Abstract
Background:
Eosinophilic gastrointestinal diseases (EGID) are defined by marked eosinophilia in the gastrointestinal (GI) tract resulting in a wide variety of GI symptoms. When accompanied by blood hypereosinophilia (HE; AEC≥1500/mm3), EGID can occur as an isolated GI disorder (HES/EGID overlap) or as part of a multisystem hypereosinophilic syndrome (Multisystem HES).
Objective:
To describe the gastrointestinal disease of patients categorized as HES/EGID overlap versus those with Multisystem HES.
Methods:
Consecutively enrolled patients on a natural history protocol to study eosinophilia with biopsy-proven EGID involving the esophagus, stomach, small-bowel and/or colon were evaluated for clinical, histopathologic, and endoscopic features by retrospective chart review.
Results:
Among the 56 patients with EGID and HE, 34 were categorized as HES/EGID overlap and 22 as Multisystem HES. Demographics, GI symptoms and associated comorbidities were similar between the two groups. Multi-segment GI eosinophilia was present in 20/30(67%) patients who underwent tissue sampling of all four GI segments. Tissue eosinophilia in all four GI segments was found in 5/30(17%) patients. Dietary therapy was more common in HES/EGID overlap patients (65% vs. 23%, p=0.0028). Multisystem HES patients were more likely to receive glucocorticoids (100% vs. 79%, p=0.0349) and non-glucocorticoid systemic therapies (77% vs. 38%, p=0.0061). One-third (8/22) of Multisystem HES patients presented with isolated GI symptoms before developing extra-intestinal manifestations at a median of 1 year (range 0.25–15).
Conclusion:
There are striking clinical similarities between Multisystem HES and HES/EGID overlap patients, despite differing treatment approaches. Moreover, Multisystem HES can present with isolated GI involvement. Larger prospective studies are needed to confirm these findings.
Keywords: Eosinophilic gastrointestinal Disorders, eosinophilia, hypereosinophilic syndrome, eosinophilic gastritis, eosinophilic gastroenteritis, eosinophilic colitis
Introduction
Hypereosinophilic syndromes (HES) are rare and heterogenous syndromes defined by elevated peripheral blood eosinophils >1500/mm3 (hypereosinophilia; HE) with associated organ and/or tissue damage. This definition has evolved over time to become more expansive and clarifying with various clinical subtypes including lymphoid, myeloid and idiopathic variants. In 2005, a consensus statement from a multi-disciplinary workshop proposed to classify patients with single organ system eosinophilic disease (EGID, chronic eosinophilic pneumonia etc.) that are accompanied by HE as a variant of HES, termed “overlap” HES and that appears to be accepted today (1–3).
Despite the increasing prevalence and recognition of both eosinophilic esophagitis (EoE) and non-EoE EGIDs, much less is known about the natural history and clinical spectrum of eosinophilic involvement of the stomach (eosinophilic gastritis (EG)), small bowel (eosinophilic enteritis (EE)), or colon and rectum (eosinophilic colitis (EC)), or how EGID with hypereosinophilia differs from EGID in the setting of HES with multiorgan system involvement. In part, the challenge of correlating reported symptoms to endoscopic and histopathologic findings is similar to the case in other inflammatory bowel diseases, such as Crohn’s disease and ulcerative colitis, where symptoms may be discordant with endoscopic or histopathologic appearance. Additionally, the rarity of these disorders(4), lack of consensus recommendations for obtaining or evaluating biopsies(5), absence of International Classification of Diseases (ICD) codes for estimating the prevalence of HES(6), and exclusion of “HES” or the presence of peripheral hypereosinophilia in active drug studies of EGIDs (Benralizumab for EG [NCT03496571]), likely play a role. Over the past 5 years, the Consortium of Eosinophilic Gastrointestinal Researchers (CEGIR) has attempted to overcome some of these hurdles through multi-institutional collaboration and pooling of patients enrolled on studies(7), yet the distinction between HES/EGID overlap and HES with multiorgan system involvement that includes the GI tract remains unresolved.
In the absence of extra-gastrointestinal clinical manifestations, most clinicians pay little attention to peripheral blood eosinophilia in treating patients with EGID. This is due, in large part, to the poor correlation between blood and tissue eosinophilia in EoE(8). Additionally, the natural history of isolated EGIDs is unknown and whether patients go on to develop Multisystem HES disease or progressive GI segment involvement has not been systematically studied. Lastly, whereas treatment for Multisystem HES patients with GI manifestations has predominantly involved systemic treatment rather than topical or dietary therapies, the rationale behind this approach has not been validated to date. As such, there is a need to understand the natural history of this cohort of patients for better treatment decision-making and prognostication. In the present study, we describe the clinical, endoscopic, histopathologic, and treatment approaches for a cohort of HES patients with either EGID as the sole organ system involvement (HES/EGID overlap) or HES with multisystemic features and a histopathologic diagnosis of EGID (Multisystem HES).
Methods
HES patients (defined as AEC≥1500/mm3 with evidence of eosinophilic end organ involvement) were recruited under an IRB-approved natural history protocol designed to study eosinophilia (NCT00001406]) and were seen between December 30th, 1994 and April 30th, 2018 at the National Institutes of Health (NIH). Every patient underwent a standard evaluation at their first NIH visit that included: history, physical exam, and blood testing that included: CBC with differential, comprehensive metabolic panel, testing for a number of genetic mutations associated with myeloid HES, serum tryptase, vitamin B12, troponin, and quantitative immunoglobulins, including IgE. To evaluate for lymphoid HES, lymphocyte immunophenotyping and TCR clonality testing were performed. End organ involvement was further assessed by transthoracic echocardiography, EKG, pulmonary function testing, CT imaging or review of outside films, biopsies of affected tissues (where possible) and review of bone marrow biopsy specimens. Additional testing, including serologic and stool testing for helminth infection, was performed if clinically indicated.
A clinical database comprised of data from patients enrolled on the above-described natural history protocol was queried to identify patients with gastrointestinal symptoms and/or a confirmed diagnosis of EGID based on the opinion of two physicians with expertise in evaluation and treatment of HES (A.K. and P.K.) and then-current knowledge of the clinical course of each patient. Patients were included if they had an absolute eosinophil count (AEC) of ≥1500/mm3 (i.e. HES) and histopathologic confirmation of EGID irrespective of other clinical manifestations. For the purposes of this study, HES/EGID Overlap is defined by AEC≥1500/mm3 and biopsy-proven gastrointestinal eosinophilia without evidence of involvement of other organ systems and Multisystem HES refers to patients with AEC≥1500/mm3 and multisystem involvement including biopsy-proven eosinophilic gastrointestinal involvement. Patients with hypereosinophilia secondary to infection, drug hypersensitivity or neoplasia were excluded from analysis, as were those with primary immune deficiency, including Loeys-Dietz and DOCK8. All patients signed informed consent.
Biopsies performed at the NIH and/or outside centers were reviewed for histopathologic confirmation of EGID diagnosis using existing NIH pathology reports. In cases where historic NIH pathology reports were suggestive of EGID (i.e., “increased” or “numerous” eosinophils, but without enumeration), original slides were re-read whenever possible by an NIH pathologist (A.P. or M.Q.). In some cases, patients were re-contacted and/or reconsented to retrieve external medical records or pathology slides for re-review.
Tissue eosinophilia in at least one GI segment was required for inclusion, and segment involvement was defined as: peak eosinophil count ≥15 eosinophils per high power field (EOS/HPF) in the esophagus, ≥30 EOS/HPF in the stomach or small bowel, and ≥60 EOS/HPF in the large bowel(9,10). Where possible, multiple levels within a tissue biopsy were examined to identify the level with the highest eosinophil concentration. Eosinophil enumeration was performed at 400x original magnification.
Retrospective chart and electronic medical record review were performed to retrieve clinical symptoms, intestinal and extra-intestinal organ involvement, comorbid atopic diseases, therapeutic interventions, laboratory, endoscopic, and histopathologic findings. This was performed by two Allergist/Immunologists (F.L.K. and P.K.) and two Gastroenterologists (B.C. and S.K.). Atopic comorbidities (asthma, allergic rhinitis, atopic dermatitis and drug, or venom hypersensitivity), IgE-mediated food allergy and specific food triggers for EGID were identified. IgE-mediated food allergy was categorized as “proven” if a convincing clinical history temporally-associated with consuming a specific food with or without testing or challenge was documented. Food allergies identified through serologic or skin testing, or suspected by patients, or with symptomology that was inconsistent with IgE-mediated food allergy were categorized as “suspected.” EGID food triggers were categorized as “proven” if clinical and/or histopathologic improvement was demonstrated after elimination of the specific food, with return of symptoms and/or histopathologic relapse (return of eosinophilia) with re-introduction of the food. Otherwise, EGID food triggers were categorized as “suspected.”
All patients who underwent endoscopy at the NIH Clinical Center were identified through Provation©, an electronic endoscopic documentation database. Reports from endoscopies performed at other institutions were also reviewed. Medical records were queried for symptoms of dysphagia, odynophagia, chest pain, regurgitation, food impaction, dyspepsia, abdominal pain, nausea, vomiting, bloating, early satiety, decreased appetite, hematemesis, melena, diarrhea, constipation, weight loss and known sequelae of EGID, including gastroparesis, food impaction or need for esophageal dilation, ascites, iron-deficiency anemia, protein-losing enteropathy, perforation, and bowel obstruction. Endoscopy reports were reviewed for the presence of abnormal findings, such as erythema, rings or furrows, ulceration, inflammation, or other abnormal appearing mucosa.
Results of numerical data are presented as median with range. The non-parametric Mann-Whitney U test was used for group comparisons with numeric or ordinal responses, and Fisher’s exact test was used for categorical responses. To depict intersections of different sets of patients who underwent biopsy in different anatomic regions, we used UpSetR plots (Figure 2A, Supplemental Figures E1 and E2) instead of Venn diagrams created using the UpSetR R package(11). When adjustments are made for multiple comparisons for apparently significant effects (unadjusted p<=0.05), we use the Holm adjustment(12) with the family of tests defined by the table. Calculations were done in Prism (version 8.2.0) or R (version 3.6.1).
Figure 2A.
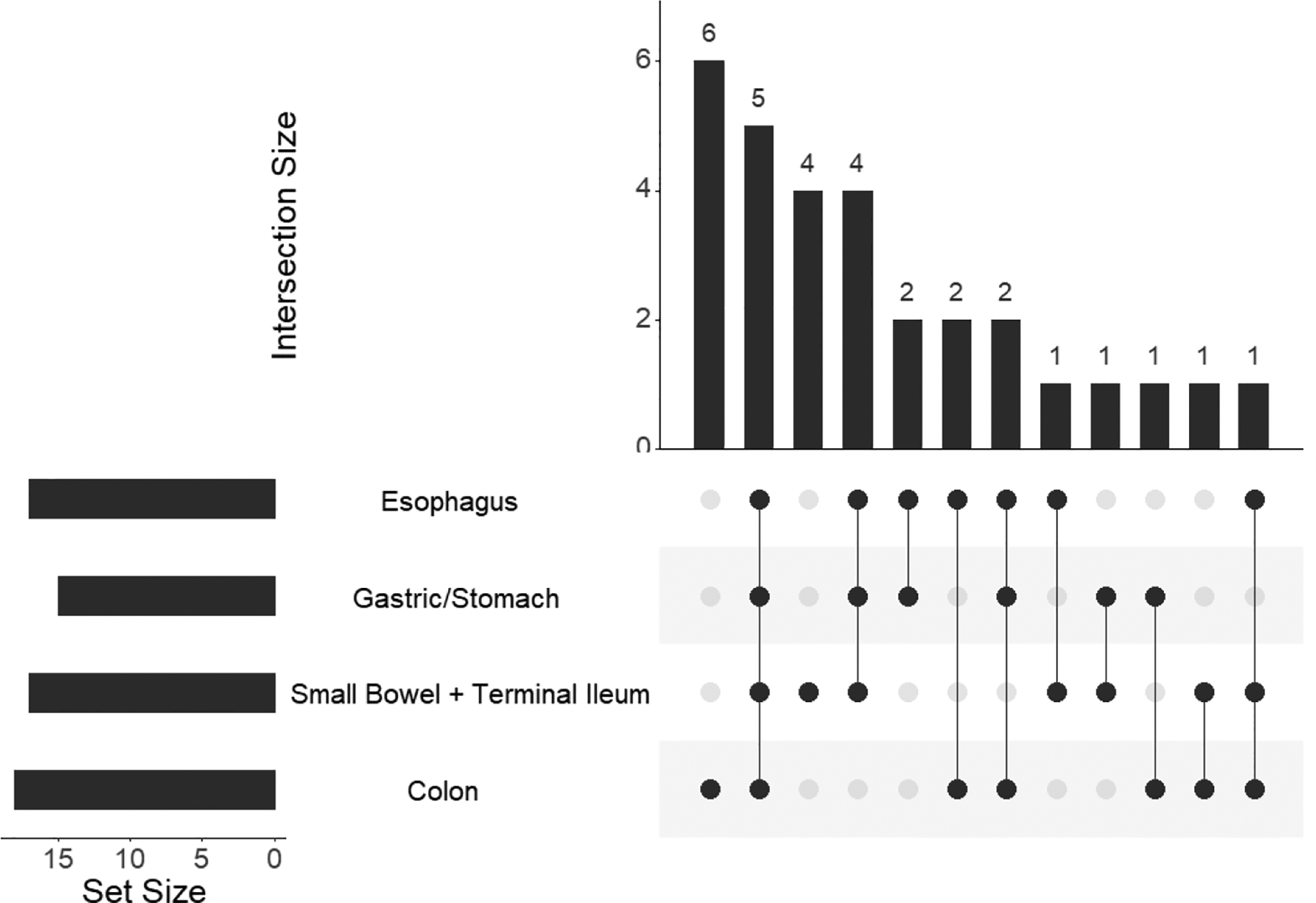
This graph represents the subset of patients who underwent biopsies in all four indicated anatomic regions (n=30). Horizontal bars indicate numbers of patients who had a biopsy meeting the eosinophil inclusion cutoff in the indicated anatomic region. Vertical bars and the numbers atop indicate patients who had a biopsy in the indicated combinations of anatomic regions meeting inclusion criteria. Black dots indicate anatomic region that met criteria for eosinophilia and combinations are connected by lines. Patients in the various groups are mutually exclusive.
Results
Patients and demographics
Eighty-four patients with AEC≥1500/mm3 and suspected EGID based on gastrointestinal symptoms were identified in the clinical database. Histopathologic evidence of gastrointestinal eosinophilia was confirmed in 56/84 (67%) (Figure 1), and these patients were included in the study. Among these, 34/56 (61%) patients met criteria for HES/EGID overlap. The remaining 22/56 (39%) were diagnosed as Multisystem HES patients with a variety of clinical subtypes including myeloid HES, lymphoid HES, idiopathic HES and other overlap HESs, such as anti-neutrophil cytoplasmic antibody (ANCA) negative eosinophilic granulomatosis with polyangiitis (EGPA) without a biopsy-proven diagnosis (Fig 1). Patients whose GI tracts were not biopsied (n=3), whose biopsies showed insufficient eosinophilia (n=10), or whose slides were not available for re-review (n=14) were excluded, as were those who were ultimately diagnosed with a disease other than HES (n=1).
Figure 1.
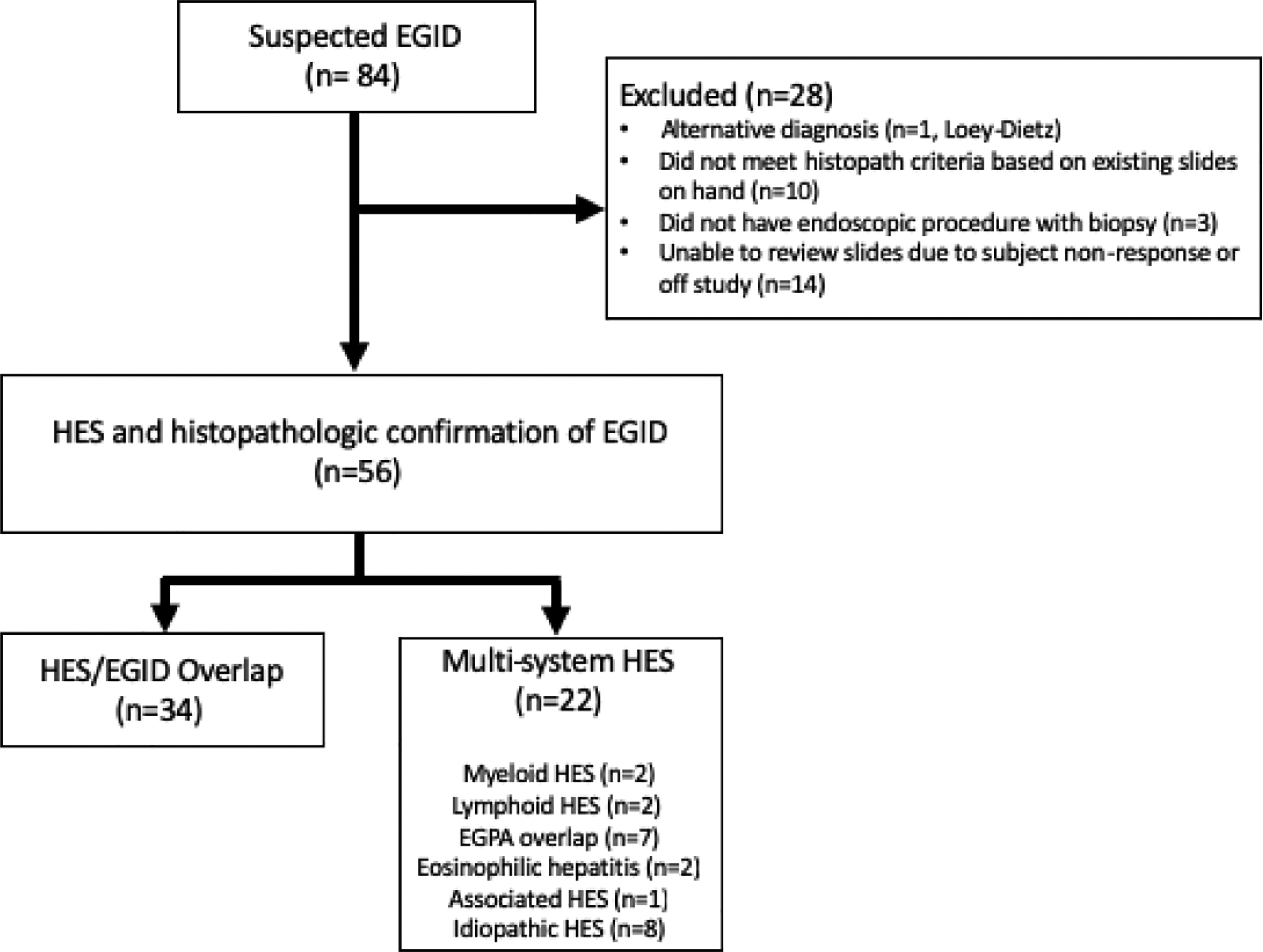
NIH study population of patients with hypereosinophilia and gastrointestinal manifestations of disease. HES - hypereosinophilic syndrome; EoE - eosinophilic esophagitis; EGID - eosinophilic gastrointestinal disease; EGPA - eosinophilic granulomatous polyangiitis.
As a group, this HES patient cohort developed GI symptoms at a median age of 35 years and presented for their initial NIH visit at a median age of 41.5 years (range 14–68), with no difference between those with HES/EGID overlap and those with Multisystem HES (Table 1). Male predominance was not noted in either HES/EGID overlap or Multisystem HES patients.
Table 1 -.
Demographic Characteristics in HES EGID Overlap and Multisystem HES Patients
| Group | HES/EGID Overlap (n=34) | Multisystem HES (n=22) | P-value |
|---|---|---|---|
| Demographics | |||
| Median Age at presentation to NIH, years (range) | 45 (14–68) | 40.5 (21–67) | p=0.49 |
| Median Age at first presentation, years (range) | 30 (33; 2–56) | 35 (22; 7–64) | p=0.54 |
| Sex, M/F (%) | 14/20 (41%/59%) | 11/11 (50%/50%) | p=0.58 |
| Race | p=0.26 | ||
| African American or Black | 1 | 3 | |
| Asian | 2 | 1 | |
| White | 31 | 17 | |
| Unknown | 0 | 1 | |
| Median BMI, kg/m2 (n; range) | 22.4 (29; 14.5 – 36.8) | 22.9 (22; 17.3 – 49.5) | p=0.22 |
| Family History of EGID | Yes 2 (5.9%) Suspected 5 (15%) No 20 (59%) Unknown 7 (21%) |
Yes 1 (4.5%) Suspected 1 (4.5%) No 18 (54%) Unknown 2 (9%) |
Fisher’s exact 2×3 p=0.38 |
BMI- Body Mass Index. EGID- eosinophilic gastrointestinal disease. HES- hypereosinophilic syndrome. Fisher’s exact test (2×2 for sex, 2×4 for race and family history of EGID) or Mann-Whitney test (all other variables) were performed.
Gastrointestinal Symptoms and Atopy are common in HES Multisystem patients
Despite reports of more serious complications, such as perforation, in EGID patients with eosinophilia(13), clinical symptoms were similar in the HES/EGID overlap and Multisystem HES groups, with the most common symptoms being abdominal pain (88.2% vs. 77.3% respectively) and diarrhea (85.3% vs.63.6%). Other common symptoms included dysphagia (44.1% vs. 31.8%), nausea (70.6% vs. 50%), vomiting (52.9% vs. 54.5%), and dyspepsia/reflux (47.1 vs. 40.9%) (Table 2).
Table 2 -.
Gastrointestinal Symptoms and Complications
| Clinical Symptoms | HES/EGID Overlap (N = 34) | Multisystem HES (N = 22) | P-value |
|---|---|---|---|
| Dysphagia | 15 (44.1%) | 7 (31.8%) | p=0.41 |
| Odynophagia | 2 (5.9%) | 2 (9.1%) | p=0.64 |
| Chest pain | 8 (23.5%) | 8 (36.4%) | p=0.37 |
| Regurgitation | 8 (23.5%) | 5 (22.7%) | p=1.00 |
| Food impaction | 2 (5.9%) | 0 | p=0.51 |
| Dyspepsia/Reflux | 16 (47.1%) | 9 (40.9%) | p=0.78 |
| Abdominal pain | 30 (88.2%) | 17 (77.3%) | p=0.29 |
| Nausea | 24 (70.6%) | 11 (50%) | p=0.16 |
| Vomiting | 18 (52.9%) | 12 (54.5%) | p=1.00 |
| Bloating | 10 (29.4%) | 5 (22.7%) | p=0.76 |
| Early Satiety | 8 (23.5%) | 8 (36.4%) | p=0.37 |
| Decreased Appetite | 11 (32.4%) | 6 (27.2%) | p=0.77 |
| Hematemesis | 1 (2.9%) | 0 | p=1.00 |
| Melena | 0 | 0 | p=1.00 |
| Diarrhea | 29 (85.3%) | 14 (63.6%) | p=0.10 |
| Constipation | 4 (11.8%) | 3 (13.6%) | p=1.00 |
| Hematochezia | 3 (8.8%) | 4 (18.2%) | p=0.41 |
| Weight loss | 12 (35.3%) | 7 (31.8%) | p=1.00 |
| Gastroparesis | 2 (5.9%) | 0 | p=0.51 |
| Esophageal Dilation | 0 | 0 | p=1.00 |
| Ascites | 2 (5.9%) | 1 (4.5%) | p=1.00 |
| Anemia | 9 (26.5%) | 5 (22.7%) | p=1.00 |
| Protein Losing Enteropathy | 3 (8.8%) | 0 | p=0.27 |
| Perforation | 0 | 0 | p=1.00 |
EGID- eosinophilic gastrointestinal disease. HES- hypereosinophilic syndrome.
There was a similar prevalence of atopic conditions, such as asthma, allergic rhinitis, atopic dermatitis, and non-food allergies, in patients with HES/EGID as compared with those with Multisystem HES with GI involvement (Table 3). The prevalence of IgE-mediated food allergies and EGID food triggers, whether suspected or proven, was also similar between the two groups. At their initial visit, patients in the two groups did not differ in peripheral blood eosinophil counts, serum IgE, or serum tryptase (Table 3), despite comparable rates of ongoing therapy. The historic peak AEC was increased in the Multisystem HES group as compared to the HES/EGID overlap group (median 9600 vs 4510 cells/mm3, adjusted p=0.0285, Table 3).
Table 3 –
Baseline Medical History and Clinical Presentation
| HES/EGID Overlap (N=34) | Multisystem HES (N=22) | P-value | |
|---|---|---|---|
| Comorbidities (n; % of group) | |||
| Asthma | 15 (32; 47%) | 12 (22; 55%) | p=0.78 |
| Allergic Rhinitis | 24 (32; 75%) | 11 (22; 50%) | p=0.08 |
| Atopic Dermatitis | 3 (32; 9%) | 1 (21; 4.5%) | p=1.00 |
| Drug/Venom Allergy | 8 (32; 25%) | 6 (22; 27%) | p=1.00 |
| Any IgE Food Allergies1,2 (n; % of group) | 9 (33; 27%) | 3 (22; 14%) | p=0.32 |
| Proven IgE FA | 7 | 3 | |
| Suspected IgE FA | 5 | 0 | |
| Any EGID Food Triggers2 (n; % of group) | 17 (33; 52%) | 6 (22; 27%) | p=0.10 |
| Proven Food Triggers | 6 (18%) | 1 (4.5%) | p=0.23 |
| Suspected Food Triggers | 15 (44%) | 5 (23%) | p=0.15 |
| On treatment at initial NIH presentation | 21 (62%) | 17 (77%) | p=0.26 |
| Laboratory Findings | |||
| Median AEC at initial NIH presentation, K/uL (n:range) | 880 (34; 40–4810) | 1360 (22; 40–6580) | p=0.094 |
| Median Historic Peak AEC K/uL (n:range) |
4510 (34; 1030–17200) | 9600 (22; 1460 – 100000) | p=0.0015 Adjusted p=0.0285* |
| IgE, IU/mL (n: range) | 156.5 (34; 11.7–3357) | 207.5 (22; 8.6–3198) | p=1.00 |
| Tryptase, ng/mL (n: range) | 5.02 (34; 2.2–15.3) | 6.5 (22; 1.3–19.7) | p=0.25 |
| CRP, mg/dL (n: range) | 0.6 (33; 0.08–6.1) | 1.8 (20; 0.08–16) | p=0.0179 |
| ESR, mm/hr (n: range) | 7(33;1–42) | 11.5 (20; 2–38) | p=0.17 |
| Hgb, g/dL (n: range) | 13.6 (34; 10.5–19.6) | 13.6 (22; 11.1–17.2) | p=1.00 |
| Albumin, g/dL (n: range) | 4.1 (34; 2.6–4.9) | 4.05 (22; 3.4–4.7) | p=0.45 |
Oral allergy syndrome was excluded as an IgE-mediated food allergy (EGID/Overlap n=3; Multisystem HES, n=1).
Patients could have had multiple IgE-mediated food allergies or EGID food triggers, either of which might have been categorized as suspected vs. proven.
AEC - absolute eosinophil count; CRP - C-reactive protein; EGID - Eosinophilic Gastrointestinal Disease; ESR - Erythrocyte sedimentation Rate; FA - food allergies; HES- hypereosinophilic syndrome; Hgb - hemoglobin.
Holm’s adjusted p-value <0.05.
Endoscopic features are similar in Multisystem HES and HES/EGID
The endoscopic manifestations of EoE, EG, EE or EC were similar in patients with HES/EGID and Multisystem HES and included esophageal furrows, rings, and plaques, mucosal erythema and ulceration (Table 4).
Table 4.
Endoscopic Findings
| Endoscopic Findings | HES/EGID Overlap (N = 26) | Multisystem HES (N = 15) | P-values |
|---|---|---|---|
| Esophageal Furrows | 3 (11.5%) | 3 (20.0%) | p=0.65 |
| Esophageal Rings | 4 (15.4%) | 2 (13.3%) | p=1.00 |
| Esophageal Plaques | 0 (0%) | 1 (6.7%) | p=0.37 |
| Mucosal Erythema | 17 (65.4%) | 10 (66.7%) | p=1.00 |
| Ulceration | 7 (26.9%) | 5 (33.3%) | p=0.73 |
| Nodularity | 2 (7.7%) | 1 (6.7%) | p=0.73 |
| Polyps | 2 (7.7%) | 0 | p=0.72 |
| Active Bleeding | 0 | 1 (6.7%) | p=0.74 |
EGID- eosinophilic gastrointestinal disease. HES- hypereosinophilic syndrome.
Multi-segment GI evaluation and eosinophilia are common among HES patients
Among the 56 patients in the study, 43 (77%) had at least one esophageal biopsy and 51 (91%) had at least one gastric biopsy. The small bowel was sampled by targeting either duodenum
(48/56; 86%) and/or jejunum (9/56; 16%) by upper endoscopy, or the terminal ileum by colonoscopy (25/ 56; 45%). At least one part of the colon was biopsied and reviewed in 41/56 (73%) of patients and 17/56 (30%) patients had a rectal biopsy. In total, 30/56 (54%) of our patient cohort had biopsies representative of all four anatomic regions: esophagus, stomach, small bowel and colon (Supplemental Figure E1, sum of bar #1, #3, #11).
Inclusion in this study required meeting tissue eosinophil thresholds in one GI segment only. However, when biopsied, many patients also demonstrated tissue eosinophilia in additional GI segments (Supplemental Figure E2). A majority of patients (53.6%; 30/56) had sampling of all four anatomic regions (esophagus, stomach, small bowel and colon or rectum), and of those 67% (20/30) had multi-segment eosinophilia with tissue eosinophilia above the respective cutoffs in all four segments in 17% (5/30). Isolated eosinophilic colitis was found in 6 patients (20%) and isolated small bowel eosinophilia in 5 patients (17%) (Figure 2A). To address whether extent of tissue eosinophilia (eg. Number of positive segments) was correlated with peripheral blood eosinophilia, we examined the cohort of 30 individuals who each had 4 GI segments tested. We looked for a correlation between the number out of 4 segments that are positive and the peak AEC, and we found Spearman correlation was not significantly different from zero (r=0.164, 95% CI −0.208, 0.495, p=0.39).
There did not appear to be a predilection for eosinophilic involvement of the upper or lower GI tracts between the HES/EGID overlap and Multisystem HES groups (Figure 2B). In fact, eosinophilic involvement was strikingly similar with upper GI (esophageal or gastric/small bowel) eosinophilia only in 56% of HES/EGID Overlap as compared with 58% of Multisystem HES patients, lower GI (colonic/rectal) eosinophilia only in 21% vs 29% and both upper and lower GI eosinophilia in 42% vs 43%.
Figure 2B.
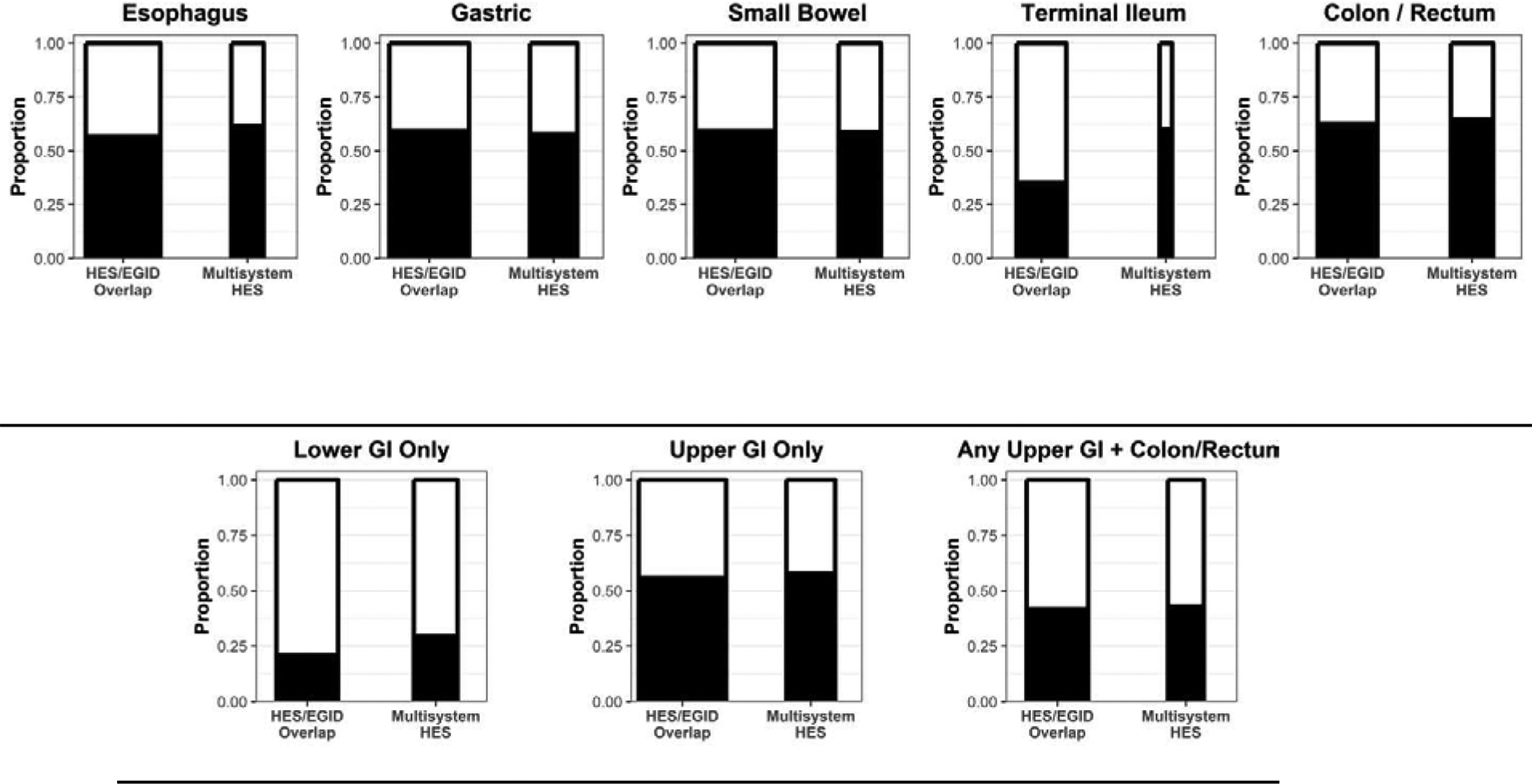
The height of the bar graphs indicates proportion of patients who had biopsies that met the inclusion cutoff in the indicated anatomic region (black) in each of the two cohorts (HES/EGID vs. Multisystem HES). The width of the bar is correlated with the number of patients who had biopsies in the indicated region. These groups are not mutually exclusive, ie. patients may be part of multiple groups. The categories “Lower GI only” and “Upper GI only” refer to observed positive biopsies in the respective region, meaning the denominator for the proportions includes patients that were not biopsied in the other region (i.e., not biopsied is interpreted as negative).
Multisystem HES patients were more likely to be treated with systemic therapies
Prior therapies were identified by chart review and included glucocorticoids (topical or systemic), dietary therapies (elemental, single or multi-food elimination regimens), and glucocorticoid-sparing agents that included biologics and experimental therapies.
HES/EGID overlap patients were more likely to have attempted some form of dietary therapy (all three types) than Multisystem HES patients (Table 5). Multisystem HES patients were more likely to have attempted glucocorticoid therapy of any kind (100% vs 79%, p=0.0349), although HES/EGID overlap patients showed a trend towards increased use of topical glucocorticoid treatment. Multisystem HES patients were also more likely to have tried non-glucocorticoid drug therapies (77% vs. 38%, p=0.0061).
Table 5 –
Therapeutic Interventions for HES/EGID
| THERAPIES | HES/EGID Overlap (n=34) | Multisystem HES (n=22) | Unadjusted p-value |
|---|---|---|---|
| Any Dietary Treatment1 (n; % of group) | 22 (33; 67%) | 5 (22; 23%) | p=0.0022* Holm’s adjusted p=0.022 |
| Elemental | 10 (33; 30%) | 1 (22; 4.5%) | p=0.0356 |
| Any Empiric- FED2 | 11 (33; 33%) | 1 (22; 4.5%) | p=0.0175 |
| Custom-FED3 | 14 (33; 42%) | 3 (22; 14%) | p=0.0364 |
| Any Steroid Treatment (% of group) | 27 (79%) | 22 (100%) | P=0.0349 |
| Topical | 22 (65%) | 8 (36%) | p=0.0554 |
| Systemic | 23 (68%) | 22 (100%) | P=0.0039* Holm’s adjusted p=0.036 |
| PPI (current or historic) | 18 (53%) | 7 (32%) | p=0.17 |
| Mast Cell | 6 (18%) | 2 (9%) | p=0.46 |
| Other Therapies4 | 13 (38%) | 17 (77%) | P=0.0061* Holm’s adjusted p=0.048 |
| hydroxychloroquine (4) 6-mercaptopurine (1), azathioprine (3), methotrexate (1), sirolimus (3), mycophenolate (1) metronidazole/albendazole,benralizumab (5), hydroxyurea (2), interferon-alpha (1), omalizumab(1), imatinib,mepolizumab (iv dosing) (1), dexpramipexole (1) | azathioprine (1), methotrexate (2), cyclophosphamide (1), interferon-alpha (3), hydroxyurea (6). cyclosporine(1) rituximab (1), mycophenolate(3), imatinib (4), dasatinib high dose mepolizumab (6, varied doses), benralizumab dexpramipexole (3) |
Individual patients may have tried multiple types of dietary therapies, glucocorticoid formulations and other systemic therapies.
Any empiric-FED includes dietary interventions where one to six or more foods chosen empirically were eliminated.
Custom-FED are defined as diets where the foods eliminated were chosen based on allergy testing.
Treatment with mepolizumab (mostly high dose), sirolimus, benralizumab and dexpramipexole was performed in the setting of clinical interventional trials.
Holm’s adjusted p-value <0.05
EGID- eosinophilic gastrointestinal disease. HES- hypereosinophilic syndrome; FED – food elimination diet; PPI – proton pump inhibitor
Among the 22 Multisystem HES patients who had been treated at some point with systemic glucocorticoid therapy, only 8 (36%) also reported use of topical glucocorticoids (Supplemental Figure E3A). This is in contrast to HES/EGID patients who had been treated with systemic glucocorticoids (n=23), of whom 18 (78%) had been treated with topical therapies (Supplemental Figure E3B, Fisher’s exact p=0.0067). Anecdotally, most patients in both groups reported a response to glucocorticoid therapy (data not shown). One notable exception was a patient with HES/EGID overlap who was a non-responder to systemic glucocorticoids and later discovered to have a PDGFRA-associated myeloid neoplasm and responded, as expected, to imatinib treatment. Use of therapies specifically targeting mast cells was uncommon in both groups.
Multisystem HES patients can present with single organ EGID at onset
A common clinical concern is whether HES/EGID overlap patients go on to develop extra-intestinal EGID or other organ system involvement. In our cohort of 22 Multisystem HES patients with GI manifestations, the initial clinical presentation consisted solely of gastrointestinal symptoms in 36% (8/22) (Table 6). These 8 patients later developed extra-intestinal organ manifestations at a median of 1 year (range 0.25 –15 years) after initial presentation. The remainder of the cohort described of non-GI symptoms or GI symptoms in conjunction with other end organ manifestations at their initial presentation (n=14). Regardless of the initial presentation (GI alone vs. non-GI symptoms or multi-system), the types of end-organ manifestations were heterogeneous and similar between the two groups (Table 6).
Table 6 –
End-Organ Manifestations in Multisystem HES patients
| Eventual End-Organ Manifestations | Initial Presentation | |
|---|---|---|
| Pure GI symptoms (n=8) | Multisystem or non-GI symptom presentation (n=14) | |
| Sclerosing Cholangitis | 1 | 1 |
| Cholangitis | 1 | 0 |
| Pancreatitis | 0 | 2 |
| Fever/Chills | 1 | 2 |
| Fatigue | 2 | 1 |
| Sinusitis | 1 | 3 |
| Nasal Polyps | 0 | 4 |
| Oral Ulcers | 0 | 1 |
| DVT/PE, vascular clots | 1 | 2 |
| Splenic Infarct | 1 | 0 |
| Angioedema | 1 | 3 |
| Rash, undefined | 2 | 4 |
| Respiratory Symptoms | 4 | 5 |
| Pulmonary infiltrates | 0 | 3 |
| Arthralgias | 1 | 2 |
| Myalgias/Myositis | 0 | 1 |
| Other | Neuropathy (2) | Cystitis (1) |
Multisystem HES patients are divided into two groups: those whose initial clinical presentation consisted solely of gastrointestinal (GI) symptoms, versus those with presented with non-GI symptoms, or GI symptoms concurrent with other end-organ manifestations. The ultimate end-organ manifestations of patients in each group are presented. Median time to first non-GI presentation is 1 year (range: 0.25 – 15 years) in those who initially presented with only GI symptoms.
Discussion
Prior studies have described an association between the manifestations of EGID and the presence of peripheral eosinophilia in the range of 500–600 cells/mm3 (15–17). Other studies either do not report eosinophilia(4,18,19) or report peripheral eosinophilia without assessing the relationship to disease manifestations(13,20–23). Moreover, several studies explicitly exclude HES, EGPA, or those with “secondary causes of eosinophilia” (16,19,24,25). One retrospective study compared the characteristics of EGID patients with HE (n=15) to those without (n=24) but excluded patients with a diagnosis of HES (which presumably excluded those with multi-organ involvement), and found an association with atopy and a greater extent of GI segmental involvement (25).
To our knowledge, our retrospective study is the first to directly examine all HES patients with gastrointestinal manifestations and compare those with HES/EGID overlap to those with multi-organ manifestations. Due to the retrospective design and small sample size, the evaluation prior to treatment was not uniform across patients and resulted in missing data and variability in the assessment of treatment responses. Additionally, patients with subserosal or muscular involvement were excluded by the requirement for certain levels of eosinophils in mucosal biopsies.
The overall clinical presentation of HES patients with GI organ involvement in the present study is consistent with data from previous studies (4,24), and found that EGID patients, excluding those with solitary EoE, have no male gender predilection, but do have significant atopic comorbidities and a broad range of gastrointestinal symptoms and multi-segment eosinophilic involvement in the gastrointestinal tract. The most common gastrointestinal symptoms were abdominal pain, nausea with or without vomiting and diarrhea, which were similar to previous reports. We found a significantly higher percentage of asthma, allergic rhinitis or drug and venom allergies (50%, 65%, and 26% respectively) as compared to the largest series reported to date (25%, 23%, and 11% respectively)(4), but no difference in reported atopic dermatitis and IgE-mediated food allergy. These differences may be explained by the fact that subspecialists in allergy/immunology evaluated the patients rather than gastroenterologists, although the adult predominance in this cohort and/or enrichment of these conditions in patients with AEC ≥1500/mm3 cannot entirely be excluded.
Multi-segment eosinophilia has been described in several case series (20% to 88% of cases) and in the recent multicenter CEGIR cohort (40%). However, in all but one Malaysian study(13), it is unclear whether patients underwent evaluation of both upper and lower gastrointestinal segments. In the present study, the majority of patients (53.6%; 30/56) had diagnostic biopsies taken throughout the GI tract and 67% (20/30) had evidence of multi-segment involvement, as compared to 14% (9/64) in the Malaysian cohort (Fisher’s exact p<0.001). In our study, 5 patients had eosinophilic involvement throughout the entire gastrointestinal tract (esophagus, stomach, small bowel and large colon). These 5 patients represent 9% of the entire study cohort and 17% of the 30 patients who had biopsies spanning all segments, in contrast to 1% of patients with involvement of all segments reported in the CEGIR cohort(4) and to the 0% (0/64) in the Malaysian cohort(13). This may reflect more broad tissue involvement in HES patients and/or more biopsies taken, but patchy involvement may also play a role. Ultimately, the selective involvement of distinct segments suggests that, similar to EoE, tissue involvement is determined, at least in part, by as yet undetermined local epithelial factors rather than a non-specific increase in tissue eosinophilia in the setting of hypereosinophilia.
With respect to demographics, symptoms, clinical presentation, complications, and segment involvement, patients with EGID/HES were more similar to those with Multisystem HES than expected. A recent study described elevated serum tryptase > 11 ng/mL as a marker of extra-intestinal disease in EoE (14). This finding could not be confirmed in our study due to the fact that only 5 subjects had tryptase levels ≥11 ng/mL. In both HES/EGID and Multisystem HES patients, symptoms referable to the involved segment were often lacking, such that patients with colitis may have only complained of abdominal pain and bloating, and some patients with diarrhea were found to have only isolated small bowel involvement. As such, screening patients for both upper and lower gastrointestinal involvement, especially if symptoms persist despite treatment, should be considered in patients with EGID and peripheral hypereosinophilia. Surprisingly, 36% (8/22) of the Multisystem HES patients presented with only gastrointestinal symptoms, for a range of 6 months to 15 years prior to the development of other organ-system involvement. This finding suggests that periodic clinical monitoring and assessment for other organ involvement may be warranted in patients with EGID and peripheral hypereosinophilia. No obvious predictors of progression were identified in this small retrospective study.
Despite similarities in clinical signs and symptoms, therapeutic approaches to treatment of EGID were different between the two groups. Patients with single-organ HES/EGID overlap were more often treated with dietary therapies, and topical glucocorticoids. In contrast, in Multisystem HES, systemic glucocorticoids and glucocorticoid-sparing therapies were more often initiated without a prior trial or concomitant use of topical glucocorticoid therapy, in contrast to the approach to other atopic conditions such as asthma or atopic dermatitis. Whether this approach reflects the bias of the treating physician or is in fact warranted is not known.
This study highlights the striking similarities between EGID patients with peripheral hypereosinophilia, irrespective of the presence of multi-organ involvement. Although rare in our cohort, the observation that EGID with peripheral hypereosinophilia may progress into Multisystem HES has not been previously reported, likely because in other studies such patients were excluded a priori. These finding should be confirmed in larger prospective and retrospective cohorts that do not exclude peripheral eosinophilia or “HES”, as they have important implications for follow-up diagnostic testing in this patient group.
Supplementary Material
Highlights Box:
-
What is already known about this topic?
Very little is currently known about the differences between patients with EGID and hypereosinophilia (HES/EGID overlap) and those with EGID in the setting of Multisystem HES.
-
What does this article add to our knowledge?
Patients with eosinophilic gastrointestinal disease and hypereosinophilia share common characteristics irrespective of single or multi-system involvement. Patients with Multisystem HES may present with isolated GI involvement prior to development of other organ involvement.
-
How does this study impact current management guidelines?
There are no current standardized diagnostic, prognostic or treatment guidelines for patients with hypereosinophilia with GI involvement other than expert opinion. This article adds to the collective knowledge of this rare patient population.
Acknowledgments
We would like to thank the NIH Clinical Center staff and the research subjects for their participation.
Funding: This work was supported in part by the Division of Intramural Research at NIAID and NIDDK. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations.
- AEC
absolute eosinophil count
- ANCA
anti-neutrophil cytoplasmic antibody
- EE
eosinophilic enteritis
- EG
Eosinophilic gastritis
- EGE
Eosinophilic gastroenteritis
- EGID
eosinophilic gastrointestinal disorder
- EGPA
eosinophilic granulomatosis with polyangiitis
- EoE
eosinophilic esophagitis
- EOS
eosinophils
- EC
Eosinophilic colitis
- CEGIR
Consortium of Eosinophilic Gastrointestinal Researchers
- HE
Hypereosinophilia
- HES
hypereosinophilic syndromes
- HPF
High powered field
- ICD
International Classification of Diseases
- GI
gastrointestinal
- NIH
National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors declare they have no conflicts of interest to disclose.
References
- 1.Klion AD. How I treat hypereosinophilic syndromes. Blood. 2015. August 27;126(9):1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klion AD, Bochner BS, Gleich GJ, Nutman TB, Rothenberg ME, Simon H-U, et al. Approaches to the treatment of hypereosinophilic syndromes: a workshop summary report. J Allergy Clin Immunol. 2006. June;117(6):1292–302. [DOI] [PubMed] [Google Scholar]
- 3.Kahn JE, Groh M, Lefèvre G. (A critical appraisal of) classification of hypereosinophilic disorders. Front Med (Lausanne). 2017. December 5;4:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pesek RD, Reed CC, Muir AB, Fulkerson PC, Menard-Katcher C, Falk GW, et al. Increasing Rates of Diagnosis, Substantial Co-Occurrence, and Variable Treatment Patterns of Eosinophilic Gastritis, Gastroenteritis, and Colitis Based on 10-Year Data Across a Multicenter Consortium. Am J Gastroenterol. 2019. April 16; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins MH, Capocelli K, Yang G-Y. Eosinophilic gastrointestinal disorders pathology. Front Med (Lausanne). 2017;4:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoury P, Akuthota P, Ackerman SJ, Arron JR, Bochner BS, Collins MH, et al. Revisiting the NIH Taskforce on the Research needs of Eosinophil-Associated Diseases (RE-TREAD). J Leukoc Biol. 2018. April 19;104(1):69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta SK, Falk GW, Aceves SS, Chehade M, Collins MH, Dellon ES, et al. Consortium of eosinophilic gastrointestinal disease researchers: advancing the field of eosinophilic GI disorders through collaboration. Gastroenterology. 2019;156(4):838–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoepfer AM, Simko A, Bussmann C, Safroneeva E, Zwahlen M, Greuter T, et al. Eosinophilic esophagitis: relationship of subepithelial eosinophilic inflammation with epithelial histology, endoscopy, blood eosinophils, and symptoms. Am J Gastroenterol. 2018. March;113(3):348–57. [DOI] [PubMed] [Google Scholar]
- 9.Lwin T, Melton SD, Genta RM. Eosinophilic gastritis: histopathological characterization and quantification of the normal gastric eosinophil content. Mod Pathol. 2011. April;24(4):556–63. [DOI] [PubMed] [Google Scholar]
- 10.DeBrosse CW, Case JW, Putnam PE, Collins MH, Rothenberg ME. Quantity and distribution of eosinophils in the gastrointestinal tract of children. Pediatr Dev Pathol. 2006. June;9(3):210–8. [DOI] [PubMed] [Google Scholar]
- 11.Conway JR, Lex A, Gehlenborg N. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics. 2017. September 15;33(18):2938–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright SP. Adjusted p-values for simultaneous inference. Biometrics. 1992. p. 1005–13. [Google Scholar]
- 13.Hui CK, Hui NK. A Prospective Study on the Prevalence, Extent of Disease and Outcome of Eosinophilic Gastroenteritis in Patients Presenting with Lower Abdominal Symptoms. Gut Liver. 2017. December 8;12(3):288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutty GR, Downs-Kelly E, Crispin HT, Peterson KA. Elevated Tryptase in EoE Is an Independent Phenomenon Associated with Extra-Esophageal Symptoms. Dig Dis Sci. 2018. September 28; [DOI] [PubMed] [Google Scholar]
- 15.Kinoshita Y, Furuta K, Ishimaura N, Ishihara S, Sato S, Maruyama R, et al. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. J Gastroenterol. 2013. March;48(3):333–9. [DOI] [PubMed] [Google Scholar]
- 16.Choi JS, Choi SJ, Lee KJ, Kim A, Yoo JK, Yang HR, et al. Clinical manifestations and treatment outcomes of eosinophilic gastroenteritis in children. Pediatr Gastroenterol Hepatol Nutr. 2015. Dec 23;18(4):253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alfadda AA, Shaffer EA, Urbanski SJ, Storr MA. Eosinophilic colitis is a sporadic self-limited disease of middle-aged people: a population-based study. Colorectal Dis. 2014. February;16(2):123–9. [DOI] [PubMed] [Google Scholar]
- 18.Mansoor E, Saleh MA, Cooper GS. Prevalence of Eosinophilic Gastroenteritis and Colitis in a Population-Based Study, From 2012 to 2017. Clin Gastroenterol Hepatol. 2017. November;15(11):1733–41. [DOI] [PubMed] [Google Scholar]
- 19.Gaertner WB, Macdonald JE, Kwaan MR, Shepela C, Madoff R, Jessurun J, et al. Eosinophilic colitis: university of Minnesota experience and literature review. Gastroenterol Res Pract. 2011. July 10;2011:857508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed C, Woosley JT, Dellon ES. Clinical characteristics, treatment outcomes, and resource utilization in children and adults with eosinophilic gastroenteritis. Dig Liver Dis. 2015. March;47(3):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caldwell JM, Collins MH, Stucke EM, Putnam PE, Franciosi JP, Kushner JP, et al. Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome. J Allergy Clin Immunol. 2014. November;134(5):1114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang JY, Choung RS, Lee RM, Locke GR, Schleck CD, Zinsmeister AR, et al. A shift in the clinical spectrum of eosinophilic gastroenteritis toward the mucosal disease type. Clin Gastroenterol Hepatol. 2010. August;8(8):669–75; quiz e88. [DOI] [PubMed] [Google Scholar]
- 23.Wong GW, Lim KH, Wan WK, Low SC, Kong SC. Eosinophilic gastroenteritis: Clinical profiles and treatment outcomes, a retrospective study of 18 adult patients in a Singapore Tertiary Hospital. Med J Malaysia. 2015. August;70(4):232–7. [PubMed] [Google Scholar]
- 24.Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990. January;31(1):54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, Dierkhising R, Wu T-T, Alexander J, Weiler C. Eosinophilic gastrointestinal disorders (EGID) with peripheral eosinophilia: a retrospective review at Mayo Clinic. Dig Dis Sci. 2011. November;56(11):3254–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


