Abstract
Purpose:
We investigated the ability of prostate MRI to detect Gleason Grade Group (GG) ≥2 cancer in a standardized, multi-institutional active surveillance cohort.
Material and Methods:
We evaluated men enrolled in Canary Prostate Active Surveillance Study (PASS) with GG<2 and who underwent a biopsy within 12 months of a multiparametric MRI. Our primary outcome was biopsy reclassification to GG2 or greater. We evaluated the performance of MRI PIRADS score and clinical factors. Multivariable logistic regression models were fit with MRI and clinical factors and used to perform receiver operating curve analyses.
Results:
There were 361 participants with 395 prostate MRIs with a median follow-up of 4.1 (IQR: 2.0–7.6) years. Overall, 108/395 (27%) biopsies showed reclassification. Defining positive MRI as PIRADS 3–5, the NPV and PPV for detecting GG ≥2 cancer was 83% (95% CI: 76–90%) and 31% (95% CI: 26–37%), respectively. PIRADS was significantly associated with reclassification (PIRADS 5 versus 1 and 2: OR = 2.71; 95% CI: 1.21–6.17, p = 0.016) in a multivariable model but did not improve upon a model with only clinical factors (AUC 0.768 versus 0.762). In 194 fusion biopsies, higher grade cancer was found in targeted cores in 21 (11%) instances, while 25 (13%) had higher grade cancer found in the systematic cores.
Conclusions:
This study adds the largest cohort data to the body of literature for MRI in active surveillance, recommending systematic biopsy in patients with a negative MRI and the inclusion of systematic biopsy in patients with a positive MRI.
Introduction
Active surveillance (AS) for low-risk prostate cancer is considered the preferred initial management strategy by national guidelines.1–3 Providers and patients remain concerned that apparent low-grade prostate cancer may harbor occult higher grade disease that would warrant treatment, and that the delay in primary curative therapy may introduce unnecessary risk of cancer progression.4 Furthermore, active surveillance involves prostate biopsies which are costly and invasive, with risks of significant pain, bleeding, infection, and anxiety.5 Tools that improve outcomes of active surveillance would have great benefit.
MRI has been promoted for the initial diagnostic workup for elevated PSA, particularly in men with previous negative biopsies.6, 7 Multiple studies have reported that MRI targeted biopsies increase detection of clinically significant prostate cancer and can reduce unnecessary biopsies and over detection of indolent cancers.8, 9 Widespread adoption of MRI has been hampered by variations in scan performance characteristics, high interobserver variability, and cost.10, 11 Most active surveillance studies investigating MRI are small, single-institution case series reporting variable sensitivity/specificity, even in tertiary care centers of excellence.12–14 One notable randomized clinical trial of MRI in active surveillance reported that the addition of MRI with targeted biopsies to systematic biopsies did not significantly increase the upgrading rate compared with systematic biopsy alone.15
Against this background, we report the operating characteristics of multiparametric MRI (mpMRI) in a cohort of men with low-grade prostate cancer who are participants in a prospective, multi-institutional active surveillance clinical study. We specifically examined the association of mpMRI lesions with disease reclassification at protocol-directed surveillance biopsies. We further studied the utility of MRI fusion biopsy techniques when compared to systematic biopsies alone.
Methods
Study sample and analytic cohort:
The Canary Prostate Cancer Active Surveillance Study (PASS) is an IRB approved multicenter prospective study (clinicaltrials.gov NCT00756665) of men who elected AS for prostate cancer management and has been described in detail.16, 17 Briefly, follow-up included quarterly PSA measures, semi-annual digital rectal examinations, and surveillance prostate biopsies at one and two years after initial diagnosis and then every two years. Use of prostate MRI as a component of surveillance was not mandated by this protocol and left to each clinician’s discretion. For this analysis we captured clinical data up through February 2019 on participants from a central database. We excluded men if they had no MRI, an MRI before initial diagnosis, MRI with no subsequent biopsy within 12 months, or Gleason Grade Group (GG) > 1 before MRI. Details are depicted in Supplemental Figure 1. In this analysis, data were included from 9 of the 10 PASS sites.
Exposures and outcomes of interest:
Images were from July 2010 to January 2019, IQR: November 2015 – August 2017. Images were interpreted according to the procedure at each site, and all were graded based on PIRADS, with approximately 80% using PIRADS v2.0. We defined MRIs as “normal” if no lesions were noted, or if suspicious regions were graded as PIRADS 1/2. If no lesion was found, for analysis the MRI was treated as a negative MRI and assigned as PIRADS 1. There was no standard protocol in terms of magnet strength (1.5T vs 3T) or use of endorectal coil, although the large majority of images were obtained with 3T magnets, with the exception of early images at 2 sites (<10% of images) that were obtained with 1.5T magnet using endorectal coil. Our primary outcome of interest was biopsy reclassification defined as any upgrade to Gleason GG ≥ 2 on the post-MRI biopsy. Biopsies were performed at the discretion of the clinician, including cognitive or MR-US fusion-image guidance, as well as the amount of systematic sampling added to targeted biopsy cores.
Statistical Analysis:
For our assessment of the diagnostic performance of prostate MRI, we considered an MRI as “negative” if no lesion or a PIRADS 1/2 lesion was present. A separate analysis was performed in which PIRADS 1–3 were grouped together in the definition of negative MRI. Biopsy results were considered the gold standard result, with presence of GG2 and greater cancer considered a positive result. Negative and positive predictive values (NPV and PPV), true and false positive rates (TPR and FPR) were calculated at the MRI-level to evaluate the accuracy of MRI (positive versus negative) for classifying reclassification status.
We then fit multivariable logistic regression models based on previously established models using available clinical information to estimate the odds of our outcome of interest. A base model, consistent with previous studies18, included age, body mass index (BMI), number of negative biopsies after diagnosis, percent of total biopsy cores containing cancer from the previous biopsy, log-transformed prostate size, and log-transformed serum PSA. We then added PIRADS score (categorical) to a second model. We then used receiver operating curve (ROC) analyses to calculate the areas under the curve (AUC) for both models to assess the discriminatory capacity for separating patients with and without biopsy reclassification.
In a separate analysis, we compared targeted versus systematic biopsy approaches for detection of high-grade cancer in the subset of men who had concurrent systematic and targeted MR/ultrasound fusion biopsy. All analyses were conducted using R version 3.1.1 (http://www.r-project.org/).
Results
Of the 361 AS patients who had an MRI and biopsy, 306 (85%) were Caucasian, 25 (7%) African American, and 30 (8%) other race (Table 1). The median PSA at the time of MRI was 5.6 ng/ml (IQR: 3.9–8.2), the median prostate size was 43.8 cm3 (IQR: 32.1–60.3), and the median percent of total biopsy cores with cancer was 8.3 (IQR: 7.1–17.4). Participant characteristics were very similar between participants in the PASS cohort who did and did not receive a prostate MRI (Supplemental Table 1). The median follow-up time for participants in the analysis who did not upgrade at biopsy was 4.1 years (IQR: 2.0–7.6). There were 395 MRI studies performed at a median of 25 (IQR: 7.4 – 57) months from diagnosis; 186 (47%) of MRI’s were obtained prior to the first surveillance biopsy after initial diagnosis, 77 (20%) before the second surveillance biopsy, 67 (17%) before the third surveillance biopsy, and 65 (16%) before later biopsies.
Table 1.
Participant characteristics at time of first MRI (N = 361).
| Characteristic | N (%) or Median (IQR) |
|---|---|
| Race | |
| Caucasian | 306 (85%) |
| African American | 25 (7%) |
| Other | 30 (8%) |
| Age (yrs) | 65 (59–69) |
| BMI (kg/m2) | 27.3 (25.1–30.4) |
| PSA (ng/ml) | 5.6 (3.9–8.2) |
| Prostate Size (cm3) | 43.8 (32.1–60.3) |
| PSA Density | 0.12 (0.08–0.16) |
| % positive cores | 8.3 (7.1–17.4) |
The 361 participants had a total of 395 MRI’s performed, 111 (28%) of which were negative (PIRADS 1–2), and 284 were positive, 87 (22%) of which were PIRADS 3, 145 (37%) PIRADS 4, and 52 (13%) PIRADS 5 (Table 2). In the biopsies following MRI, 287 (73%) had either no cancer or GG 1 detected, and 108 (27%) had reclassification to GG ≥ 2. Of the negative MRI studies, 17% (19/111) upgraded or reclassified to GG2 prostate cancer. The NPV of a negative MRI (PIRADS 1–2) was 83% (95% CI: 76–90%), and the PPV of a positive MRI was 31% (95% CI: 26–37%).The FPR and TPR were 68% (95% CI: 63–73%) and 82% (95% CI: 75–89%) respectively. If a negative MRI was defined as PIRADS 1–3, the NPV was 82% (Supplemental Table 2).
Table 2.
MRI PIRADS association with Gleason upgrading. Results are shown A) categorically by PIRADS and GG, and B) dichotomous in a 2×2 table used to calculate NPV, PPV, FPR and TPR.
| A. | |||||||
|---|---|---|---|---|---|---|---|
| PIRADS | Gleason Grade Group | Total (%) | |||||
| No cancer | 1 | 2 | 3 | 4 | 5 | ||
| 1 | 23 | 29 | 8 | 1 | 0 | 0 | 61 (15) |
| 2 | 21 | 19 | 7 | 1 | 1 | 1 | 50 (13) |
| 3 | 29 | 42 | 15 | 0 | 0 | 1 | 87 (22) |
| 4 | 23 | 75 | 32 | 10 | 3 | 2 | 145 (37) |
| 5 | 6 | 20 | 17 | 6 | 2 | 1 | 52 (13) |
| Total (%) | 102 (26) | 185 (47) | 79 (20) | 18 (5) | 6 (1) | 5 (1) | 395 |
| B. | |||
|---|---|---|---|
| No Gleason pattern 4 | Gleason pattern ≥4 | Total | |
| MRI Negative | 92 | 19 | 111 |
| MRI Positive | 195 | 89 | 284 |
| Total | 287 | 108 | 395 |
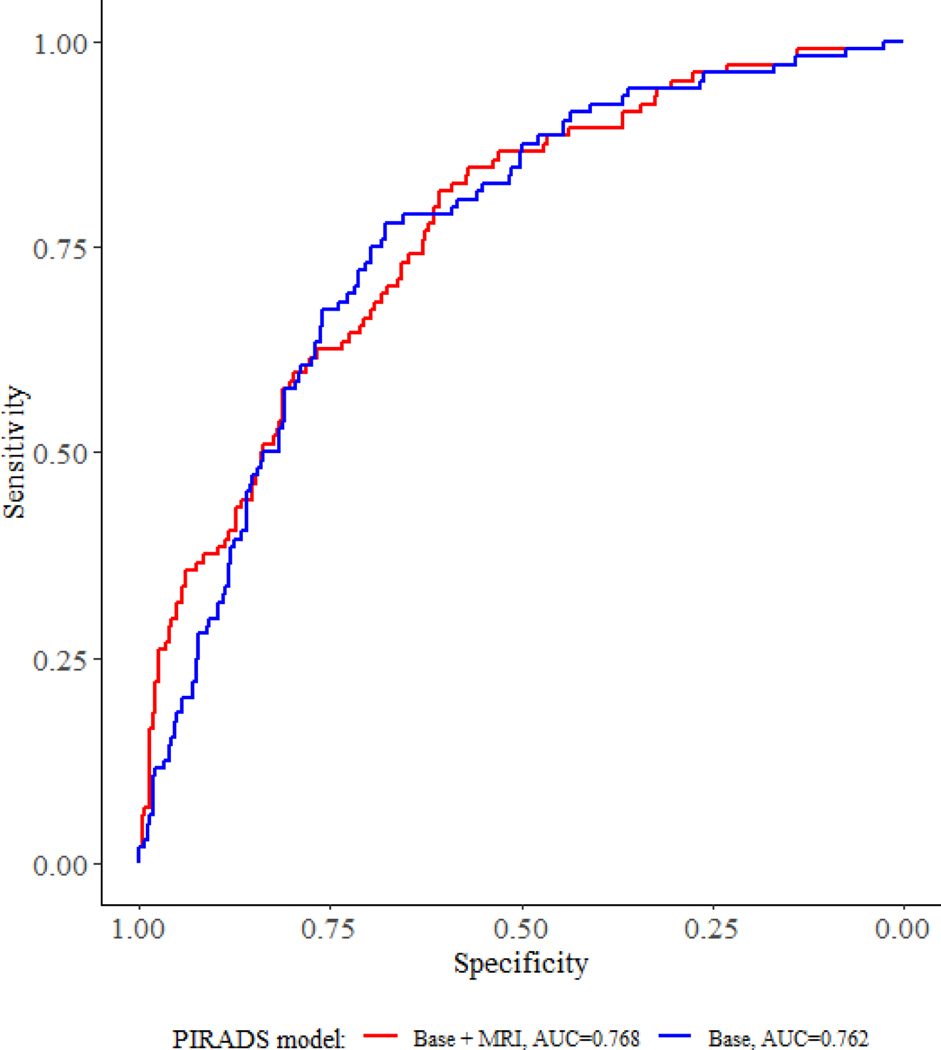
When PIRADS was included in a base multivariable model of clinically available data, MRI remained a significant predictor of upgrading (PIRADS 5 versus PIRADS 1 and 2: OR = 2.71; 95% CI: 1.21–6.17, p = 0.016; Table 3). In ROC analysis for prediction of biopsy upgrading, the AUC for a model including PIRADS was minimally larger than for a base clinical model without PIRADS (0.768 and 0.762 respectively; Figure 1).
Table 3.
Regression models for biopsy reclassification (n = 395).
| Univariate | Base Model | Base + MRI Model | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | 1.05 (1.02, 1.09) | 0.003 | 1.08 (1.04, 1.12) | <0.001 | 1.07 (1.03, 1.12) | <0.001 |
| BMI | 1.01 (0.96, 1.07) | 0.708 | 1.06 (1.00, 1.13) | 0.062 | 1.06 (0.99, 1.13) | 0.075 |
| % positive cores | 1.03 (1.02, 1.05) | <0.001 | 1.02 (1.00, 1.04) | 0.031 | 1.02 (1.00, 1.04) | 0.062 |
| Log(Prostate size) | 0.34 (0.19, 0.58) | <0.001 | 0.19 (0.09, 0.36) | <0.001 | 0.20 (0.10, 0.39) | <0.001 |
| Log(PSA) | 1.64 (1.16, 2.34) | 0.006 | 2.17 (1.47, 3.30) | <0.001 | 2.08 (1.38, 3.20) | <0.001 |
| ≥2 Prior Neg Bx | 0.34 (0.05, 1.24) | 0.159 | 0.35 (0.05, 1.41) | 0.19 | 0.32 (0.05, 1.34) | 0.167 |
| PIRADS1 (1–2 reference) | ||||||
| 3 | 1.09 (0.52, 2.27) | 0.816 | 0.74 (0.33, 1.65) | 0.47 | ||
| 4 | 2.32 (1.29, 4.33) | 0.006 | 1.54 (0.81, 3.02) | 0.20 | ||
| 5 | 4.84 (2.34, 10.24) | <0.001 | 2.71 (1.21, 6.17) | 0.016 | ||
Likelihood Ratio Test for PIRAD p=0.014
Figure 1.
Receiver Operating Characteristic curves for prediction of reclassification.
In 194 MR/ultrasound fusion biopsies with concurrent targeted and systematic biopsy cores, 148 (76%) had either no high grade or the same grade cancer found in both targeted and systematic cores (Table 4). There were 21 (11%) biopsies that had higher grade cancer found in the targeted cores relative to the systematic cores, and 25 (13%) that had higher grade cancer found in the systematic cores.
Table 4.
Results of targeted versus systematic biopsies (n = 194 fusion biopsies).
| Targeted biopsies | ||||||
|---|---|---|---|---|---|---|
| no cancer | GG 1 | GG 2 | GG 3 | GG 4–5 | ||
| Systematic biopsies | no cancer | 29 | 7 | 3 | 1 | 0 |
| GG 1 | 47 | 46 | 11 | 1 | 0 | |
| GG 2 | 7 | 7 | 16 | 2 | 3 | |
| GG 3 | 3 | 1 | 4 | 2 | 0 | |
| GG 4–5 | 1 | 0 | 1 | 1 | 1 | |
Discussion
For 361 patients who underwent a mpMRI and subsequent biopsy while on AS, a negative MRI was associated with a lower risk of upgrading on subsequent surveillance biopsy. We identified mpMRI is associated with an NPV of 83%, 6, 15, 19 suggesting that a negative MRI will still miss a substantial proportion of patients with GG≥2 disease. In addition, systematic biopsies detected a similar number of unique GG≥2 cancer as targeted MRI cores. Thus, if the goal of surveillance biopsy is to identify higher-grade disease, both systematic and targeted biopsies should be obtained for men with a region of interest (ROI) identified on MRI. Furthermore, we also found that while PIRADS 5 lesions were significantly associated with upgrading or reclassification when compared to PIRADS 1 and 2, models including PIRADS scores were only minimally improved over models that contain clinical variables alone.
While there appears to be consensus that MRI is associated with improved diagnoses of high-grade cancers and with fewer low-grade cancers upon screening prostate biopsy, there are no robust data to guide clinical practice in the active surveillance setting. For example, multiple guideline statements do not support the routine use of MRI in prostate cancer active surveillance.15, 20, 21 Our findings are consistent with studies showing that targeted biopsies in the active surveillance population may add little in terms of predicting upgrading or reclassification.15, 22–24 The translation of MRI performance in the diagnostic setting to the surveillance population is likely hampered for several reasons. Patients in AS often have had multiple biopsy sessions with more extensive gland sampling, making them less likely to have large, high grade cancers that are more likely to be evident on mpMRI.
Our findings should be considered in the context of several limitations. First, there was no standard imaging incorporated within our multi-institutional protocol, and the decision to obtain an MRI was based on physician judgment and local practice standards. Furthermore, there was no central review of imaging or biopsy results or other accounting for known inter-observer variation.25–27 Nonetheless, both of these limitations reflect real-world practice as there are no guideline-based recommendations for the frequency or need for MRI imaging during surveillance, and central radiology review is challenging in the setting of this observational cohort study. Additionally, the results of this study include data from the early adoption of MRI with the associated learning curve of MRI interpretation and evolution of the PIRADS scoring system. It is possible that contemporary interpretation and performance of MRI could lead to better performance, as could future improvements in computer-aided and artificial intelligence systems-based analyses, although artificial intelligence would require major allocation of resources for well-curated data.28–30 Finally, although a substantial proportion of the MRI’s were obtained at the time of the first surveillance biopsy, we report on all the MRI tests obtained during the course of AS in our cohort. Thus, the risk of upgrading is likely variable, depending on the time point of the imaging in relation to the initial diagnosis, which consequently affects the pre-test probability of upgrading and/or finding a clinically significant MRI lesion, although the variable performance of MRI due to cancer prevalence has been previously reported.6
Despite these limitations, our data have significant implications on the practice of AS and the use of MRI. First, we found an NPV of 83% suggesting that a negative MRI does not ensure a lack of tumor upgrading in a patient on active surveillance. Second, if an MRI is abnormal, both targeted and systematic biopsies should be performed. The practice of limited sampling, such as performed with in-gantry MRI-directed strategies, will likely miss GG≥2 cancers and have implication for clinical-decision making. While higher PIRADS scores are associated with a greater risk of a clinically significant cancer, several other clinical factors are associated with upgrading including volume/number of biopsy cores with cancer, prostate size or PSA density, and patient age. MRI has little improvement over these factors for predicting upgrading and the clinical factors should be considered when assessing risk during active surveillance.
This study adds the largest cohort data to the body of literature for MRI in active surveillance, providing awareness of the utility and shortcomings of MRI in surveillance, recommending systematic biopsy in patients with a negative MRI and the inclusion of systematic biopsy in patients with a positive MRI.
Supplementary Material
Flow chart depicting participant exclusion for this analysis. The starting cohort was the 1840 participants enrolled in Canary PASS between August 2008 and February 2019.
Descriptions of the 282 MRI that were excluded because of no subsequent biopsy are as follows: 133 (47%) of MRI in participants whose last date of follow-up was less than 12 months from MRI, and are expected to have had a biopsy after data freeze;54 (19%) were followed by a second MRI that was included in the analysis;50 (18%) were negative MRI in which the last date of follow-up was greater than 12 months; 12 (4%) were positive MRI in which the last date of follow-up was greater than 12 months; 33 (12%) were from participants who were treated (n=31) or deceased (n=2) after MRI.
MRI PIRADS association with Gleason upgrading. Results are shown by dichotomous PIRADS (1–3 vs 4–5) and GG in a 2×2 table. The NPV and PPV were 82% (95% CI: 77–88%) and 37% (95% CI: 31–44%) respectively, while the FPR and TPR were 43% (95% CI: 38–49%) and 68% (95% CI: 58–77%) respectively.
Participant characteristics at diagnosis by different groups of Canary PASS participants with and without an MRI.
Acknowledgments
Funding: Canary Foundation, Institute for Prostate Cancer Research. Dr. Liss is supported through the DOD Prostate Cancer Research Program (PCRP) Physician Research Training Award. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Prostate Cancer Research Program under Award No. W81XWH-15-1-0441. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Abbreviations
- AS
Active Surveillance
- PSA
Prostate Specific Antigen
- MRI
Magnetic Resonance Imaging
- mpMRI
Multi-parametric Magnetic Resonance Imaging
- PASS
Prostate Cancer Active Surveillance Study
- GG
Gleason Group
- PIRADS
Prostate Imaging-Reporting and Data System
- MR-US
Magnetic Resonance Imaging – Ultrasound
- ROC
Receiver operator curve
- AUC
Area under the curve
- IQR
Interquartile Range
- FPR
False Positive Rate
- TPR
True positive rate
- NPV
Negative predictive value
Footnotes
Conflicts of Interest: None
Contributor Information
Michael A. Liss, University of Texas Health Science Center San Antonio
Lisa F. Newcomb, Fred Hutchinson Cancer Research Center, University of Washington
Yingye Zheng, Fred Hutchinson Cancer Research Center.
Michael P. Garcia, Fred Hutchinson Cancer Research Center
Christopher P. Filson, Emory University School of Medicine
Hilary Boyer, Fred Hutchinson Cancer Research Center, University of Washington.
James D. Brooks, Stanford University
Peter R. Carroll, University of California at San Francisco
Matthew R. Cooperberg, University of California at San Francisco
William J. Ellis, Fred Hutchinson Cancer Research Center, University of Washington
Martin E. Gleave, University of British Columbia
Frances M. Martin, Eastern Virginia Medical School
Todd Morgan, University of Michigan.
Peter S. Nelson, Fred Hutchinson Cancer Research Center, University of Washington
Andrew A. Wagner, Beth Israel Deaconess
Ian M. Thompson, Jr., University of Texas Health Science Center San Antonio CHRISTUS Santa Rosa Medical Center, San Antonio.
Daniel W. Lin, Fred Hutchinson Cancer Research Center, University of Washington
References
- 1.Mohler JL, Antonarakis ES, Armstrong AJ et al. : Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw, 17: 479, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Bekelman JE, Rumble RB, Chen RC et al. : Clinically Localized Prostate Cancer: ASCO Clinical Practice Guideline Endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline. J Clin Oncol: JCO1800606, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Mottet N, Bellmunt J, Bolla M et al. : EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol, 71: 618, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Hamdy FC, Donovan JL, Lane JA et al. : 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med, 375: 1415, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Loeb S, Vellekoop A, Ahmed HU et al. : Systematic review of complications of prostate biopsy. Eur Urol, 64: 876, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Moldovan PC, Van den Broeck T, Sylvester R et al. : What Is the Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in Excluding Prostate Cancer at Biopsy? A Systematic Review and Meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol, 72: 250, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Bjurlin MA, Carroll PR, Eggener S et al. : Update of the Standard Operating Procedure on the Use of Multiparametric Magnetic Resonance Imaging for the Diagnosis, Staging and Management of Prostate Cancer. J Urol: 101097JU0000000000000617, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasivisvanathan V, Rannikko AS, Borghi M et al. : MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med, 378: 1767, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed HU, El-Shater Bosaily A, Brown LC et al. : Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet, 389: 815, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Vargas HA, Akin O, Afaq A et al. : Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol, 188: 1732, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greer MD, Shih JH, Lay N et al. : Interreader Variability of Prostate Imaging Reporting and Data System Version 2 in Detecting and Assessing Prostate Cancer Lesions at Prostate MRI. AJR Am J Roentgenol: 1, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frye TP, George AK, Kilchevsky A et al. : Magnetic Resonance Imaging-Transrectal Ultrasound Guided Fusion Biopsy to Detect Progression in Patients with Existing Lesions on Active Surveillance for Low and Intermediate Risk Prostate Cancer. J Urol, 197: 640, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran GN, Leapman MS, Nguyen HG et al. : Magnetic Resonance Imaging-Ultrasound Fusion Biopsy During Prostate Cancer Active Surveillance. Eur Urol, 72: 275, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Nassiri N, Margolis DJ, Natarajan S et al. : Targeted Biopsy to Detect Gleason Score Upgrading during Active Surveillance for Men with Low versus Intermediate Risk Prostate Cancer. J Urol, 197: 632, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klotz L, Loblaw A, Sugar L et al. : Active Surveillance Magnetic Resonance Imaging Study (ASIST): Results of a Randomized Multicenter Prospective Trial. Eur Urol, 75: 300, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Newcomb LF, Brooks JD, Carroll PR et al. : Canary Prostate Active Surveillance Study: design of a multi-institutional active surveillance cohort and biorepository. Urology, 75: 407, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newcomb LF, Thompson IM Jr., Boyer HD et al. : Outcomes of active surveillance for the management of clinically localized prostate cancer in the prospective, multi-institutional Canary PASS cohort. J Urol, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin DW, Newcomb LF, Brown MD et al. : Evaluating the Four Kallikrein Panel of the 4Kscore for Prediction of High-grade Prostate Cancer in Men in the Canary Prostate Active Surveillance Study. Eur Urol, 72: 448, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turkbey B, Mani H, Aras O et al. : Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology, 268: 144, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merriel SWD, Hetherington L, Seggie A et al. : Best practice in Active Surveillance for men with prostate cancer: A Prostate Cancer UK consensus statement. BJU Int, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen RC, Rumble RB, Loblaw DA et al. : Active Surveillance for the Management of Localized Prostate Cancer (Cancer Care Ontario Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol, 34: 2182, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Ma TM, Tosoian JJ, Schaeffer EM et al. : The Role of Multiparametric Magnetic Resonance Imaging/Ultrasound Fusion Biopsy in Active Surveillance. Eur Urol, 71: 174, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Hu JC, Chang E, Natarajan S et al. : Targeted prostate biopsy in select men for active surveillance: do the Epstein criteria still apply? J Urol, 192: 385, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siddiqui MM, Rais-Bahrami S, Turkbey B et al. : Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA, 313: 390, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonn GA, Fan RE, Ghanouni P et al. : Prostate Magnetic Resonance Imaging Interpretation Varies Substantially Across Radiologists. Eur Urol Focus, 5: 592, 2019 [DOI] [PubMed] [Google Scholar]
- 26.McKenney JK, Simko J, Bonham M et al. : The potential impact of reproducibility of Gleason grading in men with early stage prostate cancer managed by active surveillance: a multi-institutional study. J Urol, 186: 465, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Greer MD, Brown AM, Shih JH et al. : Accuracy and agreement of PIRADSv2 for prostate cancer mpMRI: A multireader study. J Magn Reson Imaging, 45: 579, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panebianco V, Giganti F, Kitzing YX et al. : An update of pitfalls in prostate mpMRI: a practical approach through the lens of PI-RADS v. 2 guidelines. Insights Imaging, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCammack KC, Schenker-Ahmed NM, White NS et al. : Restriction spectrum imaging improves MRI-based prostate cancer detection. Abdom Radiol (NY), 41: 946, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harmon SA, Tuncer S, Sanford T et al. : Artificial intelligence at the intersection of pathology and radiology in prostate cancer. Diagn Interv Radiol, 25: 183, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow chart depicting participant exclusion for this analysis. The starting cohort was the 1840 participants enrolled in Canary PASS between August 2008 and February 2019.
Descriptions of the 282 MRI that were excluded because of no subsequent biopsy are as follows: 133 (47%) of MRI in participants whose last date of follow-up was less than 12 months from MRI, and are expected to have had a biopsy after data freeze;54 (19%) were followed by a second MRI that was included in the analysis;50 (18%) were negative MRI in which the last date of follow-up was greater than 12 months; 12 (4%) were positive MRI in which the last date of follow-up was greater than 12 months; 33 (12%) were from participants who were treated (n=31) or deceased (n=2) after MRI.
MRI PIRADS association with Gleason upgrading. Results are shown by dichotomous PIRADS (1–3 vs 4–5) and GG in a 2×2 table. The NPV and PPV were 82% (95% CI: 77–88%) and 37% (95% CI: 31–44%) respectively, while the FPR and TPR were 43% (95% CI: 38–49%) and 68% (95% CI: 58–77%) respectively.
Participant characteristics at diagnosis by different groups of Canary PASS participants with and without an MRI.