (43.3) |
| White | 14913 (36.9) | 9353 (37.3) | 4626 (35.8) | 934 (39.9) |
| Asian | 1049 (2.6) | 834 (3.3) | 194 (1.5) | 21 (0.9) |
| Hispanic | 6312 (15.6) | 4088 (16.3) | 1946 (15.1) | 278 (11.9) |
| AI/AN | 407 (1.0) | 250 (1.0) | 137 (1.1) | 20 (0.9) |
| Other | 1326 (3.3) | 818 (3.3) | 436 (3.4) | 72 (3.1) |
| Region - N (%) | ||||
| Midwest | 8011 (19.8) | 5003 (19.9) | 2487 (19.2) | 521 (22.3) |
| Northeast | 8375 (20.7) | 5389 (21.5) | 2622 (20.3) | 364 (15.5) |
| South | 15400 (38.1) | 9144 (36.4) | 5241 (40.5) | 1015 (43.3) |
| West | 8595 (21.3) | 5571 (22.2) | 2582 (20) | 442 (18.9) |
| SLE severity* - N (%) | ||||
| Mild | 18553 (45.9) | 12772 (50.9) | 5078 (39.3) | 703 (30.0) |
| Moderate | 14528 (36) | 8489 (33.8) | 5035 (38.9) | 1004 (42.9) |
| Severe | 7300 (18.1) | 3846 (15.3) | 2819 (21.8) | 635 (27.1) |
| Medication use - N (%) | ||||
| Hydroxychloroquine | 15554 (38.5) | 9883 (39.4) | 4911 (38) | 760 (32.5) |
| Corticosteroids | 19191 (47.5) | 10862 (43.3) | 6985 (54) | 1344 (57.4) |
| Immunosuppressives+ | 7329 (18.2) | 4361 (17.4) | 2556 (19.8) | 412 (17.6) |
| Mean (SD) # of drugs | 2.8 (3.6) | 2.4 (3.3) | 3.5 (3.9) | 4.0 (4.3) |
| Comorbidities - N (%) | ||||
| Chronic pain | 829 (2.1) | 554 (2.2) | 238 (1.8) | 37 (1.6) |
| Depression | 14685 (36.4) | 8037 (32) | 5430 (42) | 1218 (52.0) |
| Cardiovascular disease | 19215 (47.6) | 10926 (43.5) | 6849 (53) | 1440 (61.5) |
| Lupus nephritis | 5283 (13.1) | 2827 (11.3) | 2060 (15.9) | 396 (16.9) |
| Diabetes mellitus | 5660 (14) | 3147 (12.5) | 2006 (15.5) | 507 (21.7) |
| Baseline utilization - mean (SD) | ||||
| ED visits | 1.6 (4.1) | 0.7 (1.9) | 2.3 (4.3) | 6.5 (10.6) |
| Outpatient visits | 6.2 (8.1) | 5.1 (7.2) | 7.9 (8.9) | 9.6 (10.3) |
| Hospitalizations | 0.7 (1.5) | 0.4 (1) | 1 (1.7) | 2.2 (3.4) |
| # of Laboratory tests++ | 4.3 (6.4) | 3.7 (5.5) | 5.1 (7.3) | 6.2 (8.1) |
| Zip Code Median household income - median (IQR) | 41,740 (33,486, 52,557) | 41,836 (33,669, 52,565) | 41,507 (33,127, 52,557) | 40,806 (31,871, 51,628) |
All variables determined from the 12 months prior to the index date (date of 3rd ICD-9 code for SLE).
Mean number of drugs and age determined on the index date.
Determined using the Garris ICD-9 and medication-based algorithm for SLE disease severity
Includes: rituximab, leflunomide, methotrexate, tacrolimus, sulfasalazine, mycophenolate mofetil, myfortic, azathioprine, cyclosporine, cyclophosphamide
Includes dsDNA, BUN, creatinine, urinalysis, C3, C4, ESR, CRP, CBC
1.3.1. Acute Care Use for Any Cause
We examined BICs, posterior probabilities and trajectory distributions for GBTMs ranging from three to six trajectory groups with time modeled in cubic and quadratic forms (Supplemental Table 1). We found that both the three-group cubic model and the three-group quadratic models were the best fit for our data of overall acute care use among patients with prevalent SLE and chose the cubic model as it was the simpler model (Figure 1). In our three-group trajectory model, there were 25,107 (62.2%) individuals with SLE classified as infrequent acute care utilizers (group 1), 12,932 (32%) as moderate (group 2) and 2,342 (5.8%) as persistently high (group 3). During the 24-month period, the mean (SD) number of hospitalizations and ED visits was 1.14 (2.37) for infrequent utilizers (group 1), 3.33 (5.0) for moderate utilizers (group 2), and 8.7 (SD 11.3) for the recurrent utilizers (group 3). The recurrent acute care utilizers (group 3) had at least one ED visit or hospitalization during approximately 80% of the eight 90-day periods. The moderate acute care utilizers (group 2) had ED visits or hospitalizations 30–40% of the time, and the infrequent utilizers 5–10%. All three groups, but particularly the moderate utilizers (group 2) had slight declines in acute care utilization over time.
Figure 1:
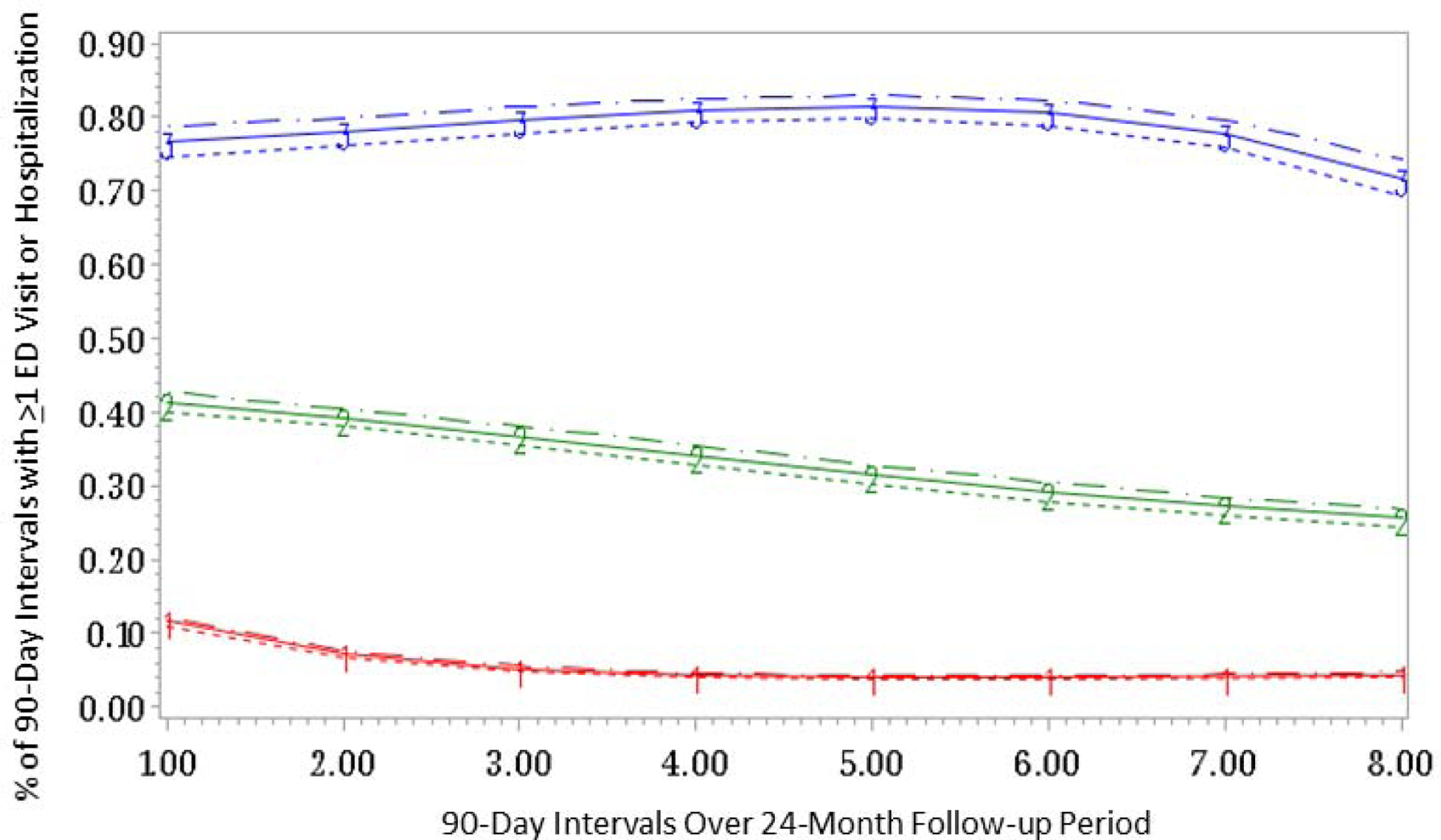
Group based trajectory model demonstrating persistently high acute care utilization for any cause (group 3, N=2,342, 5.8%), moderate acute care utilization (group 2, N=12,932, 32%) and infrequent acute care utilization (group 1, N=25,107, 62.2%) among individuals with prevalent SLE enrolled in Medicaid (N=40,381). Estimate represented by straight line; dashed lines are the 95% Confidence Intervals.
Our multivariable, multinomial model examining demographic predictors (Table 2, Model A) demonstrated strong associations between younger age (18–34 years vs. 51–65 years) and higher odds of belonging to the moderate utilization trajectory (group 2, OR 1.45, 95% CI 1.37–1.54) and to the highest utilization trajectory (group 3, OR 2.0, 95% CI 1.75–2.29) compared to the infrequent utilization trajectory (group 1). Males (vs. females) had significantly lower odds of belonging to the persistently higher utilization trajectories (OR of group 3 vs. 1 0.64, 95% CI 0.52–0.80). Asian and Hispanic individuals had lower odds of belonging to the moderate and high utilizing trajectories compared with white individuals; black individuals had a modestly higher risk of belonging to the moderate utilizing trajectory. Residing in the South (vs. the Northeast) was associated with increased odds of belonging to both the moderate and high utilizing trajectories. While individuals in Groups 2 and 3 lived in zip codes with slightly lower median household incomes (Table 1), we did not find this factor to be significant in Model A (OR (95% CI) for Group 2 vs. 1 of 1.0 (0.94–1.06) for middle income vs. lowest and 0.99 (0.94–1.05) for higher income vs. lowest, and for Group 3 vs. 1, 1.03 (0.92, 1.16) for middle vs. lowest and 1.09 (0.97–1.22) for higher income vs. lowest). We therefore did not include this variable in subsequent models. In Model B, examining SLE severity as a predictor of acute care use, both moderate and severe SLE (vs. mild) were associated with significant odds of belonging to the highest utilizing trajectories with 3.37 times greater odds (95% CI 3.0–3.78) of belonging to group 3 vs. 1 for patients with severe vs. mild SLE. Associations with demographic factors remained similar after including severity in the model.
Table 2:
Multinomial logistic regression models examining the odds (OR, 95% CI) of belonging to Group 2 (moderate acute care utilizers for any cause) or Group 3 (highest acute care utilizers for any cause) compared to Group 1 (lowest acute care utilizers for any cause, reference) by demographic, disease-, medication-, comorbidity- and health care utilization-related predictors among Medicaid beneficiaries with prevalent SLE
| Predictors | Model A OR (95% CI); ref=Group 1 |
Model B OR (95% CI); ref=Group 1 |
Model C OR (95% CI); ref=Group 1 |
Model D OR (95% CI); ref=Group 1 |
Model E OR (95% CI); ref=Group 1 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group 2 | Group 3 | Group 2 | Group 3 | Group 2 | Group 3 | Group 2 | Group 3 | Group 2 | Group 3 | |
| Age group (ref=51–65) | ||||||||||
| 18–34 years | 1.45 (1.37–1.54) | 2.00 (1.75–2.29) | 1.42 (1.33–1.51) | 1.93 (1.69–2.20) | 1.54 (1.45–1.64) | 2.25 (1.96–2.59) | 1.80 (1.69–1.93) | 3.28 (2.84–3.78) | 1.41 (1.33–1.5) | 1.9 (1.66–2.17) |
| 35–50 years | 1.18 (1.11–1.25) | 1.82 (1.60–2.07) | 1.16 (1.10–1.23) | 1.79 (1.58–2.04) | 1.21 (1.14–1.28) | 1.92 (1.68–2.18) | 1.3 (1.22–1.38) | 2.26 (1.98–2.57) | 1.16 (1.1–1.23) | 1.79 (1.57–2.03) |
| Male sex (ref=Female) | 0.90 (0.82–0.98) | 0.64 (0.52–0.80) | 0.84 (0.76–0.92) | 0.57 (0.46–0.70) | 0.89 (0.81–0.98) | 0.63 (0.51–0.79) | 0.87 (0.79–0.95) | 0.63 (0.51–0.78) | 0.86 (0.79–0.95) | 0.59 (0.48–0.73) |
| Race/ethnicity (ref=White) | ||||||||||
| Black | 1.10 (1.04–1.15) | 0.95 (0.86–1.04) | 1.06 (1.01–1.12) | 0.89 (0.81–0.99) | 1.12 (1.06–1.18) | 0.99 (0.90–1.09) | 1.08 (1.03–1.14) | 0.95 (0.85–1.05) | 1.10 (1.05–1.16) | 0.95 (0.86–1.04) |
| AI/AN | 1.08 (0.87–1.34) | 0.76 (0.48–1.22) | 1.06 (0.86–1.32) | 0.74 (0.46–1.18) | 1.1 (0.89–1.37) | 0.80 (0.50–1.27) | 1.09 (0.88–1.36) | 0.77 (0.48–1.23) | 1.11 (0.90–1.39) | 0.8 (0.5–1.27) |
| Asian | 0.46 (0.39–0.54) | 0.25 (0.16–0.39) | 0.43 (0.37–0.51) | 0.23 (0.14–0.35) | 0.46 (0.39–0.55) | 0.26 (0.17–0.41) | 0.47 (0.4–0.55) | 0.27 (0.17–0.42) | 0.43 (0.36–0.51) | 0.22 (0.14–0.34) |
| Hispanic | 0.92 (0.86–0.98) | 0.66 (0.57–0.76) | 0.89 (0.83–0.95) | 0.62 (0.54–0.72) | 0.93 (0.87–1.0) | 0.70 (0.60–0.80) | 0.92 (0.86–0.99) | 0.68 (0.59–0.79) | 0.9 (0.84–0.97) | 0.63 (0.55–0.73) |
| Region (ref=Northeast) | ||||||||||
| Midwest | 1.04 (0.97–1.11) | 1.52 (1.32–1.76) | 1.03 (0.96–1.10) | 1.50 (1.30–1.73) | 1.09 (1.01–1.17) | 1.61 (1.39–1.86) | 0.99 (0.92–1.06) | 1.39 (1.2–1.6) | 1.0 (0.93–1.07) | 1.43 (1.23–1.65) |
| South | 1.21 (1.13–1.28) | 1.71 (1.51–1.94) | 1.20 (1.13–1.28) | 1.71 (1.51–1.95) | 1.2 (1.13–1.27) | 1.66 (1.46–1.89) | 1.14 (1.07–1.21) | 1.54 (1.35–1.75) | 1.18 (1.11–1.25) | 1.66 (1.46–1.88) |
| West | 0.99 (0.92–1.06) | 1.25 (1.08–1.45) | 1.0 (0.93–1.07) | 1.28 (1.11–1.49) | 0.99 (0.93–1.06) | 1.26 (1.09–1.46) | 0.99 (0.93–1.07) | 1.27 (1.09–1.47) | 0.94 (0.88–1.01) | 1.18 (1.01–1.36) |
| SLE severity (ref=Mild) | ||||||||||
| Moderate | 1.42 (1.35–1.49) | 2.03 (1.83–2.25) | 1.33 (1.27–1.4) | 1.82 (1.64–2.02) | ||||||
| Severe | 1.97 (1.85–2.09) | 3.37 (3.0–3.78) | 1.83 (1.72–1.94) | 2.97 (2.64–3.34) | ||||||
| Medication use (vs. no use) | ||||||||||
| Hydroxychloroquine | 0.75 (0.71–0.78) | 0.55 (0.50–0.60) | ||||||||
| Immunosuppressives* | 0.98 (0.92–1.03) | 0.81 (0.72–0.91) | ||||||||
| Corticosteroids | 1.3 (1.24–1.37) | 1.51 (1.38–1.66) | ||||||||
| Number of drugs (ref=0–5) | ||||||||||
| >5–10 | 1.25 (1.18–1.33) | 1.32 (1.19–1.48) | ||||||||
| >10 | 1.78 (1.62–1.95) | 2.37 (2.02–2.77) | ||||||||
| Comorbidities | ||||||||||
| Diabetes mellitus | 1.3 (1.22–1.39) | 1.96 (1.74–2.19) | ||||||||
| Cardiovascular disease | 1.56 (1.49–1.64) | 2.29 (2.08–2.52) | ||||||||
| Lupus nephritis | 1.28 (1.19–1.36) | 1.26 (1.12–1.43) | ||||||||
| Depression | 1.41 (1.34–1.48) | 1.9 (1.74–2.09) | ||||||||
| Chronic pain | 1.45 (1.24–1.7) | 1.63 (1.15–2.32) | ||||||||
| Utilization | ||||||||||
| Outpatient visits (ref=0–5) | ||||||||||
| >5–10 | 1.12 (1.06–1.2) | 1.18 (1.05–1.34) | ||||||||
| >10 | 1.36 (1.28–1.45) | 1.45 (1.29–1.63) | ||||||||
| Laboratory tests (ref=0–5)+ | ||||||||||
| >5–10 | 1.17 (1.11–1.25) | 1.21 (1.08–1.36) | ||||||||
| >10 | 1.27 (1.18–1.36) | 1.61 (1.42–1.82) | ||||||||
Model A: Demographic factors
Model B: Demographic factors + Garris SLE severity algorithm
Model C: Demographic factors + medications
Model D: Demographic factors + comorbidities
Model E: Demographic factors + Garris SLE severity algorithm + health care utilization
All models are additionally adjusted for calendar year of index date.
Immunosuppressives includes rituximab, leflunomide, methotrexate, tacrolimus, sulfasalazine, mycophenolate mofetil, myfortic, azathioprine, cyclosporine, cyclophosphamide
Includes dsDNA, BUN, creatinine, urinalysis, C3, C4, ESR, CRP, CBC
In Model C examining medication use, hydroxychloroquine use was associated with significantly lower odds of both moderate and high acute care utilization with 45% lower odds of belonging to group 3 vs. 1 among users vs. nonusers. Immunosuppressive use was also associated with lower odds of both moderate and high acute care use; corticosteroid use was associated with significantly higher odds. More medications at the index date was associated with higher odds of acute care use. In Model D, all of the comorbidities examined (diabetes, cardiovascular disease, lupus nephritis, depression and chronic pain) were associated with significantly higher odds of moderate and high utilization patterns with the most pronounced effect seen for cardiovascular disease. In addition, after adjusting for these comorbidities, the odds associated with younger age of belonging to the highest utilization trajectory significantly increased compared to the demographics-only model (OR 3.28, 95% CI 2.84–3.78 for group 3 vs. 1 for age 18–34 years vs. 51–65 years). In Model E, more frequent outpatient utilization and more laboratory tests were also associated with higher odds of moderate and high acute care use patterns. In a sensitivity analysis, we also conducted multivariable multinomial regression models using the three-group quadratic model and results were nearly identical.
1.3.2. SLE-specific Acute Care Use
We also examined trajectories of acute care use specifically for SLE in our prevalent SLE cohort. Here, a three-group quadratic model (Supplemental Table 1) was the best fit for our data (Figure 2) with 3.1% in the highest use trajectory with 50–65% of 90-day periods with ≥1 ED visit or hospitalization for SLE, 22.3% in the moderate use group (10–25% of 90-day periods with acute care use) and 74.6% with infrequent or no use for SLE. The mean (SD) number of ED visits or hospitalizations was 1.68 (3.79) for infrequent users (group 1), 3.51 (5.78) for moderate users (group 2), and 7.89 (9.25) for persistently high users (group 3).
Figure 2.
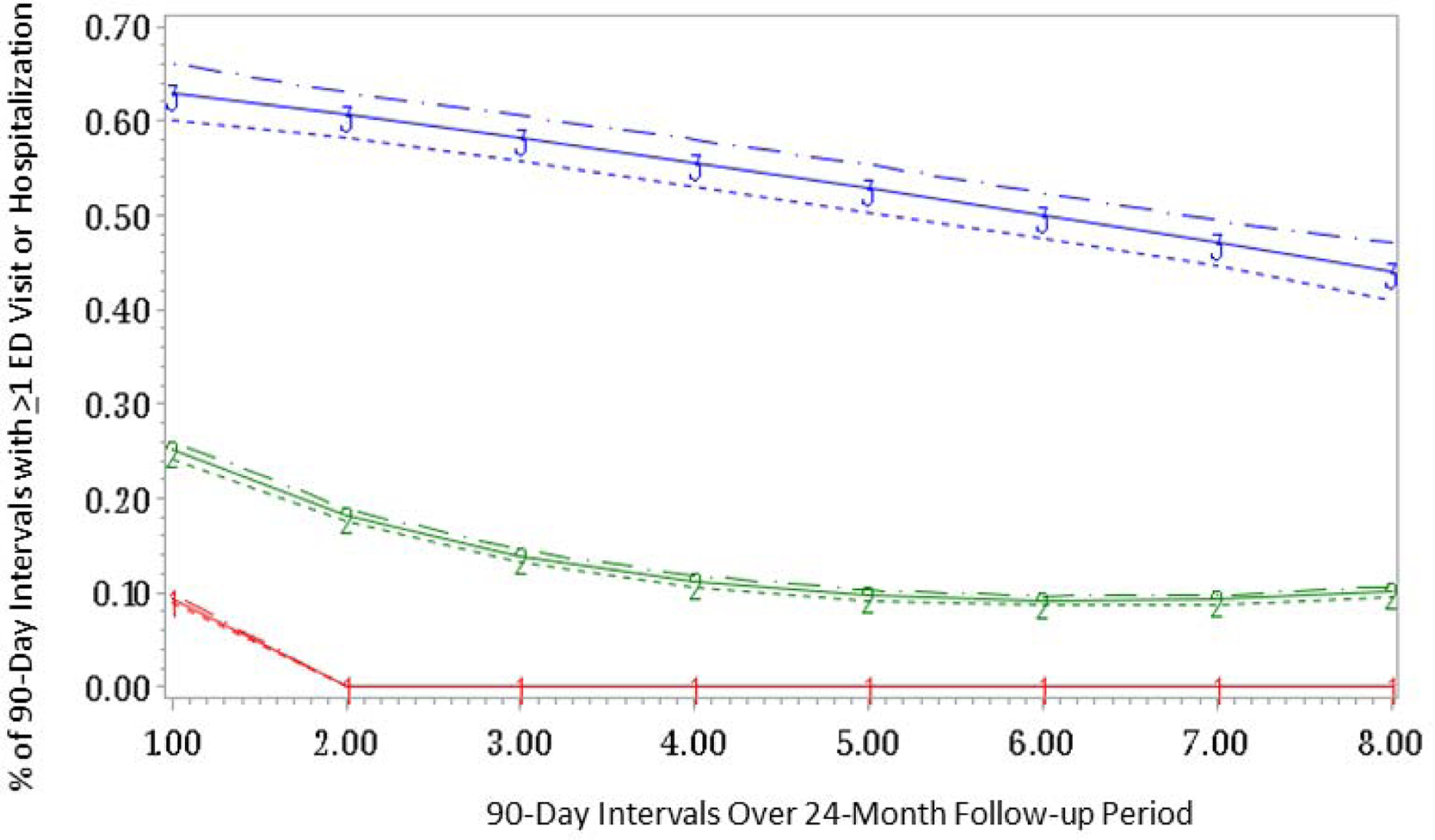
Group based trajectory model demonstrating persistently high acute care utilization for SLE (group 3, N=1,242, 3.1%), moderate acute care utilization (group 2, N=9,020, 22.3%) and infrequent acute care utilization (group 1, N=30,119, 74.6%) among individuals with prevalent SLE enrolled in Medicaid (N=40,381). Estimate represented by straight line; dashed lines are the 95% Confidence Intervals.
In multinomial models, like overall acute care use, younger age was associated with greater odds of belonging to a persistently high SLE-specific acute care use trajectory, and this was particularly pronounced after adjusting for comorbidities (Table 3). Male sex was associated with lower odds of belonging to a high acute care use trajectory. Similar to the overall acute care use model, zip code median household income was not significant in the demographic model for SLE-specific acute care use and therefore was not included in subsequent models. Associations by race/ethnicity differed in the SLE-specific trajectory models compared to the overall acute care use models. Here, black race was associated with significantly higher odds across all models of belonging to a higher vs. lower SLE-specific acute care use trajectory. While Hispanic ethnicity was associated with a lower odds of overall acute care use, we observed either higher odds, or no significant association, depending on the model, with higher SLE-specific acute care use. Similar to the overall acute care use model, more severe SLE resulted in higher odds of belonging to a persistently high acute care use SLE-specific trajectory and here, cardiovascular disease, lupus nephritis and depression also contributed significantly. Chronic pain and diabetes, however, were not significant in this model. Hydroxychloroquine use was associated with lower odds of SLE-specific acute care use and corticosteroids with higher odds.
Table 3.
Multinomial logistic regression models of SLE-specific acute care use# examining the odds (OR, 95% CI) of belonging to Group 2 (moderate acute care utilizers for SLE) or Group 3 (highest acute care utilizers for SLE) compared to Group 1 (lowest acute care utilizers for SLE, reference) by demographic, disease-, medication-, comorbidity- and health care utilization-related predictors among Medicaid beneficiaries with prevalent SLE
| Predictors | Model A OR (95% CI); ref=Group 1 |
Model B OR (95% CI); ref=Group 1 |
Model C OR (95% CI); ref=Group 1 |
Model D OR (95% CI); ref=Group 1 |
Model E OR (95% CI); ref=Group 1 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group 2 | Group 3 | Group 2 | Group 3 | Group 2 | Group 3 | Group 2 | Group 3 | Group 2 | Group 3 | |
| Age group (ref=51–65) | ||||||||||
| 18–34 years | 2.04 (1.90–2.18) | 4.86 (3.90–6.06) | 1.99 (1.86–2.14) | 4.71 (3.78–5.88) | 2.01 (1.87–2.17) | 4.75 (3.79–5.95) | 2.26 (2.10–2.43) | 6.28 (5.0–7.91) | 1.97 (1.83–2.11) | 4.55 (3.65–5.69) |
| 35–50 years | 1.49 (1.39–1.59) | 2.90 (2.33–3.63) | 1.47 (1.38–1.58) | 2.87 (2.30–3.59) | 1.48 (1.38–1.58) | 2.87 (2.29–3.59) | 1.57 (1.46–1.68) | 3.24 (2.59–4.05) | 1.47 (1.37–1.57) | 2.84 (2.27–3.55) |
| Male sex (ref=Female) | 0.80 (0.72–0.89) | 0.80 (0.61–1.04) | 0.75 (0.68–0.84) | 0.71 (0.55–0.93) | 0.79 (0.71–0.88) | 0.78 (0.60–1.02) | 0.77 (0.69–0.85) | 0.75 (0.58–0.98) | 0.76 (0.68–0.85) | 0.72 (0.55–0.93) |
| Race/ethnicity (ref=White) | ||||||||||
| Black | 1.32 (1.24–1.39) | 1.63 (1.42–1.86) | 1.28 (1.21–1.36) | 1.56 (1.36–1.79) | 1.30 (1.23–1.38) | 1.59 (1.39–1.82) | 1.28 (1.21–1.36) | 1.62 (1.41–1.86) | 1.30 (1.23–1.38) | 1.58 (1.38–1.81) |
| AI/AN | 0.99 (0.77–1.27) | 0.67 (0.31–1.44) | 0.97 (0.75–1.25) | 0.66 (0.31–1.41) | 0.98 (0.76–1.26) | 0.67 (0.31–1.44) | 0.99 (0.77–1.28) | 0.67 (0.31–1.45) | 0.99 (0.77–1.28) | 0.68 (0.32–1.45) |
| Asian | 0.77 (0.65–0.92) | 0.56 (0.33–0.93) | 0.74 (0.62–0.88) | 0.52 (0.31–0.86) | 0.74 (0.62–0.88) | 0.53 (0.32–0.88) | 0.77 (0.64–0.91) | 0.58 (0.35–0.97) | 0.73 (0.62–0.87) | 0.50 (0.30–0.84) |
| Hispanic | 1.12 (1.04–1.21) | 1.01 (0.84–1.23) | 1.09 (1.01–1.18) | 0.97 (0.80–1.18) | 1.10 (1.02–1.18) | 0.99 (0.81–1.20) | 1.12 (1.04–1.21) | 1.05 (0.86–1.28) | 1.10 (1.02–1.18) | 0.97 (0.79–1.17) |
| Region (ref=Northeast) | ||||||||||
| Midwest | 1.00 (0.92–1.08) | 1.24 (1.03–1.49) | 0.99 (0.92–1.07) | 1.22 (1.02–1.47) | 1.06 (0.98–1.15) | 1.36 (1.13–1.63) | 0.97 (0.90–1.04) | 1.16 (0.96–1.39) | 0.98 (0.90–1.05) | 1.21 (1.00–1.45) |
| South | 0.97 (0.91–1.04) | 1.17 (0.99–1.38) | 0.97 (0.91–1.04) | 1.16 (0.99–1.37) | 0.98 (0.92–1.05) | 1.16 (0.98–1.36) | 0.94 (0.88–1.01) | 1.09 (0.93–1.29) | 0.95 (0.89–1.02) | 1.14 (0.97–1.35) |
| West | 0.89 (0.83–0.96) | 1.03 (0.85–1.24) | 0.90 (0.83–0.97) | 1.04 (0.86–1.26) | 0.91 (0.84–0.98) | 1.05 (0.87–1.27) | 0.90 (0.83–0.97) | 1.04 (0.86–1.26) | 0.87 (0.80–0.94) | 0.99 (0.81–1.20) |
| SLE severity (ref=Mild) | ||||||||||
| Moderate | 1.47 (1.39–1.55) | 1.75 (1.53–2.01) | 1.41 (1.34–1.49) | 1.66 (1.44–1.90) | ||||||
| Severe | 1.71 (1.60–1.82) | 2.61 (2.24–3.04) | 1.63 (1.52–1.74) | 2.43 (2.08–2.84) | ||||||
| Medication use (vs. no use) | ||||||||||
| Hydroxychloroquine | 0.92 (0.88–0.97) | 0.78 (0.69–0.88) | ||||||||
| Immunosuppressives* | 1.09 (1.02–1.16) | 1.0 (0.86–1.15) | ||||||||
| Corticosteroids | 1.44 (1.37–1.52) | 1.89 (1.66–2.16) | ||||||||
| Number of drugs (ref=0–5) | ||||||||||
| >5–10 | 1.11 (1.05–1.19) | 1.05 (0.91–1.22) | ||||||||
| >10 | 1.35 (1.22–1.49) | 1.44 (1.15–1.80) | ||||||||
| Comorbidities | ||||||||||
| Diabetes mellitus | 1.03 (0.95–1.11) | 1.17 (0.98–1.39) | ||||||||
| Cardiovascular disease | 1.37 (1.30–1.44) | 1.83 (1.61–2.07) | ||||||||
| Lupus nephritis | 1.27 (1.18–1.36) | 1.42 (1.22–1.66) | ||||||||
| Depression | 1.18 (1.12–1.25) | 1.64 (1.45–1.85) | ||||||||
| Chronic pain | 1.12 (0.92–1.37) | 1.09 (0.59–2.02) | ||||||||
| Utilization | ||||||||||
| Outpatient visits (ref=0–5) | ||||||||||
| >5–10 | 1.02 (0.95–1.09) | 0.95 (0.81–1.11) | ||||||||
| >10 | 1.06 (0.99–1.13) | 0.95 (0.82–1.11) | ||||||||
| Laboratory tests (ref=0–5)+ | ||||||||||
| >5–10 | 1.10 (1.03–1.17) | 1.10 (0.94–1.28) | ||||||||
| >10 | 1.26 (1.16–1.35) | 1.55 (1.32–1.83) | ||||||||
SLE-specific acute care use defined as ED visits or hospitalizations with ICD-9 code for SLE (710.0) in the first or second discharge diagnosis position
Model A: Demographic factors
Model B: Demographic factors + Garris SLE severity algorithm
Model C: Demographic factors + medications
Model D: Demographic factors + comorbidities
Model E: Demographic factors + Garris SLE severity algorithm + health care utilization
All models are additionally adjusted for calendar year of index date.
Immunosuppressives includes rituximab, leflunomide, methotrexate, tacrolimus, sulfasalazine, mycophenolate mofetil, myfortic, azathioprine, cyclosporine, cyclophosphamide
Includes dsDNA, BUN, creatinine, urinalysis, C3, C4, ESR, CRP, CBC
1.3.3. Mortality
Following the 24-month utilization study period, over the remaining course of Medicaid enrollment for the prevalent SLE cohort, there were 256 deaths (10.9%, incidence rate [IR] 2.2 per 100 person-years) among the recurrent acute care utilizers for any cause (group 3), 965 deaths (7.5%, IR 1.7 per 100 person-years) among the moderate utilizers (group 2), and 968 deaths (3.9%, IR 1.2 per 100 person-years) among the infrequent utilizers (group 1). In Cox proportional hazards models adjusted for age, sex, region, race/ethnicity and calendar year, the hazard ratio (HR) of death for recurrent acute care utilizers (group 3) compared to infrequent utilizers (group 1) was 2.31 (95% CI 2.00–2.66) and the HR for group 2 vs. 1 was 1.61 (95% CI 1.47–1.76). Estimates remained similar after additionally adjusting for baseline SLE severity (for group 3 vs. 1, HR 2.08, 95% CI 1.80–2.40, for group 2 vs. 1 HR 1.52, 95% CI 1.39–1.67).
1.3.4. Secondary Analysis: Acute Care Use among Patients with Incident SLE
We identified 6,069 individuals with incident SLE. In this cohort, 95% were female, the mean (SD) age was 43 (SD 11.6) and the racial/ethnic and geographic distribution was similar to the prevalent cohort. We identified 3,821 individuals with mild SLE (63%), 1,605 (26.5%) with moderate and 643 (10.6%) with severe. 22.7% of incident SLE patients received HCQ, 10.7% immunosuppressives and 32.4% corticosteroids. CVD and depression were similarly common among incident SLE patients with 44.1% and 35.9% of the cohort respectively, with these comorbidities.
We found that a 3-group cubic model was the best fit for overall acute care use trajectories (Supplemental Figure 1). There were 4,085 infrequent users (group 1), 1,606 moderate users (group 2) and 378 recurrent users (group 3). The overall pattern and the distributions in each trajectory were similar to the prevalent SLE cohort with a slightly higher percent of incident SLE patients with recurrent acute care use (6.2%, compared to 5.8% of prevalent SLE patients). There was also a slightly higher percent of infrequent utilizers among incident SLE patients (67.3% compared to 62.2% of prevalent SLE patients).
For most multinomial models, like the prevalent cohort, among incident SLE patients, younger patients (age 18–34 years) had significant higher odds of belonging to the moderate (group 2) or recurrent acute care use (group 1) trajectory compared to the infrequent use trajectory (group 1) (Supplemental Table 2). Hispanic ethnicity was associated with lower odds of belonging to a higher acute care use trajectory in all models and Asian race was associated with lower odds as well in most models. Similar to the prevalent cohort, there were lower odds of recurrent acute care use among hydroxychloroquine users and higher odds among corticosteroids users as well as among those with more severe SLE. While diabetes, cardiovascular disease, lupus nephritis and depression were associated with higher odds, chronic pain was not among incident SLE patients however the sample size per group was smaller. We examined trajectory models for SLE-specific acute care use however the mean posterior probabilities in all model permutations were low (<80%) for at least one trajectory in each model due to the smaller sample size and therefore we did not pursue multivariable multinomial models to assess predictors.
Among patients with incident SLE, there were 25 deaths (6.6%, IR 2.5 per 100 person-years) after the 24-month follow-up period for group 3, 113 (7%, IR 2.0 per 100 person-years) for group 2 and 128 (3.1%, IR 1.3 per 100 person-years) for group 1. In age, sex, race/ethnicity, year and region-adjusted Cox models, the HR of death for group 3 vs. 1 was 2.43 (95% CI 1.56–3.80) and for group 2 vs. 1, 1.60 (95% CI 1.23–2.09).
1.3.5. Sensitivity Analyses
Sensitivity analyses conducted among prevalent SLE patients requiring ≥90 days of follow-up beginning at the index date rather than ≥24 months (N=59,034) and among incident SLE patients with ≥90 days of follow-up (N=25,931) resulted in trajectory models that closely paralleled the ≥24-month models with similar distributions across groups. In addition, multinomial models showed statistically significant findings of similar magnitude for the same predictors as the respective prevalent and incident cohort main analysis models.
1.4. Discussion
In this U.S.-based cohort of Medicaid beneficiaries with SLE for the 29 most populated states, 38% of patients with prevalent SLE and 33% of patients with incident SLE had persistent patterns of moderate-to-high acute care use over a 24-month period. The high percentages of Medicaid beneficiaries we observed with SLE with repeated hospitalizations and ED visits and associated high mortality, are in line with prior studies that have shown that Medicaid insurance is associated with high readmission rates, more ED visits, and higher in-hospital mortality compared with commercially insured patients.(2, 3, 16, 17) Medicaid insurance serves as a proxy for low socioeconomic status, which has been associated both with receipt of poorer quality ambulatory care and with higher rates of avoidable hospitalizations among SLE patients.(18, 19) In the general population, Medicaid patients have been shown to have the highest ED revisit rates compared to Medicare and privately insured patients and patients living in the poorest communities have significantly higher hospital revisit rates compared to those living in the wealthiest areas.(20) In our study, we found that zip code median household income was modestly lower among individuals in the more frequent acute care use trajectories compared to the least frequent, however this variable was not statistically significant in our models. We hypothesize that the lack of significance may in part be due to an overall lack of variability in zip code median household income in this cohort. It is also possible that zip code may not fully capture a true neighborhood as well as more granular measures (e.g. census block), which were not available to us in this dataset.
While prior smaller studies have similarly demonstrated that SLE patients are frequent utilizers of the ED and are at high risk for readmission, to our knowledge, large and diverse cohort study in a particularly vulnerable population, is the first to demonstrate the persistent and distinct longitudinal patterns of acute care use both among patients with new and existing SLE.(3) Both among prevalent and incident SLE patients, there was a modest trend towards reduced overall acute care use over the 24-month follow-up period among the moderate users but quite consistent repeated use over time among the highest utilizers for overall acute care use. The trend towards decreased utilization over time was more pronounced for SLE-specific acute care use. Our analyses were adjusted for calendar year and we found that the earlier years (2000–2005, and particularly 2000–2002), were associated with increased odds of more frequent acute care use compared to later years. Both the Centers for Medicare and Medicaid Services as well as the Agency for Healthcare Research and Quality, increased their focus on readmissions among vulnerable patient populations, and on potentially avoidable hospitalizations, in the early 2000s.(21) It is possible that our findings may reflect increased attention and policy changes to help these higher use groups. Treatments including mycophenolate mofetil and hydroxychloroquine were also more commonly used in the later years of our study. The more pronounced decrease in SLE-specific acute care use may be due to improved access to subspecialty care established during an earlier ED visit or hospitalization.
More severe SLE was a strong risk factor for persistently high acute care use in our study, and those with the highest acute care use also had the highest risk of subsequent mortality. This finding is in line with prior studies as well. In an analysis of 807 SLE patients in the San Francisco Lupus Outcomes Study, 10% of SLE patients self-reported ≥3 ED visits in the prior 12 months and more frequent use was associated both with greater disease activity and with Medicaid insurance.(3) In a study of 2,990 SLE patients in a large managed care plan, increased SLE severity and more severe flares were also associated with higher healthcare utilization and higher costs.(5) One study among 223 patients with SLE over a one-year period found a higher risk of readmission to be associated with more active and severe SLE, and younger patients with frequent readmissions had the highest risk of mortality.(22) In our analyses, younger age (18–34 years) was a strong and independent risk factor for higher, persistent acute care use, even after adjusting for disease severity. This association was even more pronounced after adjusting for comorbidities, more likely to have accumulated in older patients. An association between younger age and higher probability of 30-day readmission was demonstrated in a prior study among 31,903 patients with SLE from five U.S. states and our finding of recurrent acute care use among younger individuals is in line with this.(2)
Not surprisingly, we found strong associations between comorbidities and SLE-related complications, notably lupus nephritis, cardiovascular disease, chronic pain and depression and higher odds of persistent acute are use. Prior studies have demonstrated high rates of hospitalization for cardiovascular events among SLE patients and increased risk of hospitalization and increased healthcare costs among patients with lupus nephritis.(22–25) Chronic pain was similarly found to be a significant factor in repeated ED visits among SLE patients at one academic medical center.(6) It is possible that better ambulatory care prevention strategies could mitigate both ED visits as well as avoidable adverse outcomes associated with these conditions.
In addition to age, we found associations between certain sociodemographic factors and persistent acute care use. For all-cause utilization, after adjusting for disease severity, comorbidities and medication use, compared to white individuals, we observed reduced odds among Asian and Hispanic individuals, and depending on adjustment factors, a modestly increased odds among black individuals. These associations were somewhat different for SLE-specific acute care use where we observed more pronounced and consistently significant increased odds among black individuals compared to white, modestly increased odds among Hispanic individuals, even after adjusting for SLE severity. One study examining all-cause 30-day readmissions found increased risk of readmission among black and Hispanic patients compared to white patients, however these associations were similarly modest (OR 1.18, 95% CI 1.09–1.28 for black vs. white patients and OR 1.12, 95% CI 1.02–1.22 for Hispanic vs. white patients).(2) The different associations observed between all-cause acute care use and SLE-specific acute care use by race/ethnicity suggest that may not be patient preference for care utilization by racial/ethnic group, or quality of care delivered within racial/ethnic groups and may be more related to the activity of SLE, or to access to subspecialty care, neither of which we could measure directly. The consistently lower odds of acute care use among Asian individuals is in line with prior studies demonstrating possibly less severe disease and lower mortality among Asian Medicaid beneficiaries, however the small sample size, and lack of other studies in the literature in this population, suggest that further research is needed to help explain this association.(8) For all-cause acute care use, we also observed higher odds of persistent use associated with living in the South and in the West (vs. the Northeast). These findings differed for SLE-specific acute care use with no significant difference in odds for the South vs. Northeast and lower odds for the West. It is possible that there are fewer geographic differences in quality of SLE-specific ambulatory care but more pronounced differences in ambulatory care access and quality for other comorbidities.
In all analyses, we found a significant, protective association between hydroxychloroquine use and lower rates of persistent acute care use. In a prior study conducted by our group in the Medicaid population, we similarly demonstrated a significant association with hydroxychloroquine nonadherence and increased odds of an acute care visit.(4) Another study, among 180 SLE patients seen in one ED, similarly found that antimalarial use was associated with a reduced risk of acute care use.(26) Hydroxychloroquine use, which is standard-of-care for SLE management, is an indicator of receipt of appropriate quality ambulatory care, which in turn would reduce the need for acute care use. We have previously reported, and again observe in this study that 38.5% baseline hydroxychloroquine use does not meet the suggested standard of care for SLE.(18) It is also plausible that hydroxychloroquine use is a proxy for access to subspecialty care, which we could not measure directly using our data source. It is unlikely that this medication would be prescribed in an acute care setting, or by a primary care provider, and therefore receipt of this medication likely suggests access to a rheumatologist, or to a nephrologist. Corticosteroid use on the other hand, was associated with significantly increased acute care use, whereas immunosuppressive use was not, suggesting that corticosteroid use may be more a reflection of fragmented and poorer quality ambulatory care than of more severe SLE.
Our study has several strengths. We used nationwide data from Medicaid, the largest public health insurance program in the U.S., which captures a low-income, vulnerable population with a high burden of SLE, and examined our study question both among >40,000 patients with prevalent SLE and among >6000 patients with incident SLE. Acute care use was determined from claims and not from self-report or from a single institution’s electronic medical record data ensuring that most if not all events were captured. We explored both overall acute care use, which is particularly relevant to SLE patients who have many comorbidities many of which may be related to SLE or to the treatment but may not be billed for as such, as well as use specifically for SLE. We also examined the role of both nonmodifiable (e.g. demographic) risk factors as well as modifiable risk factors to understand whether receipt of high quality care (e.g. hydroxychloroquine prescriptions) was associated with less frequent acute care use. We applied a novel method, group-based trajectory modeling, to examine dynamic acute care use patterns over time rather than counting number of ED visits or of readmissions, with the hope of capturing trends in behavior rather than discrete and potentially isolated periods of significant illness.
There are also limitations to this work. While surrogate markers were used to determine SLE severity (e.g. a published algorithm by Garris et al., number of SLE-related lab tests and medications), we lack direct patient or physician-reported measures or actual lab results for SLE severity or activity. We did require a ≥36-month period of enrollment in Medicaid (12 months for baseline variable assessment and ≥24 months of follow-up) for prevalent SLE patients for these analyses and these patients may be different from individuals who are enrolled in Medicaid for short periods of time, or who move in and out of Medicaid coverage. However, in a sensitivity analysis requiring only ≥3 months of follow-up time after the baseline period, the patterns of acute care use we observed were nearly identical to our main analyses requiring ≥24 months. We did not explore all potential comorbidity-related risk factors, however the conditions we chose to explore, notably cardiovascular disease, diabetes, depression and chronic pain, have been shown either to be particularly prevalent among SLE patients, especially those enrolled in Medicaid, or significant risk factors for acute care use.(2, 4, 6, 25) We also lacked information on the addresses of Medicaid beneficiaries, which would have allowed us to geocode our data and examine the role of potentially important neighborhood-level factors such as socioeconomic status and neighborhood safety.
1.5. Conclusions
In this large study of Medicaid beneficiaries with incident and prevalent SLE, we found high rates of persistent all-cause and SLE-specific acute care use over a 24-month period. Our analyses demonstrated that certain factors that are potentially modifiable, notably increasing hydroxychloroquine use and limiting corticosteroid use, and enhancing screening and preventive care for comorbidities including lupus nephritis, depression and cardiovascular disease, may reduce the need for repeated readmissions and ED visits. Improving the quality of ambulatory care for all patients with SLE, particularly lower socioeconomic status individuals with a high burden of chronic disease and adverse outcomes, will hopefully begin to reduce the disparities we observe in avoidable acute care use.
Supplementary Material
Highlights.
Nearly 40% of Medicaid beneficiaries with SLE have recurrent all-cause acute care
25% have recurrent acute care use for SLE
Comorbidities, chronic pain, female sex and younger age associated with higher use
Modifiable factors like increasing hydroxychloroquine use may reduce acute care use
Funding:
This work was supported by NIH/NIAMS K23 AR071500 (Feldman).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: The authors have no competing interests to declare.
1.6 References
- 1.Kan HJ, Song X, Johnson BH, Bechtel B, O’Sullivan D, Molta CT. Healthcare utilization and costs of systemic lupus erythematosus in Medicaid. Biomed Res Int. 2013;2013:808391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yazdany J, Marafino BJ, Dean ML, Bardach NS, Duseja R, Ward MM, et al. Thirty-day hospital readmissions in systemic lupus erythematosus: predictors and hospital- and state-level variation. Arthritis Rheumatol. 2014;66(10):2828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panopalis P, Gillis JZ, Yazdany J, Trupin L, Hersh A, Julian L, et al. Frequent use of the emergency department among persons with systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010;62(3):401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman CH, Yazdany J, Guan H, Solomon DH, Costenbader KH. Medication Nonadherence Is Associated With Increased Subsequent Acute Care Utilization Among Medicaid Beneficiaries With Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken). 2015;67(12):1712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garris C, Jhingran P, Bass D, Engel-Nitz NM, Riedel A, Dennis G. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J Med Econ. 2013;16(5):667–77. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Lin J, Suter LG, Fraenkel L. Persistently Frequent Emergency Department Utilization Among Persons With Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken). 2019;71(11):1410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 2013;65(3):753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Puerta JA, Barbhaiya M, Guan H, Feldman CH, Alarcon GS, Costenbader KH. Racial/Ethnic variation in all-cause mortality among United States medicaid recipients with systemic lupus erythematosus: a Hispanic and asian paradox. Arthritis Rheumatol. 2015;67(3):752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bureau USC. United States Census 2000. 2000.
- 10.Manson S, Schroeder J, Van Riper D, Ruggles S. IPUMS National Historical Geographic Information System: Version 12.0 [Database]. In: Minnesota Uo, editor. Minneapolis: 2017. [Google Scholar]
- 11.Li D, Yoshida K, Feldman CH, Speyer C, Barbhaiya M, Guan H, et al. Initial disease severity, cardiovascular events and all-cause mortality among patients with systemic lupus erythematosus. Rheumatology (Oxford). 2019. [Google Scholar]
- 12.Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus. 2010;19(6):741–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagin DS. Group-based modeling of development. Cambridge, MA: Harvard University Press; 2005. 2005. [Google Scholar]
- 14.Franklin JM, Shrank WH, Pakes J, Sanfelix-Gimeno G, Matlin OS, Brennan TA, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med Care. 2013;51(9):789–96. [DOI] [PubMed] [Google Scholar]
- 15.Feldman CH, Collins J, Zhang Z, Subramanian SV, Solomon DH, Kawachi I, et al. Dynamic patterns and predictors of hydroxychloroquine nonadherence among Medicaid beneficiaries with systemic lupus erythematosus. Semin Arthritis Rheum. 2018;48(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward MM. Hospital experience and mortality in patients with systemic lupus erythematosus: which patients benefit most from treatment at highly experienced hospitals? J Rheumatol. 2002;29(6):1198–206. [PubMed] [Google Scholar]
- 17.Gillis JZ, Yazdany J, Trupin L, Julian L, Panopalis P, Criswell LA, et al. Medicaid and access to care among persons with systemic lupus erythematosus. Arthritis Rheum. 2007;57(4):601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yazdany J, Feldman CH, Liu J, Ward MM, Fischer MA, Costenbader KH. Quality of care for incident lupus nephritis among Medicaid beneficiaries in the United States. Arthritis Care Res (Hoboken). 2014;66(4):617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward M. Avoidable hospitalizations in patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2008;59(2):162–8. [DOI] [PubMed] [Google Scholar]
- 20.Steiner C, Barrett M, Hunter K. Hospital Readmissions and Multiple Emergency Department Visits, in Selected States, 2006–2007: Stastical Brief #90. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 21.AHRQ Quality Indicators - Guide to Prevention Quality Indicators: Hospital Admission for Ambulatory Care Sensitive Conditions. In: Quality AfHRa, editor. Rockville, MD: 2001. [Google Scholar]
- 22.Edwards CJ, Lian TY, Badsha H, Teh CL, Arden N, Chng HH. Hospitalization of individuals with systemic lupus erythematosus: characteristics and predictors of outcome. Lupus. 2003;12(9):672–6. [DOI] [PubMed] [Google Scholar]
- 23.Pelletier EM, Ogale S, Yu E, Brunetta P, Garg J. Economic outcomes in patients diagnosed with systemic lupus erythematosus with versus without nephritis: results from an analysis of data from a US claims database. Clin Ther. 2009;31(11):2653–64. [DOI] [PubMed] [Google Scholar]
- 24.Tektonidou MG, Wang Z, Ward MM. Brief Report: Trends in Hospitalizations Due to Acute Coronary Syndromes and Stroke in Patients With Systemic Lupus Erythematosus, 1996 to 2012. Arthritis Rheumatol. 2016;68(11):2680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbhaiya M, Feldman CH, Guan H, Gomez-Puerta JA, Fischer MA, Solomon DH, et al. Race/Ethnicity and Cardiovascular Events among Patients with Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rojas-Serrano J, Cardiel MH. Lupus patients in an emergency unit. Causes of consultation, hospitalization and outcome. A cohort study. Lupus. 2000;9(8):601–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


