Abstract
I have spent over 40 years studying the behavior of our closest living relatives, the apes. In this paper, I review my research on the spacing, mating, and vocal behavior of gibbons and orangutans (Pongo pygmaeus) and the vocal and social behavior of chimpanzees (Pan troglodytes). I devote special attention to results derived from a 25-year-long study of a remarkable and extraordinarily large group of chimpanzees that has recently fissioned at Ngogo in Kibale National Park, Uganda. I conclude with some advice for the next generation of field primatologists.
Keywords: apes, behavior, field experiments
INTRODUCTION
I have been extraordinarily lucky and given a unique opportunity to study our closest living relatives, the apes. As a graduate student and postdoctoral researcher, I conducted fieldwork on gibbons and orangutans in Indonesia. While making a transition to teach at the University of Michigan, I started research on chimpanzees in Tanzania. I subsequently initiated a long-term study of a community of chimpanzees at Ngogo in Kibale National Park, Uganda. I have also conducted fieldwork with the two other African apes, bonobos (Pan paniscus) in the Democratic Republic of Congo and mountain gorillas (Gorilla gorilla) in Rwanda. My research has been eclectic, with no consistent theoretical or empirical theme. Instead, I have taken advantage of opportunities as they have arisen, and if there is any coherence to my research program, it is accidental because my story emphasizes the role that serendipity plays in science.
In this paper I furnish a review of my research on apes during the past 41 years. I consider matters chronologically starting with my work on the spacing, mating, and vocal behavior of gibbons and orangutans before moving on to an account of my studies of chimpanzees. I conclude with some advice for the next generation of aspiring primatologists.
Ethics statement
Research reported in this paper adhered to the ASP Principles for Ethical Treatment of Non-Human Primates, was approved by the Institutional Animal Care and Use Committee of the University of Michigan, and complied with the legal requirements of the countries in which fieldwork was conducted.
Data sharing
The data that support the findings of this study are available from the corresponding author upon reasonable request.
BEGINNINGS
I did not have a keen interest in science or the study of animal behavior when young, but things changed during my senior year in high school with a first serendipitous event. A book arrived in the mail. My parents had purchased The Marvels of Animal Behavior for my younger brother, who is now an architect. I stole it, and it remains on my bookshelf to this day. The book was published by the National Geographic Society and in keeping with the tradition established by its magazine, the book was full of beautiful photos of exotic animals in equally exotic locales. The chapters were written by a who’s who list in the study of animal behavior: Dick Alexander, Jack Bradbury, John Eisenberg, Steve Emlen, Gordon Orians, and George Schaller, pioneers in the modern study of animal behavior in the United States. The chapter that intrigued me the most was the last one on baboons entitled, “Quest for the roots of society” by Irv DeVore. I digested what I could from the book and went to college at the University of California, Berkeley. DeVore’s mentor, Sherry Washburn, the founding father of American primatology, was teaching there at the time. I took a few classes from Washburn and got hooked.
I was fortunate to have gone to school in the 1970’s. These were heady times in the study of animal behavior as Karl von Frisch, Konrad Lorenz, and Niko Tinbergen had been awarded the Nobel Prize in Medicine or Physiology in 1973 for their seminal work that established the discipline of ethology. It was also the same time that E.O. Wilson (1975) was writing Sociobiology. In Sociobiology, Wilson resurrected Bill Hamilton’s (1963) theory of kin selection and renewed interest in this heretofore underappreciated evolutionary process that influences what animals do and why. As I continued my studies at Berkeley, I started to wonder whether one could apply some recently developed techniques to assay genetic variation in natural populations (Hubby & Lewontin, 1966) to investigate whether who’s related to whom affects who does what with whom in primates. Remembering DeVore’s (1972) paper in The Marvels of Animal Behavior, I had baboons in mind as subjects.
GRADUATE STUDY
I took my ideas and potential project to graduate school at the University of California, Davis, where I worked under the supervision of Peter Rodman. Peter had completed one of the first successful field studies of orangutans (Rodman, 1973) and having been trained at Harvard by DeVore, he had been influenced by Wilson and the emerging field of Sociobiology, which was to have a profound impact on primate field studies in the ensuing years. Peter had a singularly important impact on my career. He had many good ideas (e.g. Rodman & McHenry, 1980) and trained several talented graduate students. My plans started to change in my first year of graduate school due to a second serendipitous event. As I listened to Peter lecture one day in his primate behavior and ecology class, he outlined an intriguing idea about how easy or difficult it would be for primates to defend their territories. Peter’s steady guidance helped me turn his ideas into my first paper (Mitani & Rodman, 1979). In it, we proposed an “index of defendability,” the ratio of a group’s average daily path length to the diameter of their range. We reasoned when this ratio was large, it would be relatively easy for a group of primates to defend their range. In contrast, when it was small, the costs of defense were likely to be prohibitive. In the pre-internet era, I went to the library to comb through books and journals to dig up what I could with regard to how far different primate species moved each day and the size of their ranges. Our analysis indicated that observations generally conformed to predictions, though there were a few exceptions.
Looking back, this paper helped introduce cost-benefit analyses to the study of primate behavior, as it was one of the first to explicitly recognize that there were costs as well as benefits to primate behavior (see also Clutton-Brock & Harvey, 1976). The paper also deepened my interest in how to conduct comparative analyses to investigate functional questions about behavior. Comparative analyses to study adaptation were to feature in some of my later work on the number of males in primate groups (Mitani, Gros-Louis, & Manson, 1996), the role that sexual selection plays in the evolution of sexual size dimorphism (Mitani, Gros-Louis, & Richards, 1996), how primate allomothers help the mothers of their charges in fitness enhancing ways (Mitani & Watts, 1997), and whether intraspecific variation influences results derived from such analyses (Patterson, Sandel, Miller, & Mitani, 2014; Sandel et al., 2016).
As previously noted, I went to graduate school with the idea of conducting a study of kin selection and primate behavior with baboons as subjects. Things changed with a third serendipitous event. Peter came to me one day at the end of my first year of graduate school. He had a NSF grant to study the comparative socioecology of Bornean primates at the time and asked whether I would be interested in going to Borneo with him. I of course said, “yes,” and that launched my field career.
My first field season in Borneo gave me a chance to get my feet wet. With the passage of time, I do not recall what I did during that initial trip beyond taking in the splendor of the Bornean tropical rainforest. Peter was exceedingly patient and sent me back for a second, longer period. This time, I had a vague notion of studying the behavior and locomotion of long-tailed macaques (Macaca fascicularis) to fit in Peter’s comparative study of Bornean primates. But after six months I returned home failing miserably on an utterly forgettable project.
THESIS RESEARCH
I regrouped during my third year of graduate study. Perhaps as a result of the paper that we had just published on territoriality in primates, my thoughts turned to a comparative study of territoriality. Steve Emlen and Lew Oring (1977) had recently published a ground-breaking paper noting that polygynous males might defend their territories for different reasons, and I proposed to investigate whether socially monogamous gibbons and polygynous leaf monkeys were defending space or their mates. As an undergraduate student, I was taken by the work of Niko Tinbergen, who pioneered the use of field experiments, to address functional questions about animal behavior (Tinbergen, 1958). Taking a lead from him, I adopted an experimental playback technique using tape recorded calls to investigate the nature of Müller’s gibbon (Hylobates muelleri) and Bornean leaf monkey (Presbytis hosei) territoriality. The procedure had recently been implemented successfully for the first time in the field by Peter Waser (1975). He used the playback technique to show that male grey-cheeked mangabeys (Lophocebus albigena) employed their loud call, the whoop-gobble, to mediate intergroup spacing. Peter Waser was a very generous and important mentor. He provided sage advice while I planned my study and lent me his expensive portable speaker to use during fieldwork. He also offered constructive comments on my thesis when he later served as a member of my PhD committee.
In my own work, I used playbacks to simulate interactions between groups of gibbons and leaf monkeys in different parts of their range. In my study of gibbons, I found that their approach and vocal responses waned as simulated encounters moved from the center of their territory to the periphery and when calls were played back from the territories of their neighbors. These results were consistent with the hypothesis that gibbons were defending space per se (Mitani 1985a).
While these were satisfying results, I was not making progress on my work with leaf monkeys. This component of the project was designed to test the hypothesis that socially polygynous males were defending females instead of space, and I predicted that they would have strong reactions to any potential intruder irrespective of where they were in their territory. The suggestion that male leaf monkeys engaged in “female defense polygyny” was subsequently validated by Carel van Schaik and colleagues (van Schaik, Assink, & Salafsky, 1992). I never performed the proposed experiments because I was unable to habituate the monkeys. They were shy, lived at fairly low densities, and I could not find them very often. Instead, I frequently met orangutans. In addition, whenever I met orangutans, they were often together! I knew from reading the literature and from Peter Rodman’s work that orangutans had not been seen together often by previous researchers (Rodman, 1973; MacKinnon, 1974; Horr, 1975). In yet another serendipitous turn after nine months attempting to habituate leaf monkeys, I realized that it would be more productive to shift my efforts to study orangutans. The obvious question was why were they together so often?
It did not take long to discover that the orangutans who I had been encountering were females and males, and pairs who invariably mated. At a time when few, if any, matings between orangutans had been observed in the wild, I doubled down on the fieldwork, spent as much time with the animals as I could, and let them tell me their story. In the process, I described alternative mating tactics employed by two different types of male orangutans, large and small. During these early years of study, these large and small males were thought to be individuals who differed in age. The large individuals were considered adults, and the small ones were believed to be subadults. We now know that some of the small males never grow up, a proposal first made by Peter Rodman and me (Rodman & Mitani, 1987). The small males regardless of whether they are old or young face a competitive disadvantage due their size. To compensate, they adopt a mating tactic of first finding a female, staying with her as long as possible, and mating her repeatedly and often forcibly (Mitani, 1985b; Table 1). In contrast, the larger males stay with females for much shorter periods, mating them forcibly as often as times they don’t (Table 1). The results of this study were made possible because I made a renewed commitment to fieldwork and behavioral observations, a commitment that was to feature prominently in my subsequent research on chimpanzees (see below).
Table 1.
Alternative mating tactics by male orangutans. Adapted from Mitani 1985a.
| forced matings | unforced matings | median length of associations (days) | |
|---|---|---|---|
| small males | 144 | 7 | 6.7 |
| large males | 13 | 15 | 1.5 |
The theme of male orangutan sexual coercion emerged later after I joined the faculty at the University of Michigan. There the first class I taught was a seminar with Barb Smuts, who had become interested in the phenomenon of male sexual coercion. She took some of the things we learned in that class to publish a seminal paper on the topic with her late father, Bob (Smuts & Smuts, 1993). Shortly after publication of this paper, the issue of sexual coercion began to receive significant theoretical attention (Clutton-Brock & Parker 1995), and during the past several years, there has been renewed empirical interest in the topic (e.g. Muller, Kahlenberg, Emery Thompson, & Wrangham, 2007; Palombit, 2014). The latter is noteworthy as it coincides with recent high-profile events concerning our own behavior, making male sexual coercion a compelling topic for continued study.
My playback studies with gibbons were proceeding nicely, and I decided to employ the technique to investigate the “long calls” emitted by male orangutans. These loud, distinctive calls start with a series of low amplitude rumbles followed by a series of roars that reach a crescendo before falling off again into rumbles. Playback experiments revealed that orangutans employed the calls as a spacing mechanism, with males avoiding the calls made by the highest-ranking male in the area (Mitani, 1985c). In contrast, males did not appear to use these calls to attract females to them.
One’s research interests often shift as unexpected findings emerge, and my work on orangutan mating led me to ask questions about gibbon monogamy. Monogamy in mammals is theoretically unexpected and rare (Lukas & Clutton-Brock, 2013), and the central problem has always been why males accept monogamous relationships. Taking a lead from some work conducted by Rich Tenaza (1975) on Kloss’s gibbon (Hylobates klossi), I again used playbacks to simulate encounters between solitary females and mated pairs. These experiments revealed that female gibbons force these relationships on males through the sex-specific aggression they display to female intruders (Mitani, 1984). I later replicated these results in a playback study with another gibbon species (Mitani, 1987a). These findings have taken on renewed significance as they bear on a recent controversy regarding the evolution of monogamy in mammals. They fit nicely with Dieter Lukas and Tim Clutton-Brock’s (2013) hypothesis that social monogamy evolves when males are unable to defend multiple females.
POSTDOCTORAL RESEARCH
In my PhD research, I used vocal signals as a tool to pose questions to the animals themselves about why they were behaving in certain ways (Mitani, 1990). In the process, it was impossible not to become interested in the signals themselves. As a consequence, I pursued postdoctoral research on animal vocal communication working in the lab of Peter Marler, the leading expert in the study of animal communication (e.g., Marler, 1961, 1967). Peter coincidently had edited The Marvels of Animal Behavior, the book that originally sparked my interest in animals and primates! Peter was a giant in the study of animal behavior, and I was fortunate to work under his supervision. A life-long ornithologist, he wrote extensively about the song learning process in male birds (Marler, 1970, 1981, 1989). Peter spent some time during the middle of his career, however, conducting field research on the vocal and social behavior of primates (e.g., Marler, 1969, 1973). After he resumed his studies of male birdsong, Peter maintained his interest in primate vocal behavior by supervising a series of postdocs in his lab (e.g., Seyfarth, Cheney, & Marler 1980; Gouzoules, Gouzoules & Marler, 1984; Hauser & Marler, 1993).
My postdoctoral research focused on the songs of male agile gibbons (Hylobates agilis). I initially set out to determine how these songs were structured acoustically and the function they performed. To accomplish the former, I recorded songs by several males and brought them back to the lab to conduct acoustic analyses using state-of-the art digital signal processing techniques (Mitani 1987b, 1988; Mitani & Marler, 1989). These analyses indicated that male gibbons engaged in prolonged singing performances that can last up to two hours during which they emitted several different song types. Singing typically began with short songs consisting of a series of simple frequency upsweeps. As performances continued, songs became louder, longer, and more elaborate with more and different note types.
Males frequently countersing with their neighbors, an observation that clearly suggests a territorial spacing function (Mitani, 1988). Song complexity also increases during vocal interactions with territorial neighbors, with males singing faster, louder, longer, and with more elaborate songs than when singing alone. Results of playback experiments indicated that males responded more strongly to elaborate songs sung fast and loud compared to simple songs sung slower and at relatively low amplitude (Mitani, 1988). These findings suggest that increasing song complexity during performances represent a series of graded signals, with long complex songs more effective territorial stimuli than short simple songs.
My time as a postdoctoral researcher working in the Marler lab culminated in the publication of a paper on how gibbons create their songs using constituent note types (Mitani & Marler, 1989). In this paper, we described the different note types males used to construct their songs. We then carried out a mind-numbing set of analyses to determine how they combined these note types into songs. We then artificially rearranged those songs and played them back to gibbons comparing their responses to normal, unaltered songs serving as controls. We found that the order of note types mattered to the gibbons as they responded to the rearranged songs in a different way than to the controls. This study was one of the first to show that animal calls possess a phonological structure in the same way we use a limited number of meaningless phonemes to construct an infinite number of meaningful words. Subsequent research by Klaus Zuberbuler and colleagues (Ouattara, Lemasson, & Zuberbühler, 2009; Coye, Zuberbühler, & Lemasson, 2016) extended this finding considerably by showing that Cercopithecus monkeys combine calls in a syntactic-like way to produce sequences that differ from the meaning of the individual calls themselves.
I conducted my postdoctoral field research on gibbons at the newly established Cabang Panti Research Station in Gunung Palung National Park, Indonesia. I found the site with Mark Leighton and Rhys Bowen during a memorable summer in 1984. Mark was looking for a place to establish a long-term field research station where he could resume his comparative studies of vertebrate feeding ecology (Leighton & Leighton 1983). I continued to carry out orangutan fieldwork at Gunung Palung briefly (Mitani, Grether, Rodman, & Priatna, 1991), and Cheryl Knott has ably continued long-term research there to this day (e.g. Knott, 1998, O’Connell, Susanto, & Knott, 2019).
THE TRANSITION TO CHIMPANZEES
Toward the end of my time in the Marler lab, I felt that I was ready for a new challenge. Peter, who had studied chimpanzees with Jane Goodall earlier (Marler, 1969; Marler & Hobbett, 1975), provided the challenge by nudging me to the study of chimpanzees. Toshisada Nishida facilitated the transition. I had met Toshi earlier as a graduate student during a visit to Japan. We kept in touch thereafter, and I asked him whether I could work with the chimpanzees he had studied for many years at the Mahale Mountains National Park in Tanzania (Nishida, 1968). He agreed, and I morphed into a chimpanzee field researcher. As time elapsed, Toshi was to become much more than a facilitator of my chimpanzee research. We bonded like two male chimps, and he became a mentor, colleague, collaborator, and friend (Mitani, 2011). Toshi was above all else passionate about the study of wild chimpanzees. He was an astute observer, picking up details of their lives that others would miss. I learned a lot about chimpanzee behavior through his patient instruction and tutelage. He passed away several years ago (Mitani et al., 2012), and I still miss him to this day.
In my first project, I investigated an enduring problem in the study of primate vocal communication, namely whether learning affected the vocal acquisition process. To do so, I made a tactical decision. I took my experimentalist hat off and conducted an observational study of dialectal variation. This was an indirect way to address the problem, as the formation of dialects is one correlate of the vocal learning process (cf. Marler and Tamura 1964). We examined the long-distance pant hoots emitted by male chimpanzees, and compared calls produced by males living at Gombe and Mahale Mountains National Parks in western Tanzania (Mitani et al. 1992). We documented some subtle temporal and frequency differences between the calls produced by males at the two sites, a finding that furnished preliminary evidence of vocal dialects. In this study, we attempted to exclude other sources of variation that might explain the acoustic differences we uncovered. This so-called “method of exclusion” was adopted in subsequent studies of culture in chimpanzees (Whiten et al., 1999).
My initial study of the Mahale chimpanzees was an eye-opening experience. Having worked with socially monogamous gibbons and relatively asocial orangutans, whose group structure constrained the form, frequency, and patterns of interactions between individuals, chimpanzees were quite different. Their rich social lives opened new avenues of inquiry. One that I immediately pursued involved their vocal behavior. Early on, it became clear to me that individual males did not call equally often. Intrigued by this observation, I started to collect data regarding who called, when, and under what circumstances.
During my time as a postdoctoral researcher, a fellow postdoc, Marcel Gyger, conducted an ingenious set of experiments documenting the “audience effect” in male jungle fowl (Gallus gallus). When presented with models of aerial predators, males modulated their production of alarm calls when alone and in the presence of conspecifics, suggesting that calling behavior was under the voluntary control of signalers (Gyger, Karakashian, & Marler, 1986). I remembered that work as I was becoming aware of the importance of social relationships and long-term alliances between male chimpanzees. This led me to ask whether male chimpanzees altered their production of pant hoots in the presence and absence of their friends (Mitani & Nishida, 1993). We found that males increased the frequency of their calls when their friends were in the party and presumably nearby compared to when they were not together (Mitani & Nishida, 1993). In contrast, these same males did not alter the number of calls they produced when a randomly selected control male was in the party or absent (Mitani & Nishida, 1993). In sum, male chimpanzees appeared to be using these calls to keep in contact with specific individuals. This initial foray into the social behavior of male chimpanzees laid the groundwork for my subsequent studies of adult male chimpanzees in Uganda (see next section).
My study of how social relationships affected call production set the stage for another project created by another chance event. Specifically, I began to wonder whether social relationships influenced the vocal plasticity we possibly uncovered in the dialect study. Male chimpanzees frequently produce pant hoots together while chorusing. Analyzing these calls acoustically is hard because it is difficult to determine who is calling when they overlap in this way. As a consequence, I was typically setting aside these calls as too “messy” to analyze. While examining some audio spectrograms of these choruses in my lab one day, however, I started to notice a peculiar phenomenon. The calls produced by different males during choruses appeared to resemble each other! Using this observation as a springboard, Julie Gros-Louis laboriously analyzed calls given by males while chorusing together. By doing so, we showed that males actively modified their calls when chorusing (Mitani & Gros-Louis, 1998). We suggested that this phenomenon is similar to the process of vocal accommodation observed in humans and that in chimpanzees call convergence serves to strengthen social bonds between individuals. This line of investigation has been elaborated recently through work conducted by Pawel Fedurek and colleagues (Fedurek, Schel, & Slocombe, 2013; Fedurek, Machanda, Schel, & Slocombe, 2013), who showed that male chimpanzees flexibly adjust other parts of pant hoots when chorusing with others and that chorusing behavior reflects both long- and short-term affiliative relationships between callers.
I supported my fieldwork with the Mahale chimpanzees with a grant from the National Science Foundation. The study was designed to compare the loud calls produced by male great apes. I originally intended to limit this study to orangutans and chimpanzees, but my friend, Steve Nowicki, urged me to incorporate gorillas and bonobos into the proposal. This gave me an opportunity to conduct fieldwork with them as well (Mitani, 1996).
THE NGOGO CHIMPANZEE PROJECT
I recruited Dick Byrne to the chimpanzee dialect study, as he had made some recordings of the Mahale chimpanzees before. I visited Dick in Scotland to collect these calls and met Bill McGrew. While weaving down the streets of St. Andrews late one night, I asked Bill whether the time was ripe to bring ape researchers together as had been done 20 years before at a Wenner-Gren conference in Austria (Hamburg & McCown, 1979). That suggestion led to the publication of Great Ape Societies a few years later (McGrew, Marchant, & Nishida, 1996).
The book itself was the result of a meeting sponsored by the Wenner-Gren Foundation and led to another serendipitous turn in my career. David Watts was at the meeting. Until then, he had conducted research on mountain gorillas at the Karisoke Research Station in Rwanda (e.g. Watts, 1989), but he was interested in making a transition to the study of chimpanzees. Acting on a suggestion by Richard Wrangham, David had set his sights on Ngogo in Kibale National Park, Uganda, where one of my fellow graduate students at Davis, Mike Ghiglieri had worked on chimpanzees before (Ghiglieri, 1984). David intended to visit Ngogo the following year. Because I had no plans to return to Mahale then, I asked David whether I could tag along. I had a keen interest to do so because Kibale was well known as a premier primate research site. It had been established by Tom Struhsaker several years before (Struhsaker, 1975). I knew Tom because he was one of Peter Marler’s first graduate students, so I already felt part of the Kibale family.
My first summer at Ngogo in 1995 was a revelation. It took only a few days to realize that something was different there. There were chimps, and they were everywhere! In another serendipitous turn, Tom Struhsaker visited while we were at Ngogo. I spent several days wandering the forest with Tom attempting to glean everything he could tell me about Ngogo, Kibale, and Uganda. I remember those days as some of the most rewarding I have ever spent in the field.
I planned this to be just a visit. I had every intention of continuing my work at Mahale where I had built a productive collaboration with Nishida. But the extraordinary number of chimpanzees that were revealing themselves at Ngogo was too much to resist. Having Tom there was also an inspiration. At the end of the summer, I asked David whether he wanted to work together on a long-term project at Ngogo. He agreed. Now 26 years later, it has been an immensely satisfying and productive collaboration.
Kinship and male chimpanzee behavior
I began my studies of the Ngogo chimpanzees carrying on where I had left off at Mahale with research on their vocal behavior (Mitani, Hunley, & Murdoch, 1999). From the start, however, unsuspected findings emerged, such as the unusual subgrouping behavior of males (Mitani & Amsler, 2003), which is likely to have played a role in a recent fission event (see below). I also realized that I had an opportunity to fulfill my original plan investigating the role kin selection played in primate behavior. After several fits and starts, and difficulties obtaining the requisite genetic data from the chimps, we used information regarding mtDNA relationships between male chimpanzees to publish a few results (Mitani, Merriwether, & Zhang, 2000; Mitani, Watts, Pepper, & Merriwether, 2002). These findings were not entirely satisfying because they were based on only a single gene. It took recruiting a brilliant and hard-working graduate student, Kevin Langergraber, to fulfil my original dream of determining whether who’s related to whom actually affects who does what with whom.
Working with Linda Vigilant, Kevin conducted a heroic study in which he typed an incredibly large number of different genes from many chimpanzees (Langergraber, Mitani, & Vigilant 2007). These data gave us a reasonably good idea of who was related to whom. We combined those data with my behavioral observations to show that kinship matters. Adult male chimpanzees who were maternal half-siblings affiliated and cooperated with each other more often than did unrelated males. I subsequently used Kevin’s hard-earned genetic data to extend these results to show that male chimpanzees form long-lasting and enduring social bonds with each other (Mitani, 2009). While these results are consistent with the hypothesis that kin selection affects male chimpanzee behavior (see also Morin, Moore, Chakraborty, Jin, Goodall, & Woodruff, 1994), our study also indicated that unrelated individuals cooperate frequently (Langergraber, et al., 2007). In fact, most pairs who cooperated above levels predicted by chance were not closely related. In addition, we found no evidence that paternal half siblings cooperated more than did unrelated dyads, a finding reinforced by the recent fission of the Ngogo community (see below).
Chimpanzee hunting behavior
Our studies at Ngogo have furnished additional insights into other aspects of chimpanzee behavior, including their hunting behavior. As is the case at other chimpanzee field sites, red colobus monkeys are the favored prey of the Ngogo chimpanzees (Mitani & Watts, 1999; Watts & Mitani, 2002). The Ngogo chimpanzees hunt these monkeys with remarkable success, capturing them over 80% of the time attempts are made. Multiple kills are typically made during each successful hunt, and as our study continued, it became obvious to even a casual observer that the number of red colobus monkeys at Ngogo were dwindling. We used census data compiled by Tom Struhsaker in the 1970’s and compared his counts of red colobus monkeys (Procolobus rufomitratus) at Ngogo to those made through the early 2000’s. We found that the monkeys had suffered a precipitous 80 – 90% decline in numbers over the years (Lwanga, Struhsaker, Struhsaker, Butynski, & Mitani, 2011). As the number of potential red colobus prey has plummeted, the Ngogo chimpanzees have shifted their hunting efforts to other species (Watts & Mitani, 2015).
We have also used our observations of the Ngogo chimpanzees to address two age-old questions about chimpanzee hunting behavior, namely why they hunt and why they share meat. In the first systematic study of chimpanzee hunting behavior, Geza Teleki (1973) made the reasonable suggestion that chimpanzees hunt when they are hungry. Reasoning that chimpanzees are frugivores living in a seasonal environment where fruit availability fluctuates, Teleki hypothesized that chimpanzees hunt to compensate for the nutritional shortfall they suffer during periods of low fruit availability. In our work, however, we showed that chimpanzees do just the opposite; they hunt during periods of high fruit availability (Mitani & Watts, 2001).
We also tested a hypothesis with which I was familiar having worked with Nishida, who several years before had proposed that male chimpanzees share meat selectively with other males to develop and maintain social bonds (Nishida, Hasegawa, Hayaki, Takahata, & Uehara, 1992). Our observations of the Ngogo chimpanzees were largely consistent with this hypothesis. Male chimpanzees at Ngogo share meat non-randomly with each other, they do so reciprocally, and most importantly, males exchange meat for coalitionary support (Mitani & Watts, 2001).
Chimpanzee territoriality
Our studies of the Ngogo chimpanzees have also shed light on chimpanzee territorial behavior, leading me full circle back to my first paper on primate territoriality. Like many other primates, chimpanzees are territorial. Interactions between neighboring groups are typically hostile. Occasionally, these hostilities escalate and an individual is killed, as male chimpanzees make lethal coalitionary attacks on their neighbors (e.g. Watts, Muller, Amsler, Mbabazi, & Mitani, 2006).
Boundary patrol behavior is an integral part of chimpanzee territoriality. This is one of the most distinctive aspects of chimpanzee behavior, with individuals, typically male, gathering together to move to the boundary of their range. As they do so, their behavior changes drastically. All of the chimpanzees, who are usually noisy and boisterous, fall completely silent. They start to scan the environment and sniff the ground. Patrollers will often make a deep incursion into the territories of their neighbors. The chimpanzees appear to be seeking signs, if not contact, with their neighbors while on patrol.
We have spent considerable time documenting several aspects of boundary patrols, including who participates, what transpires, how long they last, when they occur, and why they occur (Watts & Mitani, 2001; Mitani & Watts, 2005; Amsler, 2010; Mitani, Amsler, & Watts, 2010; Sobowlewski, 2011; Sobolewski, Brown, & Mitani, 2012; Langergraber, Watts, Vigilant, & Mitani, 2017). With regard to the question of who participates, we have recently addressed an issue that has puzzled us for a long time (see Watts & Mitani, 2001). Male chimpanzees do not participate equally (Langergraber et al., 2017). Some males patrol quite frequently, while others do so far less often. This observation presents a classic cooperation or collective action problem, as defined several years ago by the economist Mancur Olson (1965). Olson proposed that collective action problems such as the ones posed by boundary patrols could be solved by what he called the “exploitation of the great by the small.” His idea was that some individuals are likely to be motivated to provide the collective good, in this case defense of the territory, because they stand the most to gain or are in a good position to pay the costs of cooperation. Our observations support this hypothesis (Langergraber et al., 2017). Males who have fathered the most infants and therefore have a strong interest in protecting the territory, not only for themselves but their offspring, patrol more frequently than do males who have sired few infants. In addition, high ranking males, who are likely to be in good physical condition and can thus pay the energetic costs of patrols (Amsler 2010), do so often.
Our studies of chimpanzee territorial behavior have also shed light on why males patrol and kill their neighbors. Since 1999, we have observed the Ngogo chimpanzees kill over 30 of their neighbors (Ngogo Chimpanzee Project unpublished data). During the early part of our study, most of the victims were from a group that inhabits an area to the northeast of the Ngogo chimpanzee territory. After killing 13 individuals from this group, the Ngogo chimpanzees had reduced the coalitionary strength of their neighbors considerably. After doing so, the Ngogo chimpanzees expanded their territory into an area once previously occupied by their neighbors (Mitani et al., 2010). The area of expansion was 6.4 km,2 representing a 22% increase in the size of the Ngogo chimpanzee territory.
Chimpanzee life history
Our long-term study of the unusually large group of chimpanzees at Ngogo gives us an opportunity to address some important questions about what makes us human. For instance, our extremely slow life histories, with individuals growing up slowly, giving birth for the first time late in life, and living a long time, has been suggested to be uniquely human (e.g. Kaplan, Hill, Lancaster, & Hurtado, 2000). Working together with Brian Wood, however, we have recently shown that chimpanzee survivorship across the life course differs dramatically at Ngogo compared to chimpanzees living elsewhere (Wood, Watts, Langergraber, & Mitani, 2017). In fact, the survivorship curve for the Ngogo chimpanzees resembles that of humans living in natural fertility populations more closely than it does those for chimpanzees at different sites (Wood, Watts, Langergraber, & Mitani, 2017). These results close the gap between chimpanzees and us with respect to how we live our lives.
Group fission
Group fission occurs in many primate species (e.g. Dittus, 1988; van Horn, Buchan, Altmann, & Alberts, 2007; Cords, 2012; Perry, Godoy, & Lammers, 2012), and in what might be the most surprising and unexpected finding of our study of the Ngogo chimpanzees, this community has recently fissioned. The fission occurred gradually with aggression between members of the two groups starting in 2015. Aggressive interactions continued for the next three years, although some males continued to move between the two incipient groups, spending time with each other in close proximity and grooming. The split was marked by males from one of the groups killing a young adult male from the other group in 2018. Since that time, males of the two groups have not associated in temporary subgroups or parties (sensu Sugiyama, 1968), and affiliative social interactions between them no longer occur. Instead, interactions between members of the two groups are aggressive and similar to the hostile intergroup encounters observed between them and their other neighboring groups.
The split that we have observed is complicated because it involves demographic, ecological, genetic, and social factors. Briefly, an unprecedented increase in the size of the community (Figure 1), fueled by a secular increase in the food supply (Potts, Watts, Langergraber, & Mitani, in press) and access to more resources due to forest regeneration (Lwanga, 2003), is likely to have led to the group fission. The territorial expansion in 2009 (Mitani et al., 2010) possibly contributed as well. Social and additional demographic factors, including the unusual male subgrouping behavior of chimpanzees (Mitani & Amsler, 2003), increased male mating competition, and the opportunity for males of one of the communities to increase the operational sex ratio (sensu Emlen & Oring, 1977), might also have played a role in the split. As is the case in some fission events in other primate species (e.g. Dittus, 1988; van Horn, Buchan, Altmann, & Alberts, 2007), male chimpanzees (the philopatric sex) at Ngogo have largely split along lines of maternal kinship, although there are a few notable exceptions (cf. Cords, 2012; Perry, Godoy, & Lammers. 2012). Many males are now fighting with their paternal brothers and fathers. We will address these and other issues in the future.
Figure 1.
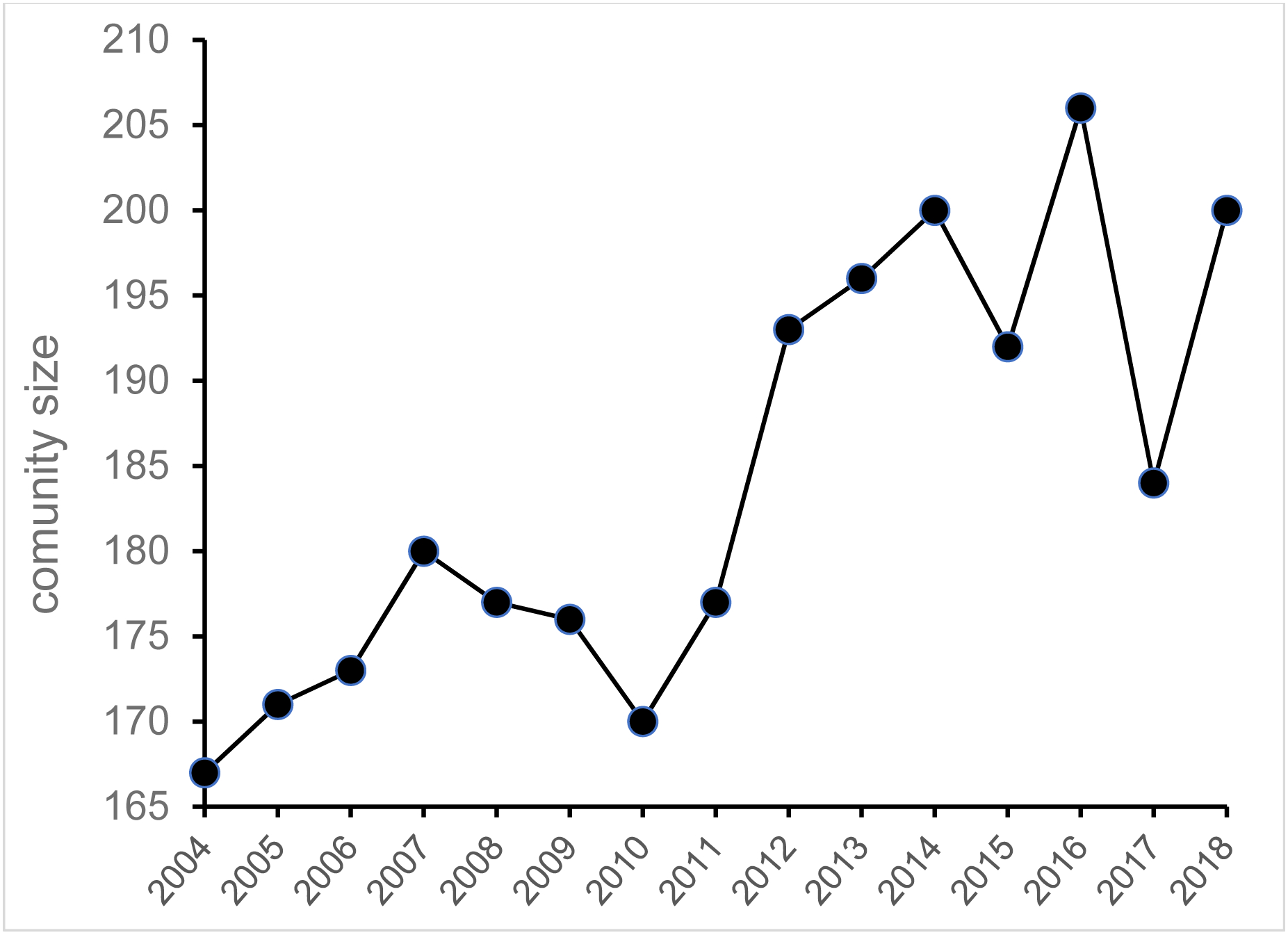
Increase in the Ngogo chimpanzee community size over 15 years between 2004 – 2018.
CONSERVATION
I would be remiss if I failed to comment on the conservation status of the apes. Apes are in decline everywhere (Estrada et al., 2017). All five types of apes are classified as endangered by the IUCN. The reasons for this have been well known for a long time (Mittermeier & Cheney, 1987). Habitat destruction due to the hand of man, hunting, and recurrent outbreaks of infectious continue to decimate populations of wild apes in Asia and Africa. Here I highlight two issues, what we do know and what we don’t know, that complicate efforts to conserve populations of wild apes.
I have conducted fieldwork for a long time, and during the past 40 years there has been a proliferation in the number of recognized species of primates. The ground-breaking book Primate Societies published in 1987 recognized 172 species of primates. In contrast, when my colleagues and I put together a follow up volume, The Evolution of Primate Societies 25 years later in 2012, the number of primate species had more than doubled to 404. There are good biological reasons for splitting primates into different species as new information becomes available, some of which is related to justifiable conservation issues. I fear, however, that this exercise will backfire if it is not supported by scientific evidence. My work with gibbons illustrates matters.
When I studied gibbons over 30 years ago, there were only 5 – 9 recognized species of gibbons. That number now stands at 18. I worked with one species that has been split, the former agile gibbon in southwestern Borneo (Mitani, 1987a, b, 1988). It has now been reclassified and renamed Hylobates albibaris, the Bornean white-bearded gibbon (Groves 2001). In the 1980’s when I worked with Hylobates agilis, it had a curious biogeographical distribution with members on the islands of Borneo and Sumatra as well continental Asia on the Malaysian peninsula. This distribution, however, makes sense given the geological history of the area, with these present-day disparate areas linked in the past (Hall 1998). To determine whether gibbons living in these different locations considered themselves to be the same or different, I asked them using the experimental playback technique I had implemented in other studies (Mitani, 1987b). The goal was to determine whether male song played a role as a species-isolating mechanism, as it does in other animals such as songbirds (e.g. Payne, 1986; Grant & Grant, 2010).
For these experiments I recorded calls made by male agile gibbons in south Sumatra. I also had recordings of what were then recognized as agile gibbons in southwestern Borneo from Gunung Palung. In addition, I had a library of songs sung by males of an entirely different species, Müller’s gibbon, by virtue of prior research elsewhere on Borneo (Mitani, 1985a). I played these songs to a target population of males at Gunung Palung and found that they failed to discriminate the songs of males from Gunung Palung and south Sumatra (Mitani, 1987b). They reacted differently, however, to the songs of the other species, Müller’s gibbon (Mitani, 1987b). These results indicate that when asked themselves, the gibbons believed that members of the southwestern Borneo and south Sumatra populations were the same. This example indicates that we should base taxonomic classifications on sound scientific evidence. Splitting primates into more species in an effort to conserve them will fail if valid biological reasons do not exist to justify new taxonomic designations.
I have been fortunate to have worked at Ngogo in Kibale National Park, Uganda. Primate field research in Kibale was initiated 50 years ago in 1971 by Tom Struhsaker, who conducted pioneering research on red colobus and redtail monkeys (Struhsaker, 1975, 1997). Kibale Park is home to several primate species, who live together at very high densities. Tom’s initial efforts put Kibale on the map and made it a world-renowned primate research site. Over the years, many researchers have flocked there to conduct research on the ecology, behavior, and conservation of primates. With so many people in the area for so long, we should know how many chimpanzees live in the Park. Nevertheless, we had no idea until recently.
The reason for this state of affairs is not hard to understand. Chimpanzees live at very low densities wherever they are found and range over extremely large areas. Happily, the question of how many chimpanzees reside in Kibale is now being determined through some innovative and hard work by Kevin Langergraber. Using a novel genetic capture-recapture methodology, Kevin has shown that there are many more chimpanzees in Kibale than anyone would have ever imagined (Langergraber, unpublished data). Many of them are likely to live in communities that exceed 100 individuals. This is an astonishing result given the fact that so many researchers have been working in the Park for such a long time.
When it comes to the fundamental questions about where chimpanzees live and at what densities, we often lack basic information. Yet without these data, it will be impossible to design and implement effective conservation strategies. At the end of the day, we have a lot of work to do. It will require time, effort, and boots on the ground.
CONCLUDING COMMENTS
The preceding makes clear that my career has taken many twists and turns and that I have simply followed the path made available to me. What can be learned from this, and is there anything worthwhile to pass on to those starting their own primate fieldwork?
I began this paper by noting that my story emphasizes the role that serendipity plays in science. I could claim that my entire career was planned, but that would not be true. As scientists we are trained to design and execute careful, controlled studies, but more often than not, things do not work out. So my first piece of advice is: be opportunistic and follow the leads your animals provide.
Second, some might view my career path as odd. I started as an experimentalist, but morphed into a simple observer of the natural world. There is a great deal we can learn from both approaches. For example, there are some things we cannot address without experimentation because implementing controls and excluding extraneous confounds are essential to test some hypotheses rigorously. This applies to work conducted in the lab as well as the field. Alternatively, documenting the behavior of wild animals reveals what they have been “designed” to do during the course of evolution and provides information required to design ecologically valid experiments. In sum, both approaches are necessary if we want to develop a complete understanding of the behavior of our subjects (sensu Tinbergen, 1963). This realization could have helped dampen some of the needless controversy between psychologists and ethologists that ushered in the modern study animal behavior (see Lehrman 1953; Lorenz 1965).
My own transition from conducting field experiments to observing animals was due to three factors. First, although I was learning a few things about the behavior of gibbons and orangutans by conducting experiments, I started to worry whether the payoffs offset the potential costs. This is because my experimental interventions disrupted the lives of the animals in important ways. My growing ethical concerns about what I was doing made me rethink matters. Second, I noted previously that I made a calculated decision to devote more time to watching animals during my transition to chimpanzee fieldwork. My early studies of orangutans taught me the importance of collecting behavioral observations. Toshisada Nishida reinforced this commitment by showing me how much we can learn by paying close attention to what animals actually do. At the end of the day, my decision to make the switch paid off scientifically and personally. With regard to the latter, there is nothing that gives me greater pleasure than spending long hours with chimpanzees, watching them, and letting them tell me their stories. Those stories often reveal themselves only after many years because chimpanzees and our other primate subjects live a long time and reproduce slowly. It is immensely satisfying, though, when that rare moment occurs, and they give up a few secrets of their lives to us. As a consequence, my second piece of advice is to spend time with your animals and watch them closely. They are bound to inform, as well as delight and surprise.
Finally, I have been lucky. I have been to places and seen and done things that most people can only dream about. Along the way, I have had the great and good fortune to be helped by many mentors, friends, and colleagues. And I have also been generously supported by several public and private funding agencies. While I have been lucky, all others who belong to this profession are lucky too. We form a small collective that has been given a chance to study a remarkable group of animals, our closest living relatives, the primates. I have studied the cooperative behavior of male chimpanzees for the past several years, and one thing I have learned has been obvious from the start: the collective is stronger when everyone contributes. With this in mind, I urge you to work for the collective that is primatology to ensure that others in the future will continue to have the same life-altering chances that we have been given.
Acknowledgments
This paper was based on a talk presented at the “Pioneers in Primatology” symposium at the 2019 annual meeting of the American Society of Primatologists in Madison, Wisconsin. I thank Sarah Brosnan for inviting me to give this talk, and I am grateful to her, Marilyn Norconk, and Karen Strier for soliciting this review. As revealed in this paper, I owe a debt to many mentors, colleagues, collaborators and friends. There are too many to list individually, but Kevin Langergraber, Peter Rodman, Barb Smuts, Tom Struhsaker, David Watts, and the late Jeremiah Lwanga, Peter Marler, and Toshisada Nishida require mention. Sarah Brosnan, Peter Rodman, Aaron Sandel, and Karen Strier reviewed the paper. I thank them for their comments, but I take full responsibility for any errors, omissions, and faulty recollections. My fieldwork in Indonesia, Tanzania, and Uganda has been sponsored by the Indonesian Institute of Sciences, the Indonesian Directorate of Nature Conservation and Wildlife Management, the Indonesian National Biological Institute, the Indonesian Association of Zoological Parks, the Tanzania Commission for Science and Technology, the Serengeti Wildlife Research Institute, the Uganda Wildlife Authority, the Ugandan National Council for Science and Technology, and Makerere University Biological Field Station. I am indebted to the many public and private funding agencies that have generously supported my field research. They include the Detroit Zoological Society, Discovery International, Harry Frank Guggenheim Foundation, L.S.B. Leakey Foundation, National Academy of Sciences, National Geographic Society, National Institutes of Health (F32 NS07670 and RO1AG049395), National Science Foundation (BNS-8022764, BNS-8919726, BNS-9021682, SBR-9253590, BCS-0256622, and IOB-0516644), Silverback Films, University of California, Davis, University of Michigan, and the Wenner-Gren Foundation for Anthropological Research. I have no conflicts of interest to declare. I dedicate this paper to my wife, Sally, who makes everything worthwhile and possible.
REFERENCES
- Amsler SJ (2010). Energetic costs of territorial boundary patrols by wild chimpanzees. American Journal of Primatology, 72, 93–103. DOI: 10.1002/ajp.20757 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T & Harvey P (1976). Evolutionary rules and primate societies. In Bateson P & Hinde R (Eds.), Growing Points in Ethology (pp. 195–238). Cambridge: Cambridge University Press. [Google Scholar]
- Clutton-Brock T & Parker G (1995). Sexual coercion in animal societies. Animal Behaviour, 49, 1345–1365. DOI: 10.1006/anbe.1995.0166 [DOI] [Google Scholar]
- Cords M (2012). The 30-Year Blues: What We Know and Don’t Know About Life History, Group Size, and Group Fission of Blue Monkeys in the Kakamega Forest, Kenya. In: Kappeler P (Ed.), Long-term Field Studies of Primates (pp. 289–311). Heidelberg: Springer. [Google Scholar]
- Coye C, Zuberbühler K, & Lemasson A (2016). Morphologically structured vocalizations in female Diana monkeys, Animal Behaviour, 115, 97–105. DOI: 10.1016/j.anbehav.2016.03.010 [DOI] [Google Scholar]
- DeVore I (1972). Quest for the roots of society. In Marler P (Ed.), The Marvels of Animal Behavior (pp.. 393–408). Washington, D.C.: National Geographic Society. [Google Scholar]
- Dittus W (1988). Group fission among wild toque macaques as a consequence of female resource competition and environmental stress. Animal Behaviour, 36, 1626–1645. DOI: 10.1016/S0003-3472(88)80104-0 [DOI] [Google Scholar]
- Emlen S & Oring L (1977). Ecology, sexual selection and the evolution of mating systems. Science, 197, 215–223. DOI: 10.1126/science.327542 [DOI] [PubMed] [Google Scholar]
- Estrada A, Garber P, Rylands A, Roos C, Fernandez-Duque E, Di Fiore A, …Li B (2017). Impending extinction crisis of the world’s primates: Why primates matter. Science Advances, 3: e1600946. DOI: 10.1126/sciadv.1600946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedurek P, Schel A, & Slocombe K (2013). The acoustic structure of chimpanzee pant-hooting facilitates chorusing. Behavioral Ecology and Sociobiology, 67, 1781–1789. DOI: 10.1007/s00265-013-1585-7 [DOI] [Google Scholar]
- Fedurek P, Machanda Z, Schel A, & Slocombe K (2013). Pant hoot chorusing and social bonds in male chimpanzees. Animal Behaviour, 86, 189–196. DOI: 10.1016/j.anbehav.2013.05.010 [DOI] [Google Scholar]
- Ghiglieri M (1984). The Chimpanzees of the Kibale Forest. New York:Columbia University Press. [Google Scholar]
- Gouzoules S, Gouzoules H, & Marler P (1984). Rhesus monkey (Macaca mulatta) screams: Representational signaling in the recruitment of agonistic aid. Animal Behaviour, 32, 182–193. DOI: 10.1016/S0003-3472(84)80336-X [DOI] [Google Scholar]
- Grant R & Grant P (2010). Songs of Darwin’s finches diverge when a new species enters the community. Proceedings of the National Academy of Sciences, 107, 20156–20163. DOI: 10.1073/pnas.101511510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves C (2001). Primate Taxonomy. Washington, D.C.: Smithsonian Institution Press. [Google Scholar]
- Gyger M, Karakashian S, & Marler P (1986). Avian alarm calling: is there an audience effect? Animal Behaviour, 34, 1570–1572. DOI: 10.1016/S0003-3472(86)80229-9 [DOI] [Google Scholar]
- Hall R (1998). The plate tectonics of Cenozoic SE Asia and the distribution of land and sea. In Hall R and Holloway JD (Eds.), Biogeography and Geological Evolution of SE Asia. (pp. 99–131). Leiden: Backhuys Publishers. [Google Scholar]
- Hamburg D & McCown E (1979). The Great Apes. Menlo Park, CA: Benjamin Cummings. [Google Scholar]
- Hamilton WD (1963). The evolution of altruistic behavior. American Naturalist, 97, 354–356. DOI: 10.1086/49711 [DOI] [Google Scholar]
- Hauser M & Marler P 1993. Food-associated calls in rhesus macaques (Macaca mulatta): I. Socioecological factors. Behavioral Ecology, 4, 194–205. DOI: 10.1093/beheco/4.3.194 [DOI] [Google Scholar]
- Horr DA (1975). The Borneo orangutan: population structure and dynamics in relationship to ecology and reproductive strategy. In Rosenblum L (Ed.), Primate Behavior: Developments in Field and Laboratory Research (Volume. 4). (pp. 307–323). New York: Academic Press. [Google Scholar]
- Hubby J & Lewontin R (1966). A molecular approach to the study of genic heterozygosity in natural populations. I. The number of alleles at different loci in Drosophila pseudoobscura. Genetics 54, 577–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H, Hill K, Lancaster J, & Hurtado AM (2000). A theory of human life history evolution: Diet, intelligence, and longevity. Evolutionary Anthropology, 4, 156–185. DOI: [DOI] [Google Scholar]
- Knott C (1998). Changes in orangutan diet, caloric intake, and ketones in response to fluctuating fruit availability. International Journal of Primatology, 19, 1061–1079. DOI: 10.1023/A:1020330404983 [DOI] [Google Scholar]
- Langergraber KE, Mitani J, & Vigilant L (2007). The limited impact of kinship on cooperation in wild chimpanzees. Proceedings of the National Academy of Sciences, 104, 7786–7790. DOI: 10.1073/pnas.0611449104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langergraber K, Watts D, Vigilant L & Mitani J (2017). Group augmentation, collective action, and territorial boundary patrols by male chimpanzees. Proceedings of the National Academy of Science, 114, 7337–7342. DOI: 10.1073/pnas.1701582114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman D (1953). A critique of Konrad Lorenz’s theory of instinctive behavior. Quarterly Review of Biology, 28, 337–368. DOI: 10.1086/399858 [DOI] [PubMed] [Google Scholar]
- Leighton M & Leighton D (1983). Vertebrate responses to fruiting seasonality within a Bornean tropical rainforest. In Sutton S, Whitmore T, & Chadwick A (Eds.), Tropical Rainforests: Ecology and Management (pp. 181–196). Oxford: Blackwell Scientific. [Google Scholar]
- Lorenz K (1965). Evolution and the Modification of Behavior. Chicago: University of Chicago Press. [Google Scholar]
- Lukas D & Clutton-Brock T (2013). The evolution of social monogamy in mammals. Science, 341, 526–530. DOI: 10.1126/science.1238677 [DOI] [PubMed] [Google Scholar]
- Lwanga JS (2003). Forest succession in Kibale National Park, Uganda: implications for forest restoration and management. African Journal of Ecology, 41, 9–22. DOI: 10.1046/j.1365-2028.2003.00428.x [DOI] [Google Scholar]
- Lwanga JS, Struhsaker T, Struhsaker P, Butynski T & Mitani J (2011). Primate population dynamics over 32.9 years at Ngogo, Kibale National Park, Uganda. American Journal of Primatology, 73, 997–1011. DOI: 10.1002/ajp.20965 [DOI] [PubMed] [Google Scholar]
- MacKinnon J (1974). The behaviour and ecology of wild orangutans (Pongo pygmaeus). Animal Behaviour, 22, 3–74. DOI: 10.1016/S0003-3472(74)80054-0 [DOI] [Google Scholar]
- Marler P (1967). Animal communication signals. Science, 157, 769–774. DOI: 10.1126/science.157.3790.769 [DOI] [PubMed] [Google Scholar]
- Marler P (1969). Colobus guereza: Territoriality and group composition. Science, 163, 93–95. DOI: 10.1126/science.163.3862.93 [DOI] [PubMed] [Google Scholar]
- Marler P (1969). Vocalizations of wild chimpanzees: an introduction. In Carpenter CR (Ed.), Proceedings of the Second International Congress of Primatology. Volume 1. (pp. 94–100). Basel, Switzerland: Karger. [Google Scholar]
- Marler P (1970). Birdsong and speech development: could there be parallels? American Scientist, 58, 669–673. [PubMed] [Google Scholar]
- Marler P (1973). A comparison of vocalizations of red-tail monkeys and blue monkeys, Cercopithecus ascanius and C. mitis, in Uganda. Zeitschrift fur Tierpsychologie, 33, 223–247. [DOI] [PubMed] [Google Scholar]
- Marler P (1981). Birdsong: The acquisition of a learned motor skill. Trends in Neuroscience, 4, 88–94. DOI: 10.1016/0166-2236(81)90029-1 [DOI] [Google Scholar]
- Marler P (1989). Learning by instinct: birdsong. American Speech-Language-Hearing, 31, 75–79. [PubMed] [Google Scholar]
- Marler P; Hobbett L (1975). Individuality in a long-range vocalization of wild chimpanzees. Zeitshcrift fur Tierpsychologie, 38, 97–109. [PubMed] [Google Scholar]
- Marler P & Tamura M (1964). Culturally transmitted patterns of vocal behavior in sparrows. Science, 146, 1483–1486. DOI: 10.1126/science.146.3650.1483 [DOI] [PubMed] [Google Scholar]
- McGrew W, Marchant L & Nishida T (1996). Great Ape Societies. Cambridge: Cambridge University Press. [Google Scholar]
- Mitani J (1984). The behavioral regulation of monogamy in gibbons (Hylobates muelleri). Behavioral Ecology and Sociobiology, 15, 225–229. DOI: 10.1007/BF00292979 [DOI] [Google Scholar]
- Mitani J (1985a). Gibbon song duets and intergroup spacing. Behaviour, 92, 59–96. DOI: 10.1163/156853985X00389 [DOI] [Google Scholar]
- Mitani J (1985b). Mating behavior of male orangutans in the Kutai Game Reserve, Indonesia. Animal Behaviour, 33, 392–402. DOI: 10.1016/S0003-3472(85)80063-4 [DOI] [Google Scholar]
- Mitani J (1985c). Sexual selection and adult male orangutan long calls. Animal Behaviour, 33, 272–283. DOI: 10.1016/S0003-3472(85)80141-X [DOI] [Google Scholar]
- Mitani J (1987a). Territoriality and monogamy among agile gibbons (Hylobates agilis). Behavioral Ecology and Sociobiology, 20, 265–269. DOI: 10.1007/BF00292179 [DOI] [Google Scholar]
- Mitani J (1987b). Species discrimination of male song in gibbons. American Journal of Primatology, 13, 413–423. DOI: 10.1002/ajp.1350130406 [DOI] [PubMed] [Google Scholar]
- Mitani J (1988). Male gibbon singing behavior (Hylobates agilis): Natural history, song variations and function. Ethology, 79:177–194. [Google Scholar]
- Mitani J (1990). Experimental field studies of Asian ape social systems. International Journal of Primatology, 11, 103–126. DOI: 10.1007/BF02192784 [DOI] [Google Scholar]
- Mitani J (1996). Comparative field studies of African ape vocal behavior. In McGrew W, Marchant L, & Nishida T (Eds.), Great Ape Societies. (pp. 241–254). Cambridge: Cambridge University Press. [Google Scholar]
- Mitani J (2009). Male chimpanzees form enduring and equitable social bonds. Animal Behaviour, 77, 633–640. DOI: 10.1016/j.anbehav.2008.11.021 [DOI] [Google Scholar]
- Mitani J (2011). Toshisada Nishida: colleague, mentor, collaborator, and friend. Primates, 52, 303–304. DOI: 10.1007/s10329-011-0262-6 [DOI] [Google Scholar]
- Mitani J & Amsler S (2003). Social and spatial aspects of male subgrouping in a community of wild chimpanzees. Behaviour, 140, 869–884. DOI: 10.1163/156853903770238355 [DOI] [Google Scholar]
- Mitani J & Gros-Louis J (1998). Chorusing and call convergence in chimpanzees: Tests of three hypotheses. Behaviour, 135, 1041–1064. DOI: 10.1163/156853998792913483 [DOI] [Google Scholar]
- Mitani J & Marler P. (1989). A phonological analysis of male gibbon singing behavior. Behaviour, 109.5, 20–45. DOI: 10.1163/156853989X00141 [DOI] [Google Scholar]
- Mitani J & Nishida T. (1993). Contexts and social correlates of long distance calling by male chimpanzees. Animal Behaviour, 45, 735–746. DOI: 10.1006/anbe.1993.1088 [DOI] [Google Scholar]
- Mitani J & Rodman PS (1979). Territoriality: The relationship of ranging pattern and home range size to defendability, with an analysis of territoriality in primates. Behavioral Ecology and Sociobiology, 5, 241–251. DOI: 10.1007/BF00293673 [DOI] [Google Scholar]
- Mitani J & Watts D (1997). The evolution of non-maternal caretaking in anthropoid primates: Do helpers help? Behavioral Ecology and Sociobiology, 40, 213–220. DOI: 10.1007/s002650050335 [DOI] [Google Scholar]
- Mitani J & Watts D (1999). Demographic influences on the hunting behavior of chimpanzees. American Journal of Physical Anthropology, 109, 439–454. DOI: [DOI] [PubMed] [Google Scholar]
- Mitani J & Watts D (2001). Why do chimpanzees hunt and share meat? Animal Behaviour, 61, 915–924. DOI: 10.1006/anbe.2000.1681 [DOI] [Google Scholar]
- Mitani J and Watts D (2005). Correlates of territorial boundary patrol behavior in wild chimpanzees. Animal Behaviour, 70, 1079–1086. DOI: 10.1016/j.anbehav.2005.02.012 [DOI] [Google Scholar]
- Mitani J, Gros-Louis J & Manson J (1996). The number of males in primate groups: Comparative tests of competing hypotheses. American Journal of Primatology, 38, 315–332. DOI: [DOI] [PubMed] [Google Scholar]
- Mitani J, Gros-Louis J, & Richards A (1996). Sexual dimorphism, the operational sex ratio, and the intensity of male competition among polygynous primates. American Naturalist, 147, 966–980. DOI: 10.1086/285888 [DOI] [Google Scholar]
- Mitani J, Hunley K & Murdoch ME (1999). Geographic variation in the calls of wild chimpanzees: A re-assessment. American Journal of Primatology, 47, 133–152. DOI: [DOI] [PubMed] [Google Scholar]
- Mitani J, Hasegawa T, Gros-Louis J, Marler P, & Byrne R (1992). Dialects in wild chimpanzees? American Journal of Primatology, 27, 233–243. DOI: 10.1002/ajp.1350270402 [DOI] [PubMed] [Google Scholar]
- Mitani J, Grether GF, Rodman PS & Priatna D (1991). Associations among wild orangutans: sociality, passive aggregations or chance? Animal Behaviour, 42, 33–46. DOI: 10.1016/S0003-3472(05)80603-7 [DOI] [Google Scholar]
- Mitani J, Merriwether DA, & Zhang C (2000). Male affiliation, cooperation, and kinship in wild chimpanzees. Animal Behaviour, 59, 885–893. DOI: 10.1006/anbe.1999.1389 [DOI] [PubMed] [Google Scholar]
- Mitani J, Watts D, Pepper J, & Merriwether DA (2002). Demographic and social constraints on male chimpanzee behaviour. Animal Behaviour, 63, 727–737. DOI: 10.1006/anbe.2002.4014 [DOI] [Google Scholar]
- Mitani J, Watts D, & Amsler S (2010). Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Current Biology, 20, R507–R508. DOI: 10.1016/j.cub.2010.04.021 [DOI] [PubMed] [Google Scholar]
- Mitani J, de Waal F, Hosaka K, McGrew W, Nakamura M, Nishimura A, Wrangham R, & Yamigiwa J (2012). Obituary: Toshisada Nishida. International Journal of Primatology 33, 10–18. DOI: 10.1007/s10764-011-9571-2 [DOI] [Google Scholar]
- Mittermeier R & Cheney D (1987). Conservation of primates and their habitats. In Smuts B, Cheney D, Seyfarth R, Struhsaker T, & Wrangham R (Eds.), Primate Societies (pp. 477–490). Chicago: University of Chicago Press. [Google Scholar]
- Morin P, Moore J, Chakraborty R, Jin L, Goodall J, & Woodruff D (1994). Kin selection, social structure, gene flow, and the evolution of chimpanzees. Science, 265, 1145–1332. DOI: 10.1126/science.7915048 [DOI] [PubMed] [Google Scholar]
- Muller M, Kahlenberg S,, Emery Thompson M, & Wrangham R (2007). Male coercion and the costs of promiscuous mating for female chimpanzees. Proceedings of the Royal Society B – Biological Sciences, 274, 1009–1014. DOI: 10.1098/rspb.2006.0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T (1968). The social group of wild chimpanzees in the Mahale Mountains. Primates, 9, 167–224. [Google Scholar]
- Nishida T, Hasegawa T, Hayaki H, Takahata Y & Uehara S (1992). Meat-sharing as a coalition strategy by an alpha male chimpanzee? In Nishida T, McGrew W, Marler P, Pickford M & deWaal F (Eds.), Topics in Primatology. Volume 1. Human Origins (pp. 159–174). Tokyo: Tokyo University Press. [Google Scholar]
- O’Connell C, Susanto T, & Knott C (2019). Sociosexual behavioral patterns involving nulliparous female orangutans (Pongo sp.) reflect unique challenges during the adolescent period, American Journal of Primatology, DOI: 10.1002/ajp.23058 [DOI] [PubMed] [Google Scholar]
- Olson M (1965). The Logic of Collective Action. Cambridge, MA: Harvard University Press. [Google Scholar]
- Ouattara K, Lemasson A, & Zuberbühler K (2009). Cambell’s monkeys use affixation to alter call meaning, PLoS ONE, 4, e7808. DOI: 10.1371/journal.pone.0007808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombit R (2014). Sexual conflict in nonhuman primates. Advances in the Study of Behavior, 46, 191–280. DOI: 10.1016/B978-0-12-800286-5.00005-5 [DOI] [Google Scholar]
- Patterson S, Sandel A, Miller J, Mitani J 2014. Data quality and the comparative method: the case of primate group size. International Journal of Primatology, 35, 990–1003. DOI: 10.1007/s10764-014-9777-1 [DOI] [Google Scholar]
- Payne R (1986). Bird songs and avian systematics. In Johnston R (Ed.), Current Ornithology Volume 3 (pp. 87–126). New York: Plenum Press. [Google Scholar]
- Perry S, Godoy I, & Lammers W (2012). The Lomas Barbudal Monkey Project: two decades of research on Cebus capucinus. In: Kappeler P (Ed.), Long-term Field Studies of Primates (pp.141–163). Heidelberg: Springer. [Google Scholar]
- Potts K, Watts D, Langergraber K, & Mitani J in press. Trends in tree fruiting phenology and their implications for chimpanzees at Ngogo, Kibale National Park, Uganda. Biotropica DOI: 10.1111/btp.12764 [DOI] [Google Scholar]
- Rodman P (1973). Population composition and adaptive organisation among orangutans of the Kutai Reserve. In Michael R & Crook J (Eds.), Comparative Ecology and Behaviour of Primates (pp. 171–209). London: Academic Press. [Google Scholar]
- Rodman P & McHenry H (1980). Bioenergetics and origins of bipedalism. American Journal of Physical Anthropology, 52, 103–106. DOI: 10.1002/ajpa.1330520113 [DOI] [PubMed] [Google Scholar]
- Rodman P & Mitani J (1987). Orangutans: sexual dimorphism in a solitary species. In Smuts B, Cheney D, Seyfarth R, Wrangham R & Struhsaker T (Eds.), Primate Societies (pp. 145–154). Chicago: University of Chicago Press. [Google Scholar]
- Sandel A, Miller J, Mitani J, Nunn C, Patterson S, & Garamszegi L 2016. Assessing sources of error in comparative analyses of primate behavior: Intraspecific variation in group size and the social brain hypothesis. Journal of Human Evolution, 94, 126–133. DOI: 10.1016/j.jhevol.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth RM, Cheney DL, & Marler P (1980). Monkey responses to three different alarm calls: Evidence for predator classification and semantic communication. Science, 210, 801–803. DOI: 10.1016/S0003-3472(80)80097-2 [DOI] [PubMed] [Google Scholar]
- Smuts B & Smuts R (1993). Male aggression and sexual coercion of females in nonhuman primates and other mammals: Evidence and theoretical implications. Advances in the Study of Behavior, 22: 1–63. DOI: 10.1016/S0065-3454(08)60404-0 [DOI] [Google Scholar]
- Sobolewski M, Brown J, & Mitani J (2012). Territoriality, tolerance, and testosterone in wild chimpanzees. Animal Behaviour, 84, 1469–1474. DOI: 10.1016/j.anbehav.2012.09.018 [DOI] [Google Scholar]
- Struhsaker T (1975). The Red Colobus Monkey. Chicago: University of Chicago Press. [Google Scholar]
- Struhsaker T (1997). The Ecology of an African Rainforest. Gainesville, FL: University of Florida Press. [Google Scholar]
- Sugiyama Y (1968). Social organization of chimpanzees in the Budongo Forest, Uganda. Primates, 9, 225–258. [Google Scholar]
- Teleki G (1973). The Predatory Behavior of Wild Chimpanzees. Lewisburg, PA: Bucknell University Press. [Google Scholar]
- Tenaza R (1975). Territory and monogamy among Kloss’ gibbons (Hylobates klossi) in Siberut Island, Indonesia. Folia Primatologica, 24, 60–80. DOI: 10.1159/000155685 [DOI] [PubMed] [Google Scholar]
- Tinbergen N (1958). Curious Naturalists. New York: Basic Books. [Google Scholar]
- Tinbergen N (1963). On the aims and methods of ethology. Zeitschrift für Tierpsychologie 20, 410–433. [Google Scholar]
- van Horn R, Buchan J, Altmann J, Alberts S (2007). Divided destinies: group choice by female savannah baboons during social group fission. Behavioral Ecology and Sociobiology, 61,1823–1837. DOI: 10.1007/s00265-007-0415-1 [DOI] [Google Scholar]
- van Schaik C, Assink P, & Salafsky N (1992). Territorial behavior in Southeast Asian langurs: Resource defense or mate defense? American Journal of Primatology, 26(4), 233–242. DOI: 10.1002/ajp.1350260402 [DOI] [PubMed] [Google Scholar]
- Waser P (1975). Experimental playbacks show vocal mediation of intergroup avoidance in a forest monkey. Nature, 255, 56–58. DOI: 10.1038/255056a0 [DOI] [Google Scholar]
- Watts D (1989). Infanticide in mountain gorillas: New cases and a reconsideration of the evidence. Ethology, 81, 1–18. [Google Scholar]
- Watts D & Mitani J (2001). Boundary patrols and intergroup encounters in wild chimpanzees. Behaviour, 138, 299–327. DOI: 10.1163/15685390152032488 [DOI] [Google Scholar]
- Watts D & Mitani J (2002). Hunting behavior of chimpanzees at Ngogo, Kibale National Park, Uganda. International Journal of Primatology, 23, 1–28. DOI: 10.1023/A:1013270606320 [DOI] [Google Scholar]
- Watts D & Mitani J (2015). Hunting and prey switching by wild chimpanzees at Ngogo. International Journal of Primatology, 36, 728–748. DOI: 10.1007/s10764-015-9851-3 [DOI] [Google Scholar]
- Watts D, Muller M, Amsler S, Mbabazi G & Mitani J (2006). Lethal intergroup aggression by chimpanzees in the Kibale National Park, Uganda. American Journal of Primatology, 68, 161–180. DOI: 10.1002/ajp.20214 [DOI] [PubMed] [Google Scholar]
- Whiten A, Goodall J, McGrew W, Nishida T, Reynolds V, Sugiyama Y, Tutin C, Wrangham R, & Boesch C (1999). Cultures in chimpanzees. Nature, 399, 682–685. DOI: 10.1038/21415 [DOI] [PubMed] [Google Scholar]
- Wilson EO (1975). Sociobiology: The New Synthesis. Cambridge, MA: Belknap Press. [Google Scholar]
- Wood B, Watts D, Mitani J, & Langergraber K (2017). Favorable ecological circumstances promote life expectancy in chimpanzees similar to that of human hunter-gatherers. Journal of Human Evolution, 105, 41–56. DOI: 10.1016/j.jhevol.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]


