Abstract
Neurons release membrane-bound extracellular vesicles (EVs) carrying proteins, nucleic acids, and other cargoes to mediate neuronal development, plasticity, inflammation, regeneration, and degeneration. Functional studies and therapeutic interventions into EV-dependent processes will require a deep understanding of how neuronal EVs are formed and released. However, unraveling EV biogenesis and trafficking mechanisms is challenging, since there are multiple pathways governing generation of different types of EVs, which overlap mechanistically with each other, as well as with intracellular endolysosomal trafficking pathways. Further, neurons present special considerations for EVs due to their extreme morphologies and specialization for membrane traffic. Here, we review recent work elucidating neuronal pathways that regulate EV biogenesis and release, with the goal of identifying directed strategies for experimental and therapeutic targeting of specific types of EVs.
Graphical Abstract
Introduction
EVs are a heterogeneous population of 40–1000 nm membrane compartments that are released from donor cells, and carry diverse protein, nucleic acid, and lipid cargoes to recipient cells [1–4]. An explosion of recent evidence shows that EVs mediate intercellular communication between different cell types in the nervous system, disposal of unwanted neuronal and glial components, and propagation as well as clearance of toxic factors in the brain [5, 6]. Further, since circulating EVs are specific to both donor and recipient cells, they are being developed for the diagnosis and treatment of neurological disease [5–10].
To experimentally interrogate and therapeutically intervene in EV functions, we need well-validated approaches to specifically manipulate EVs in the nervous system. EVs fall into two broad categories: microvesicles, which form via direct budding of cargo-enriched subdomains from the plasma membrane, and exosomes, which arise when endosomal multivesicular bodies (MVBs) fuse with the plasma membrane, releasing their intralumenal vesicles (ILVs). Even within these broad categories, multiple pathways exist for cargo packaging and EV biogenesis, leading to the diversity of released EVs [1–4]. The field has developed rigorous standards for isolating EV subtypes, establishing their functional activities, and defining their biogenesis mechanisms [11–13]. However, several challenges remain. First, different EV biogenesis and release mechanisms share overlapping membrane trafficking machinery, and also intersect with canonical endocytic and endolysosomal transport pathways, making it difficult to design specific manipulations [1–4]. Second, the majority of EV research has been conducted on mixed populations of EVs collected from body fluids, homogenized tissues, and cell culture supernatants. These bulk approaches do not resolve the heterogeneity of EVs, and are poorly suited for understanding the cellular origin and biogenesis mechanisms of EVs derived from complex tissues like the brain [8, 9]. Third, most of our understanding of EV traffic comes from non-neuronal (primarily cancer) cells [3]. Presumably, many of the EV biogenesis and release pathways identified in non-neuronal cell types also function in neurons. However, neurons present unique considerations for EV trafficking due to their extreme morphologies and compartmentalization, and because they are highly specialized for membrane traffic. Open questions resulting from these unique properties include: Where are EVs generated within these highly elongated and compartmentalized cells? How do neuron-specific adaptations of membrane traffic machinery apply to different EV pathways? How is EV traffic controlled acutely and chronically in response to neuronal activity? This review will discuss new insights into neuron-specific EV biogenesis and release pathways. We refer readers to recent reviews on the related topics of EV uptake in recipient cells [4, 14], and distinct cellular mechanisms regulating glial EVs [15, 16].
Populating EV precursor compartments
Cargoes transit to EVs via the plasma membrane and endosomes [1, 17], and a critical step diverting these cargoes from canonical endolysosomal traffic is their accumulation at membranes permissive for EV formation. Neurons add an extra layer of complexity, since these EV precursors could be locally populated with cargo in axons, dendrites, or cell bodies, or trafficked to these locations after they are loaded with cargoes. Pioneering studies at the Drosophila neuromuscular junction (NMJ) identified membrane trafficking mutants, including Rab11 (a GTPase controlling endosome-to-plasma membrane recycling), that decrease EV levels of cargoes such as the signaling protein Evi/Wntless, the calcium sensor Synaptotagmin 4, and the retroviral Gag-like protein Arc [18–21]. Notably, loss of Rab11 also led to decreased levels of at least one of these cargoes (Arc) in the donor presynaptic terminal [21], suggesting that Rab11 could promote cargo loading into an EV-permissive membrane compartment, and/or protect cargo from lysosomal degradation (Fig. 1A). Similarly, in sympathetic neurons, MVB localization and activity-dependent EV release of the p75 neurotrophin receptor correlates with its accumulation in Rab11-positive compartments [22]. In cancer cells, Munc13–4 controls Ca2+-dependent interaction of Rab11-positive endosomes with MVBs, rendering them competent for EV release [23]; it will be interesting to test if similar mechanisms drive activity-dependent release of EVs in neurons. Together, these results suggest that traffic via the recycling endosome populates neuronal EV precursor compartments.
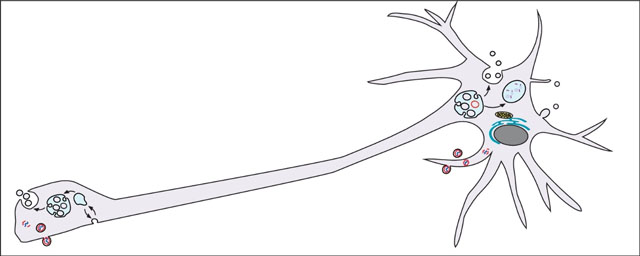
Figure 1. Membrane trafficking pathways controlling neuronal EV biogenesis and release.

Blue text indicates subcellular compartments; Red text indicates membrane traffic machinery; Black text indicates EV cargoes. (A) Packaging and release of EV cargoes at the Drosophila larval neuromuscular junction. (B) Diverse mechanisms for generating ILVs may work together or independently. It remains unknown if distinct EV subtypes form within a single MVB, or in MVBs dedicated to specific cargoes. (C) Alternative pathways for EV release from neurons. Abbreviations: Cargoes (ART: AXL, RAB18, and TMED10 EVs (contain SHH), APP-CTF: Amyloid precursor protein C-terminal fragment, 2-AG: endocannabinoid 2-arachadonoylglycerol, BMP: bis(monoacylglycero)phosphate). Compartments (EE: early endosome, MVB: multivesicular body, ILV: intralumenal vesicle, EV: extracellular vesicle, ER: endoplasmic reticulum).
Generation of intralumenal vesicles in endosomes
Most studies of neuronal EVs suggest that they are derived from the MVB-dependent exosome pathway, which utilizes multiple (possibly overlapping) mechanisms for budding of vesicles into the endosomal lumen [1–4] (Fig. 1B). The best-studied mechanisms rely on Endosomal Sorting Complex Required for Traffic (ESCRT) proteins [24]. In the canonical ESCRT pathway, ESCRT-0, -I, and -II components cluster ubiquitinated cargoes while curving membranes, and then recruit ESCRT-III components, which form a helical polymer that drives fission of the ILV bud into the MVB. The VPS4 ATPase then recycles these filaments [25]. An alternative mechanism for ILV generation bypasses early ESCRT proteins, by linking EV cargoes to ESCRT-III polymers through a complex consisting of ALIX (ALG-2-interacting protein) and syntenin, a cytosolic PDZ domain adaptor protein [26]. Though syntenin has known functions in the nervous system [27], its role in neuronal EVs, via the tetraspanin TSPAN6, has only recently been explored [28]. Overexpression of TSPAN6 in non-neuronal cells increases endosome size and ILV number in a syntenin-dependent manner, and increases the number of EVs containing amyloid precursor protein C-terminal fragments (APP-CTFs). Similarly, in neurons in culture and in vivo, TSPAN6 promotes accumulation of APP fragments [28]. Thus, ESCRT-III cargo adaptors may be valuable and as-yet-unexplored points of manipulation for specific EV trafficking pathways.
Related to this idea, a longstanding question has been why there are so many ESCRT III proteins (e.g. 12 in humans) [25]. An elegant in vivo study in mice [29] demonstrated a specific requirement for the ESCRT-III protein CHMP1A in generating brain EVs containing the morphogen Sonic Hedgehog (SHH). Loss of CHMP1A resulted in aberrant MVBs in the choroid plexus and Purkinje cell layer, reduced SHH release, and therefore diminished proliferation of cerebellar granule cell precursors. Further, knockout of Chmp1a in human iPSC-derived organoids led to loss of progenitors and premature neuron differentiation. Together, these results provide a molecular explanation for why loss of CHMP1A in humans causes microcephaly. Interestingly, SHH EVs were not enriched for the canonical EV markers CD63, CD9, and Tsg101, suggesting that they define a novel EV subtype termed ART-EV (AXL, RAB18, and TMED10 EV). Thus, diverse ESCRT-III filaments may specify unique EV subtypes. Given the existence of multiple distinct EV populations from a single cell type (e.g. CD63 versus APP-EVs in hippocampal neurons [37], CD63/CD9/syntenin versus SHH/ART-EVs in SVG-A cells [29], and Syt4 versus Evi-EVs at the Drosophila NMJ [20]), the question remains whether these cargoes are packaged in distinct ILVs within the same MVB [38], or in dedicated MVBs that could be released independently. Visualization of multiple EV cargoes by super resolution imaging or immunogold electron microscopy may resolve this question.
While ESCRT molecular activities are appealing for understanding ILV formation, ESCRT components are in some cases not necessary for EV release [24]. A lipid-directed pathway, mediated by neutral sphingomyelinase (nSMase), may operate together with or in parallel to ESCRT to generate EVs. nSMase cleaves sphingomyelin to liberate ceramide, which clusters into membrane microdomains that favor inward budding [1, 24, 30]. EV release of many neuronal cargoes is sensitive to nSMase depletion or inhibition; these include Tau [31], α synuclein [32], Prion protein (PrP) [33], CD63 [34], PTEN [35], and microRNAs [34]. In addition, pathological effects of EVs can be suppressed by inhibiting the nSMase pathway [36].
Finally, control of MVB formation depends on competition between the ILV-generating machinery and other cargo-sorting domains of the early endosome, such as those defined by the retromer complex [39]. Reduced ubiquitination of the late endosome GTPase Rab7, in patient-derived cells lacking the Parkinson’s Disease-associated ubiquitin ligase Parkin, leads to dissociation of retromer from endosomes, and increased ILV formation and EV release [40]. Future studies should resolve how ESCRT-directed, lipid-directed, and competing endosomal sorting mechanisms work in concert or independently in MVB formation, while also exploring additional pathways that may be involved (Fig. 1B).
A novel lysosome damage-dependent pathway for EV biogenesis
Due to their extreme morphologies and longevity, neurons face unique challenges in efficiently disposing of unwanted materials [41]. A novel pathway, distinct from that mediating EV release from healthy cells, relieves endolysosomal damage and stress via EV release. Lysosomal damage, induced by inhibition of the phosphatidylinositol-3-kinase Vps34 in neurons in culture and in vivo, results in release of nSMase-dependent neuronal EVs enriched for APP CTFs and the endosomal phospholipid bis(monoacylglycero)phosphate (BMP), which is not normally found in EVs [38]. This effect could be recapitulated by inhibiting lysosome acidification [42], but not by simple accumulation of APP-CTFs, or by inhibiting other Vps34-dependent pathways such as autophagy. Together, these data indicate that this EV release mechanism occurs specifically in response to lysosome dysfunction. However, though endolysosomal damage-induced release may be protective for the donor cell, the released EVs may propagate neurodegenerative-disease-associated pathological proteins to other cells; therefore, EV release through this mechanism could produce competing protective and neurotoxic effects.
Targeting and fusion of MVBs with the neuronal plasma membrane
Several recent studies provide insight into the question of how EV-specific MVBs are targeted for fusion with the plasma membrane rather than the lysosome. In one example, the adaptor muskelin associates with PrP transport carriers, and promotes their retrograde traffic in both axons and dendrites [43]. Loss of muskelin-dependent traffic decreases lysosomal degradation of PrP; instead it accumulates at the plasma membrane and is released in EVs, worsening PrP pathology in vivo. In another example, Rab27a, which promotes MVB fusion with the plasma membrane [44], is protected from ubiquitination and proteasomal degradation by the brain-enriched scaffolding protein KIBRA [45]. In the absence of KIBRA, MVBs are retained intracellularly, and KIBRA knockout mice exhibit fewer brain EVs. Finally, a novel membrane remodeling function for Hsp90 was recently discovered, independent of its canonical chaperone activity [46]. Hsp90 promotes fusion of MVBs with the plasma membrane in a reconstituted system, and is required for release of Evi/Wntless-containing EVs at the Drosophila NMJ, but notably has no effect on lysosomal degradation. Importantly, trafficking machinery that specifically affects the EV release step (such as KIBRA and Hsp90) will enable uncoupling of potentially separable roles of EV cargoes in donor cells versus recipient cells.
Alternative pathways for neuronal EV biogenesis and release
While most studies of neuronal EVs focus on MVB-derived exosomes, neurons exhibit several unique categories of EVs (Fig. 1C). One such example highlights a novel mechanism by which lipid signaling molecules such as endocannabinoids are released in EVs [47]. In response to cocaine administration, midbrain dopaminergic neurons release EVs containing the endocannabinoid 2-arachadonoylglycerol (2-AG), leading to retrograde inhibition of GABA release. Cocaine disrupts the interactions between sigma-1 receptor (Sig-1R), a chaperone protein that is tethered to mitochondrion-associated ER membrane (MAM), and the GTPase Arf6. Freeing Arf6 from MAM promotes a myosin light chain kinase (MLCK)-dependent EV release pathway. Interestingly, a similar Arf6-MLCK pathway is specifically attributed to microvesicle release in tumor cells [48], suggesting that 2-AG may also be released in microvesicles.
EVs are also released from primary cilia [49]. Ciliated sensory neurons in C. elegans release EVs that modulate male mating behavior, via an ESCRT-0, ESCRT-I, and Alixindependent mechanism, suggesting that they are not generated by the canonical MVB pathway [49, 50]. Interestingly, following their release these EVs require intraflagellar transport for movement away from the donor cell [50]. Conversely, failure to shed EVs from the specialized cilia of mammalian photoreceptors causes defects in their morphogenesis [51]. At a much larger scale, C. elegans neurons can also shed micron-sized “exophers” from their soma to dispose of toxic materials [52]. Whether these mechanisms are restricted to specialized or stressed neurons remains unknown.
Finally, two complementary studies, in mouse hippocampal neurons [53] and at the Drosophila NMJ [21], describe a novel EV pathway mediated by Arc (activity-regulated cytoskeleton-associated protein), a key regulator of synaptic plasticity and scaling [54]. Arc bears homology to retroviral Gag proteins, and can enclose and release its own and other abundant mRNAs in viral-like capsids that are taken up and translated in recipient cells. Little is known about Arc-EV biogenesis, but there may be some commonalities with other EV pathways; for example, Drosophila Arc mRNA and protein traffic at the NMJ depend on Rab11 [21]. It will be interesting to explore the EV-related roles of regulators of Arc oligomerization, such as Arc ligands [55] or phosphorylation [56]. Remarkably, vertebrate and Drosophila Arc arose from independent retroviral repurposing events, suggesting that this may be a common adaptation for intercellular communication, and that other such carriers may exist.
Subcellular location of neuronal EV release
Given the extreme morphology and compartmentalization of neurons, understanding EV functions requires knowing from where in the neuron EVs are released. Several studies demonstrate accumulation of EV cargoes and/or MVBs in the somatodendritic compartment. In C. elegans, manipulating the small GTPase RAB-5 causes release of EFF-1 positive EVs near the soma of mechanosensory neurons [57]. Mammalian cortical and hippocampal neurons accumulate the EV cargoes CD63, CD9, Flotillin, tetanus toxin heavy chain, and Tau in the somatodendritic compartment [17, 34, 58, 59]. Further, electron microscopy shows MVB accumulation within pyramidal, Purkinje, and cortical neuron dendrites, suggesting release from these sites [17, 29]. Notably, mammalian Arc mRNA is transported to and locally translated in dendrites in response to activity-dependent cues [54], suggesting a direct mechanism for its preferential release from dendrites.
There is also extensive evidence for release of neuronal EVs from other subcellular compartments, including cilia (described above), and axons. For example, though EV cargoes CD9 and Tau accumulate in the somatodendritic region of cultured neurons, microfluidic compartmentalization shows that they are also trafficked anterogradely and released from axons [58, 59]. In similar experiments, oligomers or aggregates of the ALS-associated protein TDP-43 (Transactive response DNA-binding protein-43) are released by axons, and efficiently transmitted in EVs to recipient cells [60]. Likewise, in Drosophila motor neurons, EV cargoes are released from axon terminals [18–21]. It will be important to define compartment-specific release mechanisms for EVs.
Next challenges
While it is clear that neurons release heterogenous EVs with important functions, we are only beginning to unravel the complexities of neuronal EV traffic. In particular, EVs are released from neurons in response to stimulation or depolarization [17, 53, 59, 61–67], but the mechanisms and (in most cases) functions of activity-dependent release are unknown. Understanding these diverse pathways and their activity-dependent regulation may allow manipulation of the specific steps in neuron-specific EV biogenesis and release without impacting other trafficking pathways or other cell types. This would be a significant step towards another future major challenge: deciphering EV-mediated crosstalk between different cells within the nervous system. EVs are targeted to and taken up by specific recipient cells [37, 59, 64, 68], but it remains unclear whether this is determined by the EV cell of origin, the target cell, the specific cargo carried by the vesicle, or a context-specific combination of these factors. To answer these questions, in vivo models for EV traffic and physiological function will be transformative for the field [13, 19, 29, 34, 69]. Sorting out the specific pathways of EV cargo sorting, biogenesis, release, targeting, and uptake in vivo will be essential for interrogating EV function for basic science, for developing EV-directed therapeutics, and for understanding the origins and significance of EV biomarkers.
Highlights.
Extracellular vesicles (EVs) serve important functions in the nervous system, are misregulated in multiple neurological diseases, and are being developed as biomarkers and drug delivery vehicles to diagnose and treat these diseases.
Growing evidence suggests that there are numerous distinct cargo trafficking and release pathways for EVs in neurons.
Defining these specific trafficking routes will be critical for causal studies that distinguish the functions of EVs from other intertwined signaling and endosomal trafficking pathways.
Acknowledgements
The authors would like to thank Steve Del Signore, Erica Dresselhaus, and Biljana Ermanoska for critical review of the text. This work was supported by the National Institutes of Health (grant numbers F32 NS110123 to C.R.B. and R01 NS103967 to A.A.R)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.van Niel G, D’Angelo G, and Raposo G (2018). Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19, 213–228. [DOI] [PubMed] [Google Scholar]
- 2.Hessvik NP, and Llorente A (2018). Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75, 193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latifkar A, Hur YH, Sanchez JC, Cerione RA, and Antonyak MA (2019). New insights into extracellular vesicle biogenesis and function. J Cell Sci 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathieu M, Martin-Jaular L, Lavieu G, and Thery C (2019). Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21, 9–17. [DOI] [PubMed] [Google Scholar]
- 5.Budnik V, Ruiz-Canada C, and Wendler F (2016). Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci 17, 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holm MM, Kaiser J, and Schwab ME (2018). Extracellular Vesicles: Multimodal Envoys in Neural Maintenance and Repair. Trends Neurosci 41, 360–372. [DOI] [PubMed] [Google Scholar]
- 7.Liu W, Bai X, Zhang A, Huang J, Xu S, and Zhang J (2019). Role of Exosomes in Central Nervous System Diseases. Front Mol Neurosci 12, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill AF (2019). Extracellular Vesicles and Neurodegenerative Diseases. J Neurosci 39, 9269–9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowler CD (2019). NeuroEVs: Characterizing Extracellular Vesicles Generated in the Neural Domain. J Neurosci 39, 9262–9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciregia F, Urbani A, and Palmisano G (2017). Extracellular Vesicles in Brain Tumors and Neurodegenerative Diseases. Front Mol Neurosci 10, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7, 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, et al. (2019). Reassessment of Exosome Composition. Cell 177, 428–445 e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tkach M, Kowal J, and Thery C (2018). Why the need and how to approach the functional diversity of extracellular vesicles. Philos Trans R Soc Lond B Biol Sci 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.French KC, Antonyak MA, and Cerione RA (2017). Extracellular vesicle docking at the cellular port: Extracellular vesicle binding and uptake. Semin Cell Dev Biol 67, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delpech JC, Herron S, Botros MB, and Ikezu T (2019). Neuroimmune Crosstalk through Extracellular Vesicles in Health and Disease. Trends Neurosci 42, 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paolicelli RC, Bergamini G, and Rajendran L (2019). Cell-to-cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience 405, 148–157. [DOI] [PubMed] [Google Scholar]
- 17.Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Blot B, Haase G, Goldberg Y, and Sadoul R (2011). Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci 46, 409–418. [DOI] [PubMed] [Google Scholar]
- 18.Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, Leszyk J, Zhang B, and Budnik V (2012). Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem 287, 16820–16834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, and Budnik V (2009). Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 139, 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korkut C, Li Y, Koles K, Brewer C, Ashley J, Yoshihara M, and Budnik V (2013). Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron 77, 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashley J, Cordy B, Lucia D, Fradkin LG, Budnik V, and Thomson T (2018). Retrovirus-like Gag Protein Arc1 Binds RNA and Traffics across Synaptic Boutons. Cell 172, 262–274 e211. [DOI] [PMC free article] [PubMed] [Google Scholar]; Drosophila Arc1 forms viral-like capsids that encapsulate Arc1 mRNA, promoting its transynaptic traffic in EVs across the neuromuscular junction to regulate activity-dependent synaptic plasticity.
- 22.Escudero CA, Lazo OM, Galleguillos C, Parraguez JI, Lopez-Verrilli MA, Cabeza C, Leon L, Saeed U, Retamal C, Gonzalez A, et al. (2014). The p75 neurotrophin receptor evades the endolysosomal route in neuronal cells, favouring multivesicular bodies specialised for exosomal release. J Cell Sci 127, 1966–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messenger SW, Woo SS, Sun Z, and Martin TFJ (2018). A Ca(2+)-stimulated exosome release pathway in cancer cells is regulated by Munc13–4. J Cell Biol 217, 2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juan T, and Furthauer M (2018). Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol 74, 66–77. [DOI] [PubMed] [Google Scholar]
- 25.Gatta AT, and Carlton JG (2019). The ESCRT-machinery: closing holes and expanding roles. Curr Opin Cell Biol 59, 121–132. [DOI] [PubMed] [Google Scholar]
- 26.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, et al. (2012). Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14, 677–685. [DOI] [PubMed] [Google Scholar]
- 27.Shimada T, Yasuda S, Sugiura H, and Yamagata K (2019). Syntenin: PDZ Protein Regulating Signaling Pathways and Cellular Functions. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guix FX, Sannerud R, Berditchevski F, Arranz AM, Horre K, Snellinx A, Thathiah A, Saido T, Saito T, Rajesh S, et al. (2017). Tetraspanin 6: a pivotal protein of the multiple vesicular body determining exosome release and lysosomal degradation of amyloid precursor protein fragments. Mol Neurodegener 12, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]; TSPAN6 promotes syntenin-dependent ILV formation and enhances release of EVs containing amyloid precursor protein fragments.
- 29.Coulter ME, Dorobantu CM, Lodewijk GA, Delalande F, Cianferani S, Ganesh VS, Smith RS, Lim ET, Xu CS, Pang S, et al. (2018). The ESCRT-III Protein CHMP1A Mediates Secretion of Sonic Hedgehog on a Distinctive Subtype of Extracellular Vesicles. Cell Rep 24, 973–986 e978. [DOI] [PMC free article] [PubMed] [Google Scholar]; The ESCRT-III protein CHMP1A is required for ILV formation and EV secretion of the morphogen Sonic Hedgehog, regulating neuroprogenitor proliferation.
- 30.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, and Simons M (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247. [DOI] [PubMed] [Google Scholar]
- 31.Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kugler S, and Ikezu T (2015). Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci 18, 1584–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sackmann V, Sinha MS, Sackmann C, Civitelli L, Bergstrom J, Ansell-Schultz A, and Hallbeck M (2019). Inhibition of nSMase2 Reduces the Transfer of Oligomeric alpha-Synuclein Irrespective of Hypoxia. Front Mol Neurosci 12, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo BB, Bellingham SA, and Hill AF (2015). The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J Biol Chem 290, 3455–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Men Y, Yelick J, Jin S, Tian Y, Chiang MSR, Higashimori H, Brown E, Jarvis R, and Yang Y (2019). Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat Commun 10, 4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goncalves MB, Malmqvist T, Clarke E, Hubens CJ, Grist J, Hobbs C, Trigo D, Risling M, Angeria M, Damberg P, et al. (2015). Neuronal RARbeta Signaling Modulates PTEN Activity Directly in Neurons and via Exosome Transfer in Astrocytes to Prevent Glial Scar Formation and Induce Spinal Cord Regeneration. J Neurosci 35, 15731–15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinkins MB, Enasko J, Hernandez C, Wang G, Kong J, Helwa I, Liu Y, Terry AV Jr., and Bieberich E (2016). Neutral Sphingomyelinase-2 Deficiency Ameliorates Alzheimer’s Disease Pathology and Improves Cognition in the 5XFAD Mouse. J Neurosci 36, 8653–8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laulagnier K, Javalet C, Hemming FJ, Chivet M, Lachenal G, Blot B, Chatellard C, and Sadoul R (2018). Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell Mol Life Sci 75, 757–773. [DOI] [PMC free article] [PubMed] [Google Scholar]; N2a cells release a heterogenous mixture of EVs containing discrete sub-populations of CD63+ APP-, CD63- APP+ EVs that have distinct targeting specificities.
- 38.Miranda AM, Lasiecka ZM, Xu Y, Neufeld J, Shahriar S, Simoes S, Chan RB, Oliveira TG, Small SA, and Di Paolo G (2018). Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures. Nat Commun 9, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]; Inhibition of neuronal Vps34 leads to lysosomal membrane damage, promoting nSmase-dependent release of bis(monoacylglycero)phosphate EVs as a mechanism to rid the neuron of toxic endolysosomal cargo.
- 39.McNally KE, and Cullen PJ (2018). Endosomal Retrieval of Cargo: Retromer Is Not Alone. Trends Cell Biol 28, 807–822. [DOI] [PubMed] [Google Scholar]
- 40.Song P, Trajkovic K, Tsunemi T, and Krainc D (2016). Parkin Modulates Endosomal Organization and Function of the Endo-Lysosomal Pathway. J Neurosci 36, 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferguson SM (2018). Axonal transport and maturation of lysosomes. Curr Opin Neurobiol 51, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vingtdeux V, Hamdane M, Loyens A, Gele P, Drobeck H, Begard S, Galas MC, Delacourte A, Beauvillain JC, Buee L, et al. (2007). Alkalizing drugs induce accumulation of amyloid precursor protein by-products in luminal vesicles of multivesicular bodies. J Biol Chem 282, 18197–18205. [DOI] [PubMed] [Google Scholar]
- 43.Heisler FF, Pechmann Y, Wieser I, Altmeppen HC, Veenendaal L, Muhia M, Schweizer M, Glatzel M, Krasemann S, and Kneussel M (2018). Muskelin Coordinates PrP(C) Lysosome versus Exosome Targeting and Impacts Prion Disease Progression. Neuron 99, 1155–1169 e1159. [DOI] [PubMed] [Google Scholar]; Muskelin acts as a motor-vesicle adaptor, regulating dynein and kinesin to shift the balance between lysosomal degradation and EV targeting of Prion protein.
- 44.Song L, Tang S, Han X, Jiang Z, Dong L, Liu C, Liang X, Dong J, Qiu C, Wang Y, et al. (2019). KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat Commun 10, 1639. [DOI] [PMC free article] [PubMed] [Google Scholar]; KIBRA-mediated protection of Rab27a from proteasomal degradation impacts MVB size and number and regulates EV release.
- 45.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et al. (2010). Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12, 19–30; sup pp 11–13. [DOI] [PubMed] [Google Scholar]
- 46.Lauwers E, Wang YC, Gallardo R, Van der Kant R, Michiels E, Swerts J, Baatsen P, Zaiter SS, McAlpine SR, Gounko NV, et al. (2018). Hsp90 Mediates Membrane Deformation and Exosome Release. Mol Cell 71, 689–702 e689. [DOI] [PubMed] [Google Scholar]; Hsp90 promotes fusion of MVBs with the plasma membrane to release EVs, independent of its chaperone function.
- 47.Nakamura Y, Dryanovski DI, Kimura Y, Jackson SN, Woods AS, Yasui Y, Tsai SY, Patel S, Covey DP, Su TP, et al. (2019). Cocaine-induced endocannabinoid signaling mediated by sigma-1 receptors and extracellular vesicle secretion. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cocaine-induced disruption of a Sigma-1 receptor-Arf6 interaction promotes release of endocannabinoid-containing EVs from dopaminergic neurons, resulting in decreased GABAergic inhibition.
- 48.Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, and D’Souza-Schorey C (2009). ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol 19, 1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, and Barr MM (2018). Cell-cell communication via ciliary extracellular vesicles: clues from model systems. Essays Biochem 62, 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Silva M, Haas LA, Morsci NS, Nguyen KC, Hall DH, and Barr MM (2014). C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol 24, 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salinas RY, Pearring JN, Ding JD, Spencer WJ, Hao Y, and Arshavsky VY (2017). Photoreceptor discs form through peripherin-dependent suppression of ciliary ectosome release. J Cell Biol 216, 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melentijevic I, Toth ML, Arnold ML, Guasp RJ, Harinath G, Nguyen KC, Taub D, Parker JA, Neri C, Gabel CV, et al. (2017). C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 542, 367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pastuzyn ED, Day CE, Kearns RB, Kyrke-Smith M, Taibi AV, McCormick J, Yoder N, Belnap DM, Erlendsson S, Morado DR, et al. (2018). The Neuronal Gene Arc Encodes a Repurposed Retrotransposon Gag Protein that Mediates Intercellular RNA Transfer. Cell 172, 275–288 e218. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mammalian Arc protein forms viral-like capsids containing Arc mRNA. These are released in EVs from neurons and endocytosed by target cells, where translation of Arc mRNA can occur.
- 54.Wilkerson JR, Albanesi JP, and Huber KM (2018). Roles for Arc in metabotropic glutamate receptor-dependent LTD and synapse elimination: Implications in health and disease. Semin Cell Dev Biol 77, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nielsen LD, Pedersen CP, Erlendsson S, and Teilum K (2019). The Capsid Domain of Arc Changes Its Oligomerization Propensity through Direct Interaction with the NMDA Receptor. Structure 27, 1071–1081 e1075. [DOI] [PubMed] [Google Scholar]
- 56.Zhang W, Chuang YA, Na Y, Ye Z, Yang L, Lin R, Zhou J, Wu J, Qiu J, Savonenko A, et al. (2019). Arc Oligomerization Is Regulated by CaMKII Phosphorylation of the GAG Domain: An Essential Mechanism for Plasticity and Memory Formation. Mol Cell 75, 13–25 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linton C, Riyadh MA, Ho XY, Neumann B, Giordano-Santini R, and Hilliard MA (2019). Disruption of RAB-5 Increases EFF-1 Fusogen Availability at the Cell Surface and Promotes the Regenerative Axonal Fusion Capacity of the Neuron. J Neurosci 39, 2823–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polanco JC, Li C, Durisic N, Sullivan R, and Gotz J (2018). Exosomes taken up by neurons hijack the endosomal pathway to spread to interconnected neurons. Acta Neuropathol Commun 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Balaji V, Kaniyappan S, Kruger L, Irsen S, Tepper K, Chandupatla R, Maetzler W, Schneider A, Mandelkow E, et al. (2017). The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener 12, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feiler MS, Strobel B, Freischmidt A, Helferich AM, Kappel J, Brewer BM, Li D, Thal DR, Walther P, Ludolph AC, et al. (2015). TDP-43 is intercellularly transmitted across axon terminals. J Cell Biol 211, 897–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, et al. (2006). Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 31, 642–648. [DOI] [PubMed] [Google Scholar]
- 62.Frohlich D, Kuo WP, Fruhbeis C, Sun JJ, Zehendner CM, Luhmann HJ, Pinto S, Toedling J, Trotter J, and Kramer-Albers EM (2014). Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA, et al. (2013). Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol 11, e1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, and Sadoul R (2014). Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles 3, 24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goldie BJ, Dun MD, Lin M, Smith ND, Verrills NM, Dayas CV, and Cairns MJ (2014). Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res 42, 9195–9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ataman B, Ashley J, Gorczyca M, Ramachandran P, Fouquet W, Sigrist SJ, and Budnik V (2008). Rapid activity-dependent modifications in synaptic structure and function require bidirectional Wnt signaling. Neuron 57, 705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee SH, Shin SM, Zhong P, Kim HT, Kim DI, Kim JM, Do Heo W, Kim DW, Yeo CY, Kim CH, et al. (2018). Reciprocal control of excitatory synapse numbers by Wnt and Wnt inhibitor PRR7 secreted on exosomes. Nat Commun 9, 3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prada I, Amin L, Furlan R, Legname G, Verderio C, and Cojoc D (2016). A new approach to follow a single extracellular vesicle-cell interaction using optical tweezers. Biotechniques 60, 35–41. [DOI] [PubMed] [Google Scholar]
- 69.Verweij FJ, Hyenne V, Van Niel G, and Goetz JG (2019). Extracellular Vesicles: Catching the Light in Zebrafish. Trends Cell Biol 29, 770–776. [DOI] [PubMed] [Google Scholar]


