Abstract
Background & Aims:
Anesthesia services for endoscopic procedures have proliferated with the promise of increased comfort and safety. Cirrhosis patients are higher risk for sedation, yet limited data are available describing anesthesia complications in this population.
Approach & Results:
This cross-sectional study utilized the National Anesthesia Clinical Outcomes Registry, a multi-center quality improvement database from 2010 to 2015. Cirrhosis patients undergoing an endoscopy were identified by ICD 9/CPT codes. The outcome of interest was serious anesthesia-related complication defined as cardiovascular, respiratory, neurologic, drug-related, patient injury, death, or unexpected admission. A mixed effects multivariate logistic regression model determined odds ratios between variables and serious complications adjusting for potential confounders. In total, 9,007 endoscopic procedures were performed among cirrhosis patients; 92% were esophagogastroduodenoscopies. A majority (81%) were American Society of Anesthesiologists (ASA) class >=3 and 72% had a history of hepatic encephalopathy, ascites, varices, hepatorenal syndrome, or spontaneous bacterial peritonitis identified by ICD-9/CPT codes. In total, 87 complications were reported, 33 of which were serious. The frequency of serious complications was 0.4% or 378.6 per 100,000 procedures (95% CI 260.8, 531.3). A majority of serious complications were cardiovascular (21/33) including 15 cardiac arrests. Serious complications were significantly associated with ASA4/5 (OR 3.84; 95% CI 1.09, 13.57) and general anesthesia (OR 4.71; 95% CI 1.20, 18.50) adjusting for age, sex, ASA class, anesthesia type, inpatient status, portal hypertension history, and variable complication reporting practices.
Conclusions:
Anesthesia complications among endoscopic procedures in cirrhosis are rare overall. Serious complications were predominantly cardiac and associated with sicker patients undergoing general anesthesia. The complexity of end stage liver disease may warrant more intensive care during endoscopic procedures including anesthesia monitoring.
Keywords: anesthesia-directed sedation, general anesthesia, gastrointestinal endoscopy, complication, cirrhosis
Introduction
Anesthesia services for gastrointestinal (GI) endoscopic procedures have risen dramatically over the past decade (1,2) with the promise of increased safety, patient comfort, and efficiency (2,3). Propofol anesthesia has been shown to be safe and effective in patients with cirrhosis undergoing esophagogastroduodenoscopy (EGD)(4), colonoscopy, and endoscopic retrograde cholangiopancreatography (ERCP) (5). However, limited data are available describing specific anesthesia-associated complications in this population. Moreover, it remains unclear whether anesthesia directed sedation (ADS), predominantly using propofol as compared to a combination or opioids and benzodiazepines, is associated with increased complications in this population (6).
Professional guidelines recommend that any individual with the diagnosis of cirrhosis, significant hepatic fibrosis (>20 kPa on elastography) and thrombocytopenia (platelets < 150,000) should undergo variceal screening with an upper endoscopy (7). Variceal screening or surveillance is then recommended every 1–3 years depending on the individual. Patients with cirrhosis also undergo colonoscopy and other endoscopic procedures for screening, diagnostic, and therapeutic purposes. Given that patients with cirrhosis are considered higher risk for sedation and undergo endoscopic procedures on a routine basis, often with the assistance of anesthesia administered procedural sedation, it is imperative to understand their risk for anesthesia-associated complications.
Among all patients undergoing endoscopic procedures, some studies have suggested increased risk of aspiration pneumonia (8,9), colonic perforation, bleeding, and cardiovascular events with ADS, as compared to endoscopist-directed sedation (10). Other studies have shown no increased risk of complication after accounting for disease severity and case complexity, suggesting that these factors may confound this relationship (11–14). Because sicker patients, including those with end-stage liver disease, are more likely to require anesthesiologist support, it is difficult to interpret the current literature in terms of true risks associated with deep sedation for cirrhosis patients undergoing endoscopic procedures.
The aim of this study was to identify the prevalence of anesthesia-associated complications among cirrhosis patients undergoing endoscopic procedures with ADS, and to investigate potential risk factors for serious complications including patient, facility, and procedural factors adjusting for potential confounders.
Methods
Database and Population
This cross-sectional study utilized the National Anesthesia Clinical Outcomes Registry (NACOR), a national registry comprised of inpatient and outpatient surgical, as well as anesthesia procedure data conducted at over 2700 facilities across the U.S. since January 1st 2010. NACOR comprises the largest anesthesia database in the U.S. and was developed as a quality improvement initiative sponsored by the Anesthesia Quality Institute (AQI) under the auspices of the American Society of Anesthesiologists (ASA). Over 150 variables are reported including preoperative risk factors (e.g., patient data including age, sex, International Classification of Diseases (ICD) 9 codes, ASA classification), intraoperative variables (e.g., procedure type as defined by surgical and anesthesia current procedural terminology (CPT) codes, procedure duration) for patients undergoing both surgical and other procedures with anesthesia support in various inpatient and outpatient settings. Data are automatically imported from each clinical site’s electronic medical record (EMR) and audited regularly by the AQI. Outcomes data including complications are available only if reported by the anesthesia provider at a clinical site. NACOR has been used previously for other epidemiological studies of anesthesia utilization, complications, and quality (15,16).
The population of interest included individuals with cirrhosis or portal hypertension complications as identified by ICD-9 or CPT codes. To identify cirrhosis patients, validated ICD-9 codes for cirrhosis were utilized (ICD-9: 571.2, 571.5, 571.6) (17). Portal hypertensive complications were identified by ICD-9 or CPT codes including, hepatic encephalopathy (ICD-9: 572.2, 572.3, 572.4), variceal hemorrhage (ICD-9: 456.0, 456.1, 456.2, 456.21 or CPT: 43243, 43244), spontaneous bacterial peritonitis (ICD-9: 567.23) or ascites (ICD-9: 789.59) (Figure 1) (18–20). Other large database studies have shown that the use of at least two or more ICD-9 codes for cirrhosis had a high positive predictive value in identifying individuals with cirrhosis (18,21,22). Given that this anesthesia database recorded only diagnosis codes relevant to the GI procedure at hand, in order to optimize the sensitivity of the case definition, we considered a single ICD-9 code for cirrhosis or portal hypertensive complication sufficient to identify individuals with cirrhosis. The population was therefore defined as having one or more ICD-9 or CPT code for cirrhosis or a portal hypertensive complication (Supplemental Table 1).
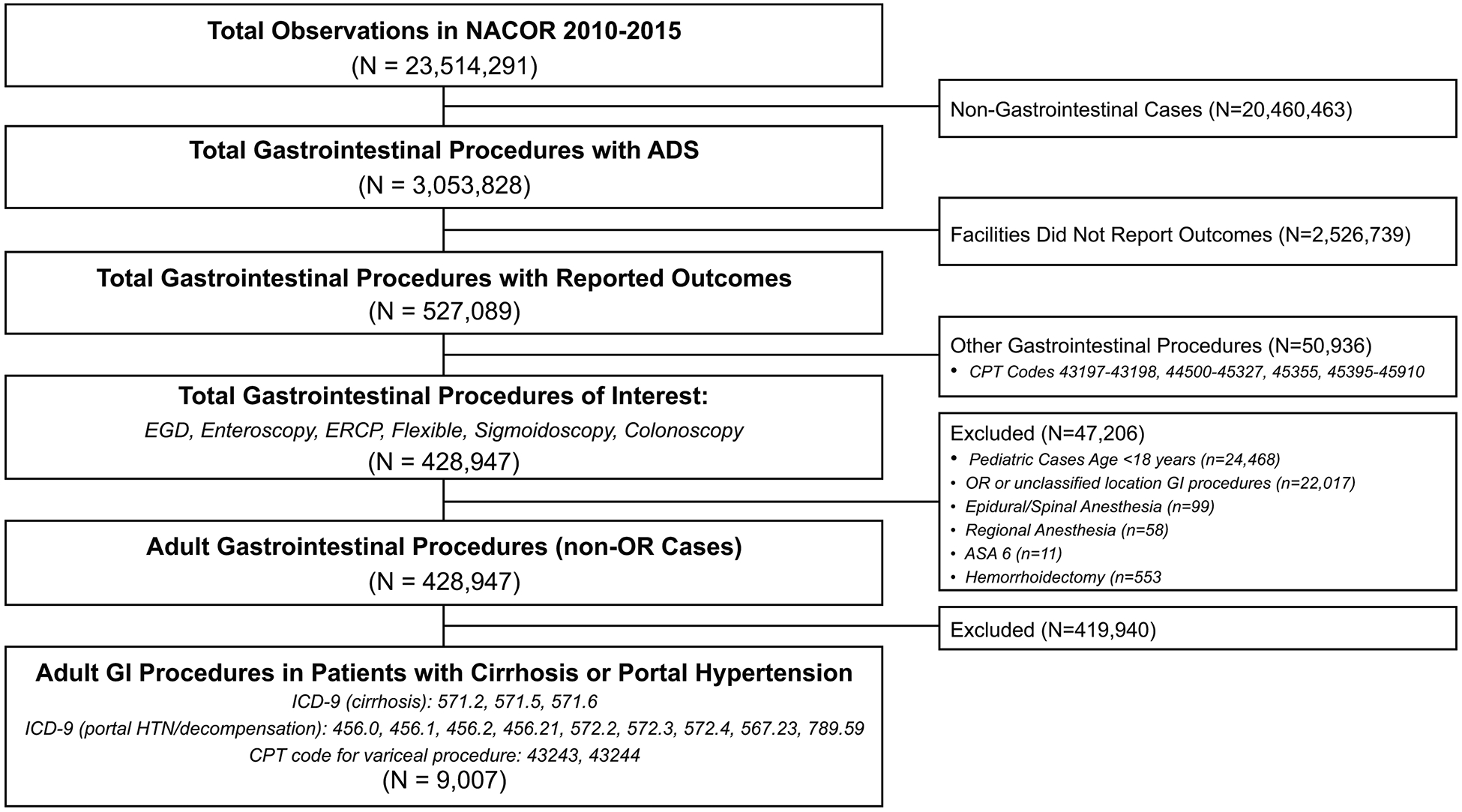
Endoscopic procedures included in this study were EGD with or without endoscopic ultrasound (EUS), enteroscopy, flexible sigmoidoscopy, colonoscopy, and endoscopic retrograde cholangiopancreatography (ERCP). Pediatric cases (<18 years) were excluded, as well as gastrointestinal surgical cases performed in the operating room. Anesthesia techniques including regional or spinal/epidural sedation were excluded given they would unlikely be performed during endoscopic GI procedures. This study received exemption from the University of North Carolina Institutional Review Board due to its use of deidentified data.
Variables and Outcomes of Interest
For all endoscopic procedures, we investigated the following variables: patient characteristics (age, sex, ASA classification), facility characteristics (U.S. region, hospital/center type, facility volume), procedure type (EGD/enteroscopy/EUS, colonoscopy/flexible sigmoidoscopy, ERCP), other procedural characteristics (day/night shift, emergent/inpatient status, weekday/holiday status), and anesthesia characteristics (case duration, sedation type). If more than one sedation type was listed, general anesthesia took precedence and was reported as the primary anesthesia type. Facility volume was defined as the number of concurrent cases taking place with anesthesia services at the time of procedure, and was categorized via tertiles as low volume (<= 16 concurrent cases), medium volume (17–45 concurrent cases), and high volume (>46 concurrent cases). These could include non-GI procedures with anesthesia services. Case duration was defined as anesthesia start to finish time (and therefore is generally longer and not equal to endoscopy procedure duration). Case duration was also categorized in tertiles as short (<25 minutes), medium (25–37 minutes), and long (>37 minutes).
A total of 47 different anesthetic complication outcomes can be recorded by an anesthesia provider at the end of a case and reported in NACOR. Examples of complications include arrhythmia, cardiac arrest, hypotension, death, aspiration, hemodynamic instability, admission, and extended post-procedure recovery unit stay, among others. These complications were grouped into 7 categories: 1) respiratory, 2) cardiovascular, 3) drug-related, 4) neurologic, 5) patient injury, 6) unplanned admission, and 7) death (Table 1). Because the NACOR database primarily collects data on anesthesia and its complications, no specific GI or endoscopic procedural outcomes are recorded in this database. For example, rates of perforation are not available in this database. Our primary outcome of interest was a composite outcome of any serious complication defined as a respiratory, cardiovascular, drug, neurologic, patient injury, unplanned admission and/or death complication. We also determine the frequency of each anesthesia-related complication individually.
Table 1.
Definition of Serious Anesthetic Complication During Endoscopic Procedures
| COMPLICATION CATEGORY | EXAMPLES |
|---|---|
| CARDIOVASCULAR | Arrhythmia |
| Cardiac arrest | |
| Hemodynamic instability | |
| Hypotension | |
| Myocardial infarction | |
| Myocardial ischemia | |
| Transfusion | |
| DEATH | Death due to any cause |
| DRUG | Adverse reaction |
| Anaphylaxis | |
| Malignant hyperthermia | |
| Medication error | |
| NEUROLOGIC | Awareness |
| Emergence | |
| Neurologic deficit | |
| Seizure | |
| Stroke | |
| Vision loss | |
| PATIENT INJURY | Eye injury |
| Infection | |
| Other bodily injury | |
| RESPIRATORY | Airway obstruction |
| Aspiration | |
| Difficult airway | |
| Pneumothorax | |
| Pulmonary embolus | |
| Reintubation | |
| Respiratory arrest | |
| UNPLANNED ADMISSION | Admission to hospital or intensive care unit |
Statistical Analysis
We examined means, standard deviations, and shapes of distributions for each continuous variable (case duration and facility volume), and frequencies for each categorical variable (patient, facility, procedural, and anesthesia variables described above). Variables including procedure type, day of the week, month, shift, holiday status had no missing data. For certain variables indicating rare events that were likely to be reported with high specificity, missing data were imputed as negative. For example, cases not classified as ‘emergent’ were assumed to be non-emergent or elective cases.
We performed bivariate comparisons of each variable with the dichotomous outcome of serious complication using Pearson’s chi-square tests for categorical predictors, and t-tests or individual logistic regression models for continuous predictors. We also examined relationships among the predictor variables for any instances of collinearity that could affect the validity of modeling estimates. Frequency of complications was calculated by dividing the number of complications by the total number of procedures with complete complication data reported (defined as the total cases at risk for complication).
We fit a logistic regression model to determine risk factors that best predicted the probability of developing a serious anesthetic complication reported as odds ratios. Mixed effects modeling was used to account for variability in practice, facility, and provider reporting of complications. More specifically, an identification code for practice, facility, and provider was used in the final hierarchical model to account for potential reporting bias. Our final multivariable logistic regression analysis was restricted to observations that had non-missing data. The final mixed effects model consisted of variables that were reported to be associated with complications in the literature (e.g. ASA, sedation type), potential predictors of complications that could be clinically relevant (e.g. age, sex), as well as those variables that were statistically significant on bivariate analysis (e.g. geographic region, facility type, facility volume, shift). This model was further reduced by eliminating variables that were not significant via likelihood ratio testing. Interaction was tested between procedure type and all other variables. Two-tailed p values are reported and p < 0.05 was considered statistically significant. Stata 15 (Stata Corporation, College Station, TX, USA) was used for all analyses.
Results
Patient and Facility Characteristics
The NACOR database comprises over 20 million procedures performed from 2010 to 2015 across the U.S. including 2,756 participating facilities. Our sample consisted of a total 9,007 endoscopic procedures performed in individuals with cirrhosis identified by ICD-9/CPT code (Figure 1). On univariate analysis, the population was 37% female with a median age of 58 years (Table 2). A majority of cases (81%) were ASA class 3 or greater (i.e. ASA 3: a patient with severe systemic disease; ASA 4: severe systemic disease that is a constant threat to life; ASA 5: a moribund patient who is not expected to survive without the procedure). Over half had an ICD-9 or CPT code identifying a decompensation/complication of cirrhosis including varices (59.6%), portal hypertension (8.9%), ascites (2.6%), hepatic encephalopathy (0.4%), or hepatorenal syndrome/spontaneous bacterial peritonitis (0.5%).
Table 2.
Patient, Facility, Procedure, and Anesthesia Characteristics of Endoscopic Procedures Performed in Cirrhosis from 2010 to 2015 in NACOR Database (N= 9,007)
| PATIENT CHARACTERISTICS | ||
|---|---|---|
| VARIABLE | FREQUENCY n (%) | |
| 18 – 49 | 1,849 (20.5) | |
| 50 – 64 | 4,817 (53.5) | |
| 65 – 79 | 2,049 (22.8) | |
| > 80 | 288 (3.2) | |
| Missing | 4 (0.04) | |
| Female | 3,348 (37.2) | |
| Male | 5,616 (62.4) | |
| Missing | 43 (0.4) | |
| 1/2 | 1,418 (15.7) | |
| 3 | 5,974 (66.3) | |
| >=4 | 1,365 (15.2) | |
| Missing | 250 (2.8) | |
| Varices | 5,364 (59.6) | |
| Ascites | 233 (2.6) | |
| Hepatic Encephalopathy | 35 (0.4) | |
| SBP or Hepatorenal | 42 (0.5) | |
| Portal Hypertension NOS | 803 (8.9) | |
| Unknown/Missing | 2,530 (28.1) | |
| FACILITY CHARACTERISTICS | ||
| Northeast | 4,693 (52.1) | |
| Midwest | 2,214 (24.6) | |
| South | 1,685 (18.7) | |
| West | 359 (4.0) | |
| Missing | 56 (0.6) | |
| University Hospital | 4,573 (50.8) | |
| Community Hospital | 2,626 (29.2) | |
| Surgical Center | 83 (0.9) | |
| Other Unspecified | 1,669 (18.5) | |
| Missing | 56 (0.6) | |
| Low (<=16) | 3,027 (33.6) | |
| Medium (17–45) | 2,958 (32.8) | |
| High (>46) | 2,731 (30.3) | |
| Missing | 291 (3.2) | |
| PROCEDURE CHARACTERISTICS | ||
| EGD | 8,326 (92.4) | |
| Colonoscopy | 399 (4.4) | |
| ERCP | 233 (2.6) | |
| Enteroscopy | 33 (0.4) | |
| Flexible Sigmoidoscopy | 16 (0.2) | |
| Yes | 2,857 (31.7) | |
| No | 6,150 (68.3) | |
| Outpatient | 3,344 (37.1) | |
| Inpatient | 4,150 (46.1) | |
| Missing | 1,513 (16.8) | |
| Elective | 8,637 (95.9) | |
| Emergent | 370 (4.1) | |
| Daytime (7:00–17:00) | 8,458 (93.9) | |
| After hours (17:01–06:59) | 549 (6.1) | |
| Weekday | 8,523 (94.6) | |
| Weekend | 484 (5.4) | |
| ANESTHESIA CHARACTERISTICS | ||
| Short (<25) | 3,023 (33.6) | |
| Medium (25–37) | 2,786 (30.9) | |
| Long (>37) | 2,728 (30.3) | |
| Missing | 291 (5.2) | |
| General | 2,070 (25.3) | |
| MAC / Sedation | 6,113 (74.6) | |
| Missing | 6 (0.1) | |
Portal hypertension complication history was defined as having at least one ICD-9 or CPT code for: hepatic encephalopathy (ICD-9: 572.2), variceal hemorrhage (ICD-9: 456.0, 456.1, 456.2 or CPT: 43243, 43244), ascites (ICD-9: 789.59, portal hypertension not otherwise specified (ICD-9: 572.3), and other complication including spontaneous bacterial peritonitis (ICD-9: 576.23) or hepatorenal syndrome (ICD-9: 572.4).
Facility case volume defined as number of concurrent cases with anesthesia services taking place at the facility at the time of procedure.
Case duration defined as anesthesia start time to end time.
Abbreviations: American Society of Anesthesiologists (ASA); Current Procedural Terminology (CPT); Esophagogastroduodenoscopy (EGD); Endoscopic retrograde cholangiopancreatography (ERCP); Endoscopic ultrasound (EUS); International Classification of Diseases (ICD); Monitored anesthesia care (MAC); Not otherwise specified (NOS); Spontaneous bacterial peritonitis (SBP)
About half of procedures were performed in the Northeast (52.1%), with just under a quarter in the Midwest (24.6%). Over half of cases were performed in a University hospital (50.8%), followed by just under a third of cases at community hospitals (29.2%). Regarding case volume, 34% of procedures were performed in low volume facilities, and 33% and 30% were performed in medium and high volume facilities, respectively.
Procedural and Anesthesia Characteristics
EGDs comprised the vast majority of procedures (92.4%), and about a third (31.7%) of all cases had a CPT code associated with varices or band ligation (i.e. CPT codes 43243, 43244). A majority of cases were non-emergent (95.9%) and performed during daytime hours (93.9%), with just under half of cases performed in an inpatient setting (46.1%). Regarding anesthesia type, a majority of cases were performed with MAC/moderate sedation (74.6%) as compared to general anesthesia (25.3%).
Prevalence of Anesthesia-Associated Complications
Among a total of 87 complications recorded from 2010 to 2015, the most common type of complication was post-procedural (61/87; 70%) including nausea, vomiting, or post-procedural pain symptoms. A total of 33 serious complications (0.38%) occurred, a majority of which were cardiovascular (21/33; 64%) including 15 cardiac arrests, 3 episodes of hemodynamic instability, and 3 episodes of clinically significant hypotension (Table 3). The frequency of serious complications was 0.4% or 378.6 per 100,000 procedures (95% CI 260.8, 531.3). This is equivalent to roughly 1 serious complication per 264 cases. A total of 3 deaths, 6 respiratory complications, and 1 neurologic complication occurred. The frequency of death was 40.0 per 100,000 procedures (95% CI 8.2, 116.7) or 1 in 2500 cases.
Table 3.
Prevalence of Anesthesia Complications Among Endoscopic Procedures (2010–2015)
| COMPLICATION TYPE | FREQUENCY (n) | TOTAL CASES (N) | RISK (%) | POINT PREVALENCE* PER 100,000 PROCEDURES (95% CI) |
|---|---|---|---|---|
| Serious Complication | 33 | 8,716 | 0.38 | 378.6 (260.8 – 531.3) |
| Any Complication | 87 | 8,764 | 0.99 | 992.7 (795.9 – 1223.1) |
| Post-Procedure^ | 61 | 7,422 | 0.82 | 821.9 (629.2 – 1054.5) |
| Unplanned Admission | 6 | 7,197 | 0.08 | 83.3 (30.6 – 181.4) |
| Cardiovascular | 21 | 8,194 | 0.26 | 256.3 (158.7 – 391.5) |
| Respiratory | 6 | 8,351 | 0.07 | 71.9 (26.4 – 156.3) |
| Death | 3 | 7,509 | 0.04 | 40.0 (8.2 – 116.7) |
| Neurologic | 1 | 6,059 | 0.02 | 16.5 (4.2 – 91.9) |
Prevalence calculated as # of complications (frequency) divided by total # of procedures with complete complications reported (total cases at risk for complications).
Post-procedure complications include post-procedural pain, nausea, vomiting, or other symptoms, as well as admission to medical floor or intensive care unit.
Given the high proportion of serious cardiac complications seen, we examined the relationship between cardiac complications and comorbidities including heart disease and non-alcoholic fatty liver disease (NAFLD) identified by ICD-9 codes (Supplemental Table 3). Out of the total population, 291 (3%) had a cardiac comorbidity, 46 of whom had a diagnosis of congestive heart failure. Only 1 serious complication (cardiac arrest) was identified among those with a cardiac comorbidity. We separately identified 1,884 cases of NAFLD using previously described methods (23). Among them, a total of 7 serious complications occurred, 6 of which were cardiac (5 cardiac arrests and 1 clinically significant episode of hypotension).
Predictors of Serious Complications: Unadjusted Bivariate Analysis
On unadjusted bivariate analysis, sex, ASA, US region, facility type, shift time, and anesthesia type were associated with serious complications (Supplementary Table 2). Associations between these variables and specific types of complication (e.g. cardiovascular, respiratory) are depicted in Supplementary Table 2. Among cardiovascular complications, higher ASA, Southern U.S. region, non-University hospitals, outpatient status, and general anesthesia were associated with increased proportion of cardiovascular complications.
Predictors of Serious Complications: Mixed Effects Multivariable Model
Among the 9,007 endoscopic procedures patients with cirrhosis or portal hypertensive complication, 7,025 observations had complete outcomes data to be included in the final mixed effects multivariable model. Table 4 depicts the unadjusted and adjusted odds ratios for serious complications associated with variables age, sex, ASA status, anesthesia type, inpatient status, and history of portal hypertensive complication. On multivariable analysis, serious complications were significantly associated with ASA 4/5 (OR 3.84; 95% CI 1.09, 13.57) and general anesthesia (OR 4.71; 95% CI 1.20, 18.50) adjusting for age, sex, ASA class, anesthesia type, inpatient status, history of portal hypertension complication, as well as facility, practice and provider ID using hierarchical/mixed-effects modeling to account for varying complication reporting practices (Table 4). Non-significant variables such as age, sex and history of portal hypertensive complication were kept in the final model given their suspected clinical importance. Other variables were removed given large number of missing observations or non-significance.
Table 4.
Associations Between Patient, Procedure, Facility, and Anesthesia Characteristics of Endoscopic Procedures and Serious Anesthesia Complications—Multivariable Mixed-Effects Logistic Regression Model (N=7,025)
| VARIABLE | UNADJUSTED OR (95% CI) | ADJUSTED OR (95% CI)* | |
|---|---|---|---|
| > 50 | 1.09 (0.51, 2.35) | 1.53 (0.54, 4.33) | |
| Female | 0.45 (0.19, 1.04) | 0.39 (0.13, 1.12) | |
| ASA 4/5 | 4.97 (2.01, 12.25) | 3.84 (1.09, 13.57) | |
| General | 12.41 (5.05, 30.53) | 4.71 (1.20, 18.50) | |
| Inpatient | 0.66 (0.32, 1.34) | 0.41 (0.12, 1.39) | |
| Yes | 1.28 (0.39, 4.19) | 2.35 (0.65, 8.46) | |
Adjusted for age, sex, ASA class, anesthesia type, inpatient status, and history of portal hypertension complication as indicated by ICD 9 code, as well as facility, practice and provider ID using hierarchical/mixed-effects modeling to account for varying complication reporting practices.
Abbreviations: American Society of Anesthesiologists (ASA); Monitored anesthesia care (MAC)
Discussion
This large, multi-center study of endoscopic procedures with anesthesiology services is the first to report the prevalence of complications among individuals with cirrhosis. Overall, serious complications were uncommon (0.38%), but were higher than that calculated for the general population (0.34%) (24). A majority of serious complications were cardiovascular in nature. Serious complications were significantly associated with higher ASA and general anesthesia, suggesting that complexity and severity of disease may drive the risk of complication.
With the rise of anesthesia-directed sedation (ADS) for endoscopy and the need for frequent variceal screening or surveillance among individuals with cirrhosis, it is imperative to understand the safety of deep sedation and general anesthesia among this population. Propofol is one of the most commonly used medications during GI endoscopic procedures performed with ADS. Among patients with cirrhosis, the pharmacokinetic profile of propofol is particularly appealing given its short duration of action, quick metabolism, and no dose adjustments required in patients with liver disease (25). A meta-analysis demonstrated that it has more rapid sedation and recovery, without a statistically significant increase in hypotension, hypoxemia, bradycardia, or worsening of encephalopathy, as compared to midazolam in this patient population (25). One of the major concerns of propofol anesthesia in patients who are heavy drinkers is the risk of a paradoxical reaction. This is more common and more severe in patients with alcoholic liver disease, and has been identified as a risk factor for procedural interruption in cases of variceal bleeding (26). However, at least one study found that in cirrhosis patients undergoing endoscopic sclerotherapy, propofol sedation actually led to lower frequency of body movements and higher operator satisfaction than midazolam (27). While there are concerns regarding propofol’s effect on delaying psychomotor performance in cirrhotic patients, at least one study has found this not to be the case (28).
Our findings also suggest that ADS is safe in cirrhosis with a low rate of serious complications. Our calculated frequency of any complication was 1.0% (992.7 complications per 100,000 endoscopic procedures) with serious complications accounting for 0.38% of cases (378.6 complications per 100,000 procedures). While these prevalence calculations may underestimate the actual risk of an anesthesia-related complication given under-reporting of complications, they were actually higher than estimates of complication prevalence in the general population undergoing endoscopic procedures. In a large retrospective observational cohort of individuals undergoing endoscopy, propensity-adjusted serious adverse event risks were calculated and found to be 0.20% of ADS colonoscopies and 0.39% of ADS endoscopies (12). These estimates were comparable to frequencies of SAEs among cases with endoscopist-directed sedation in that study. Similarly, using the same NACOR database, we calculated an overall frequency of serious complications to be 0.34% for the general population (24). Our slightly higher estimate of 0.38% serious complications (primarily among upper endoscopies) may indeed reflect an increased risk of complication in cirrhosis that can be explained by disease complexity including coagulopathy, as well as tenuous fluid and mental status changes.
Interestingly, cardiac complications comprised a majority of serious complications, suggesting that individuals with cirrhosis are higher risk for cardiac events with ADS. There is evidence to suggest that cirrhosis in and of itself is a risk factor for cardiovascular disease. Features of chronic liver disease including hepatic steatosis, insulin resistance, and lipid dysregulation have been shown to amplify cardiovascular risk regardless of lipid profile, especially in alcoholic, NAFLD, chronic hepatitis C infection (29). Moreover, hemodynamic changes related to vasodilation and hyperdynamic circulation are unique features of cirrhosis that can lead to cardiac dysfunction and cardiomyopathy (30). With the growing population of NAFLD and concomitant metabolic syndrome, cardiovascular disease is certainly expected to become more prevalent among individuals with cirrhosis. Interestingly we found that among 1,884 NAFLD patients identified in our population, serious complications were predominantly cardiac (6/7) and included 5 cardiac arrests.
The increased risk of serious complications with higher ASA and general anesthesia can be explained by the fact that sicker individuals are at higher risk for complications. However, given the inability to assess Model for End Stage Liver Disease (MELD) or Child-Pugh scores, it is difficult to assess precisely the degree to which severity of liver disease contributed to this risk. General anesthesia involves a deeper level of anesthesia that consequentially may lead to more medication-related complications such as hypotension, hypoxia, aspiration, and so forth, regardless of patient disease severity or case complexity, and regardless of whether the patient is endotracheally intubated or not. It should be acknowledged that cases initiated using MAC/sedation are likely to be converted to general anesthesia if certain complications arise, and therefore, reverse causation could also explain this observed association, as well. However, our suspicion is that the higher odds of serious complications among general anesthesia may be reflective of a sicker, more complex patient population receiving this anesthetic type.
This study has several limitations, many of which are related to using a database such as NACOR, and identifying a cohort based on ICD-9 and CPT codes. While NACOR is the largest anesthesia QI database in the US, there is still a large portion of data missing from institutions that do not submit information to the database. Cases were selected based on ICD-9 and CPT codes and may have been subject to incorrect coding. Moreover, while validated ICD-9 codes were used to identify individuals with cirrhosis, these ICD-9 codes were recorded in the context of an endoscopic procedure; hence, cases among cirrhosis patients may have been missed, especially if the GI procedure was unrelated to their liver care (e.g. screening colonoscopy for colorectal cancer, diagnostic endoscopy for non-variceal bleeding). For this reason, inpatient procedures may have been better captured than outpatient procedures in this dataset.
While markers of disease severity, such as ASA status and clinical features of decompensated cirrhosis were investigated, specific data such as laboratory values and body mass index were not available in this database. This limited our ability to more fully account for disease severity and case complexity including not being able to assess MELD or Child-Pugh score. Lastly, how anesthesia providers chart anesthetics may differ at various institutions. For example, an anesthetic where propofol is the main drug utilized, but the patient is not intubated, may be charted as MAC at one institution, but charted as a general anesthetic elsewhere. While this is likely consistent at specific locations, there is potential variation across sites in this regard.
Many of the limitations of this study were mitigated by the volume of endoscopic cases (>9,000) and the numerous risk factors investigated. Our data source includes a diverse array of inpatient and outpatient practices throughout multiple geographic regions, and the geographic variability of procedures performed with ADS was similar to that reported using a nationally representative data sample of Americans with employer-based health insurance (10). This study explored novel predictors of complications including patient, facility, procedure, and anesthesia-specific variables that have not been investigated previously. More specifically facility type, daytime/overnight shift, and case complexity as measured by case duration and facility volume have never been characterized before. We also tried to account for patient disease severity by including variables such as ASA class and inpatient vs. outpatient location for endoscopic procedures. Given that complications are voluntarily reported by anesthesia providers, there may be significant under-reporting and potential reporting bias given certain providers may be less inclined to report their complications (31). To account for this variability in reporting practices, we used mixed effects modeling and adjusted our analysis by facility, practice, and provider ID. In this sense, we were able to distinguish a true association with increased serious complications that did not merely reflect the excellent reporting of complications by certain practices, facilities, or providers. Even if the frequency of complications was under-reported in this study, we uncovered significant independent risk factors for serious complications, namely ASA class 4/5 and general anesthesia cases.
In conclusion, this large, multi-center study investigated anesthesia complications among endoscopic procedures in cirrhosis. The prevalence of serious complications in cirrhosis was low overall. Serious complications were predominantly cardiac in nature, and were associated with sicker patients (higher ASA) undergoing procedures in outpatient settings, suggesting the potential need for higher level, tertiary care for these patients given the complexity of their liver disease. This study suggests that anesthesia services in endoscopy are safe in cirrhotic patients; however, patient and providers should be aware of potential higher risks of cardiac complications in this population.
Supplementary Material
Grant Support:
This research was supported, in part, by grants from the National Institutes of Health (T32DK007634, P30 DK034987, and KL2-TR001109).
Abbreviations:
- ADS
Anesthesia directed sedation
- ASA
American Society of Anesthesia
- CRNA
Certified registered nurse anesthetist
- EDS
Endoscopist directed sedation
- EGD
Esophagogastroduodenoscopy
- ERCP
Endoscopic retrograde cholangiopancreatography
- ICD
International Classification of Diseases
- MAC
Monitored anesthesia care
- NACOR
National Anesthesia Clinical Outcomes Registry
Footnotes
Disclosures:
The authors have no conflicts of interest to disclose.
References
- 1.Cohen LB, Wecsler JS, Gaetano JN, Benson AA, Miller KM, Durkalski V, et al. Endoscopic sedation in the United States: results from a nationwide survey. Am. J. Gastroenterol 2006;101:967–74. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Waxman DA, Main R, Mattke S. Utilization of Anesthesia Services During Outpatient Endoscopies and Colonoscopies and Associated Spending in 2003–2009. JAMA. 2012;307:1178. [DOI] [PubMed] [Google Scholar]
- 3.Inadomi JM, Gunnarsson CL, Rizzo JA, Fang H. Projected increased growth rate of anesthesia professional–delivered sedation for colonoscopy and EGD in the United States: 2009 to 2015. Gastrointest. Endosc 2010;72:580–586. [DOI] [PubMed] [Google Scholar]
- 4.Correia LM, Bonilha DQ, Gomes GF, Brito JR, Nakao FS, Lenz L, et al. Sedation during upper GI endoscopy in cirrhotic outpatients: a randomized, controlled trial comparing propofol and fentanyl with midazolam and fentanyl. Gastrointest. Endosc 2011;73:45–51.e1. [DOI] [PubMed] [Google Scholar]
- 5.Fagà E, De Cento M, Giordanino C, Barletti C, Bruno M, Carucci P, et al. Safety of propofol in cirrhotic patients undergoing colonoscopy and endoscopic retrograde cholangiography. Eur. J. Gastroenterol. Hepatol. [Internet] 2012. [cited 2018 Dec 10];24:70–76. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21941187 [DOI] [PubMed] [Google Scholar]
- 6.Wahab EA, Hamed EF, Ahmad HS, Abdel Monem SM, Fathy T. Conscious sedation using propofol versus midazolam in cirrhotic patients during upper GI endoscopy: A comparative study. JGH open an open access J. Gastroenterol. Hepatol 2019;3:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal Hypertensive Bleeding in Cirrhosis: Risk Stratification, Diagnosis, and Management: 2016 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2016;65:310–335. [DOI] [PubMed] [Google Scholar]
- 8.Cooper GS, Kou TD, Rex DK. Complications Following Colonoscopy With Anesthesia Assistance. JAMA Intern. Med 2013;173:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bielawska B, Hookey LC, Sutradhar R, Whitehead M, Xu J, Paszat LF, et al. Anesthesia Assistance in Outpatient Colonoscopy and Risk of Aspiration Pneumonia, Bowel Perforation, and Splenic Injury. Gastroenterology [Internet]. 2018. [cited 2018 Dec 10];154:77–85.e3. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0016508517360766 [DOI] [PubMed] [Google Scholar]
- 10.Wernli KJ, Brenner AT, Rutter CM, Inadomi JM. Risks Associated With Anesthesia Services During Colonoscopy. Gastroenterology. 2016;150:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bielawska B, Day AG, Lieberman DA, Hookey LC. Risk Factors for Early Colonoscopic Perforation Include Non-Gastroenterologist Endoscopists: A Multivariable Analysis. Clin. Gastroenterol. Hepatol 2014;12:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vargo JJ, Niklewski PJ, Williams JL, Martin JF, Faigel DO. Patient safety during sedation by anesthesia professionals during routine upper endoscopy and colonoscopy: an analysis of 1.38 million procedures. Gastrointest. Endosc 2017;85:101–108. [DOI] [PubMed] [Google Scholar]
- 13.Wadhwa V, Issa D, Garg S, Lopez R, Sanaka MR, Vargo JJ. Similar Risk of Cardiopulmonary Adverse Events Between Propofol and Traditional Anesthesia for Gastrointestinal Endoscopy: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol 2017;15:194–206. [DOI] [PubMed] [Google Scholar]
- 14.Adeyemo A, Bannazadeh M, Riggs T, Shellnut J, Barkel D, Wasvary H. Does Sedation Type Affect Colonoscopy Perforation Rates? Dis. Colon Rectum 2014;57:110–114. [DOI] [PubMed] [Google Scholar]
- 15.Tillquist MN, Gabriel RA, Dutton RP, Urman RD. Incidence and risk factors for early postoperative reintubations. J. Clin. Anesth 2016;31:80–89. [DOI] [PubMed] [Google Scholar]
- 16.Nagrebetsky A, Dutton RP, Ehrenfeld JM, Urman RD. Variation in Frequency of Intraoperative Arterial, Central Venous and Pulmonary Artery Catheter Placement During Kidney Transplantation: An Analysis of Invasive Monitoring Trends. J. Med. Syst 2018;42:66. [DOI] [PubMed] [Google Scholar]
- 17.Kanwal F, Kramer JR, Buchanan P, Asch SM, Assioun Y, Bacon BR, et al. The quality of care provided to patients with cirrhosis and ascites in the department of veterans affairs. Gastroenterology. 2012;143:70–77. [DOI] [PubMed] [Google Scholar]
- 18.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J. Clin. Gastroenterol 2013;47:e50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barritt AS, Jiang Y, Schmidt M, Hayashi PH, Bataller R. Charges for Alcoholic Cirrhosis Exceed All Other Etiologies of Cirrhosis Combined: A National and State Inpatient Survey Analysis. Dig. Dis. Sci. [Internet] 2019. [cited 2019 May 24];64:1460–1469. Available from: http://link.springer.com/10.1007/s10620-019-5471-7 [DOI] [PubMed] [Google Scholar]
- 20.Kramer JR, Puenpatom A, Erickson KF, Cao Y, Smith D, El-Serag HB, et al. Real-world effectiveness of elbasvir/grazoprevir In HCV-infected patients in the US veterans affairs healthcare system. J. Viral Hepat 2018;25:1270–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mapakshi S, Kramer JR, Richardson P, El-Serag HB, Kanwal F. Positive Predictive Value of International Classification of Diseases, 10th Revision, Codes for Cirrhosis and Its Related Complications. Clin. Gastroenterol. Hepatol 2018;16:1677–1678. [DOI] [PubMed] [Google Scholar]
- 22.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment. Pharmacol. Ther 2007;27:274–282. [DOI] [PubMed] [Google Scholar]
- 23.Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare Cost and Utilization in Nonalcoholic Fatty Liver Disease: Real-World Data From a Large U.S. Claims Database. Hepatology [Internet]. 2018. [cited 2020 Jan 23];68:2230–2238. Available from: http://doi.wiley.com/10.1002/hep.30094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieber SR, Heller BJ, Martin CF, Howard CW, Crockett S. Complications of Anesthesia Services in Gastrointestinal Endoscopic Procedures. Clin. Gastroenterol. Hepatol 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai H-C, Lin Y-C, Ko C-L, Lou H-Y, Chen T-L, Tam K-W, et al. Propofol versus Midazolam for Upper Gastrointestinal Endoscopy in Cirrhotic Patients: A Meta-Analysis of Randomized Controlled Trials. PLoS One. 2015;10:e0117585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park CH, Han DS, Jeong JY, Eun CS, Yoo K-S, Jeon YC, et al. Outcomes of Propofol Sedation During Emergency Endoscopy Performed for Upper Gastrointestinal Bleeding. Dig. Dis. Sci 2016;61:825–834. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe K, Hikichi T, Takagi T, Suzuki R, Nakamura J, Sugimoto M, et al. Propofol is a more effective and safer sedative agent than midazolam in endoscopic injection sclerotherapy for esophageal varices in patients with liver cirrhosis: a randomized controlled trial. FUKUSHIMA J. Med. Sci 2018;64:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suh SJ, Yim HJ, Yoon EL, Lee BJ, Hyun JJ, Jung SW, et al. Is propofol safe when administered to cirrhotic patients during sedative endoscopy? Korean J. Intern. Med 2014;29:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loria P, Marchesini G, Nascimbeni F, Ballestri S, Maurantonio M, Carubbi F, et al. Cardiovascular risk, lipidemic phenotype and steatosis. A comparative analysis of cirrhotic and non-cirrhotic liver disease due to varying etiology. Atherosclerosis. 2014;232:99–109. [DOI] [PubMed] [Google Scholar]
- 30.Moller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. 2008;57:268–278. [DOI] [PubMed] [Google Scholar]
- 31.Dutton RP, Dukatz A. Quality improvement using automated data sources: the anesthesia quality institute. Anesthesiol. Clin 2011;29:439–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


