Abstract
Raspberry ketone (RK; [4-(4-hydroxyphenyl)-2-butanone]) is used by the food and cosmetic industry as a flavoring agent. RK is also marketed as a dietary supplement for weight maintenance and appetite control. The purpose of the study was to characterize the acute feeding suppression with RK (64–640 mg/kg) by oral gavage in male and female C57BL/6J mice. Cumulative 24 h food intake was reduced at 200 mg/kg (24% feeding suppression) in males and reliably reduced at 640 mg/kg (49–77% feeding suppression). Feeding suppression was not associated with pica behavior over the range of doses or conditioned taste aversion. In a separate experiment, a single oral gavage of RK (640 mg/kg) resulted in approximate 43% mortality rate (6 out 14 male mice) within 2 days. Atrophy of white adipose tissue, splenic abnormalities, and thymus involution were noted after 2 to 4 days after oral gavage RK. Total white blood cell count, lymphocytes, monocytes, eosinophils were significantly lower, while mean red blood cells, hemoglobin, and hematocrit were significantly higher with RK treatment. Our findings indicated a dose-dependent feeding suppression with acute RK, but doses that reliable suppress food intake are associated with pathological changes.
Introduction
Raspberry ketone (RK; [4-(4-hydroxyphenyl)-2-butanone]) is the principle natural flavor aromatic of red raspberries (Rubus idaeus L.) and other berries species (Borejsza-Wysocki et al., 1992; Borejsza-Wysocki and Hrazdina, 1994; Gora and Brud, 1983). Naturally derived RK content is directly associated with raspberry flavor profile and RK has been used as natural attractant lure for fly species (Borejsza-Wysocki et al., 1992; Khan et al., 2017; Stringer et al., 2019). Nonetheless, the biological yield of RK from natural sources is considerable low (Borejsza-Wysocki et al., 1992; Yuan et al., 2018). RK is commonly prepared chemically by the condensation of p-hydroxybenzaldehyde and acetone with hydrogenation, but other biological synthetic methods have been reported (Boker et al., 2001; Opdyke, 1978; Pedapudi et al., 2000; Silverman and John, 2015; Wang et al., 2019). RK is designated by the United States Food and Drug Administration (FDA) as a synthetic additive permitted for direct addition to food for human consumption (Administration, 2019). However, RK has an unauthorized “novel food” status in the United Kingdom (UK) and European Union (EU), but is still available online in the EU and UK (Rapid Alert System for Food and Feed (RASFF), 2015; Steffensen, 2018). As a flavoring or aroma agent, RK is generally regarded as safe without any adverse effects at a reference doses of 5 −25 mg/kg/day for domesticated animals or ≥1% (reference dose of 0.7 mg/kg/day) finished products for human use ((FEEDAP), 2016; Api et al., 2019; Opdyke, 1978). In recent years, RK has been marketed and sold on the internet and health food outlets as a natural “appetite suppressant” or “weight loss” agent as a single source or part of a multi-ingredient dietary supplement (Arent et al., 2018; Bredsdorff et al., 2015; Lee, 2016; Lopez et al., 2013). As a dietary supplement, the recommended daily dose of RK is much higher than the flavoring agent reference dose and can be as much as 2000 mg/day (Bredsdorff et al., 2015; Lee, 2016; Steffensen, 2018).
A few studies have supported the use of RK for the management of obesity. Human studies using RK as a weight loss dietary supplement have been limited though. A multi-ingredient supplement containing RK promoted short-term (≤ 8 weeks) weight loss in a small number (n ≤ 45) of overweight subjects on an exercise program (Arent et al., 2018; Lopez et al., 2013). Studies using rodents have indicated that RK specifically reduces weight gain associated with high-fat diet exposure and suggest that RK reduces lipid accumulation in white adipose tissue (WAT) (Cotten et al., 2017; Kshatriya et al., 2019; Leu et al., 2017; Leu et al., 2018; Morimoto et al., 2005; Tsai et al., 2017). Bioavailability and tissue distribution studies have revealed that oral RK reaches maximal concentration in the blood within 15 minutes and is distributed to the brain and WAT within 1 h after dosing (Zhao et al., 2020). In vitro studies have suggested the RK inhibits genes regulating adipogenesis, but the exact pathways have not been characterized (Park, 2015; Tsai et al., 2017). Besides the prevention of fat accumulation, RK can manage obesity by preventing excessive calorie intake or reducing feeding behavior (Cotten et al., 2017; Yimam et al., 2019). In one study, C57BL/6 male mice fed a high fat diet (HFD) adulterated with RK (5 g/kg/diet) had a similar prevention of weight gain and metabolic endpoints as mice pair-fed HFD without RK(Cotten et al., 2017). In another study, a single oral dose of RK (500 mg/kg) reduced food intake for 6 h in male rats fed a HFD (Yimam et al., 2019). While these experiments suggest RK reduces food intake, it is unclear whether RK-induced feeding suppression is by associated aversion or gastrointestinal distress. The present experiments were designed to further characterize the dose-dependent effects of RK experiments on acute feeding. We also included an assessment of pica behavior and conditioned taste aversion induced by RK. Our hypothesis was RK would dose-dependently reduce acute food intake without resulting in an associated aversion. Because we observed mortality with acute RK dosing, additional experiments were performed to evaluate histopathological endpoints.
Material and Methods
Mice.
Male and female C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were ad libitum fed standard chow (Purina Mouse Diet 5015, 25.34% fat, 19.81% protein, 54.86% CHO, 3.7 Kcal/g; Lab Diet, St. Louis, MO, USA) and water, unless otherwise noted. Mice were pair-housed, unless otherwise noted, and maintained on a 12 h light/dark cycle with lights on from 0700 h to 1900 h. The animal care protocol was approved by the Institutional animal Care and Use Committee of Rutgers University (OLAW #A3262-01).
Oral dosing of compounds.
Dosing was performed using single-use, sterile plastic feeding tubes (20 ga × 30 mm; cat# FTP-20–30, Instech Laboratories, Plymouth Meeting, PA, USA). Each mouse was oral dosed by gavage with vehicle (VEH; 50% propylene glycol, 40% water, and 10% DMSO) or raspberry ketone (RK; 4[4-hydroxyphenyl]-2-butanone; 99%; cat#178519; Sigma-Aldrich Corp, St. Louis, MO, USA). Dose volumes were 0.1 cc per 10 g bodyweight for vehicle or RK. A reference sample (~1 g) from each lot of raspberry ketone were deposited in a secure, climate-controlled repository (Yuan et al., 2019).
Feeding suppression.
A group of 10-week old male (n=20) and female (n=20) mice were single housed on arrival and acclimated for 7 days. All mice were fed standard chow and given access to pelleted kaolin (Research Diets, New Brunswick, NJ, USA) during acclimation. Mice were assigned to receive either ad libitum food or 24 h food deprivation. All mice received an ascending dose of vehicle, 64, 200 and 640 mg/kg RK by oral gavage in four consecutive weeks with a 7-day washout between dosing. Food and kaolin intake were measured at 1, 2, 4 and 24 h post-gavage. After the 24 h feeding suppression study, all mice were kept in their home cages for 7 days for survival observation.
Conditioned taste aversion (CTA).
A separate group of 10-week old male (n = 20) and female (n = 20) C57BL/6J mice were single housed for 7 days prior to beginning to the start of the experiment. All training and testing were performed in the home cage (Rauhut et al., 2008). To initiate training, mice were water restricted for 24 h and were then given a 20 min access to water. Twenty-four hours later, mice were given a pre-test 20 min access of 0.15% sodium saccharin solution (MP Biomedicals, LLC, Solon OH, USA). Sodium saccharin at 0.01–2.05% concentrations are preferred by B6 mice (Bachmanov et al., 2001). Sodium saccharin was used in these experiments because it is a gustatory stimulus with minimal post-oral consequences compared with sucrose and other saccharides (Sclafani, 2006). After the saccharin pre-test, mice were given ad libitum access to water for 24 h. Mice were then divided into groups based on their saccharin consumption at the pre-test phase. The conditioning phase was 8 consecutive sessions, consisting of 4 alternating pairing and non-pairing sessions. There was a 24 h washout between sessions (i.e., 1 session per day). On the pairing sessions mice were dosed with raspberry ketone (400 mg/kg) or vehicle by oral gavage 5 min after a 60-min period of saccharin (0.15%) access. On the intervening non-pairing sessions, mice were allowed 60 min access to water. The final test phase occurred 24 h after the last non-pairing session. On the first test session, half of the mice received 60 min access to the 0.15% saccharin whereas the other half received 60 min access to water. The counterbalance access conditions were provided on the second test session.
Two bottle preference tests.
Following the CTA test, all mice were given 24 h access to water. After which, a two-bottle choice test was performed as previously described (Gupta et al., 2018). Mice were allowed access to the two bottles for 48 h. One bottle was filtered tap water and the other contained sodium saccharin (0.15%). In the middle of 48 h access period, after a period of 24 h, mice were given new bottles and the position of bottles was switched in order to avoid place preference. Mice were not orally dosed during the two-bottle test. Percent preference was expressed by taking the volume of sodium saccharin consumed divided by the total volume of fluid consumed (taste stimulus and water) multiplied by 100.
Acute toxicology of tissues.
A separate group of 10-week old male C57BL/6J mice (n =24) were single housed and were equally divided by bodyweight. Following a 24 h food deprivation, each body weight-matched mouse received either raspberry ketone (640 mg/kg) or vehicle by oral gavage. Dose volume was 0.1 cc per 10 g bodyweight across both experimental groups. Standard chow was provided immediately after dosing. The mice were monitored for signs of distress, and their body weight was recorded daily. Samples were collected either 2 days or 4 days after the respective dosing. Blood was collected by cardiac puncture under deep isoflurane anesthesia, which was followed by exsanguination with 0.9% saline, and perfused with 4% paraformaldehyde in phosphate buffer with saline (PBS). Perfused carcasses were stored in 10% formalin for histopathological processing. Epididymal fat, mesenteric fat, brain, heart, kidney, liver, lung, pancreas, sciatic nerve, stomach, small intestine, large intestine, skeletal muscle, spleen and thymus were collected. Tissue sections were stained with hematoxylin and eosin (H& E) and evaluated by a board-certified veterinary pathologist.
Hematological analyses and liver transaminases.
A separate group of 10-week old male C57BL/6J mice (n = 24) were single housed and were equally divided by bodyweight. Following a 24 h food deprivation, each body weight-matched mouse received either a single dose of raspberry ketone (640 mg/kg) or vehicle by oral gavage. Dose volume was 0.1 cc per 10 g bodyweight across both experimental groups. Standard chow was provided immediately after dosing. The mice were monitored for signs of distress, and their body weight was recorded daily. Samples were collected 2 days after the respective dosing. Blood was collected by cardiac puncture under deep isoflurane anesthesia, animals were euthanized by decapitation following exsanguination. Blood was collected in EDTA tubes and samples were analyzed by Element HT5 Hematology Analyzer (Heska Corporation, Loveland, CO, USA). The remaining plasma was used to evaluate alanine aminotransferase (ALT) and aspartate aminotransferase (AST) by mouse-specific ELISA (MyBiosource, Inc., San Diego, CA, USA). ALT detection range were 0.25 −8 ng/ml (sensitivity: 0.1 ng/ml) and AST detection range were 0.16 −10 ng/mL (sensitivity: 0.1 ng/mL).
Statistical Analyses.
Intakes were recorded nearest 0.1 ml or 0.1 g and expressed as mean ± SEM. For feeding, CTA, or 2 bottle preference tests separate analysis of variance (ANOVA) or ANOVA with repeated measures were performed. When justified, Newman-Keuls post-hoc tests were performed. For the pathology measurements, individual independent t-test were performed. All statistical were performed using Statistica 7.1 software (StatSoft; Dell Inc, Round Rock, TX, USA), significance was set at α = 0.05.
Results
Raspberry ketone suppresses acute food intake in ad libitum and deprivation-induced refeeding.
Under ad libitum feeding conditions, males demonstrated effects for dose [F (3, 27) = 13.6, p < 0.005], time [F(3, 27) = 357.4, p < 0.001], and dose X time [F(9, 81) = 29.5, p < 0.0001]. The 64 mg/kg dose, compared with vehicle, reduced 24 h cumulative food intake (p < 0.05). The 200 mg/kg and 640 mg/kg doses, compared with vehicle, reduced cumulative food intake at 4 h and 24 h (p < 0.005 for both doses and time points), see Figure 1A. Cumulative kaolin intake at 24 h was 0.09 ± 0.04 g for vehicle, 0.20 ± 0.05 g for 64 mg/kg, 0.43 ± 0.18 g for 200 mg/kg, and 0.30 ± 0.17 g for 640 mg/kg, which were not significantly different. For females, there were effects for dose [F(3, 27) = 4.2, p < 0.05], time [F(3, 27) = 1259.4, p < 0.005], and dose X time [F(9, 81) = 36.4, p < 0.005]. Post-hoc testing revealed the cumulative food intake following the 640 mg/kg RK dose, was significantly reduced from vehicle at the 24 h intake (p < 0.005), see Figure 1 A. Cumulative kaolin intake at 24 h was 0.2 ± 0.08 g for vehicle, 0.25 ± 0.08 g for 64 mg/kg, 0.47 ± 0.15 g for 200 mg/kg, and 0.10 ± 0.06 g for 640 mg/kg, which were not significantly different. Four mice (2 males and 2 females) were found dead within 72 hours after the 640 mg/kg dose. No additional mortalities were observed following this period, the remaining mice were euthanized 7 days following the 640 mg/kg.
Figure 1. Feeding suppression of raspberry ketone.
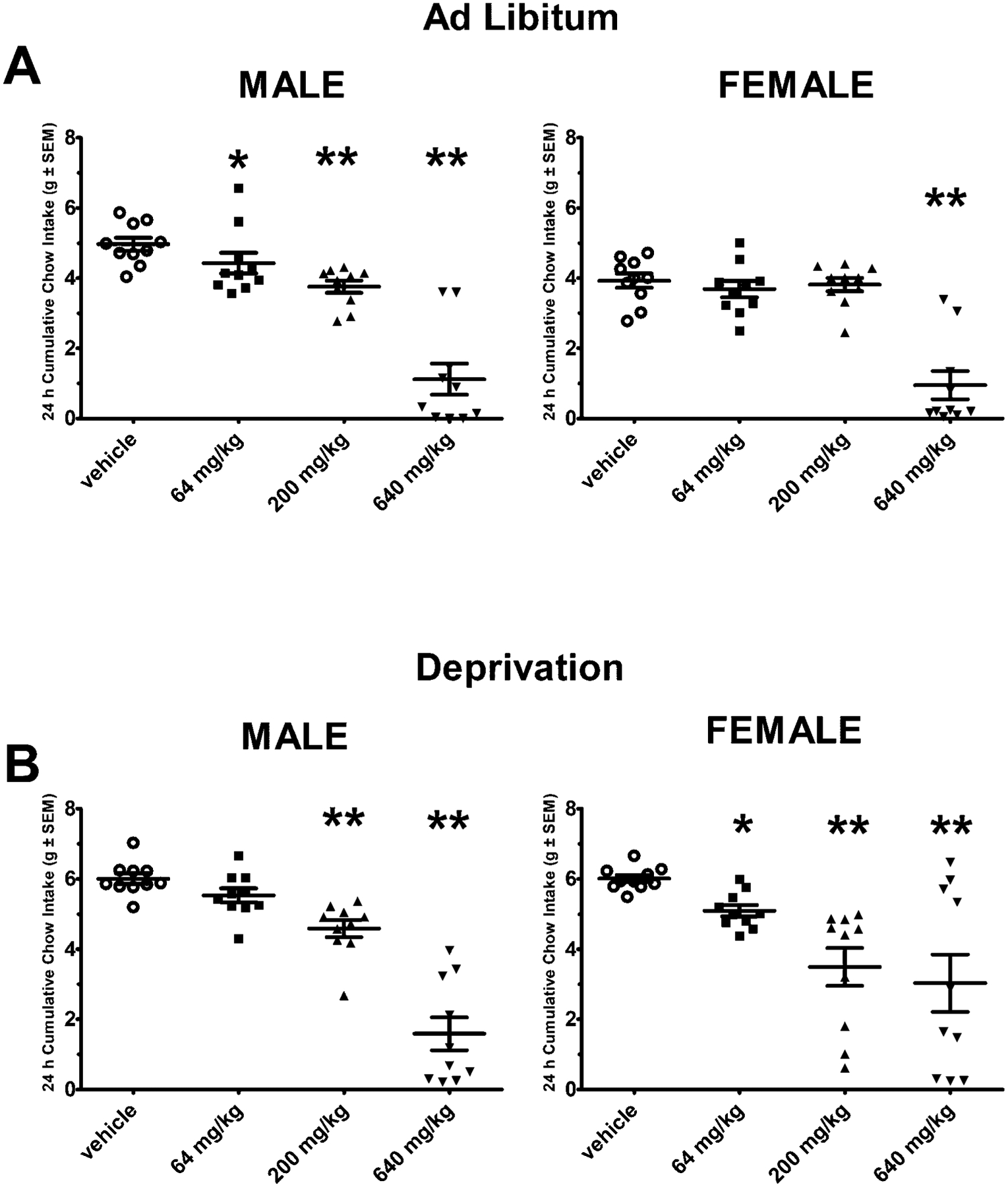
Standard chow was measured following doses of raspberry ketone (RK; 64 mg/kg, 200 mg/kg, and 640 mg/kg) or vehicle in C57Bl/6J (10 weeks old) male and female mice. Data are represented as means ± SEM. A single dose of raspberry ketone was administered by oral gavage in an ascending order in each mouse. There was a 7-day washout between doses. A: Cumulative 24 h intake was measured under ad libitum feeding conditions (n = 10/sex) B: In a separate group of mice, cumulative 24 h intake was measure following a 24 h food deprivation (n = 10/sex). In addition to chow, non-nutritive kaolin was available to assess pica behavior as indicator for nausea. There were no differences in kaolin intake in the doses of raspberry ketone tested. * indicated p < 0.05 from vehicle, ** indicated p < 0.005 from vehicle.
Under deprivation-induced feeding conditions, males demonstrated effects for dose [F(3, 27) = 5.2, p< 0.01], [F(3, 27) = 1099, p < 0.001], and dose X time [F(9, 81) = 10.2, p < 0.0001]. The 200 mg/kg and 640 mg/kg doses, compared with vehicle, reduced cumulative food intake at 24 h (p < 0.005 for both), see Figure 1B. Cumulative kaolin intake at 24 h was 0.05 ± 0.02 g for vehicle, 0.02 ± 0.01 g for 64 mg/kg, 0.10 ± 0.09 g for 200 mg/kg, and 0.06 ± 0.05 g for 640 mg/kg, which were not significantly different. For females there were demonstrated effects for dose [F(3, 27) = 5.3, p< 0.01], [F(3, 27) = 485, p < 0.001], and dose X time [F(9, 81) = 7.2, p < 0.0001]. The 64 mg/kg, 200 mg/kg, and 640 mg/kg doses, compared with vehicle, reduced cumulative food intake at 24 h (p < 0.005), see Figure 1B. Cumulative kaolin intake at 24 h was 0.07 ± 0.03 g for vehicle, 0.06 ± 0.03 g for 64 mg/kg, 0.19 ± 0.11 g for 200 mg/kg, and 0.04 ± 0.03 g for 640 mg/kg, which were not significantly different. Similar to the ad libitum condition, 4 mice (2 males and 2 females) were found dead within 72 hours after 640 mg/kg dose. No additional mortalities were observed following this period, the remaining mice were euthanized 7 days following the 640 mg/kg.
No effects of raspberry ketone (200 mg/kg) on conditioned taste aversion (CTA).
For the males, the 0.15% saccharin intakes for the pre-test were 0.52 ± 0.12 and 0.48 ± 0.16 ml for the vehicle-and raspberry ketone-assigned groups, respectively. For the testing phase, there was only an effect for solution [F (1, 18) = 13.6, p< 0.005] with mice consuming more saccharin than water, 1.79 ± 0.13 ml of 0.15% saccharin compared with 1.45 ± 0.9 ml of water (p < 0.05). There were no significant effects for pairing treatment (RK vs. vehicle) or pairing treatment X solution. For the females, the 0.15% saccharin intakes for the pre-test were 0.49 ± 0.07 and 0.49 ± 0.10 ml for the vehicle- and RK-assigned groups, respectively. For the testing phase, there were no significant effects for pairing treatment, solution, or pairing treatment X solution, Figure 2A.
Figure 2. Conditioned taste aversion (CTA) and 2-bottle preference test to 0.15% saccharin paired with raspberry ketone (400 mg/kg) or vehicle.
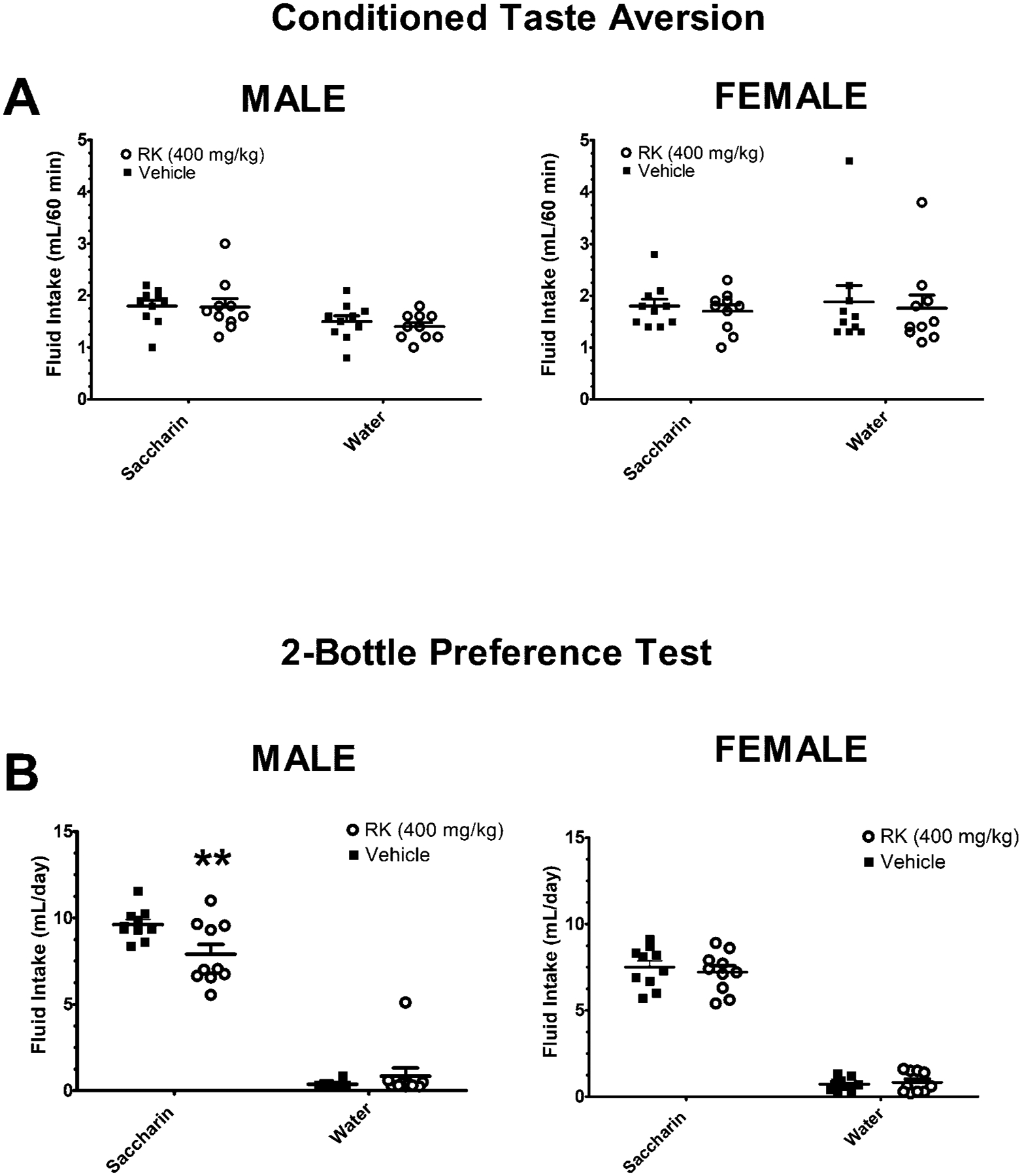
All mice were 10 weeks old at the onset of the experiment. For the CTA, there were 8-session conditioning phase under water-restricted conditions. For 4 sessions, a 60-min access of 0.15% saccharin was paired with oral gavage dosed raspberry ketone (RK; 400 mg/kg) or vehicle. The intervening 4 sessions (non-pairing) were water only. A: Water or saccharin consumption was measured in two testing sessions (60 min) randomly presented in the absence of RK or vehicle. Data are represented as means ± SEM. The two-bottle test occurred 24 h after the CTA test. Mice were not dosed during the 2-bottle test. B: Mice were given one bottle containing water and the other bottle contained 0.15% saccharin for 48 h. All groups were (n = 10). ** indicated p < 0.005 from previously vehicle-paired mice.
Previous pairing raspberry ketone (400 mg/kg) reduced intake during a two-bottle preference test with saccharin in male mice.
Following a 24 h ad libitum water access period, mice were present with a two-bottle test of 0.15% saccharin compared with water for 48 h. For the male mice, there was an effect of solution [F (1, 18) = 357, p < 0.0005], with male mice consuming more 0.15% saccharin than water (p < 0.005). There was also an effect of pairing treatment X solution [F (1, 18) = 6.4, p < 0.05]. Mice that had RK-paired with saccharin had a reduced saccharin intake during the two-bottle test, compared with vehicle-paired mice (p < 0.005). Percent preference was 96.2 ± 0.5% for vehicle-paired and 91.6 ± 3.8% for RK-paired, respectively. For the female mice, there was an effect of solution [F (1, 18) = 518.9, p < 0.0005], with female mice consuming more saccharin than water (p < 0.005). There were no significant effects for previously paired treatment or pairing treatment X solution, Figure 2B. Percent preference was 91.1 ± 1.3% for vehicle-paired and 89.9 ± 2.2% for RK-paired, respectively. There were no mortalities in either the CTA or two-bottle preference tests.
Acute toxicity of raspberry ketone (640 mg/kg).
Fourteen mice were dosed with a single dose of RK (640 mg/kg), whereas 10 mice received a single dose of vehicle. Within 48 h after the single dose of raspberry ketone, 6 mice died and 3 additional mice were euthanized for pathological examination after they had outward signs of lethargy, rough coat, prolonged inappetence (> 24 h), and hunched posture. The remaining 5 mice were euthanized for pathology on day 4 after the single dose. There were no deaths or outward distress signs noted in vehicle-treated mice. Compared with vehicle-treated mice, there were no RK-related brain, spinal cord, sciatic nerve, liver, kidney, heart, stomach, small intestine, large intestine, pancreas, lung or skeletal muscle pathologies, see table 1. There were no statistical differences in histopathological evaluation between day 2 and day 4 for any of the tissue evaluated, so scores for both days were combined for table 1.
Table 1.
Tabulated histopathological score of tissue. Sections were scored for pathological abnormalities on a scale 0–5. A score of 5 indicates the most severe pathology, whereas 0 indicates no noteworthy findings or within range of background. A total of 24 male mice (10-weeks old) received a single dose by oral gavage of vehicle (n = 10) or raspberry ketone (RK; 640 mg/kg; n=14). 4 days post-treatment 6 mice died. No noteworthy findings were found in the vehicle treated animals. Of the 8 mice that survived, 3 were euthanized on Day 2 and 5 were euthanized on Day 4.
| Histological Grade | |||||||
|---|---|---|---|---|---|---|---|
| RK (640 mg/kg) | 0 | 1 | 2 | 3 | 4 | 5 | % Survival |
| Adipose atrophy, Epididymal^ | 0 | 3 | 3 | 1 | 0 | 0 | 57.14% (8/14) |
| Adipose atrophy, Mesenteric | 0 | 1 | 1 | 1 | 5 | 0 | |
| Brain | 8 | 0 | 0 | 0 | 0 | 0 | |
| Heart | 8 | 0 | 0 | 0 | 0 | 0 | |
| Kidney | 8 | 0 | 0 | 0 | 0 | 0 | |
| Liver | 8 | 0 | 0 | 0 | 0 | 0 | |
| Lung | 8 | 0 | 0 | 0 | 0 | 0 | |
| Pancreas | 8 | 0 | 0 | 0 | 0 | 0 | |
| Sciatic Nerve | 8 | 0 | 0 | 0 | 0 | 0 | |
| Skeletal Muscle | 8 | 0 | 0 | 0 | 0 | 0 | |
| Small Intestine | 8 | 0 | 0 | 0 | 0 | 0 | |
| Spinal Cord | 8 | 0 | 0 | 0 | 0 | 0 | |
| Spleen, White Pulp Hypocellularity | 3 | 2 | 3 | 0 | 0 | 0 | |
| Spleen, Red Pulp, EMH | 2 | 0 | 4 | 1 | 1 | 0 | |
| Stomach | 8 | 0 | 0 | 0 | 0 | 0 | |
| Thymus, Involution # | 0 | 0 | 0 | 0 | 3 | 2 | |
indicated 1 sample was not of adequate size or quality for pathologist evaluation,
indicated 3 samples were not of adequate size or quality for pathologist evaluation. EMH: Extramedullary hematopoiesis.
For both epididymal and mesenteric adipose tissue, there was noted raspberry ketone-related atrophy of adipose tissue, compared with vehicle-treated tissue. Vehicle-treated mice adipose tissue was relatively hypocellular with few interstitial cells and broad fields of large uniformly sized round to hexagonal, signet ring-shaped adipocytes filled with lipid. Raspberry ketone-treated mice had multifocal areas of hypercellularity with variably sized and irregularly shaped adipocytes. Affected areas appeared hypercellular due to a higher ratio of adipocyte nuclei to reduced lipid area. Adipocytes were smaller with numerous small vacuoles with amphophilic margins, see figure 4A and 4B. There were also noted splenic findings, specifically white pulp hypocellularity severity was associated with RK treatment, see figure 4C and 4D. There were also remarkable findings in the thymic tissue with RK-treated mice having complete involution of the thymus. Vehicle-treated mice had normal thymus with abundant cortical lymphocytes, while raspberry ketone-treated mice had fewer to almost no remaining lymphocytes and prominent medullary interstitial thymic tissue, see figure 4E and 4F. All other tissues were not remarkably different from vehicle-treated mice.
Elevated levels of hematological values and reduced ALT with raspberry ketone (640 mg/kg).
For the hematological analysis mice received a single dose of RK (640 mg/kg; n = 16) or vehicle (n = 8). Within 48 h after the single dose of raspberry ketone, 4 mice died and additional 2 mice were euthanized at the recommendation of the veterinarian clinical staff due to overt pathological signs (e.g., severe lethargy, insensitive to touch, and hunched posture). No vehicle-treated mice died or displayed pathological signs. The data, therefore, represents 10 mice dosed with RK and 8 mice dosed with vehicle. Hematological values were significant lower in RK-treated mice for white blood cell (WBC) count [t(16) = −3.9, p < 0.005], lymphocytes (LYM) [t(16) = −3.9, p < 0.005], monocytes (MONO) [t(16) = −4.9, p < 0.005], eosinophils (EOS) [t(16) = −4.9, p < 0.005], LYM% [t(16) = 3.4, p < 0.005] , mean corpuscular volume (MCV) [t (16) = −3.1, p < 0.005], and red blood cell (RBS) distribution width coefficient of variation (RDW) % [t (16) = −2.62; p < 0.05] compared with vehicle-treated mice. Hematological values were significant higher in RK-treated mice for neutrophil % (NEU %) [t (16) = 3.4, p < 0.005], mean red blood cells (RBC) [t (16) = 5.5, p < 0.005], hemoglobin (HGB) [t (16) = 5.2, p < 0.005], hematocrit (HCT) [t (16) = 4.9, p < 0.005], and mean corpuscular hemoglobin concentration (MCHC) [t (16) = 3.0, p < 0.05]. For hepatic transaminases 2 days following treatment, there were no group differences in AST. However, ALT values were significantly lower in RK compared with vehicle-treated mice [t (16) = −4.01, p < 0.005] see table 2. ALT and AST levels were also measured from mice (n= 5 for each treatment) euthanized on Day 4. ALT values were 0.32 ± 0.08 ng/mL for vehicle- and 0.33 ± 0.07 ng/mL for raspberry ketone- treated mice, which were not significantly different. However, AST were elevated at Day 4 in RK-treated mice. AST values were 0.33 ± 0.03 ng/mL for vehicle- and 0.649 ± 0.09 ng/mL for raspberry ketone-treated mice [t (8) = 3.4, p < 0.01].
Table 2.
Blood parameters 2 days following a single dose by oral gavage of vehicle (n = 8) or raspberry ketone (640 mg/kg; n = 10). Values are means ± SE.
| Parameters | Vehicle | Raspberry Ketone |
|---|---|---|
| White Blood Cell Count (WBC); 103/μL | 1.48 ± 0.26 | 0.43 ± 0.12** |
| Neutrophils (NEU); 103/μL | 0.22 ± 0.04 | 0.17± 0.03 |
| Lymphocytes (LYM); 103/μL | 1.11 ± 0.22 | 0.23 ± 0.09** |
| Monocytes (Mono); 103/μL | 0.093 ± 0.01 | 0.024 ± 0.01** |
| Eosinophils (EOS); 103/μL | 0.038 ± 0.006 | 0.007 ± 0.0036** |
| NEU% | 16.26 ± 1.9 | 44.99 ± 7.4** |
| LYM% | 72.62 ± 3.4 | 44.34 ± 7.1** |
| MON0% | 7.14 ± 1.2 | 5.42 ± 0.87 |
| EOS% | 3.16 ± 0.6 | 3.05 ± 0.89 |
| Red Blood Cell Count (RBC); 106/μL | 8.23 ± 0.24 | 10.5 ± 0.34** |
| Hemoglobin Concentration (HGB); g/dL | 13.26 ± 0.41 | 16.94 ± 0.53** |
| Hematocrit (HCT); % | 40.93 ± 1.2 | 49.55 ± 1.23** |
| Mean Corpuscular Volume (MCV); fL | 49.68 ± 0.26 | 47.27 ± 0.65** |
| Mean Corpuscular Hemoglobin (MCH); pg | 16.11 ± 0.08 | 16.13 ± 0.05** |
| MCH Concentration (MCHC); g/dL | 32.45 ± 0.26 | 34.18 ± 0.46** |
| RBC Distribution Width Coefficient of variation (RDW)% | 12.79 ± 0.19 | 12.19 ± 0.15* |
| Platelet Count (PLT); 103/μL | 850.75 ± 62.6 | 1070 ± 105.2 |
| Mean Platelet Volume (MPV); fL | 5.12 ± 0.12 | 4.85 0.07 |
| Alanine Aminotransferase (ALT); ng/mL | 0.28 ± 0.03 | 0.16 ± 0.01** |
| Aspartate Aminotransferase (AST); ng/mL | 0.69 ± 0.08 | 0.66 ± 0.12 |
indicates p < 0.005 from vehicle,
indicates p < 0.05 from vehicle
Discussion
The initial objective of this study was to investigate the acute feeding suppression of standard chow over a range of RK doses in normal weight mice. Overall, our findings indicate that oral RK at 640 mg/kg effectively reduced cumulative 24 h intake. There were differential responses to the doses across feeding conditions. With ad libitum standard chow, there was a dose-dependent feeding suppression in males, but feeding suppression was only observed at 640 mg/kg in females. In the deprivation-induced feeding, the 200 mg/kg and 640 mg/kg RK doses effectively produced feeding suppression in males and females. Previously studies in rats observed a 6 h feeding suppression following a single oral dose of 500 mg/kg of RK in males under food deprived conditions (Yimam et al., 2019). Our studies suggest that the 640 mg/kg RK dose reliably produces a feeding suppression in C57BL/6J male and female mice under ad libitum and food deprived conditions.
In all feeding conditions in our experiments, non-nutritive kaolin was concurrently available with standard chow to assess pica behavior. Pica behavior in mice is used as a metric to determine gastrointestinal distress associated with experimental conditions (Yamamoto and Yamatodani, 2018). We did not observe any increase in cumulative 24 h kaolin intake at any RK dose tested. In order to further assess any additional generalized illness associated with RK we performed a conditioned taste aversion (CTA) with saccharin as the tastant (Rauhut et al., 2008). For the CTA we used an RK dose of 400 mg/kg, even though this dose was not tested in the acute feeding studies. The reason for using the 400 mg/kg dose was that we observed mortality associated with 640 mg/kg dose in the acute feeding studies and was a doubling of the 200 mg/kg dose. The 200 mg/kg dose produced a feeding suppression in all conditions, except the ad libitum feeding in female mice. Although we did not observe a RK-associated reduction in saccharin intake during the CTA test, we did observe a reduction in saccharin intake during a 48 h two-bottle test in male mice that had previously saccharin paired with RK. The rationale for performing a two-bottle test following the CTA, was because the training and testing were performed under water-restricted conditions and the CTA test was rather brief access (1 h) to saccharin. The two-bottle test was performed under water-replete conditions with a prolonged access to both saccharin and water. Our findings that previous pairing RK reduced intake during a two-bottle preference test with saccharin in male mice suggest that RK could be producing a mild associated taste avoidance. In addition, it possible at human equivalent doses a taste aversion or avoidance could be experienced. Further sensory studies are needed to fully characterize the dose-dependent taste profile of RK in rodents and humans.
In the acute feeding experiments, we observed a 20% mortality in both sexes under both feeding conditions following the 640 mg/kg dose. Consequently, we performed a histopathological evaluation following an acute single oral dose of 640 mg/kg in male mice. In order to determine more severe acute pathology, mice were dosed following a 24 h food deprivation. Of the 14 mice dosed only 8 mice (57.14%) survived for pathological examination. The only toxicological findings were in the epididymal white adipose, spleen, and thymus tissues. For the epididymal white adipose tissue, there were higher ratio of adipocyte nuclei and reduced intracellular lipid. Chronic (4 to 8 week) daily dosing with RK coincident with high-fat diet exposure results in a decrease in white adipose tissue levels, compared with vehicle treated animals(Kshatriya et al., 2019; Mehanna et al., 2018; Morimoto et al., 2005). In vitro studies demonstrate that RK dose dependently inhibits peroxisome proliferator-activated receptor-γ (PPARγ), CCAAT enhancer binding protein-α (C/EBPα), fatty acid binding protein 4 (FABP 4) and possibly through heme-oxygenase-1 (HO-1) pathways to prevent adipogenesis and lipid accumulation (Chen et al., 2011; Park, 2010, 2015; Tsai et al., 2017). Taken together, this suggests that the acute effects on epididymal white adipose tissue could contribute to the known beneficial effects of RK to prevent high-fat diet-induced fat accumulation. Blood parameters suggest possible intravascular hemolysis, but this could be masked by dehydration. It has been reported that acute doses of RK as high as 1000 mg/kg were administer to CD-1 mice without an increase in polychromatic erythrocytes in bone marrow (Api et al., 2019). Future work will be needed to fully support the involvement of spleen and hematological parameters in the pathology of RK. The liver has been suggested as one target of the RK pathology. One previous study in male ND-4 obese mice dosed with RK for 10 days found an increase in ALT with RK 500 mg/kg and 330 mg/kg compared with vehicle (Mir et al., 2019). In our present study, we did not observe RK-induced hepatic histopathological changes nor ALT and AST elevations after 2 days of treatment. We did notice an elevation in AST on day 4, suggesting the hepatic pathology observed with RK could be secondary to other pathological changes in other tissues, such as spleen and thymus.
This study was the first to identify mortality and histopathological changes with acute RK doses that suppress standard chow intake in male and female C57BL/6J mice. The RK dose that reliably suppressed food intake was 640 mg/kg. One major advantage of our study was that we examined the histopathological changes associated with mortality and examine the severity of those changes in major organ systems. A limitation of our study was that doses that produce biological effects in rodents are not easily converted to human equivalent doses. However, using the allometric scaling, based on the ratio of body surface area, the human equivalent dose for feeding suppression with RK would be estimated to be 50 mg/kg or 3000 mg for a 60 kg individual(Nair and Jacob, 2016). Based on our toxicological findings, RK should be not be marketed as an “appetite suppressant” without further evaluation.
Figure 3. Representative hematoxylin and eosin staining of tissue from mice receiving a single dose by oral gavage of raspberry ketone (640 mg/kg) or vehicle.
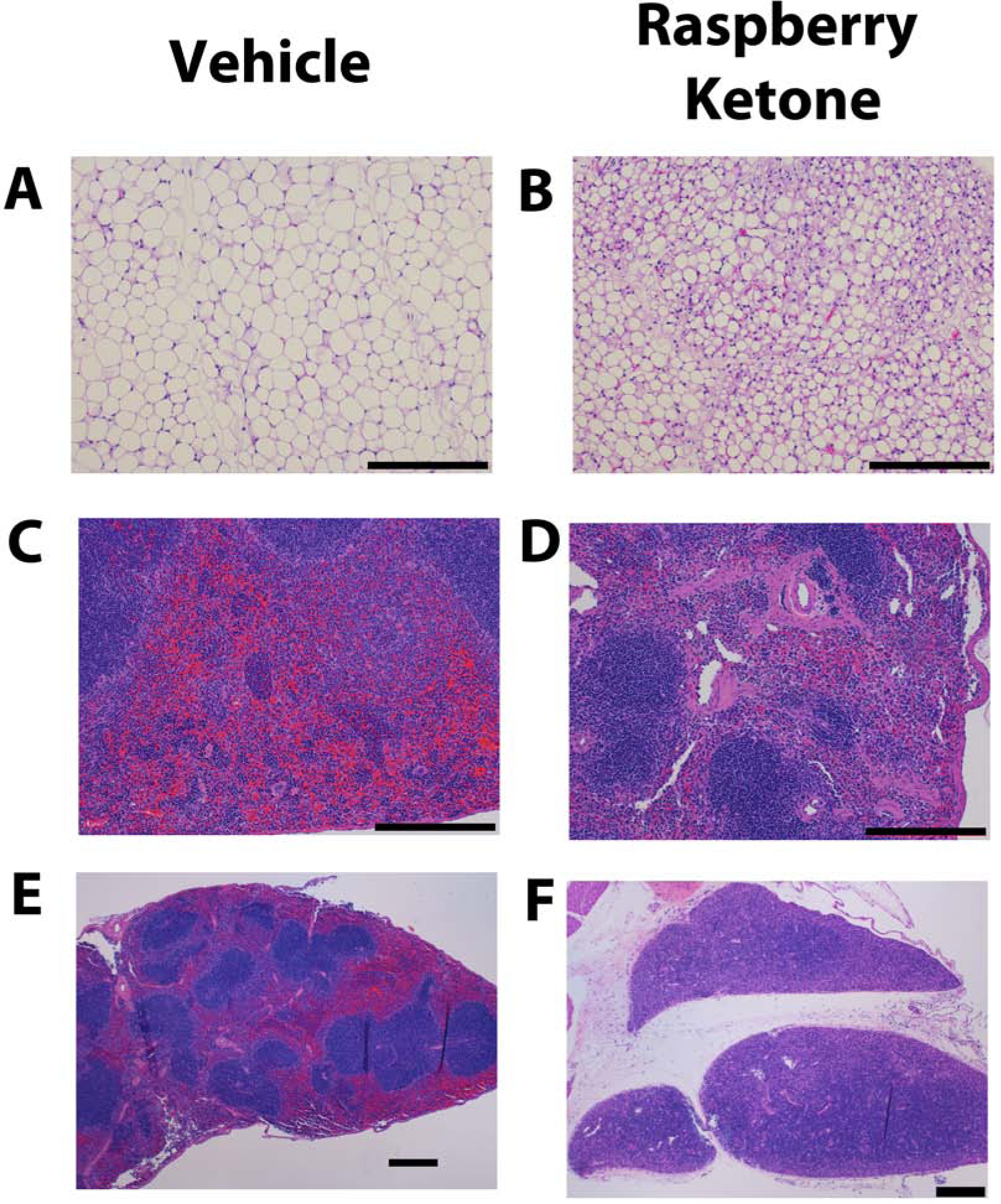
Male mice (10 weeks old) were 24 h food deprived prior to receiving treatment and euthanized on 2 or 4 days after treatment. Representative tissues are from mice euthanized on day 4. Vehicle tissue is on the left (A, C, E) and raspberry ketone (B, D, F) is on the right. A & B: epididymal adipose tissue, C & D: spleen, E & F: thymus. Tissue from vehicle-treated mice were scored as a 0 (No noteworthy pathology). Representative raspberry ketone-treated mice epididymal fat (B) was score as 3 (Moderate pathology), spleen (D) was scored as a 2 (Mild pathology), and thymus was scored as 4 (Marked pathology). Black bars on each figure represents 250 μm. There were no observable histopathological changes in other organs systems and tissue in raspberry ketone-treated compared with vehicle-treated mice.
Highlights.
Raspberry ketone (RK) is a flavor agent and dietary supplement for weight loss
Oral 640 mg/kg RK suppressed acute (24 h) food intake in C57BL/6J mice
Single oral 640 mg/kg resulted in 43% mortality within 2 days
Toxicology indicated adipose atrophy, splenic abnormalities, and thymus involution
Acknowledgments
This research was supported by the NIH-NCCIH grant R01AT008933 and USDA (NIFA) NJ06280.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None of the authors have any conflict of interests. All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- (FEEDAP), E.P.o.A.a.P.o.S.u.i.F., 2016. Safety and efficacy of aromatic ketones, secondaryalcohols and related esters belonging to chemical group21 when used asflavourings for all animal species. EFSA Journal 14, 4557. [Google Scholar]
- Administration, U.F.a.D., 2019. Food Additives Permitted For Direct Addition To Food For Human Consumption. Code of Federal Regulations Title 21 3, 1–20. [Google Scholar]
- Api AM, Belsito D, Biserta S, Botelho D, Bruze M, Burton GA Jr., Buschmann J, Cancellieri MA, Dagli ML, Date M, Dekant W, Deodhar C, Fryer AD, Gadhia S, Jones L, Joshi K, Lapczynski A, Lavelle M, Liebler DC, Na M, O’Brien D, Patel A, Penning TM, Ritacco G, Rodriguez-Ropero F, Romine J, Sadekar N, Salvito D, Schultz TW, Siddiqi F, Sipes IG, Sullivan G, Thakkar Y, Tokura Y, Tsang S, 2019. RIFM fragrance ingredient safety assessment, 4-(p-hydroxyphenyl)-2-butanone, CAS Registry Number 5471-51-2. Food Chem Toxicol 134 Suppl 2, 110948. [DOI] [PubMed] [Google Scholar]
- Arent SM, Walker AJ, Pellegrino JK, Sanders DJ, McFadden BA, Ziegenfuss TN, Lopez HL, 2018. The Combined Effects of Exercise, Diet, and a Multi-Ingredient Dietary Supplement on Body Composition and Adipokine Changes in Overweight Adults. J Am Coll Nutr 37, 111–120. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK, 2001. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses 26, 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker A, Fischer M, Berger RG, 2001. Raspberry ketone from submerged cultured cells of the basidiomycete Nidula niveo-tomentosa. Biotechnol Prog 17, 568–572. [DOI] [PubMed] [Google Scholar]
- Borejsza-Wysocki W, Goers SK, McArdle RN, Hrazdina G, 1992. (p-Hydroxyphenyl)butan-2-one Levels in Raspberries Determined by Chromatographic and Organoleptic Methods. J. Agric. Food Chem 40, 1176–1177. [Google Scholar]
- Borejsza-Wysocki W, Hrazdina G, 1994. Biosynthesis of p-Hydroxyphenylbutan-2-one in raspberry fruits and tissue culture. Phytochemistry 35, 623–628. [Google Scholar]
- Bredsdorff L, Wedebye EB, Nikolov NG, Hallas-Moller T, Pilegaard K, 2015. Raspberry ketone in food supplements--High intake, few toxicity data--A cause for safety concern? Regul Toxicol Pharmacol 73, 196–200. [DOI] [PubMed] [Google Scholar]
- Chen HS, Liu M, Shi LJ, Zhao JL, Zhang CP, Lin LQ, Liu Y, Zhang SJ, Jin JC, Wang L, Shen BZ, Liu JR, 2011. Effects of raspberry phytochemical extract on cell proliferation, apoptosis, and serum proteomics in a rat model. J Food Sci 76, T192–198. [DOI] [PubMed] [Google Scholar]
- Cotten BM, Diamond SA, Banh T, Hsiao YH, Cole RM, Li J, Simons CT, Bruno RS, Belury MA, Vodovotz Y, 2017. Raspberry ketone fails to reduce adiposity beyond decreasing food intake in C57BL/6 mice fed a high-fat diet. Food Funct 8, 1512–1518. [DOI] [PubMed] [Google Scholar]
- Gora J, Brud W, 1983. Progress in synthesis of sensory important trace components of essential oils and natural flavours. Nahrung 27, 413–428. [DOI] [PubMed] [Google Scholar]
- Gupta A, Li X, DiCicco-Bloom E, Bello NT, 2018. Altered salt taste response and increased tongue epithelium Scnna1 expression in adult Engrailed-2 null mice. Physiol Behav 194, 410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MAM, Manoukis NC, Osborne T, Barchia IM, Gurr GM, Reynolds OL, 2017. Semiochemical mediated enhancement of males to complement sterile insect technique in management of the tephritid pest Bactrocera tryoni (Froggatt). Sci Rep 7, 13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kshatriya D, Li X, Giunta GM, Yuan B, Zhao D, Simon JE, Wu Q, Bello NT, 2019. Phenolic-enriched raspberry fruit extract (Rubus idaeus) resulted in lower weight gain, increased ambulatory activity, and elevated hepatic lipoprotein lipase and heme oxygenase-1 expression in male mice fed a high-fat diet. Nutr Res 68, 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, 2016. Further research on the biological activities and the safety of raspberry ketone are needed. NFS Journal. 2:15–18. NFS Journal 2, 15–18. [Google Scholar]
- Leu SY, Chen YC, Tsai YC, Hung YW, Hsu CH, Lee YM, Cheng PY, 2017. Raspberry Ketone Reduced Lipid Accumulation in 3T3-L1 Cells and Ovariectomy-Induced Obesity in Wistar Rats by Regulating Autophagy Mechanisms. J Agric Food Chem 65, 10907–10914. [DOI] [PubMed] [Google Scholar]
- Leu SY, Tsai YC, Chen WC, Hsu CH, Lee YM, Cheng PY, 2018. Raspberry ketone induces brown-like adipocyte formation through suppression of autophagy in adipocytes and adipose tissue. J Nutr Biochem 56, 116–125. [DOI] [PubMed] [Google Scholar]
- Lopez HL, Ziegenfuss TN, Hofheins JE, Habowski SM, Arent SM, Weir JP, Ferrando AA, 2013. Eight weeks of supplementation with a multi-ingredient weight loss product enhances body composition, reduces hip and waist girth, and increases energy levels in overweight men and women. J Int Soc Sports Nutr 10, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehanna ET, Barakat BM, ElSayed MH, Tawfik MK, 2018. An optimized dose of raspberry ketones controls hyperlipidemia and insulin resistance in male obese rats: Effect on adipose tissue expression of adipocytokines and Aquaporin 7. Eur J Pharmacol 832, 81–89. [DOI] [PubMed] [Google Scholar]
- Mir TM, Ma G, Ali Z, Khan IA, Ashfaq MK, 2019. Effect of Raspberry Ketone on Normal, Obese and Health-Compromised Obese Mice: A Preliminary Study. J Diet Suppl, 1–16. [DOI] [PubMed] [Google Scholar]
- Morimoto C, Satoh Y, Hara M, Inoue S, Tsujita T, Okuda H, 2005. Anti-obese action of raspberry ketone. Life Sci 77, 194–204. [DOI] [PubMed] [Google Scholar]
- Nair AB, Jacob S, 2016. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdyke DLJ, 1978. 4-(p-Hydroxyphenyl)-2-butanone. Food. Cosmet. Toxicol 16, 781–782. [Google Scholar]
- Park KS, 2010. Raspberry ketone increases both lipolysis and fatty acid oxidation in 3T3-L1 adipocytes. Planta Med 76, 1654–1658. [DOI] [PubMed] [Google Scholar]
- Park KS, 2015. Raspberry ketone, a naturally occurring phenolic compound, inhibits adipogenic and lipogenic gene expression in 3T3-L1 adipocytes. Pharm Biol 53, 870–875. [DOI] [PubMed] [Google Scholar]
- Pedapudi S, Chin CK, Pedersen H, 2000. Production and elicitation of benzalacetone and the raspberry ketone in cell suspension cultures of Rubus idaeus. Biotechnol Prog 16, 346–349. [DOI] [PubMed] [Google Scholar]
- Rapid Alert System for Food and Feed (RASFF), E.C., 2015. Notification details - 2015.0281 Portal Last updated 12/13/2017.
- Rauhut AS, Hawrylak M, Mardekian SK, 2008. Bupropion differentially alters the aversive, locomotor and rewarding properties of nicotine in CD-1 mice. Pharmacol Biochem Behav 90, 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, 2006. Oral, post-oral and genetic interactions in sweet appetite. Physiol Behav 89, 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JR, John G, 2015. Biobased Fat Mimicking Molecular Structuring Agents for Medium-Chain Triglycerides (MCTs) and Other Edible Oils. J Agric Food Chem 63, 10536–10542. [DOI] [PubMed] [Google Scholar]
- Steffensen I-L, 2018. Safer food supplements in the Nordic countries. NORDIC WORKING PAPERS, 1–29. [Google Scholar]
- Stringer LD, Soopaya R, Butler RC, Vargas RI, Souder SK, Jessup AJ, Woods B, Cook PJ, Suckling DM, 2019. Effect of Lure Combination on Fruit Fly Surveillance Sensitivity. Sci Rep 9, 2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YC, Yang BC, Peng WH, Lee YM, Yen MH, Cheng PY, 2017. Heme oxygenase-1 mediates anti-adipogenesis effect of raspberry ketone in 3T3-L1 cells. Phytomedicine 31, 11–17. [DOI] [PubMed] [Google Scholar]
- Wang C, Zheng P, Chen P, 2019. Construction of synthetic pathways for raspberry ketone production in engineered Escherichia coli. Appl Microbiol Biotechnol 103, 3715–3725. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Yamatodani A, 2018. Strain differences in the development of cisplatin-induced pica behavior in mice. J Pharmacol Toxicol Methods 91, 66–71. [DOI] [PubMed] [Google Scholar]
- Yimam M, Jiao P, Hong M, Brownell L, Lee YC, Hyun EJ, Kim HJ, Kim TW, Nam JB, Kim MR, Jia Q, 2019. Evaluation of Natural Product Compositions for Appetite Suppression. J Diet Suppl 16, 86–104. [DOI] [PubMed] [Google Scholar]
- Yuan B, Zhao D, Du R, Kshatriya D, Bello NT, Simon JE, Li Q, 2018. A highly sensitive ultra-high performance liquid chromatography/tandem mass spectrometry method with in-source fragmentation for rapid quantification of raspberry ketone. Journal of Food and Drug Analysis In Press, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Zhao D, Du R, Kshatriya D, Bello NT, Simon JE, Wu Q, 2019. A highly sensitive ultra-high performance liquid chromatography/tandem mass spectrometry method with in-source fragmentation for rapid quantification of raspberry ketone. J Food Drug Anal 27, 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Yuan B, Kshatriya D, Polyak A, Simon JE, Bello NT, Wu Q, 2020. Influence of Diet-Induced Obesity on the Bioavailability and Metabolism of Raspberry Ketone (4-(4-Hydroxyphenyl)-2-Butanone) in Mice. Mol Nutr Food Res, e1900907. [DOI] [PMC free article] [PubMed] [Google Scholar]


