Abstract
The intense effort of investigators, in particular during the past decade, has highlighted the importance of extracellular vesicles (EVs) such as exosomes in regulating both innate and adaptive immunity in the course of a variety of infections, with clear implications for development of novel vaccines, therapeutics, and diagnostics. Current and future efforts now need to focus strongly on teasing apart the intricate and complex molecular mechanisms that operate during EV regulation of immunity. In this review, we discuss recent advances that bear on our current understanding of how EVs, including exosomes, can contribute to the innate immune functions of microglia within the central nervous system (CNS), and we also highlight future important mechanistic questions that need to be addressed. In particular, recent findings that highlight the crosstalk between autophagy and exosome pathways and their implications for innate immune functions of microglia will be presented. Microglial activation has been shown to play a key role in neuroAIDS, a neuro-infectious disease for which the importance of exosome functions, including exosome-autophagy interplay, has been reported. The importance of exosomes and exosome-autophagy crosstalk involving microglia has also been shown for the Parkinson’s disease (PD), a neurodegenerative disease that is thought to be linked with immune dysfunction and involve infectious agents as trigger. Considering the accumulation of recent findings and the vibrancy of the EV field, we anticipate that future studies will continue to have a deep impact on our understanding of the CNS pathologies that are influenced by the functions of microglia and of the infectious disease mechanisms in general.
Graphical Abstract
BRIEF INTRODUCTION
Extracellular vesicles (EV), including exosomes (EX), have attracted significant attention among scientists during the last decade as the influence of their communicative effects in a number of different human pathologies, including infectious diseases, have become apparent (Delorme-Axford et al. 2013). Their role in regulating immunity first became a subject of interest when it was discovered that certain cells secretes EVs harboring major histocompatibility complex class II (MHC class II), which can induce antigen specific MHC class II T cell responses (Raposo et al. 1996; Schorey and Harding 2016). Since this discovery, many studies have highlighted the ability of EVs to influence both innate and adaptive immune responses. However, a detailed understanding of the complexity involved remains to be attained. In this regard, the unfolding knowledge of the EV regulation of innate immune responses, including the exosome-autophagy interplay as part of this regulation, has opened new windows of understanding in the field and is therefore of intense current interest. In this review, we present a synthesis of the emerging evidence that bears on EV/EX regulation of innate immune responses in microglia and its influence on central nervous system (CNS) interactions, with a particular emphasis on the latest findings for exosome-autophagy crosstalk mechanisms as a component of this response. We also discuss some challenges that lie ahead, and propose areas of investigation to advance our understanding of the mechanisms by which EV modulation of innate immune response, including microglial response, occurs. From the accumulation of evidence discussed here, including work from our own laboratory, a central hypothesis emerges that EVs, including exosomes, released from infected microglia may induce specific innate immune responses in target naïve recipient microglia and astrocytes to fight off infection efficiently when they encounter the invading pathogen. Such responses may include induction of proinflammatory cytokine release and modulation of major signaling pathways such as p38 MAPK to allow improved pathogen clearance. We also hypothesize that autophagy mechanisms may influence this process by affecting the number of released vesicles as well as their content, or through EV regulation of autophagy responses in recipient cells. In this review, we begin by providing a brief introduction to the general properties of exosomes and other EVs, including some of the known mechanisms that govern their cargo selection that in turn determines the nature and magnitude of recipient cell responses. We next describe the state of knowledge about how EV release regulates microglial immune functions in the CNS. As part of this, studies of the closely related macrophage/monocyte populations are also informative to consider and are discussed. Finally, we highlight an emerging aspect of how the EV regulation of microglial response to infection, including microbial clearance, may be influenced; namely, the contributions of exosome-autophagy interactions. We conclude the review by a discussion of important mechanistic questions that remain to be thoroughly investigated in future studies and some of the challenges and unique opportunities that lie ahead.
PROPERTIES OF EXOSOMES AND OTHER EV SUBTYPES
EVs are nanosized vesicles released from cells into extracellular milieu (Liao et al. 2017). EVs consist of a mixed population of various vesicles, which include microvesicles (ectosomes), exosomes, and apoptotic bodies (Raposo and Stoorvogel 2013). These vesicles are distinguished mainly based on their size/density, biogenesis, and mode of release (Zaborowski et al. 2015), although due to overlap in size, composition, and protein markers, making absolute assignment of vesicle identities remains a challenge (Harties et al. 2019). The purification schemes designed for isolation of macrovesicles and exosomes have been discussed in detail in other review articles (Konoshenko et al. 2018; Zhang et al. 2019). Apoptotic bodies are released from dying cells, with sizes ranging from 50 nanometer (nm) to 5000 nm in diameter (Hartjes et al. 2019). Microvesicles are released directly from the plasma membrane, with sizes ranging from 100 nm to 1000 nm (Heijnen et al. 1999). Exosomes, the third common subtype of EVs, are endocytic in origin and have a diameter range of approximately 30 nm to 150 nm (Hessvik and Llorente 2018). They are released from the cell by the fusion of multivesicular bodies (MVBs) with the plasma membrane. As with microvesicles, exosomes elicit specific responses in recipient cells, such as changes in cytokine release profile, changes in signaling activities of immune cells, or alteration of susceptibility to infection (Fleming et al. 2014 Barclay et al. 2017; Madison and Okeoma 2015; Sampey et al. 2016). Although they were first identified in reticulocyte culture media (Johnstone et al.1987), exosomes are released from almost all eukaryotic cells in different organisms and can be isolated from different biological fluids such as plasma, urine, saliva, seminal fluid, nasal secretions, breast milk, and amniotic fluid (Caby et al. 2005; Keller et al. 2011; Lasser et al. 2011; Pisitkun et al. 2004; Poliakov et al. 2009). Overall, much still remains to be uncovered regarding the functions of exosomes, and a significantly more in-depth understanding of the signaling pathways and external stimuli that regulate their packaging and secretion is required (Zhang et al. 2019). For the studies discussed in this review, we will use the general term “EV” unless the authors have provided evidence for working with exosome-enriched materials based on a combination of size analysis (e.g., electron microscopy, or nanoparticle tracking assay), protein marker analysis (e.g., Western blot, or immunostaining), and use of appropriate purification schemes for exosome recovery.
The specific content of exosomes and other EVs is influenced by environmental factors, as well as the types of cells that produce them (Pallet et al. 2013; Tauro et al. 2013). In addition, the pathological and physiological states of the cell affect EV content, including protein cargo (Fleming et al. 2014; Sampey et al. 2014; Schwab et al. 2015). As such, EV-associated proteins can serve as indicators of disease, in addition to contributing to EV formation and trafficking (Andreu and Yanez 2014; Raposo and Stoorvogel 2013; Zhang et al. 2014). Work from different laboratories, including studies from our laboratory, have demonstrated that specific pathogen components get packaged within host EVs such as exosomes during infection (Ahsan et al. 2016; Chen et al. 2018; Fleming et al. 2014; Fleming et al. under review). For instance, we have shown the presence of viral RNA genome and the viral nucleocapsid protein in exosomes derived from cells infected with the Rift Valley fever virus, and the presence of specific bacterial proteins in exosomes of infection origin has been shown for different bacterial infections. Some of the mechanisms by which pathogenic components are packaged into exosomes have been described although much remains to be discovered. Evidence suggests that trafficking of EVs, including exosomes, shares many properties with viral assembly processes (Meckes 2015). For instance, MVBs can contain both EVs and virions (retroviruses) that are released to the extracellular milieu after fusion of MVBs to the plasma membrane, reflecting similarities in the biogenesis of EVs and retroviruses (Nolte-’t Hoen et al. 2016). Therefore, the intersection of the EV pathways with viruses is one mechanism contributing to sorting viral components into EVs (Lenassi et al. 2010; Nolte-’t Hoen et al. 2016). In support of this notion, the HIV virus Gag protein associates with CD81 and CD63 tetraspanins in endosome-like domains of the plasma membrane and is released in EVs (Booth et al. 2006). Another example is the involvement of the endosomal sorting complex (ESCRT) machinery and annexin A2 in packaging HCV RNA into EVs (Dreux et al. 2012). Some of the signals involved in incorporation of nucleic acids into EVs have been identified although a great deal is still unknown. For instance, it has been proposed that miRNA strands are transported into the MVBs by RNA binding proteins (RBPs), enabling their secretion via exosome release (Janas et al. 2015). More recently, possible RNA-loading motifs that allow miRNA packaging into EVs have also been proposed (Gao et al. 2018). Some of the mechanisms for incorporation of bacterial components have also been identified. Studies have shown that host E3 ligase-dependent ubiquitination machinery is involved in trafficking mycobacterial proteins into EVs (Smith et al. 2015). Besides ubiquitination, other specific posttranslational modifications (PTMs) have also been shown to serve as selective protein sorting mechanisms (Villarroya-Beltri et al. 2014), and therefore merit detailed investigation of their contributions. These provide some examples of the rules governing cargo loading of EV/EX; for an extensive coverage of this topic, the readers are referred to a recent review article by Anand and colleagues (Anand et al. 2019).
In recent years, exosomes have become the most investigated extracellular vesicles due to their diagnostic and therapeutic potential for a variety of human pathologies, including infectious diseases. Aside from being pivotal for physiological crosstalk between cells (Pant et al. 2012), exosomes may also affect the progression of different disease conditions through the function of components that get specifically packaged into them (Cobb et al. 2018; Huang et al. 2019; Smith et al., 2017). These range from various neurological disorders to cancer, infectious diseases, and cardiovascular diseases (Ailawadi et al., 2015; Janas et al. 2016). In the context of infectious diseases, exosomes can either inhibit or activate the immune system depending on the type of pathogen, hence providing potential therapeutic and diagnostic opportunities (Kruh-Garcia et al. 2014). However, the specific signaling pathways altered by these vesicles during innate immune response remain largely unknown and significantly more work is required in this area. Furthermore, future experiments should also aim at determining more extensively the pleiotropic effects of exosomes versus those that are immune cell specific.
MICROGLIA AND EV REGULATION OF CELLULAR INNATE IMMUNITY IN THE CNS
EV regulation of microglial immune functions
The function of EVs in relation to immunity became a subject of interest in 1996 when it was discovered that B lymphoblastoid cells secrete EVs harboring MHC class II molecules and that EVs derived from B lymphocytes induce antigen-specific MHC class II-restricted T cell responses (Raposo et al. 1996). Since this seminal report, many studies have demonstrated the ability of EVs to mediate both activation and suppression of innate and adaptive immune responses (Bobrie et al. 2011; Obregon et al. 2009; Schorey and Harding 2016; Thery et al. 2002 Zitvogel et al. 1998; Smyth et al. 2013). As resident macrophage cells, microglia provide critical innate immune function in the CNS. Similar to the oligodendrocytes (OLs), astrocytes, and neurons within the CNS, microglia secret EVs, including exosomes, with distinct characteristics and functional properties that include immune-related activities. Functional relationships between components of the innate immune system and microglial EVs have been documented. For instance, microglia transfected with the Nef protein (Negative Factor) of HIV secrete EVs that contain Nef and promote secretion of Toll-like receptor-induced cytokines and chemokines in recipient microglia (Raymond et al. 2016). This is consistent with our stated hypothesis that exosomes derived from infected microglia may induce protective immune responses in recipient CNS innate immune cells. It has also been shown that stimulation of microglia with interferon gamma (IFN-γ) leads to release of EVs that express elevated levels of MHC class II (Potolicchio et al. 2005). Interestingly, IFN-γ can also induce formation of extracellular traps (ETs) in Listeria-infected microglia, which release ET carrying EVs in response to Listeria infection (Wang et al. 2019). These microglial extracellular traps (MiET) consist mainly of extracellular DNA (eDNA), matrix metallopeptidases, citrullinated histone H3, and peptidyl arginine deiminase 2, and serve to protect the CNS against Listeria infection by arresting or killing the bacteria (Wang et al. 2019). These findings are consistent with our hypothesis that exosomes from infected microglia can prompt naïve recipient cells to fight off an invading pathogen more effectively in the event of becoming infected. The inflammatory cytokine IL-1β is also released in association with microglial EVs (Bianco et al. 2005). Intriguingly, it has been demonstrated that multitudes of cytokines can become packaged into EVs (Fitzgerald et al. 2018). Therefore, it is conceivable that EVs derived from microglia, including exosomes, may serve as delivery vehicles for cytokines that regulate inflammatory response or neurotransmission (Prada et al. 2013; Turola et al. 2012). Recent studies indicate that RNA components of microglial exosomes can also carry out important functions, including effects that are part of response to infection. It has been shown that let-7a and let-7b miRNAs associated with exosomes released from microglial cells that are infected with the Japanese encephalitis virus (JEV) cause caspase activation in recipient neuronal cells (Mukherjee et al. 2019). Additionally, it has been demonstrated that microglia secrete exosomes that are highly enriched in miR-124–3p, providing protection to the brain by inhibiting neuronal inflammation (Huang et al. 2017). This effect occurs through miR-124–3p inhibition of phosphodiesterase 4B (PDE4B) gene expression and thus suppression of the mTOR signaling activity (Huang et al. 2017). As inhibition of PDE4B is known to suppresses inflammation in response to bacterial infections (Komatsu et al. 2013), miR-124–3p delivery by microglial EVs may be one of the mechanisms by which inflammatory response to bacterial infections of the CNS is regulated. Again, these support our stated hypothesis of immune protection by exosomes released from infected microglia. The demonstration of microglial EVs regulating inflammatory response points to their potential utility as markers of brain inflammation. In a related note, a fascinating, albeit challenging, aspect that is critical to address in future work is how the complex microenvironment of the CNS impacts both the packaging and release of microglial EVs and how that in turn influences their functions in recipient cells. Factors such as the enrichment of particular CNS cell types that interact with microglia in various regions and the influence of external stimuli such as invading pathogens need to be extensively studied.
Several mechanisms that stimulate EV release from microglia have been identified. The dependence of EV release from immune-activated microglia on glutamine metabolism has been reported, as the inhibition of glutaminase, an enzyme involved in the metabolism of glutamine, reduces EV release from immune-activated microglia while α-ketoglutarate is associated with an opposite effect (Wu et al. 2018). Neuroinflammatory cytokines such as TNF‐α, IFN‐γ, and IL1β have also been shown to enhance vesicle shedding from microglia (Verderio et al. 2012). In another study, ATP activation of the P2X7R receptor has been shown to induce the release of both exosomes and MVs from microglia, likely as a means of transporting GAPDH to the outside of the cell (Takenouchi et al. 2015). These findings are consistent with previous observations that ATP-activated P2X7R induces exosome release from macrophages through exocytosis of multivesicular endosomes (Qu et al. 2007), and that P2X7R receptor activation by astrocyte-derived ATP induces MV release from microglial cells (Bianco et al. 2005). ATP not only acts as a stimulant for MV/exosome shedding but also modifies the content of these microglial EVs, enriching for proteins involved in extracellular matrix organization, cell adhesion, cellular metabolism, and autophagy lysosomal pathway, thus influencing microglial EV signaling to astrocytes that also serve innate immune functions within the CNS (Drago et al. 2017). The lipopolysaccharide (LPS) of gram-negative bacteria also stimulate microglial EV release (Kumar et al. 2017). The LPS-induced microvesicles are enriched in miR-155 and IL-1β, both of which are proinflammatory in function (Kumar et al. 2017). Furthermore, it has been demonstrated that serotonin can stimulate vesicle release from microglial cells, mediated by microglia 5-HTRs and involving the elevation of calcium levels (Glebov et al. 2014). While the functions of these vesicles were not analyzed, the presence of neurotrophic factors in exosomes has been demonstrated by proteomic analysis (Mathivanan et al. 2012), suggesting that microglia might use vesicle release as another means of promoting neuronal health and differentiation. How these stimulatory factors may play a role during response to infection in the CNS remains to be investigated; such studies will provide a wealth of information on how EV release from microglia is regulated as part of the infection process
Besides secreting EVs such as exosomes to carry out specific functions, microglia also respond to EVs secreted by other cell types within the CNS, including astrocytes. In the context of infection, HIV Tat protein has been shown to induce the release of astrocyte exosomes that contain miR-9 and promote microglial migration (Yang et al. 2018). Recent findings show that astrocyte-derived exosomes can also regulate phagocytotic activity in microglial cells that receive them (Hu et al. 2018), an effect that could influence cellular response to infection. Modulation of microglial phagocytic activity has also been observed in response to exosomes derived from motor neuronal cells that are transfected with mutant superoxide dismutase 1 (mSOD1) (Pinto et al. 2017). Additionally, alterations in microglial polarization were observed. The findings suggest that inflammatory-associated miR-124 packaged in motor neuron-derived exosomes determines these phenotypic alterations in the recipient microglial cells (Pinto et al. 2017).
EV regulation of monocyte/macrophage immune functions
According to the mononuclear phagocyte system (MPS) model, committed marrow progenitors give rise to blood monocytes that upon migration to tissues differentiate to macrophages (Hume 2006). Microglia are considered to represent the resident macrophage population within the CNS. Similar to macrophages, they migrate to the site of injury or infection where they may proliferate and become phagocytic (Gonzalez-Scarano and Baltuch 1999). Furthermore, they release inflammatory cytokines that induce the recruitment of cells to the site of infection or trauma, and similar to macrophages they can release tumor necrosis factor alpha and other potential neurotoxins that are associated with infectious diseases such as AIDS (Gonzalez-Scarano and Baltuch 1999). The macrophage population, which includes microglia, and the precursor monocyte population are members of the mononuclear phagocyte system (MPS) that share many overlapping characteristics (Guilliams et al. 2014). Therefore, for assessing EV contributions to microglial functions, and for considering areas of exploration in future EV studies of microglia, it is informative and relevant to also discuss EV regulatory aspects for monocytes/macrophages.
Several EV studies of macrophages infected with Mycobacterium tuberculosis (M.tb) have been reported to date. Macrophages infected with M.tb release exosomes that promote innate and acquired immunity both in vitro and in vivo (Singh et al. 2015; Smith et al. 2017). The M.tb RNA packaged into these exosomes can be directly translated into protein upon delivery into host cells (Singh et al. 2015). In addition, the mycobacterial RNA and pathogen-associated molecular patterns (PAMPs) packaged within these exosomes can promote production of proinflammatory cytokines (Singh et al. 2015; Smith et al. 2017), an effect that also supports our hypothesis that exosomes from infected microglia provide protection against infection in part by inducing proinflammatory cytokine release. Several additional mechanisms of regulating innate immunity by exosomes released from macrophages have also been reported to date. Exosome trafficking of IL-1β, which is a major driver of innate immune response, has been proposed as an important transport mode by which IL-1β is released by macrophages and DCs through the non-classical secretion pathway (Qu et al. 2007), reminiscent of the findings by Bianco and colleagues discussed above that microglial EVs can carry IL-1β. Another example is the finding that in the presence of oxidized low-density lipoprotein-containing immune complexes, macrophages release exosomes carrying IL-1β, in addition to HSP70 and sphingomyelinase, that can drive the induction of atherosclerotic plaques (Truman et al. 2012). Studies of macrophage response to EVs from infected cells has also been reported. It has been shown that EVs released from HBV-infected hepatocytes package viral RNA within them that are sensed by macrophages and dendritic cells via TLRs and play an important role in innate immune response against HBV (Kouwaki et al. 2016). Our own laboratory has been investigating the mechanisms by which monocytes respond to exosomes of infection origin, which we designate as EXi for short. Our focus has been investigation of highly pathogenic agents that are classified by the CDC and NIAID as Category A pathogens, or pathogens of highest concern. Specifically, we have been studying EXi that are derived from cells infected with either Yersinia pestis (Yp) bacteria or Rift Valley fever virus (RVFV). In both cases, we have found that the EXi are packaged with specific protein and nucleic acid components from the infecting pathogen (Ahsan et al. 2016; Fleming et al. 2014; Fleming et al. 2018; Fleming et al. manuscript under review; Alem et al. manuscript under review). In both infection models, the EXi activate recipient monocytes, inducing strong anti-viral response in monocytes treated with EXi-RVFV and significant reduction of intracellular bacterial load following pretreatment with EXi-Yp (Fleming et al. 2018; Fleming et al. manuscript under review; Hobbs et al. manuscript under review). These studies demonstrate that the EXi can serve a protective role for the host during infection, highlighting the potential for these exosomes to serve as novel vaccination vehicles. In concurrence with several studies alluded to above, our observations further support our stated hypothesis for how exosomes derived from infected microglia may influence their recipient cells; namely, activation of upstream signaling pathways that induce proinflammatory cytokine release, which could in turn enable the cells to reduce pathogen load in the event of becoming infected. Interestingly, in contrast to our RVFV findings, for a number of other virus infections the EVs released from infected cells produce the opposite effect and contribute instead to facilitate viral spread (Arakelyan et al. 2017; Margolis and Sadovsky 2019). Future work is needed to tease apart the mechanisms that determine whether EVs released during a particular infection aid the host or instead assist viral amplification or spread. Questions such as whether this is primarily determined by the type of cargo packaged into EVs, or whether other factors such as the type or quantities of EVs delivered to recipient cells or multiplicity of infection (MOI) are also main determinants, should be investigated. Due to the highlighted similarities between tissue macrophages and microglia, it is very likely that at least some of the attributes observed for exosomes derived from infected macrophages are also relevant for microglial response to infection. Therefore, future studies such as whether exosomes from infected microglia also package PAMPs that can promote the production of proinflammatory cytokines, or carry pathogen-derived RNA that can be translated in recipient cells, will be quite informative.
IMPLICATIONS OF AUTOPHAGY-EXOSOME INTERPALY FOR MICROGLIAL RESPONSE TO INFECTION
Signaling mechanisms regulating autophagy
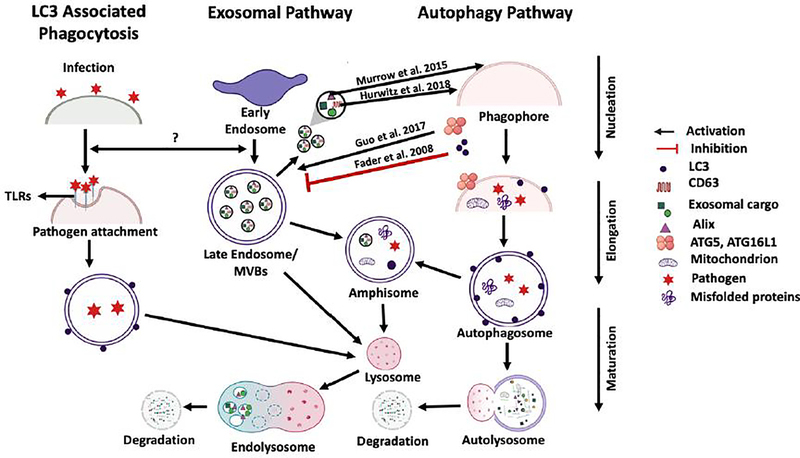
Autophagy is a well-established mechanism used by phagocytic innate immune cells such as microglia to defend against infection by eliminating pathogens (Plaza-Zabala et al. 2017). Of direct relevance to this review article are the recent findings that this important defense mechanism shows cross regulatory interactions with the exosome biogenesis and release pathways (Figure 1). As we stated earlier, we have found that pretreatment of naïve recipient monocytes with exosomes of infection origin can lower pathogen burden in these cells during a subsequent infection, and hypothesize that a similar mechanism may be involved for microglia. Clearly, this effect may be due to EV-associated modulation of autophagy in the recipient cells, which has been demonstrated for microglia by several recent studies that we discuss in the next section. To provide the necessary background for full appreciation of this crosstalk, in this section we describe the autophagy pathway and signaling network interactions that regulate it. There are three major pathways for mammalian autophagy: microautophagy, macroautophagy, and chaperone-mediated autophagy (Ke 2018; Parzych and Klionsky 2014). Although each of these three pathways differ in the way their functions are carried out, the endpoint for all three is fusion with lysosomes in order to break down and recycle materials for cellular use or destroy engulfed pathogens (Ke 2018; Parzych and Klionsky 2014). The focus of this review relates to macroautophagy (referred to simply as autophagy here), which is involved in the engulfment of bacterial and viral pathogens. Key steps in the process of autophagy include the nucleation step to form a phagophore, elongation of the phagophore membrane and vesicle completion to produce an autophagosome, and fusion of the autophagosome with lysosomes that results in vesicle breakdown and degradation of the inside cargo (Bhattacharya and Eissa 2015). As depicted in Figure 1, during infection, the process of autophagy results in engulfment of intracellular pathogen into autophagosomes followed by fusion with lysosomes to form the autolysosome, which carries hydrolases that help degrade the pathogen.
Figure 1: Exosome-Autophagy Interplay.
The pathways for LC3-associated phagocytosis (LAP autophagy), exosome biogenesis and release, and macroautophagy are indicated. The last stage of progression for all three pathways can be fusion with lysosomes for degradation of vesicular components. For the exosome pathway, the MVB can either be shuttled to the plasma membrane to release exosomes or fuse with autophagosome from the autophagy pathway to form amphisomes that subsequently fuse with lysosomes. Arrows between the pathways indicate the nature of the crosstalk between them, with the red line indicating inhibition and black arrows indicating activation or interaction. LC3 protein has been shown to negatively regulate exosome release while ATG5 and ATG16L1 proteins show positive regulation. CD63 and Alix proteins associated with released exosomes induce autophagy. It is not clear at this time whether regulatory cross talk exists between the LAP process and exosome biogenesis and release pathways.
Progression of autophagy through its multiple steps is mediated by specific protein complexes that contain autophagy-related (ATG) proteins. For instance, the UNC-51-like-kinase complex (ULK), which consists of ATG101, ATG13, FIP200 and ULK1/2, and the vacuolar protein sorting mutant 34 (Vps34) type I complex, which contains BECLIN1, VPS34, p150 and ATG14, participate in the process of phagophore formation and elongation (Chen et al. 2014; Ktistakis and Tooze 2016). As part of this, ULK is recruited through binding of ATG16L1 protein within the ATG12–ATG5-ATG16L1 complex to reinforce the activity of the Vps34 complex (Ktistakis and Tooze 2016). Another example is the ATG7–ATG3 complex, which similar to the ATG5– ATG12 complex is a ubiquitin-like conjugation system and allows the covalent linkage of ATG8 (referred to as LC3B in mammals) to phosphatidylethanolamine (PE) on the growing autophagosomal membrane (Bhattacharya and Eissa 2015; Ichimura et al. 2000; Mizushima et al. 1998 ). The last stage, which is fusion of autophagosome with the lysosome, is mediated by SNARE and Rab7 proteins. This fusion result in the formation of autolysosomes (Huotari and Helenius 2011; Hyttinen et al. 2013). ATG14, which is part of the Vps34 complex, binds the SNARE core domain of STX17 to stabilize the STX17-SNAP29 complex on the surface of the autophagosomes and priming it to interact with VAMP8 in order to promote autophagosome-lysosome fusion (Ktistakis and Tooze 2016).
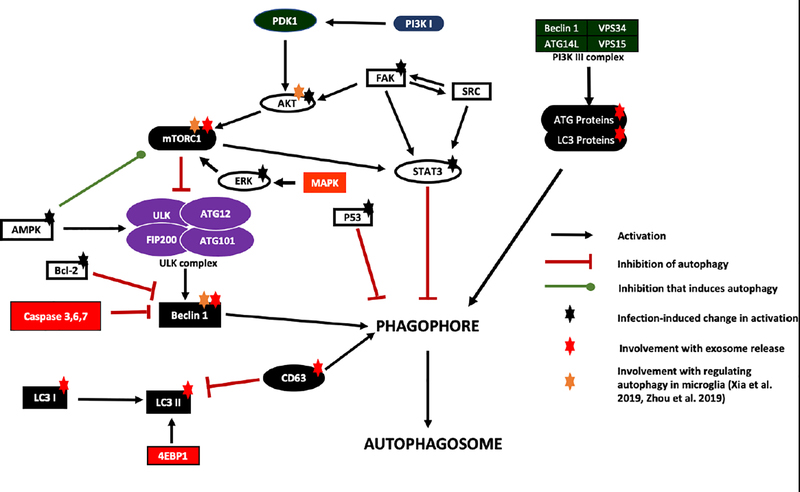
In order to understand autophagy regulation at a molecular level, an in-depth comprehension of the signaling network mechanisms is essential. Multiple host signaling pathways have been identified that regulate autophagy. The main pathways and their connectivity, including the proteins that affect autophagy in microglia and are involved in response to infection, are shown in Figure 2. For instance, AKT activation leads to activation of mammalian target of rapamycin complex 1 mTORC1 through phosphorylation of ATG13 and ULK1/2 (Bestebroer et al. 2013), resulting in negative regulation of autophagy (Kim and Guan 2015). AKT itself can get activated by focal adhesion kinase (FAK), which can also activate STAT-3 to cause autophagy inhibition (Figure 2). Work from our group has demonstrated that at 8 hours post infection with Yersinia pestis (Yp) bacteria, a coordinated response to downregulate autophagy occurs that involves the functions of several proteins depicted in Figure 2 (Alem et al. 2015), including AKT, p53 (Alem et al. 2015), FAK, STAT3, and ERK1/2 (Alem et al., manuscript in preparation). The FAK-Src-STAT3 signaling network has also been shown to regulate autophagy in response to some other infections, including Toxoplasma gondii (Portillo et al. 2017) and HIV-1 (Van Grol et al. 2010). Interestingly, some pathogens rely exclusively on activation of autophagy in the host cell for survival. For instance, recent studies show that JEV activates autophagy in order to grow and replicate (Ke 2018). Recent work also shows that methamphetamine along with HIV-Tat induce autophagy in the presence of ATG5 and ATG7 as indicated by increased protein levels of Beclin-1 and LC3II, increasing the risk of exposure to HIV-1 and contributing to neuronal dysfunction in HIV-associated neurocognitive disorders (Li et al. 2018). Given that some of the signaling proteins shown in Figure 2 such as mTORC1 or Beclin 1 are known to regulate autophagy in microglia and also play a role in exosome-autophagy interactions, analysis of their contributions to exosome-autophagy crosstalk during microglial response to infection are important to perform in future studies.
Figure 2: Protein signaling networks regulating autophagy.
Main signaling proteins and pathway networks that carry the task of regulating autophagy through effects on phagophore formation or autophagosome formation are shown. Proteins that undergo activity changes during infection, resulting in modulation of the autophagy response, are designated with a black star. Proteins involved in the exosome pathway that also regulate autophagy are designated with a red star. The regulatory proteins that have been shown to influence autophagy response in microglia are designated with a gold star. Red lines indicate inhibition. Green line designates inhibition that ultimately results in induction of autophagy. Black arrows indicate activation.
Autophagy-exosome interactions
Recent discoveries have highlighted the presence of cross regulatory mechanisms between exosome and autophagy pathways (Figure 1). Considering this interplay and the fact that autophagy is a major mechanism for regulating activation of microglia during infection (Su et al. 2016), such as during response to neuroAIDS (El-Hage et al. 2015), it is of great interest to understand how exosome-autophagy crosstalk influences microglial immune function. Several recent findings have demonstrated the importance of this crosstalk in microglial response. In one study, it was shown that neuronal cell line SH-SY5Y transfected with α-synuclein released exosomes that display overexpression of miR-19a-3p. Upon release, these exosomes stimulate recipient microglia to express miR-19a-3p. The increased expression of miR-19a-3p in exosomes also suppresses autophagy in recipient microglia, as confirmed by increased expression of P62 and decreased expression of LC3-II (Zhou et al. 2019). The modulation of autophagy by the miRNA is by targeting PTEN/AKT/mTOR signaling pathway in microglia (Zhou et al. 2019). Another recent study has shown that increased levels of miR-124–3P in microglial exosomes provides neuroprotection against traumatic brain injury through inhibition of neuronal autophagy (Li et al. 2019), an effect that may also play a role during neuronal infection. Influence on autophagy by exosome-associated miRNA has also been shown during response to infection by macrophages, the lineage to which microglia belong. Macrophages infected with M.tb secrete exosomes that are rich in miR-18a, which promotes intracellular survival of M.tb through inhibition of autophagy (Yuan et al. 2019). It has been shown that miR-18a prevents autophagy through inhibition of Ataxia-Talangiectasia Mutated (ATM) kinase, which is known to activate autophagy by suppressing mTORC1 (Tripathi et al. 2013). Furthermore, another very recent report has shown that autophagy has a role in the exosome-associated inflammatory responses by microglia (Xia et al. 2019). In this in vivo study, it was shown that exosomes derived from PD patients preferentially target microglial BV2 cells in mouse brain. Transfer of the exosomes led to inhibition of autophagy in the BV2 cells, as indicated by decreased Beclin1 and LC3-II protein levels, and accumulation of α-synuclein. It was shown that the inhibition of autophagy is achieved through activation of the Akt-mTOR signaling pathway in BV2 cells (Xia et al. 2019). Following the uptake of PD-derived exosomes, BV2 cells showed increased proliferation and increased secretion of proinflammatory cytokines and nitric oxide.
Both the autophagy pathway and the exosome biogenesis pathway are well conserved across different cell types. Exosomal biogenesis processes use highly conserved complexes that have been given different names depending on their cellular origin (Li et al. 2019). Similarly, the complexes and mechanisms involved during the autophagy process are highly conserved across all eukaryotes (Parzych et al. 2014). Consequently, the autophagy-exosome interaction mechanisms in other cell types are also directly relevant to what may occur in microglia and their consideration is quite informative. Emerging evidence shows that several ATG proteins are involved in regulation of exosome release or biogenesis in multiple cell types, including mouse embryonic fibroblast cells (MEFs), MDA-MB-231 breast cancer cells, and HCT116 colorectal cancer cells (Guo et al. 2017; Murrow et al. 2015). Of special note is the contributions of ATG16L1 and ATG5 to exosome production. ATG5 mediates the detachment of a regulatory component of vacuolar proton pumps from the MVBs, preventing the creation of an acidic environment inside the lumen of MVBs and thereby allowing exosome release (Guo et al. 2017). This was demonstrated in part by the observation that knocking out ATG5 and ATG16L1 genes leads to a significant reduction in exosome release and alteration of exosomal content. Furthermore, when treated with V-ATPase inhibitor, ATG5 knockout cells regained the ability for efficient exosome release. This affirms the importance of luminal pH influence on the ultimate fate of the MVBs and whether they fuse with plasma membrane to release their content or fuse with lysosomes for degradation. It has been shown that treatment with alkaline agents that inhibit lysosomes increases EV secretion, and inhibition of lysosomes by Bafilomycin A treatment of neuronal cell line SH-SY5Y overexpressing α-synuclein increases the levels of α-synuclein released in EVs (Alvarez-Erviti et al. 2011). Bafilomycin A treatment of human bladder epithelial cells in which autophagy is already activated by either infection with E. coli K-12 or by rapamycin treatment also increases EV secretion (Miao et al. 2015). These and other similar findings (Eitan et al. 2016) suggest that vesicle accumulation due to inhibition of fusion with lysosomes forces the cell to extrude EVs such as exosomes in order to maintain vesicular homeostasis and dispose of unwanted materials. Indeed, findings suggest that the ability of HIV-1 to inhibit autophagosome degradation shifts the virus removal process during autophagy towards exosome export in cells within the CNS (Ojha et al. 2017). The function of ATG7 protein for potential effects on the exosome pathway has also been analyzed. ATG7 knockdown does not affect exosome release, suggesting that autophagosome formation is not involved (Jing et al. 2018). However, knockdown of ATG7 or Beclin1 in human hepatocytes infected with HCV significantly decreases the release of exosomes that carry the virus, suggesting that both of these core autophagy proteins are important for HCV incorporation into vesicles (Shrivastava et al. 2016). These findings suggest that while ATG7 does not affect EV release it may nevertheless have a role in packaging of EVs. On the other hand, the ATG9 protein may be involved in regulating EV release as ATG9 has been implicated in the formation of intraluminal vesicles (ILVs) in Drosophila (Bader et al. 2015). While several of the ATG proteins function to induce EV release, modulation of autophagy can also lead to its inhibition. Conditions that induce autophagy such as rapamycin treatment and starvation cause the fusion of autophagosomes with MVBs to form amphisomes, thereby preventing release of exosomes (Fader et al. 2008). Furthermore, recent findings show that induction of autophagy reduces exosome release of the pathological isoform of prion (PrPSc) in both infected central and peripheral neuronal cells ScCAD5 and ScN2a (Abdulrahman et al. 2018). Upon activation of autophagy in the infected cells by treatment with rapamycin, a significant decrease in exosome release is observed, along with an alteration in exosomal content (Abdulrahman et al. 2018). This reduction in exosome release is so significant that it nearly parallels the level of reduction observed in cells that are treated with GW4869, an established and widely used inhibitor of exosome release (Abdulrahman et al. 2018). On the other hand, downregulation of autophagy through inhibition of class III PI3K leads only to a slight increase in exosome release that is not statistically significant although the exosomal content of PrPSc and infectivity of ScCAD5 are significantly increased under these conditions (Abdulrahman et al. 2018).
Although the importance of LC3 for exosome pathway is not fully understood, recent investigations demonstrate that exosomes are preferentially enriched in lipid-modified LC3-II (Guo et al. 2017). Also, the ATG12-ATG3 complex, which is involved in catalysis of LC3-II conjugation, interacts with Alix (ALG-2 interacting protein X) to regulate exosome biogenesis in mouse embryonic fibroblasts (MEF) (Murrow et al. 2015). Knockdown of Alix reduces autophagy flux in nutrient-rich conditions, indicating regulatory relationships between exosome biogenesis and autophagy (Murrow et al. 2015).
In the exosome-autophagy interplay, components associated with the exosome pathway have also been found to regulate autophagy. The tetraspanin CD63, a well-known exosomal marker, has been shown to play a key role in regulation of autophagy. CD63 downregulates Epstein-Barr virus LMP1-mediated mTORC1 activation, resulting in autophagy induction (Hurwitz et al. 2018). Furthermore, mTORC1, a known inhibitor of autophagy, also inhibits exosome release both in vivo (hepatocytes) and in vitro (MEFs and HeLa cells) (Zou et al., 2019). mTORC1 inhibition rescues exosome release without altering exosomal cargo, suggesting that while mTORC1 modulates exosome release it does not influence exosome formation.
Considering the above findings, the implications of autophagy-exosome interplay for microglial response to infection within the CNS merit strong attention in future studies. Aspects discovered in other cell types and discussed above, such as involvement of specific ATGs in exosome packaging during infection, or the influence of exosome-associated tetraspanins on autophagy response during infection, are likely to apply to microglia as well and need to be addressed in future investigations. As described earlier, the influence of exosome-autophagy crosstalk in microglia has been shown for PD, a neurodegenerative disease that is thought to be triggered by infectious agents. Microglial function is also important for neuroAIDS, another major neurological disease of concern that is caused by infection and for which the relevance of exosome functions and autophagy pathway has been amply demonstrated. A deep understanding of how different ATG proteins may be influencing microglia-derived exosomal release and content, and how in reverse the contents of microglia-derived exosomes may regulate autophagy response in infected host, will provide key insights into the mechanisms of CNS pathologies such as PD and neuroAIDS that are impacted by microglia.
FINAL REMARKS AND FUTURE DIRECTIONS
For over a decade, the study of the communicative roles of exosomes and other EVs have enjoyed tremendous attention and growth. While much excellent work has been done in the EV field, many unanswered questions and significant gaps in knowledge still remain given the complexity of these vesicles and also the complexity of their interactions with their surrounding milieu. In particular, their role in regulation of innate immune response is still in relative infancy. One aspect of this is understanding EV/EX functions related to microglia as part of their innate immune roles in the CNS. For instance, a great deal remains to be elucidated as to how EV-based communication between microglia and other cell types of the CNS such OLs, neurons, and astrocytes coordinate responses to pathogenic infections. In vitro co-culture experiments in the context of infection and in vivo approaches that target specific CNS cell types or molecular pathways in infected animal models will provide important insights. Mouse models such as CX3CR1CreER or CD11b-HSVTKmt−30, which allow microglial ablation in adult mice CNS, or mice deficient in specific components of the autophagy machinery (e.g., conditional brain-specific Atg knockout mice), provide unique opportunities for in vivo mechanistic investigations. In addition, proteomic approaches that allow quantitative analysis of the signaling events that form the foundation of EV effects during pathogenic infections need to be applied extensively in order to form a more in-depth picture of the underlying complexity. Platforms such as reverse-phase protein microarrays (RPMA), which we have successfully applied for high-throughput quantitative profiling of signaling changes in response to infection or exosome treatments (Alem et al. 2015; Fleming et al. 2018; Fleming et al. Under review) can be applied to provide a wealth of knowledge about the molecular mechanisms involved. The work by our laboratory and others have identified specific innate immune responses that are induced by exosomes derived from infected monocytes or macrophages (Figure 3). In this regard, it will be of interest to study whether some of the same responses occur in the CNS cells that receive exosomes released by infected microglia (Figure 3). Understandably, in general, there also exists a continuing need to develop new assays that allow better differentiation of exosomes from other EV subtypes. Comprehensive proteomic analysis of the EV subtypes to identify set of marker proteins specific to each will be worthwhile to perform. This information would also allow development of antibody-based multiplexed platforms for improved separation of different EV subtypes.
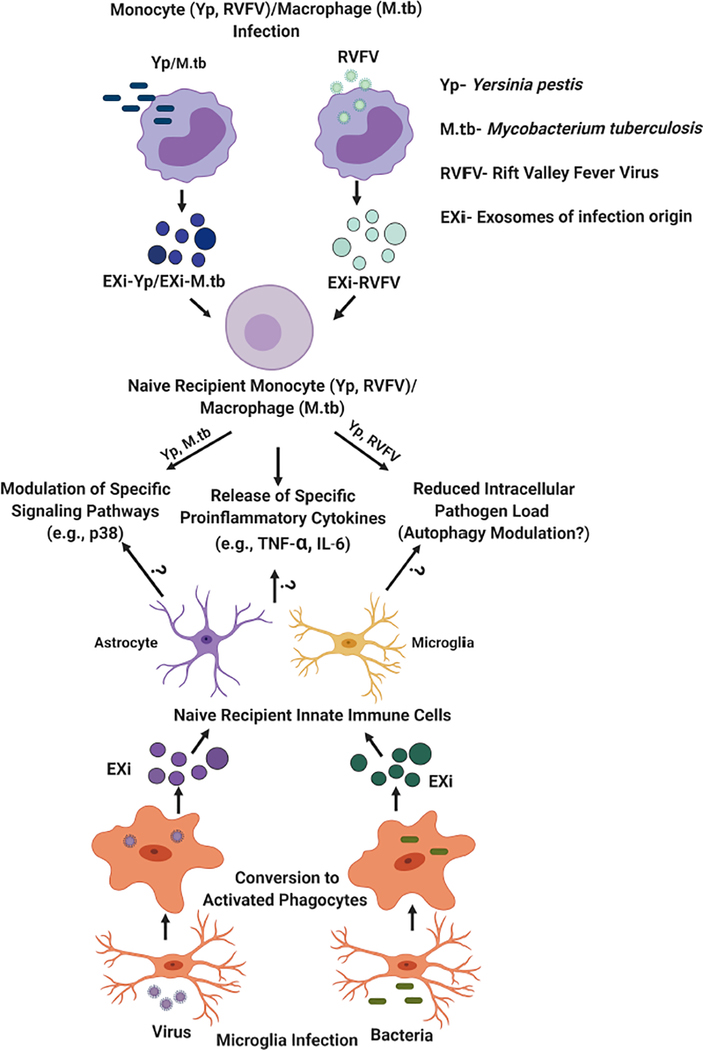
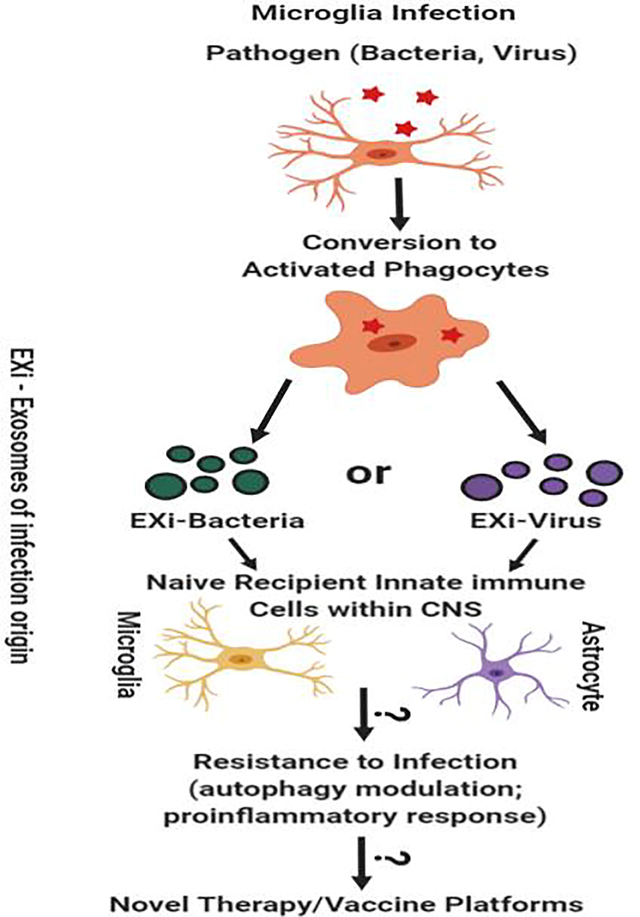
Figure 3: Regulation of innate immune response by exosomes derived from infected monocytes/macrophages (EXi) and potential implications for functions of exosomes produced by infected microglia.
As summarized by the upper half of this figure, the work from our laboratory and published studies from other groups, such as findings for M. tuberculosis infection (Bhatnagar et al. 2007; Singh et al., 2012), have demonstrated that exosomes derived from human monocytes/macrophages infected with bacteria such as Y. pestis or M. tuberculosis, or with the RVFV virus, regulate innate immune responses to infection in specific ways. These include inducing naïve recipient monocytes/macrophages to release proinflammatory cytokines such as TNF-α or IL-6, and inducing significant changes in the activation states of specific signaling proteins in these cells such as the p38 MAP kinase. Our work with both Yp and RVFV has also demonstrated that these exosomes make the naïve recipient cells refractory to subsequent infection, an effect that is likely to involve autophagy since significant changes in autophagy occur as part of host response to both Yp and RVFV (Alem et al. 2015; Moy et al. 2014). The bottom half of this figure summarizes our speculation/hypothesis about microglia, given their role as resident macrophages of the CNS, the findings summarized in the top half of the figure, and the published evidence discussed in the manuscript on effects of exosomes released from infected microglia. We propose that exosomes produced by infected microglia may also have very similar effects on recipient innate immune cells within the CNS (uninfected microglia and astrocytes) as part of mounting an effective response. These may include induction of proinflammatory cytokine release, induction of specific signaling changes, and induction of increased ability for pathogen clearance through modulation of the autophagy pathway.
One emerging and fascinating facet of exosomal regulation of innate immune response to infections is the exosome-autophagy cross regulatory mechanisms. The full nature of signals that determine the fate of the MVBs, dictating whether they fuse with the plasma membrane to release exosomes or are used to make amphisomes, and how this influences infectious diseases, need to become determined. As it was discussed in this review, several of the proteins that are involved in autophagy also affect exosome biogenesis and release. How such interplay influences infectious disease progression remains to be fully investigated. This includes an understanding of how autophagy-exosome interaction impacts microglial function as resident macrophages of the CNS.
Exosomes present with attractive features for development of novel therapeutic and vaccine platforms, and the knowledge from the current and future studies of how they regulate immune response will certainly aid such endeavors. In addition to having the advantage of being naturally produced, their excellent target interaction properties and ability to shield their cargo from immune recognition makes them attractive vehicles for drug and nucleic acid delivery. This is particularly attractive for CNS applications as exosomes derived from brain cells display brain-specific surface proteins, a feature that together with the small size of exosomes allows them to cross the blood‐brain barrier (BBB) for targeted drug delivery to the brain. The next decade promises to be an extremely exciting time for the EV field and many breakthrough advances are anticipated.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- Abdulrahman BA, Abdelaziz DH, and Schatzl HM (2018) Autophagy regulates exosomal release of prions in neuronal cells. J Biol Chem 293(23):8956–8968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahsan NA, Sampey GC, Lepene B et al. (2016) Presence of Viral RNA and Proteins in Exosomes from Cellular Clones Resistant to Rift Valley Fever Virus Infection. Front Microbiol 7:139. doi: 10.3389/fmicb.2016.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailawadi S, Wang X, Gu H, & Fan G C (2015) Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochimica et Biophysica Acta 1852(1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alem F, Yao K, Lane D, Calvert V, Petricoin EF, Kramer L, Hale ML, Bavari S, Panchal RG, Hakami RM (2015) Host response during Yersinia pestis infection of human bronchial epithelial cells involves negative regulation of autophagy and suggests a modulation of survival-related and cellular growth pathways. Front Microbiol 6:50. doi: 10.3389/fmicb.2015.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Schapira AH et al. (2011) Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis 42(3):360–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S, Samuel M, Kumar S, Mathivanan S (2019) Ticket to a bubble ride: Cargo sorting into exosomes and extracellular vesicles. Biochim Biophys Acta Proteins Proteom pii:S15709639(19)30041-X. doi: 10.1016/j.bbapap.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Andreu Z, Yanez-Mo M (2014) Tetraspanins in extracellular vesicle formation and function. Front Immunol 5:442. doi: 10.3389/fimmu.2014.00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakelyan A, Fitzgerald W, Zicari S, Vanpouille C, Margolis L (2017) Extracellular Vesicles Carry HIV Env and Facilitate Hiv Infection of Human Lymphoid Tissue. Sci Rep 7(1):1695. doi: 10.1038/s41598-017-01739-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader CA, Shandala T, Ng YS, Johnson IR, Brooks DA (2015) Atg9 is required for intraluminal vesicles in amphisomes and autolysosomes. Biol Open 4(11):1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglio SR, van Eijndhoven MA, Koppers-Lalic D et al. (2016) Sensing of latent EBV infection through exosomal transfer of 5’pppRNA. Proc Natl Acad Sci U S A.113(5):E587–E596. doi: 10.1073/pnas.1518130113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhti M, Winter C, and Simons M (2011) Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome- like vesicles. J Biol Chem 286(1):787–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay RA, Schwab A, DeMarino C et al. (2017) Exosomes from uninfected cells activate transcription of latent HIV-1. J Biol Chem 292(28):11682–11701. doi: 10.1074/jbc.M117.793521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestebroer J, V’Kovski P, Mauthe M, Reggiori F (2013) Hidden behind autophagy: the unconventional roles of ATG proteins. Traffic 14(10):1029–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS (2007) Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 110(9):3234–3244. doi: 10.1182/blood-2007-03-079152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A and Eissa NT (2015) Autophagy as a Stress Response Pathway in the Immune System. Int Rev Immunol 34(5):382–402. doi: 10.3109/08830185.2014.999156 [DOI] [PubMed] [Google Scholar]
- Bianco F, Pravettoni E, Colombo A, Schenk U, Mӧller T, Matteoli M, Verderio C (2005) Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol 174(11):7268–7277 [DOI] [PubMed] [Google Scholar]
- Bobrie A, Colombo M, Raposo G. and Théry C (2011) Exosome Secretion: Molecular Mechanisms and Roles in Immune Responses. Traffic 12(12):1659–1668. doi: 10.1111/j.16000854.2011.01225.x [DOI] [PubMed] [Google Scholar]
- Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ (2006) Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol 172(6):923–935. doi: 10.1083/jcb.200508014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C (2005) Exosomal-like vesicles are present in human blood plasma. Int Immunol 17(7):879–87 [DOI] [PubMed] [Google Scholar]
- Chen L, Feng Z, Yue H, Bazdar D, Mbonye U, Zender C, Harding CV, Bruggeman L, Karn J, Sieg SF, Wang B, Jin G (2018) Exosomes derived from HIV-1-infected cells promote growth and progression of cancer via HIV TAR RNA. Nat Commun 9(1):4585. doi: 10.1038/s41467018-07006-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, He J, Tian M, Zhang SY, Guo MR, Kasimu R, Wang JH, Ouyang L (2014) UNC51-like kinase 1, autophagic regulator and cancer therapeutic target. Cell Prolif 47(6):494–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb DA, Kim OK, Golden-Mason L, Rosen HR, Hahn YS (2018) Hepatocyte-derived exosomes promote T follicular regulatory cell expansion during hepatitis C virus infection. Hepatology 67(1):71–85. doi: 10.1002/hep.29409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme-Axford E, Donker RB, Mouillet JF, Chu T, Bayer A, Ouyang Y, Wang T, Stolz DB, Sarkar SN, Morelli AE, Sadovsky Y, Coyne CB (2013) Human placental trophoblasts confer viral resistance to recipient cells. Proc Natl Acad Sci U S A 110(29):12048–53. doi: 10.1073/pnas.1304718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago F, Lombardi M, Prada I, Gabrielli M, Joshi P, Cojoc D, Franck J, Fournier I, Vizioli J, Verderio C (2017) ATP modifies the proteome of extracellular vesicles released by microglia and influences their action on astrocytes. Front Pharmacol 8:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreux M et al. (2012) Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe 12(4):558–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitan E, Suire C, Zhang S, Mattson MP (2016) Impact of lysosome status on extracellular vesicle content and release. Ageing Res Rev 32:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage N, Rodriguez M, Dever SM, Masvekar RR, Gewirtz DA, Shacka JJ (2015) HIV-1 and morphine regulation of autophagy in microglia: limited interactions in the context of HIV-1 infection and opioid abuse. J Virol 89(2):1024–1035. doi: 10.1128/JVI.02022-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader CM, Sánchez D, Furlán M and Colombo MI (2008) Induction of Autophagy Promotes Fusion of Multivesicular Bodies with Autophagic Vacuoles in K562 Cells. Traffic 9:230–250. doi: 10.1111/j.1600-0854.2007.00677.x [DOI] [PubMed] [Google Scholar]
- Fitzgerald W et al. (2018) A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci Rep 8(1):8973. doi: 10.1038/s41598-018-27190-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A, Hobbs H, Giroux V, Zhou W, Calvert V, Salvador-Morales C, Agrawal N, Petricoin E, Hakami RM (2018) Amplifying host innate immunity through modulation of p38, Jak2 and ALK activities and induction of macrophage differentiation and IL-6 dependent bacterial clearance by exosomes released from Yersinia pestis-infected monocytes. Journal of Extracellular Vesicles 7:179–179 [Google Scholar]
- Fleming A, Sampey G, Chung MC, Bailey C, Hoek ML, van Kashanchi F et al. (2014) The carrying pigeons of the cell: exosomes and their role in infectious diseases caused by human pathogens. Pathog Dis 71(2):109–120. doi: 10.1111/2049-632X.12135 [DOI] [PubMed] [Google Scholar]
- Frühbeis C, Fröhlich D and Krämer-Albers EM (2012) Emerging roles of exosomes in neuron– glia communication. Front Physio 3:119. doi: 10.3389/fphys.2012.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Shu J, Cui JA (2018) systematic approach to RNA-associated motif discovery. BMC Genomics 19(1):146. doi: 10.1186/s12864-018-4528-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebov K, Löchner M, Jabs R, Lau T, Merkel O, Schloss P, Steinhäuser C, Walter J (2014) Serotonin stimulates secretion of exosomes from microglia cells. Glia 63:626–634 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F and Baltuch G (1999) Microglia as a mediators of inflammatory and degenerative diseases. Annu Rev Neurosci 22: 219–240. 10.1146/annurev.neuro.22.1.219 [DOI] [PubMed] [Google Scholar]
- Guilliams M et al. (2014) Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 14(8): 571–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Chitiprolu M, Roncevic L, Javalet C, Hemming FJ, Trung MT, Meng L, Latreille E, Tanese De Souza C, McCulloch D et al. (2017) Atg5 disassociates the V 1 V 0 -ATPase to promote exosome production and tumor metastasis independent of canonical macroautophagy. Dev Cell 43(6):716–730.e7 [DOI] [PubMed] [Google Scholar]
- Hartjes TA, Mytnyk S, Jenster GW, van Steijn V, van Royen ME (2019) Extracellular Vesicle Quantification and Characterization: Common Methods and Emerging Approaches. Bioengineering (Basel) 6(1):7. doi: 10.3390/bioengineering6010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ (1999) Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94(11):3791–9 [PubMed] [Google Scholar]
- Hessvik NP and Llorente A (2018) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75(2):193–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Liao K, Niu F et al. (2018) Astrocyte EV-Induced lincRNA-Cox2 Regulates Microglial Phagocytosis: Implications for Morphine-Mediated Neurodegeneration. Mol Ther Nucleic Acids 13:450–463. doi: 10.1016/j.omtn.2018.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Jiaming W et al. (2019) HSV-1 miR-H28 exported to uninfected cells in exosomes restricts cell-to-cell virus spread by inducing IFNγ mRNAs. J Virol 01005–19. doi.org/ 10.1128/JVI.01005-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Xintong G, Jinwen Y et al. (2017) Increased miR-124–3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. Faseb J 32(1):512–528. doi.org/ 10.1096/fj.201700673R [DOI] [PubMed] [Google Scholar]
- Hume DA (2006) The mononuclear phagocyte system. Curr Opin Immunol 18(1):49–53 [DOI] [PubMed] [Google Scholar]
- Huotari J and Helenius A (2011) Endosome maturation. Embo J 30(17):3481–3500. doi: 10.1038/emboj.2011.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz SN, Cheerathodi MR, Nkosi D, York SB, Meckes DG (2018) Tetraspanin CD63 Bridges Autophagic and Endosomal Processes to Regulate Exosomal Secretion and Intracellular Signaling of Epstein-Barr Virus LMP1. J Virol. 92(5):e01969–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyttinen JM, Niittykoski M, Salminen A, and Kaarniranta K (2013) Maturation of autophagosomes and endosomes: a key role for Rab7. Biochim Biophys Acta 1833(3):503–510. doi: 10.1016/j.bbamcr.2012.11.018 [DOI] [PubMed] [Google Scholar]
- Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M et al. (2000) An ubiquitin-like system mediates protein lipidation. Nature 408(6811):488–492. doi: 10.1038/35044114 [DOI] [PubMed] [Google Scholar]
- Janas AM, Sapoń K, Janas T, Stowell MHB, Janas T (2016) Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochim Biophys Acta 1858(6):11391151. [DOI] [PubMed] [Google Scholar]
- Janas T, Janas MM., Sapoń K and Janas T (2015) Mechanisms of RNA loading into exosomes. Febs Lett 589(13):1391–1398. doi: 10.1016/j.febslet.2015.04.036 [DOI] [PubMed] [Google Scholar]
- Jing X, Robert C, Sharon M (2018) The interplay between exosomes and autophagy – partners in crime. J Cell Sci 131(15) pii: jcs215210. doi: 10.1242/jcs.215210 [DOI] [PubMed] [Google Scholar]
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C (1987) Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262:9412–9420 [PubMed] [Google Scholar]
- Ke PY (2018) The Multifaceted Roles of Autophagy in Flavivirus-Host Interactions. Int J Mol Sci 19(12):3940. doi: 10.3390/ijms19123940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P (2011) Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med 9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Guan KL (2015) mTOR: a pharmacologic target for autophagy regulation. J Clin Invest 125(1):25–32. 10.1172/JCI73939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu K, Lee J, Miyata M, Lim J, Jono H, Koga T, Xu H, Yan C, Kai H, Li J (2013) Inhibition of PDE4B suppresses inflammation by increasing expression of the deubiquitinase CYLD. Nature Communications 4:1684. doi: 10.1038/ncomms2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP (2018) Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res Int 8545347 Doi: 10.1155/2018/8545347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouwaki T, Fukushima Y, Daito T, et al. (2016) Extracellular Vesicles Including Exosomes Regulate Innate Immune Responses to Hepatitis B Virus Infection. Front Immunol 7:335.. doi: 10.3389/fimmu.2016.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer-Albers EM, Bretz N, Tenzer S, Winterstein C, Mobius W, Berger H, Nave KA, Schild H, and Trotter J (2007) Oligodendrocytes secrete exosomes containing major myelin and stressprotective proteins: trophic support for axons. Proteomics Clin Appl 1(11): 1446–1461 [DOI] [PubMed] [Google Scholar]
- Kruh-Garcia N, Wolfe M, Chaisson H et al. (2014) Detection of Mycobacterium tuberculosis peptides in the exosomes of patients with active and latent M. tuberculosis infection using MRM-MS. PLoS One 9(7):e103811. doi: 10.1371/journal.pone.0103811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Stoica BA, Loane DJ, Yang M, Abulwerdi G, Khan N, Kumar A, Thom SR, Faden AI (2017) Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation 14(1):47. doi: 10.1186/s12974-017-0819-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistakis NT and Tooze SA (2016) Digesting the expanding mechanisms of autophagy. Trends Cell Biol 26(8):624–635. doi: 10.1016/j.tcb.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Lasser C, O’Neil SE, Ekerljung L, Ekstrom K, Sjostrand M, Lotvall J (2011) RNA-containing exosomes in human nasal secretions. Am J Rhinol Allergy 25(2):89–93 [DOI] [PubMed] [Google Scholar]
- Lenassi M et al. (2010) HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 11(1):110–122. doi: 10.1111/j.1600-0854.2009.01006.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Corbett AL, Taatizadeh E, et al. (2019) Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng 3(1):011503. doi: 10.1063/1.5087122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Huang S, Yin Z et al. (2019) Increases in miR-124–3p in Microglial Exosomes Confer Neuroprotective Effects by Targeting FIP200-Mediated Neuronal Autophagy Following Traumatic Brain Injury. Neurochem Res 1573–6903. doi.org/ 10.1007/s11064-019-02825-1 [DOI] [PubMed] [Google Scholar]
- Liao Z, Muth DC, Eitan E et al. (2017) Serum extracellular vesicle depletion processes affect release and infectivity of HIV-1 in culture. Sci Rep 7(1):2558. doi: 10.1038/s41598-017-02908-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison MN, Okeoma CM (2015) Exosomes: Implications in HIV-1 Pathogenesis. Viruses 7(7):4093–4118. doi: 10.3390/v7072810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis L and Sadovsky Y (2019) The biology of extracellular vesicles: The known unknowns. PLoS Biol 17(7): e3000363 10.1371/journal.pbio.3000363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan S and Fahner CJ, Reid GE, Simpson RJ (2012) Database of exosomal proteins, RNA and lipids. Nucleic Acids Res 40 D1241–D1244. doi: 10.1093/nar/gkr828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckes DG (2015) Exosomal communication goes viral. J Virol 89(10):5200–5203. doi: 10.1128/JVI.02470-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Li G, Zhang X, Xu H, Abraham SN (2015) A TRP Channel Senses Lysosome Neutralization by Pathogens to Trigger Their Expulsion. Cell 161(6):1306–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, Klionsky DJ, Ohsumi M, Ohsumi Y (1998) A protein conjugation system essential for autophagy. Nature 395(6700):395398. doi: 10.1038/26506 [DOI] [PubMed] [Google Scholar]
- Moy RH, Gold B, Molleston JM, Schad V, Yanger K, Salzano MV, Yagi Y, Fitzgerald KA, Stanger BZ, Soldan SS, Cherry S (2014) Antiviral autophagy restricts Rift Valley fever virus infection and is conserved from flies to mammals. Immunity 40(1):51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Akbar I, Kumari B, Vrati S, Basu A, and Banerjee A (2019) Japanese Encephalitis Virus‐induced let‐7a/b interacted with the NOTCH‐TLR7 pathway in microglia and facilitated neuronal death via caspase activation. J Neurochem 149(4):518–534. doi: 10.1111/jnc.14645 [DOI] [PubMed] [Google Scholar]
- Murrow L, Malhotra R. and Debnath J (2015) ATG12–ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat Cell Biol 17(3):300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte-’t Hoen E, Cremer T, Gallo RC, Margolis LB (2016) Extracellular vesicles and viruses: Are they close relatives? Proc Natl Acad Sci 113(33):9155–9161. doi: 10.1073/pnas.1605146113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obregon C, Rothen-Rutishauser B, Gerber P, Gehr P, Nicod LP (2009) Active uptake of dendritic cell-derived exovesicles by epithelial cells induces the release of inflammatory mediators through a TNF-alpha-mediated pathway. Am J Pathol 175(2):696–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojha CR, Lapierre J, Rodriguez M et al. (2017) Interplay between Autophagy, Exosomes and HIV-1 Associated Neurological Disorders: New Insights for Diagnosis and Therapeutic Applications. Viruses 9(7):176. doi: 10.3390/v9070176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallet N, Sirois I, Bell C, Hanafi LA, Hamelin K, Dieude M et al. (2013) A comprehensive characterization of membrane vesicles released by autophagic human endothelial cells. Proteomics 13(7):1108–20. doi: 10.1002/pmic.201200531 [DOI] [PubMed] [Google Scholar]
- Pant S, Hilton H, Burczynski ME (2012) The multifaceted exosome: Biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol 83(11):1484–1494. doi: 10.1016/j.bcp.2011.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parzych KR and Klionsky DJ (2014) An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal 20(3):460–473. doi: 10.1089/ars.2013.5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, Cunha C, Barbosa M, Vaz AR, Brites D (2017) Exosomes from NSC-34 Cells Transfected with hSOD1-G93A Are Enriched in miR-124 and Drive Alterations in Microglia Phenotype. Front Neurosci 11:273. doi: 10.3389/fnins.2017.00273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisitkun T, Shen RF, Knepper MA (2004) Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101(36):13368–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Sierra-Torre V, Sierra A (2017) Autophagy and Microglia: Novel Partners in Neurodegeneration and Aging. Int J Mol Sci 18(3):598. doi: 10.3390/ijms18030598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakov A, Spilman M, Dokland T, Amling CL, Mobley JA (2009) Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate 69(2):159–67 [DOI] [PubMed] [Google Scholar]
- Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ, Santambrogio L (2005) Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol 175(4):2237–2243 [DOI] [PubMed] [Google Scholar]
- Portillo JC, Muniz-Feliciano L, Lopez Corcino Y, Lee SJ, Van Grol J, Parsons SJ, Schiemman W P, Subauste CS (2017) Toxoplasma gondii induces FAK-Src-STAT3 signaling during infection of host cells that prevents parasite targeting by autophagy. PLoS Pathog 13(10):e1006671. doi: 10.1371/journal.ppat.1006671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada I, Furlan R, Matteoli M, Verderio C (2013) Classical and unconventional pathways of vesicular release in microglia. Glia 61(7):1003–1017 [DOI] [PubMed] [Google Scholar]
- Qu Y, Franchi L, Nunez G, Dubyak GR (2007) Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol 179(3):1913–1925 [DOI] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183(3):1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G and Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200(4):373–383. doi: 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond AD, Diaz P, Chevelon S, Agudelo M, Yndart-Arias A, Ding H, Kaushik A, Jayant RD, Nikkhah-Moshaie R, Roy U, Pilakka- Kanthikeel S, Nair MP (2016) Microglia-derived HIV Nef+ exosome impairment of the blood-brain barrier is treatable by nanomedicine-based delivery of Nef peptides. J Neurovirol 22:129–139 [DOI] [PubMed] [Google Scholar]
- Sampey GC, Meyering SS, Zadeh MA, Saifuddin M, Hakami RM, Kashanchi F (2014) Exosomes and their role in CNS viral infections. J Neurovirol 20(3):199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampey GC, Saifuddin M, Schwab A, et al. (2016) Exosomes from HIV-1-infected Cells Stimulate Production of Pro-inflammatory Cytokines through Trans-activating Response (TAR) RNA. J Biol Chem 291(3):1251–1266. doi: 10.1074/jbc.M115.662171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorey JS and Harding CV (2016) Extracellular vesicles and infectious diseases: new complexity to an old story. J Clin Invest 126(4):1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab A, Meyering SS, Lepene B et al. (2015) Extracellular vesicles from infected cells: potential for direct pathogenesis. Front Microbiol 6:1132. doi: 10.3389/fmicb.2015.01132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava S, Devhare P, Sujijantarat N, Steele R, Kwon YC, Ray R, Ray RB (2016) Knockdown of autophagy inhibits infectious hepatitis C virus release by the exosomal pathway. J Virol 90(3):1387–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PP, Li L, Schorey JS (2015) Exosomal RNA from Mycobacterium tuberculosis-infected cells is functional in recipient macrophages. Traffic 16(6):555–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PP, Smith VL, Karakousis PC, Schorey JS (2012) Exosomes isolated from mycobacteria-infected mice or cultured macrophages can recruit and activate immune cells in vitro and in vivo. J Immunol 189(2):777–785. doi: 10.4049/jimmunol.1103638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VL, Cheng Y, Bryant BR, Schorey JS (2017) Exosomes function in antigen presentation during an in vivo Mycobacterium tuberculosis infection. Sci Rep 7:43578.doi: 10.1038/srep43578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VL, Jackson L, Schorey JS (2015) Ubiquitination as a mechanism to transport soluble mycobacterial and eukaryotic proteins to exosomes. J Immunol 195(6):2722–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth LA, Ratnasothy K, Tsang JY, Boardman D, Warley A, Lechler R, Lombardi G (2013) CD73 expression on extracellular vesicles derived from CD4+CD25+Foxp3+ T cells contributes to their regulatory function. Eur J Immunol 43(9): 2430–2440. doi: 10.1002/eji.201242909 [DOI] [PubMed] [Google Scholar]
- Su P, Zhang J, Wang D, Zhao F, Cao Z, Aschner M, Luo W (2016) The role of autophagy in modulation of neuroinflammation in microglia. Neuroscience 319: 155–167. doi: 10.1016/j.neuroscience.2016.01.035 [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Tsukimoto M, Iwamaru Y, Sugama S, Sekiyama K, Sato M, Kojima S, Hashimoto M, Kitani H (2015) Extracellular ATP induces unconventional release of glyceraldehyde-3-phosphate dehydrogenase from microglial cells. Immunol Lett 167(2):116–124 [DOI] [PubMed] [Google Scholar]
- Tauro BJ, Greening DW, Mathias RA, Mathivanan S, Ji H, Simpson RJ (2013) Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics 12(3):587–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S (2002) Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol 3 (12):1156–1162 [DOI] [PubMed] [Google Scholar]
- Tripathi DN, Chowdhury R, Trudel LJ et al. (2013) Reactive nitrogen species regulate autophagy through ATM-AMPK-TSC2-mediated suppression of mTORC1. Proc Natl Acad Sci U S A 110(32):E2950–E2957. doi: 10.1073/pnas.1307736110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman JP, Al Gadban MM, Smith KJ, Jenkins RW, Mayroo N, Virella G, Lopes-Virella MF, Bielawska A, Hannun YA, Hammad SM (2012) Differential regulation of acid sphingomyelinase in macrophages stimulated with oxidized low-density lipoprotein (LDL) and oxidized LDL immune complexes: role in phagocytosis and cytokine release. Immunology 136(1):30–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turola E, Furlan R, Bianco F, Matteoli M, Verderio C (2012) Microglial micro- vesicle secretion and intercellular signaling. Front Physiol 3:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Grol J, Subauste C, Andrade RM, Fujinaga K, Nelson J, Subauste CS (2010) HIV-1 inhibits autophagy in bystander macrophage/monocytic cells through Src-Akt and STAT3. PloS One 5(7): e11733. doi: 10.1371/journal.pone.0011733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Muzio L, Turola E, Bergami A, Novellino L, Ruffini F, Riganti L, Corradini I, Francolini M, Garzetti L, Maiorino C, Servida F, Vercelli A, Rocca M, Libera D, Martinelli V, Comi G, Martino G, Matteoli M, Furlan R (2012) Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann Neurol 72(4): 610–624. doi: 10.1002/ana.23627 [DOI] [PubMed] [Google Scholar]
- Villarroya-Beltri C, Baixauli F, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M (2014) Sorting it out: regulation of exosome loading. Semin Cancer Biol 28:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wang Y, Shi X et al. (2019) The TRAPs From Microglial Vesicles Protect Against Listeria Infection in the CNS. Front Cell Neurosci 13:199. doi: 10.3389/fncel.2019.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Liu J, Zhao R et al. (2018) Glutaminase 1 regulates the release of extracellular vesicles during neuroinflammation through key metabolic intermediate alpha-ketoglutarate. J Neuroinflammation 15(1):79. doi: 10.1186/s12974-018-1120-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Zhang G, Han C et al. (2019) Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis 10(3):174. doi: 10.1038/s41419-019-1404-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Niu F, Yao H et al. (2018) Exosomal miR-9 Released from HIV Tat Stimulated Astrocytes Mediates Microglial Migration. J Neuroimmune Pharmacol 13(3): 330. doi.org/ 10.1007/s11481-018-9779-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Chen H, Yang Y, Fu Y, Yi Z (2019) miR-18a promotes Mycobacterial survival in macrophages via inhibiting autophagy by down-regulation of ATM. J Cell Mol Med 00:1–9. 10.1111/jcmm.14899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborowski MP, Balaj L, Breakefield XO, Lai CP (2015) Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 65(8):783–797. doi: 10.1093/biosci/biv084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HG, Grizzle WE (2014) Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol 184(1):28–41. doi: 10.1016/j.ajpath.2013.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Yeo J and Lim C (2019) Advances in Technologies for Purification and Enrichment of Extracellular Vesicles. SLAS technolo 24(5)72630319846877. doi: 10.1177/2472630319846877 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Liu H, Tang WH (2019) Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9:19. doi: 10.1186/s13578-019-0282-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Lin D, Chen Y, Peng S, Jing X, Lei M, Tao E, Liang Y (2019) α-synuclein accumulation in SH-SY5Y cell impairs autophagy in microglia by exosomes overloading miR19a-3p. Epigenomics 11(15):1661–1677. doi: 10.2217/epi-2019-0222 [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4(5):594–600 [DOI] [PubMed] [Google Scholar]
- Zou W, Lai M, Zhang Y et al. (2019) Exosome Release Is Regulated by mTORC1. Adv Sci (Weinh) 6(3):1801313. doi: 10.1002/advs.201801313 [DOI] [PMC free article] [PubMed] [Google Scholar]