Abstract
Despite the importance of cortico-striatal circuits to cognition, investigation of age effects on the structural circuitry connecting these regions is limited. The current study examined age effects on frontostriatal white matter connectivity, and identified associations with both executive function performance and dynamic modulation of blood-oxygen-level-dependent (BOLD) activation to task difficulty in a lifespan sample of 169 healthy humans aged 20–94 years. Greater frontostriatal diffusivity was associated with poorer executive function and this negative association strengthened with increasing age. Whole-brain functional magnetic resonance imaging (fMRI) analyses additionally indicated an association between frontostriatal mean diffusivity and BOLD modulation to difficulty selectively in the striatum across two independent fMRI tasks. This association was moderated by age, such that younger- and middle-aged individuals showed reduced dynamic range of difficulty modulation as a function of increasing frontostriatal diffusivity. Together these results demonstrate the importance of age-related degradation of frontostriatal circuitry on executive functioning across the lifespan, and highlight the need to capture brain changes occurring in early- to middle-adulthood.
1. Introduction
Frontostriatal circuitry in both animals and humans is important for several regulatory activities and is generally separated into five major parallel loops supporting integration of information in both motor and non-motor systems (Alexander, 1986; Lehéricy et al., 2004; Middleton & Strick, 2000). Among these loops, the dorsal-prefrontal – dorsal-striatal pathway is of specific interest in the study of cognitive aging as it supports aspects of higher-order cognitive control, with cognitive performance mediated by dopaminergic signaling within this network (Bäckman, et al., 2010). Both prefrontal cortex (PFC) and striatum show pronounced vulnerability to age-related volumetric loss relative to other cortical and subcortical regions (Kennedy & Raz, 2015). Longitudinal evidence estimates shrinkage between 3–5% per decade for both PFC and striatal gray matter across the adult lifespan (Raz et al., 2003, 2005, 2010). Weakened white matter microstructural integrity is also evident in older age, especially in anterior regions and in white matter innervating the PFC (Bartzokis, 2004; Madden et al., 2009; O’Sullivan et al., 2001). Specifically, fractional anisotropy (FA) tends to exhibit an inverted U-shaped trend, increasing into adulthood and then decreasing into older age, while mean diffusivity (MD) tends to decrease into adulthood and increase exponentially into older age (Hsu et al., 2010; Kennedy & Raz, 2015; Madden et al., 2012). These age-related structural declines in gray and white matter are moreover associated with cognitive deficits across a variety of domains, including executive function (EF), working memory, and cognitive control (Madden et al., 2012; Raz & Kennedy, 2009; Raz & Rodrigue, 2006).
In addition to regional volumetric shrinkage, evidence from functional magnetic resonance imaging (fMRI) studies consistently demonstrates age-related functional disruptions of PFC blood-oxygen-level-dependent (BOLD) activation, with older adults typically exhibiting either over- or under-activation of PFC regions compared to younger adults (for review: Cabeza & Dennis, 2002; Park & Reuter-Lorenz, 2009). Additionally, age negatively affects the ability to flexibly regulate BOLD activation in response to increasing cognitive demands (e.g., in response to increasing working memory load). Specifically, up-modulation (greater activation as a function of increasing task demands) and down-modulation (greater deactivation as a function of increasing task demands) tend to decrease across the lifespan, and this reduction in modulation is associated with both poorer task performance and general cognitive performance (Hakun & Johnson, 2017; Kennedy et al., 2017; Rieck et al., 2017; Schneider-Garces et al., 2010). This age-related disruption of functional activation may be accompanied by altered integrity of structural white matter properties (Bennett & Rypma, 2013; Brown et al., 2015; Hakun et al., 2015; Zhu et al., 2015). In line with the idea that structural disconnection in neural systems plays a role in cognitive dysfunction (Bartzokis, 2004; O’Sullivan et al., 2001), white matter alterations may represent a plausible mechanism underlying dysregulation of BOLD activation (Warbrick et al., 2017), that then has cognitive consequences. In relation to modulation of BOLD activation in response to difficulty, recent evidence suggests age-related degradation of major white matter tracts mediates reduced BOLD difficulty modulation in frontoparietal networks, contributing to poorer cognitive performance (Webb, et al., 2020). Together this evidence indicates that integrity of white matter microstructure is related to both neural function and cognition, and that age-related differences in the structure of the brain affects its function. Here, we sought to address whether age differences in frontostriatal white matter connectivity, specifically, are associated with altered BOLD modulation of cortical or subcortical regions connected by this pathway, an association which, to our knowledge, has not been previously reported.
Despite the importance of both prefrontal and striatal regions to cognitive aging, the study of the white matter pathways connecting these structures is limited and age effects on frontostriatal structural white matter connectivity have not been fully characterized. Several studies illustrate the contribution of frontostriatal white matter to cognitive control and reward processing in developmental (Darki & Klingberg, 2015) and patient (Riley et al., 2011) populations, yet only a few studies have investigated healthy aging. For example, Samanez-Larkin, et al. (2012) report linear decreases in cortico-striatal fractional anisotropy (FA) across age, as well as associations between FA and probabilistic reward learning. Longitudinal decline in frontostriatal white matter FA has also been observed in middle- and older-aged individuals (Vik et al., 2015); although no association between change in FA and cognition was reported. Additionally, Ystad et al. (2011) demonstrated that FA of frontostriatal white matter connecting cortical resting-state networks predicted EF in middle- and older-age. However, a comprehensive examination of lifespan differences in cortico-striatal white matter microstructure and their association with both cognition and task-related BOLD function has not been conducted to date.
Therefore, the goals of the current study were to 1) characterize lifespan differences in frontostriatal white matter connectivity, 2) assess frontostriatal microstructure influences on EF performance, and 3) examine associations between frontostriatal white matter connectivity and modulation of BOLD activity to increasing cognitive demand in two independent fMRI tasks. The first task consisted of a digit n-back working memory paradigm that manipulated the working memory load (0- to 4-back), and the second task consisted of a spatial distance judgment paradigm in which the difficulty of the relative distance decision was manipulated (easy to hard). In both tasks, greater demands imposed by the varying difficulty of task conditions should challenge executive decision-making processes, allowing for the systematic measurement of increases and decreases in BOLD activation as a function of increasing task difficulty. Examining these issues in two separate tasks that both modulate the degree of cognitive difficulty provides the unique opportunity to gauge whether the frontal-striatal brain responses that are associated with white matter microstructural integrity might reflect a more general, task-independent response to cognitive challenge. We hypothesized that age-related degradation of frontostriatal tract white matter connectivity would predict executive function performance and reduced BOLD modulation to task difficulty.
2. Materials and Methods
2.1. Participants
Participants from the full sample included 185 healthy adults (110 women) aged 20–94 years (Mage = 53.26, SD = 18.95) recruited from the Dallas/Fort Worth metroplex via flyers and media advertisements who were compensated for their time. All participants were right-handed, native English speakers, with a minimum of high school education or equivalent and screened to be free from neurological, metabolic, cardiovascular, psychiatric disorders, substance abuse, or head injury with loss of consciousness > 5 minutes. Participants were tested at normal or corrected-to-normal vision and had no contraindications to MRI scanning. MRI-safe prescription goggles were fitted for use during scanning. Participants were screened for dementia and depression using the Mini-Mental Status Exam (MMSE; Folstein, Folstein, & McHugh, 1975) and the Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977) with cutoffs of 26 and 16, respectively. A subset of 169 healthy adults from the full study sample with complete and vetted diffusion tractography data were included in the current diffusion analyses (96 women; M age = 51.66, SD age = 18.5). Additionally, participants from this sample with fMRI data for either of two tasks [distance judgment task: 146 adults (82 women); n-back task: 155 adults (86 women)] were included in analyses examining associations between white matter connectivity and BOLD activation. Written informed consent was obtained from all participants in accord with local institutional review board guidelines. See Table 1 for sample demographic information.
Table 1.
Participant demographics, task performance, and frontostriatal tract diffusion metric
| Younger (20–35) Mean (SD) | Middle (36–55) Mean (SD) | Older (56–69) Mean (SD) | Oldest (70–94) Mean (SD) | |
|---|---|---|---|---|
| Demographics | N = 44 | N = 49 | N = 39 | N = 37 |
| Sex N (M/W) | 20/24 | 22/27 | 16/23 | 15/22 |
| Education (years) | 15.55 (2.19) | 15.22 (2.51) | 15.69 (2.45) | 15.49 (2.82) |
| MMSE | 29.16 (0.96) | 29.22 (0.82) | 28.85 (0.84) | 28.76 (0.68) |
| CES-D | 4.66 (3.61) | 4.76 (4.33) | 3.69 (3.27) | 4.08 (3.88) |
| EF Performance | ||||
| CWI (sec) | 27.26 (12.73) | 30.74 (7.96) | 32.94 (10.01) | 42.87 (16.76) |
| TMT (sec) | 34.34 (16.22) | 42.73 (22.47) | 50.10 (23.53) | 58.30 (28.16) |
| Verbal Fluency (# correct) | 14.23 (3.08) | 13.94 (3.21) | 14.23 (2.84) | 11.16 (3.48) |
| Frontostriatal Diffusion Metrics | ||||
| FA | 0.37 (0.01) | 0.36 (0.02) | 0.36 (0.01) | 0.35 (0.02) |
| MD (mm2/sec)*1000 | 0.76 (0.02) | 0.76 (0.02) | 0.77 (0.02) | 0.81 (0.04) |
| DJ Task Accuracya | N = 41 | N = 43 | N = 34 | N = 28 |
| Easy | 0.97 (0.07) | 0.97 (0.05) | 0.95 (0.07) | 0.96 (0.06) |
| Medium | 0.95 (0.08) | 0.93 (0.08) | 0.88 (0.14) | 0.86 (0.14) |
| Hard | 0.69 (0.15) | 0.73 (0.18) | 0.71 (0.23) | 0.78 (0.17) |
| n-back Task Accuracyb | N = 41 | N = 46 | N = 35 | N = 33 |
| n0 | 0.97 (0.05) | 0.93 (0.11) | 0.95 (0.10) | 0.93 (0.10) |
| n2 | 0.93 (0.05) | 0.85 (0.12) | 0.84 (0.12) | 0.79 (0.12) |
| n3 | 0.86 (0.06) | 0.78 (0.10) | 0.76 (0.09) | 0.73 (0.08) |
| n4 | 0.83 (0.06) | 0.76 (0.10) | 0.75 (0.08) | 0.72 (0.07) |
Note: In all analyses, age was used as a continuous predictor, but is shown by arbitrary groups for demographic and behavioral data reporting.
N = 146 subset of participants with DJ (distance judgment task) fMRI data;
N = 155 subset of participants with n-back fMRI data; SD = standard deviation; M = men; W = women; Education is presented in years; MMSE = Mini-Mental Status Exam; CES-D = Center for Epidemiological Studies Depression Scale; EF = executive function; CWI = Color-Word Interference, measured as time (sec) to complete switching and inhibition conditions, adjusted for time to complete simple color naming and word reading; TMT = Trail Making Test, measured as time (sec) to complete number/letter switching, adjusted for time to complete simple number and letter sequencing; Verbal fluency measured as accuracy on the category switching trials; FA = fractional anisotropy; MD = mean diffusivity, represented as mm2/sec*1000.
2.2. MRI Acquisition Protocol
All participants were scanned on the same 3T Philips Achieva MRI scanner equipped with a 32-channel head coil using SENSE encoding. A T1-weighted MP-RAGE sequence was used to collect high-resolution anatomical images with 160 sagittal slices and the following parameters: 1 mm isotropic voxels, FOV = 256 × 204 × 160 mm, TR/TE = 8.3 ms/3.8 ms, 12° flip angle, total time = 4:19 min. A diffusion-weighted single-shot EPI sequence was acquired with 65 axial slices and the following parameters: 2 × 2 × 2.2 mm3 voxel size (reconstructed to 0.85 × 0.85 × 2.2 mm3), 30 diffusion-weighted directions (b-value = 1000s/mm2) with 1 non-diffusion weighted b0 (0 s/mm2), TR/TE = 5608 ms/51 ms, FOV = 224 × 224, matrix = 112 × 112, total time = 4:19 min. Two functional imaging sequences were collected using identical acquisition parameters: T2*-weighted echo-planar imaging (EPI) sequence was used to obtain BOLD data with 29 interleaved axial slices per volume acquired parallel to the AC-PC line and the following parameters: 64 × 64 matrix, 3.44 × 3.44 × 5 mm3 voxel size, FOV = 220 × 220 × 145 mm, TR/TE = 1500 ms/30 ms, total time = ~35 minutes for both scans.
2.3. MRI Data Processing
2.3.1. Diffusion Image Processing and Tractography Protocol
Diffusion imaging data were processed with the quality control software suite DTIPrep v1.2.4 to identify potential susceptibility or eddy current artifacts and distortions from participant movement (Oguz et al., 2014). Gradients with intensity distortions, as well as those of insufficient quality caused by participant head motion, were detected and removed from subsequent analyses using the default thresholds in DTIPrep (mean of 4 gradients removed per subject; 18–30 gradients remaining1). Remaining gradients were registered to the non-weighted b0image. Diffusion directions were adjusted to account for independent rotations of any gradient relative to the original encoding direction (Leemans & Jones, 2009). Diffusion tensors were estimated using the DSI Studio software build from September 26th, 2014 (Yeh, Verstynen, Wang, Fernández-Miranda, & Tseng, 2013; http://dsi-studio.labsolver.org).
DSI Studio was also used to execute deterministic tractography to construct a dorsal prefrontal – dorsal striatal white matter tract (see Figure 1A for frontostriatal tract in a representative 37-year-old participant; see also Supplemental Figure 1 for additional representative participants). Tracking of this frontostriatal tract was accomplished using regions of interest in the striatum (caudate + putamen) and lateral prefrontal cortex for the left and right hemispheres separately. The following parameters were used for the deterministic tracking algorithm: maximum turning angle of 35 °, step size of 1.5 mm, minimum length of 20 mm, maximum length of 500 mm, and a fractional anisotropy (FA) threshold of 0.15. To avoid inclusion of unreliable tracking output we determined a streamline cutoff via a combination of qualitative visual inspection and a quantitative streamline cutoff that sufficiently captured those with reduced quality of tracking. Individuals with left or right hemisphere streamline counts < 1.5 standard deviations below the mean of each hemisphere (i.e., < 275 streams) were excluded from further analyses (N = 13)2.
Figure 1.
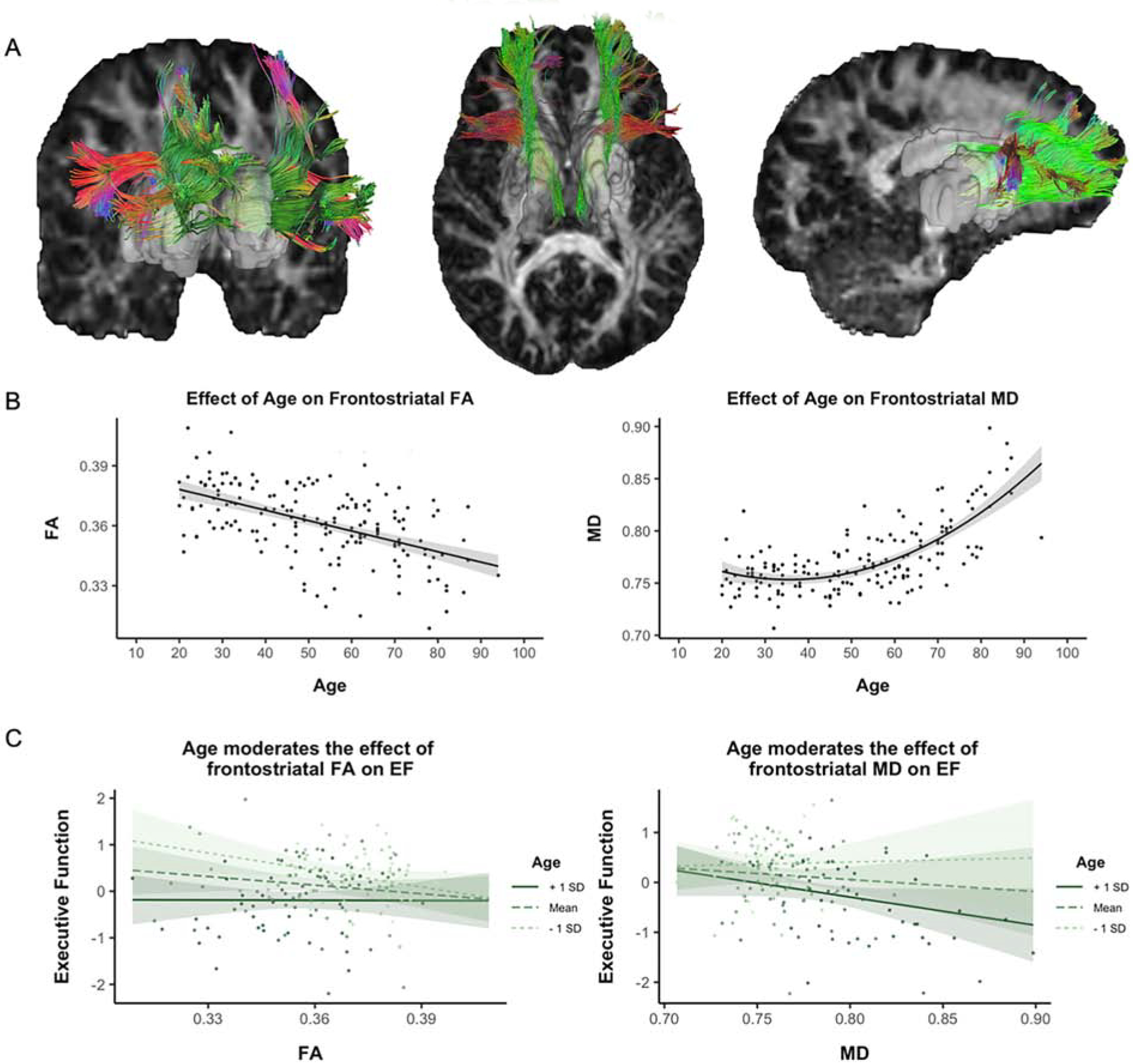
A) Frontostriatal tracts obtained from diffusion tractography in an example participant. Gray 3D regions depict caudate and putamen masks used as seeding points and colored streamlines depict the resulting frontostriatal tracts projecting into prefrontal cortex. Colors represent direction of tracts: Green = anterior-posterior; Red = left-right; Blue = superior-inferior; B) Effects of age on frontostriatal white matter fractional anisotropy (FA; left) and mean diffusivity (MD; right) where FA evidences a linear worsening with age and MD an accelerated worsening with increasing age; C) Age moderation of frontostriatal FA (left) and MD (right) associations with executive function (EF) composite performance, with higher values of EF corresponding to better executive functioning. Note: Data points in Panel C represent partial residuals covarying for sex and global FA or MD, respectively. Depicted MD values are expressed in mm2/sec multiplied by 1000.
Striatal and prefrontal regions of interest (ROIs) were defined as seeding points for the frontostriatal diffusion tractography analysis. These ROIs were defined based on individual subject parcellations obtained through Freesurfer v 5.3.0 (Fischl, 2012) using the Desikan atlas (Desikan et al., 2006) that were then warped to subject diffusion space. The striatal ROI included both the caudate and putamen subcortical segmentations, which were dilated by the dimensions of one resampled voxel to capture white matter adjacent to the subcortical structures. To isolate tracking to prefrontal regions implicated in cognitive frontostriatal loops, Freesurfer parcellations from lateral prefrontal cortex (superior frontal, rostral and caudal middle frontal, pars opercularis, pars triangularis, and pars orbitalis) were combined to create one prefrontal ROI.
Regions of avoidance (ROAs) included the motor and premotor cortices using an axial exclusionary plane at z = 111. This plane was created on the 1mm MNI template and then warped to subject diffusion space to exclude any fibers extending into the premotor/motor cortices and to isolate prefrontal projections to those implicated in cognitive frontostriatal networks. Additional ROAs were included to prevent inferior projections into the brainstem and posterior projections into the parietal or occipital cortices, as well as a ROA in the retrolenticular part of the internal capsule to restrict fornix and/or lateral protrusions from the tail of the striatum. Lastly, an interhemispheric plane was included at x = 91 so that tracking could be isolated to each hemisphere and to avoid inclusion of callosal fibers. After tracking bilaterally, binarized images from each hemisphere were combined and multiplied by the track density image to obtain the mean of all voxels in the frontostriatal tract. Descriptive statistics of the diffusion metrics, including fractional anisotropy (a scalar value ranging from 0–1), mean diffusivity, radial diffusivity, and axial diffusivity (FA, MD, RD, AD) were then obtained from these images. Diffusivities are expressed as mm2/sec*1000. Mean global white matter metrics (averaged from all white matter voxels in the brain) were also calculated to serve as a control variable in regression models to account for the general influence of white matter.
2.3.2. fMRI Data Processing
Preprocessing and statistical analysis of fMRI data was conducted in SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK) using in-house Matlab 2012b (Mathworks) scripts. Identification of motion outliers and preprocessing was identical for both fMRI tasks. Outlier EPI volumes with > 2 mm motion displacement or > 3% deviation from the mean in global intensity spikes were identified using ArtRepair toolbox (Mazaika et al., 2007), and runs with > 15% of volumes (~40 volumes) flagged as outliers were removed from analyses. Data from 3 participants were excluded from the n-back task and data from 4 participants were excluded from the DJ task due to excessive motion in 2 or more runs. These ArtRepair estimates of motion (6 directions) were included as nuisance regressors. Each participant’s functional map was first co-registered to their high-resolution T1 image, and then normalized to standard stereotaxic MNI space. Lastly, normalized data were smoothed with an 8mm isotropic FWHM Gaussian smoothing kernel.
2.4. Experimental Design and Statistical Analysis
2.4.1. Executive Function Measures
During a separate visit to the lab, prior to MRI scanning, participants completed several tests of cognitive function, including EF measures from the Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan, & Kramer, 2001). These measures included the Color-Word Interference (Stroop) task, Trail Making Test, and a Verbal Fluency task that requires producing the names of words, while switching between categories (i.e., furniture, fruit). The index of performance from the Color-Word Interference task was calculated as the time taken (in sec) on the switching and inhibition conditions, adjusted for baseline rates of simple color naming and word reading (in sec). Trails performance was indexed by the time taken to complete number/letter switching (in sec), adjusted for time to complete simple number and letter sequencing tasks alone (in sec). Verbal fluency performance was indexed by accuracy on the category switching trials. Scores on the Color-Word Interference and Trail Making tasks were multiplied by −1, so that higher scores reflect better EF. A unit-weighted composite z-score was then calculated from scores on the three D-KEFS measures to serve as an executive function composite (Cronbach’s α = .63).
2.4.2. fMRI Task Procedures
Two functional tasks, described separately below, were acquired in the same scanning session in the same order for each participant at the beginning of the scanning session, followed by the structural sequences. Both tasks were designed to measure the BOLD response to incremental increases in task difficulty. Each task was presented separately in a blocked design using PsychoPy v1.77.02 (Peirce, 2007, 2008).
2.4.2.1. Distance Judgment (DJ) Task
Participants performed a spatial distance judgment task (Rieck et al., 2017) that included both control judgment and coordinate judgment conditions that parametrically varied in difficulty (easy, medium, hard) based on spatial proximity (see Supplemental Figure 2 for a depiction of the task). At the beginning of a block participants were presented with a vertical reference line after which followed several trials of either the control or distance judgment condition. In the control condition, participants were presented with a dot appearing either to the left or right of a horizontal bar and indicated with a button press on which side the dot was presented (LEFT/RIGHT). In the remainder of the trials, participants were presented with a dot appearing above or below a horizontal bar, with the distance of the dot from the bar parametrically varying relative to the distance of the vertical reference line to establish easy, medium, and hard conditions. Participants indicated with a button press whether the dot was nearer or farther from the length of the cued reference line relative to the horizontal line (NEARER/FARTHER). Thus, this task taps both spatial processes, as well as working memory and executive decision processes. Each of the three 5-minute runs consisted of 20 task blocks that contained 5 trials of each of the four conditions (control, easy, medium, hard), presented in a pseudorandom order. Functional imaging data including this set of participants has been previously presented (Kennedy et al., 2017; Rieck et al., 2017) and can be referenced for further details of the task.
2.4.2.2. n-back Working Memory Task
Participants additionally performed a digit n-back working memory task containing four levels of difficulty (0-back, 2-back, 3-back, 4-back). Using a blocked design, participants were presented with digits (‘2’-’9’) and indicated whether the currently presented stimulus matched the one presented n trials previously using a button press (SAME/DIFFERENT; see Supplemental Figure 2 for a depiction of the task). At the beginning of each block participants were prompted with a 5 sec cue indicating the n-back condition for that block (0-, 2-, 3-, or 4-back), followed by a 2 sec fixation, and then presentation of the digit stimuli for 500 ms. Each of the three runs consisted of 8 blocks, including 2 blocks for each level of difficulty. Functional imaging data including this set of participants has been previously presented in (Kennedy et al., 2017); refer to this article for further details of the task.
2.4.3. fMRI Data Analysis
2.4.3.1. DJ Task
Individual subject- and group-level statistical analyses of fMRI data were conducted in the general linear model (GLM) framework in SPM8. Individual-level contrasts representing each level of difficulty (easy, medium, hard) were computed by convolving the BOLD response during each block of that condition with a canonical hemodynamic response function (HRF). A linear contrast (using mean centered contrast weightings of −1, 0, 1) was also computed for each participant that measured parametrically increasing task difficulty (easy < medium < hard). This linear contrast allows for examination of both up-modulation and down-modulation of the BOLD response to increasing task difficulty (i.e., greater activation or deactivation, respectively, as a function of task difficulty).
At the group-level, voxel-wise linear regression was performed to test for effects of frontostriatal diffusion metrics, age, and their interaction on BOLD modulation to task difficulty. The voxel-wise linear regression models included an intercept term, continuous FA or MD (expressed here and in all subsequent analyses as mm2/sec multiplied by 1000) terms, a continuous age term, and an interaction between the diffusion metric and age, controlling for sex (dummy-coded) and a continuous measure of global white matter FA or MD. Mean global white matter diffusion was included in the model to test whether any observed frontostriatal effects on BOLD modulation or interactions with age were regionally specific. In these and all following models, continuous variables included in interactions were standardized.
2.4.3.2. n-back Task
For the n-back task, individual-level contrasts representing each level of task difficulty (0-back, 2-back, 3-back, 4-back) were computed by convolving the BOLD response during each block of that condition with a canonical HRF. Similar to the DJ task, a linear contrast measuring parametrically increasing task difficulty (0-back < 2-back < 3-back < 4-back) was computed for each participant (using mean-centered contrast weights of −2.25, −0.25, 0.75, 1.75). These contrast weights were chosen to reflect the presumed step-wise increases in difficulty of the n-back task from 0-2-3-4-back. This linear contrast allows for examination of positive and negative modulation of the BOLD response to increasing working memory load. Identical to that conducted with the DJ task, group-level voxel-wise linear regression was performed to test for effects of frontostriatal diffusion metrics, age, and their interaction on BOLD modulation to increasing n-back load. These models, again, included an intercept term, continuous FA or MD terms, a continuous age term, and an interaction between the diffusion metric and age, controlling for sex (dummy-coded) and a continuous measure of global white matter FA or MD.
fMRI results for both tasks were thresholded at cluster-corrected FWE p < .05 and a whole-brain voxel-wise height threshold of p < .001, and the minimum critical extent threshold reported in SPM was used to identify significant clusters. For each task, the MarsBaR toolbox (Brett et al., 2002) was used to extract individual participant mean parameter estimates from identified clusters to be used in follow-up analyses conducted in R v3.5.2 (R Core Team, 2018). fMRI activation results were overlaid on a template brain using MRIcron (Rorden & Brett, 2000).
2.4.4. Statistical Analysis Approach
Separate linear regressions were performed to test for age effects on frontostriatal diffusion metrics. In each model, the diffusion metric of interest (FA or MD) served as the dependent variable and continuous age was the independent variable, controlling for effects of sex. Because FA and MD often show non-linear associations with age at different points in the lifespan (Hsu et al., 2010; Kennedy & Raz, 2015; Lebel et al., 2012; Madden et al., 2012; Simmonds et al., 2014), these models also included squared age to test for quadratic effects of age. To test the effects of frontostriatal diffusion metrics and age on EF, an additional set of linear regressions were conducted that included continuous age and diffusion metric (FA or MD), along with their interaction, predicting individual differences in composite EF scores, controlling for sex and global FA or MD.
Separate linear regressions were conducted to test whether BOLD modulation identified in the previous functional analyses relates to EF. As no significant BOLD associations were observed with frontostriatal FA, the following models were only run for MD. Both models included continuous age, frontostriatal MD, and BOLD modulation to task difficulty (during either the DJ or n-back task) and all interactions, predicting performance on the EF composite. Additionally, to examine effects of BOLD modulation to difficulty on fMRI task accuracy, separate mixed-effects models were estimated for the DJ and n-back tasks using the lmer function in R. For the DJ task a model predicting mean DJ task accuracy was estimated that included fixed effects of continuous age, frontostriatal MD, and striatal BOLD modulation to difficulty in the DJ task, along with a random intercept on participant and random slope on difficulty level, and all interactions. Similarly, for the n-back task a mixed-effects model predicting mean n-back accuracy was estimated that included fixed effects of continuous age, frontostriatal MD, and BOLD modulation to working memory load, along with a random intercept on participant and random slope on memory load and all interactions.
3. Results
3.1. Task Performance
Means and standard deviations of executive function performance and accuracy on both fMRI tasks are included in Table 1. The number of participants contributing to each variable are also listed, as the number of participants with both DTI and fMRI data for each fMRI task varied slightly.
3.2. Effects of Age on Frontostriatal Diffusion Metrics
Table 1 includes means and standard deviations of the white matter diffusion metrics in the frontostriatal tract. Because FA and MD are the most commonly reported metrics in the extant literature, we focus on those metrics in subsequent reporting of results; however, patterns of results were similar to that of MD in all analyses with the other diffusivity measures (axial and radial diffusivity). A linear regression with age and age-squared predicting FA, controlling for sex, indicated that there was a significant linear effect of age on FA (F(1,165) = 68.44, p < .001, ƞp2 = 0.29), such that frontostriatal FA decreased linearly with increasing age (Figure 1B, left panel); yet there was no significant quadratic age association with FA (F(1,165) = 0.18, p = 0.67, ƞp2 = 0.001). A similar model with age and age-squared predicting MD demonstrated significant linear (F(1,165) = 123.95, p < .001, ƞp2 = 0.43) and quadratic (F(1,165) = 39.60, p < .001, ƞp2 = 0.19) associations with age, such that the negative effect of age on frontostriatal MD accelerates with increasing age (Figure 1B, right panel).
3.3. Effects of Age-related Diffusion Metrics on Executive Function
Linear regressions with FA, age, and their interaction, controlling for sex and global FA yielded a main effect of age on EF (F(1,163) = 27.63, p < .001, ƞp2 = 0.15), no main effect of FA on EF (F(1,163) = 1.62, p = 0.206 , ƞp2 = 0.01), and importantly a significant age by FA interaction on EF performance (F(1,163) = 4.67, p = 0.032, ƞp2 = 0.03). Specifically, higher tract FA values were associated with poorer EF scores, but only for younger ages. Simple slopes analyses and the Johnson-Neyman procedure (Preacher et al., 2006) were used to probe this and all following interactions between continuous variables. This procedure allows for estimation of regions of significance of the effect of the independent variable on the dependent variable at different points of the moderator. Results indicated that the association between FA and EF was significant up to approximately age 37, and became non-significant from middle-age on, suggesting a potential protracted developmental effect of frontostriatal white matter on EF. When modeling the effects of MD and age on EF performance, covarying for sex and global MD, we again found a significant main effect of age on EF (F(1,163) = 16.92, p < .001 , ƞp2 = 0.09), no main effect of MD on EF (F(1,163) = 0.51, p = 0.475 , ƞp2 = 0.003), and a significant age by MD interaction on EF (F(1,163) = 4.16, p = .043 , ƞp2 = 0.02). Results indicated that higher MD values were associated with poorer EF scores only in older age (i.e., a significant association was observed for those > 73 years of age). See Figure 1C for visualization of these effects of age on frontostriatal metrics and EF performance (see also Supplemental Figure 3A–B for regions of significance plots for the interactions).
3.4. Association Between Frontostriatal Diffusion Metrics and BOLD Modulation
Results of the whole-brain analyses did not identify any significant regions demonstrating associations between FA and BOLD modulation or any interactions between age and FA on modulation in either fMRI task. Rather, the whole-brain analyses investigating effects of MD on modulation indicated that age significantly moderated the association between frontostriatal MD and BOLD modulation across the two independent fMRI tasks. Interestingly, in this whole-brain search, activation was selective to clusters spanning the left and right basal ganglia in the DJ and n-back tasks, respectively (see Figure 2A; Table 2). Specifically, age moderation was evident in the left dorsal and ventral caudate and ventral putamen, extending into globus pallidus and nucleus accumbens in the DJ task, and in the right dorsal caudate and putamen, extending into globus pallidus and insular cortex for the n-back task3. To determine whether BOLD modulation to task difficulty across the two tasks (DJ and n-back) were related to each other, we correlated the parameter estimates extracted from the striatal clusters evidencing significant associations between MD and difficulty modulation of BOLD activation in both tasks. There was a significant positive correlation between BOLD modulation in the striatum across the two tasks (r = 0.21, p = 0.012), providing evidence of concordance in modulation across the two hemispheres and the two independent fMRI tasks.
Figure 2.
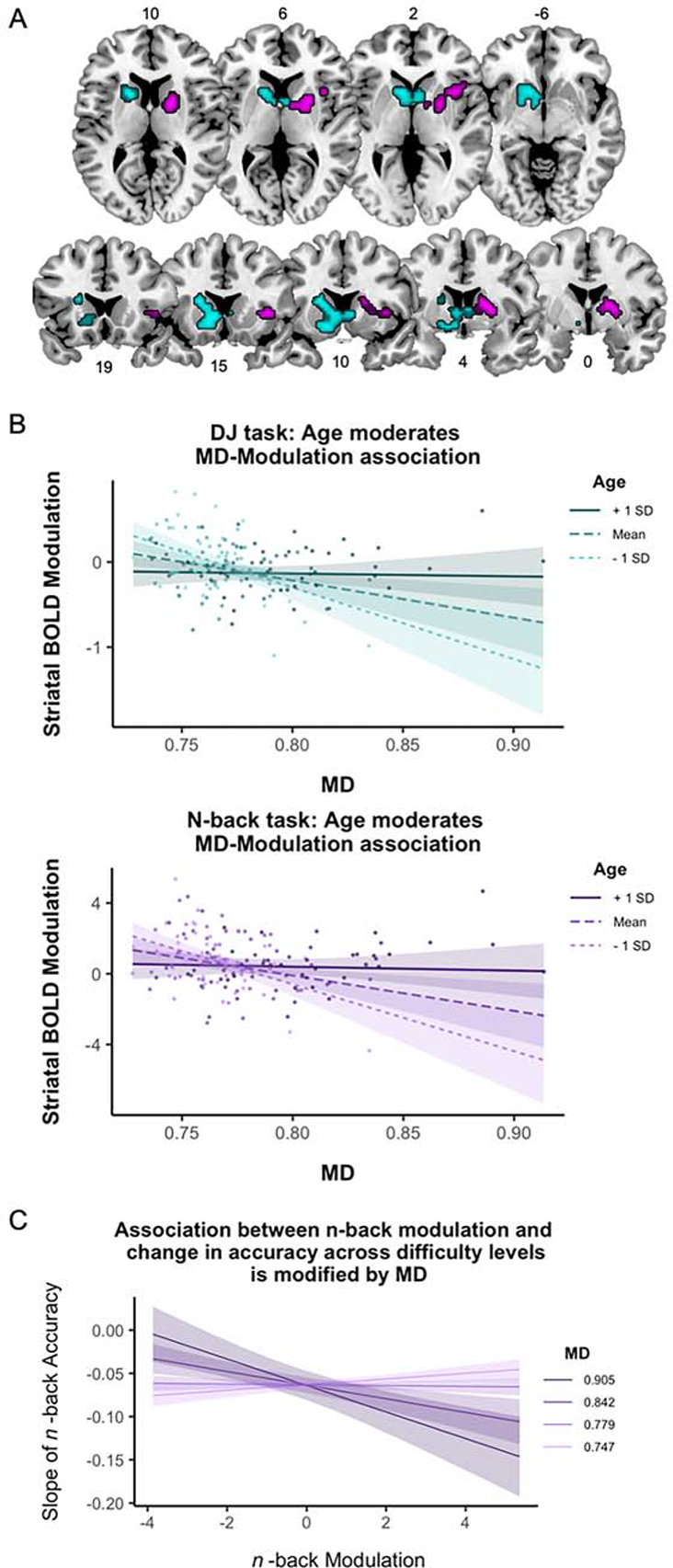
A) Age moderates frontostriatal diffusivity effects on BOLD modulation in the striatum during both the DJ task (teal) and the n-back task (purple); B) Age moderates associations between frontostriatal mean diffusivity (MD) and striatal BOLD modulation on the DJ task (top) and n-back task (bottom); C) The association between modulation to difficulty on the n-back task and change in n-back accuracy across working memory levels is modified by frontostriatal integrity. Note: Data points in Panel B represent partial residuals covarying for sex and global MD. Depicted MD values are expressed as mm2/sec multiplied by 1000.
Table 2.
Cluster peaks associated with interactions between age and frontostriatal mean diffusivity
| H | k | x | y | z | t-value | p-value (cluster-wise FWE) | |
| DJ task | |||||||
| striatum (dorsal and ventral caudate, ventral putamen, globus pallidus, nucleus accumbens) | L | 213 | −21 | 12 | 12 | 4.28 | 0.003 |
| n-back task | |||||||
| striatum (dorsal caudate and putamen, globus pallidus, insula) | R | 118 | 24 | 6 | 6 | 4.46 | 0.031 |
Note: DJ = distance judgment task; H = hemisphere, k = cluster extent, number of voxels; x, y, z MNI coordinates, FWE cluster-corrected at p < .001, whole-brain search
3.5. Decomposition of Age x MD Interaction on BOLD Modulation
3.5.1. DJ Task
The whole-brain analysis revealed a significant interaction between age and MD on BOLD modulation to task difficulty in the distance judgment task. This effect on modulation was localized to a cluster in the left striatum, including dorsal and ventral caudate and ventral putamen (see Table 2 for coordinates and statistics). Mean parameter estimates from the cluster were extracted to further interrogate the nature of the interaction. This analysis, combined with subsequent simple slopes analyses using the Johnson-Neyman procedure, indicated that there was a significant negative association between MD and BOLD up until age 58 where it became non-significant. (see Figure 2B; see also Supplemental Figure 3C for regions of significance plot). More specifically, younger- and middle-aged individuals showed reduced dynamic range in response to greater task difficulty as a function of increasing frontostriatal diffusivity. Younger individuals with lower levels of MD in frontostriatal white matter showed greater positive modulation to task difficulty in the striatum, and this decreased as a function of increasing MD, suggesting a disruption of striatal modulation early in the lifespan in individuals with more degraded frontostriatal white matter. Importantly, both positive and negative modulation (i.e., increased and decreased activation in response to task difficulty, respectively) decreased with age, and was less related to frontostriatal MD levels.
3.5.2. n-back Task
The whole-brain analysis in the n-back fMRI task also indicated a significant interaction between age and MD on BOLD modulation to increasing working memory load. This effect was localized to a cluster in the right striatum, encompassing dorsal caudate and putamen, and extending into insula (see Table 2 for coordinates and statistics and Figure 2A for activation map). Similar to that for the DJ task, simple slopes analyses indicated a significant negative association between MD and BOLD up until age 60 where it became non-significant, with younger- and middle-aged adults showing reduced dynamic range of BOLD response to increased working memory load as a function of increasing frontostriatal diffusivity (see Figure 2B; see also Supplemental Figure 3D for regions of significance plot). Younger individuals with lower frontostriatal MD showed greater positive modulation to task difficulty in the striatum, which decreased as a function of increasing MD, and this range of modulation decreased with increasing age.
3.6. Association of BOLD Modulation with Cognition
3.6.1. DJ task
Lastly, we sought to identify associations between striatal modulation and executive function and/or performance on each associated fMRI task. A significant three-way interaction was found among age, frontostriatal MD, and striatal BOLD modulation to task difficulty when predicting EF performance (F(1,138) = 4.64, p = 0.033, ƞp2 = 0.03), such that MD and age moderated the association between BOLD modulation and EF. Interrogation of this interaction suggested that at lower levels of MD (−1 SD below the mean), greater modulation to increasing difficulty in the DJ task was associated with better EF performance, only in older ages. A simple slopes analysis suggested that this association was significant only for those aged 60 and above. Therefore, at higher levels of MD (mean and above), there was no significant association between modulation and executive function at any point in the lifespan (see Supplemental Figure 4A–B for depiction of this interaction and regions of significance plots). The mixed-effects model on change in DJ task accuracy across level of difficulty identified no significant main effects of age, MD, or modulation, nor any interactions between these variables.
3.6.2. n-back Task
The linear regression including interactions between age, frontostriatal MD, and striatal BOLD modulation to n-back working memory load predicting EF revealed only a main effect of age (F(1,147) = 14.21, p < .001, ƞp2 = 0.09; all other Fs < 2.62, all ps > 0.11). However, the mixed-effects model on n-back accuracy revealed a significant interaction between age, MD, and change in working memory load on accuracy (F(1,147) = 5.73, p = 0.018). In younger ages, increasing frontostriatal MD was associated with a steeper decline in accuracy across increasing levels of working memory load. This association flipped in older ages, where greater frontostriatal MD was associated with less steep decrease in accuracy across level of task difficulty. The mixed-effects model also revealed a significant interaction among MD, modulation, and change in working memory load on mean accuracy (F(1,147) = 4.24, p = 0.041; see Figure 2C). While there is little association between striatal BOLD modulation and differences in accuracy across working memory loads at lower frontostriatal MD, the negative association between modulation and change in accuracy increases as a function of increasing MD. Less negative modulation (i.e., less deactivation with increasing load) is associated with greater decline in n-back accuracy as working memory load increases, only at higher levels of frontostriatal MD, regardless of age.
4. Discussion
Frontostriatal circuitry is critical to a variety of cognitive functions across human and animal species. In humans, gray matter regions within this network are among the most sensitive to aging, yet investigation of lifespan age effects on the structural circuitry connecting these regions is still limited. To our knowledge, the present study is the first to relate frontostriatal white matter measured in a lifespan sample to age-related alterations in both executive function and BOLD modulation. The results support theories of cortical disconnection, demonstrating that aging is accompanied by degraded frontostriatal white matter microstructure, and that these age-related alterations are associated with individual differences in EF performance, as well as a reduced range of functional modulation to cognitive challenge in early- to middle-adulthood.
In line with the few studies that have examined aging effects on cortico-striatal white matter FA (Samanez-Larkin et al., 2012; Vik et al., 2015; Ystad et al., 2011), we observed linear decreases in FA across the adult age span. We additionally demonstrated that effects of age on overall diffusivity (MD) accelerate quadratically with increasing cross-sectional age. Together, these results indicate the negative effect of age on microstructural properties of frontostriatal white matter, in terms of both directionality and rate of diffusion of molecular water in tracts innervating the frontal cortex and striatum. While these white matter properties serve as indirect measures of underlying cellular architecture, an abundance of research shows age-related alterations in striatal and extrastriatal dopaminergic neuromodulation (Kaasinen et al., 2000; Rinne et al., 1990), which affects the efficiency of information processing and communication in the human brain (Li et al., 2001). In fact, dopamine transporter availability has been linked to white matter health in older adults (Rieckmann et al., 2016), indicating one possible mechanism of age-related white matter degradation. This, however, was not localized to frontostriatal white matter, highlighting a future need to examine associations between dopaminergic signaling and frontostriatal structural circuitry across the lifespan.
Age effects on dopaminergic processing and striatal function also contribute to age differences in performance on tasks engaging cognitive control processes and executive functioning (for review: Bäckman, Nyberg, & Farde, 2006). Consistent with this previous observation, age differences in frontostriatal white matter in our sample were related to individual differences in a composite EF measure. Higher MD was associated with reduced EF performance as a function of increasing age, indicating a negative influence of age-related alterations in frontostriatal diffusivity on EF. Higher FA, however, was associated with reduced EF performance only in an earlier portion of the lifespan. This may be indicative of the protracted development of white matter tracts innervating frontal regions, which is commonly observed to increase in adolescence and into adulthood, peaking somewhere between age 20–40 (Klingberg, et al., 1999; Lebel et al., 2012). Whereas lower FA in older adults (and in various types of patients) is generally associated with poorer white matter quality, in development lower FA is seen as beneficial, perhaps reflecting proper pruning and increased organization and coherence. Thus, this result may reflect the fact that our younger adult participants are still in a protracted developmental stage where higher FA is associated with developmental alterations to frontostriatal white matter microstructure and poorer EF (Simmonds et al., 2014). This result, however, requires further replication in lifespan samples that span earlier childhood and developmental periods and include higher order EF tasks. Together, these results not only demonstrate the relevance of this specific tract for executive functioning, but also highlight that different white matter metrics may have divergent influences on executive function at different points in the lifespan.
Frontostriatal white matter measures were not only related to EF, but also showed age-varying associations with modulation of BOLD activation to increasing task demands, and further moderated associations between BOLD and behavior. Specifically, results of a whole-brain search indicated that greater MD was associated with reduced dynamic range of activation exclusively in the striatum, and this effect was strongest in young- to middle-adulthood (up until around age 60). Interestingly, we did not observe associations between frontostriatal white matter connectivity and BOLD activation in older individuals, potentially suggesting white matter alterations have more of an effect on functional modulation in middle, rather than older, age. It is also likely the case that by old age additional salient factors (e.g., cortical volume/thickness, beta-amyloid or iron accumulation, vascular health, etc.) converge to influence brain function, and these factors may be as, if not more, relevant to individual differences in BOLD modulation. These results highlight the relevance of age-related differences in structural white matter properties to regulation of neural function and cognition, supporting the idea that the structure of the brain affects its function differentially across the lifespan (Warbrick et al., 2017; Webb, et al., 2020). Importantly, the pattern of age effects on this structure-function association was evident for both the n-back and the distance judgment tasks that involved manipulations of difficulty. This highlights a main strength of the current study in that we had the unique opportunity to measure within-person effects across tasks on neural responses to cognitive challenge. We demonstrated converging effects of frontostriatal white matter microstructure on BOLD modulation to difficulty across two independent fMRI tasks within an individual. Thus, frontostriatal structural white matter influences on striatal response to difficulty manipulations appear, at least in these paradigms, to be somewhat task-general.
The effect of frontostriatal white matter on striatal activation in both tasks was mostly driven by reductions in positive modulation to cognitive demand in adults middle-aged and younger. Using structural equation modeling, we have previously shown that age-related degradation of a latent white matter factor (including major heteromodal and association tracts) is associated with reduced positive modulation to task demand across a working memory-related network (Webb et al., 2020). Here, we illustrate specificity in this association demonstrating that frontostriatal white matter diffusivity influences the degree to which activation in the striatum is modulated in response to increased cognitive load. Striatal activation is consistently evident in tasks involving executive control and decision-making across a variety of contexts (Grahn et al., 2008; Postle & D’Esposito, 1999), and is thought to reflect a gating mechanism that exerts control over attentional and executive processes. Moreover, areas of the striatum are often coactive with dorsal prefrontal cortex in such tasks (Postuma & Dagher, 2006), and studies have linked both altered striatal-prefrontal resting state and functional connectivity with age differences in decision-making, executive function, and memory performance (e.g., Su et al., 2018; Ystad et al., 2010, 2011). While we did not detect associations between frontostriatal white matter and functional modulation in the frontal cortex, the present results suggest that the white matter connecting frontostriatal networks influences the functioning of subcortical gray matter within this network. Further studies investigating how lifespan age differences in white matter are related to functional connectivity within this network both during task and at rest are needed. While our data cannot directly inform understanding of dopamine neuromodulation, reduced striatal response may reflect altered striatal dopaminergic functioning in younger individuals evidencing more degraded frontostriatal white matter. It may also be the case that frontostriatal white matter is a reflection of less efficient dopaminergic modulation that contributes to greater noise and reduced fidelity of neuronal function (Li et al., 2001). In fact, Samanez-Larkin et al. (2010) demonstrated a link between age-related alterations in temporal variability of striatal neural activation and financial decision-making. Further elucidating these differences requires future examination of neuromodulation combined with structural and functional imaging of frontostriatal networks across the lifespan.
Previous work from our lab and others additionally demonstrates that the degree of BOLD modulation, either positive or negative, is related to both fMRI task and out-of-scanner cognitive performance (e.g., Hakun & Johnson, 2017; Kennedy et al., 2017; Rieck et al., 2017). In the current study, older individuals with more intact frontostriatal microstructure showed a positive association between striatal modulation and EF performance in the DJ task, yet there was no association between modulation and EF in individuals of any age exhibiting poorer white matter connectivity. Therefore, intact frontostriatal microstructure in older age may serve to support striatal responses to cognitive challenge, where this ability to modulate is associated with better general EF ability. While we did not observe associations between BOLD modulation and EF performance in the n-back task, we did identify a relationship with in-scanner working memory performance. Regardless of age, individuals exhibiting poorer white matter microstructure showed steeper declines in n-back performance across working memory load as a function of striatal modulation. This finding suggests that frontostriatal white matter connectivity may play a role in modifying the degree to which functional modulation supports task accuracy across increasing difficulty, with more degraded white matter exerting a greater influence on activation-behavior associations across age.
The present results should be considered in the context of study strengths and limitations. It is important to acknowledge that a different selection of seed regions (e.g., inclusion of portions of anterior cingulate) could elicit different associations between diffusion metrics and EF. Future studies including high-resolution structural (e.g., biophysical diffusion models) and functional (e.g., of PFC and striatum) imaging would help further disentangle how different portions of frontostriatal networks differentially relate to age and various components of executive function. Additionally, in samples that span the adult lifespan there is the potential for age-related volumetric biases in fMRI spatial registration when using a standard template. Inclusion of whole brain gray and white matter volume covariates (residualized for intracranial volume), as an attempt to account for this possible issue did not alter the fMRI findings in the present study, which helps mitigate against this bias. Lastly, longitudinal data are necessary to quantify individual age-related progression of frontostriatal white matter integrity degradation, as well as to identify associations between different white matter indices and executive function performance across time.
Overall, the present findings illustrate that age-related alterations in frontostriatal white matter negatively affect both executive function and dynamic range of functional modulation to cognitive challenge, supporting theories positing that cortical disconnection contributes to cognitive dysfunction. While performance on EF tasks is sensitive to frontostriatal white matter alterations across age, frontostriatal circuity influences the ability to flexibly modulate striatal BOLD activity earlier in the lifespan, and this has consequences for EF and task performance. Our multi-task fMRI results indicate that white matter is important for dynamic functional modulation to increasing task demands in early- and middle-adulthood broadly across different cognitive tasks. Collectively, the present results lend support to cortical disconnection theories, suggesting that structural degradation contributes to alterations in brain and cognitive function (Bartzokis, 2004; O’Sullivan et al., 2001), and inform our understanding about how white matter tracts innervating cortical and subcortical structures influence cognitive and brain function across the lifespan. However, the current results indicate that these associations might be most evident in younger- to middle-age, highlighting the need to capture brain changes occurring earlier in the age span.
Supplementary Material
Highlights.
Aging is accompanied by degraded frontostriatal (FS) white matter microstructure
FS microstructure is associated with individual differences in executive function
Affects range of functional modulation to cognitive challenge early in the lifespan
Acknowledgments:
This work was supported in part by grants from the National Institutes of Health (AG036848, AG-036818, AG-056535) to KMK and KMR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note that including number of gradients and age x gradient interactions in analyses did not alter the main results for any analyses.
Analyses were repeated including these 13 participants (total N = 182) and all primary results remained, with the exception of the age x FA interaction on executive function which became marginal (p = 0.063).
Inclusion of whole brain gray and white matter volume covariates (residualized for intracranial volume), to adjust for age-related differences in brain volume, did not alter the results in either task or for either covariate.
Verification
The authors declare no conflicts of interest or competing financial interests.
References
- Alexander G (1986). Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annual Review of Neuroscience, 9(1), 357–381. 10.1146/annurev.neuro.9.1.357 [DOI] [PubMed] [Google Scholar]
- Bäckman L, Lindenberger U, Li S-C, & Nyberg L (2010). Linking cognitive aging to alterations in dopamine neurotransmitter functioning: Recent data and future avenues. Neuroscience & Biobehavioral Reviews, 34(5), 670–677. 10.1016/j.neubiorev.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li S-C, & Farde L (2006). The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neuroscience & Biobehavioral Reviews, 30(6), 791–807. 10.1016/j.neubiorev.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Bartzokis G (2004). Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiology of Aging, 25(1), 5–18. 10.1016/j.neurobiolaging.2003.03.001 [DOI] [PubMed] [Google Scholar]
- Bennett IJ, & Rypma B (2013). Advances in functional neuroanatomy: A review of combined DTI and fMRI studies in healthy younger and older adults. Neuroscience & Biobehavioral Reviews, 37(7), 1201–1210. 10.1016/j.neubiorev.2013.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, & Poline JB (2002). Imaging techniques. NeuroImage, 16(2), 37–258. 10.1016/S1053-8119(02)90010-8 [DOI] [Google Scholar]
- Brown CA, Hakun JG, Zhu Z, Johnson NF, & Gold BT (2015). White matter microstructure contributes to age-related declines in task-induced deactivation of the default mode network. Frontiers in Aging Neuroscience, 7, 194 10.3389/fnagi.2015.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, & Dennis NA (2002). Principles of Frontal Lobe Function In Stuss DT & Knight RT (Eds.), Principles of frontal lobe function. Oxford University Press; 10.1093/acprof:oso/9780195134971.001.0001 [DOI] [Google Scholar]
- Darki F, & Klingberg T (2015). The Role of Fronto-Parietal and Fronto-Striatal Networks in the Development of Working Memory: A Longitudinal Study. Cerebral Cortex, 25(6), 1587–1595. 10.1093/cercor/bht352 [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001). Delis-Kaplan executive function system. San Antonio, TX: The Psychological Corporation; 10.3109/02770903.2012.715704 [DOI] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, & Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Fischl B (2012). FreeSurfer. NeuroImage, 62(2), 774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state.” Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, & Owen AM (2008). The cognitive functions of the caudate nucleus. Progress in Neurobiology, 86(3), 141–155. 10.1016/j.pneurobio.2008.09.004 [DOI] [PubMed] [Google Scholar]
- Hakun JG, & Johnson NF (2017). Dynamic range of frontoparietal functional modulation is associated with working memory capacity limitations in older adults. Brain and Cognition, 118, 128–136. 10.1016/j.bandc.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakun JG, Zhu Z, Brown CA, Johnson NF, & Gold BT (2015). Longitudinal alterations to brain function, structure, and cognitive performance in healthy older adults: A fMRI-DTI study. Neuropsychologia, 71, 225–235. 10.1016/j.neuropsychologia.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J-L, Van Hecke W, Bai C-H, Lee C-H, Tsai Y-F, Chiu H-C, Jaw F-S, Hsu C-Y, Leu J-G, Chen W-H, & Leemans A (2010). Microstructural white matter changes in normal aging: A diffusion tensor imaging study with higher-order polynomial regression models. NeuroImage, 49(1), 32–43. 10.1016/j.neuroimage.2009.08.031 [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, Någren K, Helenius H, Olsson H, Farde L, & Rinne J (2000). Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiology of Aging, 21(5), 683–688. 10.1016/s0197-4580(00)00149-4 [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Boylan MA, Rieck JR, Foster CM, & Rodrigue KM (2017). Dynamic range in BOLD modulation: lifespan aging trajectories and association with performance. Neurobiology of Aging, 60, 153–163. 10.1016/j.neurobiolaging.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, & Raz N (2015). Normal Aging of the Brain In Brain Mapping (pp. 603–617). Elsevier. 10.1016/B978-0-12-397025-1.00068-3 [DOI] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JDE, Moseley ME, & Hedehus M (1999). Myelination and organization of the frontal white matter in children. NeuroReport, 10(13), 2817–2821. 10.1097/00001756-199909090-00022 [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, & Beaulieu C (2012). Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage, 60(1), 340– 352. 10.1016/j.neuroimage.2011.11.094 [DOI] [PubMed] [Google Scholar]
- Leemans A, & Jones DK (2009). The B -matrix must be rotated when correcting for subject motion in DTI data. Magnetic Resonance in Medicine, 61(6), 1336–1349. 10.1002/mrm.21890 [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Van De Moortele P-F, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, & Kim D-S (2004). Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Annals of Neurology, 55(4), 522–529. 10.1002/ana.20030 [DOI] [PubMed] [Google Scholar]
- Li S-C, Lindenberger U, & Sikström S (2001). Aging cognition: from neuromodulation to representation. Trends in Cognitive Sciences, 5(11), 479–486. 10.1016/S1364-6613(00)01769-1 [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen N, & Song AW (2012). Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1822(3), 386–400. 10.1016/j.bbadis.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, & Song AW (2009). Cerebral White Matter Integrity and Cognitive Aging: Contributions from Diffusion Tensor Imaging. Neuropsychology Review, 19(4), 415–435. 10.1007/s11065-009-9113-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika P, Whitfield-Gabrieli S, & Reiss A (2007). Artifact repair for fMRI data from high motion clinical subjects. [Google Scholar]
- Middleton FA, & Strick PL (2000). Basal Ganglia Output and Cognition: Evidence from Anatomical, Behavioral, and Clinical Studies. Brain and Cognition, 42(2), 183–200. 10.1006/brcg1999.1099. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, & Markus HS (2001). Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology, 57(4), 632–638. 10.1212/WNL.57.4.632 [DOI] [PubMed] [Google Scholar]
- Oguz I, Farzinfar M, Matsui J, Budin F, Liu Z, Gerig G, Johnson HJ, & Styner M (2014). DTIPrep: quality control of diffusion-weighted images. Frontiers in Neuroinformatics, 8, 4 10.3389/fninf.2014.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, & Reuter-Lorenz P (2009). The Adaptive Brain: Aging and Neurocognitive Scaffolding. Annual Review of Psychology, 60(1), 173–196. 10.1146/annurev.psych.59.103006.093656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW (2007). PsychoPy—Psychophysics software in Python. Journal of Neuroscience Methods, 162(1–2), 8–13. 10.1016/j.jneumeth.2006.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JW (2008). Generating stimuli for neuroscience using PsychoPy. Frontiers in Neuroinformatics, 2 10.3389/neuro.11.010.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, & D’Esposito M (1999). Dissociation of human caudate nucleus activity in spatial and nonspatial working memory: an event-related fMRI study. Cognitive Brain Research, 8(2), 107–115. 10.1016/S0926-6410(99)00010-5 [DOI] [PubMed] [Google Scholar]
- Postuma RB, & Dagher A (2006). Basal Ganglia Functional Connectivity Based on a Meta-Analysis of 126 Positron Emission Tomography and Functional Magnetic Resonance Imaging Publications. Cerebral Cortex, 16(10), 1508–1521. 10.1093/cercor/bhj088 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2006). Computational Tools for Probing Interactions in Multiple Linear Regression, Multilevel Modeling, and Latent Curve Analysis. Journal of Educational and Behavioral Statistics, 31(4), 437–448. 10.3102/10769986031004437 [DOI] [Google Scholar]
- R Core Team. (2018). R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing. https://www.r-project.org/ [Google Scholar]
- Radloff LS (1977). The CES-D Scale. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, & Lindenberger U (2010). Trajectories of brain aging in middle-aged and older adults: Regional and individual differences. NeuroImage, 51(2), 501–511. 10.1016/j.neuroimage.2010.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, & Kennedy KM (2009). A Systems Approach to the Aging Brain: Neuroanatomic Changes, Their Modifiers, and Cognitive Correlates In Imaging the Aging Brain (pp. 43–70). Oxford University Press; 10.1093/acprof:oso/9780195328875.003.0004 [DOI] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, & Acker JD (2005). Regional Brain Changes in Aging Healthy Adults: General Trends, Individual Differences and Modifiers. Cerebral Cortex, 15(11), 1676– 1689. 10.1093/cercor/bhi044 [DOI] [PubMed] [Google Scholar]
- Raz N, & Rodrigue KM (2006). Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Review s, 30(6), 730–748. 10.1016/j.neubiorev.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon F, & Acker JD (2003). Differential aging of the human striatum: longitudinal evidence. AJNR American Journal of Neuroradiology, 24(9), 1849–1856. [PMC free article] [PubMed] [Google Scholar]
- Rieck JR, Rodrigue KM, Boylan MA, & Kennedy KM (2017). Age-related reduction of BOLD modulation to cognitive difficulty predicts poorer task accuracy and poorer fluid reasoning ability. NeuroImage, 147, 262–271. 10.1016/j.neuroimage.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann A, Hedden T, Younger AP, Sperling RA, Johnson KA, & Buckner RL (2016). Dopamine transporter availability in clinically normal aging is associated with individual differences in white matter integrity. Human Brain Mapping, 37(2), 621–631. 10.1002/hbm.23054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JD, Moore S, Cramer SC, & Lin JJ (2011). Caudate atrophy and impaired frontostriatal connections are linked to executive dysfunction in temporal lobe epilepsy. Epilepsy & Behavior, 21(1), 80–87. 10.1016/j.yebeh.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JO, Lönnberg P, & Marjamäki P (1990). Age-dependent decline in human brain dopamine D1 and D2 receptors. Brain Research, 508(2), 349–352. 10.1016/0006-8993(90)90423-9 [DOI] [PubMed] [Google Scholar]
- Rorden C, & Brett M (2000). Stereotaxic Display of Brain Lesions. Behavioural Neurology, 12(4), 191–200. 10.1155/2000/421719 [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Kuhnen CM, Yoo DJ, & Knutson B (2010). Variability in Nucleus Accumbens Activity Mediates Age-Related Suboptimal Financial Risk Taking. Journal of Neuroscience, 30(4), 1426–1434. 10.1523/JNEUROSCI.4902-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Levens SM, Perry LM, Dougherty RF, & Knutson B (2012). Frontostriatal White Matter Integrity Mediates Adult Age Differences in Probabilistic Reward Learning. Journal of Neuroscience, 32(15), 5333–5337. 10.1523/JNEUROSCI.5756-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, Maclin EL, Gratton G, & Fabiani M (2010). Span, CRUNCH, and Beyond: Working Memory Capacity and the Aging Brain. Journal of Cognitive Neuroscience, 22(4), 655– 669. 10.1162/jocn.2009.21230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, & Luna B (2014). Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. NeuroImage, 92, 356–368. 10.1016/j.neuroimage.2013.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y-S, Chen J-T, Tang Y-J, Yuan S-Y, McCarrey AC, & Goh JOS (2018). Age-related differences in striatal, medial temporal, and frontal involvement during value-based decision processing. Neurobiology of Aging, 69, 185–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vik A, Hodneland E, Haász J, Ystad M, Lundervold AJ, & Lundervold A (2015). Fractional anisotropy shows differential reduction in frontal-subcortical fiber bundles—A longitudinal MRI study of 76 middle-aged and older adults. Frontiers in Aging Neuroscience, 7 10.3389/fnagi.2015.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warbrick T, Rosenberg J, & Shah NJ (2017). The relationship between BOLD fMRI response and the underlying white matter as measured by fractional anisotropy (FA): A systematic review. NeuroImage, 153, 369–381. 10.1016/j.neuroimage.2016.12.075 [DOI] [PubMed] [Google Scholar]
- Webb CE, Rodrigue KM, Hoagey DA, Foster CM, & Kennedy KM (2020). Contributions of White Matter Connectivity and BOLD Modulation to Cognitive Aging: A Lifespan Structure-Function Association Study. Cerebral Cortex, 30(3), 1649–1661. 10.1093/cercor/bhz193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F-C, Verstynen TD, Wang Y, Fernández-Miranda JC, & Tseng W-YI (2013). Deterministic Diffusion Fiber Tracking Improved by Quantitative Anisotropy. PLoS ONE, 8(11), e80713 10.1371/journal.pone.0080713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ystad M, Eichele T, Lundervold AJ, & Lundervold A (2010). Subcortical functional connectivity and verbal episodic memory in healthy elderly—A resting state fMRI study. NeuroImage, 52(1), 379–388. 10.1016/j.neuroimage.2010.03.062 [DOI] [PubMed] [Google Scholar]
- Ystad M, Hodneland E, Adolfsdottir S, Haász J, Lundervold AJ, Eichele T, & Lundervold A (2011). Cortico-striatal connectivity and cognition in normal aging: A combined DTI and resting state fMRI study. NeuroImage, 55(1), 24–31. 10.1016/j.neuroimage.2010.11.016 [DOI] [PubMed] [Google Scholar]
- Zhu Z, Johnson NF, Kim C, & Gold BT (2015). Reduced Frontal Cortex Efficiency is Associated with Lower White Matter Integrity in Aging. Cerebral Cortex, 25(1), 138–146. 10.1093/cercor/bht212 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


